Abstract
The repressive activity of ancestral histone-like proteins helps integrate transcription of foreign genes with discrepant AT content into existing regulatory networks. Our investigations indicate that the AT-rich discriminator region located between the −10 promoter element and the transcription start site of the regulatory gene ssrA plays a distinct role in the balanced expression of the Salmonella pathogenicity island-2 (SPI2) type III secretion system. The RNA polymerase-binding protein DksA activates the ssrAB regulon post-transcriptionally, whereas the alarmone guanosine tetraphosphate (ppGpp) relieves the negative regulation imposed by the AT-rich ssrA discriminator region. An increase in the GC-content of the ssrA discriminator region enhances ssrAB transcription and SsrB translation, thus activating the expression of downstream SPI2 genes. A Salmonella strain expressing a GC-rich ssrA discriminator region is attenuated in mice and grows poorly intracellularly. The combined actions of ppGpp and DksA on SPI2 expression enable Salmonella to grow intracellularly, and cause disease in a murine model of infection. Collectively, these findings indicate that (p)ppGpp relieves the negative regulation associated with the AT-rich discriminator region in the promoter of the horizontally-acquired ssrA gene, whereas DksA activates ssrB gene expression post-transcriptionally. The combined effects of (p)ppGpp and DksA on the ssrAB locus facilitate a balanced SPI2 virulence gene transcription that is essential for Salmonella pathogenesis.
Introduction
Nontyphoidal Salmonella enterica serovar Typhimurium is a common cause of gastroenteritis in immunocompetent individuals and a life-threatening disseminated complication in immunocompromised hosts unable to mount CD4+ T cell immunity or IFNγ host responses1,2. This intracellular pathogen replicates within Salmonella-containing vacuoles (SCV) of epithelial and phagocytic cells in part due to the activity of a type III secretion system that is encoded within the horizontally-acquired Salmonella pathogenicity island-2 (SPI2)3–5. Effector proteins translocated through the SPI2 type III secretion system minimize contact of SCV with lysosomes and cell host vesicles harboring NADPH phagocyte oxidase or inducible nitric oxide synthase (iNOS)6–10. By redirecting SCVs to the trans-Golgi network and exocytic pathway, the SPI2 type III secretion system also aids Salmonella in overcoming the nutritional restrictions found in vesicles of the degradative pathway11,12.
Salmonella initiate SPI2 gene transcription as the transforming SCV microenvironment acidifies and becomes limiting for iron and other divalent cations13–16. These signals activate the EnvZ and PhoQ sensor kinases, which catalyze phosphotransfer reactions to their cognate response regulators OmpR and PhoP, respectively17,18. PhoP competes with histone-like proteins for binding to the ssrA promoter, counter-silencing the repressive activity of these nucleoid-structuring proteins19,20. The sensor kinase encoded by the ssrA gene senses acidification via several histidine residues in the periplasmic domain21. Activated SsrA phosphorylates its cognate SsrB response regulator, which in turn recruits the RNA polymerase to SPI2 genes encoding components of the secretion apparatus, chaperones, and effectors5,22. The negative regulation of SPI2 genes is also an important aspect in Salmonella pathogenesis. For example, EIIANtr, which prevents binding of SsrB to promoters of SPI2 target genes, is required for Salmonella virulence23. Also, the inactivation of SsrB via oxidation or S-nitrosation of Cys203 contributes to Salmonella virulence24. Moreover, binding of the histone-like proteins H-NS and YdgT to AT-rich SPI2 genes represses SPI2 transcription during non-inducing conditions25,26. The absence of these histone-like proteins attenuates Salmonella in spite of SPI2 overexpression23,24,26.
The AT-rich composition of the discriminator region, which is located between the −10 element and transcriptional start site, could be an additional repressive element to transcription of horizontally-acquired genes. Promoters with AT-rich discriminator regions often produce stable, long-lived, open complexes that become saturated with RNA polymerase, aborting initiation of transcription27,28. The stringent response is controlled by the RNA polymerase-binding protein DksA and the nucleotide alarmones guanosine tetra/pentaphosphate [(p)ppGpp] that are synthesized in Salmonella by the RelA and SpoT proteins. DksA binds to the secondary channel of the RNA polymerase, whereas two molecules of (p)ppGpp bind between the ω and β’ subunits and at the interface of RNA polymerase and DksA29,30. DksA and (p)ppGpp exert transcriptional regulation by reducing the half-life of RNA polymerase-DNA open complexes27,28,31,32. The stringent response generally activates or represses gene transcription from promoters with AT- or GC-rich discriminator regions, respectively27,28,31,32. The preservation of AT-rich discriminator regions in horizontally-acquired genes suggests that the negative control associated with AT-rich discriminators provides a selective advantage to bacterial pathogens.
Microarrays and differential RNA sequencing indicate that the stringent response regulators DksA and (p)ppGpp are required for the activation of SPI2 gene transcription33–38. However, the mechanism by which DksA and (p)ppGpp regulate expression of the SPI2 virulence program remains unknown. Herein, we tested the hypothesis that the stringent response regulators DksA and (p)ppGpp contribute to Salmonella virulence by relieving the negative regulation imposed by the AT-rich discriminator region of the ssrAB locus encoding the SPI2 master two-component regulatory system.
Results
DksA and (p)ppGpp promote intracellular replication of Salmonella
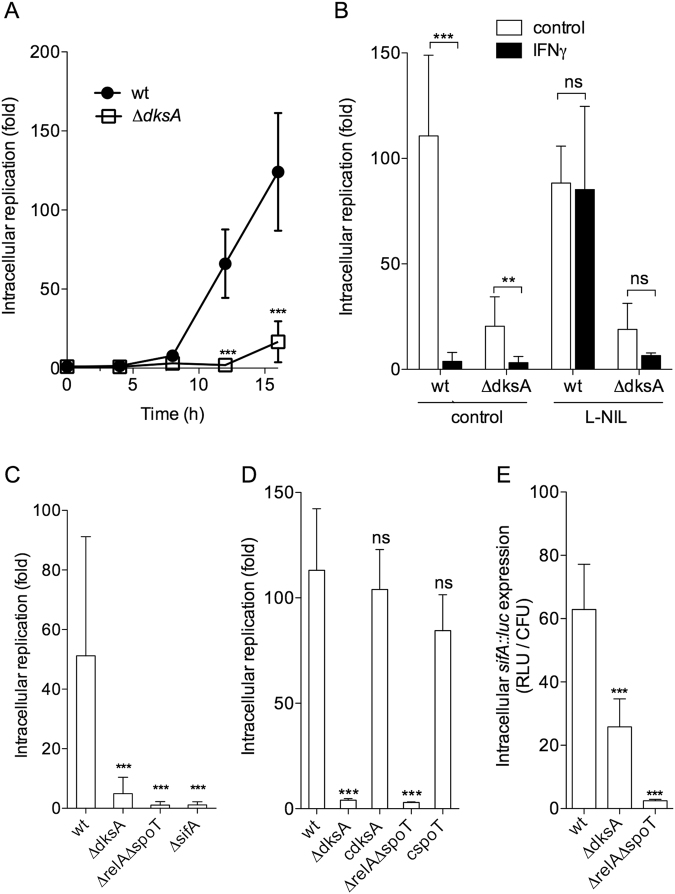
By regulating the expression of gene products that maintain NADPH/NADP+ and GSH/GSSG redox homeostasis, DksA and (p)ppGpp protect Salmonella against the antimicrobial activity associated with NADPH phagocyte oxidase and iNOS hemoproteins39–43. To examine whether DksA and (p)ppGpp play additional roles during the intracellular growth of Salmonella, we measured the replication of ΔdksA and ΔrelA ΔspoT Salmonella in J774A.1 macrophage-like cells. Wild-type Salmonella began to replicate 8 h post-infection, reaching over 100-fold higher bacterial burdens 16 h after the initial infection (Fig. 1A). In contrast, ΔdksA Salmonella grew poorly (Fig. 1A). Under the experimental conditions tested, our J774A.1 cells do not generate detectable amounts of reactive oxygen species in response to Salmonella43. These findings suggest that the growth defect of ΔdksA Salmonella in this population of J774A.1 cells cannot be attributed to its reported hypersusceptibility to oxidative stress39–43. Treatment of J774A.1 cells with IFNγ arrested growth of both wild-type and ΔdksA Salmonella (Fig. 1B). Wild-type bacteria, but not the ΔdksA mutant, grew in IFNγ-activated macrophages treated with the iNOS specific inhibitor N-iminoethyl-L-lysine (L-NIL) (Fig. 1B). As expected, L-NIL inhibited NO synthesis (Fig. S1A). Together, these investigations suggest that DksA can aid in the intracellular replication of Salmonella independently of its promotion of antioxidant and antinitrosative defenses.
Figure 1.
Contributions of DksA and (p)ppGpp to the ability of Salmonella to replicate intracellularly. Intracellular replication of wild-type (wt) and mutant Salmonella was quantified over time (A) or 18 h post-infection (B–D) in J774A.1 macrophage-like cells. Where indicated, J774A.1 cells were stimulated with 200 U/ml IFNγ 24 h prior to infection, or treated since the time of infection with 960 μM of the selective iNOS inhibitor L-NIL. Intracellular expression of sifA::luc 8 h after J774A.1 cells were infected with Salmonella (E). Non-significant (ns), *p < 0.05, **p < 0.01, or ***p < 0.001 compared to wild-type controls. The data represent the mean ± S.D. from 3–19 biological replicates.
Since DksA and (p)ppGpp often coregulate the RNA polymerase, we also tested the intracellular growth of a ΔrelA ΔspoT strain. A ΔrelA ΔspoT Salmonella strain exhibited profound intracellular growth defects (Fig. 1C). The failure of ΔdksA and ΔrelA ΔspoT Salmonella strains to replicate intracellularly resembles phenotypes reported for strains deficient in SPI2 genes3–5. Accordingly, an isogenic strain lacking the SPI2 effector sifA, whose product is necessary for maintaining integrity of the SCV44, grew as poorly in J774A.1 cells as ΔdksA and ΔrelA ΔspoT Salmonella controls (Fig. 1C). The growth defect of ΔdksA and ΔrelA ΔspoT Salmonella could be complemented by dksA and spoT alleles expressed in the chromosome (Fig. 1D). We next tested whether the stringent response regulators DksA and (p)ppGpp contribute to the intracellular expression of sifA. Compared to wild-type controls, both ΔdksA and ΔrelA ΔspoT Salmonella expressed low levels of the SPI2 effector sifA in J774A.1 macrophage-like cells (Fig. 1E). As shown previously33–35,37,38, wild-type Salmonella grown for 3 h in 8 μM MgCl2 N9 medium expressed all SPI2 promoters tested; however, ΔdksA or ΔrelA ΔspoT Salmonella did not stimulate expression of any SPI2 genes examined (Fig. S1B).
Collectively, these observations raise the possibility that the stringent response regulators DksA and (p)ppGpp help Salmonella grow in macrophages by controlling the expression of the SPI2 type III secretion system.
Contributions of DksA, (p)ppGpp, and SsrB to Salmonella pathogenesis
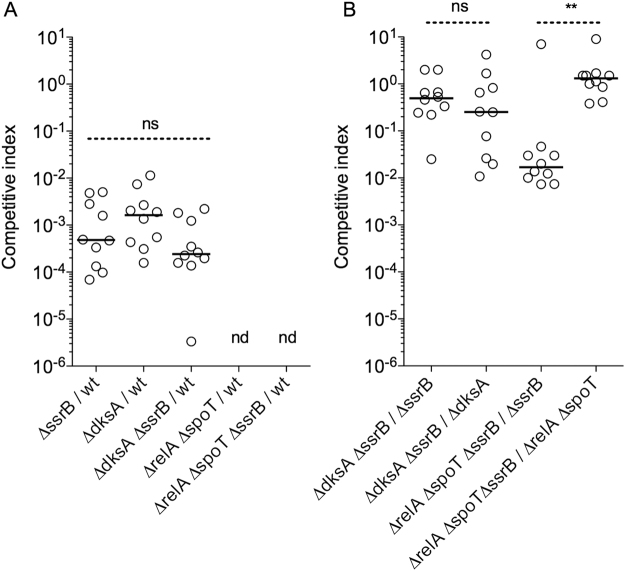
Since DksA and (p)ppGpp play broad roles in gene transcription33,38,39,45,46, we deemed it important to quantify the extent that these stringent response regulators rely on the SPI2 type III secretion system to promote Salmonella pathogenesis. When compared to wild-type controls, the number of ΔssrB, ΔdksA, or ΔdksA ΔssrB Salmonella was about 1,000-fold lower in spleens (Fig. 2A) and livers (Fig. S2A) of C57BL/6 mice. Strains unable to generate (p)ppGpp were more attenuated than ΔssrB or ΔdksA Salmonella, as demonstrated by their complete elimination from spleens and livers 3 days after intraperitoneal inoculation (Figs 2A and S2A). To determine fitness of ΔrelA ΔspoT Salmonella, C57BL/6 mice were inoculated with 105 CFU of each ΔrelA ΔspoT and ΔrelA ΔspoT ΔssrB Salmonella. The ΔrelA ΔspoT ΔssrB mutant had a competitive index of ~1 when compared to ΔrelA ΔspoT Salmonella, but showed a 100-fold lower competitive index than ΔssrB Salmonella (Figs 2B and S2B). These data suggest that (p)ppGpp can participate in Salmonella virulence in SPI2-dependent and -independent ways. To better calculate the apparent codependency of SsrB and DksA, we used the method described by Beuzon and Holden to quantify virulence gene interactions in vivo44,47. Groups of C57BL/6 mice were inoculated with 105 CFU of ΔssrB ΔdksA in combination with ΔssrB or ΔdksA Salmonella. The ΔdksA ΔssrB double mutant strain was isolated from spleen and liver tissue in similar numbers to ΔssrB or ΔdksA single mutants (Figs 2B and S2B), suggesting that the role played by this RNA polymerase-binding protein in Salmonella pathogenesis appears to be strongly co-dependent on the SPI2 master regulator SsrB.
Figure 2.
Codependence of SsrB, ppGpp, and DksA in Salmonella pathogenesis. Competitive indexes of Salmonella strains recovered from spleens of C57BL/6 mice 3 days after infection. Mice were inoculated intraperitoneally with 102 (A) or 105 (B) CFU of the indicated Salmonella strains. No detectable (nd) CFU were isolated for the ΔrelA ΔspoT strain under the experimental conditions used in panel A. Competitive index was determined according to the equation: (strain 1/strain 2)output/(strain 1/strain 2)input. Non-significant (ns), **p < 0.01.
Requirement of DksA and (p)ppGpp for the activation of ssrAB transcription
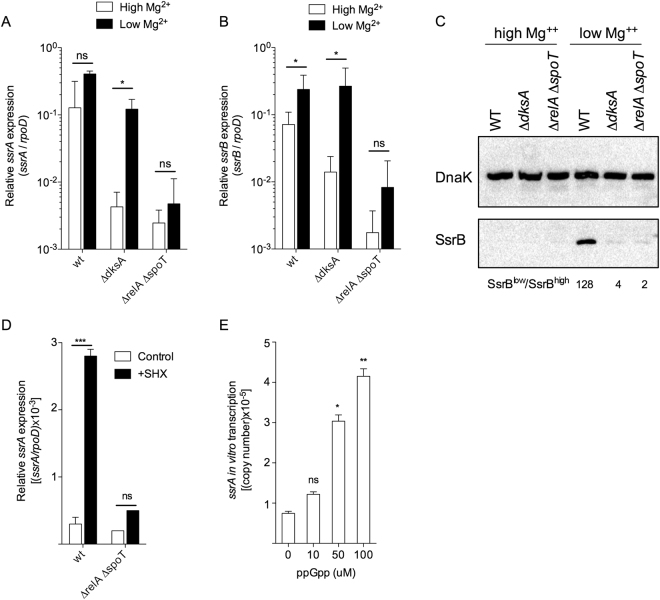
We examined whether DksA and (p)ppGpp participate in the transcriptional activation of the ssrA and ssrB genes that encode the master two-component regulatory system that activates SPI2 expression. Wild-type Salmonella up-regulated the expression of ssrA (Fig. 3A) and ssrB mRNA (Fig. 3B) 3 h after culture in 8 μM MgCl2 N9 medium. We also observed that ΔdksA Salmonella induced excellent ssrA and ssrB expression upon culture in 8 μM MgCl2 N9 medium (Fig. 3A,B). These findings indicate that DksA does not appear to regulate ssrA or ssrB gene transcription. Since ΔdksA Salmonella induced ssrB expression but failed to globally activate SPI2 transcription, Western blotting was used to visualize the amount of SsrB protein in wild-type and ΔdksA Salmonella. Wild-type Salmonella harbored low concentrations of SsrB protein when grown under non-inducing 10 mM MgCl2 N9 medium, but harbored high concentrations of this response regulator 3 h after growth in 8 μM MgCl2 N9 medium (Fig. 3C). Compared to wild-type controls, ΔdksA Salmonella expressed much lower concentrations of SsrB protein upon culture in 8 μM MgCl2 N9 medium. Expression of a dksA allele reestablished production of SsrB protein in ΔdksA Salmonella (Fig. S3A). In view of the abundant ssrB mRNA seen in ΔdksA Salmonella, deficient production of SsrB protein indicates that DksA may regulate the expression of this response regulator post-transcriptionally.
Figure 3.
The alarmone ppGpp directly activates ssrA gene transcription. The abundance of ssrA (A) and ssrB (B) mRNA was quantified by qRT-PCR in Salmonella grown for 3 h in 8 μM (low) or 10 mM (high) MgCl2 N9 media. The data, which are from 4 biological replicates and are plotted as the mean ± S.D., represent transcripts levels normalized to the housekeeping gene rpoD. Western blot of SsrB-FLAG in Salmonella grown for 3 h in high or low MgCl2 N9 medium (C). The blot is representative of 4 biological replicates. The abundance of the DnaK internal control was measured for comparison. The relative amounts of SsrB protein were compared between Salmonella grown in low and high Mg++ (SsrBlow/SsrBhigh). The amount of ssrA mRNA induced by serine hydroxamate (SHX) was quantified by qRT-PCR in Salmonella grown to log-phase in M9 minimal medium (D). Data represent mean ± S.D. transcripts levels normalized to the housekeeping gene rpoD. Effects of increasing ppGpp concentrations on PssrA in vitro transcription using the pTIM-ssrA template (E). The data, which are plotted as the mean ± S.D., represent absolute copy number determined by qRT-PCR from 6–8 biological replicates. Non-significant (ns), *p < 0.05, **p < 0.01, or ***p < 0.001 as compared to high Mg2+ (A,B) or untreated (D,E) controls. An independent, uncropped, blot of panel C can be seen in Fig. S6A.
Compared to ΔdksA Salmonella and wild-type controls, ΔrelA ΔspoT Salmonella had significantly lower basal levels of ssrA and ssrB mRNA in non-inducing 10 mM MgCl2 N9 medium (Fig. 3A,B). Growth of ΔrelA ΔspoT Salmonella in 8 μM MgCl2 N9 medium did not stimulate ssrA or ssrB expression. As predicted from these transcriptional profiles, ΔrelA ΔspoT Salmonella contained extremely low amounts of the SsrB protein (Fig. 3C). The lack of ssrA and ssrB expression in ΔrelA ΔspoT Salmonella raises the possibility that (p)ppGpp may directly activate ssrAB gene transcription. To test this idea, serine hydroxamate (SHX) was added to Salmonella grown to log phase in M9 minimal media. Addition of SHX to rapidly growing bacteria inhibits seryl-tRNA synthetase; the resulting accumulation of deacylated tRNAs stimulates (p)ppGpp synthesis from RelA48. The expression of ssrA mRNA was induced after the addition of SHX (Fig. 3D). SHX, however, did not induce ssrA transcription in ΔrelA ΔspoT Salmonella. To further examine the possibility that (p)ppGpp directly activates ssrA transcription, ppGpp was added to in vitro transcription reactions containing the pTIM-ssrA plasmid template (Fig. S4). ssrA transcripts were quantified by combining in vitro transcription reactions with a highly sensitive and specific qRT-PCR method49,50. This approach revealed that ppGpp directly stimulates ssrA in vitro transcription in a concentration-dependent manner (Fig. 3E). These data indicate that (p)ppGpp suffices to activate ssrA transcription in Salmonella.
The ssrA AT-rich discriminator region facilitates Salmonella virulence
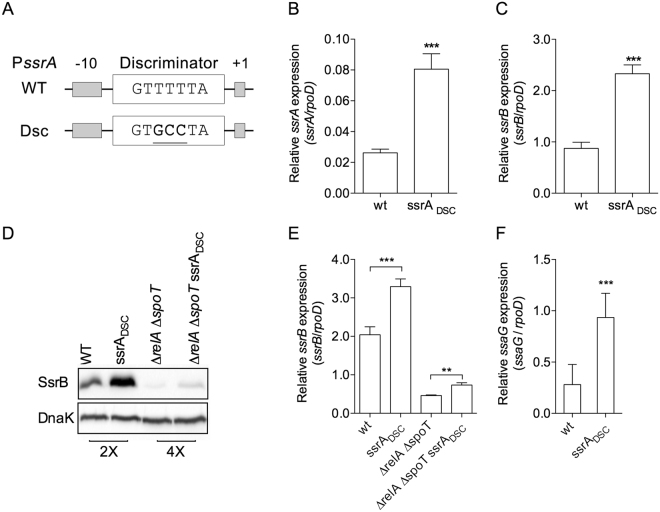
Since (p)ppGpp often activates gene transcription from AT-rich discriminator regions that form stable, long-lived, open complexes with RNA polymerase27,28,31, we focused our attention on the AT-rich PssrA discriminator region. We reasoned that increasing the GC-content would modulate the negative regulation associated with the AT-rich discriminator region of PssrA. To test this model, we engineered three substitutions at the native locus in the Salmonella chromosome that increased the GC content in the ssrA discriminator region, yielding the ssrADsc Salmonella strain (Figs 4A and S5A). Transcription of ssrA (Fig. 4B) and ssrB (Fig. 4C) was markedly higher in ssrADsc Salmonella than wild-type controls grown in LB broth to early stationary phase. Consistent with higher levels of ssrA and ssrB mRNA, the concentration of SsrB protein was higher in ssrADsc Salmonella grown in stationary phase in LB broth than in wild-type isogenic controls (Fig. 4D). The concentration of SsrB was also higher in ssrADsc Salmonella than wild-type controls grown in N9 low Mg2+ media (Fig. S5B). Expression of ssrADsc allele in the ΔrelA ΔspoT background dramatically reduced the amount of intracellular SsrB protein in early stationary phase Salmonella, suggesting that the derepression of ssrAB transcription associated with a GC-rich ssrA discriminatory region is dependent on (p)ppGpp. It should be noted that ΔrelA ΔspoT ssrADsc Salmonella expressed more SsrB than ΔrelA ΔspoT controls (Fig. 4D), but less than wild-type and ssrADsc controls. The concentration of SsrB protein (Fig. 4D) reflected ssrB mRNA levels (Fig. 4C,E). Transcription of the SsrB-regulated ssaG gene was also upregulated (p < 0.001) in ssrADsc Salmonella compared to isogenic wild-type bacteria (Fig. 4F).
Figure 4.
The AT-rich discriminator region of ssrA serves as a negative regulator of SPI2 expression. A mutant discriminator region of the ssrA promoter was expressed from the native locus in the Salmonella chromosome (A). The abundance of ssrA (B), ssrB (C,E) and ssaG (F) transcripts was measured by qRT-PCR in Salmonella grown for the indicated times in LB broth. Data, which are depicted as mean ± S.D. from 6–8 biological replicates, were normalized to the mRNA levels of the rpoD housekeeping gene. Western blot analysis the SsrB protein in Salmonella grown in LB broth for 5 h (D). **p < 0.01; ***p < 0.001. The abundance of the DnaK chaperone was measured as an internal control. Data are representative of 3 independent experiments. An independent, uncropped blot, of panel D can be seen in Fig. S6B.
Virulence of ssrADscSalmonella
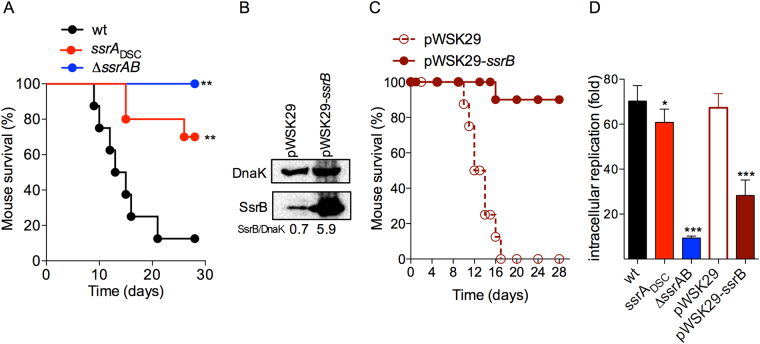
Because some SPI2-dependent phenotypes, such as the one associated with an SsrB C203S variant, were revealed in a C3H/HeN model of oral salmonellosis24, we chose this model to test the virulence of ssrADsc Salmonella. Moreover, the oral mucosa is the natural route of Salmonella infection. We found that ssrADsc Salmonella appear to be as attenuated as ΔssrAB isogenic bacteria when compared to wild-type Salmonella (Fig. 5A). These data suggest that the overexpression of SPI2 attenuates ssrADsc Salmonella in a murine model of oral salmonellosis. To test this idea, we evaluated the virulence of a Salmonella strain overexpressing the SsrB protein (Fig. 5B). Salmonella expressing pWSK29-ssrB, not the empty vector, were attenuated when inoculated p.o. into C3H/HeN mice (Fig. 5C). Salmonella strains expressing the ssrADSC allele (p < 0.05) or the pWSK29-ssrB plasmid (p < 0.001) grew to lower densities in J774 macrophage-like cells than wild-type controls (Fig. 5D). Collectively, these findings indicate that overexpression of SsrB diminishes Salmonella virulence.
Figure 5.
Importance of the AT-rich discriminator region of ssrA in Salmonella virulence. (A) The virulence of wild-type (wt) and mutant Salmonella was recorded in a C3H/HeN murine model of oral infection. (B) SsrB protein in Salmonella expressing the pWSK29 or pWSK29-ssrB plasmids. Virulence of pWSK29-ssrB+ Salmonella in a C3H/HeN murine model of acute oral infection. Data in A and C are from 9–10 mice per group. Growth of the indicated Salmonella strains in J774 A.1 cells after 20 h of infection (D). The data are shown as the mean ± S.D. of 6 biological replicates **p < 0.01, or ***p < 0.001 compared to wild-type controls.
Discussion
Horizontally-acquired and ancestral genes often contain considerable differences in base composition, as exemplified by the SPI2 genes of Salmonella51. This enteric pathogen has resolved potential difficulties of regulating the SPI2 virulence program by silencing AT-rich promoters with histone-like proteins such as H-NS and YdgT25,26. The inhibitory effects of H-NS are counter-silenced by transcription factors such as PhoP and SsrB19,52. The AT-rich composition of the discriminator region located between the −10 element and transcription start site can also impose a considerable burden to transcriptional initiation by forming stable, long-lived, open complexes that become saturated with free RNA polymerase27,28. Despite the potential burden to transcription, maintenance of an AT-rich discriminator region on ssrA suggests that this regulatory element provides a selective advantage to Salmonella pathogenesis. Herein, we tested the intriguing possibility that the AT-rich discriminator region serves as a negative regulatory element that is essential for both the appropriate expression of SPI2 gene transcription and Salmonella virulence.
In order to investigate whether the ssrA AT-rich discriminator region serves as a negative regulatory element, we constructed an ssrADsc Salmonella strain with increased GC-content in the discriminator region. Salmonella expressing ssrADsc overexpressed ssrA and ssrB genes, which led to enhanced expression of SsrB protein and the downstream ssaG gene. The amount of SsrB protein recorded in ssrADsc Salmonella was dramatically reduced when combined with ΔrelA ΔspoT mutations. These findings demonstrate that (p)ppGpp controls the ssrA discriminator region and that preservation of an AT-rich discriminator region places control of SPI2 gene transcription under the stimulatory effects of (p)ppGpp. The conversion of the discriminator from GTTTTTA to GTCCCTA may have also affected the repression of ssrA locus via the nucleoid proteins H-NS and YdgT. However, the increases in ssrB mRNA and SsrB protein noted in the ssrADsc Salmonella strain were significantly suppressed when the GC-rich discriminator region was express in ΔrelA ΔspoT Salmonella. These findings strongly argue that the derepression of ssrB expression seen in the GC-rich ssrADsc Salmonella is dependent on (p)ppGpp rather than through the relief of HN-S, YdgT or StpA binding.
Although appropriate SPI2 expression enables the intracellular replication of Salmonella, misregulation of SPI2 transcription seems to diminish the virulence potential of this enteropathogen in a murine model of infection and in macrophage-like J774 cells (herein and23–26). The attenuation of Salmonella overexpressing SPI-2 genes is analogous to attenuation of Salmonella expressing a constitutively active PhoP allele53. At present, we don’t know why the overexpression of SsrB attenuates Salmonella. In addition to activating SPI-2 gene transcription, SsrB activates the expression of ancestral genes54. The overexpression of ancestral or horizontally-acquired genes may be detrimental to Salmonella pathogenesis. Together, our investigations emphasize the importance that the repression associated with the AT-rich ssrA discriminator region plays in Salmonella pathogenesis.
Our investigations indicate that ΔrelA ΔspoT Salmonella are about 100-fold more attenuated than a ΔssrB mutant strain, suggesting roles for (p)ppGpp that are independent of SPI2 gene transcription. This result might reflect the fact that (p)ppGpp regulates 34% of coding RNA transcripts, including SPI1-dependent invasion genes and adaptive stress response programs38,46,55,56. Important ways by which this alarmone may contribute to Salmonella virulence independently of SPI2 activation include the stringent response to nutritional limitation, alternative sigma factor utilization, mRNA stability, and modulation of translation34,45,48,55–57. Nonetheless, the (p)ppGpp-dependent activation of SPI2 transcription might play a sizable role in Salmonella pathogenesis as suggested by the fact that the ΔrelA ΔspoT Salmonella is 100-fold more attenuated than ΔssrB controls but over 106-fold more attenuated than wild-type Salmonella. A ΔrelA ΔspoT Salmonella strain does not express ssrAB mRNA or SsrB protein, indicating (p)ppGpp is fundamental to transcriptional activation of ssrAB. Our in vitro and in vivo transcriptional analyses demonstrate that ssrAB is activated through the regulatory effects of (p)ppGpp on the AT-rich ssrA discriminator region.
DksA has also been shown to affect open complex stability and often works synergistically with (p)ppGpp29,31. It would have been reasonable to predict similar mechanisms in the regulation of SPI2 transcription for both (p)ppGpp and DksA. However, in contrast to (p)ppGpp, our data suggest that DksA does not regulate ssrAB transcription. Our biochemical analyses indicate that the amount of SsrB protein, not ssrB mRNA, is highly reduced in ΔdksA Salmonella, suggesting that DksA regulates ssrB expression post-transcriptionally. Transcriptional control of a small RNA could mediate the DksA-dependent post-transcriptional activation of ssrB. For example, DksA regulates σS post-transcriptionally via the small RNA DsrA58. Further investigations are needed to elucidate whether DsrA or a small RNA contribute to the DksA-dependent activation of ssrB.
Because DksA regulates approximately 10% of the Salmonella transcriptome45, we were surprised by the remarkable degree of co-dependency between DksA and the SPI2 master regulator SsrB in Salmonella pathogenesis. DksA has also been shown to play a major role in the antioxidant and antinitrosative defenses of Salmonella40–43. By regulating the expression of gene products of central metabolism, cysteine and glutathione biosynthesis, and iron and redox homeostasis, DksA promotes resistance to oxidative and nitrosative stress39–42. Our findings herein raise the possibility that the antioxidant and antinitrosative defenses associated with DksA Salmonella are not limited to the regulation of NADPH/NADP+ and GSH/GSSG redox homeostasis40–42. Given the effects that the SPI2 type III secretion system has on vesicular trafficking of NADPH oxidase and iNOS hemoproteins6,7,10, it is possible that the regulation of SPI2 gene transcription is a sizable component by which DksA promotes antioxidant and antinitrosative defenses of intracellular Salmonella.
Our investigations shed light into the molecular mechanisms by which the stringent response regulators DksA and (p)ppGpp activate the expression of bacterial virulence programs. The stringent response regulators control intracellular spread of Shigella flexneri59, motility of Pseudomonas putida60, adherence and virulence of Haemophilus ducreyi61, and avoidance of lysosomes by Legionella pneumophila62. DksA and/or (p)ppGpp also regulate the transcription of genes encoding type III secretion systems of Bordetella pertussis, Erwinia amylovora, L. pneumophila, and Pseudomonas syringae62–65. In Salmonella, DksA activates motility66 as well as SPI-1 and SPI-2 type III secretion systems38. In most cases, the mechanisms by which these virulence programs are regulated remain unknown. Employing the broadly conserved stringent response regulator (p)ppGpp to overcome the inhibitory barrier imposed by the AT-rich discriminator region of horizontally-acquired pathogenicity islands provides new insights into the regulation of virulence programs in pathogenic bacteria.
Experimental Procedures
Ethics Statement
All methods and experimental procedures were carried out in accordance to protocols approved by the University of Colorado School of Medicine (UCSOM) Institutional Biosafety Committee, authorization number 01–028. Mouse experiments were performed at Animal Care Facility of the UCSOM in accordance to the guidelines established by the UCSOM Institutional Animal Care and Use Committee (IACUC) protocol # 56413(07)1E.
Bacterial strains and growth conditions
Salmonella enterica serovar Typhimurium strain 14028 s (ATCC, Manassas, VA) and derivative strains are described in Table S1. A 1916-bp DNA fragment, including a 352-bp of the promoter region of the ssrB gene, was amplified by PCR from genomic DNA of strain AV07104. The PCR product was directionally cloned into EcoRI/PstI sites of pWSK29, generating the pWSK29-ssrB 3 × -FLAG plasmid. E. coli strain DH5α (ATCC) was used in molecular cloning. Mutations and plasmids were confirmed by sequencing. Unless specified, bacteria were grown in Luria-Bertani (LB) broth at 37 °C with continuous shaking. When applicable, 20 μg/mL chloramphenicol, 100 μg/mL penicillin, 100 μg/mL ampicillin, 100 μg/mL streptomycin, 20 μg/mL tetracycline, or 50 μg/mL kanamycin were added to the cultures.
Construction of ssrADscSalmonella
Segments of a 6.1-kb DNA fragment containing the ssrAB operon and a chloramphenicol resistant cassette were amplified from Salmonella ssrB-3xFLAG genomic DNA by PCR using the primers described in Table S3 and Figure S5A. PCR products were digested and ligated into pBluescript SK(+) to generate pSK-ssrAB-3xFLAG::cm. To introduce the discriminator mutations into the ssrA promoter, primer ssrA5-F and ssrADsc-R containing the mutations in the discriminator region were used to generate part ssrADsc-P1. The 5′ end of ssrA was amplified using primers ssrADsc-F and ssrA4-R to generate part ssrADsc-P2. The two ssrA segments, ssrADsc-P1 and ssrADsc-P2, were stitched together by PCR elongation. This fragment was ligated into pSK-ssrAB-3xFLAG::cm after digestion with EcoRI and NdeI. The 6.1-kb DNA fragment was digested with EcoRI and SacI out of pSK-ssrAB-3xFLAG::cm and introduced into ΔssrAB::FRT Salmonella by allelic replacement.
Allelic replacement
Salmonella strains generated in this study followed the method previously described by Datsenko and Wanner67 (Table S1). To generate Salmonella mutant strains, the plasmids pKD13 and pSK::cm containing a flippase recognition target (FRT)-flanked chloramphenicol cassette was used as a template to generate amplicons with 60-base-pair long primers containing 40-base-pair regions of homology to the gene locus. Salmonella strains containing the plasmid pTP223, which expresses the λ Red recombinase from an isopropyl β-D-1-thiogalactopyranoside (IPTG) inducible promoter, were grown in LB broth containing 20 μg/mL tetracycline for 16 h at 37 °C in a shaker incubator. Cells were subcultured 1:100 in LB broth containing 20 μg/mL tetracycline and 1 mg/mL IPTG. Cells were grown for 3 h in a shaker incubator followed by incubation on ice for 30 min. Cells were washed 3-times with 10% glycerol. Approximately 100 ng of DNA were electroporated into bacterial strains using an ECM 399 Exponential Decay Wave Electroporation System (BTX Harvard Apparatus Inc., Holliston, Ma) at 1800 volts for 5 milliseconds. Chromosomal genes were replaced by phage λ Red homologous recombination of electroporated PCR products67. Translational fusions containing the promoters of SPI2 genes and lacZY68 or luciferase69 reporter genes were transduced into ΔdksA and ΔrelA ΔspoT Salmonella using P22 phage. The strain ΔrelA ΔspoT put::spoT was generated by amplifying the genetic locus encompassing spoT with spoT pSK primers (Table S3) and cloning into pSK::Cm by digestion with ApaI and XhoI. The construct was amplified with put::spoT primers (Table S3) and recombined into the Salmonella put site by allelic replacement
Intracellular replication of Salmonella
J774A.1 macrophage-like cells (ATCC) were grown in RPMI+ medium [RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom), 15 mM Hepes, 2 mM L-glutamine, 1 mM sodium pyruvate (Sigma-Aldrich, St. Louis, MO)] at 37 °C in a 5% CO2 incubator. J774A.1 cells were infected with stationary phase Salmonella that had been grown in LB broth for 20 h at 37 °C in a shaker incubator. Selected groups of macrophages were treated with 200 U/ml IFNγ 20 h before Salmonella infection, and where specified, some of the cultures were treated with 960 μM of the iNOS inhibitor L-NIL (Cayman Chemical, Ann Arbor, MI) at the time of infection. J774A.1 cells were infected with stationary phase Salmonella at an MOI of 2. Cells were then incubated in RPMI+ medium containing 10 μg/ml gentamicin. At 2 h and 18 h post infection, cells were lysed with 0.25% deoxycholic acid and intracellular Salmonella were enumerated by dilution plating on LB agar.
Quantification of intracellular sifA::luc expression
J774A.1 macrophage-like cells were infected at an MOI of 20 with sifA::luc-expressing Salmonella grown to stationary phase in LB broth for 20 h at 37 °C in a shaker incubator. Extracellular bacteria were removed from the monolayers 25 min after challenge by washing with pre-warmed RPMI+ medium containing 50 μg/ml gentamicin. At 8 h post infection, the macrophages were treated with lysis buffer (Promega, Madison, WI) containing 5 mg/mL lysozyme. In parallel, selected macrophages were lysed with 0.25% deoxycholic acid and intracellular bacteria were enumerated on LB agar. Gene expression was measured by following luciferase activity according to the instructions provided by the One-Glo luciferase kit (Promega). Luciferase activity was measured by a Glomax multi-detection system after 5 sec integration in a Lumistar chemiluminometer (Promega). The amount of sifA::luc expression is represented as relative light units (RLU) per colony forming unit (CFU).
SPI2 induction
Salmonella SPI2 induction was performed as previously described26,68. Salmonella strains grown in LB broth for 16 h at 37 °C in a shaker incubator were subcultured 1:100 in N9 medium [5 mM KCl, 7.5 mM (NH4)2SO4, 0.5 mM K2SO4, 1 mM KH2PO4, 38 mM glycerol, 0.1% casamino acids and 100 mM Tris-HCl], pH 7.6 supplemented with 10 mM MgCl2 until they reached an OD600 of 0.5. The specimens were washed 3 times with 8 μM MgCl2 N9 medium, pH 5.8, and then diluted to an OD600 of 0.25 in 8 μM MgCl2 N9 medium, pH 5.8. After 3 h, cells were pelleted for quantification of SPI2 expression. Alternatively, SPI2 gene expression was induced as Salmonella entered into stationary phase in LB broth as previously described34,38. Briefly, Salmonella grown in LB broth for 16 h at 37 °C in a shaker incubator were subcultured 1:100 into fresh LB broth and grown for 2.5 h or 5 h at 37 °C in a shaker incubator. Independently, SPI2 gene expression was measured in Salmonella grown to OD600 of 0.5 in M9 minimum medium (7 mg/ml Na2HPO4, 3 mg/ml KH2PO4, 0.5 mg/ml NaCI, 1 mg/ml NH4Cl, 5 μg/ml thiamine, 0.12 mg/ml MgSO4, 0.015 mg/ml CaCl2) containing 2 mg/ml glucose and 100 μg/ml casamino acids. Selected samples grown in M9 medium were treated for 30 min with 0.4 mg/mL serine hydroxamate (SHX) previously demonstrated to induce (p)ppGpp accumulation48.
Quantification of ssrA and ssrB transcripts by real-time qPCR
Salmonella cultures growth in 8 μM MgCl2 N9 medium for 3 h were mixed 1:5 (v/v) with an ice-cold solution containing 5% phenol and 95% ethanol. The specimens were placed on ice for 20 min for RNA stabilization. Isolation of bacterial RNA, synthesis of cDNA, and qRT-PCR was performed as previously described41. Briefly, RNA was purified using the high pure RNA isolation kit (Roche) according to the instructions provided by the manufacturer. One microgram of total RNA was used to generate cDNA in reactions that contained 100 U MMLV reverse transcriptase (Promega), 0.45 μM N6 random hexamer primers (ThermoFisher Scientific), and 20 U RNAsin Plus RNase inhibitor (Promega). Reverse transcription was performed for 1 h at 42 °C. The primers and probes used for the qRT-PCR are listed in Table S4. Reactions prepared using TaqMan Gene Expression Master Mix (ThermoFisher Scientific) were incubated for 2 min at 50 °C, followed by 10 min at 95 °C, 40 cycles for 15 sec at 95 °C, and 57 °C for 1 min. Data are expressed as relative expression over the rpoD housekeeping gene copy number.
ssrA in vitro transcription and quantitative RT-PCR
To measure ssrA in vitro transcription, we combined in vitro transcription reactions with non-radioactive qPCR analysis49,50. Briefly, 5 nM pTIM-ssrA plasmid was mixed with increasing concentrations of ppGpp (Trilink) in reaction buffer (40 mM HEPES, pH 7.4, 2 mM MgCl2, 60 mM potassium glutamate, 0.05% NP-40, 200 μM ATP, 200 μM GTP, 200 μM CTP, 200 μM UTP, and 1 mM DTT). Upon addition of 5 nM E. coli RNA polymerase σ70 holoenzyme (NEB, Ipswich, MA) to a 10 μl reaction mixture, the in vitro transcription reaction was carried out at 37 °C for 10 min, and then terminated at 70 °C for 10 min. DNA-free DNA Removal kit (ThermoFisher) removed template DNA and DNaseI (ThermoFisher). The resulting materials were used as templates to generate cDNA with 100 U M-MLV reverse transcriptase (Promega), 0.45 μM N6 random hexamer primers (ThermoFisher), and 20 U RNase inhibitor (Promega). The amount of cDNA synthesized for 1 h at 42 °C was quantified by real-time PCR (qRT-PCR) using the primers and probe described in Table S4. The ssrA specific transcripts were normalized to the standard curve generated with known ssrA gene copy concentrations.
Western blotting
Salmonella expressing ssrB with a C-terminal FLAG epitope24 were cultured in SPI2-inducing 8 μM MgCl2 N9 medium as described above. After 3 h, cultures were centrifuged at 10,000 g for 5 min and bacterial pellets were stored at −80 °C. Samples were lysed by sonication in 125 mM NaCl Tris buffer, pH 7.0. Cellular debris was pelleted upon centrifugation at 16,000 g for 5 min. The protein concentration was determined with a Pierce 660 nm Protein Assay Reagent (ThermoFisher Scientific). Total soluble proteins (500 ng) resolved in 12% (v/v) SDS-PAGE gels were transferred electrophoretically to nitrocellulose membranes. The membranes were blocked with 5% milk, and immunoblotted with a 1:500 dilution of mouse IgG1 anti-FLAG M2 (Sigma-Aldrich) or a 1:2500 dilution of mouse IgG anti-DnaK (MBL International Corporation, Woburn, MA) monoclonal antibodies. The membranes were probed with 1:5,000 of HRP-conjugated sheep anti-mouse IgG secondary antibody (GE Healthcare). The signals in the membranes, developed with an Amersham ECL Prime Western Blotting Detection Reagent (GE Healthcare), were visualized with a Molecular Imager ChemiDoc XRS + system (Bio-Rad).
Competitive index assay
The relative contribution of ssrB, dksA, and (p)ppGpp to Salmonella virulence was quantified by recording the competitive index of mutant and wild-type isogenic controls. Briefly, C57BL/6 J (The Jackson Laboratory, Mount Desert Island, ME) mice bred in our animal facility according to institutional guidelines were infected i.p. with about 102 or 105 CFU of Salmonella grown to stationary phase in LB broth for 20 h at 37 °C in a shaker incubator. The bacteria used for inoculation were prepared in PBS. Spleens and livers collected 3 days after infection were macerated in PBS, and the amount of Salmonella present in the tissues was enumerated by dilution replica-plating on LB agar containing the appropriate antibiotics. The competitive index was calculated as:
(3) (strain 1/strain 2)output/(strain 1/strain 2)input.
Mouse survival
The virulence of ssrADsc-expressing Salmonella was investigated in C3H/HeN mice (The Jackson Laboratory) that were bred in the CU Anschutz animal facility. Briefly, C3H/HeN mice were infected orally with 107 CFU of the indicated Salmonella strains that had been grown in LB broth for 20 h at 37 °C in a shaker incubator. The bacteria used for inoculation were prepared in PBS. Mice survival was monitored for 28 days. The data are from 10 mice.
Statistical Analysis
Statistical analysis and graphing were performed using GraphPad Prism 4.0 software. Determination of statistical significance between two comparisons was achieved using an unpaired t-test. Determination of statistical significance between multiple comparisons was done using a one-way analysis of variance (ANOVA) followed by Bonferroni or Dunnett’s multiple comparison post-test with respective isogenic strain as control. To determine statistical significance for competitive indexes, one-way ANOVA or Mann-Whitney tests were used. Statistical significance for C3H/HeN mice survival curves was determined using log-rank test, comparing mutant Salmonella strain to wild-type controls.
Electronic supplementary material
Acknowledgements
This project was supported by the National Institutes of Health grants R01 AI54959, T32 GM008730, T32 AI052066, F31 AI118223, and F32 AI108249; the Veterans Administration grant IO1BX002073; and the Burroughs Wellcome Fund. We thank Dr. Sangeeta Chakraborty for discussions.
Author Contributions
T.T. and A.V.T. wrote the main manuscript text; T.T., J.S.K., M.A.C., L.F., L.L., and J.J.C. performed experiments; T.T., J.S.K., L.L., and A.V.T. prepared the figures and tables. All authors reviewed the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Timothy Tapscott and Ju-Sim Kim contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-27780-9.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gordon MA, et al. Non-typhoidal Salmonella bacteraemia among HIV-infected Malawian adults: high mortality and frequent recrudescence. AIDS. 2002;16:1633–1641. doi: 10.1097/00002030-200208160-00009. [DOI] [PubMed] [Google Scholar]
- 2.de Jong R, et al. Severe mycobacterial and Salmonella infections in interleukin-12 receptor-deficient patients. Science. 1998;280:1435–1438. doi: 10.1126/science.280.5368.1435. [DOI] [PubMed] [Google Scholar]
- 3.Cirillo DM, Valdivia RH, Monack DM, Falkow S. Macrophage-dependent induction of the Salmonella pathogenicity island 2 type III secretion system and its role in intracellular survival. Mol Microbiol. 1998;30:175–188. doi: 10.1046/j.1365-2958.1998.01048.x. [DOI] [PubMed] [Google Scholar]
- 4.Ochman H, Soncini FC, Solomon F, Groisman EA. Identification of a pathogenicity island required for Salmonella survival in host cells. Proc Natl Acad Sci USA. 1996;93:7800–7804. doi: 10.1073/pnas.93.15.7800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hensel M, et al. Genes encoding putative effector proteins of the type III secretion system of Salmonella pathogenicity island 2 are required for bacterial virulence and proliferation in macrophages. Mol Microbiol. 1998;30:163–174. doi: 10.1046/j.1365-2958.1998.01047.x. [DOI] [PubMed] [Google Scholar]
- 6.Vazquez-Torres A, et al. Salmonella pathogenicity island 2-dependent evasion of the phagocyte NADPH oxidase. Science. 2000;287:1655–1658. doi: 10.1126/science.287.5458.1655. [DOI] [PubMed] [Google Scholar]
- 7.Gallois A, Klein JR, Allen LA, Jones BD, Nauseef WM. Salmonella pathogenicity island 2-encoded type III secretion system mediates exclusion of NADPH oxidase assembly from the phagosomal membrane. J Immunol. 2001;166:5741–5748. doi: 10.4049/jimmunol.166.9.5741. [DOI] [PubMed] [Google Scholar]
- 8.Uchiya K, et al. A Salmonella virulence protein that inhibits cellular trafficking. EMBO J. 1999;18:3924–3933. doi: 10.1093/emboj/18.14.3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McGourty K, et al. Salmonella inhibits retrograde trafficking of mannose-6-phosphate receptors and lysosome function. Science. 2012;338:963–967. doi: 10.1126/science.1227037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chakravortty D, Hansen-Wester I, Hensel M. Salmonella pathogenicity island 2 mediates protection of intracellular Salmonella from reactive nitrogen intermediates. J Exp Med. 2002;195:1155–1166. doi: 10.1084/jem.20011547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuhle V, Abrahams GL, Hensel M. Intracellular Salmonella enterica redirect exocytic transport processes in a Salmonella pathogenicity island 2-dependent manner. Traffic. 2006;7:716–730. doi: 10.1111/j.1600-0854.2006.00422.x. [DOI] [PubMed] [Google Scholar]
- 12.Salcedo SP, Holden DW. SseG, a virulence protein that targets Salmonella to the Golgi network. EMBO J. 2003;22:5003–5014. doi: 10.1093/emboj/cdg517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rathman M, Sjaastad MD, Falkow S. Acidification of phagosomes containing Salmonella typhimurium in murine macrophages. Infect Immun. 1996;64:2765–2773. doi: 10.1128/iai.64.7.2765-2773.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia-del Portillo F, Foster JW, Maguire ME, Finlay BB. Characterization of the micro-environment of Salmonella typhimurium-containing vacuoles within MDCK epithelial cells. Mol Microbiol. 1992;6:3289–3297. doi: 10.1111/j.1365-2958.1992.tb02197.x. [DOI] [PubMed] [Google Scholar]
- 15.Eriksson S, Lucchini S, Thompson A, Rhen M, Hinton JCD. Unravelling the biology of macrophage infection by gene expression profiling of intracellular Salmonella enterica. Mol Microbiol. 2003;47:103–118. doi: 10.1046/j.1365-2958.2003.03313.x. [DOI] [PubMed] [Google Scholar]
- 16.Jabado N, Cuellar-Mata P, Grinstein S, Gros P. Iron chelators modulate the fusogenic properties of Salmonella-containing phagosomes. Proc Natl Acad Sci USA. 2003;100:6127–6132. doi: 10.1073/pnas.0937287100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forst S, Delgado J, Inouye M. Phosphorylation of OmpR by the osmosensor EnvZ modulates expression of the ompF and ompC genes in Escherichia coli. Proc Natl Acad Sci USA. 1989;86:6052–6056. doi: 10.1073/pnas.86.16.6052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Véscovi EG, Soncini FC, Groisman EA. Mg2+ as an extracellular signal: environmental regulation of Salmonella virulence. Cell. 1996;84:165–174. doi: 10.1016/S0092-8674(00)81003-X. [DOI] [PubMed] [Google Scholar]
- 19.Perez JC, Latifi T, Groisman EA. Overcoming H-NS-mediated transcriptional silencing of horizontally acquired genes by the PhoP and SlyA proteins in Salmonella enterica. J Biol Chem. 2008;283:10773–10783. doi: 10.1074/jbc.M709843200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garmendia J, Beuzón CR, Ruiz-Albert J, Holden DW. The roles of SsrA–SsrB and OmpR–EnvZ in the regulation of genes encoding the Salmonella typhimurium SPI-2 type III secretion system. Microbiology. 2003;149:2385–2396. doi: 10.1099/mic.0.26397-0. [DOI] [PubMed] [Google Scholar]
- 21.Mulder DT, et al. Multiple histidines in the periplasmic domain of the Salmonella enterica sensor kinase SsrA enhance signaling in response to extracellular acidification. Mol Microbiol. 2014;95:678–691. doi: 10.1111/mmi.12895. [DOI] [PubMed] [Google Scholar]
- 22.Feng X, Walthers D, Oropeza R, Kenney LJ. The response regulator SsrB activates transcription and binds to a region overlapping OmpR binding sites at Salmonella pathogenicity island 2. Mol Microbiol. 2004;54:823–835. doi: 10.1111/j.1365-2958.2004.04317.x. [DOI] [PubMed] [Google Scholar]
- 23.Choi J, et al. Salmonella pathogenicity island 2 expression negatively controlled by EIIANtr–SsrB interaction is required for Salmonella virulence. Proc Natl Acad Sci USA. 2010;107:20506–20511. doi: 10.1073/pnas.1000759107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Husain M, et al. Redox sensor SsrB Cys203 enhances Salmonella fitness against nitric oxide generated in the host immune response to oral infection. Proc Natl Acad Sci USA. 2010;107:14396–14401. doi: 10.1073/pnas.1005299107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Navarre WW, et al. Selective silencing of foreign DNA with low GC content by the H-NS protein in Salmonella. Science. 2006;313:236–238. doi: 10.1126/science.1128794. [DOI] [PubMed] [Google Scholar]
- 26.Coombes BK, Wickham ME, Lowden MJ, Brown NF, Finlay BB. Negative regulation of Salmonella pathogenicity island 2 is required for contextual control of virulence during typhoid. Proc Natl Acad Sci USA. 2005;102:17460–17465. doi: 10.1073/pnas.0505401102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barker MM, Gaal T, Josaitis CA, Gourse RL. Mechanism of regulation of transcription initiation by ppGpp. I. Effects of ppGpp on transcription initiation in vivo and in vitro. J Mol Biol. 2001;305:673–688. doi: 10.1006/jmbi.2000.4327. [DOI] [PubMed] [Google Scholar]
- 28.Gummesson B, Lovmar M, Nystrom T. A proximal promoter element required for positive transcriptional control by guanosine tetraphosphate and DksA protein during the stringent response. J Biol Chem. 2013;288:21055–21064. doi: 10.1074/jbc.M113.479998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ross W, Vrentas CatherineE, Sanchez-Vazquez P, Gaal T, Gourse RichardL. The magic spot: a ppGpp binding site on E. coli RNA polymerase responsible for regulation of transcription initiation. Mol Cell. 2013;50:420–429. doi: 10.1016/j.molcel.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lennon CW, et al. Direct interactions between the coiled-coil tip of DksA and the trigger loop of RNA polymerase mediate transcriptional regulation. Genes Dev. 2012;26:2634–2646. doi: 10.1101/gad.204693.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paul BJ, Berkmen MB, Gourse RL. DksA potentiates direct activation of amino acid promoters by ppGpp. Proc Natl Acad Sci USA. 2005;102:7823–7828. doi: 10.1073/pnas.0501170102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paul BJ, et al. DksA: a critical component of the transcription initiation machinery that potentiates the regulation of rRNA promoters by ppGpp and the initiating NTP. Cell. 2004;118:311–322. doi: 10.1016/j.cell.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 33.Ramachandran VK, Shearer N, Thompson A. The primary transcriptome of Salmonella enterica serovar Typhimurium and its dependence on ppGpp during late stationary phase. PLoS One. 2014;9:e92690. doi: 10.1371/journal.pone.0092690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song M, et al. ppGpp-mediated stationary phase induction of the genes encoded by horizontally acquired pathogenicity islands and cob/pdu locus in Salmonella enterica serovar Typhimurium. J Microbiol. 2010;48:89–95. doi: 10.1007/s12275-009-0179-6. [DOI] [PubMed] [Google Scholar]
- 35.Azriel S, Goren A, Rahav G, Gal-Mor O. The Stringent Response Regulator DksA Is Required for Salmonella enterica Serovar Typhimurium Growth in Minimal Medium, Motility, Biofilm Formation, and Intestinal Colonization. Infect Immun. 2016;84:375–384. doi: 10.1128/IAI.01135-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Webb C, Moreno M, Wilmes-Riesenberg M, Curtiss Iii R, Foster JW. Effects of DksA and ClpP protease on sigma S production and virulence in Salmonella typhimurium. Mol Microbiol. 1999;34:112–123. doi: 10.1046/j.1365-2958.1999.01581.x. [DOI] [PubMed] [Google Scholar]
- 37.Thompson A, et al. The bacterial signal molecule, ppGpp, mediates the environmental regulation of both the invasion and intracellular virulence gene programs of Salmonella. J Biol Chem. 2006;281:30112–30121. doi: 10.1074/jbc.M605616200. [DOI] [PubMed] [Google Scholar]
- 38.Rice CJ, Ramachandran VK, Shearer N, Thompson A. Transcriptional and Post-Transcriptional Modulation of SPI1 and SPI2 Expression by ppGpp, RpoS and DksA in Salmonella enterica sv Typhimurium. PLoS One. 2015;10:e0127523. doi: 10.1371/journal.pone.0127523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crawford MA, et al. DksA-dependent transcriptional regulation in Salmonella experiencing nitrosative stress. Front Microbiol. 2016;7:444. doi: 10.3389/fmicb.2016.00444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Henard CA, Bourret TJ, Song M, Vazquez-Torres A. Control of redox balance by the stringent response regulatory protein promotes antioxidant defenses of Salmonella. J Biol Chem. 2010;285:36785–36793. doi: 10.1074/jbc.M110.160960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Henard CA, et al. The 4-cysteine zinc-finger motif of the RNA polymerase regulator DksA serves as a thiol switch for sensing oxidative and nitrosative stress. Mol Microbiol. 2014;91:790–804. doi: 10.1111/mmi.12498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Henard CA, Vazquez-Torres A. DksA-dependent resistance of Salmonella enterica serovar Typhimurium against the antimicrobial activity of inducible nitric oxide synthase. Infect Immun. 2012;80:1373–1380. doi: 10.1128/IAI.06316-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Crawford MA, et al. Redox-active sensing by bacterial DksA transcription factors is determined by cysteine and zinc content. mBio. 2016;7:e02161–02115. doi: 10.1128/mBio.02161-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beuzon CR, et al. Salmonella maintains the integrity of its intracellular vacuole through the action of SifA. EMBO J. 2000;19:3235–3249. doi: 10.1093/emboj/19.13.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Åberg A, Fernández-Vázquez J, Cabrer-Panes JD, Sánchez A, Balsalobre C. Similar and divergent effects of ppGpp and DksA deficiencies on transcription in. Escherichia coli. J Bacteriol. 2009;191:3226–3236. doi: 10.1128/JB.01410-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramachandran VK, Shearer N, Jacob JJ, Sharma CM, Thompson A. The architecture and ppGpp-dependent expression of the primary transcriptome of Salmonella typhimurium during invasion gene expression. BMC Genomics. 2012;13:25. doi: 10.1186/1471-2164-13-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beuzon CR, Holden DW. Use of mixed infections with Salmonella strains to study virulence genes and their interactions in vivo. Microbes Infect. 2001;3:1345–1352. doi: 10.1016/S1286-4579(01)01496-4. [DOI] [PubMed] [Google Scholar]
- 48.Durfee T, Hansen A-M, Zhi H, Blattner FR, Jin DJ. Transcription Profiling of the Stringent Response in. Escherichia coli. J Bacteriol. 2008;190:1084–1096. doi: 10.1128/JB.01092-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Park JH, Magan N. Reverse transcriptase-coupled quantitative real time PCR analysis of cell-free transcription on the chromatin-assembledp21 promoter. PloS One. 2011;6:e23617. doi: 10.1371/journal.pone.0023617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang J, Zhao S, Zhou Y, Wei Y, Deng W. Establishment and validation of a non-radioactive method for in vitro transcription assay using primer extension and quantitative real time PCR. PLoS One. 2015;10:e0135317. doi: 10.1371/journal.pone.0135317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shea JE, Hensel M, Gleeson C, Holden DW. Identification of a virulence locus encoding a second type III secretion system in Salmonella typhimurium. Proc Natl Acad Sci USA. 1996;93:2593–2597. doi: 10.1073/pnas.93.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carroll RK, et al. Structural and functional analysis of the C-terminal DNA binding domain of the Salmonella typhimurium SPI-2 response regulator SsrB. The Journal of biological chemistry. 2009;284:12008–12019. doi: 10.1074/jbc.M806261200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miller SI, Mekalanos JJ. Constitutive expression of the PhoP regulon attenuates Salmonella virulence and survival within macrophages. J Bacteriol. 1990;172:2485–2490. doi: 10.1128/jb.172.5.2485-2490.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tomljenovic-Berube AM, Mulder DT, Whiteside MD, Brinkman FSL, Coombes BK. Identification of the Regulatory Logic Controlling Salmonella Pathoadaptation by the SsrA-SsrB Two-Component System. PLoS Genet. 2010;6:e1000875. doi: 10.1371/journal.pgen.1000875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Potrykus K, Cashel M. (p)ppGpp: still magical? Annu Rev Microbiol. 2008;62:35–51. doi: 10.1146/annurev.micro.62.081307.162903. [DOI] [PubMed] [Google Scholar]
- 56.Dalebroux ZD, Swanson M. S. ppGpp: magic beyond RNA polymerase. Nat Rev Microbiol. 2012;10:203–212. doi: 10.1038/nrmicro2720. [DOI] [PubMed] [Google Scholar]
- 57.Jishage M, Kvint K, Shingler V, Nyström T. Regulation of σ factor competition by the alarmone ppGpp. Genes Dev. 2002;16:1260–1270. doi: 10.1101/gad.227902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Girard, M. E. et al. DksA and ppGpp Regulate the σS Stress Response by Activating Promoters for the Small RNA DsrA and the Anti-Adapter Protein IraP. J Bacteriol. 200 (2018). [DOI] [PMC free article] [PubMed]
- 59.Mogull SA, Runyen-Janecky LJ, Hong M, Payne SM. dksA is required for intercellular spread of Shigella flexneri via an RpoS-independent mechanism. Infect Immun. 2001;69:5742–5751. doi: 10.1128/IAI.69.9.5742-5751.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Osterberg S, Skarfstad E, Shingler V. The sigma-factor FliA, ppGpp and DksA coordinate transcriptional control of theaer2 gene of Pseudomonas putida. Environ Microbiol. 2010;12:1439–1451. doi: 10.1111/j.1462-2920.2009.02139.x. [DOI] [PubMed] [Google Scholar]
- 61.Holley CL, et al. DksA and (p)ppGpp have unique and overlapping contributions to Haemophilus ducreyi pathogenesis in humans. Infect Immun. 2015;83:3281–3292. doi: 10.1128/IAI.00692-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dalebroux ZD, Yagi BF, Sahr T, Buchrieser C, Swanson MS. Distinct roles of ppGpp and DksA in Legionella pneumophila differentiation. Mol Microbiol. 2010;76:200–219. doi: 10.1111/j.1365-2958.2010.07094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chatnaparat T, Li Z, Korban SS, Zhao Y. The Stringent Response Mediated by (p)ppGpp Is Required for Virulence of Pseudomonas syringae pv. tomato and Its Survival on Tomato. Mol Plant Microbe Interact. 2015;28:776–789. doi: 10.1094/MPMI-11-14-0378-R. [DOI] [PubMed] [Google Scholar]
- 64.Ancona V, et al. The bacterial alarmone (p)ppGpp activates the type III secretion system in Erwinia amylovora. J Bacteriol. 2015;197:1433–1443. doi: 10.1128/JB.02551-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hanawa T, et al. Glutamate limitation, BvgAS activation and (p)ppGpp regulate the expression of the Bordetella pertussis type 3 secretion system. J Bacteriol. 2015;198:343–351. doi: 10.1128/JB.00596-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Azriel S, Goren A, Rahav G, Gal-Mor O. The stringent response regulator DksA is required for Salmonella enterica serovar Typhimurium growth in minimal medium, motility, biofilm formation and intestinal colonization. Infect. Immun. 2015;84:375–384. doi: 10.1128/IAI.01135-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McCollister BD, Bourret TJ, Gill R, Jones-Carson J, Vázquez-Torres A. Repression of SPI2 transcription by nitric oxide-producing, IFNg-activated macrophages promotes maturation of Salmonella phagosomes. J Exp Med. 2005;202:625–635. doi: 10.1084/jem.20050246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xu X, Hensel M. Systematic Analysis of the SsrAB Virulon of Salmonella enterica. Infect Immun. 2010;78:49–58. doi: 10.1128/IAI.00931-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.