Abstract
Frequenin, a Ca2+-binding protein, has previously been implicated in the regulation of neurotransmission, possibly by affecting ion channel function. Here, we provide direct evidence that frequenin is a potent and specific modulator of Kv4 channels, the principal molecular components of subthreshold activating A-type K+ currents. Frequenin increases Kv4.2 current amplitudes (partly by enhancing surface expression of Kv4.2 proteins) and it slows the inactivation time course in a Ca2+-dependent manner. It also accelerates recovery from inactivation. Closely related Ca2+-binding proteins, such as neurocalcin and visinin-like protein (VILIP)-1 have no such effects. Specificity for Kv4 currents is suggested because frequenin does not modulate Kv1.4 or Kv3.4 currents. Frequenin has negligible effects on Kv4.1 current inactivation time course. By using chimeras made from Kv4.2 and Kv4.1 subunits, we determined that the differential effects of frequenin are mediated by means of the Kv4 N terminus. Immunohistochemical analysis demonstrates that frequenin and Kv4.2 channel proteins are coexpressed in similar neuronal populations and have overlapping subcellular localizations in brain. Coimmunoprecipitation experiments demonstrate that a physical interaction occurs between these two proteins in brain membranes. Together, our data provide strong support for the concept that frequenin may be an important Ca2+-sensitive regulatory component of native A-type K+ currents.
Frequenin belongs to the EF-hand family of Ca2+ sensing proteins (1), which includes recoverin, calmodulin, neurocalcin, KChIPs, visinin-like proteins (VILIPs), and hippocalcin. Although the functional roles of these Ca2+-binding proteins are highly diverse, accumulating evidence suggests that many of these proteins modulate ion channels and other proteins, often bestowing Ca2+ senstivitity to the function of their interacting partners (1, 2). The functional role of frequenin (also termed neuronal calcium sensor 1; NCS-1) is largely unknown. Early reports suggested an involvement in exocytosis, because overexpression of frequenin results in enhanced neurotransmission in neurons and exocytosis in neuroendocrine cells (3–5). Frequenin also interacts with phosphotidyl-4-OH kinase (PI-4-K) in yeast (6) and mammals (7), which in turn has been implicated in Golgi transport (8). Biochemical assays have shown that frequenin binds to multiple proteins in both a Ca2+-dependent and -indpependent manner (9); frequenin may therefore have a diverse array of target proteins, some of which may be channel proteins. Indeed, experiments with the V7 Drosophila mutant, which overexpresses frequenin, suggest a role for frequenin in neurotransmission, possibly by affecting ion channel activity (3). Further support for a direct role of frequenin in the modulation of specific ion channels comes from the observation that the modulation of A-type K+ currents by intracellular Ca2+ was altered in the V7 mutant fly (10). In this study, we provide direct evidence that frequenin strongly and specifically modulates Kv4 channels (the molecular components of subthreshold-activating A-type K+ currents). This action may be physiologically relevant because frequenin colocalizes with Kv4.2 in some areas of the brain, such as the hippocampus and cerebellar cortex, and physically interacts with native brain Kv4.2 proteins.
Methods
Molecular Biology.
The following cDNAs were obtained as expressed sequence tags from Image Consortium and sequenced to confirm their integrity and identity with published sequences (GenBank accession numbers in brackets): human frequenin (W81153), human KChIP1 (AW007011), human calmodulin (AW161277), mouse VILIP (AA734202), and human hippocalcin (AI878898). Mouse neurocalcin (a gift from K. Hashimoto, National Institute of Infectious Diseases, Tokyo) was subcloned into pSGEM. The coding region of mouse Kv4.1 (a gift from M. Covarrubias, Thomas Jefferson University, Philadelphia) was subcloned into pcDNA3. We constructed green fluorescent protein (GFP)-tagged and myc-tagged frequenin by respectively subcloning the coding region into pEGFP-N3 (CLONTECH) or the pCS2 + MT vector, which contains 6 myc epitope copies (11). Two copies of the hemagglutinin (HA)-epitope were inserted immediately before the Kv4.2 stop codon to obtain Kv4.2-HA. Kv4 chimeric constructs were generated as described (12).
We synthesized cRNA by using run-off in vitro transcription (Ambion, Austin, TX). The integrity and size of all cRNAs were verified by denaturing agarose gel electrophoresis. The cRNA concentration was quantified by densitometry (Scion Image, Frederick, MD) and by comparison to a known RNA size marker (GIBCO).
Electrophysiology.
For microinjection of in vitro transcribed cRNA (50 nl; 0.1–18 ng), stage V–VI oocytes were harvested from Xenopus laevis. Two to three days after the injection, oocytes were voltage clamped by using standard two-electrode voltage-clamp techniques as described (13). Recordings were obtained by using a Geneclamp 500 amplifier (Axon Instruments, Foster City, CA) with data sampled at 5 kHz and filtered at 1 kHz. Currents were elicited by depolarizing steps from −100 to +60 mV in 10-mV increments every 15 s from a holding potential of −120 mV. To avoid contamination by endogenous Cl− currents, we superfused (1 ml/min; 20 ± 2°C) oocytes with a low Cl− recording solution containing 96 mM Na-glutamate, 2 mM K-glutamate, 1.8 mM CaCl2, 1 mM MgCl2, and 5 mM Hepes (pH 7.5, adjusted with NaOH).
Data Analysis.
Data were analyzed by using the PCLAMP suite of software (Axon Instruments) and ORIGIN for Windows (Microcal Software, Northampton, MA). Leak subtraction was performed for each trace. Results are expressed as means ± SEM.
Recovery from inactivation was examined by applying a double pulse protocol consisting of two depolarizing pulses to +20 mV, separated by intervals of increasing duration (from 20 to 460 ms in 40-ms increments) at −120 mV. The peak current amplitude during the test pulse (I) was normalized to that recorded during the first pulse (Io) and plotted as a function of the interpulse duration. The best-fit exponential function was used to derive the time constant of recovery from inactivation.
Steady-state inactivation was measured by using a voltage protocol in which the membrane was held at voltages ranging between −150 mV and −10mV for a period of 10 s (to obtain complete steady-state inactivation) followed by a 1-s test pulse to +20 mV, during which peak current amplitude (I) was measured. Data were normalized to the peak current amplitude following a prepulse at −150 mV (Imax), and plotted as a function of prepulse potential. The best-fit Boltzmann function was used to derive the midpoint voltage of inactivation and slope factor.
Coimmunoprecipitation.
HEK293 cells were transfected (Fugene6, Roche Diagnostics) with either HA-tagged Kv4.2 or GFP-tagged frequenin cDNA, or cotransfected with both cDNAs. Two days after transfection, cells were solubilized and total cell lysates were obtained. Clarified lysates were immunoprecipitated with a polyclonal anti-HA (Covance) or anti-Kv4.2 antibody (14), or anti-GFP antibody (CLONTECH) and incubated with protein A/G Sepharose (Amersham Pharmacia). The immunoprecipitated proteins (or cell lysates) were separated by SDS/12% PAGE, and Western analysis was performed by using an anti-GFP or anti-HA (clone 12CA5) monoclonal antibody, and a secondary anti-mouse IgG antibody (Amersham Pharmacia). Proteins were visualized by chemiluminescence (Pierce).
Total membrane fractions were obtained from homogenized mouse brain (15). Membrane fractions were immunoprecipitated as described above by using polyclonal Kv4.2 antibody or chicken antifrequenin antibodies (Research Diagnostics), and subjected to Western analysis. The secondary antibody was donkey anti-chicken IgG antibody (Jackson ImmunoResearch) or donkey anti-rabbit IgG (Amersham Pharmacia).
Immunofluorescence Microscopy.
COS cells were transiently transfected with Kv4.2 or GFP-tagged frequenin cDNA, or cotransfected with both cDNA and subjected to immunocytochemistry (16). Mouse brain sections (40 μm) were obtained and processed as described (17). The primary antibodies and concentration used were rabbit anti-Kv4.2 (Sigma; 1:500) and chicken antifrequenin (1:1000). The latter antibodies specifically recognize frequenin because immunoblotting shows that frequenin (but not KChIP1) is detected in lysates from frequenin- or KChIP1-transfected cells (data not shown). Secondary antibodies (Jackson ImmunoResearch) were goat Cy2- or Cy3-conjugated antirabbit IgG and donkey Cy2-conjugated antichicken IgG. Images for presentation were acquired on an Olympus AX-70 with 4× 0.3 numerical aperture and 60× 1.25 numerical aperture objectives by using a MagnaFire-cooled charge-coupled device camera with Olympus MAGNAFIRE and Adobe PHOTOSHOP software (Adobe Systems, Mountain View, CA).
Statistics.
Comparisons between two groups were performed by using Student's t test or the Rank Sum test. Values of P < 0.05 were considered statistically significant.
Results
Frequenin Modulates Kv4.2 Currents in Xenopus Oocytes.
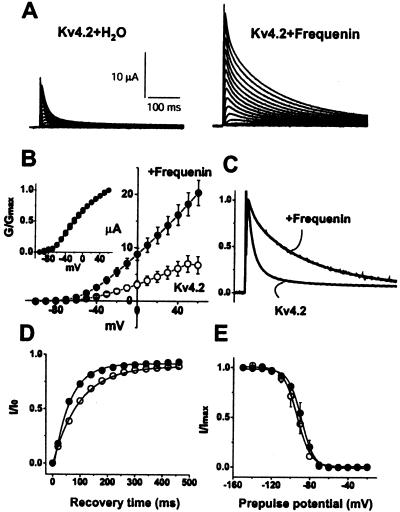
When Xenopus oocytes were coinjected with Kv4.2 and frequenin cRNAs, the expressed outward currents were substantially larger than in oocytes injected with only Kv4.2 cRNA (plus H2O to assure a constant injection volume). Peak Kv4.2 currents were, respectively, 18.1 ± 1.9 and 7.0 ± 1.6 μA at +50 mV (Fig. 1A). Although the magnitude of the increase varied amongst different batches of oocytes (range: 1.4–2.6 times larger than Kv4.2 alone), it occurred at all test potentials examined without a significant shift in voltage dependence of activation as shown in the normalized conductance–voltage relationships (Fig. 1 B and Inset). Frequenin also markedly slowed the time course of inactivation of Kv4.2 currents, as illustrated by normalizing Kv4.2 current traces in the presence and absence of frequenin (Fig. 1C; see also Table 1).
Figure 1.
Effects of frequenin on Kv4.2 currents expressed in Xenopus oocytes. (A) Representative traces of Kv4.2 currents expressed with or without frequenin. (B) Averaged current–voltage (I–V) relationship of peak Kv4.2 current with (●) or without (○) frequenin. (Inset) The conductance–voltage curves that were constructed by dividing the current by the driving force (assuming a K+ equilibrium potential of −99 mV), and normalizing to the maximal conductance (Gmax) at +60 mV. (C) Kv4.2 and Kv4.2/frequenin currents, normalized to the maximal peak amplitudes at +50 mV, to illustrate the effects of frequenin on the inactivation time course. (D) Time course of the recovery from inactivation. Time constants of recovery from inactivation were, respectively, 68.8 ± 3.4 ms and 97.7 ± 4.0 ms (P < 0.05) with (●) and without frequenin (○). (E) Voltage dependence of steady-state inactivation. The voltage at which half-maximal steady-state inactivation occurred (V1/2) were, respectively, −76 ± 3.8 and −71 ± 1.7 mV for Kv4.2 (○) and Kv4.2/frequenin currents (●), and the slope factors (k) were 8.0 ± 0.6 and 7.6 ± 0.7, respectively. (n = 6–8 for these experiments.)
Table 1.
Effects of frequenin on the inactivation parameters of Kv4.2, Kv4.3, and Kv4.1 currents
| Median inactivation time, ms | τfast, ms | τmiddle, ms | τslow, ms | |
|---|---|---|---|---|
| Kv4.2 + H2O (n = 23) | 33 ± 1.9 | 21 ± 0.6 (80% ± 0.6%) | 188 ± 2 (13% ± 0.9%) | |
| Kv4.2 + Frequenin (n = 22) | 75 ± 7.4* | 23 ± 1.5 (36% ± 3.6%*) | 178 ± 4 (61% ± 3.5%*) | |
| Kv4.3 + H2O (n = 10) | 50 ± 1.6 | 33 ± 2.6 (78% ± 1.4%) | 220 ± 13 (15% ± 1.7%) | |
| Kv4.3 + Frequenin (n = 10) | 74 ± 3.4* | 40 ± 2.9 (64% ± 3.1%*) | 196 ± 11 (28% ± 3.2%*) | |
| Kv4.1 + H2O (n = 14) | 80 ± 5.6 | 16 ± 0.9 (18% ± 1.8%) | 84 ± 2.6 (37% ± 1.7%) | 252 ± 7.7 (42% ± 2.1%) |
| Kv4.1 + Frequenin (n = 12) | 78 ± 5.0 | 20 ± 1.8 (20% ± 1.8%) | 89 ± 3.8 (37% ± 2.2%) | 249 ± 7.9 (40% ± 1.9%) |
The median inactivation times were obtained by measuring the time until half-maximal inactivation has occurred. The inactivation time constants were obtained by fitting individual current traces to either a sum of two (for Kv4.2 and Kv4.3 currents) or three (for Kv4.1 current) exponential functions. The relative contributions of the individual components were calculated (as a percentage of the total component) and shown in brackets.
, P < 0.05 (Student's t test) compared to the values without frequenin. Currents were measured at +50 mV.
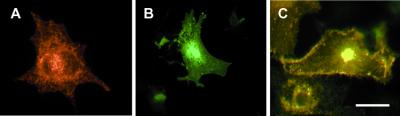
The increase in Kv4.2 current amplitude may be explained in part by the ability of frequenin to enhance the trafficking of Kv4.2 proteins to the plasma membrane (Fig. 2). When COS cells were transfected with Kv4.2 cDNA alone, Kv4.2 proteins were predominantly expressed in perinuclear regions (Fig. 2A). In the presence of frequenin, however, more Kv4.2 immunoreactivity was apparent in the outer margins of the cells (Fig. 2C).
Figure 2.
Frequenin increases the surface expression level of Kv4.2. COS cells were transiently transfected with Kv4.2 cDNA alone (A), GFP-tagged frequenin alone (B), or Kv4.2 and GFP-frequenin (C). Kv4.2 and frequenin are detected with anti-Kv4.2 antibody and GFP fluorescence, respectively. (Scale bar, 20 μm.)
To determine how frequenin alters inactivation kinetics, we examined the decay of individual outward currents assuming a sum of exponential terms. Kv4.2 current traces were best described as a sum of two exponential functions (13). Frequenin altered neither the fast nor the slow time constants of inactivation, but instead reversed their relative contribution (Table 1). In the absence of frequenin, the ratio of fast to slow components of Kv4.2 inactivation was 80:13, but with frequenin, the ratio was 36:61 (Table 1). From this analysis, we conclude that the slower inactivation of Kv4.2 currents in the presence of frequenin was due to an enhanced relative contribution of the slow inactivation process. Other Kv4.2 inactivation parameters were affected less by frequenin: there was a small but significant acceleration of recovery from inactivation (Fig. 1D), but the voltage dependence of steady-state inactivation was largely unaffected (Fig. 1E).
Modulation of Kv4 Currents by Frequenin Requires Intracellular Ca2+.
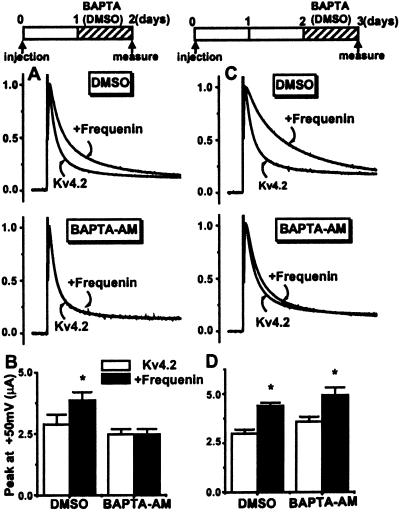
Frequenin binds Ca2+ through an interaction with its three EF-hand domains (1). We examined whether manipulation of cytosolic Ca2+ might influence the effects that frequenin has on Kv4.2 currents. After injecting Xenopus oocytes with frequenin and/or Kv4.2 cRNAs (molar ratio of 4:1), they were incubated for a period of 20–26 h with the membrane-permeable Ca2+ chelator, BAPTA-AM (10 μM; or only with the solvent DMSO as a control). The incubation with BAPTA-AM was started either soon after injection (immediately or 1-day after injection, when protein synthesis and/or trafficking is expected to occur at high rates), or at a later stage (2 days after injection, when significant protein synthesis has already occurred and significant amounts of channel proteins have already trafficked to the membrane; indeed, we have observed that the biggest increase of Kv4.2 currents occurred within the first 2 days after injection; data not shown).
The effects of frequenin on Kv4.2 currents were prevented when oocytes were incubated with BAPTA-AM during the first day after injection (Fig. 3 A and B). However, when BAPTA-AM incubation was started 2 days after injection, it only prevented the effects of frequenin on inactivation time course but not on current amplitude (Fig. 3 C and D). These data show that cytosolic Ca2+ is important in mediating the effects of frequenin on Kv4.2 currents and suggest that different mechanisms are involved in the regulation of the two parameters.
Figure 3.
Intracellular Ca2+ is required for the effects of frequenin on Kv4.2 currents. (A and B) One day after injection, oocytes were incubated for 20–26 h with BAPTA-AM (10 μM in 0.1% DMSO) or 0.1% DMSO alone (as a control; hatched bars). Currents were measured 2 days after the injection. Shown are Kv4.2 and Kv4.2/frequenin current traces normalized to their peak amplitudes at +50 mV. Under control conditions (DMSO only), the median inactivation times of Kv4.2 currents were 32 ± 1.0 ms in the absence and 64 ± 5.9 ms in the presence of frequenin (n = 6 each, P < 0.05). When incubated with BAPTA-AM, the median inactivation times were 37 ± 2.7 ms and 32 ± 0.7 ms, respectively, with and without frequenin (n = 6 each). (C and D) Incubation with BAPTA-AM (or DMSO) commenced 2 days after injection. When oocytes were incubated with DMSO, the median inactivation time course values were, respectively, 105 ± 3.3 ms and 40 ± 2.1 ms with and without frequenin (n = 7 each, P < 0.05), with BAPTA-AM incubation the corresponding values were 55 ± 3.5 ms and 43 ± 0.9 ms (n = 6 each). *, P < 0.05
Effects of Other Ca2+-Binding Proteins on Kv4.2 Currents.
The effects of frequenin on Kv4.2 currents resemble those recently reported for a family of Ca2+ binding proteins known as KChIPs, identified by using yeast two-hybrid methods (18). KChIPs are distantly related to frequenin (only 31–37% amino acid identity) as compared with other members of the Ca2+ binding family such as VILIPs, neurocalcin, and hippocalcin (with ≈60% identity in amino acid sequence). We examined whether these, more closely related, Ca2+ binding proteins affected Kv4.2 currents. Unlike frequenin or KChIP1, these proteins had no significant effects on the amplitude or time course of Kv4.2 currents (Table 2). Similarly, the more distantly related Ca2+ binding protein, calmodulin, had no effects on Kv4.2 currents. These results demonstrate that interaction with Kv4 proteins is not a general feature of Ca2+ binding proteins.
Table 2.
Effects of other Ca2+-binding proteins closely related to frequenin on Kv4.2 currents
| Median inactivation time, ms
|
Peak amplitude, μA
|
|||
|---|---|---|---|---|
| Kv4.2 only (+H2O) | +Ca2+-binding protein | Kv4.2 only (+H2O) | +Ca2+-binding protein | |
| KChIP1 (n = 7) | 26 ± 0.8 | 37 ± 5.5* | 7.1 ± 1.6 | 31.4 ± 2.8* |
| VILIP1 (n = 5) | 46 ± 3.5 | 47 ± 2.8 | 10.2 ± 1.3 | 11.5 ± 2.5 |
| Hippocalcin (n = 4) | 42 ± 3.9 | 54 ± 1.2 | 9.4 ± 1.4 | 11.5 ± 2.5 |
| Neurocalcin (n = 4) | 38 ± 5.5 | 40 ± 1.6 | 5.9 ± 0.4 | 7.2 ± 0.5 |
| Calmodulin (n = 6) | 27 ± 1.1 | 27 ± 0.8 | 7.1 ± 1.6 | 6.8 ± 1.1 |
Kv4.2 currents were measured at +50 mV.
, P < 0.05 (Student's t test) compared to the values without KChIP1.
Frequenin Specifically Modulates Kv4 Channels.
To investigate the specificity in modulating Kv4 currents, we examined whether frequenin also affected other Kv subfamily members. We chose Kv1.4 and Kv3.4, both of which also express rapidly inactivating voltage-activated K+ currents (the molar ratio of frequenin to Kv-channel cRNA was 4:1). Frequenin had no effect on these two currents. For Kv1.4 currents, the average median inactivation times were 47 ± 1.0 ms and 50 ± 1.5 ms (n = 6 each; P was not significant) with and without frequenin (the corresponding amplitudes were 6.2 ± 1.6 μA and 7.5 ± 1.8 μA). Kv3.4 currents were also unaffected; the median inactivation times were 16 ± 1.2 ms and 16 ± 0.7 ms (n = 6 each; P was not significant) with and without frequenin (the corresponding amplitudes were 4.3 ± 0.2 μA and 4.2 ± 1.0 μA). We performed similar experiments with Kir2.1, a structurally unrelated K+ channel subunit of inward rectifying K+ channels (19), and found it was also unaffected by frequenin (data not shown). Thus, the effect of frequenin may be specific to the Kv4 subfamily.
Effects of Frequenin on Other Kv4 Subfamily Members.
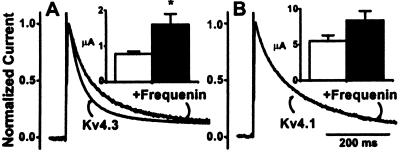
As we observed for Kv4.2 currents, when coexpressed with frequenin, Kv4.3 cRNAs produced significantly larger currents. The Kv4.3 inactivation time course was also considerably slower (Fig. 4A, Table 1), which (as was the case for Kv4.2 currents) was because of an increase in the relative contribution of the slow inactivation process (Table 1). In contrast, frequenin had relatively modest effects on Kv4.1 currents. Although in some batches of oocytes frequenin increased Kv4.1 current amplitudes, the overall effect was not statistically significant and the inactivation kinetics were largely unaffected (Fig. 4B, Table 1). Curve fitting of Kv4.1 current traces to a sum of three exponentials showed that frequenin affected neither the time constants nor the relative contribution of inactivation components (Table 1).
Figure 4.
Effects of frequenin on Kv4.3 (A) and Kv4.1 (B) currents, depicted as normalized currents and peak current amplitudes (at +50 mV) in the presence (closed column) or absence (open column) of frequenin. *, P < 0.05
The Kv4.2 N Terminus Is Responsible for the Differential Effects of Frequenin on Kv4.2 and Kv4.1 Channels.
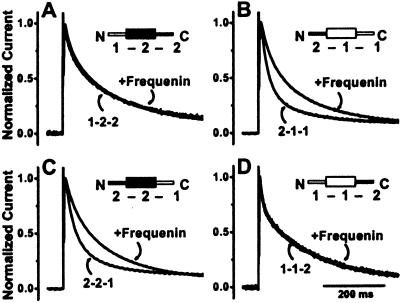
We took advantage of the fact that frequenin had different effects on the inactivation time course of Kv4.1 and Kv4.2 currents to determine the structural elements of Kv4 proteins that are responsible for these differences. We constructed Kv4 chimeras by swapping the divergent N termini and C termini of Kv4.2 and Kv4.1 subunits. We refer to the construct containing the Kv4.1 N terminus, the Kv4.2 core (S1-S6), and Kv4.2 C terminus as 1–2–2 and conversely; chimera 2–1–1 is the Kv4.1 subunit having a Kv4.2 N terminus.
Interestingly, frequenin had no discernable effect on the time course of currents produced by the 1–2–2 chimera (Fig. 5A), despite the fact that wild-type Kv4.2 currents were radically slowed. In contrast, the presence of the Kv4.2 N terminus bestows frequenin-sensitivity to the otherwise insensitive Kv4.1 current (frequenin slows 2–1–1 current inactivation; Fig. 5B). These effects of the Kv4.2 N terminus were independent of which C-terminal sequence was present, because C-terminal chimeras 2–2–1 and 1–1–2 were modulated in a similar manner as wild-type currents (Fig. 5 C and D). Collectively, these results provide strong support for the notion that the Kv4.2 N terminus (and likely also the Kv4.3 N terminus) contains structural elements that mediate the differential effects of frequenin on these currents.
Figure 5.
The Kv4.2 N terminus mediates the effects of frequenin on inactivation time course. We used chimeras of Kv4.1 and Kv4.2, in which the corresponding N-terminal or C-terminal ends were swapped. Depicted are results from N-terminal chimeras 1–2–2 (A) and 2–1–1 (B), and C-terminal chimeras 2–2–1 (C) and 1–1–2 (D). In the presence or absence of frequenin, the respective median inactivation times were 78 ± 5.6 ms and 82 ± 3.0 ms for 1–2–2 currents (P was not significant), 75 ± 3.0 ms and 38 ± 2.9 ms for 2–1–1 current (P < 0.05), 77 ± 2.2 ms and 43 ± 1.4 ms for 2–2–1 currents (P < 0.05) and 69 ± 3.7 ms and 70 ± 4.5 ms for 1–1–2 currents (P was not significant; n = 6 each for all of these experiments).
Physical Interaction of Frequenin with Kv4.2 Channel Proteins.
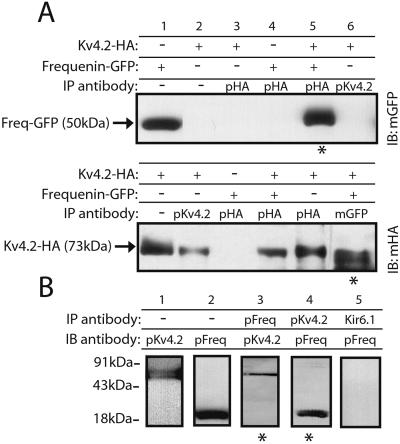
Physical interaction occurs between frequenin and Kv4.2 channels as demonstrated by using coimmunoprecipitation analysis from HEK293 cells cotransfected with HA-tagged Kv4.2 and GFP-tagged frequenin cDNAs. Immunoprecipitation of Kv4.2 proteins with anti-HA antibodies resulted in detection of frequenin in immunoblots when using anti-GFP antibodies (Fig. 6A Top). Conversely, Kv4.2 subunits were detected (using anti-HA antibodies) when frequenin was immunoprecipitated with anti-GFP antibodies (Fig. 6A Bottom). To determine whether this association also occurs in native tissue, we immunoprecipitated protein complexes with anti-Kv4.2 antibodies from mouse brain membrane fractions. Frequenin could be detected in Kv4.2 immunoprecipitates, and reciprocally, Kv4.2 proteins were present when immunoprecipitating with antifrequenin antibodies (Fig. 6B). These results demonstrate that Kv4.2 and frequenin proteins are associated in brain tissue.
Figure 6.
Frequenin physically associates with Kv4.2 proteins in transfected cells and in mouse brain. (A) Coimmunoprecipitation in transfected cells. Total cell lysates of HEK293 cells transfected with Kv4.2-HA and/or GFP-frequenin (as indicated) were immunoprecipitated (IP) by using polyclonal anti-HA antibody (pHA), anti-Kv4.2 antibody (pKv4.2), or monoclonal anti-GFP antibody (mGFP). The immunoprecipitation products were separated by SDS/12% PAGE and the resulting immunoblots (IB) were probed by using monoclonal anti-GFP antibody (mGFP; Upper) or anti-HA antibody (mHA; Lower). Lanes without an IP antibody (−) indicate that lysates were subjected to immunoblotting. (B) Mouse brain membrane fractions were immunoprecipitated by using rabbit anti-Kv4.2 (pKv4.2), chicken antifrequenin (pFreq), or rabbit anti-Kir6.1 antibodies (as a negative control). The resulting immunoblots were probed with chicken antifrequenin antibody (lanes 2, 4, and 5) or rabbit anti-Kv4.2 antibody (lanes 1 and 3). The Kv4.2 antibodies were specific in our assays (see also ref. 14) because they immunoprecipitate Kv4.2-HA but not GFP-frequenin proteins (A). The antifrequenin antibodies also specifically recognize frequenin but not KChIP1 proteins expressed in HEK293 cells (data not shown). Lanes indicated by stars denote experimental lanes.
Frequenin Colocalizes with Kv4.2 Proteins in Mouse Brain.
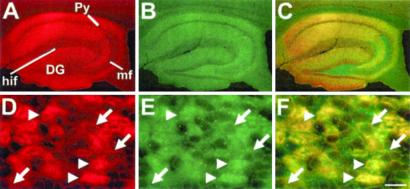
mRNA transcripts encoding Kv4.2 subunits are expressed in specific neuronal populations in the brain, and most prominently in excitatory hippocampal neurons and in cerebellar granule cells (20). In these neurons, the protein products are highly enriched in dendritic processes. They are also present in somatic membrane, but not in axons (21). Frequenin has been reported to be expressed in both axonal and dendritic processes and shown to be expressed in the hippocampus and cerebellum (22–24). We performed dual labeling experiments to determine whether Kv4.2 and frequenin are colocalized in neurons as would be predicted if the interactions reported here are of physiological relevance. We used a commercial chicken antifrequenin antibody, which in immunoblots of mouse heart and brain lysates recognizes a single band of ≈22 kDa, corresponding to the expected molecular weight of frequenin. Multiple higher molecular weight bands, as would be expected if these antibodies crossreact with KChIPs, were not observed (data not shown). This suggests that this commercial antibody reacts specifically with frequenin and provides support for the immunohistochemical observations. Both Kv4.2 and frequenin antibodies produced intense immunostaining of the dendritic processes of pyramidal and granule cells of the hippocampal formation (Fig. 7 A and B), as well as the dendrites of cerebellar granule cells (Fig. 7 D and E). There is extensive overlap localization of both proteins as illustrated by the superimposition of images taken following immunostaining with one or the other antibody (Fig. 7 C and F). Both proteins are also colocalized in somatic membrane; however, frequenin (but not Kv4.2 antibodies) also produced strong immunostaining of the mossy fiber in the hippocampus (Fig. 7 A–C). This result confirms the expression of frequenin in axonal processes and suggests that frequenin must have Kv4-related and -unrelated functions.
Figure 7.
Colocalization of Kv4.2 and frequenin in mouse brain. Mouse brain sections were double stained with Kv4.2 (A and D) and frequenin (B and E) antibodies. Over-imposed images (C and F) showed the colocalization of both proteins in the hippocampal formation (C) and in the granular layer of the cerebellar cortex. Note that the glomeruli in the granular layer (arrowheads) as well as the granular cell membrane (arrows) show immunoreactivity for both antibodies (F). DG, dentate gyrus; hif, hippocampal fissure; mf, mossy fiber; Py, pyramidal cell layer. (Scale bar: 300 μm for A–C, and 10 μm for D–F.)
Discussion
In neuronal cells, A-type K+ channels operate in the subthreshold voltage range to control the timing, duration, and frequency of action potentials. In heart, their functional correlate is the transient outward K+ current (Ito) that contributes to repolarization of the action potential. The principal molecular components of these rapidly inactivating K+ currents are Kv4 subunits (13, 25–29). However, several discrepancies exist between heterologously expressed Kv4 currents and native A-type currents (13, 26, 30) and there is also significant diversity in the functional characteristics of native A-type K+ currents in various tissue types (31). These differences might be due to the presence of regulatory (β) subunits that may alter Kv4 channel kinetics. The existence of such putative regulatory subunits was demonstrated by the ability of certain brain mRNA fractions to affect the functional properties of A-type K+ currents expressed by Kv4 transcripts or Kv4-containing brain mRNA fractions (26, 32, 33). A set of proteins named KChIPs that specifically interact with the N terminus of Kv4.2 and Kv4.3 subunits were only recently identified by using yeast two-hybrid methods (17). These proteins are Ca2+-binding proteins that are distantly related to frequenin (1) and were shown to potently modify Kv4.2 and Kv4.3 currents (18). We report here that frequenin also exhibits properties that make it a strong candidate as an additional regulatory subunit of Kv4 currents. Frequenin (i) specifically increased Kv4 current amplitudes and modified their time course, (ii) increased surface trafficking of Kv4.2 proteins, (iii) coimmunoprecipitated with native Kv4.2 proteins, and (iv) colocalizes with Kv4.2 proteins in mouse brain. Qualitatively, the effects of frequenin and KChIP1 on Kv4 currents are similar. However, frequenin seems to have a larger effect on the inactivation time course, whereas KChIPs appears to have a bigger effect on current amplitude and recovery from inactivation (see Tables 1 and 2 and Fig. 1; see also refs. 12 and 18). It is intriguing that both KChIPs and frequenin affect Kv4 currents, because they are distantly related members of the Ca2+-binding protein family (the sequence identity between frequenin and KChIPs is only 31–37%) as compared with family members that are more closely related (VILIPs, neurocalcin, and hippocalcin; with around 60% amino acid identity) but had no effect on Kv4.2 currents. Even more intriguing is the observation that neurocalcin, which has a very similar three-dimensional structure to that of frequenin (34), also lacked effects on Kv4 currents. Because frequenin may be the evolutionary ancestor of the Ca2+-binding protein family (1), it is possible that the ability of frequenin to interact and/or modify Kv4 currents was transmitted to some (such as the KChIPs), but not all of the Ca2+-binding protein family members.
A Requirement for Intracellular Calcium.
Our results show that the effects of frequenin on Kv4.2 currents were Ca2+-dependent. The crystal structure of human frequenin reveals that, like neurocalcin and recoverin, frequenin possesses four EF-hand motifs, but that only three of these (EF 2–4) bind Ca2+ (34). EF-3 and EF-4 bind Ca2+ with an affinity well within the biological range of Ca2+ concentrations (KEF3 = KEF4 = 0.4 μM), whereas EF-2 binds Ca2+ rather weakly (KEF2 = 10 μM; ref. 35). The fact that frequenin affects Kv4.2 currents under resting conditions in Xenopus oocytes suggests that frequenin was at least partially activated at the normal resting Ca2+ levels (≈90 nM in Xenopus oocytes; see ref.36). It also suggests that frequenin may be partially activated at rest in mammalian cells, such as those in heart and brain, which have resting Ca2+ levels in a similar range. Because the Ca2+ binding of frequenin is cooperative (i.e., the binding of one Ca2+ vastly increases the Ca2+ affinity of the remaining Ca2+ binding sites), there is a steep dependence of frequenin activity on Ca2+ levels throughout the range of biological Ca2+ concentrations (100 nM to 1 μM; ref. 35), suggesting that frequenin may affect its target proteins under diverse physiological and pathophysiological conditions.
Frequenin Enhances Surface Trafficking of Kv4 Channels.
Our immunocytochemistry data show that when frequenin was expressed by itself in COS cells, it was diffusely distributed throughout the cytoplasm. Similarly, Kv4.2 protein expression was confined to perinuclear regions (most likely in the endoplasmic reticulum and Golgi apparatus; Fig. 2 A and B). When coexpressed, both Kv4.2 and frequenin proteins were detected at the cell periphery, suggesting that frequenin increases membrane trafficking of Kv4 proteins. This may explain the observation that frequenin increases Kv4 current amplitude (although other mechanisms such as an increased single-channel conductance may also be responsible for this increase). Because frequenin trafficking is also affected by the presence of Kv4 proteins (Fig. 2), the possibility is raised that frequenin and Kv4 proteins associate and that the efficiency of membrane trafficking of the resulting protein complex is enhanced, as is the case for Kir6 and SUR subunits (37). Our coimmunoprecipitation data support the concept that association occurs between Kv4 and frequenin proteins. The physiological relevance of this association is demonstrated by the observations that (i) coimmunoprecipitation also occurs with native Kv4 and frequenin proteins isolated from mouse brain membranes, and (ii) that frequenin and Kv4 proteins are colocalized in some neurons in the mouse brain.
Kv4 Current Inactivation Time Course.
The mechanisms of inactivation of Kv4 channels appear to differ from the better characterized N-type or C-type inactivation (38). When expressed in Xenopus oocytes, Kv4.1 currents inactivate slower than either Kv4.2 or Kv4.3 currents. Curve fitting of the inactivating portion of current traces reveals both rapid and slow components of inactivation. The rapid components predominate in Kv4.2/4.3 currents and the slower components have a dominant role in Kv4.1 inactivation (38). The effects of frequenin were larger on those currents having a large contribution of rapid inactivation (Kv4.2, Kv4.3, and chimeras 2–1–1 & 2–2–1) and negligible on currents having a small component of rapid inactivation (those produced by Kv4.1 or chimeras 1–2–2 or 1–1–2; Table 1). One interesting possibility is that frequenin does not have the same effect on the two components of inactivation, and supports the concept that these two components arise from different molecular mechanisms (e.g., closed-state vs. open-state inactivation; see refs. 38 and 39).
Physiological and Pathophysiological Implications.
Our data demonstrating an association between native Kv4.2 and frequenin proteins in immunoprecipitable complexes in mouse brain membranes strongly suggest that the ability of frequenin to regulate A-type K+ current is physiologically relevant. This suggestion is further underscored by their colocalization. In addition to KChIPs and frequenin, reports exist that regulatory subunits of other voltage-gated K+ channels (including KChAP, Kvβ2, and MiRP1) may also affect Kv4 currents (40–42). Because the latter proteins lack specificity for Kv4 proteins, studies of the dynamics of protein–protein interactions will be required to understand the native system, which seems quite complex given the number of potential interacting proteins. Although the relative importance of frequenin in modulating native Kv4 proteins (and A-currents in brain and heart) remains to be determined, it is likely that KChIPs and frequenin (which are Ca2+-binding proteins) may have a more prominent role in physiological settings with altered intracellular Ca2+ concentrations. For example, the association of frequenin with A-type channels in neurons containing Ca2+-permeable receptors such as NMDA-type glutamate receptors may allow for feedback control of A-type current function and, hence, signaling. If expressed in heart, it is possible that frequenin may bestow activity-dependent modulation of the transient outward current, and, hence, may partly be responsible for the frequency-dependent decrease of the action potential duration. In addition, our data suggest that elevated levels of Ca2+, as might occur during ischemia and stroke, might also affect A-currents and excitability under pathophysiological conditions.
Acknowledgments
This work was supported in part by American Heart Association Scientist Development Award 013019N (to T.Y.N.), American Heart Association Established Investigator Award 0040193N (to W.A.C.), National Institutes of Health Grant NS30989 (to B.R.), and National Science Foundation Grant 0078297 (to B.R.).
Abbreviations
- VILIP
visinin-like protein
- HA
hemagglutinin
- GFP
green fluorescent protein
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Burgoyne R D, Weiss J L. Biochem J. 2001;353:1–12. [PMC free article] [PubMed] [Google Scholar]
- 2.Braunewell K H, Gundelfinger E D. Cell Tissue Res. 1999;295:1–12. doi: 10.1007/s004410051207. [DOI] [PubMed] [Google Scholar]
- 3.Pongs O, Lindemeier J, Zhu X R, Theil T, Engelkamp D, Krah-Jentgens I, Lambrecht H G, Koch K W, Schwemer J, Rivosecchi R. Neuron. 1993;11:15–28. doi: 10.1016/0896-6273(93)90267-u. [DOI] [PubMed] [Google Scholar]
- 4.Olafsson P, Wang T, Lu B. Proc Natl Acad Sci USA. 1995;92:8001–8005. doi: 10.1073/pnas.92.17.8001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McFerran B W, Graham M E, Burgoyne R D. J Biol Chem. 1998;273:22768–22772. doi: 10.1074/jbc.273.35.22768. [DOI] [PubMed] [Google Scholar]
- 6.Hendricks K B, Wang B Q, Schnieders E A, Thorner J. Nat Cell Biol. 1999;1:234–241. doi: 10.1038/12058. [DOI] [PubMed] [Google Scholar]
- 7.Weisz O A, Gibson G A, Leung S M, Roder J, Jeromin A. J Biol Chem. 2000;275:24341–24347. doi: 10.1074/jbc.M000671200. [DOI] [PubMed] [Google Scholar]
- 8.Walch-Solimena C, Novick P. Nat Cell Biol. 1999;1:523–525. doi: 10.1038/70319. [DOI] [PubMed] [Google Scholar]
- 9.McFerran B W, Weiss J L, Burgoyne R D. J Biol Chem. 1999;274:30258–30265. doi: 10.1074/jbc.274.42.30258. [DOI] [PubMed] [Google Scholar]
- 10.Poulain C, Ferrus A, Mallart A. Pflugers Arch. 1994;427:71–79. doi: 10.1007/BF00585944. [DOI] [PubMed] [Google Scholar]
- 11.Roth M B, Zahler A M, Stolk J A. J Cell Biol. 1991;115:587–596. doi: 10.1083/jcb.115.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakamura T Y, Nandi S, Pountney D J, Artman M, Rudy B, Coetzee W A. FEBS Lett. 2001;499:205–209. doi: 10.1016/s0014-5793(01)02560-1. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura T Y, Coetzee W A, Vega-Saenz D M, Artman M, Rudy B. Am J Physiol. 1997;273:H1775–H1786. doi: 10.1152/ajpheart.1997.273.4.H1775. [DOI] [PubMed] [Google Scholar]
- 14.Yao J A, Jiang M, Fan J S, Zhou Y Y, Tseng G N. Cardiovasc Res. 1999;44:132–145. doi: 10.1016/s0008-6363(99)00154-6. [DOI] [PubMed] [Google Scholar]
- 15.Pond A L, Scheve B K, Benedict A T, Petrecca K, Van Wagoner D R, Shrier A, Nerbonne J M. J Biol Chem. 2000;275:5997–6006. doi: 10.1074/jbc.275.8.5997. [DOI] [PubMed] [Google Scholar]
- 16.Bahring R, Dannenberg J, Peters H C, Leicher T, Pongs O, Isbrandt D. J Biol Chem. 2001;276:23888–23894. doi: 10.1074/jbc.M101320200. [DOI] [PubMed] [Google Scholar]
- 17.Chow A, Erisir A, Farb C, Nadal M S, Ozaita A, Lau D, Welker E, Rudy B. J Neurosci. 1999;19:9332–9345. doi: 10.1523/JNEUROSCI.19-21-09332.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.An W F, Bowlby M R, Betty M, Cao J, Ling H P, Mendoza G, Hinson J W, Mattsson K I, Strassle B W, Trimmer J S, et al. Nature (London) 2000;403:553–556. doi: 10.1038/35000592. [DOI] [PubMed] [Google Scholar]
- 19.Coetzee W A, Amarillo Y, Chiu J, Chow A, Lau D, McCormack T, Moreno H, Nadal M S, Ozaita A, Pountney D, et al. Ann NY Acad Sci. 1999;868:233–285. doi: 10.1111/j.1749-6632.1999.tb11293.x. [DOI] [PubMed] [Google Scholar]
- 20.Serodio P, Rudy B. J Neurophysiol. 1998;79:1081–1091. doi: 10.1152/jn.1998.79.2.1081. [DOI] [PubMed] [Google Scholar]
- 21.Sheng M, Tsaur M L, Jan Y N, Jan L Y. Neuron. 1992;9:271–284. doi: 10.1016/0896-6273(92)90166-b. [DOI] [PubMed] [Google Scholar]
- 22.Martone M E, Edelmann V M, Ellisman M H, Nef P. Cell Tissue Res. 1999;295:395–407. doi: 10.1007/s004410051246. [DOI] [PubMed] [Google Scholar]
- 23.Paterlini M, Revilla V, Grant A L, Wisden W. Neuroscience. 2000;99:205–216. doi: 10.1016/s0306-4522(00)00201-3. [DOI] [PubMed] [Google Scholar]
- 24.Olafsson P, Soares H D, Herzog K H, Wang T, Morgan J I, Lu B. Brain Res Mol Brain Res. 1997;44:73–82. doi: 10.1016/s0169-328x(96)00188-x. [DOI] [PubMed] [Google Scholar]
- 25.Fiset C, Clark R B, Shimoni Y, Giles W R. J Physiol (London) 1997;500:51–64. doi: 10.1113/jphysiol.1997.sp021998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Serodio P, Kentros C, Rudy B. J Neurophysiol. 1994;72:1516–1529. doi: 10.1152/jn.1994.72.4.1516. [DOI] [PubMed] [Google Scholar]
- 27.Baldwin T J, Tsaur M L, Lopez G A, Jan Y N, Jan L Y. Neuron. 1991;7:471–483. doi: 10.1016/0896-6273(91)90299-f. [DOI] [PubMed] [Google Scholar]
- 28.Xu H, Li H, Nerbonne J M. J Physiol (London) 1999;519:11–21. doi: 10.1111/j.1469-7793.1999.0011o.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johns D C, Nuss H B, Marban E. J Biol Chem. 1997;272:31598–31603. doi: 10.1074/jbc.272.50.31598. [DOI] [PubMed] [Google Scholar]
- 30.Faivre J F, Calmels T P, Rouanet S, Javre J L, Cheval B, Bril A. Cardiovasc Res. 1999;41:188–199. doi: 10.1016/s0008-6363(98)00215-6. [DOI] [PubMed] [Google Scholar]
- 31.Rudy B. Neuroscience. 1988;25:729–749. doi: 10.1016/0306-4522(88)90033-4. [DOI] [PubMed] [Google Scholar]
- 32.Rudy B, Hoger J H, Lester H A, Davidson N. Neuron. 1988;1:649–658. doi: 10.1016/0896-6273(88)90164-x. [DOI] [PubMed] [Google Scholar]
- 33.Chabala L D, Bakry N, Covarrubias M. J Gen Physiol. 1993;102:713–728. doi: 10.1085/jgp.102.4.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bourne Y, Dannenberg J, Pollmann V, Marchot P, Pongs O. J Biol Chem. 2001;276:11949–11955. doi: 10.1074/jbc.M009373200. [DOI] [PubMed] [Google Scholar]
- 35.Ames J B, Hendricks K B, Strahl T, Huttner I G, Hamasaki N, Thorner J. Biochemistry. 2000;39:12149–12161. doi: 10.1021/bi0012890. [DOI] [PubMed] [Google Scholar]
- 36.Cork R J, Cicirelli M F, Robinson K R. Dev Biol. 1987;121:41–47. doi: 10.1016/0012-1606(87)90136-9. [DOI] [PubMed] [Google Scholar]
- 37.Zerangue N, Schwappach B, Jan Y N, Jan L Y. Neuron. 1999;22:537–548. doi: 10.1016/s0896-6273(00)80708-4. [DOI] [PubMed] [Google Scholar]
- 38.Jerng H H, Covarrubias M. Biophys J. 1997;72:163–174. doi: 10.1016/S0006-3495(97)78655-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jerng H H, Shahidullah M, Covarrubias M. J Gen Physiol. 1999;113:641–660. doi: 10.1085/jgp.113.5.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuryshev Y A, Gudz T I, Brown A M, Wible B A. Am J Physiol Cell Physiol. 2000;278:C931–C941. doi: 10.1152/ajpcell.2000.278.5.C931. [DOI] [PubMed] [Google Scholar]
- 41.Yang E K, Alvira M R, Levitan E S, Takimoto K. J Biol Chem. 2001;276:4839–4844. doi: 10.1074/jbc.M004768200. [DOI] [PubMed] [Google Scholar]
- 42.Zhang M, Jiang M, Tseng G N. Circ Res. 2001;88:1012–1019. doi: 10.1161/hh1001.090839. [DOI] [PubMed] [Google Scholar]