Abstract
Purpose
Current guidelines recommend maintaining a mean arterial pressure (MAP) ≥ 65 mmHg in septic patients. However, the relationship between hypotension and major complications in septic patients remains unclear. We, therefore, evaluated associations of MAPs below various thresholds and in-hospital mortality, acute kidney injury (AKI), and myocardial injury.
Methods
We conducted a retrospective analysis using electronic health records from 110 US hospitals. We evaluated septic adults with intensive care unit (ICU) stays ≥ 24 h from 2010 to 2016. Patients were excluded with inadequate blood pressure recordings, poorly documented potential confounding factors, or renal or myocardial histories documented within 6 months of ICU admission. Hypotension exposure was defined by time-weighted average mean arterial pressure (TWA-MAP) and cumulative time below 55, 65, 75, and 85 mmHg thresholds. Multivariable logistic regressions determined the associations between hypotension exposure and in-hospital mortality, AKI, and myocardial injury.
Results
In total, 8,782 patients met study criteria. For every one unit increase in TWA-MAP < 65 mmHg, the odds of in-hospital mortality increased 11.4% (95% CI 7.8%, 15.1%, p < 0.001); the odds of AKI increased 7.0% (4.7, 9.5%, p < 0.001); and the odds of myocardial injury increased 4.5% (0.4, 8.7%, p = 0.03). For mortality and AKI, odds progressively increased as thresholds decreased from 85 to 55 mmHg.
Conclusions
Risks for mortality, AKI, and myocardial injury were apparent at 85 mmHg, and for mortality and AKI risk progressively worsened at lower thresholds. Maintaining MAP well above 65 mmHg may be prudent in septic ICU patients.
Electronic supplementary material
The online version of this article (10.1007/s00134-018-5218-5) contains supplementary material, which is available to authorized users.
Keywords: Sepsis, Hypotension, Blood pressure monitoring, Mortality, Acute kidney injury, Myocardial injury
Take-home message:
| In septic adults exposed to hypotension in the ICU, risks for in-hospital mortality, acute kidney injury (AKI) and myocardial injury were apparent by a mean arterial pressure of 85 mmHg, and for mortality and AKI risk progressively worsened at lower thresholds. Maintaining mean pressure well above 65 mmHg may be prudent in septic ICU patients. |
Introduction
Sepsis affects approximately a million people each year in the United States, and many more globally; it is the leading cause of death in intensive care patients. In 2008, an estimated $14.6 billion was spent in the US on hospitalizations for septicemia [1]. The syndrome is caused by a dysregulated inflammatory response to bacterial infections [2, 3]. Among the major risks is end-organ damage consequent to hypoperfusion and cellular/metabolic dysfunction [2, 4, 5], especially renal and myocardial injury. Since hypotension worsens tissue perfusion, it seems likely that some organ injury can be prevented by maintaining a suitable arterial pressure.
Preventing hypotension is therefore a crucial component of sepsis management [5, 6]. The Society of Critical Care Medicine’s Surviving Sepsis Guidelines [3] suggest initially maintaining mean arterial pressure (MAP) > 65 mmHg (higher for older patients and those with cardiovascular morbidities), followed by monitoring via multiple hemodynamic parameters to an endpoint of tissue perfusion [3]. Systolic blood pressure of 100 mmHg or less is a component of the quick Sequential Organ Failure Assessment score (qSOFA) which helps identify adult patients with suspected infections who are more likely to have poor outcomes typical of sepsis [7]. Despite these guidelines, relationships between various durations and depths of hypotension and serious complications remain unclear. The evidence for clearly defining blood pressure targets in septic patients is currently contentious and weak [3]. We, therefore, evaluated associations between hypotension and in-hospital mortality, acute kidney, and myocardial injury in septic patients [8].
Methods
We analyzed in-patient data from the Cerner Health Facts electronic health records database (Kansas City, MO, USA), which includes clinical and administrative data from 720 US hospitals and health systems. Our analysis of de-identified data was determined to be exempt from local institutional review board (IRB) review in advance by Western IRB (Puyallup, WA, USA).
In-patients admitted and discharged between January 1, 2010 and November 30, 2016 were analyzed. The study included adults ≥ 18 years old with a sepsis diagnosis (primary or secondary, any priority) via International Classification of Disease (ICD) 9 and 10 codes as shown in Online Resource 1 and an ICU stay ≥ 24 h. We considered only the initial episode when patients had more than one qualifying hospitalization containing an ICU admission for sepsis within the database.
Patients were excluded when they lacked at least a 6-month database history before the septic hospital admission; had more than a single ICU stay during the index admission; lacked a baseline serum creatinine measurement within 6 months before ICU admission and at least one measurement during the ICU stay; had a history of acute kidney or myocardial injury within 6 months before ICU admission (based on ICD-9 or ICD-10 codes, Online Resource 2); received dialysis within 6 months before ICU admission through the first 24 h within the ICU (time from which outcomes were analyzed); or had fewer than five valid blood pressure readings during each ICU day in which exposure was analyzed. We also excluded patients whose records contained more than two 5-h gaps between MAP recordings or were missing age, sex, diagnosis codes, or medication records for the index hospitalization.
Exact ICU admission times are not recorded in Cerner Health Facts. Consequently, we defined admission time to be when the first laboratory test or medication order was noted in an ICU care setting. Discharge times were available for a proportion of patients, but when missing, we used the order location to estimate discharge time.
Hypotension exposure extended from ICU admission through the first of: ICU discharge, development of an outcome, or 7 days. We selected MAP as our global measure of blood pressure to be consistent with existing literature [6]. Hypotension exposure was characterized by: (1) Time-weighted average of MAP (TWA-MAP) below MAP thresholds of 55, 65, 75, or 85 mmHg. TWA-MAP was calculated as the area below the MAP threshold curve divided by the total time exposure was monitored; (2) cumulative time measured in minutes during which MAP was below absolute thresholds of 55, 65, 75, or 85 mmHg.
We used absolute thresholds because a previous study showed that absolute and relative thresholds were comparably predictive of myocardial injury and AKI [9]. To calculate relative thresholds, baseline MAP is required which could not be reliably determined in this analysis. We used recorded MAP values when available, or estimated MAP from systolic and diastolic pressures using the formula: [(2 × diastolic) + systolic]/3. MAP readings were deemed invalid and excluded if diastolic blood pressure (DBP) ≤ 5 mmHg, DBP ≥ 225 mmHg, or systolic blood pressure (SBP) ≤ DBP + 5 mmHg [9]. An average of 357 MAP readings were available per patient per ICU day.
Outcomes
The primary outcome was in-hospital mortality; secondary outcomes were acute kidney injury (AKI) and myocardial injury. Mortality was defined by a discharge status of “deceased” for the hospital visit. Secondary outcomes were determined from 24 h after ICU admission until the first of: ICU discharge, 7 days, death, or diagnosis of AKI or myocardial injury (Online Resource 3). Outcomes were largely limited to the ICU to maintain proximity to the hypotension exposure.
AKI was defined as stage 1 or higher based upon serum creatinine (SCr) readings according to the Kidney Disease Improving Global Outcomes 2012 guidelines (using criteria for SCr increase over baseline [defined as the lowest reading within 6 months prior, and closest to ICU admission] and with respect to SCr values within 48 h) [10]. Urine output was not used because there were insufficient data in the registry. Myocardial injury was defined by at least one elevated troponin value > 0.03 ng/mL of “Troponin I”, “Troponin T”, or “Troponin” before onset of AKI. Myocardial injury was not evaluated past the date upon which AKI was identified because renal dysfunction might have falsely elevated troponin concentrations.
Statistical analyses
Baseline patient characteristics were summarized via counts and percentages for binary or categorical variables and with means and standard deviations, or via medians and interquartile ranges for continuous variables. For univariate inferences, Chi square test or t test were used, as appropriate. Multivariable logistic regression quantified the relationship between hypotension exposure (TWA-MAP and cumulative time measured in minutes spent below MAP thresholds) and the primary and two secondary outcomes.
Specifically, we created individual logistic regression models, each with one hypotension exposure and one of the outcomes. We assessed the need for restricted cubic splines by plotting deciles and ventiles (20 equal-sized groups) of the hypotension exposure variable versus the mean proportion of the outcome for each exposure and outcome, and looked for any substantial non-linear trend. No substantial non-linear trend was evident so the exposures were modeled as linear predictors.
Table 1 lists all the covariates included in the models to reduce potential confounding. This includes the Acute Physiology and Chronic Health Evaluation (APACHE) III score used to adjust for patient acuity and the Elixhauser comorbidities used to adjust for chronic comorbidities. For the uncommon outcome of myocardial injury, an algorithm that used bootstrapping and stepwise regression was used to determine a parsed model containing a limited set of potential confounders with hypotension exposure and the outcome [11]. To adjust for a potential lack of independence among observations within hospitals, we derived robust (Huber–White) standard errors clustered at the hospital level for all regression models [12, 13]. We plotted the results of the logistic regression models as marginal probabilities of the outcome across the observed range of the hypotension exposure variable to facilitate interpretation of the results.
Table 1.
Comprehensive list of potentially confounding variables for MAP threshold groups < 55 mmHg, < 65 mmHg, < 75 mmHg and < 85 mmHg
| Variable group | Variable type | Min. MAP < 55 mmHg (n = 3308) | Min. MAP < 65 mmHg (n = 6310) | Min. MAP < 75 mmHg (n = 8039) | Min. MAP < 85 mmHg (n = 8609) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mortality n (%)** | Mortality n (%) | Mortality n (%) | Mortality n (%) | ||||||||||
| Yes | No | p value | Yes | No | p value | Yes | No | p value | Yes | No | p value | ||
| Gender* | Male | 329 (44%) | 1079 (42%) | 0.522 | 51 (47%) | 2343 (45%) | 0.302 | 592 (48%) | 3183 (47%) | 0.473 | 617 (48%) | 3481 (48%) | 0.521 |
| Race | African American | 77 (10%) | 234 (10%) | 0.243 | 120 (11%) | 502 (10%) | 0.240 | 137 (11%) | 705 (10%) | 0.380 | 142 (11%) | 801 (11%) | 0.592 |
| Caucasian | 585 (78%) | 206 (81%) | 858 (78%) | 4164 (80%) | 964 (78%) | 5400 (79%) | 994 (78%) | 5777 (79%) | |||||
| Other | 74 (10%) | 211 (9%) | 97 (9%) | 455 (9%) | 107 (9%) | 586 (8%) | 110 (9%) | 635 (9%) | |||||
| Unknown | 19 (3%) | 48 (2%) | 26 (2%) | 88 (2%) | 28 (2%) | 112 (2%) | 28 (2%) | 122 (2%) | |||||
| Age (years)* | Mean | 70 | 65 | < 0.001 | 69 | 63 | < 0.001 | 69 | 62 | < 0.001 | 69 | 63 | < 0.001 |
| Admission type | Elective | 40 (5%) | 119 (5%) | 0.779 | 62 (6%) | 213 (4%) | 0.065 | 70 (6%) | 274 (4%) | 0.025 | 73 (6%) | 291 (4%) | 0.009 |
| Emergency | 671 (89%) | 2276 (89%) | 975 (89%) | 4711 (90%) | 1092 (88%) | 6173 (91%) | 1125 (88%) | 6672 (91%) | |||||
| Trauma Center | 5 (0.7%) | 12 (0.5%) | 3 (0.3%) | 10 (0.2%) | 4 (0.3%) | 11 (0.2%) | 4 (0.3%) | 11 (0.2%) | |||||
| Urgent | 2 (0.3%) | 4 (0.2%) | 53 (5%) | 257 (5%) | 62 (5%) | 321 (5%) | 64 (5%) | 337 (5%) | |||||
| Unknown | 37 (5%) | 142 (6%) | 8 (0.7%) | 18 (0.4%) | 8 (0.7%) | 24 (0.4%) | 8 (0.6%) | 24 (0.3%) | |||||
| Discharge year | 2010 | 3 (0.4%) | 23 (0.9%) | 0.009 | 6 (0.5%) | 44 (0.8%) | 0.009 | 9 (0.7%) | 53 (0.8%) | 0.001 | 9 (0.7%) | 55 (0.8%) | < 0.001 |
| 2011 | 31 (4.1%) | 79 (3%) | 41 (4%) | 172 (3%) | 49 (4%) | 220 (3%) | 50 (4%) | 232 (3%) | |||||
| 2012 | 82 (10.9%) | 185 (7%) | 115 (11%) | 383 (7%) | 130 (11%) | 510 (8%) | 134 (11%) | 539 (7%) | |||||
| 2013 | 141 (19%) | 434 (17%) | 196 (18%) | 848 (16%) | 215 (17%) | 1064 (16%) | 223 (18%) | 1125 (15%) | |||||
| 2014 | 183 (24%) | 679 (27%) | 270 (25%) | 1320 (25%) | 305 (25%) | 1685 (25%) | 313 (25%) | 1818 (25%) | |||||
| 2015 | 165 (22%) | 584 (23%) | 236 (21%) | 1202 (23%) | 265 (21%) | 1592 (23%) | 275 (22%) | 1733 (24%) | |||||
| 2016 | 150 (20%) | 569 (22%) | 237 (22%) | 1240 (24%) | 263 (21%) | 1679 (25%) | 270 (21%) | 1833 (25%) | |||||
| Census region | Midwest | 93 (12%) | 365 (14%) | < 0.001 | 141 (13%) | 772 (15%) | < 0.001 | 161 (13%) | 1054 (16%) | < 0.001 | 167 (13%) | 1137 (16%) | < 0.001 |
| Northeast | 308 (41%) | 793 (31%) | 430 (39%) | 1501 (29%) | 477 (39%) | 1922 (28%) | 484 (38%) | 2062 (28%) | |||||
| South | 213 (28%) | 905 (36%) | 336 (31%) | 2003 (39%) | 388 (31%) | 2654 (39%) | 404 (38%) | 2873 (39%) | |||||
| West | 141 (19%) | 490 (19%) | 194 (18%) | 933 (18%) | 210 (17%) | 1173 (17%) | 219 (17%) | 1263 (17%) | |||||
| Hospital bed size | < 100 | 44 (6%) | 208 (8%) | < 0.001 | 63 (6%) | 443 (9%) | < 0.001 | 70 (6%) | 603 (9%) | < 0.001 | 74 (6%) | 665 (9%) | < 0.001 |
| 100–199 | 91 (12%) | 403 (16%) | 141 (13%) | 871 (17%) | 159 (13%) | 1149 (17%) | 162 (13%) | 1232 (17%) | |||||
| 200–299 | 115 (15%) | 496 (19%) | 174 (16%) | 994 (19%) | 192 (16%) | 1332 (20%) | 197 (16%) | 1457 (20%) | |||||
| 300–499 | 191 (25%) | 558 (22%) | 296 (27%) | 1220 (23%) | 344 (28%) | 1612 (24%) | 356 (28%) | 1746 (24%) | |||||
| 500+ | 314 (42%) | 888 (35%) | 427 (39%) | 1681 (32%) | 471 (38%) | 2107 (31%) | 485 (38%) | 2235 (31%) | |||||
| ICU type | General ICU | 472 (63%) | 1722 (68%) | 0.001 | 675 (61%) | 3465 (67%) | 0.001 | 765 (62%) | 4549 (67%) | 0.002 | 791 (62%) | 4920 (67%) | < 0.001 |
| Medical ICU | 95 (13%) | 278 (11%) | 156 (14%) | 668 (13%) | 172 (14%) | 880 (13%) | 178 (14%) | 947 (13%) | |||||
| Surgical ICU | 46 (6%) | 181 (7%) | 69 (6%) | 309 (6%) | 75 (6%) | 382 (6%) | 75 (6%) | 407 (6%) | |||||
| Cardiac ICU | 70 (9%) | 138 (5%) | 99 (9%) | 301 (6%) | 103 (8%) | 392 (6%) | 109 (9%) | 418 (6%) | |||||
| Coronary care unit | 72 (10%) | 234 (9%) | 102 (9%) | 466 (9%) | 121 (10%) | 600 (9%) | 121 (10%) | 643 (9%) | |||||
| Drugs received | Diuretics received | 171 (23%) | 341 (13%) | < 0.001 | 264 (24%) | 653 (13%) | < 0.001 | 291 (23%) | 838 (12%) | < 0.001 | 303 (24%) | 897 (12%) | < 0.001 |
| ACE inhibitors received | 68 (9%) | 232 (9%) | 0.946 | 115 (11%) | 453 (9%) | 0.066 | 130 (11%) | 594 (9%) | 0.044 | 137 (11%) | 648 (9%) | 0.028 | |
| Beta blockers received | 157 (21%) | 378 (15%) | < 0.001 | 252 (23%) | 773 (15%) | < 0.001 | 282 (23%) | 1020 (15%) | < 0.001 | 298 (23%) | 1105 (15%) | < 0.001 | |
| Calcium channel blockers received | 82 (11%) | 156 (6%) | < 0.001 | 136 (12%) | 332 (6%) | < 0.001 | 156 (13%) | 465 (9%) | < 0.0001 | 165 (13%) | 519 (7%) | < 0.001 | |
| *Modified APACHE III score | Mean | 76 | 65 | < 0.001 | 74 | 62 | < 0.001 | 73 | 60 | < 0.001 | 72 | 59 | < 0.001 |
| Serum lactate | No reading available | 335 (44%) | 125 (49%) | < 0.001 | 505 (46%) | 2745 (53%) | < 0.001 | 592 (48%) | 3729 (55%) | < 0.001 | 614 (48%) | 4077 (56%) | < 0.001 |
| Normal < 2 mmol/L | 183 (24%) | 813 (32%) | 279 25%) | 1555 (30%) | 304 (25%) | 1934 (28%) | 309 (24%) | 2045 (28%) | |||||
| Mild 2 to < 5 mmol/L | 172 (23%) | 406 (16%) | 230 (21%) | 780 (15%) | 250 (20%) | 983 (15%) | 257 (20%) | 1050 (14%) | |||||
| Moderate 5 to < 8 mmol/L | 40 (5%) | 57 (2%) | 58 (5%) | 95 (2%) | 61 (5%) | 119 (2%) | 63 (5%) | 123 (2%) | |||||
| Severe ≥ 8 mmol/L | 25 (3%) | 20 (0.8%) | 29 (3%) | 34 (0.7%) | 29 (2%) | 38 (0.6%) | 31 (2%) | 40 (0.6%) | |||||
| Elixhauser index | Mean | 18 | 13 | < 0.001 | 18 | 13 | < 0.001 | 18 | 13 | < 0.001 | 18 | 12 | < 0.001 |
| Payer | Commercial | 102 (14%) | 403 (16%) | < 0.001 | 145 (13%) | 883 (17%) | < 0.001 | 165 (13%) | 1176 (17%) | < 0.001 | 168 (13%) | 1293 (18%) | < 0.001 |
| Medicaid | 53 (7%) | 334 (13%) | 93 (8%) | 696 (13%) | 114 (9%) | 917 (14%) | 116 (9%) | 989 (14%) | |||||
| Medicare | 494 (65%) | 145 (57%) | 700 (64%) | 2839 (55%) | 780 (63%) | 3668 (54%) | 807 (63%) | 3907 (53%) | |||||
| Other | 48 (6%) | 141 (6%) | 70 (6%) | 317 (6%) | 74 (6%) | 415 (6%) | 75 (6%) | 461 (6%) | |||||
| Unknown | 58 (7%) | 225 (9%) | 93 (9%) | 474 (9%) | 103 (8%) | 627 (9%) | 108 (9%) | 685 (9%) | |||||
| Teaching status | Yes | 516 (68%) | 1620 (64%) | 0.014 | 723 (65.7%) | 3161 (61%) | 0.002 | 803 (65%) | 4057 (60%) | < 0.001 | 824 (65%) | 4342 (59%) | 0.000 |
| Urban/rural status | Urban | 553 (73%) | 2029 (80%) | < 0.001 | 270 (25%) | 991 (19%) | < 0.001 | 930 (75%) | 5513 (81%) | < 0.001 | 963 (76%) | 5965 (81%) | < 0.001 |
| Hospital acute status | Acute | 755 (100%) | 2553 (100%) | 1101 (100%) | 5209 (100%) | 1235 (100%) | 6803 (100%) | 1273 (100%) | 7335 (100%) | ||||
| Elixhauser comorbidities | Congestive heart failure | 316 (42%) | 784 (31%) | < 0.001 | 438 (40%) | 1477 (28%) | < 0.001 | 477 (39%) | 1855 (27%) | < 0.001 | 494 (39%) | 1953 (27%) | < 0.001 |
| Valvular disease | 163 (22%) | 444 (17%) | 0.009 | 234 (21%) | 832 (16%) | < 0.001 | 259 (21%) | 1051 (16%) | < 0.001 | 264 (21%) | 1114 (15%) | < 0.001 | |
| Pulmonary circulation disease | 139 (18%) | 306 (12%) | < 0.001 | 190 (17%) | 596 (11%) | < 0.001 | 211 (17%) | 740 (11%) | < 0.001 | 218 (17%) | 777 (11%) | < 0.001 | |
| Peripheral vascular disease | 174 (23%) | 490 (19%) | 0.020 | 253 (23%) | 950 (18%) | 0.000 | 276 (22%) | 1218 (18%) | < 0.001 | 285 (22%) | 1302 (18%) | < 0.001 | |
| Paralysis | 68 (9%) | 301 (11%) | 0.033 | 101 (9%) | 588 (11%) | 0.041 | 117 (10%) | 726 (11%) | 0.203 | 125 (10%) | 766 (10%) | 0.495 | |
| Other neurological disorders | 250 (33%) | 875 (34%) | 0.554 | 368 (33%) | 1702 (33%) | 0.630 | 413 (33%) | 2202 (32%) | 0.470 | 422 (33%) | 2352 (32%) | 0.456 | |
| Chronic pulmonary disease | 333 (44%) | 1062 (42%) | 0.220 | 510 (46%) | 2159 (42%) | 0.003 | 565 (46%) | 2825 (42%) | 0.006 | 589 (46%) | 3019 (41%) | 0.00 | |
| Diabetes w/o chronic complications | 164 (22%) | 604 (24%) | 0.268 | 242 (22%) | 1223 (24%) | 0.285 | 273 (22%) | 1581 (23%) | 0.376 | 286 (23%) | 1721 (24%) | 0.430 | |
| Diabetes w/chronic complications | 117 (16%) | 341 (13%) | 0.135 | 161 (15%) | 695 (13%) | 0.260 | 184 (15%) | 950 (14%) | 0.392 | 189 (15%) | 1051 (14%) | 0.635 | |
| Hypothyroidism | 178 (24%) | 602 (24%) | 0.998 | 251 (23%) | 1182 (23%) | 0.939 | 281 (23%) | 1469 (22%) | 0.371 | 286 (23%) | 1556 (21%) | 0.321 | |
| Renal failure | 210 (28%) | 535 (21%) | < 0.001 | 304 (28%) | 1007 (19%) | < 0.001 | 336 (27%) | 1342 (20%) | < 0.001 | 344 (27%) | 1440 (20%) | < 0.001 | |
| Liver disease | 138 (18%) | 279 (11%) | < 0.001 | 210 (19%) | 587 (11%) | < 0.001 | 233 (19%) | 790 (12%) | < 0.001 | 241 (19%) | 852 (12%) | < 0.001 | |
| Peptic ulcer disease excl. bleeding | 9 (1%) | 28 (1%) | 0.827 | 15 (1%) | 53 (1%) | 0.314 | 16 (1%) | 64 (0.9%) | 0.249 | 16 (1%) | 69 (0.9%) | 0.294 | |
| Acquired immune deficiency syndrome | 6 (0.8%) | 27 (1%) | 0.523 | 10 (0.9%) | 43 (0.8%) | 0.785 | 11 (0.9%) | 58 (0.9%) | 0.896 | 12 (0.9%) | 63 (0.9%) | 0.769 | |
| Lymphoma | 29 (4%) | 72 (3%) | 0.152 | 52 (5%) | 160 (3%) | 0.006 | 59 (5%) | 188 (3%) | < 0.001 | 61 (5%) | 200 (3%) | < 0.001 | |
| Metastatic cancer | 112 (15%) | 174 (7%) | < 0.001 | 154 (14%) | 365 (7%) | < 0.001 | 184 (15%) | 458 (7%) | < 0.001 | 192 (15%) | 487 (7%) | < 0.001 | |
| Solid tumor without metastasis | 73 (10%) | 234 (9%) | 0.676 | 110 (10%) | 461 (9%) | 0.231 | 119 (10%) | 574 (8%) | 0.170 | 122 (10%) | 608 (8%) | 0.128 | |
| Rheumatoid arthritis/collagen vascular diseases | 46 (6%) | 188 (7%) | 0.231 | 71 (7%) | 367 (7%) | 0.479 | 83 (7%) | 453 (7%) | 0.942 | 84 (7%) | 494 (7%) | 0.852 | |
| Coagulopathy | 285 (38%) | 650 (26%) | < 0.001 | 410 (37%) | 1244 (24%) | < 0.001 | 462 (37%) | 1570 (23%) | < 0.001 | 474 (37%) | 1654 (23%) | < 0.001 | |
| Obesity | 137 (18%) | 590 (23%) | 0.004 | 201 (18%) | 1186 (23%) | 0.001 | 229 (19%) | 1618 (24%) | < 0.001 | 237 (19%) | 1767 (24%) | < 0.001 | |
| Weight loss | 276 (37%) | 730 (29%) | < 0.001 | 405 (37%) | 1360 (26%) | <0.001 | 447 (36%) | 1674 (25%) | < 0.001 | 458 (36%) | 1752 (24%) | < 0.001 | |
| Fluid and electrolyte disorders | 638 (85%) | 1986 (78%) | <0.001 | 922 (84%) | 4007 (77%) | < 0.001 | 1032 (84%) | 5195 (76%) | <0.001 | 1063 (83%) | 5575 (76%) | < 0.001 | |
| Chronic blood loss anemia | 41 (5%) | 122 (5%) | 0.467 | 69 (6%) | 227 (4%) | 0.007 | 76 (6%) | 285 (4%) | 0.002 | 76 (6%) | 303 (4%) | 0.003 | |
| Deficiency anemias | 448 (59%) | 1399 (55%) | 0.027 | 644 (59%) | 2759 (53%) | 0.001 | 723 (59%) | 3516 (52%) | < 0.001 | 747 (59%) | 3744 (51%) | < 0.001 | |
| Alcohol abuse | 106 (14%) | 294 (12%) | 0.062 | 156 (14%) | 579 (11%) | 0.004 | 175 (15%) | 785 (12%) | 0.009 | 179 (14%) | 869 (12%) | 0.026 | |
| Drug abuse | 65 (9%) | 272 (11%) | 0.103 | 90 (8%) | 583 (11%) | 0.003 | 100 (8%) | 772 (11%) | 0.001 | 105 (8%) | 842 (12%) | 0.001 | |
| Psychoses | 88 (12%) | 353 (14%) | 0.123 | 13 (12%) | 750 (14%) | 0.044 | 150 (12%) | 990 (15%) | 0.025 | 156 (12%) | 1066 (15%) | 0.031 | |
| Depression | 197 (26%) | 722 (28%) | 0.238 | 294 (27%) | 1464 (28%) | 0.346 | 325 (26%) | 1876 (28%) | 0.353 | 339 (27%) | 2022 (28%) | 0.480 | |
| Hypertension | 556 (74%) | 1782 (70%) | 0.042 | 808 (73%) | 3606 (69%) | 0.006 | 914 (74%) | 4741 (70%) | 0.003 | 944 (74%) | 5140 (71%) | 0.004 | |
Because of rounding, categories will not always add to 100%
APACHE III score includes physiology, chronic health investigation, and age variables [18]
*Variables included in the adjustment of regression models for myocardial injury
**Percentages for the variables types in this table are calculated by using a denominator which is the total number of patients within that particular variable group
We conservatively estimated that if the probability of in-hospital mortality was 13% with the hypotension exposure of interest at its mean and the probability of in-hospital mortality was 16% when hypotension exposure was one standard deviation above the mean, then the sample size would need to be 1766 to detect a difference as great as this or larger with 90% power and alpha = 0.05 [14–17]. These power calculations further assumed a low correlation of 0.2 between the hypotension exposure and other predictors in the model. Consequently, we concluded that the sample size would be more than adequate to detect clinically significant associations with hypotension and the primary outcome of mortality. All statistical analyses were performed using Stata/MP 15.1 for Windows (StataCorp, College Station, TX, USA).
Results
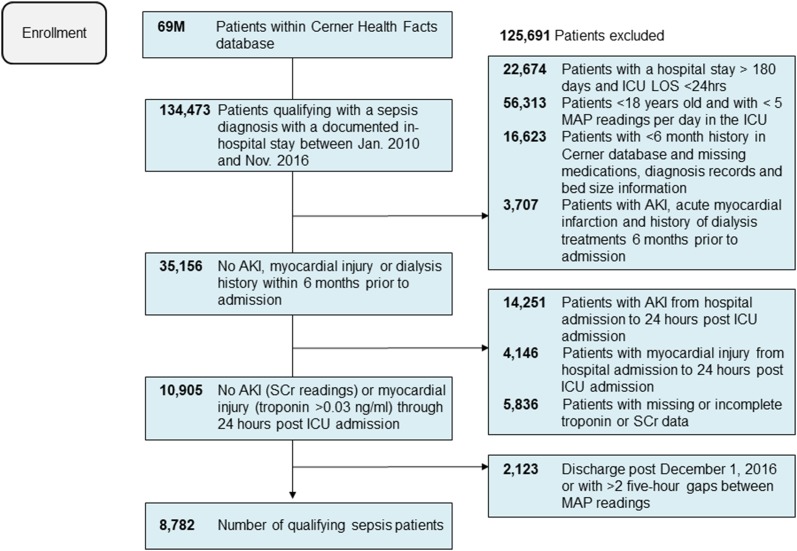
We identified 8782 patients from 110 hospitals after applying all inclusion and exclusion criteria (Fig. 1). The mean (SD) age of the patients was 63 (18) years. Of these, 79% were self-identified as Caucasian and 48% were male. The mean (SD) APACHE III score was 61 (20) [18]. The unadjusted in-hospital mortality rate was 14.6% (n = 1283). Fifteen percent (n = 1315) experienced AKI and 0.7% (n = 63) experienced myocardial injury during the study period (AKI and myocardial injury rates appear low because patients who developed AKI or myocardial injury during the initial 24 ICU hours were excluded, Fig. 1). Table 1 and Online Resource 4 list all the covariates included in the regression models for the outcomes of in-hospital mortality and AKI, respectively. For myocardial injury, the regression models adjusted for hypotension along with age, sex, APACHE III score, and the Elixhauser comorbidities of congestive heart failure, diabetes with complications, and renal failure.
Fig. 1.
Patient attrition diagram. AKI acute kidney injury
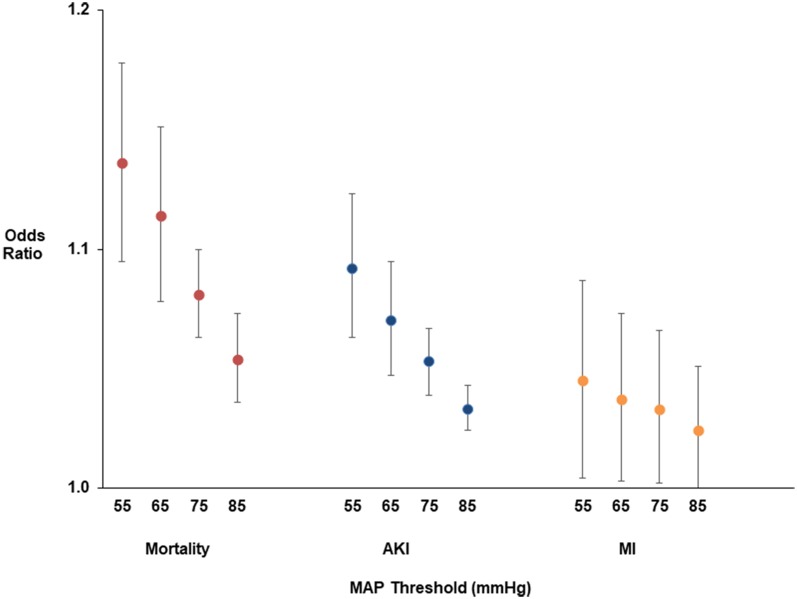
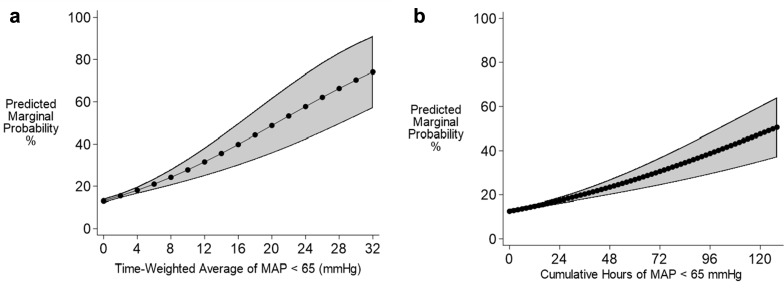
The odds ratios with 95% confidence intervals for the regression models with TWA-MAP are graphed in Fig. 2. The primary hypotension exposure of TWA-MAP < 65 mmHg was positively correlated with in-hospital mortality. The analysis indicates that for every one mmHg increase in TWA-MAP < 65 mmHg, the odds of in-hospital mortality increase by 11.4%; 95% CI (7.8, 15.1%); p < 0.001 (Fig. 2 and Online Resource 5). Sensitivity analyses show the odds ratios decreased as the MAP threshold increased from 55 to 85 mmHg. The predicted marginal probabilities of in-hospital mortality across TWA-MAP < 65 mmHg are shown Fig. 3a.
Fig. 2.
Association of hypotension exposure with in-hospital mortality, AKI and myocardial injury. Adjusted odds ratios and 95% confidence intervals for a 1 mmHg increase in TWA-MAP, below different thresholds are shown for the primary outcome of in-hospital mortality and secondary outcomes of acute kidney injury and myocardial injury
Fig. 3.
Predicted mortality outcome for time-weighted average (TWA)-MAP below 65 mmHg and cumulative hours of MAP below 65 mmHg. Predicted probability of mortality from the TWA-MAP < 65 mmHg threshold and cumulative hours of MAP < 65 mmHg are represented in panels a and b, respectively
Cumulative time below a MAP threshold of 65 mmHg revealed that every 2 h (120 min) increased the odds of in-hospital mortality by 3.6%; 95% CI (2.5, 4.8%); p < 0.001 (Online Resource 6). The predicted marginal probabilities of in-hospital mortality for cumulative time of MAP < 65 mmHg showed similar trends to probabilities for TWA-MAP (Fig. 3). Predicted marginal probabilities are shown in Online Resource 7.
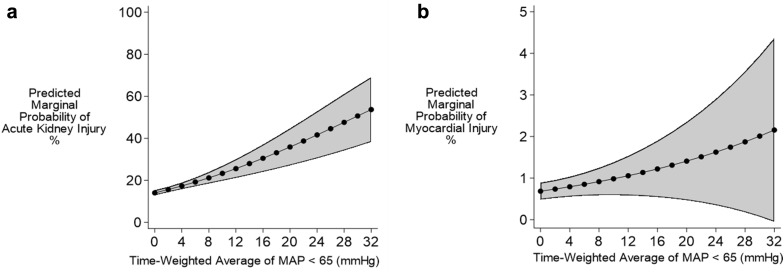
The relationship between TWA-MAP and AKI was similar to in-hospital mortality (Fig. 4a). For every one mmHg increase in TWA-MAP < 65 mmHg, the odds of developing AKI increase by 7.0%; 95% CI (4.7, 9.5%); p < 0.001 (Fig. 2). Patients who spent between 6 and 8 h in the ICU with MAP < 65 mmHg had odds of developing AKI 37% higher (95% CI 3, 82%; p = 0.031) compared to patients with no time below MAP of 65 mmHg (Online Resource 6). Although TWA-MAP below 55, 75, and 85 mmHg showed a positive correlation (p < 0.001) with developing AKI, we did not see similar trends for cumulative time below thresholds of 75 and 85 mmHg (Online Resource 6). Here, patients with the longest times below the thresholds tended to have fewer AKI events than the increasingly small number of patients with no time below these thresholds. This apparent contradiction is, in part, due to over-representation of the time below MAP threshold in the longest duration category, as these patient records had more and longer gaps between MAP readings.
Fig. 4.
Predicted marginal probability for AKI and myocardial injury for TWA-MAP below 65 mmHg threshold. AKI and myocardial injury predicted probability from the TWA-MAP below 65 mmHg threshold are shown in panels a and b, respectively. Both exposures showed a linear relationship with the secondary outcomes of AKI and myocardial injury. AKI acute kidney injury
For every one mmHg increase in TWA-MAP < 65 mmHg, the odds of developing myocardial injury increased by 3.7%; 95% CI (0.3, 7.3%), p = 0.03 (Fig. 2, Online Resource 5). We derived marginal probabilities of developing myocardial injury for TWA-MAP (Fig. 4b) and cumulative hours of MAP below 65 mmHg (Online Resource 7). Unlike the relationship for in-hospital mortality and AKI, there was no significant worsening of myocardial injury at lower MAP thresholds (Fig. 4b and Online Resource 8). A sensitivity analysis that repeated the regression modeling restricted to survivors found similar associations between hypotension at the 65 mmHg threshold and the outcomes of AKI and myocardial injury (data not shown).
Discussion
Given the complexity of defining hypotension exposure, we analyzed both time-weighted average (TWA) and the cumulative time under specific thresholds. TWA-MAP is a comprehensive measure of hypotension exposure because it measures both the degree and the duration below a threshold. Continuous time below a MAP threshold is intuitive but neglects severity of hypotension and is subject to two types of variation: the frequency of MAP readings, and the total time for MAP exposure calculation. Spurious extreme values can occur and distort the results due to (1) gaps between readings (carried forward) that can over-represent times below a given MAP threshold, especially as there were more and longer gaps in patients who spent long periods below various thresholds; and (2) patients with longer ICU stays may have been especially prone to hypotension. To minimize both sources of error, we excluded patients with fewer than five blood pressures recorded per each 24-h period, and those who had more than two gaps exceeding 5 h between readings.
We observed strong associations between the TWA-MAP below various thresholds and in-hospital mortality and kidney injury in septic patients. Substantial mortality risk was evident even among the higher thresholds, and the risk progressively increased as the MAP thresholds decreased from 85 to 55 mmHg. A similar relationship between in-hospital mortality and cumulative time < 65 mmHg was also observed. It is important to note that the TWA-MAP and cumulative time measures below a given threshold are nested and not mutually exclusive (e.g., patients with TWA-MAP < 55 mmHg are included in the analysis of TWA-MAP < 65 mmHg). We, therefore, cannot definitively determine an optimal threshold with this study design alone.
As with in-hospital mortality, we observed that odds of developing AKI was associated with hypotension characterized by TWA-MAP, with the odds of developing AKI being greatest for MAP readings < 55 mmHg and lowest for MAP < 85 mmHg. However, similar trends were not observed in the cumulative minutes of MAP below threshold groups. We theorize that is because we excluded AKI and myocardial injury before and within 24 h of ICU admission and restricted this outcome to the first 7 days of exposure. Also, myocardial injury may be undercounted when routine troponin monitoring is not performed.
Overall, our results are consistent with previous literature with notable exceptions. Prior research found that increasing the MAP from 65 to 85 mmHg with norepinephrine does not significantly affect systemic oxygen metabolism, skin microcirculatory blood flow, urine output, renal function, or splanchnic perfusion—although cardiac index increased [19, 20]. However, a prospective study of thirteen patients with septic shock found that increasing MAP to above 65 mmHg with norepinephrine increased cardiac output, improved microvascular function, and was associated with decreased blood lactate concentrations. The investigators noticed that microvascular responses varied considerably among patients, suggesting that individualization of blood pressure targets may be warranted [21].
Only limited evidence from randomized trials provides guidance on optimal thresholds. Asfar et al. [22] randomized 776 septic shock patients and reported that 28- and 90-day mortality did not differ significantly between those who were treated to reach a target MAP of 80–85 mmHg and those who were treated to reach a target of 65–70 mmHg [19]. However, even in the lower MAP target group the blood pressure was maintained at 70–75 mmHg and authors noticed a lower than expected death rate, which supports our findings using a much larger cohort and suggests a threshold above 65 mmHg may be more appropriate. This study also highlights the complexity of performing randomized trials in this population and the value of our analysis.
We observed that 14.6% of patients with sepsis died during hospitalization over the period from 2010 to 2016. Presumably patients in our cohort were sicker than all sepsis patients given a required ICU stay of at least 1 day. However, two recent European studies found sepsis mortality to range from 8 to 26%. [18, 19] This is in contrast to higher in-hospital mortality of 26% reported from analysis of a German patient population from 2007 to 2013 [23]. Furthermore, Freund and colleagues [24], based on 2016 European hospital data, observed 8% in-hospital mortality in patients with suspected sepsis. These results suggest that outcomes from sepsis may be improving over time.
In general, AKI in critically ill patients affects approximately 40% of the patients at some time during their stay and one third who develop renal injury die within 90 days [16]. In a study consistent with ours, hypotensive episodes of MAP < 73 mmHg were associated with progression of AKI in critically ill patients with severe sepsis [17].
Myocardial injury, measured by troponin elevation, may be as high as 15–25% in ICU patients, but is often unrecognized because routine troponin monitoring remains uncommon [16, 25]. When troponins are routinely monitored in septic ICU patients, only 7% of biomarker elevations happened within 24 h of ICU admission [26]. While our definition of myocardial injury artificially lowered the observed rate by excluding cases within 24 h of ICU admission and after AKI development), we still observed significant association between TWA-MAP < 65 mmHg and myocardial injury. However, it is possible that a raised troponin value is present in the absence of myocardial injury [27], although raised troponin values have been tied with myocardial injury within the septic population [28–31]. Our results of myocardial injury analysis should be interpreted with caution due to lack of universal troponin screening and diverse troponin tests employed among various U.S. Hospitals.
We report associations between hypotension in ICU patients and both myocardial and kidney injury. However, we report associations which are surely at least to some degree confounded by unobserved baseline patient characteristics. Randomized trials will be required to confirm causal relationships that may benefit from intervention. Another study limitation is our inability to distinguish between untreated hypotension and hypotension that persisted despite treatment—and thus presumably indicated worse sepsis. To address this, we adjusted for medication use and other potential confounders. Nevertheless, unmeasured confounding remains likely. For example, septic patients are always given antibiotics, some of which are nephrotoxic. However, we did not attempt to link specific antibiotics to AKI. Hypotension identification and duration is dependent upon the frequency of recorded blood pressure readings. While some MAP data (up to two 5-h gaps per record) were missing, an average 357 MAP readings were available per ICU day which indicates that the exposure was generally well characterized.
We were also unable to distinguish hypotension that is a marker of severe sepsis from hypotension that directly contributed to organ dysfunction. The distinction is important because interventions to reduce hypotension will only improve the fraction of organ dysfunction that is causally related to blood pressure. Additionally, some treatments for hypotension can themselves provoke organ injury. For example, increased rates of atrial fibrillation are noted with higher vasopressor use [22]. Nonetheless, our results suggest that harm may begin to accrue well above the currently recommended initial threshold of 65 mmHg, and higher for older patients and those with cardiovascular comorbidities [3]. However, the definitive way to answer how the duration of hypotension impacts mortality and other outcomes in critically ill sepsis patients is via a prospective, randomized controlled trial that follows a standard protocol for vasopressor and intravenous fluid use. This study did not examine outcomes post ICU or hospital discharge; therefore the association with mid- to long-term outcomes are unknown. And finally, while our measure of ICU duration is based on the timing of laboratory and medication orders, given their frequency in critically ill patients, this limitation seems unlikely to bias our results substantially.
In summary, the Surviving Sepsis Guidelines suggest keeping mean arterial pressure initially above 65 mmHg, followed by individualized treatment to optimize tissue perfusion. In our analysis, risks for mortality, AKI and myocardial injury were apparent by 85 mmHg, and for mortality and AKI risk progressively worsened at lower thresholds. Until randomized trials show that the relationship between hypotension and serious complications is not causal, it would probably be prudent to keep mean arterial pressure well above 65 mmHg in septic ICU patients.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors wish to thank Dr. Seungyoung Hwang for his assistance with data analysis. This research was supported by Edwards Lifesciences, Irvine, CA.
Compliance with ethical standards
Conflicts of interest
Drs. Maheshwari and Sessler work as consultants for Edwards Lifesciences. Dr. Khanna consults for La Jolla pharmaceuticals. Drs. Khangulov, Munson and Badani work as consultants for Boston Strategic Partners, Inc. who received funds from Edwards Lifesciences to perform the research. Dr. Nathanson is an employee of OptiStatim, LLC, which received consulting fees from Boston Strategic Partners, Inc.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s00134-018-5218-5) contains supplementary material, which is available to authorized users.
References
- 1.Hall MJ, Williams SN, DeFrances CJ, Golosinskiy A. Inpatient care for septicemia or sepsis: a challenge for patients and hospitals. NCHS Data Brief. 2011;62:1–8. [PubMed] [Google Scholar]
- 2.De Backer D, Dorman T. Surviving sepsis guidelines: a continuous move toward better care of patients with sepsis. JAMA. 2017;317:807–808. doi: 10.1001/jama.2017.0059. [DOI] [PubMed] [Google Scholar]
- 3.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, Rochwerg B, Rubenfeld GD, Angus DC, Annane D, Beale RJ, Bellinghan GJ, Bernard GR, Chiche JD, Coopersmith C, De Backer DP, French CJ, Fujishima S, Gerlach H, Hidalgo JL, Hollenberg SM, Jones AE, Karnad DR, Kleinpell RM, Koh Y, Lisboa TC, Machado FR, Marini JJ, Marshall JC, Mazuski JE, McIntyre LA, McLean AS, Mehta S, Moreno RP, Myburgh J, Navalesi P, Nishida O, Osborn TM, Perner A, Plunkett CM, Ranieri M, Schorr CA, Seckel MA, Seymour CW, Shieh L, Shukri KA, Simpson SQ, Singer M, Thompson BT, Townsend SR, Van der Poll T, Vincent JL, Wiersinga WJ, Zimmerman JL, Dellinger RP. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Crit Care Med. 2017;45:486–552. doi: 10.1097/CCM.0000000000002255. [DOI] [PubMed] [Google Scholar]
- 4.Iskander KN, Osuchowski MF, Stearns-Kurosawa DJ, Kurosawa S, Stepien D, Valentine C, Remick DG. Sepsis: multiple abnormalities, heterogeneous responses, and evolving understanding. Physiol Rev. 2013;93:1247–1288. doi: 10.1152/physrev.00037.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seymour CW, Liu VX, Iwashyna TJ, Brunkhorst FM, Rea TD, Scherag A, Rubenfeld G, Kahn JM, Shankar-Hari M, Singer M, Deutschman CS, Escobar GJ, Angus DC. Assessment of clinical criteria for sepsis: for the third international consensus definitions for sepsis and septic shock (sepsis-3) JAMA. 2016;315:762–774. doi: 10.1001/jama.2016.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leone M, Asfar P, Radermacher P, Vincent JL, Martin C. Optimizing mean arterial pressure in septic shock: a critical reappraisal of the literature. Crit Care. 2015;19:101. doi: 10.1186/s13054-015-0794-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The third international consensus definitions for sepsis and septic shock (sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maheswari KS, Munson S, Nathanson B, Hwang S, Khanna A. Relationship between intensive care unit hypotension and morbidity in patients diagnosed with sepsis. Crit Care. 2018;22(Suppl):1. [Google Scholar]
- 9.Salmasi V, Maheshwari K, Yang D, Mascha EJ, Singh A, Sessler DI, Kurz A. Relationship between intraoperative hypotension, defined by either reduction from baseline or absolute thresholds, and acute kidney and myocardial injury after noncardiac surgery: a retrospective cohort analysis. Anesthesiology. 2017;126:47–65. doi: 10.1097/ALN.0000000000001432. [DOI] [PubMed] [Google Scholar]
- 10.Kellum J, Lameire N, Co-Chairs WG. Kidney disease: improving global outcomes (KDIGO). KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1–138. doi: 10.1038/kisup.2012.1. [DOI] [Google Scholar]
- 11.Austin PC, Tu, Jack V (2004) Bootstrap methods for developing predictive models. In: Book bootstrap methods for developing predictive models. American Statistical Association, pp 131–137
- 12.Williams RL. A note on robust variance estimation for cluster-correlated data. Biometrics. 2000;56:645–646. doi: 10.1111/j.0006-341X.2000.00645.x. [DOI] [PubMed] [Google Scholar]
- 13.Wooldridge JM. Econometric analysis of cross section and panel data. Cambridge: MIT Press; 2010. [Google Scholar]
- 14.Knoop ST, Skrede S, Langeland N, Flaatten HK. Epidemiology and impact on all-cause mortality of sepsis in Norwegian hospitals: a national retrospective study. PLoS ONE. 2017;12:e0187990. doi: 10.1371/journal.pone.0187990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Melville J, Ranjan S, Morgan P. ICU mortality rates in patients with sepsis before and after the Surviving Sepsis Campaign. Crit Care. 2015;19(Suppl1):P15. doi: 10.1186/cc14095. [DOI] [Google Scholar]
- 16.Pettila V, Bellomo R. Understanding acute kidney injury in sepsis. Intensive Care Med. 2014;40:1018–1020. doi: 10.1007/s00134-014-3313-9. [DOI] [PubMed] [Google Scholar]
- 17.Poukkanen M, Wilkman E, Vaara ST, Pettila V, Kaukonen KM, Korhonen AM, Uusaro A, Hovilehto S, Inkinen O, Laru-Sompa R, Hautamaki R, Kuitunen A, Karlsson S, Group FS. Hemodynamic variables and progression of acute kidney injury in critically ill patients with severe sepsis: data from the prospective observational FINNAKI study. Crit Care. 2013;17:R295. doi: 10.1186/cc13161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knaus WA, Wagner DP, Draper EA, Zimmerman JE, Bergner M, Bastos PG, Sirio CA, Murphy DJ, Lotring T, Damiano A, et al. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991;100:1619–1636. doi: 10.1378/chest.100.6.1619. [DOI] [PubMed] [Google Scholar]
- 19.Bourgoin A, Leone M, Delmas A, Garnier F, Albanese J, Martin C. Increasing mean arterial pressure in patients with septic shock: effects on oxygen variables and renal function. Crit Care Med. 2005;33:780–786. doi: 10.1097/01.CCM.0000157788.20591.23. [DOI] [PubMed] [Google Scholar]
- 20.LeDoux D, Astiz ME, Carpati CM, Rackow EC. Effects of perfusion pressure on tissue perfusion in septic shock. Crit Care Med. 2000;28:2729–2732. doi: 10.1097/00003246-200008000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Thooft A, Favory R, Salgado DR, Taccone FS, Donadello K, De Backer D, Creteur J, Vincent JL. Effects of changes in arterial pressure on organ perfusion during septic shock. Crit Care. 2011;15:R222. doi: 10.1186/cc10462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asfar P, Meziani F, Hamel JF, Grelon F, Megarbane B, Anguel N, Mira JP, Dequin PF, Gergaud S, Weiss N, Legay F, Le Tulzo Y, Conrad M, Robert R, Gonzalez F, Guitton C, Tamion F, Tonnelier JM, Guezennec P, Van Der Linden T, Vieillard-Baron A, Mariotte E, Pradel G, Lesieur O, Ricard JD, Herve F, du Cheyron D, Guerin C, Mercat A, Teboul JL, Radermacher P, Investigators S. High versus low blood-pressure target in patients with septic shock. N Engl J Med. 2014;370:1583–1593. doi: 10.1056/NEJMoa1312173. [DOI] [PubMed] [Google Scholar]
- 23.Fleischmann C, Thomas-Rueddel DO, Hartmann M, Hartog CS, Welte T, Heublein S, Dennler U, Reinhart K. Hospital incidence and mortality rates of sepsis. Dtsch Arztebl Int. 2016;113:159–166. doi: 10.3238/arztebl.2016.0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freund Y, Lemachatti N, Krastinova E, Van Laer M, Claessens YE, Avondo A, Occelli C, Feral-Pierssens AL, Truchot J, Ortega M, Carneiro B, Pernet J, Claret PG, Dami F, Bloom B, Riou B, Beaune S, French Society of Emergency Medicine Collaborators Group Prognostic Accuracy of Sepsis-3 Criteria for In-Hospital Mortality Among Patients With Suspected Infection Presenting to the Emergency Department. JAMA. 2017;317:301–308. doi: 10.1001/jama.2016.20329. [DOI] [PubMed] [Google Scholar]
- 25.Lim W, Qushmaq I, Cook DJ, Crowther MA, Heels-Ansdell D, Devereaux PJ, Troponin TTG. Elevated troponin and myocardial infarction in the intensive care unit: a prospective study. Crit Care. 2005;9:R636–R644. doi: 10.1186/cc3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frencken JF, Donker DW, Spitoni C, Koster-Brouwer ME, Soliman IW, Ong DSY, Horn J, van der Poll T, van Klei WA, Bonten MJM, Cremer OL. Myocardial injury in patients with sepsis and its association with long-term outcome. Circ Cardiovasc Qual Outcomes. 2018;11:e004040. doi: 10.1161/CIRCOUTCOMES.117.004040. [DOI] [PubMed] [Google Scholar]
- 27.Ammann P, Fehr T, Minder EI, Gunter C, Bertel O. Elevation of troponin I in sepsis and septic shock. Intensive Care Med. 2001;27:965–969. doi: 10.1007/s001340100920. [DOI] [PubMed] [Google Scholar]
- 28.Bessiere F, Khenifer S, Dubourg J, Durieu I, Lega JC. Prognostic value of troponins in sepsis: a meta-analysis. Intensive Care Med. 2013;39:1181–1189. doi: 10.1007/s00134-013-2902-3. [DOI] [PubMed] [Google Scholar]
- 29.Sheyin O, Davies O, Duan W, Perez X. The prognostic significance of troponin elevation in patients with sepsis: a meta-analysis. Heart Lung. 2015;44:75–81. doi: 10.1016/j.hrtlng.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 30.Mehta NJ, Khan IA, Gupta V, Jani K, Gowda RM, Smith PR. Cardiac troponin I predicts myocardial dysfunction and adverse outcome in septic shock. Int J Cardiol. 2004;95:13–17. doi: 10.1016/j.ijcard.2003.02.005. [DOI] [PubMed] [Google Scholar]
- 31.Jeong HS, Lee TH, Bang CH, Kim JH, Hong SJ. Risk factors and outcomes of sepsis-induced myocardial dysfunction and stress-induced cardiomyopathy in sepsis or septic shock: a comparative retrospective study. Medicine (Baltimore) 2018;97:e0263. doi: 10.1097/MD.0000000000010263. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.