Abstract
In vitro screens for nephrotoxicity are currently poorly predictive of toxicity in humans. Although the functional proteins that are expressed by nephron tubules and mediate drug susceptibility are well known, current in vitro cellular models poorly replicate both the morphology and the function of kidney tubules and therefore fail to demonstrate injury responses to drugs that would be nephrotoxic in vivo. Advances in protocols to enable the directed differentiation of pluripotent stem cells into multiple renal cell types and the development of microfluidic and 3D culture systems have opened a range of potential new platforms for evaluating drug nephrotoxicity. Many of the new in vitro culture systems have been characterized by the expression and function of transporters, enzymes, and other functional proteins that are expressed by the kidney and have been implicated in drug-induced renal injury. In vitro platforms that express these proteins and exhibit molecular biomarkers that have been used as readouts of injury demonstrate improved functional maturity compared with static 2D cultures and represent an opportunity to model injury to renal cell types that have hitherto received little attention. As nephrotoxicity screening platforms become more physiologically relevant, they will facilitate the development of safer drugs and improved clinical management of nephrotoxicants.
The specialized role of the kidney in filtering substances from the blood to maintain volume and electrolyte homeostasis, coupled with the high metabolic activity of the renal tubule epithelium, makes the kidney particularly vulnerable to drug-induced injury. A wide variety of commonly used pharmaceutical compounds are nephrotoxic; therefore, the degree of nephrotoxicity of each compound has to be balanced against its utility and is often dose limiting. For example, antibiotics (such as gentamicin and vancomycin) and immunosuppressive agents (including ciclosporin) can induce tubular injury1, whereas lithium, which is frequently prescribed for bipolar disorder, can cause damage to the collecting duct2. Several epidemiological studies have shown a strong association between the use of common drugs, such as antiretroviral agents and aminoglycoside antibiotics, and the risk of acute kidney injury (AKI)3. However, the development of drug derivatives with improved renal safety profiles has proved challenging as currently available in vitro screening methods are poorly predictive of nephrotoxicity in animal models or humans4. Of note, preclinical studies can also fail to identify nephrotoxicity owing to species-specific variations in the metabolic response to various pharmaceutical agents and in the expression of certain genes4.
The failure of in vitro drug screening methods to identify nephrotoxic activity results from a combination of factors. A major contributing factor is the lack of valid in vitro cell models of the kidney5. A second is the lack of robust markers of kidney injury in both in vitro and in vivo studies5,6. The fact that drugs can interact with each other and/or compete for detoxification enzyme complexes further complicates screening and presents difficulties in terms of predicting which drug combinations can be safely used by a patient7–9. Finally, the market has failed to develop models with which to predict drug responses of individual patients, for example, owing to genetic variations in cytochrome P450 (CYP) enzymes10.
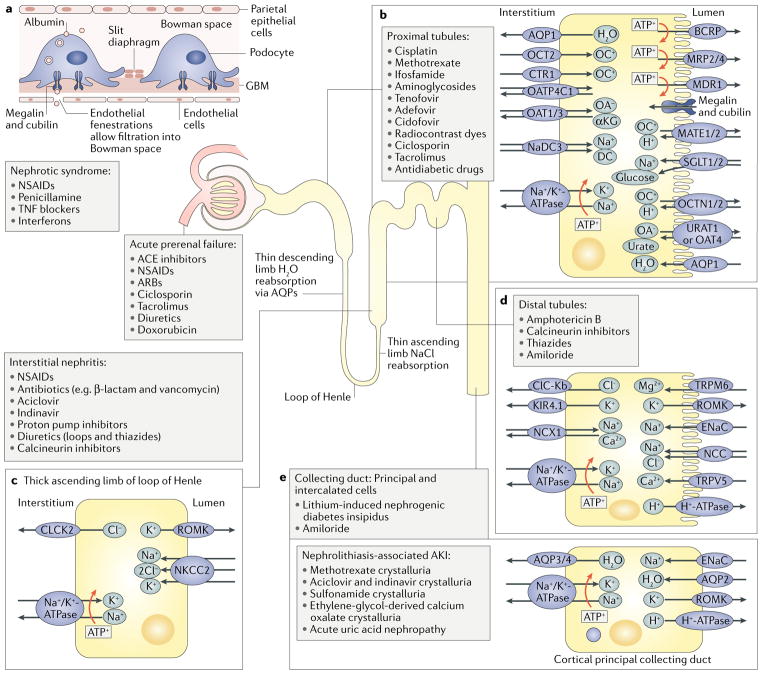
Current in vitro screens for nephrotoxic compounds have focused mostly on proximal tubule cells because this segment of the nephron is an important target of nephrotoxic injury in vivo. The proximal tubules secrete xenobiotics into the filtrate and reabsorb glucose, albumin, and various electrolytes via an array of transporters and receptors that can also transport drugs. To generate energy for these processes, proximal tubule cells are rich in mitochondria; thus, proximal tubule cells are also sensitive to disruptions in oxidative phosphorylation11. Moreover, metabolic enzymes such as β-lyase, expressed in renal proximal tubule cells, can bioactivate xenobiotics, potentiating the toxicity of these agents. However, nephrotoxic injury is not restricted to the proximal tubules, with all segments of the nephron, including the podocytes, distal nephrons, and collecting ducts, displaying specific drug sensitivities (FIG. 1). In addition, the kidney microvasculature is also susceptible to drug-induced injury, which can cause diminished blood flow, hypoxic injury, and inflammation with consequences on tubule function12.
Fig. 1. Renal transporters and targets of nephrotoxicants.
Different segments of the nephron express various transporters and receptors that affect the susceptibility of the segments to the nephrotoxic effects of different drugs. a | In addition to the specific nephrotoxic effects of agents on different transporters in the tubule (discussed below), drugs such as nonsteroidal anti-inflammatory drugs (NSAIDs) can cause nephrotic syndrome by inducing immunoglobulin deposition on the glomerular basement membrane (GBM), damaging the membrane and podocytes. b | The proximal tubule expresses many transporters and receptors that are affected by pharmaceutical agents. For example, tenofovir, cisplatin, and gentamicin have affinity for organic anion transporters (OATs), organic cation transporters (OCTs), and endocytic receptors, respectively, allowing them to accumulate within the cell and cause toxic injury. c | The loop of Henle expresses aquaporins (AQPs) and ion transporters in different segments. d | The distal tubule expresses unique ion transporters. Drugs such as ciclosporin can affect the expression of these transporters and cause adverse effects such as Mg2+ and Na+ loss. e | The collecting duct is composed of principal and intercalated cells. The principal cells express epithelial sodium channel (ENaC), which can transport lithium, contributing to lithium-induced diabetes insipidus. In addition, pharmaceutical agents can cause nonspecific nephrotoxic injury by inducing ischaemia, interstitial nephritis, or microangiopathy (not shown). αKG, α-ketoglutarate; ACE, angiotensin-converting enzyme; AKI, acute kidney injury ; ARB, angiotensin receptor blocker; BCRP, breast cancer resistance protein; CLCK2, H+/Cl− exchange transporter 5 (also known as CLCN5); ClC-Kb, chloride channel protein ClC-Kb (also known as CLCNKB); CTR1, copper transporter 1; DC, dicarboxylate cotransporter; KIR4.1, ATP-dependent inwardly rectifying potassium channel KIR4.1 (also known as KCNJ10); MATE, multidrug and toxin extrusion protein; MDR1, multidrug resistance protein 1; MRP, multidrug resistance-associated protein; NaDC3, Na+/dicarboxylate cotransporter 3; NCC, Na+/Cl− cotransporter; NCX1, Na+/Ca2+-exchange protein 1 (also known as SLC8A1); NKCC2, solute carrier family 12 member 1 (also known as SLC12A1); OA, organic anion; OATP4C1, solute carrier organic anion transporter family member 4C1 (also known as SLCO4C1); OC, organic cation; OCTN1, organic cation/carnitine transporter 1 (also known as SLC22A4); ROMK, ATP-sensitive inward rectifier potassium channel 1 (also known as KCNJ1); SGLT, sodium/glucose cotransporter; TNF, tumour necrosis factor; TRPM6, transient receptor potential cation channel subfamily M member 6; TRPV5, transient receptor potential cation channel subfamily V member 5; URAT1, urate anion exchanger 1 (also known as SLC22A12).
The development of in vitro models to reliably predict the nephrotoxicity of potential pharmacologic agents requires a solid understanding of the specific cellular targets and consequences of nephrotoxicants as well as robust and reliable mechanistic biomarkers of nephrotoxicity. Two-dimensional cultures of primary or conditionally immortalized renal epithelial cells have traditionally represented the gold standard in vitro model for nephrotoxicant assessment. However, proximal tubular epithelial cells cultured using this approach exhibit poor apical–basal polarization and low levels of key protein transporters such as organic anion transporters (OATs) and organic cation transporter 2 (OCT2), hindering their utility as tools for predicting the effects of nephrotoxic agents in vivo13,14. Advances in the directed differentiation of human pluripotent stem cells (hPSCs) to multiple renal cell types, as well as in microfluidic culture systems, have opened up a range of potential novel platforms for in vitro nephrotoxicity screening. Here, we discuss the transporters, enzymes, and proteins expressed by the many different cell types in the kidney that have been implicated in drug-induced injury as well as molecular biomarkers that have been used as readouts of injury in in vitro models. We describe mechanisms of nephrotoxic injury, the functional features of tubular cell models that are essential for predicting the toxicity of pharmaceutical compounds, and novel in vitro cell models that are currently under development.
Mechanisms of nephrotoxic injury
Nephrotoxicants cause injury by selectively injuring specific cell types or by nonselectively injuring multiple cell types within the kidney, depending on their mechanism of action1 (FIG. 1). The direct effects of nephrotoxicants have been most extensively studied for epithelial cells in the proximal tubule. However, tubular epithelial cells express a wide range of transporters, many of which are unique to specific segments of the tubule; hence, drugs with an affinity for those transporters can cause cell death in specific nephron segments. By contrast, some drugs, such as amphotericin B, cause tubular toxicity by nonspecifically damaging the membranes of epithelial cells throughout the tubule15. In addition, tubular epithelial cells can suffer injury as a result of the osmotic effects of pharmaceutical compounds, drug-induced nephrolithiasis, or from drug-induced ischaemic events. For example, contrast media used for procedures such as angiography can induce nephropathy through the induction of oxidative stress and osmotic effects as well as haemodynamic changes16.
The interstitial cells that surround the tubules are also targets of drug toxicity. Acute interstitial nephritis is most often the result of an inflammatory, non-dose-dependent response to a drug that causes immunoglobulin deposition in tubule basement membranes. By contrast, chronic interstitial nephritis is often associated with the long-term use of drugs such as ciclosporin, which can also cause acute tubular injury through the induction of inflammatory changes and interstitial fibrosis1.
Podocytes, which maintain the filtration barrier in glomeruli, are also targets of drug-induced nephrotoxicity17. Similar to acute interstitial nephritis, drug-induced podocyte injury is often mediated by immune mechanisms. However, certain drugs have direct toxic effects on podocytes. For example, puromycin, which is taken up by podocytes via the plasma membrane monoamine transporter (PMAT; also known as ENT4 and encoded by SLC29A4), and bisphosphonates18–20 are thought to cause podocyte injury by disrupting their cytoskeleton18. Once injured, podocytes undergo a dedifferentiation programme, which disrupts the glomerular filtration barrier, leading to nephrotic syndrome. The resulting proteinuria can cause secondary tubular injury (FIG. 1).
Beyond their nephrotoxic effects on specific renal cell types, drugs can exert injurious effects on the haemodynamic regulatory mechanisms of the kidney. Renal blood flow and glomerular filtration rate (GFR) are regulated by complex mechanisms, including prostaglandin and renin–angiotensin system signalling pathways1. Several drug classes, such as nonsteroidal anti-inflammatory drugs (NSAIDs) and angiotensin-converting enzyme (ACE) inhibitors, can disrupt these pathways21,22, reducing GFR and potentially causing renal ischaemia and cell death through excessive vasoconstriction.
The kidney also contains a highly diverse population of endothelial cells that form its microvasculature. These cells are also sensitive to drug-induced injury. Unlike the renal tubules, these endothelial cells lack regenerative capacity12; hence, acute injury of the renal vasculature can predispose patients to the development of chronic kidney disease23. Nephrotoxicants can directly affect vascular responsiveness by modulating endothelial barrier function, coagulation cascades, and/or inflammatory processes24. Tubulovascular crosstalk takes place through vascular endothelial growth factor A (VEGFA), which is expressed in renal tubular epithelial cells, and its receptor, VEGF receptor 2 (VEGFR2), located almost exclusively on peritubular capillary endothelial cells25. This interaction is essential for the maintenance of the peritubular microvasculature. Anticancer drugs that target the VEGF pathway can induce thrombotic microangiopathy, proteinuria, and hypertension26. Thrombotic microangiopathy — involving thrombocytopenia, microangiopathic haemolytic anaemia, and microvascular occlusion — can be immune-mediated and often leads to acute tubular necrosis. Drugs associated with thrombotic microangiopathy include quinine, ciclosporin, and tacrolimus27.
Assessment of the potential nephrotoxic effects of pharmaceutical compounds on different components of the kidney therefore requires the use of different model systems. Direct injurious effects of a compound on tubular cells are the easiest mechanisms to model in vitro, whereas injury mechanisms that require blood flow, urine or filtrate production, or immune-mediated injury — or those that involve secondary injury — are more challenging as they require a model system in which complex cellular architecture is combined with functional components. Below, we discuss current in vitro models that are used to assess the nephrotoxicity of pharmaceutical agents and the development of novel systems that more closely recapitulate the complexity of the kidney.
Characterization of cellular models
Markers of functional maturity
An in vitro model that recapitulates the in vivo response of renal cells to nephrotoxicants requires appropriate expression of the transporters and receptors that interact with the drug of interest. Much work has been done to identify the transporters and enzymes that are associated with drug-induced nephrotoxicity; the degree to which in vitro models express physiological levels of these proteins will affect the ability of the models to accurately predict drug nephrotoxicity in clinical studies. In addition to detecting expression of transporters at the transcript and protein level by quantitative PCR and immunohistochemistry, the function of transporters and endocytic receptors can be investigated by assessing the effect of fluorescent substrates and transport inhibitors (TABLE 1). Inhibition of substrate uptake routes will decrease intracellular fluorescence, whereas inhibition of efflux transporters will increase intracellular fluorescence. Of importance, these transporters, receptors, and enzymes do not act in isolation. For example, drug transporters might rely on Na+/K+-ATPase activity to generate an electrochemical gradient that is then used to transport the substrate. A drug can also act as a substrate for multiple transporters and receptors or can become a substrate after bioactivation by an enzyme. Thus, for a model to accurately predict drug nephrotoxicity, it must express appropriate levels of all key transporters and enzymes and carry out the metabolic activities required for these transporters and enzymes to function.
Table 1.
Approaches to functionally characterize renal transporters, receptors, and ion channels in vitro
| Protein (gene) | Labelled substrate | Inhibitors | Refs |
|---|---|---|---|
| Glomerulus | |||
| TRPC6 (TRPC6) | Fura-2-AM (10 μM) | 2-Aminoethoxydiphenyl borate (100 μM) | 156,157 |
| Megalin (LRP2) | FITC–human serum albumin (Km 4.5 mg/ml) | Equimolar transferrin or IgG | 158 |
| PMAT (SLC29A4) | PAN (100–250 μM, unlabelled) | Decynium-22 (2 μM) | 19 |
| Proximal tubule | |||
| OAT1 (SLC22A6) | Fluorescein (Km 0.8 ± 0.1 μM) | • Para-aminohippuric acid (IC50 18 ±4μM) • Probenecid (IC50 12.7 ±5μM) • Kynurenic acid (IC50 6 ±1μM) |
34,116 |
| OAT3 (SLC22A8) | Fluorescein (Km 3.7 ± 0.5 μM) | • Estrone sulfate (IC50 2.1 ± 0.3 μM) • Probenecid (IC50 1.9 ± 0.6 μM) • Kynurenic acid (IC50 6 ±1μM) |
34,116 |
| OCT2 (SLC22A2) | ASP+ (Km 36.4 ± 6.8 μM) | • TPA (IC50 16 ±2μM) • Metformin (IC50 3.9 ± 1.2 μM) • Cimetidine (IC50 8 ±2μM) • Verapamil (IC50 10 ±2μM) |
114,159 |
| BCRP (ABCG2) | • Hoechst 33342 (1.25 μM) • Fluorescein (1 μM) |
• KO143 (IC50 4.4 ± 0.9 μM) • MK571 (IC50 5.1 ± 0.9 μM) • PSC833 (IC50 1.3 ± 0.3 μM) |
116,160 |
| MRPs (ABCC2 and ABCC4) | • CMFDA (1.25 μM) • Fluorescein (1 μM) |
• KO143 (IC50 2.7 ± 0.2 μM) • MK571 (IC50 6.4 ± 1.3 μM) • PSC833 (IC50 1.0 ± 0.2 μM) |
116,160 |
| MDR1 (ABCB1) | Calcein-AM (1 μM) | • KO143 (IC50 2.5 ± 0.5 μM) • MK571 (IC50 10.2 ± 3.1 μM) • PSC833 (IC50 0.7 ± 0.5 μM) • Zosuquidar (0.07 μM)a |
160,161 |
| MATE2K (SLC47A2) | ND | Pyrimethamineb | 162,163 |
| Megalin (LRP2) | FITC-bovine serum albumin (Km 126 μg/ml) | RAP (IC50 0.02 μM)c | 112,164 |
| RAP–GST (2.5 μg/ml) | – | 160 | |
| Rhodamine–dextran (5 μM) | – | 123 | |
| Alexa 488–dextran (10 μg/ml) | – | 124 | |
| SGLT2 (SLC5A2) | 2-NBDG (200 μg/ml) | • Apigenin (50 μM) • Dapagliflozin (0.5 μM) |
67 |
| Distal tubule | |||
| NCC (SLC12A3) | 22Na+ (2 μCi/ml) | • Hydrochlorothiazide (100 μM) • Spironolactone (100 μM) • Metolazone (0.3 ± 0.001 μM) |
52,165 |
| TRPV5 (TRPV5) | Fluo-4 (2μM) | Ruthenium red (10 μM) | 166 |
| Collecting duct | |||
| ENaC (SCNN1A) | Lithium (10 mM, unlabelled) | Amiloride (10 μM) | 68 |
2-NBDG, 2-(N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)2-deoxyglucose; ASP+, 4-(4-(dimethylamino)styryl)-N-methylpyridinium; BCRP, breast cancer resistance protein; CMFDA, 5-chloromethylfluorescein diacetate; ENaC, epithelial Na+ channel; FITC, fluorescein isothiocyanate; IgG, immunoglobulin G; MATE2K, multidrug and toxin extrusion protein 2; MDR1, multidrug resistance protein 1; MRP, multidrug resistance-associated protein; NCC, Na+/Cl− cotransporter; ND, not done; OAT, organic anion transporter; OCT, organic cation transporter; PAN, puromycin aminonucleoside; PMAT, plasma membrane monoamine transporter; RAP–GST, receptor-associated protein–glutathione S-transferase fusion protein; SGLT2, sodium/glucose cotransporter 2; TPA, tetrapentylammonium; TRPC6, short transient receptor potential channel; TRPV5, transient receptor potential cation channel subfamily V member 5.
IC50 abacavir inhibition. Zosuquidar 1 μM is a potent inhibitor of MDR1-mediated calcein efflux (R.M., unpublished observation).
Inhibits efflux of N-methylnicotinamide and metformin in low micromolar range.
Concentration used in assay 1 μM112.
The polarized, mature proximal tubule expresses a range of transporters that have overlapping substrate affinities and allow the transcellular secretion of drugs and endogenous compounds from the interstitial compartment into the pro-urine. Major influx transporters include the family of OATs and OCT2, which are expressed on the basolateral surface of the proximal tubule. OAT1–OAT3 and OCT2 are not expressed in other nephron segments in adult humans28,29. OAT-mediated transport is driven by an α-ketoglutarate gradient generated by the Na+/K+-ATPase and Na+/dicarboxylate cotransporter 3 (NaDC3; encoded by SLC13A3), whereas OCT2 operates by electrogenic transport30,31. Multiple studies, including mouse knockout models and in vitro transfection models, have demonstrated that OAT1–OAT3 and OCT2 transporters increase sensitivity to known nephrotoxic drugs such as nucleoside analogue antivirals and cisplatin32–36. In addition to OCT2, copper transporter 1 (CTR1; encoded by SLC31A1), which is also expressed on the basolateral surface of the proximal tubule in mice, is also involved in cisplatin uptake and contributes to its toxicity37 (BOX 1). Similarly, the megalin–cubilin endocytic receptor complex expressed on the apical surface of proximal tubule cells and in podocytes increases sensitivity of these cells to amino-glycoside, polymyxin, and glycopeptide antibiotics as these drugs bind to the receptor and are endocytosed38,39.
Box 1. The many faces of cisplatin.
Cisplatin is a well-known nephrotoxic drug and is often used to study nephrotoxic injury responses in both animal and in vitro models. As it is a cationic drug, the prevailing paradigm is that cisplatin in the plasma enters proximal tubule cells via cation transporters and causes injury by inducing mitochondrial DNA damage and oxidative stress, with some efflux occurring via multidrug and toxin extrusion (MATE) transporters103,153,154. However, deletion of organic anion transporters or the endocytic receptor megalin also attenuates cisplatin toxicity in mouse models38,155, suggesting that alternative modes of cisplatin uptake exist. Cisplatin is also a substrate for γ-glutamyltransferase (GGT), which is expressed by proximal tubules, but conflicting reports exist as to whether GGT processing attenuates or exacerbates cisplatin toxicity63. Cisplatin also shows general cytotoxicity at high doses, suggesting that nonspecific toxicity responses are possible. Hence, the induction of a nephrotoxic effect in response to cisplatin cannot necessarily be used to determine which transporters and metabolic functions are present in the model.
Efflux transporters residing in the proximal tubule include the ATP-binding cassette transporters breast cancer resistance protein (BCRP; also known as ABCG2), multidrug resistance-associated proteins (MRPs), and multidrug resistance protein 1 (MDR1; also known as P-gp and ABCB1) as well as the multidrug and toxin and extrusion proteins (MATEs). According to the GUDMAP database, most of these transporters are expressed at the mRNA level before nephron formation in the developing mouse kidney40,41. If these transporters are also present in the developing human kidney, their expression in an in vitro model would not necessarily provide evidence of functional maturity and should be interpreted with caution. Indeed, HK-2 immortalized proximal tubule epithelial cells express MDR1 and demonstrate efflux function even when their influx transporter expression and function are close to zero42. Within the adult human nephron, these markers (that is, BCRP, MRPs, MDR1, and MATEs) are expressed only on the apical surface of the proximal tubule43–45. As these transporters export substances from the cell, their overexpression or upregulation attenuates renal toxicity of their substrates. Different affinities for these transporters could distinguish nephrotoxic drugs from less nephrotoxic derivatives10,46,47.
Other transporters expressed in the renal proximal tubule include the sodium/glucose cotransporter 2 (SGLT2; encoded by SLC5A2) and aquaporin 1 (encoded by AQP1). SGLT2 is a target for a new class of drug for type 2 diabetes mellitus (SGLT2 inhibitors), but concerns exist that these agents might cause AKI in some patients48,49, possibly owing to increased intratubular oxidative stress, uricosuria (with or without crystal formation), or an as-yet unidentified indirect mechanism48,49. Further insights into the underlying mechanism of SGLT2 inhibitor nephrotoxicity could potentially be provided by using an in vitro model of the renal proximal tubule that expresses SGLT2 (REF.50).
In addition to active transcellular transport via transporter proteins, ATP-independent paracellular transport also occurs in the proximal tubule. The proximal tubule epithelium has a leaky barrier, characterized by a transepithelial electrical resistance (TEER) of merely 5–7 Ω*cm2 compared with isolated renal distal tubular cells, which form a less leaky monolayer and have a TEER of 630 Ω*cm2 (REFS51,52). Ions, such as Na+, Cl−, and K+, and, to a lesser extent, compounds such as water are reabsorbed via passive diffusion and osmosis through paracellular tight junctions to the interstitial space. The tight junctions consist of a variety of proteins — including claudin 2, claudin 10a, claudin 11, and claudin 17 as well as occludin and tricellulin — that form ion-selective channels with a pore size of ~4 Å in radius51,53. Functional analyses have shown that claudin 2 channels facilitate the diffusion of cations (for example, Na+, K+, Ca2+ and H2O), whereas claudin 10a and possibly claudin 17 are involved in anion diffusion. Claudin 2 expression is regulated by components of the immune system (including IL-2, tumour necrosis factor (TNF), IL-13, IL-15, and IL-17) and growth factor signalling (including pro-epidermal growth factor (EGF), hepatocyte growth factor (HGF), extracellular signal-regulated kinase 1 (ERK1; also known as MAPK3), ERK2, and the RACα serine/threonine-protein kinase (AKT) pathways)54. Under pathophysiological conditions such as cisplatin-induced nephrotoxicity, ischaemic injury, or metabolic acidosis, claudin 2 expression is reduced, resulting in impaired paracellular transport of cations, which may contribute to disruption of the proximal tubule epithelium55,56. Tight junction injury can be visualized in vitro by tracking immunofluorescence or leakage of labelled inulin.
Proximal tubule cells also express a range of bio-transformation enzymes on their brush border and cytoplasm, including γ-glutamyltransferase (GGT) as well as CYPs, glutathione S-transferases (GSTs), flavin-containing monooxygenase (FMO), and UDP-glucuronosyltransferases (UGTs)7,57,58. These renal enzymes, especially CYP3A4 and CYP3A5 and UGT1A9 and UGT2B7, might facilitate drug detoxification and efflux10,58,59, which could lead to drug–drug interactions because substrates that inhibit these enzymes could cause other drugs to accumulate in the proximal tubule, thereby exacerbating their nephrotoxic effects60. In addition to their detoxifying effects, various studies have demonstrated that these enzymes can also bioactivate xenobiotics, potentially exacerbating their nephrotoxic effects61–63. However, the degree to which renal biotransformation contributes to the nephrotoxic effects of pharmaceutical compounds in vivo is not yet clear. GGT activity in proximal tubule models has been measured using a colorimetric assay reported in 1981 or with commercially available kits, although a difficulty in normalizing the assay readout to total protein content has been reported64–66. Acivicin, a GGT inhibitor, can be used to demonstrate the specificity of a chosen GGT assay67. Fluorometric assays for CYP enzymes, GSTs, and UGTs are also available from various suppliers.
The glomerulus, loop of Henle, distal tubule, and collecting duct also express transporters and receptors unique to those segments. Although some of these transporters have been linked to drug nephrotoxicity, less is known about the contributions of these transporters to nephrotoxicity than those of the proximal tubule. In principal cells of the collecting duct, lithium is absorbed via the epithelial sodium channel (ENaC), inducing downregulation of AQP2 expression, which leads to diabetes insipidus and with chronic exposure can lead to remodelling of the collecting duct, characterized by loss of principal cells and an increase in the number of intercalated cells68–70. Distinct transporters are also responsible for some of the adverse effects of drugs in the absence of overt renal cell injury; for example, Mg2+ and Na+ loss following ciclosporin treatment is caused by downregulation of transient receptor potential cation channel subfamily M member 6 (TRPM6) and Na+/Cl− cotransporter (NCC) in the distal tubule71.
Thus, the sensitivity of the kidney to nephrotoxicants is mediated by a multitude of unique functional proteins that are present in distinct epithelial components of the tubule. Therefore, modelling nephrotoxicity in vitro requires characterization of the functional identity of the cells under study, regardless of the cell source used. Such characterization requires substantially more than evaluation of gene expression and should also include characterization of protein localization and function.
Readouts of injury
Studies that use in vitro models to assess the nephrotoxicity of pharmaceutical agents usually rely on general cytotoxicity measures — such as cell death, the degree of cytoskeletal defects or mitochondrial function as determined by lactate production, or ATP content — as a measure of injury. The assumption is that in a relatively homogeneous population of renal cells, any compound that is cytotoxic to these cells is nephrotoxic. This assumption fails, however, when using a model that has more than one cell type by design. In addition, these cytotoxicity responses might reflect only advanced stages of injury elicited by high doses of the drug and therefore might fail to detect subtle injury that could still lead to acute or chronic kidney injury in vivo.
The past decade has witnessed a push to identify biomarkers of renal injury with improved specificity and sensitivity over the current standards of serum creatinine (SCr) and blood urea nitrogen (BUN)72. The fact that many of these biomarkers are proteins that are upregulated in response to injury led to the assumption that their in vitro expression could be used as a readout of the nephrotoxic potential of a given drug. However, not all biomarkers with potential utility in vivo are reliable indicators of nephrotoxicity in vitro.
Kidney injury molecule 1 (KIM1), also known as hepatitis A virus cellular receptor 1 (encoded by HAVCR1), is a type I membrane glycoprotein that is expressed in the liver, in activated immune cells, and in proximal tubules following injury73,74. Following AKI, KIM1 is expressed on the apical surface of proximal tubule cells where it acts as a phagocytic receptor, enabling the removal of apoptotic cell debris by surviving cells75. The KIM1 ecto-domain is shed into the urine following metalloproteinase cleavage, enabling it to be used as a biomarker of kidney injury73. The demonstration that urinary KIM1 concentration is a more sensitive and specific readout of in vivo kidney injury in rats and humans than SCr and BUN led to US Food and Drug Administration (FDA) and European Medicines Agency (EMA) approval of KIM1 as one of seven biomarkers to be used in addition to SCr and BUN for monitoring drug-induced nephrotoxicity in preclinical trials76–79. However, KIM1 has not performed as well in in vitro models, with a number of studies of primary proximal tubule cells or cell lines being unable to consistently detect significant differences in KIM1 expression between controls and cultures exposed to nephrotoxicants80–83. These inconsistent findings might suggest that additional cell types are required within the organ for KIM1 expression to be induced or that deficits exist in the in vitro cell models with respect to their cellular identity and/or response to injury.
Haem oxygenase 1 (HO1; encoded by HMOX1) is a 32 kDa enzyme that is induced in response to oxidative stress84. Within the kidney, HO1 is expressed following injury to the proximal tubules, glomeruli, or interstitial cells and is upregulated in renal mononuclear phagocytes in response to injury85. One study showed that levels of plasma and urinary HO1 are significantly higher in rats and humans with AKI than in healthy controls, although sample sizes used in that study were smaller than those of studies that have validated the specificity of other novel biomarkers, such as the 2008 and 2010 studies validating the performance of KIM177,78,86. A study that performed transcriptional profiling of an in vitro primary proximal tubule cell model exposed to nephrotoxicants showed that HMOX1 was the marker that correlated positively with dose for the largest number of nephrotoxic compounds tested87. HO1 expression also showed greater specificity and sensitivity for distinguishing between nephrotoxic and non-nephrotoxic compounds than the cell viability measures of cell death and ATP levels. Moreover, upregulation of HO1 expression was detectable by immunofluorescence in a kidney-on-a-chip model following exposure to cadmium chloride, whereas KIM1 expression was not detectable before exposure and only marginally increased after exposure, suggesting that the HO1 response to this nephrotoxic metal is more robust than that of KIM1 in vitro87.
IL-6 is a multifunctional cytokine that is expressed by macrophages, endothelial cells, and fibroblasts, whereas IL-8 attracts neutrophils and is expressed by a number of immune cells as well as by endothelial and epithelial cells88. In the kidney, tubular epithelial cells and podocytes produce these interleukins as part of an inflammatory response following most types of injury, including ischaemia–reperfusion injury and pyelonephritis89–92. The potential utility of IL-6 and IL-8 as in vivo biomarkers of AKI is unclear, but a pilot study that assessed the effects of gentamicin and cadmium chloride on primary proximal tubule cells from two different donors showed greater upregulation of IL-6 and IL-8 than KIM1 levels in response to the compounds82. Upregulation of IL-6 and IL-8 expression also occurs in conditionally immortalized proximal tubule cell lines in response to uraemic toxins93. Together, these findings suggest that IL-6 and IL-8 are used as readouts of injury in vitro.
Neutrophil gelatinase-associated lipocalin (NGAL), also known as lipocalin 2 (encoded by LCN2), is expressed in maturing granulocytes and in various epithelial tissues including the stomach, lung, and kidney94. The healthy kidney filters serum NGAL through the glomerulus, whereupon it is endocytosed by the proximal tubule95. Kidney injury leads to upregulation of NGAL levels in the distal tubules and collecting ducts and shedding of NGAL into the urine96. The utility of urinary NGAL as a clinical biomarker of AKI is, however, limited by the finding that inflammation affects NGAL levels78,97–99. NGAL is expressed at relatively high levels in proximal tubule cells in vitro, which may indicate the poor phenotype retention of proximal tubule cells when cultured using standard techniques as NGAL is not upregulated in mouse or rat proximal tubules following injury in vivo6,82,83. NGAL might be useful as a marker of distal tubule and collecting duct injury in vitro, although this possibility has not been extensively investigated.
Preclinical screens of nephrotoxicity
Animal models
Preclinical testing of drug candidates using animal models is required to study safety and efficacy on a systems biology level before moving to clinical studies. However, animal pharmacokinetic profiles often differ considerably from those of humans; hence, animal models are often poor predictors of adverse drug effects of drug disposition in humans100,101. This mismatch between animal and human outcomes is largely due to the many differences in drug transporter and metabolizing enzyme expression between species102. For example, expression levels of OCT1 and OCT2 are comparable in the rodent kidney, whereas OCT2 predominates in the human kidney103,104. Similarly, renal expression of BCRP is much higher in male FVB mice and Wistar Hanover rats than in humans44. Other transporters, such as MATE2K, have been detected only in humans103. Transporter expression and function can also differ between different non-human animal species. For example, renal expression of BCRP is lower in female Sprague-Dawley rats than in female C57BL/6J mice105. In the process of developing polymyxin analogues, a compound that was selected for further investigation on the basis of promising safety results in rats was subsequently found to be nephrotoxic in dogs4. Sex can also influence transporter expression and, hence, drug kinetics, which creates the potential for toxicity screen results to differ between sexes106,107. In rats, for example, sex differences have been observed in the expression of tubule transporters and tight junction proteins, including claudin 2, AQP1, NCC, and ENaC108.
The differences in transporter expression between animals and humans, and between different non-human species, limit the utility of animal models to study adverse drug effects. Therefore, the development of more suitable and predictive in vitro models is key to providing more reliable measures of the efficacy and safety of novel drug candidates in early stages of their development. In addition, the development of such in vitro models will greatly reduce preclinical toxicity testing in animals. Of note, in the area of cardiotoxicity, the FDA is in the process of validating induced pluripotent stem cell (iPSC)-derived human cardiomyocytes as a model in which to screen compounds for cardiac toxicity109.
In vitro models of nephrotoxicity
Primary and immortalized cell lines
In vitro models for nephrotoxicity screening commonly use primary human renal proximal tubule cells, usually acquired from cadaveric specimens and cultured in 2D. However, primary cells display large interdonor variability, have limited expansion capacity, and are prone to dedifferentiation and loss of transporter expression5,13,82,110. To overcome difficulties in expanding primary proximal tubule cells, lines of immortalized or conditionally immortalized proximal tubule cells have been developed, for example, using oncogenes (such as E6/E7 or SV40 large T antigen) or telomerase (for example TERT1)66,111,112. Although TERT1-immortalized renal proximal tubule cells can undergo at least 90 population doublings and possess several characteristics of proximal tubule cells, including microvilli, tight junctions, GGT activity, endocytic activity, and functional drug transporters, the functional characteristics of early and late passage cells have not been compared66,113. Conditionally immortalized proximal tubule cells exhibit tight junctions, endocytic activity, OCT2 and MDR1 function, and UGT activity, but like all renal proximal tubule cells in 2D culture, these conditionally immortalized proximal tubule cells lose OAT1 and OAT3 expression unless the proteins are transduced34,112,114,115.
The difficulty in maintaining transporter and metabolic function in primary cells and cell lines, and in reaching levels of transporter function comparable to those observed in vivo, are likely to result from the use of static 2D culture formats. In vitro renal transport or toxicity assays often use primary proximal tubule cells or cell lines cultured in a monolayer on a permeable support system, such as a Transwell insert, that is coated with an extracellular matrix67,87,112,116. The insert is placed in a well with media on top and underneath, enabling cells with appropriate transporters to transport substances from one side of the insert to the other. The major advantages of this system are that it is simple and that transporter and barrier function can be assayed by analysing substrate content on the top and bottom of the Transwell insert. However, the simplicity of this model also results in the loss of the environmental cues to which kidney tubules are normally exposed in vivo, such as fluid flow across the apical surface or signals from other adjacent cell types14,110,117. This deficiency has led to the development of more complex 3D culture systems and systems in which more than one renal cell type can be investigated; although, of note, Transwell inserts are still commonly used as a base for kidney organoids and renal tissue arrays, which are discussed later.
Pluripotent stem-cell-derived renal cells and reprogrammed cells
hPSCs have sparked considerable interest in terms of their potential to develop into fully functional renal cells. These cells can give rise to any cell type within the human body and can be easily expanded118. Including both embryonic stem cells and human iPSCs (hiPSCs), hPSCs have now been used to generate renal cells in vitro using combinations of growth factors and small molecules to mimic progression through normal embryonic kidney development119–127. Although the differentiation protocols developed so far vary in their duration, culture configuration, and timing of growth factor administration, most protocols involve an initial stimulation of WNT signalling followed by the use of fibroblast growth factor 9 (FGF9) with or without accompanying bone morphogenetic protein (BMP), retinoic acid, or activin A signalling (TABLE 2).
Table 2.
Protocols and characterization of iPSC-derived kidney lineages
| iPSC origin | Differentiation protocol | PTEC markers | Nephrotoxicity response | Refs | ||||
|---|---|---|---|---|---|---|---|---|
| Inducing factors | Configuration | Total time (days) | Genes | Proteins | Function | |||
| Human foreskin (ATCC); human dermal fibroblast-α (Invitrogen) | • IMa: CHIR, FGF2, and RA • MM and nephrogenesis: activin A, CHIR, and FGF9 |
2D | 11 | • AQP1 • MGLL |
• LTL • N-cadherin |
ND | ND | 119 |
| Human pluripotent stem cells; exact source unknown | IMa: activin A, BMP7 and CHIR | 2D; 3D embryonic bodies | • 2D: 20 • 3D: 3 +17b |
AQP1 | • AQP1c • LTLc |
ND | ND | 120 |
| Human pluripotent stem cells; exact source unknown | • IM: BMP4 and bFGF • UB: RA, activin A, and BMP2 |
3D chimeric aggregates (E12.5 mouse MM and human) | 4 +6 | ND | ND | ND | ND | 121 |
| Human dermal fibroblasts (RIKEN BioResource Center) | IM: activin A, BMP4, CHIR, RA and bFGF | MM: 3D embryonic bodies | 11 +3 | ND | • SALL1 • Cadherin 6 |
ND | ND | 122 |
| MM and nephrogenesis: activin A, BMP4, CHIR, RA and FGF9 | Nephrogenesis: 3D co-culture spheroids | 14 +8–12 | ||||||
| Human BJ foreskin fibroblasts (ATCC CRL-2522), human HDFα dermal fibroblasts (Invitrogen C-013-5C), human LR5-iPSCs (Hubrecht Institute, Netherlands), and human fibroblast hfib2-iPS4 and hfib2-iPS5 iPSCs (Boston Children’s Hospital) | • IMa: CHIR • MM and nephrogenesis: B27 supplement (Thermo Fisher) |
3D spheroid cultures towards organoids | 23 | ND | • LTLd • Megalin • Cubilin |
• Endocytosis using rhodamine–dextran • Organic anion transport activity detected by fluorescein methotrexate • Barrier integrity measured by Lucifer yellowe |
Organoids: KIM1 expression in response to cisplatin (50 μM) and gentamycin treatment (5 mM) | 123 |
| Human female fibroblasts (ATCC line CRL1502) | • IM: CHIR, FGF9 and heparin • MM and nephrogenesis: CHIR pulse, FGF9; after 5 days FGF9, no GF days 12–25 |
3D organoids | 7 +18 | ND | • LTL • Cubilin |
Endocytosis using dextran–Alexa 488 | Organoids: caspase 3 expression in LTL+ECAD+ PTECs in response to cisplatin (5 and 20 μM) treatment | 124 |
| Human foreskin fibroblasts (WiCell Research Institute) | IMa + MM towards HPTC-like cells: BMP2 and BMP7 | 2D | 8 | • SLC34A1 • SLC4A4 • SLC5A2 • SLC2A5 • SLC22A6 • SLC22A8 • SLC22A2 • SLC22A5 • ABCB1 • LRP2 • SLC15A1 • AQP1 • ANPEP • GGT • ATPase |
• GLUT1c • SGLTc • AQP1c • OAT3c • PEPT1c • ATPasec |
OAT activity determined by citrinin and OCT2 activity by cimetidine (indirect approaches: IL-6 and IL-8 induction) | 2D cells: NF-κB, γH2AX, and 4-HNE expression in response to cisplatin (100 μg/ ml = 333 μM) and aristolochic acid (1,000 μg/ml=2,932 μM) | 126 |
| Human dermal fibroblasts (Invitrogen C-013-5C) | • IM: CHIR, Noggin and activin A • MM and nephrogenesis: FGF9, CHIR; no GFs days 14–28 |
2D; 3D organoids | • 2D: 21 • 3D: 9 +12 |
ND | • LTL and AQP1 • N-cadherin |
ND | Organoids: KIM1 expression in response to cisplatin (5 μM) and gentamycin treatment (5 mg/ml = 10 mM); γH2AX expression in response to cisplatin (5 μM and 50 μM) | 125 |
| Human foreskin fibroblasts (System Biosciences International, SC101A-1) | • IM: activin A, CCG-1423, LY294002, and RA • MM towards renal progenitors: BMP7, FGF2, and GDNF |
2D | 19 | ND | AQP1 | ND | Regenerative effect in cisplatin AKI mice model | 127 |
4-HNE, 4-hydroxynonenal; γH2AX, phosphorylated histone H2AX; AKI, acute kidney injury; AQP1, aquaporin 1; bFGF, basic fibroblast growth factor; BMP, bone morphogenetic protein; CCG-1423, RHOA inhibitor; CHIR, CHIR99201; E12.5, embryonic day 12.5; ECAD, epithelial cadherin; FGF, fibroblast growth factor; GDNF, glial cell line-derived neurotrophic factor; GF, growth factor; GLUT1, glucose transporter type 1; HDFα, human dermal fibroblast-α; HPTC, human proximal tubule cell; IM, intermediate mesoderm; iPSC, induced pluripotent stem cell; KIM1, kidney injury molecule 1; LTL, Lotus tetragonolobus lectin; LY294002, phosphoinositide 3-kinase–RACα serine/threonine-protein kinase inhibitor; MM, metanephric mesenchyme; MRP, multidrug resistance-associated protein; N-cadherin, neural cadherin; ND, not done; NF-κB, nuclear factor-κB; OAT, organic anion transporter; OCT2, organic cation transporter 2; PEPT1, peptide transporter 1; PTEC, proximal tubular epithelial cell; RA, retinoic acid; RKi, RHO-kinase inhibitor; SALL1, Sal-like protein 1; SGLT, sodium/glucose cotransporter; UB, ureteric bud.
RKi was added to support single-cell passaging before differentiation start.
Indicates 3D aggregate formation after 3 days of 2D differentiation and additional culture in 3D for 17 days.
Specificity not shown by cellular localization or double staining with proximal tubule marker.
LTL-positive tubules remained stable upon long-term culturing up to 120 days.
Lucifer yellow is also an OAT and MRP substrate167.
In one study of hiPSCs, use of a short differentiation protocol gave rise to proximal tubule-like cells, which when cultured in 2D showed expression of the tight junction protein ZO1 and the transporters OAT3, SGLT1, glucose transporter type 1 GLUT1, AQP1, and peptide transporter 1 (PEPT1) (REF.126). However, similar to 2D cultures of renal proximal tubule cells, these hiPSC-derived cells exhibited high baseline expression of the injury marker KIM1 and the dedifferentiation marker vimentin. In addition, immunofluorescence staining for transporters did not show correct polarization of transporter expression. Transcript expression analysis also demonstrated expression of markers of nephron segments other than the proximal tubule, which could indicate the presence of a mixed population of renal tubular cells, transdifferentiation of individual cells within the cultures, or the presence of nonspecific gene expression due to a lack of clear lineage commitment. Nonetheless, the study describes the use of these cells and IL-6 and IL-8 expression levels to classify compounds as nephrotoxic or nontoxic.
Two studies have described protocols for the generation of hiPSC-derived podocytes128,129. Although one of these protocols described the production of a cell population containing >90% podocytes128, a direct comparison of the transcriptional signature of the cells with that of freshly isolated podocytes has not been presented129. In addition, in one of the studies128, immunofluorescence staining for the podocyte marker Wilms tumour protein (WT1) was not nuclear, as would be anticipated for this protein.
A 2016 paper described a protocol to enable the direct reprogramming of fibroblasts to renal epithelial cells without first reprogramming the cells to a pluripotent state130. This approach has been described for other cell types and typically involves the overexpression of transcription factors associated with the target cell type131. Kaminski et al. determined that overexpression of homeobox protein EMX2, paired box protein PAX8, and hepatocyte nuclear factors 1β and 4α could induce mouse or human fibroblasts to differentiate directly into induced renal epithelial cells (iRECs), which expressed GGT and megalin and had a transcriptional profile more similar to that of whole human kidney lysate than that of human fibroblasts. Mouse iRECs could transport albumin and demonstrated greater cytotoxic responses to cisplatin, gentamicin, and tacrolimus than mouse fibroblasts, suggesting that the cells contained functional renal transporters. The cisplatin response was ameliorated by administration of the OCT antagonist cimetidine, suggesting functional OCT2 involvement in cisplatin tubulotoxicity, although expression of OCT2 or of other proximal tubule cell transporters was not confirmed. KIM1 expression was also significantly increased in response to gentamicin; however, KIM1 was also expressed at low levels in untreated iRECs, consistent with earlier observations that KIM1 is expressed by untreated, hiPSC-derived proximal tubule-like cells cultured in 2D126 and suggesting that KIM1 is a nonspecific marker of tubule cell injury in in vitro models. In addition to the above caveats, the protocol for direct reprogramming produced a mixed population of iRECs that expressed markers of different nephron segments, with some individual cells expressing markers of multiple segments, suggesting that this approach generates pan-epithelial cells, which might represent an endogenous progenitor or tubular intermediate but might also represent nonspecific epithelial cell types that are not analogous to any cell type found in vivo. Further refinement of the protocol to control the types of cells produced and increase their functional maturity might enable the use of direct reprogramming to generate proximal tubule cells for in vitro nephrotoxicity screening.
Organoids
Several protocols now exist to enable the directed differentiation of hPSCs (human embryonic stem cells and hiPSCs) into complex multicellular kidney organoids (TABLE 2). These protocols exploit the ability of cells grown in 3D cultures to self-organize, which is reminiscent of the processes that occur during embryonic development, and can produce nephrons with evidence of glomeruli as well as segmentation of proximal and distal tubules, with some models showing additional evidence of surrounding stroma, vasculature, and collecting duct epithelium122–125,132 (FIG. 2a). As with all protocols for the directed differentiation of hPSCs into complex structures, the kidney organoids produced to date are representative of the early developing nephron and do not necessarily represent a mature functional epithelium124. Nevertheless, proximal tubules within kidney organoids developed by different groups demonstrate some characteristic functions of mature tubules. Organoids produced by Takasato et al. had proximal tubules that were capable of dextran uptake and had greater sensitivity to cisplatin (as determined by the induction of apoptosis) than other cell types within the organoid, although injury marker expression was not determined124. Using a different protocol, Morizane et al. developed organoids in which proximal tubules expressed KIM1 in response to cisplatin and gentamicin and demonstrated that cisplatin induced proximal tubule-specific DNA damage as determined by phosphorylated histone H2AX (γH2AX) staining125. These findings show that organoids could potentially be used as a nephrotoxicity screening platform, although further characterization of the functional maturity and their injury response to known nephrotoxicants is needed.
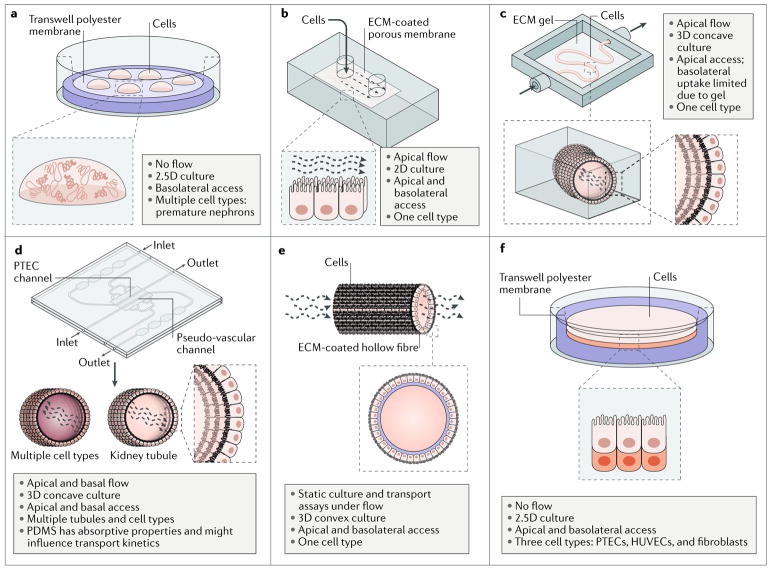
Fig. 2. Novel culture platforms for modelling nephrotoxicity in vitro.
a | Organoids consist of multiple immature nephrons and interstitial cells that self-organize in response to developmental cues and overcome the cellular simplicity of 2D cultures. b | A simple kidney-on-a-chip model allows media containing the compound of interest to flow across a cell monolayer. c | A kidney-on-a-chip that comprises a perfusable, convoluted 3D tubule within an extracellular matrix (ECM) enables fluid flow and the administration of test compounds to the apical surface of the cells. d | A kidney-on-a-chip with parallel 3D channels enables multiple cell types (for example, vasculature) to be modelled on a single chip with tubules. e | A biofunctionalized hollow fibre is coated with ECM and seeded with proximal tubule cells on its external surface, rendering both the basolateral and apical surfaces of the cells accessible for testing compounds. f | A 3D engineered renal tissue array consists of a monolayer of proximal tubule cells with an interstitial layer (comprising human umbilical vein endothelial cells (HUVECs) and fibroblasts) on their basolateral side, arranged on a Transwell. This model enables control over the spatial arrangement of cell types and media access to the basolateral and apical surfaces of the cells. PDMS, poly(dimethylsiloxane); PTEC, proximal tubule epithelial cell. Part b is reproduced with permission from REF.133, Royal Society of Chemistry. Part c is reproduced from REF.134, Macmillan Publishers Limited. Part d is reproduced with permission from REF.67, Elsevier.
Three-dimensional multicellular models are particularly valuable because they overcome the cellular simplicity of 2D cultures, wherein a single renal cell type is typically cultured in a monolayer, and instead provide a system with which to model the complex intercellular relationships involved in the response to a toxic insult. These intercellular relationships include interactions between adjacent epithelial cells and surrounding stromal cells but also include the microvasculature surrounding the tubules. Some protocols for the generation of organoids can induce the formation of vascular progenitors along with evidence of endothelial cells, perivascular cells, and pericytes124. Ideally, these would allow both for the modelling of vascular toxicity, such as that induced by VEGF inhibitors, and for studying the role of the endothelial network in tubular injury. However, the endothelial vessels in organoids arise between the forming nephrons with no convenient access point for perfusion. Thus, current kidney organoids do not yet recapitulate microvascular function to the extent required to study peritubular vasculature injury and/or downstream tubular effects in vitro.
Although organoids are a relatively straightforward way to derive and co-culture a variety of renal cell types, and the presence of distinct nephron segments should enable detection of segment-specific toxicity during screens, their use is also associated with unique challenges. For instance, the presence of multiple cell types within a single structure also means that a solid understanding of the markers of functional maturity of each cell type is required to properly characterize an organoid model. Cell-type-specific readouts of injury also become more important for identifying the mechanism of injury of a test compound. To date, none of the available organoid protocols produce an anatomically functional organoid with collecting ducts that merge into a single ureter. Moreover, tubules within the organoids cannot be directionally perfused, which makes the administration of test compounds more challenging. In addition, the lack of direct access to either the apical or basolateral surface of the tubular epithelium, as is possible with 2D cultures, could hinder the delivery of toxic compounds to the cells of interest following their addition to the medium.
Kidney-on-a-chip
We define kidneys-on-a-chip as renal cells that are seeded in a microfluidic device that allows flow of media across the cell surface and/or cell surfaces110 (FIG. 2b). One of the first studies of this technology seeded primary human proximal tubule cells on 2D chips with apical and basolateral media compartments and demonstrated that fluid flow across the apical surface stimulated MDR1 expression and function and induced the formation of tight junctions and columnar morphology133. Cells in fluidic culture were also better protected by cimetidine from cisplatin-induced apoptosis than were cells in static culture, although OCT2 function and expression were not directly assessed and injury marker expression was not determined by the researchers.
Although cells grown on 2D microfluidic chips demonstrate better proximal tubule cell function than cells grown in static cultures, 3D chip designs might more closely replicate the architecture of the proximal tubule and may offer further improvements (FIG. 2c). Indeed, a bioprinting approach has been used to fabricate a 3D chip containing a convoluted tubule filled with a fugitive ink within a gelatin–fibrinogen matrix. After flushing, the tubules were seeded with immortalized proximal tubule cells134, which, similar to the 2D chips, could then be perfused with media to provide fluid flow and administer test compounds to the apical surface of the cells. Epithelial cells cultured within these 3D models had higher expression of megalin and greater albumin uptake than cells cultured under static conditions and on 2D microfluidic chips, indicative of greater functional maturity. These bioprinted cells also showed evidence of tight junction damage following exposure to ciclosporin; however, the expression of injury markers was not assessed.
Although these 3D bioprinted chips are an improvement on static and 2D designs, further improvements are needed to develop ‘vascular’ networks that surround the tubule in order to gain access to the basolateral surface of the printed proximal tubule134. A 2014 study described the development of chips for modelling human vasculature, which contained a collagen matrix and two parallel 3D channels that could be perfused independently135. This system has since been used to culture primary human proximal tubule cells, seeding one channel with the cells and using the second channel as a cell-free, pseudovascular channel67 (FIG. 2d). Proximal tubule cells cultured in this 3D flow-directed microphysiological system demonstrated GGT activity and SGLT2-mediated transport of a fluorescent glucose analogue, indicating the presence of mature, functional proximal tubule cells. Perfusion of the OAT substrates para-aminohippurate or indoxyl sulfate through the pseudovascular channel was followed by their detection in the ‘proximal tubule’ effluent, suggesting transepithelial transport. Moreover, the amount of each substrate in the effluent relative to the inflow was decreased in the presence of probenecid (an OAT and MRP inhibitor), suggesting active transport of each substrate via OATs and/or MRPs, although the use of high concentrations of probenecid might have compromised cell metabolism136. Nevertheless, this study demonstrates that primary proximal tubule cells cultured in this chip design show characteristic functions of this cell type and that the pseudovascular channel can be used to administer compounds to the basolateral surface of these cells, although nephrotoxic injury responses were not determined in this study.
Several chips with vascular channels have been designed and can theoretically also be used to model injury to the renal and extrarenal microvasculature. For example, one microfluidic device seeded with human microvascular endothelial cells has been used for modelling haematologic diseases137. Activation of these endothelial cells with TNF resulted in decreased flow and microchannel occlusion, whereas exposure of the cells to Shiga-like toxin 2 resulted in the formation of thrombi that occluded the microchannels, indicating that this design would be suitable for studying drug-induced thrombotic microangiopathy. More recently, a protocol has been developed for isolating human kidney peritubular microvascular endothelial cells (HKMECs) and culturing them in a microfluidic device138. These HKMECs have a markedly different transcriptional profile from that of human umbilical vein endothelial cells (HUVECs) cultured under the same conditions and showed lower angiogenic potential and increased responsiveness to flow, demonstrating that the kidney microvasculature has specific properties that might not be replicated in models that use other endothelial cell types or lack physiological features such as flow.
Modelling the interactions between renal tubular cells and the microvasculature during injury requires the use of chips with dedicated renal epithelial tubules, vasculature seeded with appropriate endothelial cells, and, possibly, pericytes incorporated into the extracellular matrix of the chip139. Advances in the past couple of years have led to the development of high-throughput platforms with vascularized microorgans in a 96-well format, with the intent of recreating tissues with multiple cell types for rapid screening of large drug libraries. So far, however, the microorgans studied with these platforms have been restricted to simple architectures, such as tumour models, and renal models have not yet been produced with this design140,141.
Most kidney-on-a-chip systems to date have used primary proximal tubule cells; however, it should also be possible to seed chips with hPSC-derived proximal tubule cells or other tubular cells. Although no system has yet assessed multiple nephron cell types on a single chip, a 2017 study reported the development of a ‘glomerulus-on-a-chip’, in which hPSC-derived podocytes were seeded on one side of a porous membrane and primary endothelial cells were seeded on the other128. The chip design also permitted cyclic stretching of the membrane to model the mechanical strain of blood pulsation in glomeruli in vivo. As no specific molecular biomarker of podocyte injury has been identified, the researchers used albumin leakage across the membrane as a readout of injury following adriamycin exposure. Although extensive characterization of the hPSC-derived podocytes was not carried out, evidence for the differential retention of albumin and inulin across the chip membrane suggests the existence of barrier function.
Kidneys-on-a-chip have also been used in in vitro systems to model multiorgan toxicity and pharmacodynamics, whereby the kidney chip is functionally coupled (via the manual transfer of media) or physically coupled to in vitro models of other organ systems142,143. One 2017 study demonstrated that human proximal tubules on a chip showed increased KIM1 production and cell death in response to aristolochic acid I when a hepatocyte-containing chip was coupled upstream compared with when the proximal tubule chip was isolated, consistent with bioactivation of aristolochic acid I by hepatocytes143. This study provides proof of concept that a multiorgan in vitro model can assess the nephrotoxicity of a test compound and its metabolites. The benefits of multiorgan models and the challenges of designing them have been discussed elsewhere142.
Kidneys-on-a-chip are advantageous as they afford precise control over the spatial arrangement of different cell types and the perfusion of test compounds, making them a potentially valuable tool for nephrotoxicity screening. Further improvements in the engineering and design of these chips to enable the study of multiple ‘tubules’ in parallel will facilitate high-throughput screening110. However, additional characterization of injury marker expression of renal cells cultured on chips is required to enable rapid assessment of injury.
Biofunctionalized hollow fibres
A 2015 study showed that conditionally immortalized proximal tubule epithelial cells could be seeded onto the exterior surface of hollow fibres coated with an extracellular matrix, creating biofunctionalized hollow fibres with a monolayer of cells144 (FIG. 2e). The proximal tubule cells grown on these fibres demonstrated improved tight junction formation and OAT1 expression compared with that of the same cells cultured in 2D even in the absence of flow through the fibres, suggesting that the 3D culture itself improved the expression of these markers. The integrity of the tight junction and OAT1 function were confirmed using fluorescein isothiocyanate (FITC)-labelled inulin leakage and fluorescein uptake assays, respectively, demonstrating that both the basolateral and apical surfaces of the cells are accessible for testing compounds. Flow through the basolateral compartment is also possible. Exposure of these hollow fibres to uraemic toxins increased IL-6 expression and induced leakage of FITC-labelled inulin, indicating disruption of the epithelial tight junctions145. Notably, the convex arrangement of tubular epithelial cells on the exterior of the hollow fibre is not reflective of their arrangement in vivo — a caveat that should be addressed in future designs.
Engineered renal tissue arrays
Tissue arrays are a form of multicellular 3D culture that has been used for in vitro hepatotoxicity screening, and they have now been adapted for the assessment of nephrotoxicity in renal tissue65,146 (FIG. 2f). To generate these renal tissue arrays, a suspension of renal fibroblasts and HUVECs in a proprietary gel were bioprinted into Transwells to create a 3D interstitial layer65, after which a second suspension of primary human proximal tubule cells was bioprinted over the interstitial layer. This method produced a 3D tissue array consisting of a monolayer of proximal tubule cells with a thicker interstitial layer on their basolateral side. The proximal tubule cells in these arrays expressed polarized, functional MDR1, OAT1, OAT3, OCT2, and SGLT2 transporters and showed ACE and GGT activity. The arrays also had TEER values of 18.1 Ω*cm2, higher than those seen in proximal tubule cells in vivo but better than values typical of cells cultured in static 2D systems. The proximal tubule cells desquamated and proliferated in response to cisplatin, whereas cimetidine exhibited protective effects. Furthermore, the interstitial layer showed a fibrotic response to transforming growth factor-β (TGFβ). To our knowledge, this is one of the first proof-of-concept models of renal fibrosis in vitro.
Renal tissue arrays enable the co-culture of multiple renal cell types and enable investigation of injury to different cell types, with control over the spatial arrangement of cell types and media access to the basolateral and apical surfaces of proximal tubule cells. Current designs involve bioprinting of cells into 24-well Transwell plates, which enable moderate-throughput toxicity screens. However, rapid readouts of proximal tubule injury will be required to increase throughput; therefore, further characterization of injury markers is needed. Improved tissue array designs that permit laminar flow (possibly requiring microfabrication similar to that used for kidneys-on-a-chip) might further improve the functional maturity of the proximal tubule cells cultured in these tissue arrays65.
Future directions
The development of in vitro nephrotoxicity screens that more accurately predict the in vivo response than currently available approaches is likely to occur with improvement of the approaches described above. The development of approaches to obtain functionally mature 3D models of individual nephron segments or even entire nephrons will improve the predictive ability of screens of novel compounds. However, these models will eventually require higher-throughput screening approaches with rapid readouts of injury that can be measured by an automated system in order for these models to be adopted by industry.
Advances in automated imaging and image analysis have given rise to high-content screening, in which fluorescent probes or reporter lines are used to visualize specific cellular features (for example, specific injury markers, general apoptotic markers, or the actin cytoskeleton), followed by automated imaging and quantification of changes in those features across the test conditions147. Machine-learning algorithms, trained on a set of reference compounds, can then be used to classify test compounds as either toxic or nontoxic on the basis of the imaging readouts148. The feasibility of this approach was demonstrated in a 2016 study, in which a high-content screen and classification algorithm in primary human proximal tubule cells were used to correctly identify 15 of 24 compounds known to be nephrotoxic to proximal tubule cells and 17 of 20 compounds that are not toxic to proximal tubule cells148. Such high-content screening enables large amounts of data to be collected and interpreted, which will be useful for analysing compound responses in the above-described complex nephrotoxicity models under development.
As nephrotoxicity models become more physiologically relevant, their clinical predictive value will lie in screening not only for single drug effects but also for drug–drug interactions and the effects of individual variations such as the effects of underlying kidney disease or CYP enzyme polymorphisms on drug metabolism and toxicity100,149,150. Such analyses will require in vitro-to-in vivo extrapolations, involving mathematical models that can numerically simulate the behaviour of a drug in a complex system using results obtained experimentally in an in vitro system as the input parameter. The success of these mechanistic models is highly dependent on in-depth quantitative knowledge of renal physiology, such as relevant flow rates, the total number of nephrons and cells in a kidney, and absolute protein expression levels of transporters and enzymes100,151. In physiologically based pharmacokinetic modelling, drug pharmacokinetics or toxicokinetics can be predicted in silico by incorporating relevant drug disposition data such as renal drug metabolism and renal accumulation during excretion152. Such computational models not only might be useful for early-phase predictions of drug nephrotoxicity but also might guide the design of clinical studies and reduce reliance on animal testing. A final challenge in this area is to predict the individual response of a patient to any compound. As many drugs in clinical use have dose-limiting nephrotoxicity, understanding the capacity of an individual patient to detoxify a given drug will aid dose management. Such a precision approach will require integration of pharmacogenomics data into the computational models and might also require the development of multiorgan in vitro models to obtain relevant data from nonrenal systems152. At present, this goal is some time off.
Conclusions
The failure of existing in vitro cell and preclinical models to accurately predict nephrotoxicity highlights the need for more accurate models. Advances in our understanding of kidney development, regeneration of human kidney cell types from pluripotent stem cells, and increasingly complex 3D platforms for tissue culture have sparked the development of new approaches to address this challenge. The main goal will be to generate cellular models that demonstrate improved functional maturity and consequently improved injury responses and predictivity for known and new nephrotoxicants. Further technological advances will lead to the development of models that contain multiple renal cell types and nephron segments and enable access to both basolateral and apical sides of the model nephron, improving the physiological relevance of the models. Improved throughput in compound testing and data collection is also needed. Ultimately, the integration of information gained from these in vitro models into computational algorithms that incorporate patient-specific physiological parameters will not only reduce the late loss of drugs from the development pipeline but also facilitate the development of safer drugs and improve management of clinically important compounds with nephrotoxic adverse effects.
Key points.
Currently available in vitro and animal models of drug-induced nephrotoxicity are poorly predictive of toxicity in humans.
Functional proteins that underlie the susceptibility of various renal cell types to specific drugs, and molecular biomarkers of injury, can be used to characterize the functional maturity of in vitro models and their capacity to respond to nephrotoxicants.
In vitro models derived using new protocols for the directed differentiation of pluripotent stem cells to renal cells and new 3D in vitro culture systems demonstrate improved functional maturity over static 2D systems.
Improved functional maturity of cultured renal cells in systems that more closely replicate the physiology of the renal tubule and its supporting cells will improve the predictive ability of in vitro models of nephrotoxicity.
Acknowledgments
J.Y.-C.S. is supported by a University of Melbourne Research Scholarship and a Murdoch Children’s Research Institute Top up scholarship. J.J. is supported by the Dutch Kidney Foundation (Kolff postdoctoral fellowship abroad grant 170KK05), EMBO (short-term fellowship 6893), and by the partners of Regenerative Medicine Crossing Borders powered by Health~Holland, Top Sector Life Sciences & Health. R.M. is supported by a grant from the UK National Centre for the Replacement, Refinement and Reduction of Animals in Research (NC3Rs), the NephroTube CRACK IT Challenge, and the RegMed XB consortium. M.H.L. is an Australian National Health and Medical Research Council (NHMRC) Senior Principal Research Fellow (GNT1042093) and is supported by funding from the NHMRC (GNT1100970) and the US National Institutes of Health (DK107344).
Glossary
- Nephrotoxicants
Any compounds, natural and synthetic, that exert an adverse effect on a specific kidney cell type or mediate an unwanted event affecting kidney functioning. By contrast, a toxin is a poisonous substance produced within living cells or organisms.
- Electrogenic transport
Transport that leads to a change in net charge across a cell membrane.
- Transepithelial electrical resistance
(TEER). The electrical resistance across a cell monolayer. The higher the value, the less permeable the monolayer.
- Kidney-on-a-chip
Renal cells seeded in a 2D or 3D configuration in a microfluidic device. For proximal tubule chips, these devices typically allow flow of media across the cells’ apical surface, basolateral surface, or both. Other cell types such as endothelial cells might be included; in these cases, the organization of the different cell types is defined by the design of the chip.
- Kidney organoids
Three-dimensional aggregates of interstitial cells and nephron structures with characteristic segments, typically formed by directing pluripotent stem cells to a renal fate and aggregating these cells to enable self-organization, with or without additional extracellular matrix.
- Renal tissue arrays
Three-dimensional co-cultures of renal epithelial cells, renal fibroblasts, and endothelial cells. Cell suspensions are prepared in biocompatible gels and bioprinted. The composition of the suspensions and the spatial arrangement of the different suspensions used define the organization of the different cell types. Scaffolds composed of extracellular matrix may or may not be used.
- Fugitive ink
Biocompatible material that can be printed and later evacuated to leave a hollow space within a mould.
- Biofunctionalized hollow fibres
Hollow, porous polymer fibres coated with extracellular matrix on which renal cells can be cultured.
- Desquamated
Peeling and shedding of the top layer of an epithelium.
Footnotes
Author contributions
J.J. researched data for the article. J.Y.-C.S., J.J., and R.M. wrote the article. All authors contributed substantially to discussion of the article’s content and reviewed and edited the manuscript before submission.
Competing interests
M.H.L. holds a research contract with Organovo, Inc. The other authors declare no competing interests.
References
- 1.Nolin TD, Himmelfarb J. In: Adverse Drug Reactions. Uetrecht J, editor. Springer; Berlin, Heidelberg: 2010. pp. 111–130. [Google Scholar]
- 2.Grünfeld JP, Rossier BC. Lithium nephrotoxicity revisited. Nat Rev Nephrol. 2009;5:270–276. doi: 10.1038/nrneph.2009.43. [DOI] [PubMed] [Google Scholar]
- 3.Rewa O, Bagshaw SM. Acute kidney injury — epidemiology, outcomes and economics. Nat Rev Nephrol. 2014;10:193–207. doi: 10.1038/nrneph.2013.282. [DOI] [PubMed] [Google Scholar]
- 4.Magee TV, et al. Discovery of Dap-3 polymyxin analogues for the treatment of multidrug-resistant Gram-negative nosocomial infections. J Med Chem. 2013;56:5079–5093. doi: 10.1021/jm400416u. [DOI] [PubMed] [Google Scholar]
- 5.Tiong HY, et al. Drug-induced nephrotoxicity: clinical impact and preclinical in vitro models. Mol Pharm. 2014;11:1933–1948. doi: 10.1021/mp400720w. [DOI] [PubMed] [Google Scholar]
- 6.Huang JX, et al. Evaluation of biomarkers for in vitro prediction of drug-induced nephrotoxicity: comparison of HK-2, immortalized human proximal tubule epithelial, and primary cultures of human proximal tubular cells. Pharmacol Res Perspect. 2015;3:e00148. doi: 10.1002/prp2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dekant W, Vamvakas S. Biotransformation and membrane transport in nephrotoxicity. Crit Rev Toxicol. 1996;26:309–334. doi: 10.3109/10408449609012526. [DOI] [PubMed] [Google Scholar]
- 8.Hawksworth GM, et al. In: Toxicology — From Cells to Man. Seiler SP, Kroftová O, Eybl V, editors. Springer; Berlin, Heidelberg: 1996. pp. 184–192. [Google Scholar]
- 9.Lock EA, Reed CJ. Xenobiotic metabolizing enzymes of the kidney. Toxicol Pathol. 1998;26:18–25. doi: 10.1177/019262339802600102. [DOI] [PubMed] [Google Scholar]
- 10.Knops N, et al. The functional implications of common genetic variation in CYP3A5 and ABCB1 in human proximal tubule cells. Mol Pharm. 2015;12:758–768. doi: 10.1021/mp500590s. [DOI] [PubMed] [Google Scholar]
- 11.Bhargava P, Schnellmann RG. Mitochondrial energetics in the kidney. Nat Rev Nephrol. 2017;13:629–646. doi: 10.1038/nrneph.2017.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kramann R, Tanaka M, Humphreys BD. Fluorescence microangiography for quantitative assessment of peritubular capillary changes after AKI in mice. J Am Soc Nephrol. 2014;25:1924–1931. doi: 10.1681/ASN.2013101121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qi W, Johnson DW, Vesey DA, Pollock CA, Chen X. Isolation, propagation and characterization of primary tubule cell culture from human kidney (Methods in Renal Research) Nephrology. 2007;12:155–159. doi: 10.1111/j.1440-1797.2007.00779.x. [DOI] [PubMed] [Google Scholar]
- 14.Fisel P, Renner O, Nies AT, Schwab M, Schaeffeler E. Solute carrier transporter and drug-related nephrotoxicity: the impact of proximal tubule cell models for preclinical research. Expert Opin Drug Metab Toxicol. 2014;10:395–408. doi: 10.1517/17425255.2014.876990. [DOI] [PubMed] [Google Scholar]
- 15.Lemke A, Kiderlen AF, Kayser O. Amphotericin, B. Appl Microbiol Biotechnol. 2005;68:151–162. doi: 10.1007/s00253-005-1955-9. [DOI] [PubMed] [Google Scholar]
- 16.Mamoulakis C, et al. Contrast-induced nephropathy: Basic concepts, pathophysiological implications and prevention strategies. Pharmacol Ther. 2017;180:99–112. doi: 10.1016/j.pharmthera.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 17.Paueksakon P, Fogo AB. Drug-induced nephropathies. Histopathology. 2017;70:94–108. doi: 10.1111/his.13064. [DOI] [PubMed] [Google Scholar]
- 18.Perazella MA, Markowitz GS. Bisphosphonate nephrotoxicity. Kidney Int. 2008;74:1385–1393. doi: 10.1038/ki.2008.356. [DOI] [PubMed] [Google Scholar]
- 19.Xia L, Zhou M, Kalhorn TF, Ho HTB, Wang J. Podocyte-specific expression of organic cation transporter PMAT: implication in puromycin aminonucleoside nephrotoxicity. Am J Physiol-Ren Physiol. 2009;296:F1307–F1313. doi: 10.1152/ajprenal.00046.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yilmaz M, Taninmis H, Kara E, Ozagari A, Unsal A. Nephrotic syndrome after oral bisphosphonate (alendronate) administration in a patient with osteoporosis. Osteoporos Int. 2012;23:2059–2062. doi: 10.1007/s00198-011-1836-2. [DOI] [PubMed] [Google Scholar]
- 21.Cheng HF, Harris RC. Renal effects of non-steroidal anti-inflammatory drugs and selective cyclooxygenase-2 inhibitors. Curr Pharm Des. 2005;11:1795–1804. doi: 10.2174/1381612053764922. [DOI] [PubMed] [Google Scholar]
- 22.Perazella MA. Drug-induced renal failure: update on new medications and unique mechanisms of nephrotoxicity. Am J Med Sci. 2003;325:349–362. doi: 10.1097/00000441-200306000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Basile DP, Donohoe D, Roethe K, Osborn JL. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am J Physiol Renal Physiol. 2001;281:F887–F899. doi: 10.1152/ajprenal.2001.281.5.F887. [DOI] [PubMed] [Google Scholar]
- 24.Verma SK, Molitoris BA. Renal endothelial injury and microvascular dysfunction in acute kidney injury. Semin Nephrol. 2015;35:96–107. doi: 10.1016/j.semnephrol.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dimke H, et al. Tubulovascular cross-talk by vascular endothelial growth factor a maintains peritubular microvasculature in kidney. J Am Soc Nephrol. 2015;26:1027–1038. doi: 10.1681/ASN.2014010060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lameire N. Nephrotoxicity of recent anti-cancer agents. Clin Kidney J. 2014;7:11–22. doi: 10.1093/ckj/sft135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Al-Nouri ZL, Reese JA, Terrell DR, Vesely SK, George JN. Drug-induced thrombotic microangiopathy: a systematic review of published reports. Blood. 2015;125:616–618. doi: 10.1182/blood-2014-11-611335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Breljak D, et al. Distribution of organic anion transporters NaDC3 and OAT1-3 along the human nephron. Am J Physiol Renal Physiol. 2016;311:F227–F238. doi: 10.1152/ajprenal.00113.2016. [DOI] [PubMed] [Google Scholar]
- 29.Motohashi H, et al. Gene expression levels and immunolocalization of organic ion transporters in the human kidney. J Am Soc Nephrol. 2002;13:866–874. doi: 10.1681/ASN.V134866. [DOI] [PubMed] [Google Scholar]
- 30.Ingraham L, et al. A plasma concentration of α-ketoglutarate influences the kinetic interaction of ligands with organic anion transporter 1. Mol Pharmacol. 2014;86:86–95. doi: 10.1124/mol.114.091777. [DOI] [PubMed] [Google Scholar]
- 31.Budiman T, Bamberg E, Koepsell H, Nagel G. Mechanism of electrogenic cation transport by the cloned organic cation transporter 2 from rat. J Biol Chem. 2000;275:29413–29420. doi: 10.1074/jbc.M004645200. [DOI] [PubMed] [Google Scholar]
- 32.Cihlar T, et al. The antiviral nucleotide analogs cidofovir and adefovir are novel substrates for human and rat renal organic anion transporter 1. Mol Pharmacol. 1999;56:570–580. doi: 10.1124/mol.56.3.570. [DOI] [PubMed] [Google Scholar]
- 33.Ho ES, Lin DC, Mendel DB, Cihlar T. Cytotoxicity of antiviral nucleotides adefovir and cidofovir is induced by the expression of human renal organic anion transporter 1. J Am Soc Nephrol. 2000;11:383–393. doi: 10.1681/ASN.V113383. [DOI] [PubMed] [Google Scholar]
- 34.Nieskens TTG, et al. A human renal proximal tubule cell line with stable organic anion transporter 1 and 3 expression predictive for antiviral-induced toxicity. AAPS J. 2016;18:465–475. doi: 10.1208/s12248-016-9871-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hagos Y, Wolff NA. Assessment of the role of renal organic anion transporters in drug-induced nephrotoxicity. Toxins. 2010;2:2055–2082. doi: 10.3390/toxins2082055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ciarimboli G. Role of organic cation transporters in drug-induced toxicity. Expert Opin Drug Metab Toxicol. 2011;7:159–174. doi: 10.1517/17425255.2011.547474. [DOI] [PubMed] [Google Scholar]
- 37.Pabla N, Murphy RF, Liu K, Dong Z. The copper transporter Ctr1 contributes to cisplatin uptake by renal tubular cells during cisplatin nephrotoxicity. Am J Physiol Renal Physiol. 2009;296:F505–F511. doi: 10.1152/ajprenal.90545.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hori Y, et al. Megalin blockade with cilastatin suppresses drug-induced nephrotoxicity. J Am Soc Nephrol. 2017;28:1783–1791. doi: 10.1681/ASN.2016060606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nagai J, Takano M. Entry of aminoglycosides into renal tubular epithelial cells via endocytosis-dependent and endocytosis-independent pathways. Biochem Pharmacol. 2014;90:331–337. doi: 10.1016/j.bcp.2014.05.018. [DOI] [PubMed] [Google Scholar]
- 40.Genitourinary Development Molecular Anatomy Project. GUDMAP; 2017. [No authors listed.] http://www.gudmap.org/ [Google Scholar]
- 41.Harding SD, et al. The GUDMAP database — an online resource for genitourinary research. Development. 2011;138:2845–2853. doi: 10.1242/dev.063594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jenkinson SE, et al. The limitations of renal epithelial cell line HK-2 as a model of drug transporter expression and function in the proximal tubule. Pflüg Arch. 2012;464:601–611. doi: 10.1007/s00424-012-1163-2. [DOI] [PubMed] [Google Scholar]
- 43.van Aubel RA, Smeets PH, Peters JG, Bindels RJ, Russel FG. The MRP4/ABCC4 gene encodes a novel apical organic anion transporter in human kidney proximal tubules: putative efflux pump for urinary cAMP and cGMP. J Am Soc Nephrol. 2002;13:595–603. doi: 10.1681/ASN.V133595. [DOI] [PubMed] [Google Scholar]
- 44.Huls M, et al. The breast cancer resistance protein transporter ABCG2 is expressed in the human kidney proximal tubule apical membrane. Kidney Int. 2008;73:220–225. doi: 10.1038/sj.ki.5002645. [DOI] [PubMed] [Google Scholar]
- 45.Motohashi H, Inui K. Multidrug and toxin extrusion family SLC47: Physiological, pharmacokinetic and toxicokinetic importance of MATE1 and MATE2-K. Mol Aspects Med. 2013;34:661–668. doi: 10.1016/j.mam.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 46.Wen X, et al. MDR1 transporter protects against paraquat-induced toxicity in human and mouse proximal tubule cells. Toxicol Sci. 2014;141:475–483. doi: 10.1093/toxsci/kfu141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yokoo S, et al. Differential contribution of organic cation transporters, OCT2 and MATE1, in platinum agent-induced nephrotoxicity. Biochem Pharmacol. 2007;74:477–487. doi: 10.1016/j.bcp.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 48.Hahn K, Ejaz AA, Kanbay M, Lanaspa MA, Johnson RJ. Acute kidney injury from SGLT2 inhibitors: potential mechanisms. Nat Rev Nephrol. 2016;12:711–712. doi: 10.1038/nrneph.2016.159. [DOI] [PubMed] [Google Scholar]
- 49.Nadkarni GN, et al. Acute kidney injury in patients on SGLT2 inhibitors: a propensity-matched analysis. Diabetes Care. 2017;40:1479–1485. doi: 10.2337/dc17-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saly D, Perazella MA. Harnessing basic and clinic tools to evaluate SGLT2 inhibitor nephrotoxicity. Am J Physiol Renal Physiol. 2017;313:F951–F954. doi: 10.1152/ajprenal.00250.2017. [DOI] [PubMed] [Google Scholar]
- 51.Yu ASL. Claudins and the kidney. J Am Soc Nephrol. 2015;26:11–19. doi: 10.1681/ASN.2014030284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Markadieu N, et al. A primary culture of distal convoluted tubules expressing functional thiazide-sensitive NaCl transport. Am J Physiol Renal Physiol. 2012;303:F886–F892. doi: 10.1152/ajprenal.00114.2012. [DOI] [PubMed] [Google Scholar]
- 53.Fromm M, Piontek J, Rosenthal R, Günzel D, Krug SM. Tight junctions of the proximal tubule and their channel proteins. Pflüg Arch. 2017;469:877–887. doi: 10.1007/s00424-017-2001-3. [DOI] [PubMed] [Google Scholar]
- 54.Günzel D, Yu ASL. Claudins and the modulation of tight junction permeability. Physiol Rev. 2013;93:525–569. doi: 10.1152/physrev.00019.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trujillo J, et al. Renal tight junction proteins are decreased in cisplatin-induced nephrotoxicity in rats. Toxicol Mech Methods. 2014;24:520–528. doi: 10.3109/15376516.2014.948248. [DOI] [PubMed] [Google Scholar]
- 56.Balkovetz DF. Tight junction claudins and the kidney in sickness and in health. Biochim Biophys Acta. 2009;1788:858–863. doi: 10.1016/j.bbamem.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 57.Lash LH, Putt DA, Cai H. Drug metabolism enzyme expression and activity in primary cultures of human proximal tubular cells. Toxicology. 2008;244:56–65. doi: 10.1016/j.tox.2007.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miners J, Yang X, Knights K, Zhang L. The role of the kidney in drug elimination: transport, metabolism, and the impact of kidney disease on drug clearance. Clin Pharmacol Ther. 2017;102:436–449. doi: 10.1002/cpt.757. [DOI] [PubMed] [Google Scholar]
- 59.Bolbrinker J, et al. CYP3A5 genotype-phenotype analysis in the human kidney reveals a strong site-specific expression of CYP3A5 in the proximal tubule in carriers of the CYP3A5*1 allele. Drug Metab Dispos. 2012;40:639–641. doi: 10.1124/dmd.111.042648. [DOI] [PubMed] [Google Scholar]
- 60.Yu J, Zhou Z, Owens KH, Ritchie TK, Ragueneau-Majlessi I. What can be learned from recent new drug applications? A systematic review of drug interaction data for drugs approved by the US FDA in 2015. Drug Metab Dispos. 2017;45:86–108. doi: 10.1124/dmd.116.073411. [DOI] [PubMed] [Google Scholar]
- 61.Dekant W. The role of biotransformation and bioactivation in toxicity. EXS. 2009;99:57–86. doi: 10.1007/978-3-7643-8336-7_3. [DOI] [PubMed] [Google Scholar]
- 62.Liu S, et al. The role of renal proximal tubule P450 enzymes in chloroform-induced nephrotoxicity: Utility of renal specific P450 reductase knockout mouse models. Toxicol Appl Pharmacol. 2013;272:230–237. doi: 10.1016/j.taap.2013.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fliedl L. Controversial role of gamma-glutamyl transferase activity in cisplatin nephrotoxicity. ALTEX. 2014;31:269–278. doi: 10.14573/altex.1311152. [DOI] [PubMed] [Google Scholar]
- 64.Meister A, Tate SS, Griffith OW. Gamma-glutamyl transpeptidase. Methods Enzymol. 1981;77:237–253. doi: 10.1016/s0076-6879(81)77032-0. [DOI] [PubMed] [Google Scholar]
- 65.King SM, et al. 3D proximal tubule tissues recapitulate key aspects of renal physiology to enable nephrotoxicity testing. Front Physiol. 2017;8:123. doi: 10.3389/fphys.2017.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wieser M, et al. hTERT alone immortalizes epithelial cells of renal proximal tubules without changing their functional characteristics. Am J Physiol Renal Physiol. 2008;295:F1365–F1375. doi: 10.1152/ajprenal.90405.2008. [DOI] [PubMed] [Google Scholar]
- 67.Weber EJ, et al. Development of a microphysiological model of human kidney proximal tubule function. Kidney Int. 2016;90:627–637. doi: 10.1016/j.kint.2016.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kortenoeven MLA, et al. Amiloride blocks lithium entry through the sodium channel thereby attenuating the resultant nephrogenic diabetes insipidus. Kidney Int. 2009;76:44–53. doi: 10.1038/ki.2009.91. [DOI] [PubMed] [Google Scholar]
- 69.Thomsen K, Shirley DG. A hypothesis linking sodium and lithium reabsorption in the distal nephron. Nephrol Dial Transplant. 2006;21:869–880. doi: 10.1093/ndt/gfk029. [DOI] [PubMed] [Google Scholar]
- 70.Christensen BM, Kim YH, Kwon TH, Nielsen S. Lithium treatment induces a marked proliferation of primarily principal cells in rat kidney inner medullary collecting duct. Am J Physiol Renal Physiol. 2006;291:F39–F48. doi: 10.1152/ajprenal.00383.2005. [DOI] [PubMed] [Google Scholar]
- 71.Ledeganck KJ, et al. Expression of renal distal tubule transporters TRPM6 and NCC in a rat model of cyclosporine nephrotoxicity and effect of EGF treatment. Am J Physiol Renal Physiol. 2011;301:F486–F493. doi: 10.1152/ajprenal.00116.2011. [DOI] [PubMed] [Google Scholar]
- 72.Bonventre JV, Vaidya VS, Schmouder R, Feig P, Dieterle F. Next-generation biomarkers for detecting kidney toxicity. Nat Biotechnol. 2010;28:436–440. doi: 10.1038/nbt0510-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bailly V, et al. Shedding of kidney injury molecule-1, a putative adhesion protein involved in renal regeneration. J Biol Chem. 2002;277:39739–39748. doi: 10.1074/jbc.M200562200. [DOI] [PubMed] [Google Scholar]
- 74.Ichimura T. Kidney injury molecule-1: a tissue and urinary biomarker for nephrotoxicant-induced renal injury. Am J Physiol Renal Physiol. 2004;286:552F–563. doi: 10.1152/ajprenal.00285.2002. [DOI] [PubMed] [Google Scholar]
- 75.Ichimura T, et al. Kidney injury molecule-1 is a phosphatidylserine receptor that confers a phagocytic phenotype on epithelial cells. J Clin Invest. 2008;118:1657–1668. doi: 10.1172/JCI34487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vaidya VS. Urinary kidney injury molecule-1: a sensitive quantitative biomarker for early detection of kidney tubular injury. Am J Physiol Renal Physiol. 2006;290:F517–F529. doi: 10.1152/ajprenal.00291.2005. [DOI] [PubMed] [Google Scholar]
- 77.Vaidya VS, et al. Kidney injury molecule-1 outperforms traditional biomarkers of kidney injury in preclinical biomarker qualification studies. Nat Biotechnol. 2010;28:478–485. doi: 10.1038/nbt.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vaidya VS, et al. Urinary biomarkers for sensitive and specific detection of acute kidney injury in humans. Clin Transl Sci. 2008;1:200–208. doi: 10.1111/j.1752-8062.2008.00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dieterle F, et al. Renal biomarker qualification submission: a dialog between the FDA-EMEA and Predictive Safety Testing Consortium. Nat Biotechnol. 2010;28:455–462. doi: 10.1038/nbt.1625. [DOI] [PubMed] [Google Scholar]
- 80.Rached E, et al. Evaluation of putative biomarkers of nephrotoxicity after exposure to ochratoxin A in vivo and in vitro. Toxicol Sci. 2008;103:371–381. doi: 10.1093/toxsci/kfn040. [DOI] [PubMed] [Google Scholar]
- 81.Sohn SJ, et al. In vitro evaluation of biomarkers for cisplatin-induced nephrotoxicity using HK-2 human kidney epithelial cells. Toxicol Lett. 2013;217:235–242. doi: 10.1016/j.toxlet.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 82.Li Y, et al. An in vitro method for the prediction of renal proximal tubular toxicity in humans. Toxicol Res. 2013;2:352. [Google Scholar]
- 83.Luo QH, et al. Evaluation of KIM-1 and NGAL as early indicators for assessment of gentamycin-induced nephrotoxicity in vivo and in vitro. Kidney Blood Press Res. 2016;41:911–918. doi: 10.1159/000452592. [DOI] [PubMed] [Google Scholar]
- 84.Gozzelino R, Jeney V, Soares MP. Mechanisms of cell protection by heme oxygenase-1. Annu Rev Pharmacol Toxicol. 2010;50:323–354. doi: 10.1146/annurev.pharmtox.010909.105600. [DOI] [PubMed] [Google Scholar]
- 85.Lever JM, Boddu R, George JF, Agarwal A. Heme oxygenase-1 in kidney health and disease. Antioxid Redox Signal. 2016 doi: 10.1089/ars.2016.6659. https://doi.org/10.1089/ars.2016.6659. [DOI] [PMC free article] [PubMed]
- 86.Zager RA, Johnson ACM, Becker K. Plasma and urinary heme oxygenase-1 in AKI. J Am Soc Nephrol. 2012;23:1048–1057. doi: 10.1681/ASN.2011121147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Adler M, et al. A quantitative approach to screen for nephrotoxic compounds in vitro. J Am Soc Nephrol. 2016;27:1015–1028. doi: 10.1681/ASN.2015010060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Akdis M, et al. Interleukins, from 1 to 37, and interferon-γ: receptors, functions, and roles in diseases. J Allergy Clin Immunol. 2011;127:701–721.e70. doi: 10.1016/j.jaci.2010.11.050. [DOI] [PubMed] [Google Scholar]
- 89.Tramma D, Hatzistylianou M, Gerasimou G, Lafazanis V. Interleukin-6 and interleukin-8 levels in the urine of children with renal scarring. Pediatr Nephrol. 2012;27:1525–1530. doi: 10.1007/s00467-012-2156-2. [DOI] [PubMed] [Google Scholar]
- 90.Grigoryev DN, et al. The local and systemic inflammatory transcriptome after acute kidney injury. J Am Soc Nephrol. 2008;19:547–558. doi: 10.1681/ASN.2007040469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Araki M, et al. Expression of IL-8 during reperfusion of renal allografts is dependent on ischemic time. Transplantation. 2006;81:783–788. doi: 10.1097/01.tp.0000198736.69527.32. [DOI] [PubMed] [Google Scholar]
- 92.Su H, Lei CT, Zhang C. Interleukin-6 signaling pathway and its role in kidney disease: an update. Front Immunol. 2017;8:405. doi: 10.3389/fimmu.2017.00405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mihajlovic M, et al. Allostimulatory capacity of conditionally immortalized proximal tubule cell lines for bioartificial kidney application. Sci Rep. 2017;7:7103. doi: 10.1038/s41598-017-07582-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cowland JB, Borregaard N. Molecular characterization and pattern of tissue expression of the gene for neutrophil gelatinase-associated lipocalin from humans. Genomics. 1997;45:17–23. doi: 10.1006/geno.1997.4896. [DOI] [PubMed] [Google Scholar]
- 95.Charlton JR, Portilla D, Okusa MD. A basic science view of acute kidney injury biomarkers. Nephrol Dial Transplant. 2014;29:1301–1311. doi: 10.1093/ndt/gft510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Paragas N, et al. The Ngal reporter mouse detects the response of the kidney to injury in real time. Nat Med. 2011;17:216–222. doi: 10.1038/nm.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.McIlroy DR, Wagener G, Lee HT. Neutrophil gelatinase-associated lipocalin and acute kidney injury after cardiac surgery: the effect of baseline renal function on diagnostic performance. Clin J Am Soc Nephrol. 2010;5:211–219. doi: 10.2215/CJN.04240609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mårtensson J, Bellomo R. The rise and fall of NGAL in acute kidney injury. Blood Purif. 2014;37:304–310. doi: 10.1159/000364937. [DOI] [PubMed] [Google Scholar]
- 99.Haase M, et al. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;54:1012–1024. doi: 10.1053/j.ajkd.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 100.Scotcher D, Jones C, Posada M, Galetin A, Rostami-Hodjegan A. Key to opening kidney for in vitro-in vivo extrapolation entrance in health and disease: Part II: mechanistic models and in vitro-in vivo extrapolation. AAPS J. 2016;18:1082–1094. doi: 10.1208/s12248-016-9959-1. [DOI] [PubMed] [Google Scholar]
- 101.Hilgendorf C, et al. Expression of thirty-six drug transporter genes in human intestine, liver, kidney, and organotypic cell lines. Drug Metab Dispos. 2007;35:1333–1340. doi: 10.1124/dmd.107.014902. [DOI] [PubMed] [Google Scholar]
- 102.Chu X, Bleasby K, Evers R. Species differences in drug transporters and implications for translating preclinical findings to humans. Expert Opin Drug Metab Toxicol. 2013;9:237–252. doi: 10.1517/17425255.2013.741589. [DOI] [PubMed] [Google Scholar]
- 103.Yonezawa A, Inui K. Importance of the multidrug and toxin extrusion MATE/SLC47A family to pharmacokinetics, pharmacodynamics/toxicodynamics and pharmacogenomics. Br J Pharmacol. 2011;164:1817–1825. doi: 10.1111/j.1476-5381.2011.01394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Aoki M, et al. Kidney-specific expression of human organic cation transporter 2 (OCT2/SLC22A2) is regulated by DNA methylation. Am J Physiol Renal Physiol. 2008;295:F165–F170. doi: 10.1152/ajprenal.90257.2008. [DOI] [PubMed] [Google Scholar]
- 105.Tanaka Y, Slitt AL, Leazer TM, Maher JM, Klaassen CD. Tissue distribution and hormonal regulation of the breast cancer resistance protein (Bcrp/Abcg2) in rats and mice. Biochem Biophys Res Commun. 2004;326:181–187. doi: 10.1016/j.bbrc.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 106.Soldin OP, Mattison DR. Sex differences in pharmacokinetics and pharmacodynamics. Clin Pharmacokinet. 2009;48:143–157. doi: 10.2165/00003088-200948030-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Joseph S, et al. Expression of drug transporters in human kidney: impact of sex, age, and ethnicity. Biol Sex Differ. 2015;6:4. doi: 10.1186/s13293-015-0020-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Veiras LC, et al. Sexual dimorphic pattern of renal transporters and electrolyte homeostasis. J Am Soc Nephrol. 2017;28:3504–3517. doi: 10.1681/ASN.2017030295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Validating human stem cell cardiomyocyte technology for better predictive assessment of drug-induced cardiac toxicity. U.S. Food & Drug Administration; 2016. [No authors listed.] https://www.fda.gov/ScienceResearch/SpecialTopics/RegulatoryScience/ucm507998.htm. [Google Scholar]
- 110.Wilmer MJ, et al. Kidney-on-a-chip technology for drug-induced nephrotoxicity screening. Trends Biotechnol. 2016;34:156–170. doi: 10.1016/j.tibtech.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 111.Ryan MJ, et al. HK-2: An immortalized proximal tubule epithelial cell line from normal adult human kidney. Kidney Int. 1994;45:48–57. doi: 10.1038/ki.1994.6. [DOI] [PubMed] [Google Scholar]
- 112.Wilmer MJ, et al. Novel conditionally immortalized human proximal tubule cell line expressing functional influx and efflux transporters. Cell Tissue Res. 2010;339:449–457. doi: 10.1007/s00441-009-0882-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Aschauer L, Carta G, Vogelsang N, Schlatter E, Jennings P. Expression of xenobiotic transporters in the human renal proximal tubule cell line RPTEC/ TERT1. Toxicol In Vitro. 2015;30:95–105. doi: 10.1016/j.tiv.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 114.Schophuizen CMS, et al. Cationic uremic toxins affect human renal proximal tubule cell functioning through interaction with the organic cation transporter. Pflüg Arch. 2013;465:1701–1714. doi: 10.1007/s00424-013-1307-z. [DOI] [PubMed] [Google Scholar]
- 115.Mutsaers HAM, et al. Uremic toxins inhibit renal metabolic capacity through interference with glucuronidation and mitochondrial respiration. Biochim Biophys Acta. 2013;1832:142–150. doi: 10.1016/j.bbadis.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 116.Jansen J, et al. Bioengineered kidney tubules efficiently excrete uremic toxins. Sci Rep. 2016;6:26715. doi: 10.1038/srep26715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ivliev AE, ’t Hoen PAC, Roon-Mom WMC, van Peters DJM, Sergeeva MG. Exploring the transcriptome of ciliated cells using in silico dissection of human tissues. PLoS ONE. 2012;7:e35618. doi: 10.1371/journal.pone.0035618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ohnuki M, Takahashi K. Present and future challenges of induced pluripotent stem cells. Phil Trans R Soc B Biol Sci. 2015;370:20140367. doi: 10.1098/rstb.2014.0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lam AQ, et al. Rapid and efficient differentiation of human pluripotent stem cells into intermediate mesoderm that forms tubules expressing kidney proximal tubular markers. J Am Soc Nephrol. 2014;25:1211–1225. doi: 10.1681/ASN.2013080831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mae SI, et al. Monitoring and robust induction of nephrogenic intermediate mesoderm from human pluripotent stem cells. Nat Commun. 2013;4:1367. doi: 10.1038/ncomms2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Xia Y, et al. The generation of kidney organoids by differentiation of human pluripotent cells to ureteric bud progenitor-like cells. Nat Protoc. 2014;9:2693–2704. doi: 10.1038/nprot.2014.182. [DOI] [PubMed] [Google Scholar]
- 122.Taguchi A, et al. Redefining the in vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells. Cell Stem Cell. 2014;14:53–67. doi: 10.1016/j.stem.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 123.Freedman BS, et al. Modelling kidney disease with CRISPR-mutant kidney organoids derived from human pluripotent epiblast spheroids. Nat Commun. 2015;6:8715. doi: 10.1038/ncomms9715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Takasato M, et al. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature. 2015;526:564–568. doi: 10.1038/nature15695. [DOI] [PubMed] [Google Scholar]
- 125.Morizane R, et al. Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nat Biotechnol. 2015;33:1193–1200. doi: 10.1038/nbt.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kandasamy K, et al. Prediction of drug-induced nephrotoxicity and injury mechanisms with human induced pluripotent stem cell-derived cells and machine learning methods. Sci Rep. 2015;5:12337. doi: 10.1038/srep12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Imberti B, et al. Renal progenitors derived from human iPSCs engraft and restore function in a mouse model of acute kidney injury. Sci Rep. 2015;5:8826. doi: 10.1038/srep08826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Musah S, et al. Mature induced-pluripotent-stem-cell-derived human podocytes reconstitute kidney glomerular-capillary-wall function on a chip. Nat Biomed Eng. 2017;1:0069. doi: 10.1038/s41551-017-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Song B, et al. The directed differentiation of human iPS cells into kidney podocytes. PLoS ONE. 2012;7:e46453. doi: 10.1371/journal.pone.0046453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kaminski MM, et al. Direct reprogramming of fibroblasts into renal tubular epithelial cells by defined transcription factors. Nat Cell Biol. 2016;18:1269–1280. doi: 10.1038/ncb3437. [DOI] [PubMed] [Google Scholar]
- 131.Xu J, Du Y, Deng H. Direct lineage reprogramming: strategies, mechanisms, and applications. Cell Stem Cell. 2015;16:119–134. doi: 10.1016/j.stem.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 132.Takasato M, et al. Directing human embryonic stem cell differentiation towards a renal lineage generates a self-organizing kidney. Nat Cell Biol. 2014;16:118–126. doi: 10.1038/ncb2894. [DOI] [PubMed] [Google Scholar]
- 133.Jang KJ, et al. Human kidney proximal tubule-on-a-chip for drug transport and nephrotoxicity assessment. Integr Biol. 2013;5:1119. doi: 10.1039/c3ib40049b. [DOI] [PubMed] [Google Scholar]
- 134.Homan KA, et al. Bioprinting of 3D convoluted renal proximal tubules on perfusable chips. Sci Rep. 2016;6:34845. doi: 10.1038/srep34845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tourovskaia A, Fauver M, Kramer G, Simonson S, Neumann T. Tissue-engineered microenvironment systems for modeling human vasculature. Exp Biol Med. 2014;239:1264–1271. doi: 10.1177/1535370214539228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Masereeuw R, et al. Probenecid interferes with renal oxidative metabolism: a potential pitfall in its use as an inhibitor of drug transport. Br J Pharmacol. 2000;131:57–62. doi: 10.1038/sj.bjp.0703541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tsai M, et al. In vitro modeling of the microvascular occlusion and thrombosis that occur in hematologic diseases using microfluidic technology. J Clin Invest. 2012;122:408–418. doi: 10.1172/JCI58753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ligresti G, et al. A novel three-dimensional human peritubular microvascular system. J Am Soc Nephrol. 2016;27:2370–2381. doi: 10.1681/ASN.2015070747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kelly EJ, et al. Innovations in preclinical biology: ex vivo engineering of a human kidney tissue microperfusion system. Stem Cell Res Ther. 2013;4:S17. doi: 10.1186/scrt378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Phan DTT, et al. A vascularized and perfused organ-on-a-chip platform for large-scale drug screening applications. Lab Chip. 2017;17:511–520. doi: 10.1039/c6lc01422d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.van Duinen V, et al. 96 perfusable blood vessels to study vascular permeability in vitro. Sci Rep. 2017;7:18071. doi: 10.1038/s41598-017-14716-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Vernetti L, et al. Functional coupling of human microphysiology systems: intestine, liver, kidney proximal tubule, blood-brain barrier and skeletal muscle. Sci Rep. 2017;7:42296. doi: 10.1038/srep42296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chang SY, et al. Human liver-kidney model elucidates the mechanisms of aristolochic acid nephrotoxicity. JCI Insight. 2017;2:95978. doi: 10.1172/jci.insight.95978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jansen J, et al. Human proximal tubule epithelial cells cultured on hollow fibers: living membranes that actively transport organic cations. Sci Rep. 2015;5:16702. doi: 10.1038/srep16702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mihajlovic M, et al. Role of vitamin D in maintaining renal epithelial barrier function in uremic conditions. Int J Mol Sci. 2017;18:2531. doi: 10.3390/ijms18122531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nguyen DG, et al. Bioprinted 3D primary liver tissues allow assessment of organ-level response to clinical drug induced toxicity in vitro. PLoS ONE. 2016;11:e0158674. doi: 10.1371/journal.pone.0158674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Persson M, Hornberg JJ. Advances in predictive toxicology for discovery safety through high content screening. Chem Res Toxicol. 2016;29:1998–2007. doi: 10.1021/acs.chemrestox.6b00248. [DOI] [PubMed] [Google Scholar]
- 148.Su R, Xiong S, Zink D, Loo LH. High-throughput imaging-based nephrotoxicity prediction for xenobiotics with diverse chemical structures. Arch Toxicol. 2016;90:2793–2808. doi: 10.1007/s00204-015-1638-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Abdullah R, Alhusainy W, Woutersen J, Rietjens IMCM, Punt A. Predicting points of departure for risk assessment based on in vitro cytotoxicity data and physiologically based kinetic (PBK) modeling: The case of kidney toxicity induced by aristolochic acid I. Food Chem Toxicol. 2016;92:104–116. doi: 10.1016/j.fct.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 150.Zhou SF, Liu JP, Chowbay B. Polymorphism of human cytochrome P450 enzymes and its clinical impact. Drug Metab Rev. 2009;41:89–295. doi: 10.1080/03602530902843483. [DOI] [PubMed] [Google Scholar]
- 151.Scotcher D, et al. Microsomal and cytosolic scaling factors in dog and human kidney cortex and application for in vitro-in vivo extrapolation of renal metabolic clearance. Drug Metab Dispos. 2017;45:556–568. doi: 10.1124/dmd.117.075242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Leclerc E, Hamon J, Bois FY. Investigation of ifosfamide and chloroacetaldehyde renal toxicity through integration of in vitro liver–kidney microfluidic data and pharmacokinetic-system biology models. J Appl Toxicol. 2016;36:330–339. doi: 10.1002/jat.3191. [DOI] [PubMed] [Google Scholar]
- 153.Miller RP, Tadagavadi RK, Ramesh G, Reeves WB. Mechanisms of cisplatin nephrotoxicity. Toxins. 2010;2:2490–2518. doi: 10.3390/toxins2112490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Nakamura T, Yonezawa A, Hashimoto S, Katsura T, Inui K. Disruption of multidrug and toxin extrusion MATE1 potentiates cisplatin-induced nephrotoxicity. Biochem Pharmacol. 2010;80:1762–1767. doi: 10.1016/j.bcp.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 155.Hu S, et al. Identification of OAT1/OAT3 as contributors to cisplatin toxicity. Clin Transl Sci. 2017;10:412–420. doi: 10.1111/cts.12480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sonneveld R, et al. Glucose specifically regulates TRPC6 expression in the podocyte in an AngII-dependent manner. Am J Pathol. 2014;184:1715–1726. doi: 10.1016/j.ajpath.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 157.Ambrus L, et al. Inhibition of TRPC6 by protein kinase C isoforms in cultured human podocytes. J Cell Mol Med. 2015;19:2771–2779. doi: 10.1111/jcmm.12660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Eyre J, et al. Statin-sensitive endocytosis of albumin by glomerular podocytes. Am J Physiol Renal Physiol. 2007;292:F674–F681. doi: 10.1152/ajprenal.00272.2006. [DOI] [PubMed] [Google Scholar]
- 159.Kido Y, Matsson P, Giacomini KM. Profiling of a prescription drug library for potential renal drug–drug interactions mediated by the organic cation transporter 2. J Med Chem. 2011;54:4548–4558. doi: 10.1021/jm2001629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Caetano-Pinto P, et al. Fluorescence-based transport assays revisited in a human renal proximal tubule cell line. Mol Pharm. 2016;13:933–944. doi: 10.1021/acs.molpharmaceut.5b00821. [DOI] [PubMed] [Google Scholar]
- 161.Shaik N, Giri N, Pan G, Elmquist WF. P-Glycoprotein-mediated active efflux of the anti-HIV1 nucleoside abacavir limits cellular accumulation and brain distribution. Drug Metab Dispos. 2007;35:2076–2085. doi: 10.1124/dmd.107.017723. [DOI] [PubMed] [Google Scholar]
- 162.Kusuhara H, et al. Effects of a MATE protein inhibitor, pyrimethamine, on the renal elimination of metformin at oral microdose and at therapeutic dose in healthy subjects. Clin Pharmacol Ther. 2011;89:837–844. doi: 10.1038/clpt.2011.36. [DOI] [PubMed] [Google Scholar]
- 163.Ito S, et al. Potent and specific inhibition of mMate1-mediated efflux of type I organic cations in the liver and kidney by pyrimethamine. J Pharmacol Exp Ther. 2010;333:341–350. doi: 10.1124/jpet.109.163642. [DOI] [PubMed] [Google Scholar]
- 164.Zhai XY, et al. Cubilin- and megalin-mediated uptake of albumin in cultured proximal tubule cells of opossum kidney. Kidney Int. 2000;58:1523–1533. doi: 10.1046/j.1523-1755.2000.00314.x. [DOI] [PubMed] [Google Scholar]
- 165.Moreno E, et al. Affinity-defining domains in the Na-Cl cotransporter: a different location for Cl− and thiazide binding. J Biol Chem. 2006;281:17266–17275. doi: 10.1074/jbc.M602614200. [DOI] [PubMed] [Google Scholar]
- 166.Andrukhova O, et al. FGF23 promotes renal calcium reabsorption through the TRPV5 channel. EMBO J. 2014;33:229–246. doi: 10.1002/embj.201284188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Masereeuw R, Moons MM, Toomey BH, Russel FGM, Miller DS. Active lucifer yellow secretion in renal proximal tubule: evidence for organic anion transport system crossover. J Pharmacol Exp Ther. 1999;289:1104–1111. [PubMed] [Google Scholar]