ABSTRACT
Chitin is a polysaccharide that provides structure and rigidity to the cell walls of fungi and insects. Mammals possess multiple chitinases, which function to degrade chitin, thereby supporting a role for chitinases in immune defense. However, chitin degradation has been implicated in the pathogenesis of asthma. Here, we determined the impact of acidic mammalian chitinase (AMCase) (Chia) deficiency on host defense during acute exposure to the fungal pathogen Aspergillus fumigatus as well as its contribution to A. fumigatus-associated allergic asthma. We demonstrate that chitin in the fungal cell wall was detected at low levels in A. fumigatus conidia, which emerged at the highest level during hyphal transition. In response to acute A. fumigatus challenge, Chia−/− mice unexpectedly demonstrated lower A. fumigatus lung burdens at 2 days postchallenge. The lower fungal burden correlated with decreased lung interleukin-33 (IL-33) levels yet increased IL-1β and prostaglandin E2 (PGE2) production, a phenotype that we reported previously to promote the induction of IL-17A and IL-22. During chronic A. fumigatus exposure, AMCase deficiency resulted in lower dynamic and airway lung resistance than in wild-type mice. Improved lung physiology correlated with attenuated levels of the proallergic chemokines CCL17 and CCL22. Surprisingly, examination of inflammatory responses during chronic exposure revealed attenuated IL-17A and IL-22 responses, but not type 2 responses, in the absence of AMCase. Collectively, these data suggest that AMCase functions as a negative regulator of immune responses during acute fungal exposure and is a contributor to fungal asthma severity, putatively via the induction of proinflammatory responses.
KEYWORDS: Aspergillus fumigatus, asthma/allergy, lung defense
INTRODUCTION
The spectrum of diseases caused by the opportunistic mold Aspergillus fumigatus ranges from mild allergic disorders, such as fungal asthma, to severe invasive diseases, such as invasive aspergillosis (IA) (1). Invasive fungal infection caused by A. fumigatus remains one of the most lethal human infectious diseases. The development of IA may be a result of multiple predisposing factors, yet immunosuppression leading to neutropenia remains the predominant risk factor (2, 3). The wider use of more-aggressive treatment modalities for conditions such as hematopoietic stem cell transplantation (HSCT) and solid-organ transplantation (SOT), new chemotherapeutic agents, and new immunomodulatory agents has increased the population of immunocompromised patients at risk for invasive fungal infection. Although there are multiple genetic immunodeficiencies associated with the development of IA, there is a growing concern for the development of nosocomial IA in the intensive care unit (ICU). Studies have indicated that up to 7% of ICU patients are diagnosed with IA at autopsy, 70% of whom usually do not have a hematological malignancy (4) (5). Identified risk factors for ICU patients include chronic obstructive pulmonary disease, malnutrition, diabetes mellitus, and liver cirrhosis (reviewed in references 6 and 7).
Asthma is an increasing health concern, affecting more than 25 million individuals in the United States and more than 300 million individuals worldwide. In 2006, a new subset of asthma, termed “severe asthma with fungal sensitization” (SAFS), was described for individuals whose asthma was poorly controlled and who were sensitized to Alternaria, Aspergillus, Cladosporium, and/or Penicillium (8). Previous reports indicate that the estimated prevalence of SAFS ranges from 17 to 46% (9–12). Common characteristics of individuals having severe asthma with fungal sensitization include onset of disease at a younger age, higher IgE levels, higher steroid usage, and more-frequent exacerbations and hospitalizations (13) (9). Human studies suggest that the pro-type 2 cytokine interleukin-33 (IL-33) may be central to the pathogenesis of SAFS (11, 12). A recent study examined genetic susceptibility in patients with SAFS in comparison to atopic, nonfungal asthmatics and healthy individuals and found numerous, significant associations with single nucleotide polymorphisms (SNPs) in Toll-like receptor 3 (TLR3), TLR9, Dectin-1, IL-10, MBL2, CCL2, CCL17, plasminogen, and the adenosine A2a receptor (14), many of which have now been studied in host defense against acute A. fumigatus exposure or immunopathogenesis during chronic A. fumigatus exposure (reviewed in reference 15).
Virulence and immune recognition of A. fumigatus have been linked to its cell wall components (16). Chitin, the second most abundant polysaccharide in nature, is a critical component of the fungal cell wall (17, 18). Chitin is processed by enzymes termed chitinases, and many species, including fungi, encode their own chitinases to aid in breaking down chitin-containing substrates and changing cell wall morphologies (19). Although mammalian species do not express chitin, they express chitinases, which are thought to aid in the degradation of chitin from insects and fungi. Humans have multiple chitinases, although only two “true” human chitinases, i.e., those with chitinolytic activity, exist and include acidic mammalian chitinase (AMCase) and chitotriosidase (20). AMCase has been studied in various models of allergic asthma (21, 22), although relatively little is known regarding its host defense capabilities. In the present study, we examined the role of AMCase during experimental invasive infection and allergic fungal asthma associated with A. fumigatus. Here, we show that AMCase negatively contributes to host defense during lung fungal infection and to immunopathogenesis during fungal asthma.
RESULTS
Chitin is detected in increasing amounts as A. fumigatus conidia transition to hyphae.
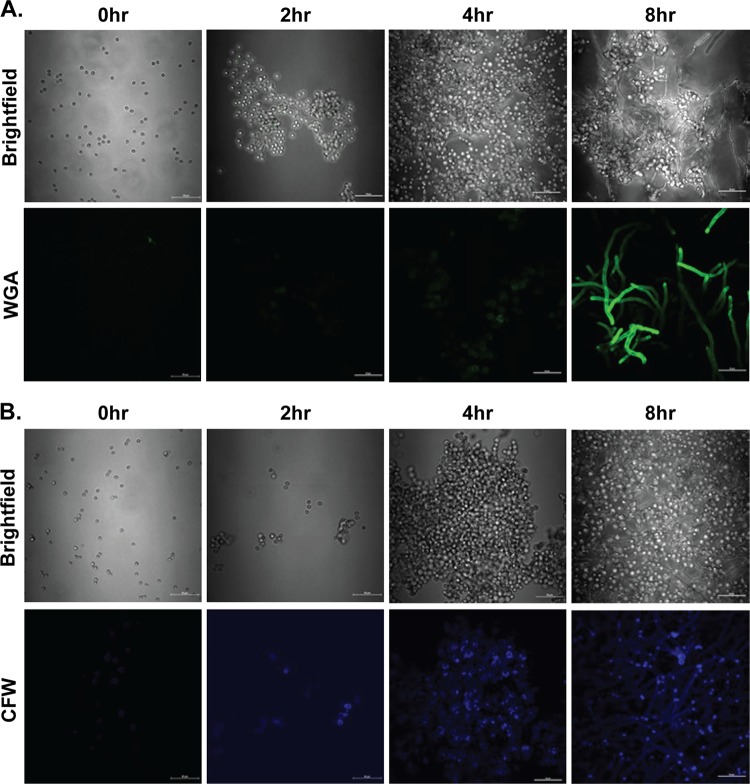
The cell wall of A. fumigatus is comprised primarily of polysaccharides, which play a vital role in providing structural rigidity (23). In addition to providing structural support, the cell wall plays important roles in fungal growth and modulation of the host immune response. A previous study employing a cell wall preparation from Aspergillus niger hyphae demonstrated chitin staining that was sensitive to degradation by microbially derived (parasitic and bacterial) chitinases (18). To better understand the natural exposure of chitin in A. fumigatus, we cultured the organism over a period of 8 h, a time course which we employed previously to identify the unmasking of beta-glucans in resting conidia, swollen conidia, germinating conidia, and hyphae (24). Initial studies employing Oregon Green 488-conjugated wheat germ agglutinin (WGA), which specifically binds N-acetylglucosaminyl residues in chitin, revealed low levels of chitin in A. fumigatus conidia after 2 h (Fig. 1A, second panel) and 4 h (Fig. 1A, third panel) of culture. Chitin staining significantly intensified in germinating conidia and hyphae at 8 h (Fig. 1A, fourth panel). We also employed calcofluor white (CFW), a widely employed reagent that reacts with cellulose and chitin, which replicated the findings with Oregon green 488-conjugated wheat germ agglutinin. However, CFW was much more sensitive at detecting lower levels of chitin with positive staining at 0 h (Fig. 1B, first panel), which increased throughout the time course (Fig. 1B, second to fourth panels). Thus, chitin emerges as A. fumigatus progresses through the transition from resting conidia to swollen conidia to hyphae.
FIG 1.
Chitin is detected in increasing amounts as A. fumigatus conidia transition to hyphae. A. fumigatus conidia were resuspended in RPMI medium supplemented with 1% penicillin-streptomycin-glutamine and 10% heat-inactivated FBS and cultured for 0, 2, 4, and 8 h on glass slides at 37°C in a 5% CO2 incubator. For the 0-h time point, conidia were incubated for 1 h at room temperature to allow adherence. Adhered conidia were washed with PBS for 5 min and stained with Oregon green 488-conjugated wheat germ agglutinin at 37°C (A) or with calcofluor white at room temperature (B). Stained conidia were washed twice with PBS before mounting. 2D fluorescent images of stained conidia were imaged by using a Nikon A1 high-speed confocal laser scanning microscope with constant parameters across samples. Corresponding bright-field images are included to aid in the visualization of the culture. Representative images are shown.
Acidic mammalian chitinase negatively affects A. fumigatus lung clearance.
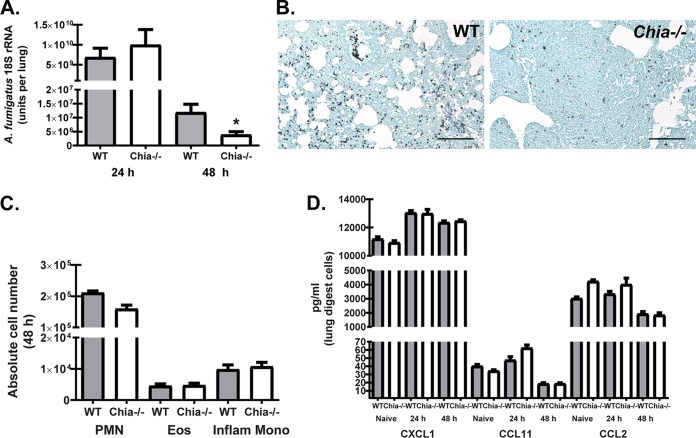
Chitin is the second must abundant carbohydrate polymer in nature after cellulose (25). As such, bacteria, parasites, fungi, insects, and mammals have developed chitinolytic enzymes, termed chitinases, that may function in diverse cellular processes (25). Mammalian chitinases, which include AMCase and chitotriosidase, were initially described as having antifungal properties against clinically relevant pathogens such as Cryptococcus neoformans and Candida albicans (reviewed in reference 26). Based on those observations, we hypothesized that AMCase-deficient (Chia−/−) mice would demonstrate increased susceptibility to lung infection with A. fumigatus. Much to our surprise, although there was no difference in early fungal clearance at 24 h postchallenge, Chia−/− mice had a >66% reduction in lung fungal burden compared to wild-type (WT) mice at 48 h, thus demonstrating enhanced fungal clearance (Fig. 2A). Grocott-Gomori's methenamine silver (GMS) staining of lung tissues also convincingly showed much higher numbers of A. fumigatus organisms in WT than in Chia−/− mice (Fig. 2B). Intriguingly, the augmented fungal clearance in Chia−/− mice at 48 h postchallenge was not a consequence of differential lung cellularity, as we observed no significant changes in neutrophils and eosinophils (gated on CD11b+ cells followed by Ly6G+ cells as neutrophils and Siglec-F+ cells as eosinophils) or inflammatory monocytes (gated on CD11b+ Ly6C+ cells followed by CCR2+ cells) (all of which have been implicated in innate lung clearance of A. fumigatus [2, 27, 28]) (Fig. 2C). This observation is supported by the lack of differences in the levels of various chemokines produced by lung digest cells that support the recruitment of neutrophils, eosinophils, and inflammatory monocytes (Fig. 2D). Thus, despite the putative antifungal activity of AMCase, clearance of A. fumigatus from the lung is enhanced in the absence of AMCase.
FIG 2.
Acidic mammalian chitinase negatively affects A. fumigatus lung clearance. (A) C57BL/6 wild-type (WT) and acidic mammalian chitinase-deficient (Chia−/−) mice were challenged intratracheally with A. fumigatus conidia, and at 24 and 48 h postexposure, lung fungal burdens were assessed by real-time PCR analysis of A. fumigatus 18S rRNA levels. Shown are cumulative data from three independent studies (n = 4 to 6 mice per group, per study). Data are expressed as mean A. fumigatus 18S rRNA levels and standard errors of the means. (B) Representative GMS-stained lung sections from wild-type (left) and Chia−/− (right) mice challenged intratracheally with A. fumigatus conidia for 48 h. Original magnification, ×200. Bar, 100 μm. (C) WT and Chia−/− mice were challenged with A. fumigatus, and 48 h after exposure, the right lungs were collected, enzymatically digested, Fc blocked, stained with a live/dead staining kit, and thereafter stained with fluorochrome-conjugated antibodies against the following cell surface markers: neutrophils and eosinophils (Eos) (gated on CD11b+ cells followed by Ly6G+ cells as neutrophils and Siglec-F+ cells as eosinophils) and inflammatory monocytes (Inflam Mono) (gated on CD11b+ Ly6C+ cells followed by gating on CCR2+ cells). Cumulative flow cytometric data are from two independent studies (n = 2 to 4 mice per group, per study). Data are expressed as absolute numbers of live cells in lung digests. PMN, polymorphonuclear leukocytes. (D) WT and Chia−/− mice were challenged intratracheally with A. fumigatus conidia, the right lungs were collected 24 h and 48 h after exposure and enzymatically digested, and unfractionated lung cells were cultured in triplicate for 24 h. The right lungs from naive WT and Chia−/− mice were also collected and processed to examine baseline mediator levels. CXCL1/KC, CCL11/eotaxin, and CCL2/MCP-1 levels in clarified coculture supernatants were quantified by Bio-Plex. Shown are cumulative data from three independent studies (n = 1 to 2 mice per group, per study). For all graphs, * represents a P value of <0.05 (unpaired two-tailed Student's t test).
Acidic mammalian chitinase regulates lung inflammatory responses after acute A. fumigatus exposure.
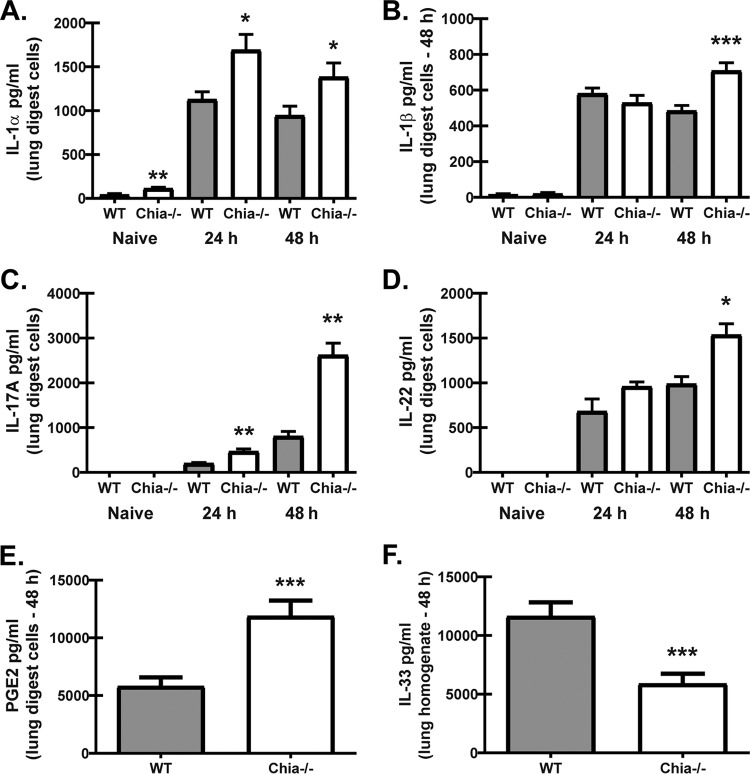
Roles for A. fumigatus chitin recognition and responsiveness in lung defense are poorly understood. Previous studies implicated A. fumigatus chitin recognition in eosinophil-mediated immunopathology during neutropenia-associated invasive infection (29). Other studies showed that A. fumigatus chitin recognition in human peripheral blood mononuclear cell (PBMC) cultures may induce the anti-inflammatory cytokine IL-1RA (30) or that A. fumigatus chitin is essentially inert (31). We recently reported that mice deficient in the receptor for the IL-1 family cytokine IL-33 demonstrated similarly enhanced A. fumigatus lung clearance in the absence of changes in lung cellularity (32). Mechanistically, we demonstrated this phenotype was a result of increased IL-1α, IL-1β, IL-6, IL-17A, and IL-22 production (32). In accordance with this, despite the lower fungal burden at 48 h, Chia−/− mice also demonstrated elevated IL-1α (Fig. 3A), IL-1β (Fig. 3B), IL-17A (Fig. 3C), and IL-22 (Fig. 3D) production at 24 h (with the exception of IL-1β) and at 48 h post-A. fumigatus challenge. In our report with IL-33 receptor-deficient mice, enhanced prostaglandin E2 (PGE2) production drove the observed elevations in IL-17A and IL-22 levels at 48 h postchallenge (32). We similarly observed elevated production of PGE2 by lung cells from Chia−/− mice compared to WT mice at 48 h postchallenge (Fig. 3E), suggesting that AMCase negatively regulates PGE2. Furthermore, we show that elevated PGE2 levels at 48 h postchallenge correlated with significantly lower levels of total IL-33 (measured in lung homogenates) in Chia−/− mice (Fig. 3F). Thus, AMCase functions as a negative regulator of inflammatory cytokine production during acute A. fumigatus exposure.
FIG 3.
Acidic mammalian chitinase regulates lung inflammatory responses after acute A. fumigatus exposure. WT and Chia−/− mice were challenged intratracheally with A. fumigatus conidia, the right lungs were collected 24 h and 48 h after exposure and enzymatically digested, and unfractionated lung cells were cultured in triplicate for 24 h. The right lungs were also collected from naive WT and Chia−/− mice and processed to examine baseline mediator levels. (A to E) IL-1α (A), IL-1β (B), and IL-17A (C) levels in clarified coculture supernatants were quantified by Bio-Plex, and IL-22 (D) and PGE2 (E) levels were quantified by an ELISA. (F) The left lungs were collected, homogenized in PBS supplemented with protease inhibitors, and clarified by centrifugation. The IL-33 level in clarified lung homogenates was quantified by an ELISA. Shown are cumulative data from three independent studies (n = 1 to 3 mice per group, per study). For all graphs, *, **, and *** represent P values of <0.05, 0.01, and 0.001, respectively (unpaired two-tailed Student's t test).
Acidic mammalian chitinase regulates chemokines associated with classical and alternative macrophage activation after acute A. fumigatus exposure.
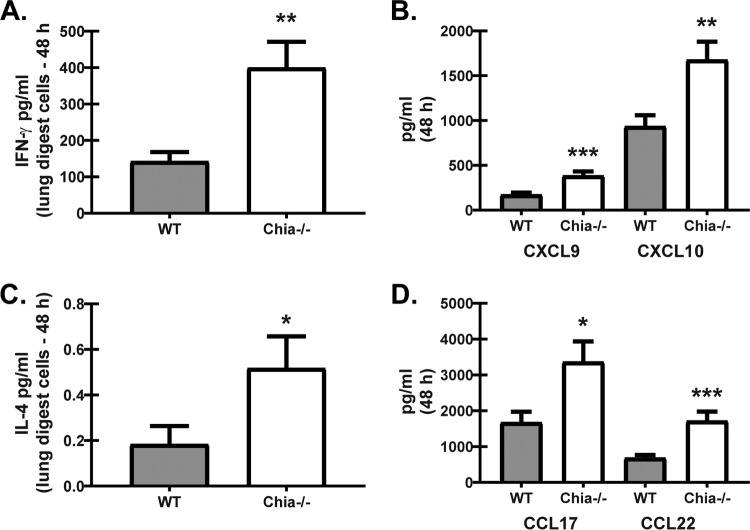
A recent study demonstrated that chemical inhibition of AMCase in human monocyte-derived macrophages enhanced the killing of the yeast Candida albicans, which was associated with reduced arginase-1 activity (33). Therefore, an additional hypothesis may be that AMCase deficiency results in enhanced classical macrophage activation that is conducive to enhanced A. fumigatus elimination. To this end, Chia−/− mice demonstrated increased production of gamma interferon (IFN-γ) 48 h after A. fumigatus challenge (Fig. 4A). Increased IFN-γ production further correlated with elevated levels of the chemokines CXCL9 and CXCL10 (Fig. 4B), suggesting that classical macrophage activation (M1) was increased in the absence of AMCase. However, a previous study demonstrated that alternative macrophage activation (M2) may also function in the clearance of acute A. fumigatus exposure (34). To this end, IL-4 levels in lung digest cell cultures, while produced at very small amounts, were significantly higher in Chia−/− mice (Fig. 4C). IL-13 production by lung digest cells was not detectable (data not shown). Increased IL-4 production further correlated with elevated levels of the chemokines CCL17 and CCL22 (Fig. 4D), suggesting that alternative macrophage activation may also be increased in the absence of AMCase. There were no differences in IFN-γ, IL-4, or IL-13 levels at baseline (data not shown). Thus, AMCase may also function as a negative regulator of macrophage activation during acute A. fumigatus exposure.
FIG 4.
Acidic mammalian chitinase regulates chemokines associated with classical and alternative macrophage activation after acute A. fumigatus exposure. WT and Chia−/− mice were challenged intratracheally with A. fumigatus conidia, the right lungs were collected 48 h after exposure and enzymatically digested, and unfractionated lung cells were cultured in triplicate for 24 h. (A to C) IFN-γ (A), CXCL9 and CXCL10 (B), and IL-4 (C) levels in clarified coculture supernatants were quantified by Bio-Plex. (D) CCL17 and CCL22 levels in clarified lung homogenates were quantified by an ELISA. Shown are cumulative data from three to four independent studies (n = 4 to 5 mice per group, per study). For all graphs, *, **, and *** represent P values of < 0.05, 0.01, and 0.001, respectively (unpaired two-tailed Student's t test).
Acidic mammalian chitinase promotes immunopathogenic responses during fungal asthma.
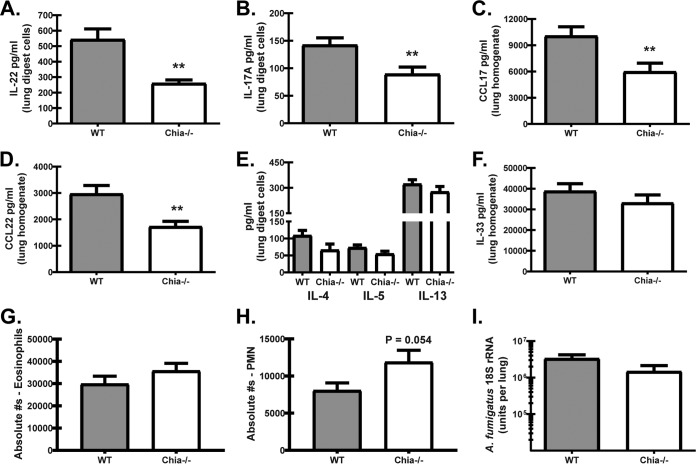
We previously reported that mice deficient in the beta-glucan receptor Dectin-1 demonstrated improved lung function during fungal asthma, which correlated with reductions in the levels of multiple proallergic and proinflammatory mediators (35). We furthermore demonstrated that Dectin-1 drove IL-22 production, and genetic deficiency or neutralization of IL-22 resulted in less-severe fungal asthma. Results in Fig. 5 show that the levels of IL-22 (Fig. 5A) as well as IL-17A (Fig. 5B) were reduced in Chia−/− mice during fungal asthma. Although this is an unexpected observation based on the increased levels of these mediators in Chia−/− mice during acute fungal exposure (Fig. 3A), these results nevertheless correlated with less-severe asthma in these mice and reproduced our previously reported results (36). Likewise, Chia−/− mice demonstrated lower levels of the proallergic chemokines CCL17 (Fig. 5C) and CCL22 (Fig. 5D), two clinically relevant biomarkers of fungal asthma severity in humans (37, 38) and in our fungal asthma model (35). However, we did not observe any differences in the production of the type 2 cytokines IL-4, IL-5, and IL-13 (Fig. 5E) or in the production of the pro-type 2 cytokine IL-33 (Fig. 5F). AMCase deficiency also did not impact the level of neutrophils (Fig. 5G) or eosinophils (Fig. 5F) in the lung. Similar to our previous study with WT versus Dectin-1-deficient mice with fungal asthma (35), there was no difference in fungal burdens between WT and Chia−/− mice (Fig. 5I). Thus, the absence of AMCase correlates with the attenuated production of IL-17A and IL-22 during fungal asthma.
FIG 5.
Acidic mammalian chitinase promotes immunopathogenic responses during fungal asthma. WT and Chia−/− mice were subjected to experimental fungal asthma. Twenty-four hours after the last organism challenge, the right lungs were collected and enzymatically digested, and unfractionated lung cells were cultured for 24 h in the presence of A. fumigatus conidia, or the left lungs were collected, homogenized, and clarified by centrifugation. (A to F) IL-22 levels in lung digest cell culture supernatants were quantified by an ELISA (A); IL-17A levels in lung digest cell culture supernatants were quantified by Bio-Plex (B); CCL17 (C) and CCL22 (D) levels in lung homogenates were quantified by an ELISA; IL-4, IL-5, and IL-13 levels in lung digest cell culture supernatants were quantified by Bio-Plex (E); and IL-33 levels in lung homogenates were quantified by an ELISA (F). Shown are cumulative data from two to four independent studies (n = 2 to 5 mice per group, per study). (G and H) WT and Chia−/− mice were subjected to experimental fungal asthma. Twenty-four hours after the last organism challenge, the right lungs were collected, enzymatically digested, Fc blocked, stained with a live/dead staining kit, and thereafter stained with fluorochrome-conjugated antibodies against the following cell surface markers: neutrophils and eosinophils (gated on CD11b+ cells followed by Ly6G+ cells as neutrophils and Siglec-F+ cells as eosinophils). Cumulative flow cytometric data from four independent studies (n = 3 to 5 mice per group, per study). Data are expressed as absolute numbers of live cells in lung digests. (I) WT and Chia−/− mice were subjected to experimental fungal asthma. Twenty-four hours after the last organism challenge, the lung fungal burden was assessed by real-time PCR analysis of A. fumigatus 18S rRNA levels. Shown are cumulative data from two independent studies (n = 4 to 5 mice per group, per study). Data are expressed as mean A. fumigatus 18S rRNA levels and standard errors of the means. For all graphs, ** represents a P value of <0.01 (unpaired two-tailed Student's t test).
Acidic mammalian chitinase contributes to worse lung function during fungal asthma.
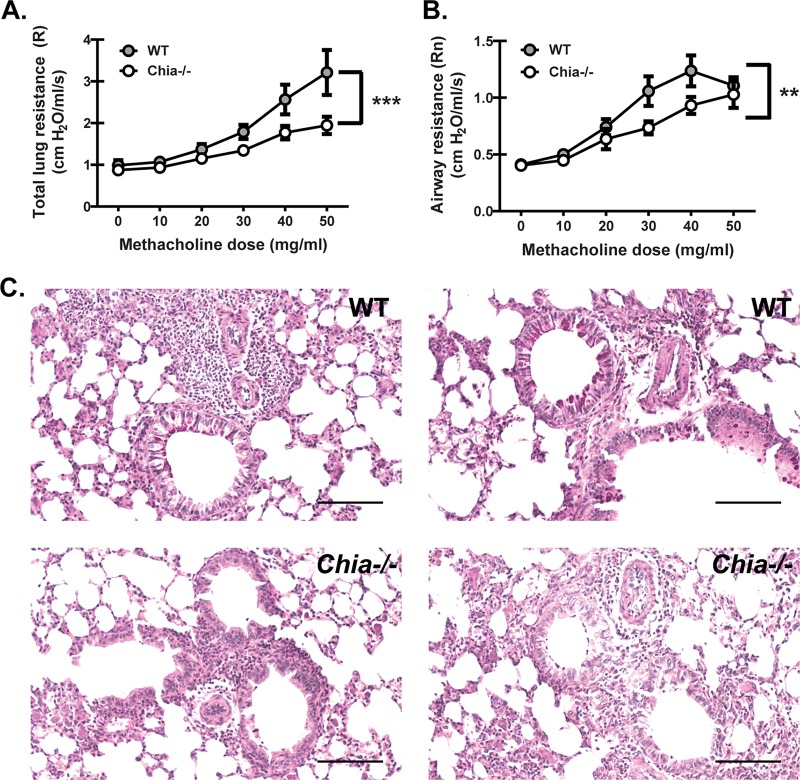
Chitinases are hypothesized to play a significant role in asthma, owing to the observation that predominant aeroallergens, such as house dust mites (HDMs), cockroaches, and fungi, have chitin in their exoskeletons or cell walls (39). However, the contribution of AMCase to asthma pathogenesis is controversial, with reports arguing for and against its role (21, 22). We previously investigated immunopathogenic mechanisms using a repetitive-challenge model of fungal asthma with live A. fumigatus conidia that reproduces some critical features of allergic disease observed in individuals who are persistently exposed to fungi (35). Employing this chronic A. fumigatus exposure, we observed significantly lower dynamic lung resistance (Fig. 6A) and airway resistance (Fig. 6B) in Chia−/− mice upon challenge with increasing methacholine concentrations than in WT mice. Histological assessment of lung tissue sections revealed that Chia−/− mice had evidence of attenuated goblet cell hyperplasia and mucus production (Fig. 6C). Thus, the absence of AMCase results in improved lung function during chronic exposure to live A. fumigatus conidia.
FIG 6.
Acidic mammalian chitinase contributes to worse lung function during fungal asthma. (A and B) WT and Chia−/− mice were subjected to experimental fungal asthma. Twenty-four hours after the last organism challenge, dynamic lung resistance (A) and airway resistance (B) were analyzed via mechanical ventilation using the flexiVent system. Shown are cumulative data from two independent studies (n = 5 mice per group, per study). Data are expressed as means ± standard errors of the means. (C) Representative periodic acid-Schiff-stained lung sections from WT (top images) and Chia−/− (bottom images) mice. Original magnification, ×200. Bar, 100 μm. For all graphs, ** and *** represent P values of <0.01 and 0.001, respectively (2-way analysis of variance).
DISCUSSION
In this report, we demonstrate that acidic mammalian chitinase negatively regulates host defense during acute exposure to A. fumigatus (invasive fungal infection model) and promotes disease severity during chronic exposure to A. fumigatus (fungal asthma model). A putative negative role for AMCase during invasive aspergillosis was an unexpected finding based on its hypothesized antifungal role. A previous study employed a disc diffusion method with both recombinant and purified AMCase and demonstrated growth inhibition of A. fumigatus (40). Another study employing conditioned medium containing a second true mammalian chitinase, chitotriosidase, demonstrated reduced A. fumigatus hyphal growth (41). Antifungal activity is not unique to mammalian chitinases, as chitinases from bacterial species have also been shown to degrade A. fumigatus alkali-insoluble cell wall fragments that contain chitin linked covalently to beta-glucan (42). However, with the exception of two reports investigating Toxoplasma gondii infection (43) and gastrointestinal nematode infection (44), the role of AMCase in in vivo host defense has not been investigated.
We initiated our studies by first examining whether A. fumigatus lung clearance was impacted by AMCase deficiency and found that clearance was surprisingly enhanced at 2 days postexposure. Although opposite our hypothesis, the ∼3-fold decrease in the fungal burden between wild-type and AMCase-deficient mice at 2 days postchallenge is of a magnitude comparable to that in our previous studies employing mice on the C57BL/6 background. Susceptible Dectin-1-deficient mice demonstrate ∼3- to 5-fold-higher burdens and significant associated mortality (45), whereas resistant IL-33 receptor (IL-33R)-deficient mice have ∼4- to 5-fold-lower burdens (32). Examination of changes in the immune responses between WT and Chia−/− mice at 2 days postexposure demonstrated the enhanced production of a specific set of inflammatory mediators. First, IL-1α and IL-1β were produced at higher levels by lung digest cells from Chia−/− mice. Renewed interest in the IL-1 family of cytokines has uncovered novel roles for IL-1α and IL-1β in A. fumigatus lung defense. IL-1 receptor deficiency results in profound susceptibility to A. fumigatus as a result of IL-1α-mediated early (day 1) neutrophil recruitment and IL-1β-mediated macrophage antifungal responses (46). More recently, IL-1α was found to be required for the elimination of highly virulent, highly germinating A. fumigatus strains (47). Likewise, we recently reported that another function of IL-1 receptor signaling during A. fumigatus exposure is the induction of IL-17A and IL-22 (32), both of which we have shown to be essential for A. fumigatus elimination from the lungs (48) (49). To this end, Chia−/− mice also demonstrated increased production of IL-17A and IL-22 by lung digest cells, which correlated with the observed increases in IL-1α and IL-1β production. However, the enhanced production of IL-1α, IL-1β, IL-17A, and IL-22 did not affect the level of inflammatory cells known to be important for A. fumigatus lung clearance. The enhanced fungal clearance in the presence of a heightened inflammatory cytokine profile, but no differences in inflammatory cell recruitment, is reminiscent of a phenotype that we recently reported for Il1rl1−/− (IL-33 receptor-deficient) mice after acute A. fumigatus exposure (32). In an effort to link these two observations, we demonstrate that lower total IL-33 levels, as measured in lung homogenates, were also observed in AMCase-deficient mice. Previous work has shown that during house dust mite-associated asthma, uncleaved chitin increases IL-33 levels in lung lavage fluid (50), which is opposite our findings here. It is possible that measurement of IL-33 levels in lung homogenates versus lung lavage fluid yields different observations. However, lower total IL-33 levels in AMCase-deficient mice (measured in total lung homogenates) occurred in the presence of enhanced PGE2 production (measured in supernatants from cultured lung digest cells), suggesting a connection between these mediators based on data from our recent report (32). The link between AMCase deficiency, lower total IL-33 levels, and enhanced PGE2 production is the subject of ongoing investigation. Another finding was that the levels of chemokines associated with macrophage activation status, CXCL9 and CXCL10 for classical activation and CCL17 and CCL22 for alternative activation (51), were also elevated in the absence of AMCase. As these increases correlated with elevated levels of the type 1 cytokine IFN-γ and the type 2 cytokine IL-4, there is a possibility that AMCase negatively regulates the innate cellular sources of these mediators. Alternatively, since alveolar macrophages are a source of AMCase (21), there is also a possibility that AMCase controls macrophage activation at some level. These observations are the basis of future studies. Overall, our data suggest that AMCase expressed during acute A. fumigatus exposure functions to limit the magnitude of the inflammatory response.
We next examined the role of AMCase in chronic exposure to A. fumigatus, which mimics severe asthma with fungal sensitization. Here, we found that AMCase deficiency resulted in overall less-severe fungal asthma. A role for AMCase in asthma pathogenesis has been debatable. The initial study describing a role for AMCase in asthma employed an ovalbumin sensitization model and demonstrated that AMCase was expressed by epithelial cells and macrophages in an IL-13-dependent manner and played a role in airway hyperreactivity (AHR) (21). Human asthma studies have identified SNPs in Chia/AMCase that were associated with disease severity (52), although other studies did not find an association (53). There has also been some debate as to whether AMCase is fully functional in the lung (44), as AMCase was thought to be most active at low pH (54). However, a recent detailed biochemical assessment of AMCase chitinolytic activity demonstrated that AMCase may work under a variety of somatic tissue pH conditions, with activity up to a pH of 8.0 (55). By use of a more relevant chitin-associated allergic asthma model that employed HDM and cockroach extracts, AMCase-deficient mice demonstrated elevated lymphocyte and neutrophil, but not eosinophil, numbers yet lower IL-13 levels. However, this phenotype had no impact on lung function (22). A study employing lower HDM allergen concentrations also demonstrated that there was no impact of AMCase deficiency on lung cellularity or type 2 responses (44). A separate study developed a different mouse strain, specifically AMCase knock-in/knockout reporter mice, which demonstrated AMCase expression in alveolar type 2 cells but not macrophages (56). When challenged with extracts from HDMs or A. niger, these mice demonstrated increased lung cellularity, including increased numbers of type 2 innate lymphoid cells (ILC2s) as well as IL-17A+ γδ T cells. A third study developed an additional mouse strain that expressed a mutant extracellular domain of AMCase, which demonstrated enhanced type 2 responses in an HDM asthma model (50). Collectively, these results suggest that AMCase regulates multiple inflammatory phenotypes (type 2 and type 17) during allergic asthma. However, at least in the case of the latter two reports (50, 56), lung physiologic responses were not measured; therefore, it is difficult to appreciate how these immunologic changes impacted lung function. One of the more striking observations in our work was the profound effect of AMCase deficiency on lung function. Our data showed that a lack of AMCase had a major impact on both total lung resistance (R) as well as airway resistance (Rn), i.e., Newtonian resistance, which is more commonly known as AHR. This is an important observation, as a previous study employing chronic exposure to an A. niger hyphal cell wall preparation demonstrated decreased eosinophil recruitment to the lungs in mice overexpressing AMCase, yet this did not result in any differences in lung physiologic measurements (18). Differences between data from our study and that study include fungal species (A. fumigatus versus A. niger), model of exposure (live organisms versus a cell wall preparation), and mouse strain (genetic deficiency versus transgenic overexpression). Overall, our results support the hypothesis that the expression of AMCase and/or the degradation of A. fumigatus chitin in vivo during fungal asthma leads to immune responses that increase the sensitivity of the airways to bronchoconstrictors.
The next question was what type of immune response facilitated the phenotype observed in AMCase-deficient mice during fungal asthma. The data described above suggest that the absence of AMCase results in more type 2 (22), less type 2 (50), or no changes in type 2 (44) responses during allergic asthma. In our live fungal challenge model, we did not observe any differences in the levels of IL-4, IL-5, or IL-13 production by unfractionated lung digest cells. We also did not observe any differences in IL-33 levels in lung homogenates between WT and AMCase-deficient mice. AMCase deficiency was also associated with increased numbers of IL-17A-producing γδ T cells during A. niger extract exposure (56). In contrast, we observed significantly lower levels of IL-17A and IL-22 production by lung digest cells from AMCase-deficient mice. Although this observation is opposite our findings with acute A. fumigatus exposure, it nevertheless agrees with data from our previous work demonstrating that IL-22 promotes immunopathology during chronic A. fumigatus exposure (35). We also observed significant reductions in CCL17 and CCL22 levels in AMCase-deficient mice. CCL17 and CCL22 have been identified as biomarkers of disease severity in human allergic bronchopulmonary aspergillosis, and we demonstrated previously that the levels of these chemokines are reduced in mice with less-severe fungal asthma (35). Our results are also supported by data from a previous report showing that lung epithelial cells transfected with AMCase induced the production of CCL17 (57). In addition, mice deficient in CCR4, the receptor for CCL17 and CCL22, have reduced airway hyperresponsiveness during A. fumigatus-associated asthma (58). The CCL17/CCL22/CCR4 axis is commonly viewed as being important for type 2-associated responses, specifically recruiting Th2 CD4 T cells (reviewed in reference 59). However, CCR4 may also be coexpressed with CCR6 on Th17 (60) and Th22 (61) CD4 T cells. Therefore, a possible mechanism is that lower CCL17 and CCL22 levels result in the decreased recruitment of cells that produce IL-17A and IL-22 during fungal asthma.
In conclusion, A. fumigatus conidia express a low level of chitin that emerges through the germination process. The absence of acidic mammalian chitinase results in an augmented IL-17A/IL-22 response during acute A. fumigatus infection, which aids in more-efficient elimination from the lung. In contrast, the absence of acidic mammalian chitinase during chronic fungal asthma results in a dramatic improvement in lung function, which paradoxically is associated with a reduction in IL-17A/IL-22 responses. These results provide intriguing insights into the in vivo effects of chitin degradation during acute versus chronic fungal exposure.
MATERIALS AND METHODS
Mice.
Male and female age-matched C57BL/6NTac mice were purchased from Taconic Farms Incorporated (Hudson, NY). Chia−/− (AMCase-deficient) mice were obtained from Lori Fitz, Pfizer Worldwide (Cambridge, MA) (22). Mice were maintained in microisolator cages in a specific-pathogen-free, Association for Assessment and Accreditation of Laboratory Animal Care-certified facility and handled according to Public Health Service Office of Laboratory Animal Welfare policies after review by the University of Alabama at Birmingham (UAB) Institutional Animal Care and Use Committee.
Preparation, exposure, and analysis of A. fumigatus.
A. fumigatus isolate ATCC 13073 (ATCC, Manassas, VA) was maintained on potato dextrose agar for 5 to 7 days at 37°C. Conidia were harvested by washing the culture flask with 50 ml of sterile phosphate-buffered saline (PBS) supplemented with 0.1% Tween 20. The conidia were then passed through a sterile 40-μm nylon membrane to remove hyphal fragments and enumerated on a hemacytometer. For acute exposure, mice were anesthetized lightly with isoflurane and administered 7 × 107 A. fumigatus conidia in a volume of 50 μl intratracheally (i.t.), as described previously (45). For chronic exposure, a repeated A. fumigatus exposure model was employed, as described previously (35). Briefly, mice were anesthetized lightly with isoflurane and administered 1 × 107 live A. fumigatus conidia in a volume of 50 μl of PBS i.t. Starting at day 7, mice were challenged i.t. with 1 × 106 live A. fumigatus conidia in 50 μl of PBS daily for 5 days, rested for 2 days, and challenged daily for another 3 days. Twenty-four hours after the final challenge, immune and physiologic measures were assessed, as described below. For lung fungal burden analysis, the left lungs were collected at 48 h postexposure and homogenized in 1 ml of PBS. Total RNA was extracted from 0.1 ml of the unclarified lung homogenate by using the MasterPure yeast RNA purification kit (Epicentre Biotechnologies, Madison, WI), which includes a DNase treatment step to eliminate genomic DNA, as described previously (45). Total RNA was also extracted from serial 1:10 dilutions of live A. fumigatus conidia (101 to 109) and DNase treated to form a standard curve. The lung A. fumigatus burden was analyzed by real-time PCR measurement of the A. fumigatus 18S rRNA level (GenBank accession number AB008401) and quantified by using a standard curve of A. fumigatus conidia, as described above. For chitin staining, A. fumigatus conidia were resuspended at 5 × 107 conidia/ml in RPMI medium (Sigma, St. Louis, MO) supplemented with 1% penicillin-streptomycin-glutamine (Mediatech, Herndon, VA) and 10% heat-inactivated fetal bovine serum (FBS) (Invitrogen, Carlsbad, CA) and cultured for 0 to 8 h on glass slides (Lab-TekII, catalog number 1544453; Thermo Fisher) at 37°C in a CO2 incubator. For the 0-h time point, conidia were incubated for 1 h at room temperature to allow adherence. Adhered conidia were washed with PBS for 5 min and stained with 5 μg/ml of Oregon green 488-conjugated wheat germ agglutinin (catalog number W7024; Invitrogen, Carlsbad, CA) for 12 min at 37°C or with calcofluor white (catalog number 18909; Sigma, St. Louis, MO) for 10 min at room temperature. Stained conidia were washed twice with PBS before mounting. Two-dimensional (2D) fluorescent images of stained conidia were imaged by using a Nikon A1 high-speed confocal laser scanning microscope, with constant parameters across samples.
Lung cell isolation and culture and inflammatory cytokine and PGE2 analyses.
Mice were anesthetized with ketamine-xylazine intraperitoneally and sacrificed by exsanguination at 48 h postinfection. Both lungs were collected and minced in Iscove's modified Dulbecco's medium (IMDM) (Sigma, St. Louis, MO) supplemented with 1% penicillin-streptomycin-glutamine (Mediatech, Herndon, VA), 10% heat-inactivated FBS (Invitrogen, Carlsbad, CA), and 0.4 mg/ml polymyxin B (Thermo Fisher), followed by incubation for 60 min with tissue culture-grade type IV collagenase (1 mg/ml; Sigma, St. Louis, MO) in a 37°C orbital shaker at 100 rpm. The cell suspension was filtered through sterile 70-μm and 40-μm nylon filters, and red blood cells were lysed with ACK buffer (Lonza, Walkersville, MD) to create lung cell digest preparations. For lung cell cultures, cells were enumerated on a hemacytometer and plated at 1 × 106 cells in a volume of 0.2 ml. Supernatants were collected after 24 h, clarified by centrifugation, and stored at −80°C. Supernatants were analyzed for protein levels of 32 cytokines and chemokines by using a Milliplex multiplex suspension cytokine array (Millipore), according to the manufacturer's instructions. The data were analyzed by using Bio-Plex Manager software (Bio-Rad Laboratories). IL-22, IL-33, CCL17, and CCL22 levels were quantified by an enzyme-linked immunosorbent assay (ELISA) (R&D Systems). PGE2 levels were quantified by using the prostaglandin E2 parameter assay kit (catalog number KGE004B; R&D Systems).
Lung cell flow cytometry.
Lung cells were isolated previously, as described above. Cells were washed, and Fc receptors were blocked with mouse BD Fc block (BD Biosciences, San Diego, CA) at 4°C for 20 min. Thereafter, cells were stained with a single-color live/dead fixable dead cell stain (Invitrogen), followed by labeling with specific immune cell surface markers. The following staining parameters were employed: eosinophils as CD11b+ Siglec-F+ Ly6G− cells, neutrophils as CD11b+ Ly6G+ cells, inflammatory monocytes as CD11b+ Ly6Chi CCR2+ cells, and T cells as CD3+ CD4+ cells (all antibodies were purchased from eBioscience and BD Biosciences). Unstained or single-color-stained cells served as compensation controls. Samples were acquired by using a four-laser, 20-parameter analytic BD LSR II cytometer (BD Biosciences), and data were analyzed by using FlowJo software (TreeStar, Ashland, OR).
Pulmonary function assessment.
Individually anesthetized A. fumigatus-exposed mice were cannulated intratracheally and attached to a computer-controlled volume ventilator (flexiVent; SciReq, Montreal, QC, Canada). Regular breathing was set at 150 bpm, with the volume and pressure controlled by the flexiVent system based on individual animal weights. Positive end-expiratory pressure (PEEP) was set to 2 cm H2O and measured during each breath stroke. Respiratory input impedance (Zrs) was measured by using the forced oscillation technique, controlled by the flexiVent system. The single-compartment model was used to describe dynamic lung resistance. All measurements were collected at baseline and after a linear dose response with methacholine challenge (10 to 40 mg/ml).
Statistics.
Data were analyzed by using GraphPad Prism version 5.0 statistical software. Comparisons between groups when data were normally distributed were made with the Student t test. Significance was accepted at a P value of <0.05.
ACKNOWLEDGMENTS
This work was supported by PHS grants HL136211 and HL122426 (to C.S.).
We have no conflicts of interest to declare.
REFERENCES
- 1.Park SJ, Mehrad B. 2009. Innate immunity to Aspergillus species. Clin Microbiol Rev 22:535–551. doi: 10.1128/CMR.00014-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herbrecht R, Bories P, Moulin JC, Ledoux MP, Letscher-Bru V. 2012. Risk stratification for invasive aspergillosis in immunocompromised patients. Ann N Y Acad Sci 1272:23–30. doi: 10.1111/j.1749-6632.2012.06829.x. [DOI] [PubMed] [Google Scholar]
- 3.De Le Rosa GR, Champlin RE, Kontoyiannis DP. 2002. Risk factors for the development of invasive fungal infections in allogeneic blood and marrow transplant recipients. Transpl Infect Dis 4:3–9. doi: 10.1034/j.1399-3062.2002.00010.x. [DOI] [PubMed] [Google Scholar]
- 4.Meersseman W, Lagrou K, Maertens J, Wilmer A, Hermans G, Vanderschueren S, Spriet I, Verbeken E, Van Wijngaerden E. 2008. Galactomannan in bronchoalveolar lavage fluid—a tool for diagnosing aspergillosis in intensive care unit patients. Am J Respir Crit Care Med 177:27–34. doi: 10.1164/rccm.200704-606OC. [DOI] [PubMed] [Google Scholar]
- 5.Bassetti M, Bouza E. 2017. Invasive mould infections in the ICU setting: complexities and solutions. J Antimicrob Chemother 72:i39–i47. doi: 10.1093/jac/dkx032. [DOI] [PubMed] [Google Scholar]
- 6.Ostrosky-Zeichner L, Al-Obaidi M. 2017. Invasive fungal infections in the intensive care unit. Infect Dis Clin North Am 31:475–487. doi: 10.1016/j.idc.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 7.Suleyman G, Alangaden GJ. 2016. Nosocomial fungal infections: epidemiology, infection control, and prevention. Infect Dis Clin North Am 30:1023–1052. doi: 10.1016/j.idc.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Denning DW, O'Driscoll BR, Hogaboam CM, Bowyer P, Niven RM. 2006. The link between fungi and severe asthma: a summary of the evidence. Eur Respir J 27:615–626. doi: 10.1183/09031936.06.00074705. [DOI] [PubMed] [Google Scholar]
- 9.Medrek SK, Kao CC, Yang DH, Hanania NA, Parulekar AD. 2017. Fungal sensitization is associated with increased risk of life-threatening asthma. J Allergy Clin Immunol Pract 5:1025.e2–1031.e2. doi: 10.1016/j.jaip.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 10.Agarwal R. 2011. Severe asthma with fungal sensitization. Curr Allergy Asthma Rep 11:403–413. doi: 10.1007/s11882-011-0217-4. [DOI] [PubMed] [Google Scholar]
- 11.Masaki K, Fukunaga K, Matsusaka M, Kabata H, Tanosaki T, Mochimaru T, Kamatani T, Ohtsuka K, Baba R, Ueda S, Suzuki Y, Sakamaki F, Oyamada Y, Inoue T, Oguma T, Sayama K, Koh H, Nakamura M, Umeda A, Kamei K, Izuhara K, Asano K, Betsuyaku T. 2017. Characteristics of severe asthma with fungal sensitization. Ann Allergy Asthma Immunol 119:253–257. doi: 10.1016/j.anai.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 12.Castanhinha S, Sherburn R, Walker S, Gupta A, Bossley CJ, Buckley J, Ullmann N, Grychtol R, Campbell G, Maglione M, Koo S, Fleming L, Gregory L, Snelgrove RJ, Bush A, Lloyd CM, Saglani S. 2015. Pediatric severe asthma with fungal sensitization is mediated by steroid-resistant IL-33. J Allergy Clin Immunol 136:312.e7–322.e7. doi: 10.1016/j.jaci.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goh KJ, Yii ACA, Lapperre TS, Chan AK, Chew FT, Chotirmall SH, Koh MS. 2017. Sensitization to Aspergillus species is associated with frequent exacerbations in severe asthma. J Asthma Allergy 10:131–140. doi: 10.2147/JAA.S130459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Overton NL, Simpson A, Bowyer P, Denning DW. 2017. Genetic susceptibility to severe asthma with fungal sensitization. Int J Immunogenet 44:93–106. doi: 10.1111/iji.12312. [DOI] [PubMed] [Google Scholar]
- 15.Maskarinec SA, Johnson MD, Perfect JR. 2016. Genetic susceptibility to fungal infections: what is in the genes? Curr Clin Microbiol Rep 3:81–91. doi: 10.1007/s40588-016-0037-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Latge JP, Beauvais A, Chamilos G. 2017. The cell wall of the human fungal pathogen Aspergillus fumigatus: biosynthesis, organization, immune response, and virulence. Annu Rev Microbiol 71:99–116. doi: 10.1146/annurev-micro-030117-020406. [DOI] [PubMed] [Google Scholar]
- 17.Wagener J, Malireddi RK, Lenardon MD, Koberle M, Vautier S, MacCallum DM, Biedermann T, Schaller M, Netea MG, Kanneganti TD, Brown GD, Brown AJ, Gow NA. 2014. Fungal chitin dampens inflammation through IL-10 induction mediated by NOD2 and TLR9 activation. PLoS Pathog 10:e1004050. doi: 10.1371/journal.ppat.1004050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Dyken SJ, Garcia D, Porter P, Huang X, Quinlan PJ, Blanc PD, Corry DB, Locksley RM. 2011. Fungal chitin from asthma-associated home environments induces eosinophilic lung infiltration. J Immunol 187:2261–2267. doi: 10.4049/jimmunol.1100972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alcazar-Fuoli L, Clavaud C, Lamarre C, Aimanianda V, Seidl-Seiboth V, Mellado E, Latge JP. 2011. Functional analysis of the fungal/plant class chitinase family in Aspergillus fumigatus. Fungal Genet Biol 48:418–429. doi: 10.1016/j.fgb.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 20.Ober C, Chupp GL. 2009. The chitinase and chitinase-like proteins: a review of genetic and functional studies in asthma and immune-mediated diseases. Curr Opin Allergy Clin Immunol 9:401–408. doi: 10.1097/ACI.0b013e3283306533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu Z, Zheng T, Homer RJ, Kim YK, Chen NY, Cohn L, Hamid Q, Elias JA. 2004. Acidic mammalian chitinase in asthmatic Th2 inflammation and IL-13 pathway activation. Science 304:1678–1682. doi: 10.1126/science.1095336. [DOI] [PubMed] [Google Scholar]
- 22.Fitz LJ, Declercq C, Brooks J, Kuang W, Bates B, Demers D, Winkler A, Nocka K, Jiao A, Greco RM, Mason LE, Fleming M, Quazi A, Wright J, Goldman S, Hubeau C, Williams CM. 2012. Acidic mammalian chitinase is not a critical target for allergic airway disease. Am J Respir Cell Mol Biol 46:71–79. doi: 10.1165/rcmb.2011-0095OC. [DOI] [PubMed] [Google Scholar]
- 23.Mellado E, Dubreucq G, Mol P, Sarfati J, Paris S, Diaquin M, Holden DW, Rodriguez-Tudela JL, Latge JP. 2003. Cell wall biogenesis in a double chitin synthase mutant (chsG−/chsE−) of Aspergillus fumigatus. Fungal Genet Biol 38:98–109. doi: 10.1016/S1087-1845(02)00516-9. [DOI] [PubMed] [Google Scholar]
- 24.Steele C, Rapaka RR, Metz A, Pop S, Williams DL, Gordon S, Kolls JK, Brown GD. 2005. The beta-glucan receptor dectin-1 recognizes specific morphologies of Aspergillus fumigatus. PLoS Pathog 1:e42. doi: 10.1371/journal.ppat.0010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adrangi S, Faramarzi MA. 2013. From bacteria to human: a journey into the world of chitinases. Biotechnol Adv 31:1786–1795. doi: 10.1016/j.biotechadv.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 26.Bussink AP, van Eijk M, Renkema GH, Aerts JM, Boot RG. 2006. The biology of the Gaucher cell: the cradle of human chitinases. Int Rev Cytol 252:71–128. doi: 10.1016/S0074-7696(06)52001-7. [DOI] [PubMed] [Google Scholar]
- 27.Espinosa V, Jhingran A, Dutta O, Kasahara S, Donnelly R, Du P, Rosenfeld J, Leiner I, Chen CC, Ron Y, Hohl TM, Rivera A. 2014. Inflammatory monocytes orchestrate innate antifungal immunity in the lung. PLoS Pathog 10:e1003940. doi: 10.1371/journal.ppat.1003940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lilly LM, Scopel M, Nelson MP, Burg AR, Dunaway CW, Steele C. 2014. Eosinophil deficiency compromises lung defense against Aspergillus fumigatus. Infect Immun 82:1315–1325. doi: 10.1128/IAI.01172-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amarsaikhan N, Sands EM, Shah A, Abdolrasouli A, Reed A, Slaven JE, Armstrong-James D, Templeton SP. 2017. Caspofungin increases fungal chitin and eosinophil and gammadelta T cell-dependent pathology in invasive aspergillosis. J Immunol 199:624–632. doi: 10.4049/jimmunol.1700078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Becker KL, Aimanianda V, Wang X, Gresnigt MS, Ammerdorffer A, Jacobs CW, Gazendam RP, Joosten LA, Netea MG, Latge JP, van de Veerdonk FL. 2016. Aspergillus cell wall chitin induces anti- and proinflammatory cytokines in human PBMCs via the Fc-gamma receptor/Syk/PI3K pathway. mBio 7:e01823-. doi: 10.1128/mBio.01823-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alvarez FJ. 2014. The effect of chitin size, shape, source and purification method on immune recognition. Molecules 19:4433–4451. doi: 10.3390/molecules19044433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garth JM, Reeder KM, Godwin MS, Mackel JJ, Dunaway CW, Blackburn JP, Steele C. 2017. IL-33 signaling regulates innate IL-17A and IL-22 production via suppression of prostaglandin E2 during lung fungal infection. J Immunol 199:2140–2148. doi: 10.4049/jimmunol.1602186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wagener J, MacCallum DM, Brown GD, Gow NA. 2017. Candida albicans chitin increases arginase-1 activity in human macrophages, with an impact on macrophage antimicrobial functions. mBio 8:e01820-. doi: 10.1128/mBio.01820-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhatia S, Fei M, Yarlagadda M, Qi Z, Akira S, Saijo S, Iwakura Y, van Rooijen N, Gibson GA, St Croix CM, Ray A, Ray P. 2011. Rapid host defense against Aspergillus fumigatus involves alveolar macrophages with a predominance of alternatively activated phenotype. PLoS One 6:e15943. doi: 10.1371/journal.pone.0015943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lilly LM, Gessner MA, Dunaway CW, Metz AE, Schwiebert L, Weaver CT, Brown GD, Steele C. 2012. The beta-glucan receptor dectin-1 promotes lung immunopathology during fungal allergy via IL-22. J Immunol 189:3653–3660. doi: 10.4049/jimmunol.1201797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aoki K, Barker C, Danthinne X, Imperiale MJ, Nabel GJ. 1999. Efficient generation of recombinant adenoviral vectors by Cre-lox recombination in vitro. Mol Med 5:224–231. [PMC free article] [PubMed] [Google Scholar]
- 37.Hartl D, Latzin P, Zissel G, Krane M, Krauss-Etschmann S, Griese M. 2006. Chemokines indicate allergic bronchopulmonary aspergillosis in patients with cystic fibrosis. Am J Respir Crit Care Med 173:1370–1376. doi: 10.1164/rccm.200508-1271OC. [DOI] [PubMed] [Google Scholar]
- 38.Latzin P, Hartl D, Regamey N, Frey U, Schoeni MH, Casaulta C. 2008. Comparison of serum markers for allergic bronchopulmonary aspergillosis in cystic fibrosis. Eur Respir J 31:36–42. doi: 10.1183/09031936.00078107. [DOI] [PubMed] [Google Scholar]
- 39.Mack I, Hector A, Ballbach M, Kohlhaufl J, Fuchs KJ, Weber A, Mall MA, Hartl D. 2015. The role of chitin, chitinases, and chitinase-like proteins in pediatric lung diseases. Mol Cell Pediatr 2:3. doi: 10.1186/s40348-015-0014-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen L, Shen Z, Wu J. 2009. Expression, purification and in vitro antifungal activity of acidic mammalian chitinase against Candida albicans, Aspergillus fumigatus and Trichophyton rubrum strains. Clin Exp Dermatol 34:55–60. doi: 10.1111/j.1365-2230.2008.03092.x. [DOI] [PubMed] [Google Scholar]
- 41.Gordon-Thomson C, Kumari A, Tomkins L, Holford P, Djordjevic JT, Wright LC, Sorrell TC, Moore GP. 2009. Chitotriosidase and gene therapy for fungal infections. Cell Mol Life Sci 66:1116–1125. doi: 10.1007/s00018-009-8765-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dubey LK, Moeller JB, Schlosser A, Sorensen GL, Holmskov U. 2014. Induction of innate immunity by Aspergillus fumigatus cell wall polysaccharides is enhanced by the composite presentation of chitin and beta-glucan. Immunobiology 219:179–188. doi: 10.1016/j.imbio.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 43.Nance JP, Vannella KM, Worth D, David C, Carter D, Noor S, Hubeau C, Fitz L, Lane TE, Wynn TA, Wilson EH. 2012. Chitinase dependent control of protozoan cyst burden in the brain. PLoS Pathog 8:e1002990. doi: 10.1371/journal.ppat.1002990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vannella KM, Ramalingam TR, Hart KM, de Queiroz Prado R, Sciurba J, Barron L, Borthwick LA, Smith AD, Mentink-Kane M, White S, Thompson RW, Cheever AW, Bock K, Moore I, Fitz LJ, Urban JF Jr, Wynn TA. 2016. Acidic chitinase primes the protective immune response to gastrointestinal nematodes. Nat Immunol 17:538–544. doi: 10.1038/ni.3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Werner J, Metz AE, Horn D, Faro-Trindade I, Schoeb TR, Hewitt MM, Schwiebert LM, Brown GD, Steele C. 2009. Requisite role for the dectin-1 beta-glucan receptor in pulmonary defense against Aspergillus fumigatus. J Immunol 182:4938–4946. doi: 10.4049/jimmunol.0804250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Caffrey AK, Lehmann MM, Zickovich JM, Espinosa V, Shepardson KM, Watschke CP, Hilmer KM, Thammahong A, Barker BM, Rivera A, Cramer RA, Obar JJ. 2015. IL-1alpha signaling is critical for leukocyte recruitment after pulmonary Aspergillus fumigatus challenge. PLoS Pathog 11:e1004625. doi: 10.1371/journal.ppat.1004625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Caffrey-Carr AK, Kowalski CH, Beattie SR, Blaseg NA, Upshaw CR, Thammahong A, Lust HE, Tang YW, Hohl TM, Cramer RA, Obar JJ. 2017. IL-1alpha is critical for resistance against highly virulent aspergillus fumigatus isolates. Infect Immun 85:e00661-17. doi: 10.1128/IAI.00661-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Werner JL, Gessner MA, Lilly LM, Nelson MP, Metz AE, Horn D, Dunaway CW, Deshane J, Chaplin DD, Weaver CT, Brown GD, Steele C. 2011. Neutrophils produce IL-17A in a dectin-1 and IL-23 dependent manner during invasive fungal infection. Infect Immun 79:3966–3977. doi: 10.1128/IAI.05493-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gessner MA, Werner JL, Lilly LM, Nelson MP, Metz AE, Dunaway CW, Chan YR, Ouyang W, Brown GD, Weaver CT, Steele C. 2012. Dectin-1-dependent interleukin-22 contributes to early innate lung defense against Aspergillus fumigatus. Infect Immun 80:410–417. doi: 10.1128/IAI.05939-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim LK, Morita R, Kobayashi Y, Eisenbarth SC, Lee CG, Elias J, Eynon EE, Flavell RA. 2015. AMCase is a crucial regulator of type 2 immune responses to inhaled house dust mites. Proc Natl Acad Sci U S A 112:E2891–E2899. doi: 10.1073/pnas.1507393112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, Locati M, Mantovani A, Martinez FO, Mege JL, Mosser DM, Natoli G, Saeij JP, Schultze JL, Shirey KA, Sica A, Suttles J, Udalova I, van Ginderachter JA, Vogel SN, Wynn TA. 2014. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chatterjee R, Batra J, Das S, Sharma SK, Ghosh B. 2008. Genetic association of acidic mammalian chitinase with atopic asthma and serum total IgE levels. J Allergy Clin Immunol 122:202.e7–208.e7. doi: 10.1016/j.jaci.2008.04.030. [DOI] [PubMed] [Google Scholar]
- 53.Wu AC, Lasky-Su J, Rogers CA, Klanderman BJ, Litonjua AA. 2010. Fungal exposure modulates the effect of polymorphisms of chitinases on emergency department visits and hospitalizations. Am J Respir Crit Care Med 182:884–889. doi: 10.1164/rccm.201003-0322OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kashimura A, Kimura M, Okawa K, Suzuki H, Ukita A, Wakita S, Okazaki K, Ohno M, Bauer PO, Sakaguchi M, Sugahara Y, Oyama F. 2015. Functional properties of the catalytic domain of mouse acidic mammalian chitinase expressed in Escherichia coli. Int J Mol Sci 16:4028–4042. doi: 10.3390/ijms16024028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wakita S, Kimura M, Kato N, Kashimura A, Kobayashi S, Kanayama N, Ohno M, Honda S, Sakaguchi M, Sugahara Y, Bauer PO, Oyama F. 2017. Improved fluorescent labeling of chitin oligomers: chitinolytic properties of acidic mammalian chitinase under somatic tissue pH conditions. Carbohydr Polym 164:145–153. doi: 10.1016/j.carbpol.2017.01.095. [DOI] [PubMed] [Google Scholar]
- 56.Van Dyken SJ, Liang HE, Naikawadi RP, Woodruff PG, Wolters PJ, Erle DJ, Locksley RM. 2017. Spontaneous chitin accumulation in airways and age-related fibrotic lung disease. Cell 169:497.e13–509.e13. doi: 10.1016/j.cell.2017.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hartl D, He CH, Koller B, Da Silva CA, Homer R, Lee CG, Elias JA. 2008. Acidic mammalian chitinase is secreted via an ADAM17/epidermal growth factor receptor-dependent pathway and stimulates chemokine production by pulmonary epithelial cells. J Biol Chem 283:33472–33482. doi: 10.1074/jbc.M805574200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schuh JM, Power C, Proudfoot AE, Kunkel SL, Lukacs NW, Hogaboam CM. 2002. Airway hyperresponsiveness, but not airway remodeling, is attenuated during chronic pulmonary allergic responses to Aspergillus in CCR4−/− mice. FASEB J 16:1313–1315. doi: 10.1096/fj.02-0193fje. [DOI] [PubMed] [Google Scholar]
- 59.Solari R, Pease JE. 2015. Targeting chemokine receptors in disease—a case study of CCR4. Eur J Pharmacol 763:169–177. doi: 10.1016/j.ejphar.2015.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lim HW, Lee J, Hillsamer P, Kim CH. 2008. Human Th17 cells share major trafficking receptors with both polarized effector T cells and FOXP3+ regulatory T cells. J Immunol 180:122–129. doi: 10.4049/jimmunol.180.1.122. [DOI] [PubMed] [Google Scholar]
- 61.Trifari S, Kaplan CD, Tran EH, Crellin NK, Spits H. 2009. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from T(H)-17, T(H)1 and T(H)2 cells. Nat Immunol 10:864–871. doi: 10.1038/ni.1770. [DOI] [PubMed] [Google Scholar]