ABSTRACT
Enteric α-defensins, termed cryptdins (Crps) in mice, and lysozymes secreted by Paneth cells contribute to innate host defense in the ileum. Antimicrobial factors, including lysozymes and β-defensins, are often embedded in luminal glycosylated colonic Muc2 mucin secreted by goblet cells that form the protective mucus layer critical for gut homeostasis and pathogen invasion. In this study, we investigated ileal innate immunity against Entamoeba histolytica, the causative agent of intestinal amebiasis, by inoculating parasites in closed ileal loops in Muc2+/+ and Muc2−/− littermates and quantifying Paneth cell localization (lysozyme expression) and function (Crp secretion). Relative to Muc2+/+ littermates, Muc2−/− littermates showed a disorganized mislocalization of Paneth cells that was diffusely distributed, with elevated lysozyme secretion in the crypts and on villi in response to E. histolytica. Inhibition of E. histolytica Gal/GalNAc lectin (Gal-lectin) binding with exogenous galactose and Entamoeba histolytica cysteine proteinase 5 (EhCP5)-negative E. histolytica had no effect on parasite-induced erratic Paneth cell lysozyme synthesis. Although the basal ileal expression of Crp genes was unaffected in Muc2−/− mice in response to E. histolytica, there was a robust release of proinflammatory cytokines and Crp peptide secretions in luminal exudates that was also present in the colon. Interestingly, E. histolytica-secreted cysteine proteinases cleaved the proregion of Crp4 but not the active form. These findings define Muc2 mucin as an essential component of ileal barrier function that regulates the localization and function of Paneth cells critical for host defense against microbes.
KEYWORDS: Entamoeba histolytica, defensins, goblet cell, protozoa, mucin, ileum, Muc2, Paneth cell, cryptdins, lysozyme
INTRODUCTION
The small intestine is organized into villus protrusions intercalated by the crypts of Lieberkuhn. Undifferentiated progenitor cells at the base of the crypts differentiate into absorptive enterocytes, goblet cells, Paneth cells, and enteroendocrine cells (1). Enterocytes absorb nutrients and secrete hydrolytic enzymes, while enteroendocrine cells secrete hormones. Goblet cells produce the mucus layer in the small intestine (2), which is loose and thinner than what is observed in the colon (3). Gel-forming Muc2 mucin is the main component of mucus in the small intestine that is O-glycosylated, although it shows a simpler O-glycan profile than that of the Muc2 mucus layers in the colon (2).
Paneth cells synthesize intestine-specific lysozymes and α-defensin peptides, termed cryptdins (Crps) in rodents. Two closely related lysozyme C genes are present in mice, although lysozyme P, also termed Lyz1, is expressed abundantly in the small intestine, while lysozyme M, or Lyz2, is mostly found in activated macrophages and expressed in lung and bone marrow (not abundant in the intestine) (4). In mice, secretory granules of Paneth cells store matrix metalloproteinase 7 (MMP7)-processed active Crp peptides (5), which are then secreted into the intestinal lumen upon bacterial stimuli (6). These secreted α-defensins kill microbes by forming anion-conductive channels in microbial cell membranes (7). While humans produce two α-defensins in the small intestine (HD-5 and HD-6) (8, 9), mouse Paneth cells express approximately 20 Crp isoforms (Crp1 to Crp20). The Crps produced at measurable peptide levels in the mouse small intestine are Crp1 to Crp3 and -Crp6, which have highly similar primary structures, and Crp4 and Crp5, which are structurally distinct (10). Although Crps are produced throughout the small intestine, the ileum, where Paneth cells occur at the highest abundance, is a particular immunological site because of the high level of production of these peptides. For instance, Crp4 synthesis is restricted to the ileum (11), and HD-5 and HD-6 show increased expression along the proximal-to-distal intestinal axis (12). Thus, the ileum is key in defining the role of Paneth cell-derived α-defensins in gut homeostasis and disease.
The regulation of Paneth cell differentiation, distribution along the crypt-villus axis, and maturation are dependent largely on Wnt signaling. This includes the Wnt receptor Frizzled-5 (Fzd5), expressed only in developing crypts of neonatal and adult mice, which confines Paneth cells to crypt cells (13). The tyrosine kinase ephrin B2 (EphB2) receptor is essential for the correct positioning of Paneth cells at the bottom of the crypts in a Wnt gradient along the crypt-villus axis (14). Other innate mechanisms, such as the mucin barrier, could also regulate Paneth cell functions. While secreted lysozymes and β-defensins are found to be embedded in the colonic mucus layer (15), it remains unclear whether Muc2 mucin in the ileum is essential for sequestering α-defensins to exert their antimicrobial functions. In this study, we investigated the role of Paneth cell lysozymes and α-defensins when the mucin barrier is altered in response to Entamoeba histolytica, a protozoan parasite that causes amebic dysentery and/or liver abscesses (16). However, for E. histolytica to colonize the colon, ingested infective cysts must first excyst in the terminal ileum. Thus, the ileum is first part of the gut that senses and responds to E. histolytica parasites that eventually migrate to the colon. Several virulence factors expressed by E. histolytica modulate host proinflammatory responses and invasion in the gut (17). The binding and adherence of E. histolytica to the intestinal mucus layer are mediated by a 170-kDa surface adhesin, the Gal/GalNAc lectin (Gal-lectin) (18, 19). In addition, cell surface E. histolytica cysteine proteinase (EhCP)-A5 has been shown to play key roles in the pathogenesis of amebiasis, inducing mucin exocytosis, degrading MUC2 mucin, and evoking acute proinflammatory responses dominated by interleukin-1β (IL-1β) secretion (20–22). How the host senses and responds to E. histolytica in the ileum is unknown, and this was the impetus for our study. Here we show that E. histolytica in the ileum of Muc2−/− but not Muc2+/+ littermates stimulated robust proinflammatory cytokines and enhanced the secretion of lysozymes and Crps. Secreted Crps were activated and resistant to proteolytic cleavage by E. histolytica cysteine proteinase. These results show that Muc2 mucin in the terminal ileum plays a major role in innate host defenses by limiting the exposure of the epithelium to inflammatory insults and regulates Paneth cell innate responses to E. histolytica.
RESULTS
Muc2 mucin-deficient mice are prone to developing ileal inflammation.
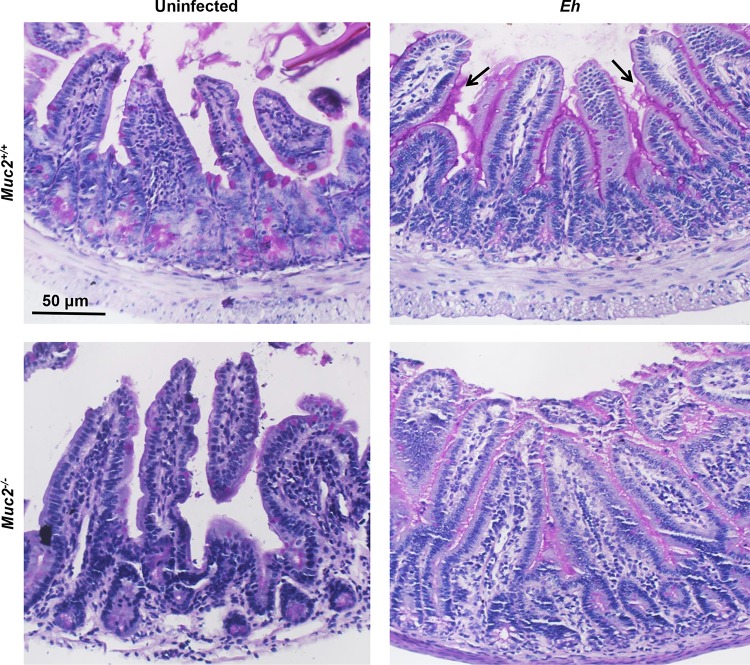
Muc2−/− animals are a reliable model to study the mucus layer in the ileum, as it shows no compensatory increases in the levels of other secretory mucins (23). To quantify the contributions of Muc2 mucin and Paneth cell antiamebic defenses, we inoculated live E. histolytica parasites in closed ileal loops into Muc2+/+ and Muc2−/− littermates for 4 h. Basally, Muc2+/+ mice showed packed periodic acid-Schiff stain-positive (PAS+) goblet cells in the crypts and sparse goblet cells on the villi (Fig. 1, top left), which were absent in Muc2−/− mice (Fig. 1, bottom left). In response to E. histolytica in Muc2+/+ mice, there was hypersecretion of mucus from villi and crypt goblet cells that formed a thick continuous coating of mucus (Fig. 1, magenta) on the mucosal surface and crypts (Fig. 1, top right, arrows). Most notably, following exposure to E. histolytica, no filled goblet cells were seen, even in the deep crypts. E. histolytica inoculated into Muc2−/− mice elicited enhanced watery secretions with a thin nonmucin layer coating the ileal surface (Fig. 1, bottom right).
FIG 1.
Histological characteristics of the ileum from Muc2+/+ and Muc2−/− littermates inoculated with E. histolytica. Ligated ileal loops were inoculated with log-phase wild-type E. histolytica (Eh) (2 × 106 trophozoites in 100 μl PBS) or PBS only (control) (100 μl) for 4 h. Paraffin-embedded sections were stained with PAS reagent to detect mucopolysaccharides and for morphological characterization. Note the hypersecretion of mucus (black arrows) in the crypts and lumen of Muc2+/+ littermates in response to E. histolytica, with very few PAS stain-filled goblet cells. Images are representative of results for one of six mice under the same conditions.
Increased synthesis of Paneth cell-specific lysozymes in Muc2−/− mice.
Paneth cells are highly specialized epithelial cells of the small intestine that exert control over enteric pathogens. For instance, mice transgenic for human Paneth cell α-defensin HD5 (DEFA5-transgenic+/+) become resistant to Salmonella enterica serovar Typhimurium (24). To determine if Paneth cells in the ileum of Muc2−/− mice are altered in their functions, immunofluorescence studies with antilysozyme antibodies were conducted. Immune lysozyme-stained cells were located at the base of the crypts (Fig. 2, arrows) in the ileum of Muc2+/+ mice, corresponding to the proper location of Paneth cells. In contrast, in Muc2−/− littermates, lysozyme-containing cells were not restricted to the crypts and were diffusely distributed in the crypts and on villi (Fig. 2, arrows). Under conditions of acute E. histolytica challenge, lysozyme immune staining was widely spread over crypts and villi in both Muc2+/+ and Muc2−/− mice (Fig. 2, arrows). Most notably, immune staining of lysozymes was abundant and localized prominently at the tip of villi (Fig. 2, bracketed area) in E. histolytica-challenged Muc2−/− mice (P < 0.05 for mean fluorescence intensity [MFI]) (Fig. 2).
FIG 2.
Distribution of Paneth cell-derived lysozymes in the ileum of Muc2+/+ and Muc2−/− littermates inoculated with E. histolytica. Ileal sections from Muc2+/+ and Muc2−/− littermates inoculated with PBS (control), E. histolytica parasites, EhCP-A5− E. histolytica parasites, or E. histolytica parasites pretreated for 15 min with 55 mM d-galactose (E. histolytica + Gal) were immunoblotted with antilysozyme (red) antibody and quantified by immunofluorescence microscopy. Nuclei were stained with DAPI (blue). IgG was used as an antibody control. The mean fluorescence intensity (MFI) (histogram) was quantified by using ImageJ software and averaged over 10 random fields of view for two to three independent slides per animal and is represented as MFI normalized to the area of the field of view. Means ± SE are shown (n = 2 independent experiments run in triplicate). P values for all significant comparisons (*, P < 0.05) are represented (one-way ANOVA followed by Bonferroni posttests). The images are from 1 of 4 independent experiments. NS, not significant.
To establish whether altered Paneth cell functions might be a consequence of innate Muc2 defects or E. histolytica virulence factors, we inhibited the binding of E. histolytica Gal-lectin to intestinal cells (25) and the engagement of EhCP5 with host cell integrins at the intercellular junction to provoke robust proinflammatory responses (25–27). Surprisingly, neither EhCP5-A5-negative (EhCP5-A5−) parasites nor E. histolytica parasites pretreated with galactose inhibited mislocalization and abundant lysozyme immune staining in ileal crypts and villi of Muc2−/− littermates, compared to E. histolytica parasites alone (Fig. 2, arrows). We did not test E. histolytica parasites pretreated with galactose in Muc2+/+ mice, as the parasites adhere to the mucus layer. Based on these results, we next determined the expression profiles of two mouse lysozyme C genes, lysozyme P (Lyz1) and lysozyme M (Lyz2), in Paneth cells. The expression of Lyz1 was slightly enhanced in Muc2+/+ mice in response to E. histolytica compared to uninfected littermate controls (Fig. 3). In stark contrast, the basal expression level of Lyz1 was significantly higher (4.5-fold; P < 0.01) in Muc2−/− mice, and following exposure to E. histolytica, Lyz1 expression was significantly reduced to the same levels as those in uninfected Muc2+/+ littermates (P < 0.01) (Fig. 3). A similar decrease in the Lyz1 expression level was noted for Muc2−/− littermates inoculated with EhCP5-A5− E. histolytica parasites and E. histolytica parasites pretreated with galactose compared to E. histolytica parasites alone (Fig. 3). The transcriptional expression of the Lyz2 gene, present mostly in macrophages (4), was modest in Muc2+/+ and Muc2−/− littermates, except in Muc2+/+ littermates in response to EhCP5-A5− parasites (P < 0.01) (Fig. 3).
FIG 3.

Transcriptional gene expression of lysozyme markers in the ileum of Muc2+/+ and Muc2−/− littermates inoculated with E. histolytica. mRNA expression levels of the mLyz1 and mLyz2 genes in the ileal mucosa of Muc2+/+ and Muc2−/− mice inoculated with PBS (control), E. histolytica parasites, EhCP-A5− E. histolytica parasites, or E. histolytica parasites pretreated with Gal, as described in the legend of Fig. 2, were quantified by qRT-PCR. Data are presented as fold changes relative to the value for the housekeeping gene Gapdh. Means ± SE are shown (n = 3 independent experiments run in triplicate). P values for all significant comparisons (**, P < 0.01) are represented (one-way ANOVA followed by Bonferroni posttests).
The migration and location of Paneth cells in ileal crypts are regulated by canonical Wnt signaling (14). Wnt signaling is transduced through beta-catenin/TCF4 and negatively regulates the expression of genes encoding tyrosine kinase EphB2/B3 receptors to maintain the correct positioning of Paneth cells (13). The expression of the Wnt receptor Frizzled-5 (Fzd5) and its binding to the Wnt5a ligand in crypt cells are also critical for Wnt-dependent Paneth cell maturation (13). To investigate whether Paneth cell responses to E. histolytica infection corresponded to an alteration of Wnt/β-catenin signaling, we quantified the transcriptional expression levels of EphB2 and Fzd5. Baseline levels of the EphB2 and Fzd5 genes did not differ between Muc2+/+ and Muc2−/− littermates (Fig. 4). However, in response to E. histolytica, the expression of EphB2 genes in the ileum of Muc2−/− mice was significantly reduced compared to that in untreated animals or Muc2−/− mice inoculated with EhCP-A5− parasites or E. histolytica parasites pretreated with Gal (P < 0.05, P < 0.01, and P < 0.001) (Fig. 4). The expression level of ileal Fzd5 was markedly reduced by E. histolytica in Muc2+/+ mice (P < 0.001) (Fig. 4) and showed a tendency to be lower in Muc2−/− mice despite E. histolytica challenge (Fig. 4). These data suggest that E. histolytica inhibits the expression of EphB2 genes in the ileum of Muc2−/− mice.
FIG 4.

Transcriptional gene expression of Paneth cell signaling and maturation markers in the ileum of Muc2+/+ and Muc2−/− littermates inoculated with E. histolytica. mRNA expression levels of the Fzd5 and Ephb2 genes in the ileal mucosa of Muc2+/+ and Muc2−/− mice inoculated with PBS only (control), E. histolytica parasites, EhCP-A5− E. histolytica parasites, or E. histolytica parasites treated with Gal, as described in the legend of Fig. 2, were quantified by qRT-PCR. Data are presented as fold changes relative to the value for the housekeeping gene Gapdh. Means ± SE are shown (n = 3 independent experiments run in triplicate). P values for all significant comparisons (*, P < 0.05; **, P < 0.01; ***, P < 0.001) are represented (one-way ANOVA followed by Bonferroni posttests).
Increased secretion of Crps in Muc2−/− mice in response to E. histolytica.
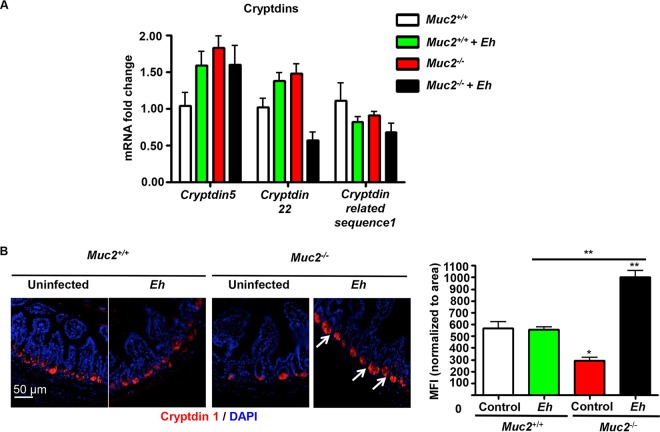
To determine whether a deficiency in luminal Muc2 mucin altered the production of α-defensins, Crps in the ileum were assessed at the gene and peptide levels. Under basal conditions, Muc2−/− mice showed no significant changes in cryptdin 5, and cryptdin 22, and in cryptdin-related sequence 1 mRNA levels in the ileum compared to those in Muc2+/+ mice (Fig. 5A). However, as assessed by immunofluorescence microscopy, cryptdin 1 peptide levels were significantly higher in Paneth cells (Fig. 5B, arrows and MFI) at the base of ileal crypts of Muc2−/− littermates in response to E. histolytica (P < 0.01).
FIG 5.
Transcriptional gene expression and immune localization of cryptdins in the ileum of Muc2+/+ and Muc2−/− littermates inoculated with E. histolytica. (A) mRNA expression levels of the cryptdin 5, cryptdin 22, and cryptdin-related sequence 1 genes in the ileum of mice inoculated with E. histolytica or PBS, as described in the legend of Fig. 1, were quantified by qRT-PCR. Data are expressed as fold changes relative to the value for the housekeeping gene Gapdh. Means ± SE are shown (n = 3 independent experiments run in triplicate). (B) Ileal sections were immunoblotted with anti-Crp1 (red) antibody and quantified by immunofluorescence microscopy. Nuclei were stained with DAPI (blue). IgG was used as an antibody control. The fluorescence intensity was quantified by using ImageJ software and averaged over 10 random fields of view in two to three independent slides per animal and is represented as MFI (histogram) normalized to the area of the field of view. Means ± SE are shown (n = 2 independent experiments run in triplicate). P values for all significant comparisons (*, P < 0.05; **, P < 0.01) are represented (one-way ANOVA followed by Bonferroni posttests). Images are from 1 of 4 independent experiments.
To further investigate whether the luminal secretion of α-defensins was altered in Muc2−/− littermates lacking an ileal mucus layer, the level of secreted cryptdin 1 was quantified across the ileum (proximal to the ileocecal junction) and large intestine (distal to the cecocolonic junction). Although basal levels of cryptdin 1 were similar in both groups of mice, in response to E. histolytica, the level of ileal cryptdin 1 was significantly increased in Muc2−/− mice, whereas it was decreased in Muc2+/+ mice (P < 0.05) (Fig. 6A). These findings suggest that deficiencies in the mucin barrier altered the production of α-defensins in response to E. histolytica, perhaps by augmenting their secretion from Paneth cells. In the cecum and colon, Muc2−/− mice showed higher basal levels of luminal Crp1 than those in Muc2+/+ mice, and these higher levels were not increased further in response to E. histolytica (Fig. 6A). Since Paneth cells are the source of enteric α-defensins, the cryptdin 1 peptides found in the large intestinal lumen were likely to have been derived from small intestinal Paneth cells (28).
FIG 6.
Secretion of Crp1 and proinflammatory cytokines in Muc2+/+ and Muc2−/− littermates inoculated with E. histolytica. The levels of luminally secreted Crp1 in the ileum, cecum, and proximal colon of Muc2+/+ and Muc2−/− mice inoculated with E. histolytica or PBS, as described in the legend of Fig. 1, were measured by a sandwich ELISA. Levels of peptides are expressed relative to total protein levels. *, P < 0.05.
Ileal challenge with E. histolytica induces rapid transcriptional synthesis of proinflammatory cytokines.
Because Muc2−/− mice develop more-severe enteritis when challenged with E. histolytica, we investigated whether proinflammatory cytokines contributed to the observed altered synthesis of Paneth cell-produced lysozymes (Fig. 2). Interferon gamma (IFN-γ) and tumor necrosis factor alpha (TNF-α) were targeted, because IFN-γ alone or in synergy with TNF-α blocks Wnt signaling by promoting the secretion of the Wnt inhibitor (Dkk1) (29). E. histolytica induced higher Ifn-γ and Tnf-α mRNA levels (30- to 50-fold) in the ileum of both Muc2+/+ and Muc2−/− littermates than those in untreated controls or mice inoculated with EhCP5-A5− parasites and E. histolytica parasites pretreated with galactose (P < 0.001) (Fig. 7). Thus, enhanced Ifn-γ and Tnf-α gene expression in response to E. histolytica could alter Wnt signaling, affecting the maturation and establishment of Paneth cells in Muc2+/+ and Muc2−/− littermates.
FIG 7.

Transcriptional gene expression of proinflammatory cytokines in the ileum of Muc2+/+ and Muc2−/− littermates inoculated with E. histolytica. mRNA expression levels of the Ifn-γ and Tnf-α genes in the ileal mucosa of Muc2+/+ and Muc2−/− mice inoculated with PBS (control), E. histolytica parasites, EhCP-A5− E. histolytica parasites, or E. histolytica parasites pretreated with Gal, as described in the legend of Fig. 2, were quantified by qRT-PCR. Data are presented as fold changes relative to the value for the housekeeping gene Gapdh. Means ± SE are shown (n = 3 independent experiments run in triplicate). P values for all significant comparisons (***, P < 0.001) are represented (one-way ANOVA followed by Bonferroni posttests).
Secreted cysteine proteinase from E. histolytica cleaves the proregions of pro-Crp4, but the active form of Crp4 is resistant to proteolytic cleavage.
As Paneth cell-derived Crps were secreted in response to E. histolytica in Muc2−/− littermates, we tested the stability of secreted Crp4 and pro-Crp4 against E. histolytica proteases. E. histolytica cysteine proteinases readily degrade components of innate defenses, including MUC2 mucin (30, 31), extracellular matrix proteins, and cathelicidins (31–33). To determine whether E. histolytica cysteine proteinases could degrade secreted α-defensins, live E. histolytica parasites and E. histolytica-secreted proteinases were incubated with recombinant mouse Crp4, a highly bactericidal α-defensin in the ileum (34). As predicted, E. histolytica-secreted proteinases and live E. histolytica parasites readily cleaved pro-Crp4 in the proregion, as visualized by acid-urea–PAGE (AU-PAGE), which was 100% inhibited with the specific cysteine proteinase inhibitor E64 (Fig. 8A). By matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) analysis, the putative sites that were cleaved by E. histolytica occurred only within the proregion of pro-Crp4 in an E64-inhibitable manner (Fig. 8B, arrows). Live E. histolytica parasites and E. histolytica-secreted proteinase did not cleave mature Crp4 peptides (Fig. 8B), consistent with the resistance of Crp4 to numerous proteinases. These results demonstrate that E. histolytica cysteine proteinases can cleave sites that convert inactive α-defensin precursors to microbicidal forms and that mature α-defensins, including Crp4, are resistant to further E. histolytica proteolysis.
FIG 8.
Proteolytic cleavage of Crp4 by E. histolytica-secreted cysteine proteinases and live E. histolytica parasites. (A) Recombinant pro-Crp4 (10 μg) and recombinant Crp4 (5 μg) were incubated for 3 h with PBS (control), E. histolytica-secreted proteinases, or live E. histolytica parasites (10 μg) with or without the protease inhibitor E64 (100 μM). Ninety-six percent of each digest was run in acid-urea–PAGE gels and stained with Coomassie blue. (B) Primary structure of pro-Crp4 with arrows inserted to depict sites of cleavage by E. histolytica cysteine proteinases, as determined by MALDI-TOF mass spectrometry on 4% of each digest. The mature Crp4 primary structure is underlined.
DISCUSSION
In this study, we describe the regulatory mechanisms in the ileal mucosa, where a deficiency of Muc2 altered the expression of Crps and the localization of Paneth cells upon E. histolytica infection, as assessed by an enzyme-linked immunosorbent assay (ELISA) and immunohistochemistry for the lysozyme Lyz1 (secreted by Paneth cells). This advances our understanding of the pathogenesis of intestinal amebiasis, as the ileum is the initial site of amebic infection where E. histolytica parasites excyst (35, 36) prior to migrating to the colon (37). Our data demonstrate that ileal Muc2 mucin plays an essential role in the restricted expression of Lyz1 (mostly at the base of the crypt) and, hence, the localization of Paneth cells. Unlike the colon, which has a mucus bilayer, the small intestine is made of a loose layer of mucus (38), allowing E. histolytica to trigger robust secretory and proinflammatory responses that are more pronounced in the absence of mucus (30, 39). Our study reinforces that Muc2 is important in the ileum and that in its absence, dysfunctional host responses against E. histolytica occur, which may predispose the host to intestinal amebiasis and concomitant infections.
Different from other differentiated epithelial cells that migrate from the crypt to the villus, Paneth cells are localized at the base of the crypt, directed by Wnt signals (13). Mature Paneth cells are the main source of the enteric lysozyme Lyz1 and hydrolases involved in digestive and microbicidal effects (4). We show that a Muc2 mucin deficiency affects Lyz1 synthesis, the location of Paneth cells, and the secretion of Crps in the ileum of E. histolytica-infected mice. We also observed increased immune staining of lysozymes widely distributed in both villi and crypts of the ileum in Muc2 mucin deficiency. Moreover, exposure of Muc2−/− littermates to E. histolytica increased lysozyme secretion at both crypts and villi. The effects of E. histolytica on abundant and erratic lysozyme expression in the ileum of Muc2−/− mice were not dependent on E. histolytica Gal-lectin and EhCP-A5. These findings indicate that an augmented release of Paneth cell-derived lysozymes can reach the apical surfaces of the ileum in the absence of a protective mucin barrier. The increased proinflammatory responses elicited by the presence of E. histolytica seem to be followed eventually by exhausted or lysed Paneth cells, as the transcriptional baseline gene expression level of Lyz1 was high in Muc2−/− littermates but drastically decreased following E. histolytica infection. The magnified presence of Paneth cell lysozymes (mostly Lyz1) may provide additional antimicrobial defenses. Although Lyz2 is more effective than Lyz1 in killing Gram-negative bacteria, Lyz1 and Lyz2 are equally effective in killing Gram-positive bacteria (4). In this study, Paneth cell secretions did not kill E. histolytica, as only live parasites were observed in ileal loop contents, and luminal secretions had no effect on the viability of E. histolytica parasites in culture.
The altered distribution of lysozyme contents across crypts and villi suggests a mispositioning of Paneth cells in Muc2-deficient mice. In this regard, the levels of two genes related to Wnt/β-catenin signaling and the maturation of Paneth cells, Ephb2 and Fzd5, were not altered in the absence of Muc2, but the level of Ephb2 was particularly decreased in response to E. histolytica. As β-catenin inversely controls the expression of the EphB2/EphB3 receptors and their ligand, ephrin B1, along the crypt-villus axis (14), when Ephb2 genes are downregulated, Paneth cells may not be guided correctly to the bottom of the crypts, and this could affect the localized secretion of their antimicrobial contents, such as Lyz1. The levels of Fzd5 mRNA were overall slightly lower in Muc2−/− littermates and were reduced even further in response to E. histolytica. The expression of the Wnt receptor Fzd5 is restricted to crypts from adult mice, and it was previously observed that a knockdown of the Fzd5 gene caused a random distribution of Paneth cells in crypts and villi (13). Alterations of other genes related to Paneth cell signaling and maturation have also been shown to conduct an erratic placement of Paneth cells. Modifications of the Gnaq and Gna11 genes that encode trimeric G proteins resulted in mislocalized Paneth cells, with increased numbers of intermediate cells in crypts and on villi and increased numbers of goblet cells on villi (40). Likewise, mice deficient in anterior gradient 2 (Agr2), a member of the endoplasmic reticulum (ER) protein disulfide isomerase, showed Paneth cell hypertrophy and a loss of viable goblet cells as a consequence of increased ER stress (41). Thus, the expression of the Ephb2 and Fzd5 genes is crucial for Wnt/β-catenin signaling for Paneth cells to migrate to the bottom of the crypts, and therefore, an increased susceptibility to deregulation in the absence of Muc2 could lead to a misplacement of Paneth cells or an augmented release of lysozyme at apical surfaces.
Muc2 deficiency in the colon or in cultured genetically knocked down goblet-like cells affects the synthesis and antimicrobial activity of cathelicidins and β-defensins (23, 42). We found that the ileal mucus did not affect the baseline genetic expression of either Crps or lumen-secreted Crps under normal homeostatic conditions. This was demonstrated by similar amounts of secreted and tissue-identified Crp1 in the ileum of uninfected Muc2+/+ and Muc2−/− littermates. However, E. histolytica provoked exacerbated ileal secretion of Crp1 in Muc2-deficient but not in Muc2-sufficient mice. This implies that the absence of a mucus barrier allows E. histolytica and its secreted components to interact freely with the intestinal mucosa to augment the secretion of α-defensins by Paneth cells. Crps in the ileal lumen of Muc2−/− mice may not be as effective as those in Muc2+/+ mice, as the mucus layer in the colon was shown to retain the physical and antimicrobial barrier of secreted factors, such as β-defensins (15). In this regard, intestinal mucus may function as a reservoir for antimicrobial defensins (15), the loss of which could perhaps impair the binding and anchoring of α-defensins to the mucin matrix. Thus, the augmented amounts of secreted “free” Crp1 in the ileum of Muc2−/− mice may be due to the lack of a mucin matrix that can retain the peptides. In agreement, mucin in the rectum was shown to reversibly bind and retain several antimicrobial peptides, including hBD-1 and cathelicidins in humans (15).
We observed larger amounts of Crp1 along the intestinal tract in Muc2−/− mice, including the cecum and colon, than in Muc2+/+ mice. This observation coincides with the presence of enteric Paneth cell-derived Crps in the mouse colonic lumen (28). Moreover, both full-length and N-terminally truncated forms of α-defensins can be present in the large intestinal lumen as they undergo luminal proteolytic activation by alternative host and microbial proteinases (43). An example is Crp4, which is processed extracellularly by a trypsin-like enzyme (44). Therefore, we emphasize the importance of the Muc2 mucin in the ileum and its matrix role in the retention of Paneth cell Crps secreted into the small intestinal lumen that persist throughout the intestinal tract.
The proinflammatory cytokines IFN-γ and TNF-α contribute to the pathogenesis of inflammatory bowel diseases and drive inflammation in dextran sulfate sodium-induced colitis (45) and E. histolytica infection (46). Intrinsic interactions between innate factors remain elusive, but it was shown previously that an impaired mucin barrier in the colon predisposes the host to increased E. histolytica-induced secretory and proinflammatory responses (39). In our acute model of ileal E. histolytica infection, a pronounced luminal inflammatory exudate and ileitis induced by E. histolytica were similarly noted for both Muc2+/+ and Muc2−/− littermates. Perhaps the innate thin mucin barrier in the ileum compared with that in the colon contributed to the exaggerated ileal inflammation and local expression of Ifn-γ and Tnf-α. Both IFN-γ and TNF-α can deregulate Wnt signaling (29), which is critical for positioning Paneth cells at the base of the crypts. Moreover, Ifn-γ provoked the elimination of Paneth cells in Toxoplasma gondii-infected mice (47) and the rapid and total degranulation of Paneth cells in primary epithelial organoids (48). Thus, the misfolding and aberrant expression of Muc2 could allow an altered localization of Paneth cells, ongoing inflammation, and the production of the proinflammatory cytokines IFN-γ and TNF-α, which are likely to perturb the location and survival of Paneth cells even more. In agreement, ileitis in mice overproducing Tnf-α (TnfΔARE/+) led to a loss of lysozyme-expressing Paneth cells and defective antimicrobial responses (49).
We found that secreted cysteine proteinase from E. histolytica cleaved Crp4 at conserved sites in the proregion, including the junction of the propeptide and α-defensin moiety. These cleavage sites in the Crp4 proregion resemble the MMP-7 (matrilysin)-mediated proteolysis of the Crp4 proregions at Ser43↓Ile44, Ala53↓Leu54, and Ser58↓Leu59 that relieve the inhibitory effects on the microbicidal activity of Crp4 (44, 50). Interestingly, we found that the mature Crp4 peptide was resistant to E. histolytica cysteine proteinases. The proteolytic stability of Crp4 against E. histolytica proteinases may be attributed to the highly conserved structure and biochemical properties of Crp4 (51). Importantly, this characteristic of Crp4 is unique among other host defense peptides susceptible to amebic enzymes. In this regard, the level of Crp1 was reduced in the ileum of Muc2+/+ littermates after infection with E. histolytica. We have shown previously that synthetic human and murine cathelicidins are cleaved by E. histolytica cysteine proteinase (32), and E. histolytica infection in mice affected the production of β-defensins (23) and cathelicidins (42) in the colon. Regarding the amebicidal effects of Crps, Crp2 showed in vitro lytic effects on E. histolytica, provoking morphological alterations in the parasitic membrane and interfering with the synthesis of DNA, RNA, and protein (52). However, it is not known if Crp4 can disrupt the membrane of E. histolytica. In general, α-defensins have shown antiparasitic roles against other protozoa, including Trypanosoma cruzi (53) and T. gondii (54), although cathelicidins did not kill E. histolytica under in vitro conditions (32). Thus, Crp4 may confer unique antimicrobial defenses in innate immunity against amebic infection. Taken together, data from our studies demonstrate that Muc2 mucin is an essential component of ileal barrier function that regulates the location and function of mature Paneth cells, and their expression of innate defense factors might prevent close encounters between microbes and the intestinal surface.
MATERIALS AND METHODS
Entamoeba histolytica.
E. histolytica (HM-1:IMSS) parasites were grown axenically in TYI-S-3 medium with 100 U ml−1 penicillin and 100 μg/ml streptomycin sulfate at 37°C in sealed 15-ml tubes and regularly passaged through gerbil livers to maintain high virulence (55). Virulence was maintained by regular subpassage in gerbil livers (56). EhCP-A5− trophozoites (kindly provided by David Mirelman, Weizmann Institute of Science, Rehovot, Israel) were cultured identically. Trophozoites were harvested during the logarithmic growth phase (48 to 72 h) by chilling on ice for 10 min, pelleted by centrifugation at 200 × g (5 min at 4°C), and resuspended in serum-free RPMI. The viability of trophozoites after incubation was >95%, as determined by a trypan blue exclusion assay. Concentrated secreted components of E. histolytica were collected from live trophozoites (2 × 107 trophozoites/ml) incubated in Hanks' balanced salt solution for 2 h at 37°C (46). The resulting secreted product was collected by centrifugation (1,000 × g for 5 min), assayed for protein amounts by using Bradford's reagent, normalized (0.1 μg/μl of E. histolytica secretions), aliquoted, and stored at −80°C. To irreversibly inhibit E. histolytica cysteine protease activity, amebae were cultured overnight in E64 (100 μM), as described previously (57). For the inhibition of adherence, E. histolytica parasites were incubated with 55 mM d-galactose for 15 min on ice prior to inoculation of mice.
E. histolytica-mediated proteolysis of recombinant Crp peptides.
Recombinant Crp4 and Crp4 proregion (positions 20 to 92) variants were prepared by site-directed PCR-based mutagenesis, expressed as N-terminal His6-tagged fusion proteins, and subsequently affinity purified (50). After cleavage of the His6 tag, the peptides were further purified by high-performance liquid chromatography (HPLC). Peptide homogeneity was assessed by analytical reverse-phase HPLC and AU-PAGE, and peptide masses were confirmed by MALDI-TOF mass spectrometry (Voyager-DE) (44). To test pro-Crp4 and Crp4 variants for proteolytic conversion by E. histolytica, 10-μg peptide samples were incubated with or without E. histolytica-secreted components or live E. histolytica parasites pretreated or not with the cysteine proteinase inhibitor E64 in HEPES buffer (1 mM HEPES, 15 mM NaCl, 0.5 mM CaCl2 [pH 7.4]) for 3 h at 37°C. Complete peptide digests were resolved by AU-PAGE and stained with Coomassie blue.
Mice.
All studies were approved by the University of Calgary Animal Care Committee. Male 10- to 12-week-old C57BL/6 wild-type mice from Charles River and Muc2−/− mice (58) of the same genetic background were bred in-house, and first filial generation Muc2+/− mice were backcrossed to generate F2 wild-type (Muc2+/+) and homozygous (Muc2−/−) mice. Mice were maintained in sterilized and filter-top cages, under specific-pathogen-free conditions, and provided food and water ad libitum.
Ileal loop studies with E. histolytica.
The use of closed intestinal loops for injecting E. histolytica trophozoites is a well-established model to study amebic pathogenesis. This model has been used to determine the role of E. histolytica in mucin exocytosis (59) and antimicrobial peptide production (23) in the colon and for biochemical characterization of rat mucins (60). Here closed ileal loops inoculated with E. histolytica were used to quantify Paneth cell localization (lysozyme expression) and function (Crp secretion). Muc2+/+ and Muc2−/− littermates fasted overnight were anesthetized by intraperitoneal injection with sodium pentobarbital (4.2 mg/kg of body weight) (Ceva Santé Animale). A laparotomy was performed to exteriorize the small intestine, the ileum was ligated with 3.0 black silk sutures (Ethicon Inc.), and E. histolytica parasites (2 × 106 parasites in log phase in 100 μl phosphate-buffered saline [PBS] [pH 7.3]) were inoculated into the lumen. Control loops were inoculated with PBS. The procedure was similar to the one used previously to inoculate E. histolytica into the colon (39). Care was taken to keep the mesenteries, blood vessels, and nerves intact. Mice were maintained for up to 4 h and then sacrificed by cervical dislocation. Samples up to 2 cm in diameter were homogenized in TRIzol reagent (Invitrogen) and immediately frozen in liquid nitrogen for quantitative PCR (qPCR) studies. In parallel, intestine tissue was fixed in either Carnoy's fixative (60% of 100% ethanol, 30% chloroform, 10% glacial acetic acid) to preserve the mucus layer or 10% neutral buffered formalin for 3 h, transferred to ethanol at 4°C, and then embedded in paraffin blocks for histological and immunofluorescence studies.
Histological staining of ileum sections from E. histolytica-infected Muc2+/+ and Muc2−/− mice.
Paraffin sections (5 μm) were deparaffinized by heating at 55°C to 65°C for 10 min, cleared with xylene, and rehydrated through an ethanol gradient to water. Periodic acid-Schiff (PAS) staining for mucus layer analysis and hematoxylin-eosin (H&E) staining for microscopic pathology were performed as described previously (23, 42).
Expression of Crps, Paneth cell markers, and proinflammatory cytokines in the ileum of E. histolytica-infected Muc2+/+ and Muc2−/− mice.
Relative levels of transcriptional mRNA synthesis of Crps, lysozymes, Ifn-γ, Tnf-α, Fzd5, and Ephb2 were quantified by real-time reverse transcriptase quantitative PCR (qRT-PCR). Total RNA was extracted from the homogenized frozen ileum by using TRIzol (Invitrogen) according to the manufacturer's specifications. cDNA was prepared from 1 μg of total RNA by using Moloney murine leukemia virus reverse transcriptase (iScript reverse transcription; Bio-Rad). The quality and quantity of the resulting RNA and cDNA were determined by using a spectrophotometer. The absence of contaminating genomic DNA from RNA preparations was checked by using a minus-reverse-transcriptase control (i.e., a sample with all RT-PCR reagents except reverse transcriptase). Real-time qRT-PCR was performed by using a CFX-96 real-time PCR system (Bio-Rad). Each reaction mixture contained 100 ng of cDNA, 1× SsoAdvanced universal SYBR green supermix (Bio-Rad), and 0.5 μM each specific primer (Table 1), in a final volume of 10 μl. The reaction mixtures were incubated at 95°C for 5 min, followed by denaturation for 5 s at 95°C and a combined annealing-extension step for 10 s at 60°C for a total of 40 cycles. Primers used in this study were experimentally verified for specificity and efficiency. The RT-qPCR efficiency (>95%) for each gene was calculated from the slope and determined by a linear regression model according to the following equation, as indicated by minimum information for publication of quantitative real-time PCR experiments (MIQE) guidelines (61): efficiency = 10 (−1/slope) − 1. Target gene mRNA values were corrected relative to the value for the glyceraldehyde-3-phosphate dehydrogenase (Gapdh) housekeeping gene. Negative controls for cDNA synthesis and PCR procedures were included in all cases. Data were analyzed by using the 2−ΔΔCT method and expressed as fold changes (means ± standard errors [SE]) between E. histolytica-treated (or not) Muc2+/+ and Muc2−/− mice.
TABLE 1.
Details of primers for relative quantification of mRNA by qRT-PCRa
| Gene | Catalogue no. | Sequence | Description |
|---|---|---|---|
| Crp5 | NA | F, 5′-GCAGCAGAATACGAAAGT-3′ | Cryptdin 5 |
| R, 5′-ACTTTCGTATTCTGCTGC-3′ | |||
| Crp22 | NA | F, 5′-GAGAGATCTGATCTGCCTTTG-3′ | Cryptdin 22 |
| R, 5′-CAGCGCAAAAAAGGTCCTGC-3′ | |||
| Crp_rs1 | NA | F, 5′-AGCAGCCATTGTGCGAAGAA-3′ | Cryptdin-related sequence 1 |
| R, 5′-TGCTGTTGTATTTGGAGCTTGG-3′ | |||
| Lyz1 | NA | F, 5′-GAGACCGAAGCACCGACTATG-3′ | Lysozyme 1 |
| R, 5′-CGGTTTTGACATTGTGTTCGC-3′ | |||
| Lyz2 | NA | F, 5′-ATGGAATGGCTGGCTACTATGG-3′ | Lysozyme 2 |
| R, 5′-ACCAGTATCGGCTATTGATCTGA-3′ | |||
| Fzd5 | NA | F, 5′-AGGATCCTCCCGAGAGTTCTGTCCT-3′ | Frizzled-5 |
| R, 5′-GGGCTGGCAACCTGTTGGTTTCTT-3′ | |||
| Ephb2 | NA | F, 5′-CCATTGAACAGGACTACAGACTACC-3′ | Ephrin type B receptor 2 |
| R, 5′-CACCGTGTTAAAGCTGGTGTAG-3′ | |||
| Ifn-γ | PPM03121Ab | NA | Interferon gamma |
| Tnf-α | PPM03113Gb | NA | Tumor necrosis factor alpha |
F, forward/sense primer sequence; R, reverse/antisense primer sequence; NA, not applicable.
The catalogue number refers to the sequence used to design the RT2 qPCR primer assay (Qiagen).
Immunofluorescence localization of Crp1 and lysozymes in the ileum of Muc2+/+ and Muc2−/− littermates.
Ileal tissue samples fixed in 10% formalin were sectioned (5 μm) and deparaffinized by using a xylene substitute (Neo-Clear, catalogue number 65351-85; Millipore), followed by decreasing concentrations of ethanol and running tap water (3 min). No antigen retrieval was conducted to avoid the release of lysosome or peptide contents during the immunostaining process. Sections were blocked with PBS-Tween (PBS-Tw), 1% bovine serum albumin (BSA), 10% goat serum, 0.3 M glycine, and 0.05% saponin for 2 h at room temperature (RT) and rinsed with PBS-Tw. Slides were exposed to affinity-purified rat monoclonal IgG anti-mouse Crp1 (clone 77 R20; T. Ayabe and K. Nakamura, Hokkaido University) or rabbit monoclonal anti-mouse lysozyme (catalogue number ab108508; Abcam) diluted 1:100 and 1:1,000, respectively, in PBS-Tw with 0.05% saponin and incubated at 4°C overnight. Sections were rinsed with PBS-Tw and incubated with fluorescently labeled secondary DyLight 488-affinipure goat anti-rat Crp1 and Alexa Fluor 594-conjugated donkey anti-mouse IgG(H+L) (catalogue number 715-585-150; Jackson ImmunoResearch) for lysozyme. Secondary antibodies were diluted 1:10,000 in PBS-Tw plus 1% BSA for 1 h at RT. Sections were rinsed in PBS-Tw, and nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) and mounted with FluorSave reagent (Calbiochem). Slides were examined by using a FluoView FV1000 confocal immunofluorescence microscope (Olympus). The fluorescence intensities for Crp1, TNF-α, and lysozyme were quantified by using ImageJ software. Ten fields of view were randomly selected per slide, and the average fluorescence intensity (normalized to the field of view) was calculated. Fluorescence quantification was obtained from two to three independent slides per animal and are represented as mean fluorescence intensities normalized to the area of the field of view.
Detection of secreted Crp peptides in luminal contents in the intestinal tract.
Specimens were obtained from the ileum (proximal to the ileocecal junction at a 20-cm length), cecum (distal to the ileocecal junction at a 2.5-cm length), and proximal colon (distal to the cecocolonic junction at a 5-cm length) of E. histolytica-treated (or not) Muc2+/+ and Muc2−/− mice. For these samples, the luminal contents were flushed from the segments with deionized distilled water, and the washed solution was collected. Samples were vortex mixed for 1 h at 4°C, centrifuged at 630 × g for 10 min to yield a clear solution, and then lyophilized. A sandwich ELISA was used to measure secreted Crp1, as described previously for Crp1 (62) and Crp4 (63). Microtiter plate wells were coated overnight at 4°C with 100 μl of the capture rat monoclonal anti-Crp1 antibody (1 μg/ml) in 50 mM sodium carbonate-bicarbonate buffer (pH 9.6). The plate was washed three times with PBS-Tw and blocked for 1 h at 25°C with 200 μl of 25% Block Ace (DS Pharma Biomedical). Next, 100 μl of luminal contents was added to the wells, and the mixture was incubated at 25°C for 2 h. After washing in PBS-Tw, 100 μl of biotinylated detection anti-Crp1 antibody (0.5 μg/ml) was added at 25°C for 1 h, and the wells were incubated with 100 μl of a streptavidin-horseradish peroxidase (HRP) conjugate (GE Healthcare Biosciences) at 1:5,000 dilutions at 25°C for 1 h. After final washes, 100 μl of 3,3′,5,5′-tetramethylbenzidine (TMB) chromogen substrate buffer was added, and the mixture was incubated at 25°C for 30 min. The reaction was stopped by the addition of 100 μl of 0.6 N H2SO4, and absorbance values were determined at 450 nm by using a microplate reader. A standard curve of a serial dilution of synthesized recombinant Crp1 was prepared in the range of 0.02 to 5 ng/ml to adjust absorbance values to the peptide concentration per total protein.
Statistical analysis.
Graphs represent normally distributed/Gaussian-distributed (parametric) results, represented as means, and bars represent standard errors of the means from a minimum of two independent experiments. Normality was assessed by using the Shapiro-Wilk (Royston) test. Differences between groups/treatments were analyzed by one-way analysis of variance (ANOVA) followed by Bonferroni posttests (GraphPad Prism 5.0 Mac; GraphPad Software). A P value of <0.05 was considered statistically significant. All statistical analyses were performed with GraphPad Prism software (GraphPad 7.0).
ACKNOWLEDGMENTS
This work was supported by grants from Crohn's and Colitis Canada (CCC) and the Canadian Institute of Health Research (CIHR) to K.C.; grants from the Margaret Gunn Endowment for Animal Research (University of Calgary), ALMA (2016E004R), and NSERC Discovery (RGPAS-2017-507827) to E.R.C.; and NIH grants (DK44632 and AI105057) and a USC Norris Cancer Center Support grant (P30CA014089) from the National Cancer Institute to A.O. This work was also supported by grants-in-aid for scientific research from the Japan Society for the Promotion of Science to K.N. and the Center of Innovation Program from the Japan Science and Technology Agency to K.N. and T.A.
Footnotes
[This article was published on 21 June 2018 with a byline that lacked Yoshihiro Eriguchi. The byline was updated in the current version, posted on 24 July 2018.]
REFERENCES
- 1.Cheng H, Leblond CP. 1974. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian theory of the origin of the four epithelial cell types. Am J Anat 141:537–561. doi: 10.1002/aja.1001410407. [DOI] [PubMed] [Google Scholar]
- 2.Holmen Larsson JM, Thomsson KA, Rodriguez-Pineiro AM, Karlsson H, Hansson GC. 2013. Studies of mucus in mouse stomach, small intestine, and colon. III. Gastrointestinal Muc5ac and Muc2 mucin O-glycan patterns reveal a regiospecific distribution. Am J Physiol Gastrointest Liver Physiol 305:G357–G363. doi: 10.1152/ajpgi.00048.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bajka BH, Rigby NM, Cross KL, Macierzanka A, Mackie AR. 2015. The influence of small intestinal mucus structure on particle transport ex vivo. Colloids Surf B Biointerfaces 135:73–80. doi: 10.1016/j.colsurfb.2015.07.038. [DOI] [PubMed] [Google Scholar]
- 4.Markart P, Faust N, Graf T, Na CL, Weaver TE, Akinbi HT. 2004. Comparison of the microbicidal and muramidase activities of mouse lysozyme M and P. Biochem J 380:385–392. doi: 10.1042/bj20031810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ayabe T, Satchell DP, Pesendorfer P, Tanabe H, Wilson CL, Hagen SJ, Ouellette AJ. 2002. Activation of Paneth cell alpha-defensins in mouse small intestine. J Biol Chem 277:5219–5228. doi: 10.1074/jbc.M109410200. [DOI] [PubMed] [Google Scholar]
- 6.Ayabe T, Satchell DP, Wilson CL, Parks WC, Selsted ME, Ouellette AJ. 2000. Secretion of microbicidal alpha-defensins by intestinal Paneth cells in response to bacteria. Nat Immunol 1:113–118. doi: 10.1038/77783. [DOI] [PubMed] [Google Scholar]
- 7.Wiesner J, Vilcinskas A. 2010. Antimicrobial peptides: the ancient arm of the human immune system. Virulence 1:440–464. doi: 10.4161/viru.1.5.12983. [DOI] [PubMed] [Google Scholar]
- 8.Jones DE, Bevins CL. 1993. Defensin-6 mRNA in human Paneth cells: implications for antimicrobial peptides in host defense of the human bowel. FEBS Lett 315:187–192. doi: 10.1016/0014-5793(93)81160-2. [DOI] [PubMed] [Google Scholar]
- 9.Porter EM, Liu L, Oren A, Anton PA, Ganz T. 1997. Localization of human intestinal defensin 5 in Paneth cell granules. Infect Immun 65:2389–2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shanahan MT, Tanabe H, Ouellette AJ. 2011. Strain-specific polymorphisms in Paneth cell alpha-defensins of C57BL/6 mice and evidence of vestigial myeloid alpha-defensin pseudogenes. Infect Immun 79:459–473. doi: 10.1128/IAI.00996-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karlsson J, Putsep K, Chu H, Kays RJ, Bevins CL, Andersson M. 2008. Regional variations in Paneth cell antimicrobial peptide expression along the mouse intestinal tract. BMC Immunol 9:37. doi: 10.1186/1471-2172-9-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wehkamp J, Chu H, Shen B, Feathers RW, Kays RJ, Lee SK, Bevins CL. 2006. Paneth cell antimicrobial peptides: topographical distribution and quantification in human gastrointestinal tissues. FEBS Lett 580:5344–5350. doi: 10.1016/j.febslet.2006.08.083. [DOI] [PubMed] [Google Scholar]
- 13.van Es JH, Jay P, Gregorieff A, van Gijn ME, Jonkheer S, Hatzis P, Thiele A, van den Born M, Begthel H, Brabletz T, Taketo MM, Clevers H. 2005. Wnt signalling induces maturation of Paneth cells in intestinal crypts. Nat Cell Biol 7:381–386. doi: 10.1038/ncb1240. [DOI] [PubMed] [Google Scholar]
- 14.Batlle E, Henderson JT, Beghtel H, van den Born MM, Sancho E, Huls G, Meeldijk J, Robertson J, van de Wetering M, Pawson T, Clevers H. 2002. Beta-catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/ephrinB. Cell 111:251–263. doi: 10.1016/S0092-8674(02)01015-2. [DOI] [PubMed] [Google Scholar]
- 15.Antoni L, Nuding S, Weller D, Gersemann M, Ott G, Wehkamp J, Stange EF. 2013. Human colonic mucus is a reservoir for antimicrobial peptides. J Crohns Colitis 7:652–664. doi: 10.1016/j.crohns.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 16.Mortimer L, Chadee K. 2010. The immunopathogenesis of Entamoeba histolytica. Exp Parasitol 126:366–380. doi: 10.1016/j.exppara.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 17.Marie C, Petri WA Jr. 2014. Regulation of virulence of Entamoeba histolytica. Annu Rev Microbiol 68:493–520. doi: 10.1146/annurev-micro-091313-103550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petri WA Jr, Smith RD, Schlesinger PH, Murphy CF, Ravdin JI. 1987. Isolation of the galactose-binding lectin that mediates the in vitro adherence of Entamoeba histolytica. J Clin Invest 80:1238–1244. doi: 10.1172/JCI113198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chadee K, Petri WA Jr, Innes DJ, Ravdin JI. 1987. Rat and human colonic mucins bind to and inhibit adherence lectin of Entamoeba histolytica. J Clin Invest 80:1245–1254. doi: 10.1172/JCI113199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ankri S, Stolarsky T, Bracha R, Padilla-Vaca F, Mirelman D. 1999. Antisense inhibition of expression of cysteine proteinases affects Entamoeba histolytica-induced formation of liver abscess in hamsters. Infect Immun 67:421–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirata KK, Que X, Melendez-Lopez SG, Debnath A, Myers S, Herdman DS, Orozco E, Bhattacharya A, McKerrow JH, Reed SL. 2007. A phagocytosis mutant of Entamoeba histolytica is less virulent due to deficient proteinase expression and release. Exp Parasitol 115:192–199. doi: 10.1016/j.exppara.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Melendez-Lopez SG, Herdman S, Hirata K, Choi MH, Choe Y, Craik C, Caffrey CR, Hansell E, Chavez-Munguia B, Chen YT, Roush WR, McKerrow J, Eckmann L, Guo J, Stanley SL Jr, Reed SL. 2007. Use of recombinant Entamoeba histolytica cysteine proteinase 1 to identify a potent inhibitor of amebic invasion in a human colonic model. Eukaryot Cell 6:1130–1136. doi: 10.1128/EC.00094-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cobo ER, Kissoon-Singh V, Moreau F, Chadee K. 2015. Colonic MUC2 mucin regulates the expression and antimicrobial activity of beta-defensin 2. Mucosal Immunol 8:1360–1372. doi: 10.1038/mi.2015.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salzman NH, Ghosh D, Huttner KM, Paterson Y, Bevins CL. 2003. Protection against enteric salmonellosis in transgenic mice expressing a human intestinal defensin. Nature 422:522–526. doi: 10.1038/nature01520. [DOI] [PubMed] [Google Scholar]
- 25.Mortimer L, Moreau F, Cornick S, Chadee K. 2014. Gal-lectin-dependent contact activates the inflammasome by invasive Entamoeba histolytica. Mucosal Immunol 7:829–841. doi: 10.1038/mi.2013.100. [DOI] [PubMed] [Google Scholar]
- 26.Mortimer L, Moreau F, Cornick S, Chadee K. 2015. The NLRP3 inflammasome is a pathogen sensor for invasive Entamoeba histolytica via activation of α5β1 integrin at the macrophage-amebae intercellular junction. PLoS Pathog 11:e1004887. doi: 10.1371/journal.ppat.1004887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cornick S, Moreau F, Chadee K. 2016. Entamoeba histolytica cysteine proteinase 5 evokes mucin exocytosis from colonic goblet cells via αvβ3 integrin. PLoS Pathog 12:e1005579. doi: 10.1371/journal.ppat.1005579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mastroianni JR, Ouellette AJ. 2009. Alpha-defensins in enteric innate immunity: functional Paneth cell alpha-defensins in mouse colonic lumen. J Biol Chem 284:27848–27856. doi: 10.1074/jbc.M109.050773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nava P, Koch S, Laukoetter MG, Lee WY, Kolegraff K, Capaldo CT, Beeman N, Addis C, Gerner-Smidt K, Neumaier I, Skerra A, Li L, Parkos CA, Nusrat A. 2010. Interferon-gamma regulates intestinal epithelial homeostasis through converging beta-catenin signaling pathways. Immunity 32:392–402. doi: 10.1016/j.immuni.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moncada D, Keller K, Chadee K. 2003. Entamoeba histolytica cysteine proteinases disrupt the polymeric structure of colonic mucin and alter its protective function. Infect Immun 71:838–844. doi: 10.1128/IAI.71.2.838-844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lidell ME, Moncada DM, Chadee K, Hansson GC. 2006. Entamoeba histolytica cysteine proteases cleave the MUC2 mucin in its C-terminal domain and dissolve the protective colonic mucus gel. Proc Natl Acad Sci U S A 103:9298–9303. doi: 10.1073/pnas.0600623103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cobo ER, He C, Hirata K, Hwang G, Tran U, Eckmann L, Gallo RL, Reed SL. 2012. Entamoeba histolytica induces intestinal cathelicidins but is resistant to cathelicidin-mediated killing. Infect Immun 80:143–149. doi: 10.1128/IAI.05029-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reed SL, Ember JA, Herdman DS, DiScipio RG, Hugli TE, Gigli I. 1995. The extracellular neutral cysteine proteinase of Entamoeba histolytica degrades anaphylatoxins C3a and C5a. J Immunol 155:266–274. [PubMed] [Google Scholar]
- 34.Ouellette AJ, Darmoul D, Tran D, Huttner KM, Yuan J, Selsted ME. 1999. Peptide localization and gene structure of cryptdin 4, a differentially expressed mouse Paneth cell alpha-defensin. Infect Immun 67:6643–6651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Espinosa-Cantellano M, Martinez-Palomo A. 2000. Pathogenesis of intestinal amebiasis: from molecules to disease. Clin Microbiol Rev 13:318–331. doi: 10.1128/CMR.13.2.318-331.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stanley SL., Jr 2003. Amoebiasis. Lancet 361:1025–1034. doi: 10.1016/S0140-6736(03)12830-9. [DOI] [PubMed] [Google Scholar]
- 37.Martinez-Palomo A, Tsutsumi V, Anaya-Velazquez F, Gonzalez-Robles A. 1989. Ultrastructure of experimental intestinal invasive amebiasis. Am J Trop Med Hyg 41:273–279. doi: 10.4269/ajtmh.1989.41.273. [DOI] [PubMed] [Google Scholar]
- 38.Johansson ME, Phillipson M, Petersson J, Velcich A, Holm L, Hansson GC. 2008. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci U S A 105:15064–15069. doi: 10.1073/pnas.0803124105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kissoon-Singh V, Moreau F, Trusevych E, Chadee K. 2013. Entamoeba histolytica exacerbates epithelial tight junction permeability and proinflammatory responses in Muc2(−/−) mice. Am J Pathol 182:852–865. doi: 10.1016/j.ajpath.2012.11.035. [DOI] [PubMed] [Google Scholar]
- 40.Watanabe N, Mashima H, Miura K, Goto T, Yoshida M, Goto A, Ohnishi H. 2016. Requirement of Galphaq/Galpha11 signaling in the preservation of mouse intestinal epithelial homeostasis. Cell Mol Gastroenterol Hepatol 2:767.e6–782.e6. doi: 10.1016/j.jcmgh.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao F, Edwards R, Dizon D, Afrasiabi K, Mastroianni JR, Geyfman M, Ouellette AJ, Andersen B, Lipkin SM. 2010. Disruption of Paneth and goblet cell homeostasis and increased endoplasmic reticulum stress in Agr2−/− mice. Dev Biol 338:270–279. doi: 10.1016/j.ydbio.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cobo ER, Kissoon-Singh V, Moreau F, Holani R, Chadee K. 2017. MUC2 mucin and butyrate contribute to the synthesis of the antimicrobial peptide cathelicidin in response to Entamoeba histolytica- and dextran sodium sulfate-induced colitis. Infect Immun 85:e00905-16. doi: 10.1128/IAI.00905-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mastroianni JR, Costales JK, Zaksheske J, Selsted ME, Salzman NH, Ouellette AJ. 2012. Alternative luminal activation mechanisms for Paneth cell alpha-defensins. J Biol Chem 287:11205–11212. doi: 10.1074/jbc.M111.333559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weeks CS, Tanabe H, Cummings JE, Crampton SP, Sheynis T, Jelinek R, Vanderlick TK, Cocco MJ, Ouellette AJ. 2006. Matrix metalloproteinase-7 activation of mouse Paneth cell pro-alpha-defensins: SER43↓ILE44 proteolysis enables membrane-disruptive activity. J Biol Chem 281:28932–28942. doi: 10.1074/jbc.M602041200. [DOI] [PubMed] [Google Scholar]
- 45.Obermeier F, Kojouharoff G, Hans W, Scholmerich J, Gross V, Falk W. 1999. Interferon-gamma (IFN-gamma)- and tumour necrosis factor (TNF)-induced nitric oxide as toxic effector molecule in chronic dextran sulphate sodium (DSS)-induced colitis in mice. Clin Exp Immunol 116:238–245. doi: 10.1046/j.1365-2249.1999.00878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu Y, Chadee K. 1997. Entamoeba histolytica stimulates interleukin 8 from human colonic epithelial cells without parasite-enterocyte contact. Gastroenterology 112:1536–1547. doi: 10.1016/S0016-5085(97)70035-0. [DOI] [PubMed] [Google Scholar]
- 47.Raetz M, Hwang SH, Wilhelm CL, Kirkland D, Benson A, Sturge CR, Mirpuri J, Vaishnava S, Hou B, Defranco AL, Gilpin CJ, Hooper LV, Yarovinsky F. 2013. Parasite-induced TH1 cells and intestinal dysbiosis cooperate in IFN-gamma-dependent elimination of Paneth cells. Nat Immunol 14:136–142. doi: 10.1038/ni.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Farin HF, Karthaus WR, Kujala P, Rakhshandehroo M, Schwank G, Vries RG, Kalkhoven E, Nieuwenhuis EE, Clevers H. 2014. Paneth cell extrusion and release of antimicrobial products is directly controlled by immune cell-derived IFN-gamma. J Exp Med 211:1393–1405. doi: 10.1084/jem.20130753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roulis M, Bongers G, Armaka M, Salviano T, He Z, Singh A, Seidler U, Becker C, Demengeot J, Furtado GC, Lira SA, Kollias G. 2016. Host and microbiota interactions are critical for development of murine Crohn's-like ileitis. Mucosal Immunol 9:787–797. doi: 10.1038/mi.2015.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shirafuji Y, Tanabe H, Satchell DP, Henschen-Edman A, Wilson CL, Ouellette AJ. 2003. Structural determinants of procryptdin recognition and cleavage by matrix metalloproteinase-7. J Biol Chem 278:7910–7919. doi: 10.1074/jbc.M210600200. [DOI] [PubMed] [Google Scholar]
- 51.Rosengren KJ, Daly NL, Fornander LM, Jonsson LM, Shirafuji Y, Qu X, Vogel HJ, Ouellette AJ, Craik DJ. 2006. Structural and functional characterization of the conserved salt bridge in mammalian Paneth cell alpha-defensins: solution structures of mouse CRYPTDIN-4 and (E15D)-CRYPTDIN-4. J Biol Chem 281:28068–28078. doi: 10.1074/jbc.M604992200. [DOI] [PubMed] [Google Scholar]
- 52.Preet S, Bharati S, Shukla G, Koul A, Rishi P. 2011. Evaluation of amoebicidal potential of Paneth cell cryptdin-2 against Entamoeba histolytica. PLoS Negl Trop Dis 5:e1386. doi: 10.1371/journal.pntd.0001386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johnson CA, Rachakonda G, Kleshchenko YY, Nde PN, Madison MN, Pratap S, Cardenas TC, Taylor C, Lima MF, Villalta F. 2013. Cellular response to Trypanosoma cruzi infection induces secretion of defensin alpha-1, which damages the flagellum, neutralizes trypanosome motility, and inhibits infection. Infect Immun 81:4139–4148. doi: 10.1128/IAI.01459-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tanaka T, Rahman MM, Battur B, Boldbaatar D, Liao M, Umemiya-Shirafuji R, Xuan X, Fujisaki K. 2010. Parasiticidal activity of human alpha-defensin-5 against Toxoplasma gondii. In Vitro Cell Dev Biol Anim 46:560–565. doi: 10.1007/s11626-009-9271-9. [DOI] [PubMed] [Google Scholar]
- 55.Belley A, Keller K, Grove J, Chadee K. 1996. Interaction of LS174T human colon cancer cell mucins with Entamoeba histolytica: an in vitro model for colonic disease. Gastroenterology 111:1484–1492. doi: 10.1016/S0016-5085(96)70009-4. [DOI] [PubMed] [Google Scholar]
- 56.Denis M, Chadee K. 1989. Cytokine activation of murine macrophages for in vitro killing of Entamoeba histolytica trophozoites. Infect Immun 57:1750–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hou Y, Mortimer L, Chadee K. 2010. Entamoeba histolytica cysteine proteinase 5 binds integrin on colonic cells and stimulates NFκB-mediated pro-inflammatory responses. J Biol Chem 285:35497–35504. doi: 10.1074/jbc.M109.066035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Velcich A, Yang W, Heyer J, Fragale A, Nicholas C, Viani S, Kucherlapati R, Lipkin M, Yang K, Augenlicht L. 2002. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science 295:1726–1729. doi: 10.1126/science.1069094. [DOI] [PubMed] [Google Scholar]
- 59.Cornick S, Moreau F, Gaisano HY, Chadee K. 2017. Entamoeba histolytica-induced mucin exocytosis is mediated by VAMP8 and is critical in mucosal innate host defense. mBio 8:e01323-17. doi: 10.1128/mBio.01323-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tse SK, Chadee K. 1992. Biochemical characterization of rat colonic mucins secreted in response to Entamoeba histolytica. Infect Immun 60:1603–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT. 2009. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- 62.Eriguchi Y, Nakamura K, Hashimoto D, Shimoda S, Shimono N, Akashi K, Ayabe T, Teshima T. 2015. Decreased secretion of Paneth cell alpha-defensins in graft-versus-host disease. Transpl Infect Dis 17:702–706. doi: 10.1111/tid.12423. [DOI] [PubMed] [Google Scholar]
- 63.Nakamura K, Sakuragi N, Ayabe T. 2013. A monoclonal antibody-based sandwich enzyme-linked immunosorbent assay for detection of secreted alpha-defensin. Anal Biochem 443:124–131. doi: 10.1016/j.ab.2013.08.021. [DOI] [PubMed] [Google Scholar]