ABSTRACT
Enterotoxigenic Escherichia coli (ETEC) is a major cause of traveler's diarrhea as well as of endemic diarrhea and stunting in children in developing areas. However, a small-mammal model has been badly needed to better understand and assess mechanisms, vaccines, and interventions. We report a murine model of ETEC diarrhea, weight loss, and enteropathy and investigate the role of zinc in the outcomes. ETEC strains producing heat-labile toxins (LT) and heat-stable toxins (ST) that were given to weaned C57BL/6 mice after antibiotic disruption of normal microbiota caused growth impairment, watery diarrhea, heavy stool shedding, and mild to moderate intestinal inflammation, the latter being worse with zinc deficiency. Zinc treatment promoted growth in zinc-deficient infected mice, and subinhibitory levels of zinc reduced expression of ETEC virulence genes cfa1, cexE, sta2, and degP but not of eltA in vitro. Zinc supplementation increased shedding and the ileal burden of wild-type (WT) ETEC but decreased shedding and the tissue burden of LT knockout (LTKO) ETEC. LTKO ETEC-infected mice had delayed disease onset and also had less inflammation by fecal myeloperoxidase (MPO) assessment. These findings provide a new murine model of ETEC infection that can help elucidate mechanisms of growth, diarrhea, and inflammatory responses as well as potential vaccines and interventions.
KEYWORDS: ETEC, murine model, zinc, LT and ST, enteropathy, diarrhea
INTRODUCTION
Enterotoxigenic Escherichia coli (ETEC) is one of the most common causes of childhood diarrhea in developing countries and is a leading cause of diarrhea deaths in infants (1). In the GEMS and MALED studies, ETEC was found to be one of the top 4 most common pathogens in children aged 0 to 59 months and was associated with diarrhea and increased risk of death (2, 3). Additionally, Lamberti et al. reported that ETEC is the leading cause of diarrhea-associated mortality and morbidity (followed by Shigella) in children who are >5 years of age in both South Asia and Africa (4).
ETEC infections are also the leading cause of traveler's diarrhea (followed by Campylobacter infections) (5). The incidence of traveler's diarrhea is estimated at 20% to 50% of all travelers to developing countries (6). ETEC infection in adults usually occurs 1 to 3 days after exposure and lasts from 3 to 5 days. Common symptoms are watery diarrhea, abdominal cramping, nausea, vomiting, and fever (7, 8).
ETEC virulence is associated with colonization of the small intestine and production of one or more toxins that induce secretion of chloride, sodium, and water into the lumen (9). The two major classes of toxins, the heat-labile toxins (LT) and heat-stable toxins (ST), act by stimulating expression of adenylate and guanylate cyclase, respectively (10, 11). ETEC strains may have one or both of the LT and ST (12). The toxins are encoded on large plasmids which also encode most of the known ETEC colonization factors (CFs) (13). There is a large variety of colonization factors. Most are fimbrial or fibrillary proteins and consist of a single antigen (colonization factor antigen I [CFA/I]) or of multiple fibrillary “colonization surface antigens” (which currently number 1 to 21, including colonization surface antigen 1 [CS1] to CS3, constituting CFA/II, and CS4 to CS6, constituting CFA/IV) (14). Sensitive quantitative PCR (qPCR) arrays have recently been developed to identify ETEC colonization factors (15). Both the toxins and colonization factors of ETEC have been targeted for vaccine development (16–18).
In the current report, we describe a robust, small-animal model of ETEC infection in young mice exhibiting diarrhea, weight loss, and mild to moderate intestinal inflammation. We also investigate the impact of zinc deficiency as well as zinc treatment on disease outcomes. This model will be valuable in testing potential interventions as well as ETEC vaccine candidates.
RESULTS
Enterotoxigenic Escherichia coli infection causes weight loss and diarrhea.
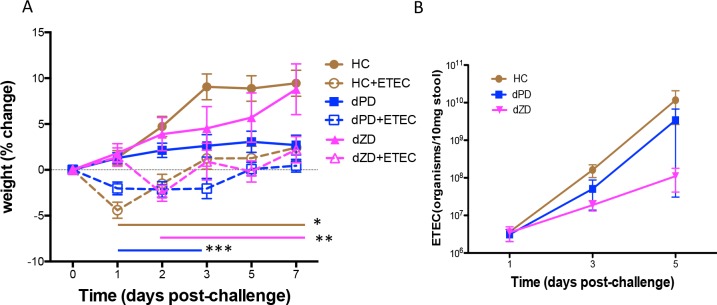
As shown in Fig. 1A, mice infected with ETEC lost weight as early as day 1 postinfection (pi) (*, P < 0.01 [standard rodent “house chow” {HC} diet versus HC plus ETEC during days 1 to 7]; **, P < 0.005 [defined protein source diet without zinc {dZD} versus dZD plus ETEC during days 2 to 7]), and the mice fed HC or dZD developed watery diarrhea. While mice fed with a defined reduced-protein diet (dPD) did not develop diarrhea, they did have significant weight loss at 3 days postinfection (***, P < 0.05 [dPD versus dPD plus ETEC during days 1 to 3]).
FIG 1.
Effects of ETEC strain H10407 infection in antibiotic-pretreated mice fed a nourished diet (HC), a protein-deficient diet (dPD), or a zinc-deficient diet (dZD). Mice were fed one of the three diets (HC, dPD, or dZD) for 2 weeks and pretreated with an antibiotic cocktail prior to infection with 108 CFU of ETEC strain H10407. (A) Body weight change postchallenge. Daily weight measures were collected after infection. *, P < 0.01 (HC versus HC plus ETEC during days 1 to 7 postinfection); **, P < 0.005 (dZD versus dZD plus ETEC during days 2 to 7 postinfection) (n = 8/group); ***, P < 0.05 (dPD versus dPD plus ETEC during days 1 to 3 postinfection). (B) Stool ETEC H10407 shedding. Quantitative PCR was performed using DNA samples that had been extracted from stool samples from ETEC-infected mice (n = 8/group).
Enterotoxigenic E. coli shedding in stool.
Antibiotic disruption of resident microbiota was required for ETEC colonization in our mouse model as there was no detectable level of ETEC in stool following infection in non-antibiotic-treated mice (data not shown). However, in mice pretreated with the antibiotic cocktail described in Materials and Methods, ETEC was shed (at 7 to 10 logs/10 mg stool) in mouse feces during days 1, 3, and 5 postinfection (Fig. 1B), effects that were not increased under conditions of zinc or protein deficiency.
Enterotoxigenic E. coli increases levels of intestinal inflammatory biomarkers in mice.
We have previously demonstrated the tight correlation of fecal biomarkers lipocalin-2 (LCN-2) and myeloperoxidase (MPO) with malnutrition and disease burden in the MAL-ED study in children (19, 20). As shown in Fig. 2, fecal levels were significantly elevated in mice at day 3 following a single dose of ETEC for both LCN-2 (*, P < 0.05 [dZD versus dZD plus ETEC and dPD versus dPD plus ETEC]) (Fig. 2A) and MPO (*, P < 0.05; **, P < 0.01 [dZD versus dZD plus ETEC]) (Fig. 2B) (n = 8/group). The exceptionally high levels of LCN-2 and MPO in the zinc-deficient infected mice led us to investigate potential zinc effects on ETEC virulence traits.
FIG 2.
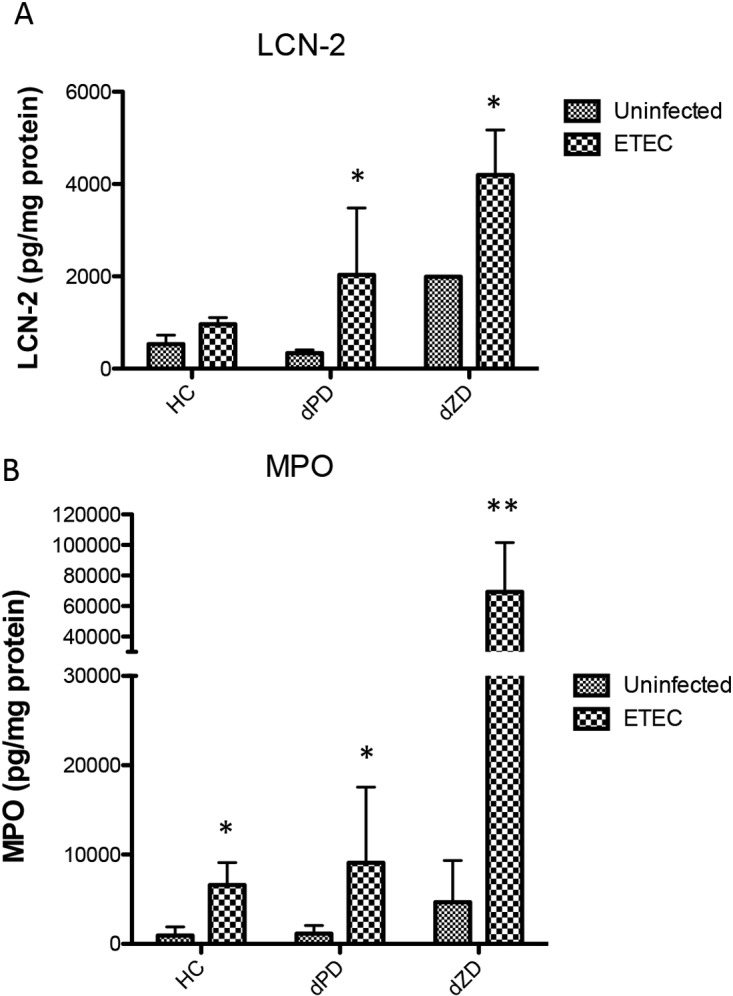
Intestinal inflammation induced by ETEC strain H10407 infection in antibiotic-pretreated mice fed a nourished diet (HC), a protein-deficient diet (dPD), or a zinc-deficient diet (dZD). Protein extracts were obtained from fecal samples at day 2 postinfection by lysing fecal specimens in RIPA buffer. Supernatants from the lysates were used for protein quantification and specific ELISAs for evaluation of myeloperoxidase (MPO) or lipocalin (LCN-2). (A) LCN-2 fecal levels after infection. *, P < 0.05 (dZD versus dZD plus ETEC and dPD versus dPD plus ETEC). (B) MPO fecal levels after infection. *, P < 0.05 (HC versus HC plus ETEC and dPD versus dPD plus ETEC); **, P < 0.01 (dZD versus dZD plus ETEC) (n = 8/group).
Influence of zinc on ETEC H10407 growth and virulence gene expression in vitro.
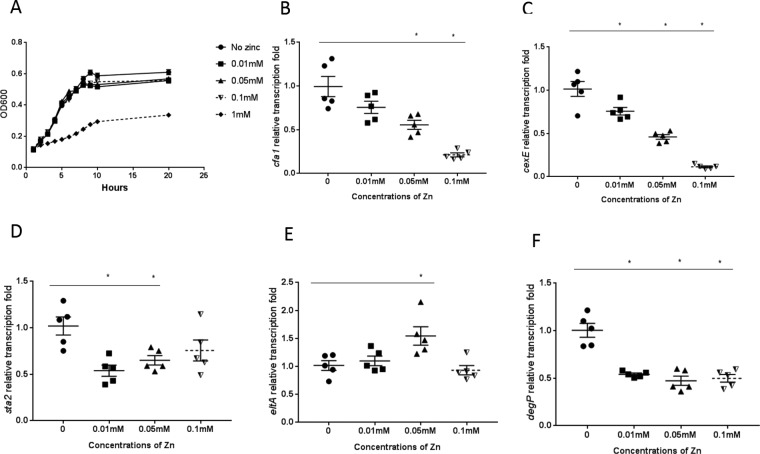
We have previously seen effects of zinc on enteroaggregative E. coli (EAEC) virulence trait expression (21, 22). To investigate zinc effects on ETEC virulence, we conducted similar in vitro growth assays. As shown in Fig. 3, supplementation of zinc at a concentration of 0.01 mM or higher (the concentrations were not inhibitory for ETEC growth until >0.1 mM was reached) (Fig. 3A) was able to alter expression of the cfaA, cexE, sta2, eltA, and degP genes (*, P < 0.05 for comparisons of the findings obtained with bacteria that had been exposed to zinc with those obtained with bacteria that had not been cultured with zinc) (n = 5/group) (Fig. 3B to F).
FIG 3.
Influence of zinc on ETEC growth and virulence gene expression in vitro. (A) Growth of ETEC strain H10407 in the presence of zinc. Bacteria were incubated with select concentrations of zinc and monitored spectrophotometrically. *, no zinc versus 1 mM zinc; OD600, optical density at 600 nm. (B to F) Bacterial mRNA was isolated from ETEC strain H10407 after incubation with non-growth-inhibitory zinc concentrations. Reverse transcription-qPCR (RT-qPCR) was performed for measurement of expression of specific genes (cfaA, cexE, sta2, eltA, and degP). *, P < 0.05 (comparing the findings seen with zinc exposure to those seen in the absence of zinc exposure) (n = 5/group).
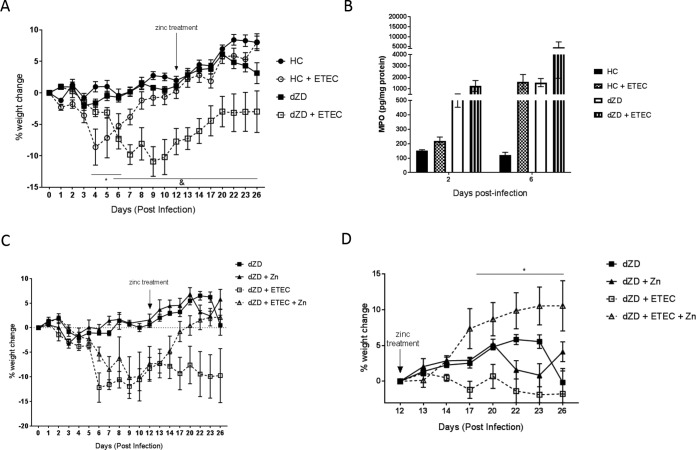
Zinc supplementation enables catchup growth in ETEC H10407-infected groups, without decreasing intestinal colonization.
At day 12 postinfection, zinc in the drinking water (150 mg/liter) was given to zinc-deficient infected mice. Figure 4A shows percentages of body weight changes during the whole experiment (before and after zinc treatment start) without discriminating the results obtained with infected zinc-deficient mice from those obtained with the mice that had received zinc. Increased fecal MPO levels were observed at day 6 pi in both “house chow” (a defined protein source diet without zinc)-deficient and zinc-deficient infected mice (Fig. 4B). By day 17 (5 days after zinc treatment), catchup growth had been enabled in the infected zinc-supplemented mice (P < 0.05) (Fig. 4C). Further, the significance of the benefit of zinc supplementation reached a P value of <0.0001 for percent body weight change values compared with those determined for the non-zinc-supplemented groups starting at day 12 as the baseline (when zinc supplementation was implemented; n = 4/group) (Fig. 4D).
FIG 4.
Zinc supplementation enables catchup growth in zinc-deficient ETEC H10407-infected C57BL/6 mice. Zinc-deficient mice received supplemental zinc sulfate in drinking water (150 mg/liter) starting from day 12 post-ETEC infection. Daily weight measures were collected from the day of infection until the end of the experiment. (A) Percentages of body weight change using day 0 as the baseline without discriminating the zinc treatment groups. *, P < 0.05 (for HC versus HC plus ETEC during days 4 to 6 postinfection and for dZD versus dZD plus ETEC during days 6 to 26 postinfection). (B) Myeloperoxidase (MPO) fecal levels after infection. Protein extracts were obtained from fecal samples at day 2 postinfection by lysing fecal specimens in RIPA buffer. Supernatants from the lysates were used for protein quantification and specific ELISAs for evaluation of MPO fecal levels. (C) Percentages of body weight change using day 0 as the baseline and discrimination of the zinc-treated groups. (D) Percentages of body weight change determined using day 12 (the initial day of zinc treatment) as the baseline. *, P < 0.0001 (dZD plus ETEC versus dZD plus WT plus Zn during days 23 to 26 postinfection) (n = 4/group).
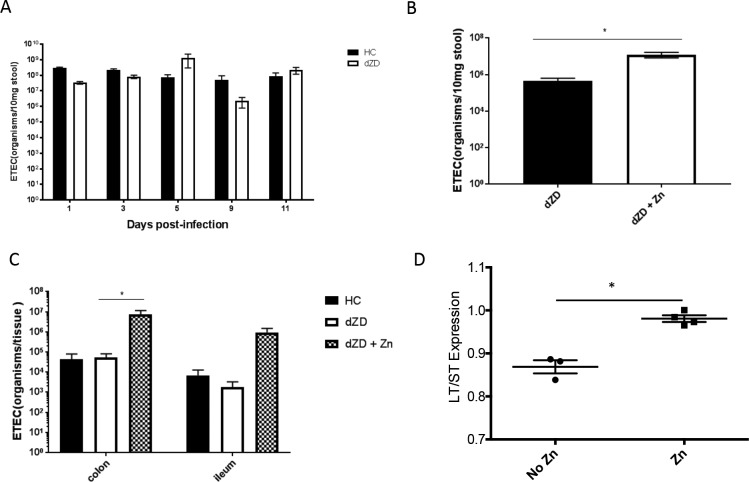
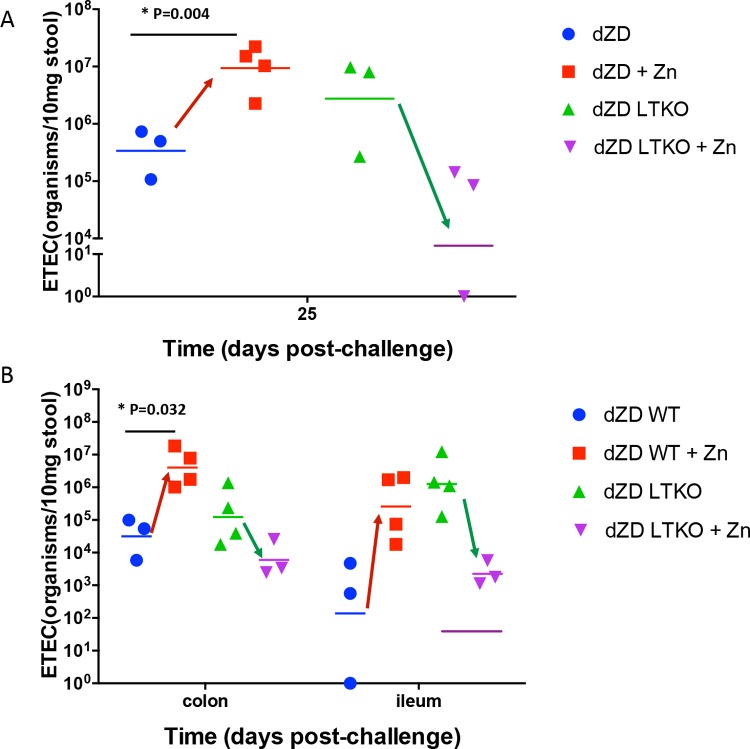
Quantitative PCR was performed on DNA that was extracted from stool samples or intestine samples from ETEC-infected mice. Interestingly, zinc supplementation significantly increased both ETEC shedding and the intestinal burden in stool samples (Fig. 5). ETEC H10407 possesses both the sta2 and eltA genes, encoding ST and LT, respectively. Considering that zinc downregulated sta2 but upregulated eltA in vitro, this suggests that ST may be more highly associated with inflammation and weight loss and that LT is more highly associated with colonization. Hence, the specific roles of ST and of LT require further investigations. As shown in Fig. 5D, the ratio of LT expression to ST expression was altered in the cecum of zinc-deficient mice receiving zinc treatment.
FIG 5.
Zinc supplementation increases ETEC H10407 intestinal colonization in zinc-deficient infected C57BL/6 mice. Zinc-deficient mice received supplemental zinc sulfate in drinking water (150 mg/liter) starting from day 12 post-ETEC infection. Quantitative PCR was performed using DNA samples that had been extracted from stool samples or intestine samples from ETEC-infected mice. (A) Stool ETEC H10407 shedding before zinc treatment. (B) Effect of zinc treatment on stool ETEC H10407 shedding after 13 days of zinc treatment (day 25 postinfection). *, P < 0.05 (dZD versus dZD plus Zn). (C) Effect of zinc treatment on colon and ileum ETEC H10407 colonization after 13 days of zinc treatment (day 25 postinfection). *, P < 0.05 (dZD versus dZD plus Zn in the colon) (n = 4/group). (D) Effect of zinc treatment on cecal ETEC H10407 toxin expression following zinc treatment. *, P = 0.0008 (no Zn versus Zn).
The effects of zinc deficiency and zinc supplementation are toxin specific.
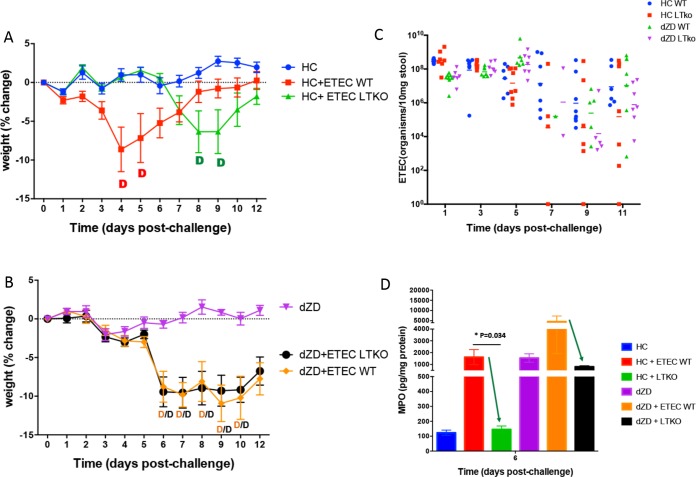
In order to dissect the different roles of ST and LT in our infection model, we created an LT-negative (LT−) (i.e., LT knockout [LTKNO]) mutant of H10407. LTKO-infected mice fed a standard diet had a delayed disease outcome similar to what was observed in zinc-deficient mice infected with wild-type (WT) H10407 (Fig. 6A), while zinc-deficient mice infected with LTKO had a disease outcome similar to that seen with wild-type-infected zinc-deficient mice (Fig. 6B). The levels of shedding of the organisms were not significantly different (Fig. 6C). LTKO-infected zinc-deficient mice showed lower levels of fecal MPO than ETEC wild-type control-challenged mice (Fig. 6D).
FIG 6.
Outcomes of infection with an ETEC LT mutant of strain H01407 (LTKO) are different from those seen with the wild type in house chow-deficient and zinc-deficient mice. House chow-deficient or zinc-deficient mice were infected with either the ETEC H10407 wild type or ETEC LTKO strains. Daily weight measures were collected from the day of infection until the end of the experiment. (A) Percentages of body weight change of house chow mice after infection. (B) Percentages of body weight change of zinc-deficient mice after infection. *, P < 0.05 (ZD versus ZD plus ETEC WT/LTKO). (C) Shedding of ETEC WT and LTKO strains in HC- and dZD-fed mice. LTKO ETEC showed a trend of lower shedding levels than WT ETEC following 1 week of infection in both diet groups. There were no significant differences between groups. (D) Myeloperoxidase (MPO) fecal levels after 6 days of infection. Protein extracts were obtained from fecal samples at day 2 postinfection by lysing fecal specimens in RIPA buffer. Supernatants from the lysates were used for protein quantification and specific ELISAs for evaluation of MPO. *, P < 0.05 (HC plus ETEC WT versus HC ETEC LTKO) (n = 8/group).
Zinc supplementations of zinc-deficient mice infected with either WT or LTKO ETEC showed very different responses. Whereas zinc supplementation increased shedding and the ileal burden of WT ETEC, zinc reduced shedding and the tissue burden of LTKO ETEC (Fig. 7). These data suggest that, while zinc supplementation may significantly reduce the expression and impact of ST as demonstrated in vitro and in the LTKO-infected mice, zinc supplementation has mixed effects on LT- and ST-expressing WT ETEC. Further studies on additional wild-type, knockout, and single-toxin-expressing strains are needed to evaluate the specific impact of zinc deficiency as well as of zinc supplementation on ETEC toxins.
FIG 7.
Zinc supplementation decreases intestinal colonization by the ETEC LT mutant of H010407 (LTKO) in zinc-deficient infected C57/BL6 mice. Zinc-deficient mice received supplemental zinc sulfate in drinking water (150 mg/liter) starting from day 12 post-ETEC infection. Quantitative PCR was performed using DNA samples that had been extracted from stool samples or intestine samples from infected mice. (A) Effect of zinc supplementation on stool ETEC LTKO shedding after 13 days of zinc treatment (day 25 postinfection). (B) Effect of zinc supplementation on colon and ileum ETEC LTKO colonization after 13 days of zinc treatment (day 25 postinfection) (n = 4/group).
In conclusion, we describe a robust C57BL/6 mouse model of ETEC infection with measurable outcomes of diarrhea, weight loss, stool shedding, tissue burden, and fecal biomarkers. This model will be useful in vaccine development as well as for further investigation into the impact of dietary deficiencies such as zinc deficiencies or of novel interventions on ETEC disease outcomes.
DISCUSSION
The need for robust and affordable animal models for studying enteric pathogens is urgent for developing and evaluating novel interventions, including therapeutics or vaccines. ETEC plays a major role in the burden of moderate to severe diarrhea as recently reported in a large multicenter study in developing countries (GEMS) (3). The potential scope of its role in milder diarrhea in community-based studies has been extended in the multicountry MAL-ED study as well (2).
Considering the frequency of causation of mild to severe diarrhea by ETEC worldwide and, from the EAEC experience, its potential to obtain additional virulence traits (23, 24), it is of critical importance that it remain a focus of vaccine development. This illustrates the need for a consistently reproducible small-animal model to test therapeutic interventions and vaccines. In this report, we demonstrate a C57BL/6 mouse model of enteropathy, with or without acute, watery diarrhea, with oral infections with ETEC strain H10407, which expresses both ST and LT as well as the CFA/1 colonization factor.
Other groups have previously used murine models as an approach for evaluating ETEC pathogenesis (25, 26). However, none have evaluated the effects of undernutrition on infection susceptibility and outcomes. Here, we examined weight, biomarker, and diarrhea outcomes with ETEC infection in the context of protein and zinc deficiencies, two common deficiencies in children in developing countries (27, 28). Differential nutritional status can modulate intestinal microbiome, metabolome, and immune responses (29–31), although these effects are currently being defined only in the context of enteric infections. Furthermore, undernourished children in low-resource settings are associated with poor vaccine responses but the mechanisms of the responses are not fully understood (32).
Antibiotics are widely used to disrupt intestinal microbiota and induce susceptibility to infection and to develop animal models for several enteropathogens in murine and other systems (33–35). Allen and colleagues reported the use of streptomycin in drinking water for a mouse model of ETEC infection, in addition to the use of cimetidine to reduce gastric acidity. Interestingly, they observed only intestinal colonization (24 h after infection) with no signs of disease or of an inflammatory response (25). In our study, we used an antibiotic cocktail which has been used for other enteropathogen mouse models by us and other groups (21, 36). This approach allowed us to see significant disease outcomes after ETEC infection and robust stool shedding for at least 5 days or longer postinfection. Different antibiotic treatments likely lead to different susceptibility conditions for the host (37). ETEC infection without antibiotic pretreatment did not induce infection or disease even in undernourished mice.
In recent years, we have gained better appreciation of the potential impacts and physiologic effects of enteric infections beyond just transient overt diarrhea (2, 20, 38). To these clinical studies, we can now add an experimental model of ETEC infections that includes growth impairment and intestinal inflammation in addition to overt watery diarrhea. Regarding growth, it is now recognized that infections by several enteropathogens, such as enteroaggregative E. coli, Campylobacter jejuni, and Giardia sp., can be associated with deficits in growth and, potentially, in cognitive development, even without causing diarrhea (39–42). Intestinal inflammation likely plays an important role in these effects (43). Although a neonatal mouse model showed death outcomes within 24 h postinfection (44), our weaned-mouse model and dietary differences can model acutely symptomatic as well as “silent” (with respect to overt diarrhea) ETEC infections given orally. Other published ETEC models that showed disease consequences (i.e., not just intestinal colonization) did not use oral administration of the bacterial inoculum (26, 45), limiting their direct clinical relevance. The model we describe here closely reflects the route of infection and potentially relevant outcomes of ETEC infection seen in humans. The growth impairment and diarrhea induced by oral administration of ETEC strain H10407 represent robust outcomes that match those specific consequences from clinical studies of ETEC infections (3, 46, 47).
In an effort to establish an affordable and reproducible ETEC infection model, we thought it important to demonstrate colonization and diarrhea in mice fed a standard rodent chow diet (HC). Unlike the dPD and dZD diets, the standard rodent chow is a very common diet produced by Harlan Teklad that is used by Jackson Laboratories. We have also used a defined protein source control diet (Research Diets), which produced results similar to those produced by the HC model (data not shown). As demonstrated previously (21), feeding with a zinc-free protein source (e.g., egg white protein) diet for 2 weeks is required in order to achieve “clinical” zinc deficiency. While this is not nearly as affordable or rapid a model as the HC model, it has been very useful in determining the effects of zinc deficiency on host microbiome and metabolome (29) as well as the effects of zinc deficiency and subsequent zinc supplementation on virulence traits of diarrheagenic bacteria (21, 22). Interestingly, we have seen only modest alterations in resident microbiotas of mice in the 2-week period of dZD feeding required to generate zinc deficiency (29). However, note that we have not extensively investigated the effects of our antibiotic cocktail (required for colonization in this model) on resident microbiota or metabolome or whether these effects are altered by protein or zinc deficiencies. Studies to address these important issues are under way.
In the current study, infected protein-deficient mice were the only infected mice that exhibited growth deficits in the absence of overt diarrhea. In addition, the greatest growth impairments induced by ETEC were seen with nourished but zinc-deficient mice. Increased fecal MPO and LCN-2 levels were seen for all infected groups regardless of the diets used. However, the strongest induction was seen with zinc-deficient mice. Both host and pathogen mechanisms affected by zinc deficiency might contribute to this outcome. Interestingly, when zinc was provided to mice in the zinc-deficient infected groups, the mice underwent catchup growth. This finding corroborates clinical studies that noted the growth-promoting effects of zinc (48) and further helps to model the benefits underlying the use of zinc for treatment of infectious diarrhea in zinc-deficient subjects.
Previous studies by our group and others have shown the antivirulence effects of zinc on several bacteria, including diarrheagenic E. coli (22, 49, 50). In the context of ETEC, zinc was already shown to inhibit ion secretion induced by LT through targeting the cyclic AMP-stimulated potassium channel (51). In addition, zinc was shown to inhibit ETEC K88 adhesion in vitro (52). However, the influence of zinc on ETEC virulence gene expression is still unclear. In order to test this, we assessed virulence gene expression of the ETEC H10407 strain incubated with a range of subinhibitory and inhibitory concentrations of zinc in vitro. Downregulation of the CFA/I colonization factor was observed.
Expression levels of the cfaI and cexE genes were decreased by administration of zinc in a dose-dependent manner. Interestingly, both genes are regulated by cfaD, a homolog of aggR in EAEC, a major transcriptional regulator. These findings suggest that zinc acts on ETEC virulence gene expression in a manner similar to that in which it influences EAEC virulence gene expression, as previously described (53). However, while zinc decreased ST gene expression, it did not decrease LT gene expression. This result shows that ST expression is more altered by zinc than LT expression (which tended to actually be increased with low zinc levels) and might help to explain our in vivo observations. Nourished mice infected by LTKO had delayed disease compared to the wild-type infection, suggesting an impaired ability of ETEC to colonize the intestinal epithelium in early infection (∼1 to ∼5 days pi), although the level of impairment was not maintained throughout the experiment. Other in vivo models have shown the importance of LT for colonization (25, 45). LT was also important for inducing fecal MPO, and the data further corroborate the importance of LT for ETEC pathogenesis. Interestingly, the influence of LT on the ETEC zinc deficiency model was not evident regarding clinical outcomes and is likely due to the significant impact of zinc deficiency on ST and potentially on other virulence traits in the pathogen and possibly in part on host immune responses (21, 54). However, zinc supplementation decreased colonization in mice infected with LTKO but not in the ETEC-wild-type-infected mice, which also suggested that zinc can even increase expression of LT. Specifically, ETEC strains producing ST are associated with moderate to severe diarrhea and increased risk of death in children (2), and this effect may account for the decreased diarrhea outcomes associated with zinc supplementation (55). On the other hand, LT− ETEC may be associated with environmental enteropathy, as suggested by in vitro studies (56). Our results suggest that the consequences of infections by ETEC strains, including both ST− ETEC and LT− ETEC strains, especially in malnourished children, warrant further assessment.
In conclusion, our findings demonstrate the establishment of a new mouse model of ETEC infection that shows growth impairment, diarrhea, and intestinal inflammation across different host nutritional states, which are features commonly seen in children in developing areas. Zinc is clearly an important determinant of clinical outcomes and differentially influences ETEC gene virulence expression. The clinically relevant outcomes seen in this murine model, which mimic several outcomes in children with ETEC infections, help advance our understanding of ETEC pathobiology and provide key tools for testing of new interventions, such as potential vaccine candidates.
MATERIALS AND METHODS
Animal husbandry.
This study included the use of mice. This study was carried out in strict accordance with the recommendations included in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Committee on the Ethics of Animal Experiments of the University of Virginia (protocol number 3315). All efforts were made to minimize suffering. This protocol was approved by the Institutional Animal Care and Use Committee of the University of Virginia and is in accordance with their policies. The University of Virginia is accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care, International (AAALAC). Mice used in this study were male 22-day-old C57BL/6 strain mice from Jackson Laboratories (Bar Harbor, ME). The mice weighed approximately 11 g on arrival and were cohoused in groups with up to five animals per cage. The vivarium was kept at a temperature of between 68 and 74°F, with 14-h light and 10-h dark cycles.
Rodent diet.
Weaned mice (22 days old) were acclimated, fed a regular diet for 2 to 5 days, and then fed a Harlan Laboratories (Indianapolis, Indiana) standard rodent “house chow” (HC) diet, a defined protein source diet without zinc (dZD), or a defined reduced-protein diet (2%) (dPD) from Research Diets (New Brunswick, New Jersey). All diets were isocaloric, and calories from fat, protein, and carbohydrates were as previously reported (29). We have previously demonstrated serum and tissue zinc deficiency in mice fed the dZD diet for 14 days (21). For experiments in which zinc supplementation was employed, zinc sulfate was dissolved in water and filtered before being provided in drinking water at 150 mg/liter. This concentration was based on the estimated dose/weight approximation equivalence of the U.S. recommended daily allowance for zinc for children (57).
Antibiotic disruption of native flora.
Four-week-old C57Bl/6J mice were placed on a standard rodent chow (HC) diet, a zinc-deficient diet (dZD), or a protein-deficient diet (dPD) for 2 weeks, following a 2-day acclimation period upon arrival. On day 10 of diet feeding, mice were given gentamicin (35 mg/liter), vancomycin (45 mg/liter), metronidazole (215 mg/liter), and colistin (850 U/ml) in drinking water to disrupt resident microbiota as previously published (21, 33). After 3 days on antibiotics in water, mice were given untreated water for 1 day and then were given a single oral challenge by gavage of 109 CFU ETEC–100 μl Dulbecco's modified Eagle's medium (DMEM) or DMEM without bacteria as a control (described below).
ETEC infection.
Enterotoxigenic Escherichia coli (H10407, a prototype strain that produces both ST and LT) was originally isolated from a patient with severe, cholera-like watery diarrhea in Bangladesh (7, 53). Cultures were grown from glycerol stocks in DMEM at 37°C in a shaking incubator. Each infected mouse received an inoculum of ∼1 × 109 CFU ETEC in 100 μl of freshly prepared DMEM; controls received 100 μl of DMEM alone.
In this study, we used the following six groups for each experiment: mice fed HC with or without ETEC, mice fed dPD with or without ETEC, and mice fed dZD with or without ETEC. Each experiment used 8 mice per experimental group, and mice were euthanized at either day 3 or day 10 postinfection.
Protein extraction from stool and tissue.
After rapid dissection of the mouse intestines, cecal contents and stool samples were flash frozen in liquid nitrogen (LN2). At the time of assay, samples were lysed in radioimmunoprecipitation assay (RIPA) buffer (20 mM Tris [pH 7.5], 150 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 1 mM EDTA, 0.1% SDS) containing protease inhibitor cocktail (Roche) and phosphatase inhibitors (1 mM sodium orthovanadate, 5 mM sodium fluoride, 1 mM microcystin-LR, and 5 mM beta-glycerophosphate). Tissue lysates were cleared by centrifugation, and the supernatant was used for total protein measurements, cytokine measurements by Luminex assay (Bio-Rad), and specific enzyme-linked immunosorbent assays (ELISAs) for evaluation of lipocalin-2 (LCN-2) and myeloperoxidase (MPO) levels as previously described (29).
DNA extraction.
DNA was isolated from fecal pellets using a QIAamp DNA stool minikit as previously described (58). DNA was extracted from frozen tissue samples using a QIAamp DNA tissue kit (Qiagen). To enhance extraction of the pathogen's DNA, we made an improvement in the original protocol, namely, vigorous homogenization of the samples with 300 mg of 1.0-mm-diameter zirconia beads (BioSpec, Bartlesville, OK, USA) using a Mini-BeadBeater (BioSpec, Bartlesville, OK, USA). DNA from cecum or stool content was extracted from the thawed stool samples using a QIAamp DNA stool kit (Qiagen), following the manufacturer's instructions. After extraction, DNA was eluted in 200 μl elution buffer and stored at −20°C.
Real-time PCR for ETEC quantification.
Stool DNA and tissue were analyzed for the ETEC-specific LTb and STh genes to determine the levels of shedding of the organism in stool and the burden in the tissue as described by Liu et al. (15).
Quantification of the infection was performed in a Bio-Rad CFX PCR detection system by interpolating threshold cycle (CT) values of each run with a standard curve of known amounts of ETEC DNA, and the data were transformed into numbers of organisms per milligram of sample. The master mix solution and primers were used as described elsewhere (3). Amplification consisted of 3 min at 95°C, followed by 40 cycles of 10 s at 95°C and 30 s at 58°C.
In vitro gene expression analysis.
Total cellular RNA was obtained from ETEC cultures using RNeasy kits (Qiagen), and cDNA was synthesized from 1 μg RNA using iScript (Bio-Rad). For quantitative PCR analyses of virulence factor mRNA abundance, cDNA was diluted 1:8; 4 μl of this dilution was used for each PCR. Bioline Sensi-Fast Sybr reagent was used for quantitative PCRs. The primer sequences used are listed in Table S1 in the supplemental material. The PCR conditions were as follows: 95°C for 5 min followed by 40 cycles of 95°C for 15 s and 60°C for 30 s followed by a melt curve analysis. Data were analyzed and are presented based on the relative-expression method (59). The formula used for the calculation was as follows: relative expression = 2−(SΔCT − CΔCT), where ΔCT is the difference in threshold cycle values between the gene of interest (i.e., the LTb gene) and the control gene (i.e., the 16S gene). In this formula, “S” represents E. coli-challenged mice and “C” represents uninfected mice.
Statistical analysis.
Data analyses were performed with GraphPad Prism 6 software (GraphPad Software). All statistical analyses were done from raw data with the use of analysis of variance, Student t tests, and Bonferroni post hoc analysis where applicable. Differences were considered significant at a P value of <0.05. Data are represented as means ± standard errors of the means.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported in part by Opportunity ID OPP1137923 (Host, pathogen and pathogen interaction determinants of environmental enteric dysfunction) from the Bill & Melinda Gates Foundation and in part by NIH award U19 AI109776 (CETR [Centers for Excellence for Translational Research]; principal investigator [PI], Myron Levine) from the National Institute of Allergy and Infectious Diseases (NIAID). S.E.L. was supported by the Global Infectious Diseases Training (GIDRT) grant D43 TW006578 from the Fogarty International Center at NIH.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00183-18.
REFERENCES
- 1.GBD 2013 Risk Factors Collaborators, Forouzanfar MH, Alexander L, Anderson HR, Bachman VF, Biryukov S, Brauer M, Burnett R, Casey D, Coates MM, Cohen A, Delwiche K, Estep K, Frostad JJ, Astha KC, Kyu HH, Moradi-Lakeh M, Ng M, Slepak EL, Thomas BA, Wagner J, Aasvang GM, Abbafati C, Abbasoglu Ozgoren A, Abd-Allah F, Abera SF, Aboyans V, Abraham B, Abraham JP, Abubakar I, Abu-Rmeileh NM, Aburto TC, Achoki T, Adelekan A, Adofo K, Adou AK, Adsuar JC, Afshin A, Agardh EE, Al Khabouri MJ, Al Lami FH, Alam SS, Alasfoor D, Albittar MI, Alegretti MA, Aleman AV, Alemu ZA, Alfonso-Cristancho R, Alhabib S, Ali R, et al. 11 September 2015. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet doi: 10.1016/S0140-6736(15)00128-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Platts-Mills JA, Babji S, Bodhidatta L, Gratz J, Haque R, Havt A, McCormick BJ, McGrath M, Olortegui MP, Samie A, Shakoor S, Mondal D, Lima IF, Hariraju D, Rayamajhi BB, Qureshi S, Kabir F, Yori PP, Mufamadi B, Amour C, Carreon JD, Richard SA, Lang D, Bessong P, Mduma E, Ahmed T, Lima AA, Mason CJ, Zaidi AK, Bhutta ZA, Kosek M, Guerrant RL, Gottlieb M, Miller M, Kang G, Houpt ER, MAL-ED Network Investigators. 2015. Pathogen-specific burdens of community diarrhoea in developing countries: a multisite birth cohort study (MAL-ED). Lancet Global Health 3:e564–e575. doi: 10.1016/S2214-109X(15)00151-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, Faruque AS, Zaidi AK, Saha D, Alonso PL, Tamboura B, Sanogo D, Onwuchekwa U, Manna B, Ramamurthy T, Kanungo S, Ochieng JB, Omore R, Oundo JO, Hossain A, Das SK, Ahmed S, Qureshi S, Quadri F, Adegbola RA, Antonio M, Hossain MJ, Akinsola A, Mandomando I, Nhampossa T, Acacio S, Biswas K, O'Reilly CE, Mintz ED, Berkeley LY, Muhsen K, Sommerfelt H, Robins-Browne RM, Levine MM. 2013. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 382:209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 4.Lamberti LM, Bourgeois AL, Fischer Walker CL, Black RE, Sack D. 2014. Estimating diarrheal illness and deaths attributable to Shigellae and enterotoxigenic Escherichia coli among older children, adolescents, and adults in South Asia and Africa. PLoS Negl Trop Dis 8:e2705. doi: 10.1371/journal.pntd.0002705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang ZD, DuPont HL. 2017. Etiology of travellers' diarrhea. J Travel Med 24:S13–S16. doi: 10.1093/jtm/tax003. [DOI] [PubMed] [Google Scholar]
- 6.Goldsmith R. 1973. Delhi belly, Hong Kong dog, Casablanca crud, Montezuma's revenge, Turista. Calif Med 118:37–38. [PMC free article] [PubMed] [Google Scholar]
- 7.Sack RB, Gorbach SL, Banwell JG, Jacobs B, Chatterjee BD, Mitra RC. 1971. Enterotoxigenic Escherichia coli isolated from patients with severe cholera-like disease. J Infect Dis 123:378–385. doi: 10.1093/infdis/123.4.378. [DOI] [PubMed] [Google Scholar]
- 8.Skrede S, Steinsland H, Sommerfelt H, Aase A, Brandtzaeg P, Langeland N, Cox RJ, Saevik M, Wallevik M, Skutlaberg DH, Tellevik MG, Sack DA, Nataro JP, Guttormsen AB. 2014. Experimental infection of healthy volunteers with enterotoxigenic Escherichia coli wild-type strain TW10598 in a hospital ward. BMC Infect Dis 14:482. doi: 10.1186/1471-2334-14-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guth BEC. 2000. Enterotoxigenic Escherichia coli—an overview. Mem Inst Oswaldo Cruz 95(Suppl 1):95–97. doi: 10.1590/S0074-02762000000700017. [DOI] [PubMed] [Google Scholar]
- 10.Guerrant RL, Ganguly U, Casper AG, Moore EJ, Pierce NF, Carpenter CC. 1973. Effect of Escherichia coli on fluid transport across canine small bowel. Mechanism and time-course with enterotoxin and whole bacterial cells. J Clin Invest 52:1707–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hughes JM, Murad F, Chang B, Guerrant RL. 1978. Role of cyclic GMP in the action of heat-stable enterotoxin of Escherichia coli. Nature 271:755–756. doi: 10.1038/271755a0. [DOI] [PubMed] [Google Scholar]
- 12.Sears CL, Kaper JB. 1996. Enteric bacterial toxins: mechanisms of action and linkage to intestinal secretion. Microbiol Rev 60:167–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.So M, Boyer HW, Betlach M, Falkow S. 1976. Molecular cloning of an Escherichia coli plasmid determinant than encodes for the production of heat-stable enterotoxin. J Bacteriol 128:463–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaper JB, Nataro JP, Mobley HL. 2004. Pathogenic Escherichia coli. Nat Rev Microbiol 2:123–140. doi: 10.1038/nrmicro818. [DOI] [PubMed] [Google Scholar]
- 15.Liu J, Gratz J, Amour C, Kibiki G, Becker S, Janaki L, Verweij JJ, Taniuchi M, Sobuz SU, Haque R, Haverstick DM, Houpt ER. 2013. A laboratory-developed TaqMan Array Card for simultaneous detection of 19 enteropathogens. J Clin Microbiol 51:472–480. doi: 10.1128/JCM.02658-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steffen R, Cramer JP, Burchard G, Jelinek T, Schwarz U, Ramdas P, Chatterjee S, Jiang ZD, DuPont HL, Dewasthaly S, Westritschnig K, Behrens RH. 2013. Efficacy of a travelers' diarrhea vaccine system in travelers to India. J Travel Med 20:374–379. doi: 10.1111/jtm.12064. [DOI] [PubMed] [Google Scholar]
- 17.Taxt A, Aasland R, Sommerfelt H, Nataro J, Puntervoll P. 2010. Heat-stable enterotoxin of enterotoxigenic Escherichia coli as a vaccine target. Infect Immun 78:1824–1831. doi: 10.1128/IAI.01397-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu M, Ruan X, Zhang C, Lawson SR, Knudsen DE, Nataro JP, Robertson DC, Zhang W. 2011. Heat-labile- and heat-stable-toxoid fusions (LTRG-STaPF) of human enterotoxigenic Escherichia coli elicit neutralizing antitoxin antibodies. Infect Immun 79:4002–4009. doi: 10.1128/IAI.00165-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prata MM, Havt A, Bolick DT, Pinkerton R, Lima A, Guerrant RL. 2016. Comparisons between myeloperoxidase, lactoferrin, calprotectin and lipocalin-2, as fecal biomarkers of intestinal inflammation in malnourished children. J Transl Sci 2:134–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guerrant RL, Leite AM, Pinkerton R, Medeiros PH, Cavalcante PA, DeBoer M, Kosek M, Duggan C, Gewirtz A, Kagan JC, Gauthier AE, Swann J, Mayneris-Perxachs J, Bolick DT, Maier EA, Guedes MM, Moore SR, Petri WA, Havt A, Lima IF, Prata MM, Michaleckyj JC, Scharf RJ, Sturgeon C, Fasano A, Lima AA. 2016. Biomarkers of environmental enteropathy, inflammation, stunting, and impaired growth in children in northeast Brazil. PLoS One 11:e0158772. doi: 10.1371/journal.pone.0158772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bolick DT, Kolling GL, Moore JH II, de Oliveira LA, Tung K, Philipson C, Viladomiu M, Hontecillas R, Bassaganya-Riera J, Guerrant RL. 2014. Zinc deficiency alters host response and pathogen virulence in a mouse model of enteroaggregative Escherichia coli-induced diarrhea. Gut Microbes 5:618–627. doi: 10.4161/19490976.2014.969642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Medeiros P, Bolick DT, Roche JK, Noronha F, Pinheiro C, Kolling GL, Lima A, Guerrant RL. 19 August 2013. The micronutrient zinc inhibits EAEC strain 042 adherence, biofilm formation, virulence gene expression, and epithelial cytokine responses benefiting the infected host. Virulence doi: 10.4161/viru.26120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rasko DA, Webster DR, Sahl JW, Bashir A, Boisen N, Scheutz F, Paxinos EE, Sebra R, Chin CS, Iliopoulos D, Klammer A, Peluso P, Lee L, Kislyuk AO, Bullard J, Kasarskis A, Wang S, Eid J, Rank D, Redman JC, Steyert SR, Frimodt-Moller J, Struve C, Petersen AM, Krogfelt KA, Nataro JP, Schadt EE, Waldor MK. 2011. Origins of the E. coli strain causing an outbreak of hemolytic-uremic syndrome in Germany. N Engl J Med 365:709–717. doi: 10.1056/NEJMoa1106920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scheutz F, Nielsen EM, Frimodt-Moller J, Boisen N, Morabito S, Tozzoli R, Nataro JP, Caprioli A. 2011. Characteristics of the enteroaggregative Shiga toxin/verotoxin-producing Escherichia coli O104:H4 strain causing the outbreak of haemolytic uraemic syndrome in Germany, May to June 2011. Euro Surveill 16:19889. doi: 10.2807/ese.16.24.19889-en. [DOI] [PubMed] [Google Scholar]
- 25.Allen KP, Randolph MM, Fleckenstein JM. 2006. Importance of heat-labile enterotoxin in colonization of the adult mouse small intestine by human enterotoxigenic Escherichia coli strains. Infect Immun 74:869–875. doi: 10.1128/IAI.74.2.869-875.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ren W, Yin J, Duan J, Liu G, Zhu X, Chen S, Li T, Wang S, Tang Y, Hardwidge PR. 2014. Mouse intestinal innate immune responses altered by enterotoxigenic Escherichia coli (ETEC) infection. Microbes Infect 16:954–961. doi: 10.1016/j.micinf.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 27.Guerrant RL, Oria RB, Moore SR, Oria MO, Lima AA. 2008. Malnutrition as an enteric infectious disease with long-term effects on child development. Nutr Rev 66:487–505. doi: 10.1111/j.1753-4887.2008.00082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Young GP, Mortimer EK, Gopalsamy GL, Alpers DH, Binder HJ, Manary MJ, Ramakrishna BS, Brown IL, Brewer TG. 2014. Zinc deficiency in children with environmental enteropathy—development of new strategies: report from an expert workshop. Am J Clin Nutr 100:1198–1207. doi: 10.3945/ajcn.113.075036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mayneris-Perxachs J, Bolick DT, Leng J, Medlock GL, Kolling GL, Papin JA, Swann JR, Guerrant RL. 12 October 2016. Protein- and zinc-deficient diets modulate the murine microbiome and metabolic phenotype. Am J Clin Nutr doi: 10.3945/ajcn.116.131797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bolick DT, Mayneris-Perxachs J, Medlock GL, Kolling GL, Papin J, Swann JR, Guerrant RL. 17 May 2017. Increased urinary trimethylamine N-oxide (TMAO) following Cryptosporidium infection and protein malnutrition independent of microbiome effects. J Infect Dis doi: 10.1093/infdis/jix234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bartelt LA, Bolick DT, Mayneris-Perxachs J, Kolling GL, Medlock GL, Zaenker EI, Donowitz J, Thomas-Beckett RV, Rogala A, Carroll IM, Singer SM, Papin J, Swann JR, Guerrant RL. 2017. Cross-modulation of pathogen-specific pathways enhances malnutrition during enteric co-infection with Giardia lamblia and enteroaggregative Escherichia coli. PLoS Pathog 13:e1006471. doi: 10.1371/journal.ppat.1006471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoest C, Seidman JC, Pan W, Ambikapathi R, Kang G, Kosek M, Knobler S, Mason CJ, Miller M, MAL-ED Network Investigators . 2014. Evaluating associations between vaccine response and malnutrition, gut function, and enteric infections in the MAL-ED cohort study: methods and challenges. Clin Infect Dis 59(Suppl 4):S273–279. doi: 10.1093/cid/ciu611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen X, Katchar K, Goldsmith JD, Nanthakumar N, Cheknis A, Gerding DN, Kelly CP. 2008. A mouse model of Clostridium difficile-associated disease. Gastroenterology 135:1984–1992. doi: 10.1053/j.gastro.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 34.Harp JA, Wannemuehler MW, Woodmansee DB, Moon HW. 1988. Susceptibility of germfree or antibiotic-treated adult mice to Cryptosporidium parvum. Infect Immun 56:2006–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iizumi T, Taniguchi T, Yamazaki W, Vilmen G, Alekseyenko AV, Gao Z, Perez Perez GI, Blaser MJ. 2016. Effect of antibiotic pre-treatment and pathogen challenge on the intestinal microbiota in mice. Gut Pathog 8:60. doi: 10.1186/s13099-016-0143-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen P, Soares AM, Lima AA, Gamble MV, Schorling JB, Conway M, Barrett LJ, Blaner WS, Guerrant RL. 2003. Association of vitamin A and zinc status with altered intestinal permeability: analyses of cohort data from northeastern Brazil. J Health Popul Nutr 21:309–315. [PubMed] [Google Scholar]
- 37.Vogt SL, Finlay BB. 2017. Gut microbiota-mediated protection against diarrheal infections. J Travel Med 24:S39–S43. doi: 10.1093/jtm/taw086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kosek MN, Lee GO, Guerrant RL, Haque R, Kang G, Ahmed T, Bessong P, Ali A, Mduma E, Peñataro Yori P, Faubion WA, Lima AAM, Paredes Olortegui M, Mason C, Babji S, Singh R, Qureshi S, Kosek PS, Samie A, Pascal J, Shrestha S, McCormick BJJ, Seidman JC, Lang DR, Zaidi A, Caulfield LE, Gottlieb M; MAL-ED Network. 1 July 2017. Age and sex normalization of intestinal permeability measures for the improved assessment of enteropathy in infancy and early childhood: results from the MAL-ED Study. J Pediatr Gastroenterol Nutr doi: 10.1097/MPG.0000000000001610. [DOI] [PubMed] [Google Scholar]
- 39.Amour C, Gratz J, Mduma E, Svensen E, Rogawski ET, McGrath M, Seidman JC, McCormick BJ, Shrestha S, Samie A, Mahfuz M, Qureshi S, Hotwani A, Babji S, Trigoso DR, Lima AA, Bodhidatta L, Bessong P, Ahmed T, Shakoor S, Kang G, Kosek M, Guerrant RL, Lang D, Gottlieb M, Houpt ER, Platts-Mills JA, tiology, Risk Factors, and Interactions of Enteric Infections and Malnutrition and the Consequences for Child Health and Development Project (MAL-ED). 2016. Epidemiology and impact of Campylobacter infection in children in 8 low-resource settings: results from the MAL-ED Study. Clin Infect Dis 63:1171–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guerrant RL, DeBoer MD, Moore SR, Scharf RJ, Lima AA. 2013. The impoverished gut–a triple burden of diarrhoea, stunting and chronic disease. Nat Rev Gastroenterol Hepatol 10:220–229. doi: 10.1038/nrgastro.2012.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nataro JP, Guerrant RL. 23 May 2017. Chronic consequences on human health induced by microbial pathogens: growth faltering among children in developing countries. Vaccine doi: 10.1016/j.vaccine.2017.05.035. [DOI] [PubMed] [Google Scholar]
- 42.Niehaus MD, Moore SR, Patrick PD, Derr LL, Lorntz B, Lima AA, Guerrant RL. 2002. Early childhood diarrhea is associated with diminished cognitive function 4 to 7 years later in children in a northeast Brazilian shantytown. Am J Trop Med Hyg 66:590–593. doi: 10.4269/ajtmh.2002.66.590. [DOI] [PubMed] [Google Scholar]
- 43.Kosek MN, MAL-ED Network Investigators. 2017. Causal pathways from enteropathogens to environmental enteropathy: findings from the MAL-ED Birth Cohort Study. EBioMedicine 18:109–117. doi: 10.1016/j.ebiom.2017.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guevara CP, Luiz WB, Sierra A, Cruz C, Qadri F, Kaushik RS, Ferreira LC, Gomez-Duarte OG. 2013. Enterotoxigenic Escherichia coli CS21 pilus contributes to adhesion to intestinal cells and to pathogenesis under in vivo conditions. Microbiology 159:1725–1735. doi: 10.1099/mic.0.065532-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kumar A, Hays M, Lim F, Foster LJ, Zhou M, Zhu G, Miesner T, Hardwidge PR. 2015. Protective enterotoxigenic Escherichia coli antigens in a murine intranasal challenge model. PLoS Negl Trop Dis 9:e0003924. doi: 10.1371/journal.pntd.0003924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Black RE, Brown KH, Becker S. 1984. Effects of diarrhea associated with specific enteropathogens on the growth of children in rural Bangladesh. Pediatrics 73:799–805. [PubMed] [Google Scholar]
- 47.Lee G, Paredes Olortegui M, Penataro Yori P, Black RE, Caulfield L, Banda Chavez C, Hall E, Pan WK, Meza R, Kosek M. 2014. Effects of Shigella-, Campylobacter- and ETEC-associated diarrhea on childhood growth. Pediatr Infect Dis J 33:1004–1009. doi: 10.1097/INF.0000000000000351. [DOI] [PubMed] [Google Scholar]
- 48.Mayo-Wilson E, Junior JA, Imdad A, Dean S, Chan XH, Chan ES, Jaswal A, Bhutta ZA. 2014. Zinc supplementation for preventing mortality, morbidity, and growth failure in children aged 6 months to 12 years of age. Cochrane Database Syst Rev 2014:CD009384. doi: 10.1002/14651858.CD009384.pub2. [DOI] [PubMed] [Google Scholar]
- 49.Mellies JL, Thomas K, Turvey M, Evans NR, Crane J, Boedeker E, Benison GC. 2012. Zinc-induced envelope stress diminishes type III secretion in enteropathogenic Escherichia coli. BMC Microbiol 12:123. doi: 10.1186/1471-2180-12-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Crane JK, Broome JE, Reddinger RM, Werth BB. 2014. Zinc protects against Shiga-toxigenic Escherichia coli by acting on host tissues as well as on bacteria. BMC Microbiol 14:145. doi: 10.1186/1471-2180-14-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hoque KM, Rajendran VM, Binder HJ. 2005. Zinc inhibits cAMP-stimulated Cl secretion via basolateral K-channel blockade in rat ileum. Am J Physiol Gastrointest Liver Physiol 288:G956–G963. doi: 10.1152/ajpgi.00441.2004. [DOI] [PubMed] [Google Scholar]
- 52.Roselli M, Finamore A, Garaguso I, Britti MS, Mengheri E. 2003. Zinc oxide protects cultured enterocytes from the damage induced by Escherichia coli. J Nutr 133:4077–4082. doi: 10.1093/jn/133.12.4077. [DOI] [PubMed] [Google Scholar]
- 53.Evans DG, Silver RP, Evans DJ Jr, Chase DG, Gorbach SL. 1975. Plasmid-controlled colonization factor associated with virulence in Esherichia coli enterotoxigenic for humans. Infect Immun 12:656–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Prasad AS. 2007. Zinc: mechanisms of host defense. J Nutr 137:1345–1349. doi: 10.1093/jn/137.5.1345. [DOI] [PubMed] [Google Scholar]
- 55.Lazzerini M, Wanzira H. 2016. Oral zinc for treating diarrhoea in children. Cochrane Database Syst Rev 12:CD005436. doi: 10.1002/14651858.CD005436.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kreisberg RB, Harper J, Strauman MC, Marohn M, Clements JD, Nataro JP. 2011. Induction of increased permeability of polarized enterocyte monolayers by enterotoxigenic Escherichia coli heat-labile enterotoxin. Am J Trop Med Hyg 84:451–455. doi: 10.4269/ajtmh.2011.10-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gibson RS, King JC, Lowe N. 2016. A review of dietary zinc recommendations. Food Nutr Bull 37:443–460. doi: 10.1177/0379572116652252. [DOI] [PubMed] [Google Scholar]
- 58.Liu J, Bolick DT, Kolling GL, Fu Z, Guerrant RL. 2016. Protein malnutrition impairs intestinal epithelial cell turnover, a potential mechanism of increased cryptosporidiosis in a murine model. Infect Immun 84:3542–3549. doi: 10.1128/IAI.00705-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.