ABSTRACT
Iron is an essential micronutrient for most microbes and their hosts. Mammalian hosts respond to infection by inducing the iron-regulatory hormone hepcidin, which causes iron sequestration and a rapid decrease in the plasma and extracellular iron concentration (hypoferremia). Previous studies showed that hepcidin regulation of iron is essential for protection from infection-associated mortality with the siderophilic pathogens Yersinia enterocolitica and Vibrio vulnificus. However, the evolutionary conservation of the hypoferremic response to infection suggests that not only rare siderophilic bacteria but also common pathogens may be targeted by this mechanism. We tested 10 clinical isolates of Escherichia coli from children with sepsis and found that both genetic iron overload (by hepcidin-1 knockout [HKO]) and iatrogenic iron overload (by intravenous iron) potentiated infection with 8 out of the 10 studied isolates: after peritoneal injection of E. coli, iron-loaded mice developed sepsis with 60% to 100% mortality within 24 h, while control wild-type mice suffered 0% mortality. Using one strain for more detailed study, we show that iron overload allows rapid bacterial multiplication and dissemination. We further found that the presence of non-transferrin-bound iron (NTBI) in the circulation is more important than total plasma or tissue iron in rendering mice susceptible to infection and mortality. Postinfection treatment of HKO mice with just two doses of the hepcidin agonist PR73 abolished NTBI and completely prevented sepsis-associated mortality. We demonstrate that the siderophilic phenotype extends to clinically common pathogens. The use of hepcidin agonists promises to be an effective early intervention in patients with infections and dysregulated iron metabolism.
KEYWORDS: iron, infection, hepcidin, Escherichia coli, sepsis, NTBI
INTRODUCTION
Iron is an essential trace element required for multiple vital functions, such as oxygen transport, cellular respiration, DNA replication and repair, and cell proliferation and differentiation (1). Consequently, iron is critical for the survival of nearly all organisms, including microbial pathogens. Host defense mechanisms evolved to target the iron dependence of invading microbes during infections, with multiple mechanisms acting to sequester iron from microbes (2). Hepcidin is a hepatic peptide hormone that acts as the principal regulator of iron homeostasis and orchestrates iron absorption and the tissue distribution of iron by inhibiting ferroportin-mediated cellular iron transport to plasma and extracellular fluid (3). Hepcidin production is strongly induced by interleukin-6 and other inflammatory signals (4), thus causing iron sequestration in enterocytes and macrophages and a rapid reduction in plasma iron levels. On the other side, pathogens have evolved to obtain iron from multiple sources even under limiting conditions, using such mechanisms as transferrin receptors (5); the breakdown of ferroproteins, including hemoglobin (6); and the secretion and reuptake of siderophores (7), which can steal iron from host molecules. However, these mechanisms, while effective, may impose a substantial metabolic burden on the invading pathogen (8). The battle for iron between the host and the invading pathogen can determine the outcome of infection (9).
Interventions or diseases that increase iron availability in the host have been linked to enhanced bacterial pathogenicity and increased susceptibility to infections (10–12). Iron supplementation in human trials in South Asia and Africa was associated with an increased risk of respiratory and gastrointestinal infections as well as malaria, leading to a greater severity of infection and even mortality (13–16). In addition, hereditary hemochromatosis patients, who are iron overloaded as a result of hepcidin deficiency, are highly susceptible to infections with several types of bacteria (17–20). Intramuscular iron dextran administration was associated with increased Escherichia coli sepsis in neonates (21).
We recently showed that hepcidin deficiency and iron overload dramatically increase infection-associated mortality in mouse models of infection with the siderophilic bacterium Yersinia enterocolitica (22) or Vibrio vulnificus (23). Studies with a common reference strain of Gram-negative bacteria, Klebsiella pneumoniae ATCC 43816, revealed similar results, although the effect of iron availability on survival after infection was less striking (24). It is therefore not clear whether the enhancement of pathogenicity by excess iron is applicable to clinically common bacteria that cause sepsis in humans.
In each of these infections, the experimental evidence implicates non-transferrin-bound iron (NTBI) as the iron species promoting infection. NTBI (25) appears in the circulation when the binding capacity of the principal plasma iron-transport protein transferrin is exceeded transiently or chronically, as seen under conditions such as hereditary hemochromatosis or β-thalassemia, acute and chronic liver failure, hematopoietic stem cell transplantation, chronic parenteral iron administration, and blood transfusions with older packed red blood cells (PRBCs) (11, 25, 26). The molecular forms of NTBI include mainly ferric citrate but also ferric salts of other organic acids and iron loosely bound to albumin.
E. coli is a Gram-negative pathogen which normally inhabits the gastrointestinal tract of humans and other animals without causing any adverse effects (27). However, it is also among the most common causes of infections in hospitalized patients, ranging from gastrointestinal infections and urinary tract infections to bacteremia and sepsis (28). Sepsis affects about 750,000 people in the United States every year and has a high mortality rate, reaching 30% to 50% (29). According to CDC, E. coli was the pathogen most commonly isolated from the blood of septic patients (30). Even though E. coli is not considered a classical siderophilic pathogen, there are a few reports suggesting a correlation between iron overload and susceptibility to E. coli infection (31, 32), but the mechanistic connection has not been further explored.
Using randomly selected clinical isolates of E. coli from pediatric patients with sepsis, we now show in mouse models that iron overload either from genetic hepcidin deficiency (hereditary hemochromatosis) or from intravenous (i.v.) iron supplementation increases the rate of sepsis-associated mortality. The pathogenicity of E. coli is enhanced not by tissue iron overload but by the generation of a specific iron species, NTBI.
We demonstrate that hepcidin has a critical role in host defense against E. coli infections by clearing NTBI and that acute postexposure treatment of susceptible hepcidin-1 knockout (HKO) mice with the hepcidin agonist PR73 abolishes NTBI and improves survival.
RESULTS
Hepcidin deficiency promotes susceptibility to E. coli sepsis.
Hepcidin-deficient mice had a much higher susceptibility to E. coli infection than wild-type (WT) mice (Table 1; see also Fig. S1 in the supplemental material). Most of the clinical E. coli isolates, 8 out of 10, caused mortality (60 to 100%) in naturally iron-loaded HKO (IL-HKO) mice within 24 h of infection at doses where none of the WT mice developed signs of disease (no significant weight loss, except after infection with isolates 3 and 10, where weight loss was minimal [Table S1], and no changes in appearance or activity). The remaining two E. coli isolates (isolates 9 and 10) were avirulent in mice, regardless of their iron status, since we observed very low mortality after infection of IL-HKO and WT mice even with high bacterial doses.
TABLE 1.
Hepcidin deficiency promotes susceptibility to infection with clinical E. coli isolatesa
| Isolate no. | Diagnosis | Ceftriaxone resistance | Dose (no. of CFU/mouse) | HKO mice |
WT mice |
P value | ||
|---|---|---|---|---|---|---|---|---|
| % mortality | No. of mice | % mortality | No. of mice | |||||
| 1 | Urosepsis | No | 104 | 100 | 6 | 0 | 6 | <0.001 |
| 2 | Liver failure | No | 104 | 100 | 6 | 0 | 6 | <0.001 |
| 3 | Leukemia | No | 104 | 100 | 5 | 0 | 6 | 0.002 |
| 4 | Sickle cell disease | Yes | 104 | 100 | 5 | 0 | 5 | 0.003 |
| 5 | Urosepsis | No | 104 | 100 | 5 | 0 | 5 | 0.003 |
| 6 | Congenital heart disease | No | 106 | 100 | 5 | 0 | 5 | 0.003 |
| 7 | Evan's syndrome | Yes | 106 | 100 | 5 | 0 | 3 | 0.012 |
| 8 | Kidney and liver transplant | No | 106 | 60 | 5 | 0 | 3 | 0.05 |
| 9 | Liver and bowel transplant | No | 106 | 30 | 4 | 0 | 3 | 0.386 |
| 10 | End-stage renal disease | Yes | 107b | 20 | 5 | 0 | 5 | 0.371 |
The clinical E. coli blood isolates were obtained from the Ronald Reagan UCLA Medical Center. WT mice suffered 0% mortality and did not develop disease signs, such as weight loss, a change in appearance, or decreased activity, after infection with any of the isolates at the described doses. However, for 8 out of 10 isolates, HKO mice suffered 60% to 100% mortality with the same inocula.
For isolate 10, we also tested a 5 × 107-CFU/mouse dose, and it caused 100% mortality in both WT and HKO mice.
Genetic as well as iatrogenic iron overload promotes sepsis-associated mortality in an E. coli infection model.
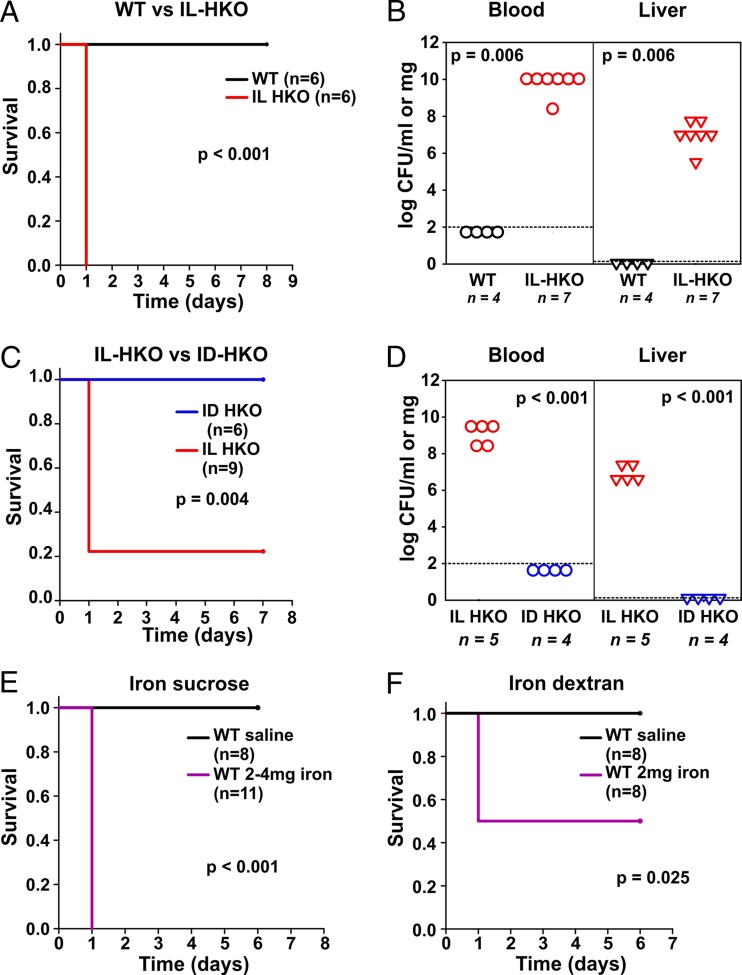
From among the five isolates that caused rapid mortality in mice at the 104-CFU inoculum, we randomly selected isolate 1 (a blood culture isolate from a patient with urosepsis) for more detailed characterization. Because they lack hepcidin, HKO mice develop spontaneous severe iron overload even when they are fed a standard diet, modeling hereditary hemochromatosis in human patients. As shown in Table 1, E. coli isolate 1 caused 100% mortality in IL-HKO mice, while none of the infected WT mice died (Fig. 1A). These results were validated using the WT littermates of HKO mice (data not shown). Further analysis showed that hepcidin deficiency and the associated iron overload promoted tissue bacterial dissemination: at 16 h after infection, WT mice had undetectable numbers of CFU, while naturally iron-loaded HKO mice had 1010 CFU/ml in their blood and 106 CFU/mg in their liver (Fig. 1B), indicating that the initial inoculum rapidly multiplied in the host. We then tested whether it was the iron overload rather than any other potential effect of hepcidin deficiency that promoted susceptibility to E. coli infection. In these experiments, iron-loaded HKO mice suffered 80% mortality within 24 h of intraperitoneal (i.p.) infection with 104 CFU of E. coli (isolate 1) per mouse. However, iron depletion of HKO mice completely prevented the mortality associated with infection with the same isolate (Fig. 1C), indicating that hepcidin deficiency promotes susceptibility to E. coli infection through iron overload. Accordingly, the bacterial burden in tissues and blood was very high for iron-loaded HKO (IL-HKO) mice, while bacteria were not detected in iron-depleted HKO (ID-HKO) mice (Fig. 1D).
FIG 1.
Genetic as well as iatrogenic iron overload enhances sepsis-associated mortality in a mouse model of E. coli infection (A and B) Survival (A) and the number of bacterial CFU in blood and liver (B) after infection of wild-type (WT) or naturally iron-loaded hepcidin-1 knockout (IL-HKO) mice with 104 CFU/mouse E. coli (isolate 1). Dashed line, limit of detection. (C and D) Survival (C) and number of bacterial CFU in blood and liver (D) after infection of IL-HKO or dietary iron-depleted HKO (ID-HKO) mice with 104 CFU of E. coli (isolate 1) per mouse. Dashed line, limit of detection. (E and F) Survival of WT mice that were injected i.v. with saline, 2 to 4 mg iron sucrose (E), or 2 mg iron dextran (F) and 4 h later infected i.p. with 104 CFU of E. coli (isolate 1) per mouse. The results of two separate experiments were combined for panels E and F. In the experiment whose results are shown in panel E, two different batches of iron sucrose were used and pretested prior to these experiments. In pilot experiments, the first batch promoted susceptibility to infection at 2 mg/mouse. In pilot studies with the second batch, 2 mg/mouse did not promote infection, while 4 mg/mouse did, consistent with the reported variability of rapid labile iron release from such preparations (44).
Intravenous (i.v.) iron administration for iron deficiency is used quite commonly in chronically ill hospitalized patients, especially if repletion is urgent or the oral route is not available because of the underlying disease processes. i.v. iron can cause iatrogenic iron overload in these patients and in some studies has been linked to higher infection rates (9). Although the iron-polysaccharide complexes that make up these preparations are formulated to be processed by macrophages before iron is delivered to transferrin, various amounts of labile forms of iron are also directly released from these complexes (33). Here we investigated whether i.v. iron supplementation affects E. coli sepsis. WT mice were injected with either 2 or 4 mg of i.v. iron sucrose from two different preparations, respectively (Fig. 1E), or 2 mg of i.v. iron dextran (Fig. 1F) through the retroorbital plexus. Control animals received sterile saline injections. At 4 h after i.v. iron administration, the mice were infected intraperitoneally (i.p.) with 104 CFU of E. coli (isolate 1) per mouse. We observed 100% mortality in iron sucrose-injected mice and 50% mortality in iron dextran-injected mice (Fig. 1E and F), while all control animals survived, suggesting that iatrogenic iron loading also promotes susceptibility to sepsis-associated mortality in WT mice. We also tested i.v. iron injection alone without infection and did not observe any adverse effects of iron administration (data not shown). Another common cause of iron overload and possible transient increase of serum iron is red blood cell (RBC) transfusion, especially when older packed RBCs are transfused (11). We developed a model in which WT mice were transfused through the retroorbital plexus with 400 μl of fresh (3-h-old) mouse packed RBCs and 4 h later were infected with E. coli. However, transfusion did not promote susceptibility to sepsis in this model (Fig. S2). It has been previously shown that a single transfusion only with old PRBCs (>14-day-old mouse PRBCs, which is equivalent to >42-day-old human packed RBCs) but not fresh packed RBCs promotes susceptibility (12) to infection, which may explain our results. Furthermore, a single transfusion in our model introduced only a moderate amount of iron (∼0.4 mg), most of which likely remained in RBC hemoglobin during the course of infection, and did not reflect the degree of clinical iron overload resulting from multiple transfusions over time (34).
In infected WT mice, the early inflammatory response induces hepcidin and subsequent hypoferremia.
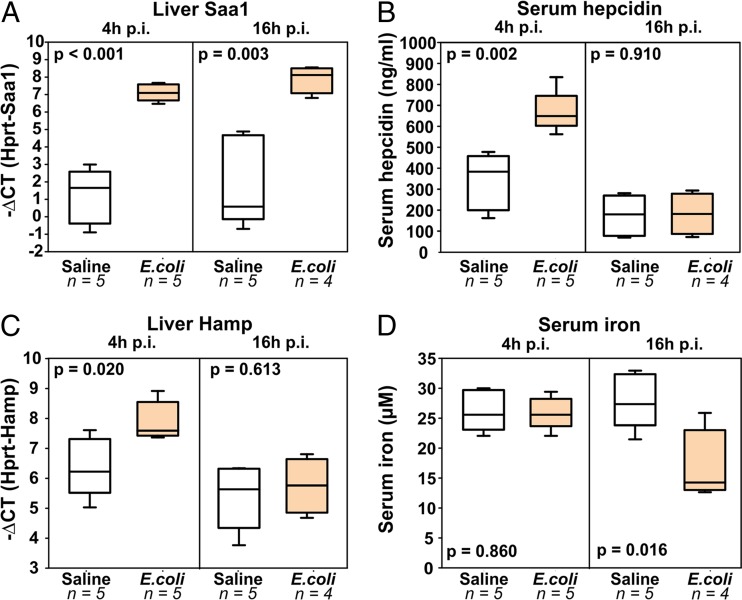
We have previously shown that hepcidin is essential for protection from early mortality after infection with certain siderophilic (iron-loving) pathogens, such as Yersinia enterocolitica and Vibrio vulnificus (22, 23). Hepcidin has two important effects, which are demonstrated, respectively, in the two models: (i) it controls baseline iron levels, allowing local defenses to abort the initial infection without eliciting a systemic inflammatory response (in the case of oral administration of Y. enterocolitica), and (ii) it induces inflammation-driven hypoferremia after the initial infection develops and thus further limits the amount of iron available for subsequent bacterial replication (seen after intraperitoneal injection of Y. enterocolitica [22] and subcutaneous injection of V. vulnificus [23]). To measure the inflammatory response in our E. coli model, we injected WT mice i.p. with saline (control) or 104 CFU of E. coli (isolate 1) per mouse and collected tissues for analysis at 4 h and 16 h postinfection (p.i.). As before, none of the infected WT mice suffered mortality or showed signs of illness. However, the amount of liver Saa1 mRNA, which is a marker of acute inflammation, was increased 64-fold both at 4 h p.i. and at 16 h p.i. (Fig. 2A), showing that an early systemic inflammatory response to the infection was mounted. Accordingly, hepcidin was induced, as we detected 2-fold higher levels of hepcidin protein (Fig. 2B) and 4-fold increased levels of hepcidin mRNA (Fig. 2C) at 4 h p.i. We often observed even greater hepcidin induction, but the mice used in this experiment were 10 to 14 weeks old and their baseline hepcidin level was already very high, limiting the fold increase. Hepcidin induction at 4 h p.i. resulted in hypoferremia (Fig. 2D), detected at 16 h p.i., since it takes time for the plasma to become depleted of iron after iron efflux is inhibited by hepcidin. It has been previously reported that inflammation can induce hypoferremia independently of hepcidin (35), but this was not the case in our model, since hepcidin-deficient mice did not have lower serum iron levels at 16 h after infection with E. coli (Fig. S3). Thus, even in mice that did not show any signs of illness, E. coli infection was sufficient to elicit a systemic inflammatory response and increased hepcidin levels and hypoferremia.
FIG 2.
E. coli infection rapidly induces systemic inflammation, increases hepcidin levels, and causes hypoferremia. WT mice were injected i.p. with either saline or 104 CFU of E. coli (isolate 1) per mouse. Tissues were collected at 4 and 16 h postinfection for analysis. We measured liver Saa1 levels as a marker of systemic inflammation (A), hepcidin protein (B), hepcidin mRNA (C), and the serum iron concentration (D). Inflammation induces the initial rise in serum hepcidin protein and mRNA levels, followed by hypoferremia and a reactive decrease in hepcidin protein and mRNA levels.
Minihepcidin PR73 prevents E. coli sepsis-associated mortality in HKO mice, a model of hereditary hemochromatosis.
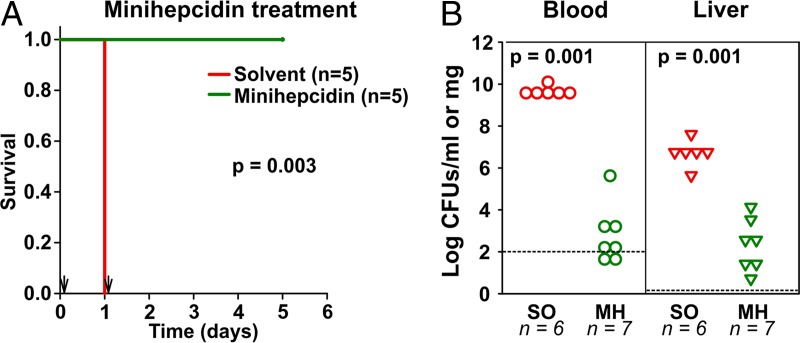
Our study so far showed that hepcidin is essential for protection against E. coli sepsis. We therefore tested for the therapeutic effect of the hepcidin analog PR73 in infected mice. Naturally iron-loaded HKO mice were infected with 104 CFU of E. coli (isolate 1) per mouse and treated i.p. with solvent (control) or 100 nmol of PR73 at 3 h and 24 h postinfection. As before, 100% of the control animals died within 24 h of infection and solvent treatment. However, treatment with only two doses of minihepcidin was sufficient to completely prevent mortality after infection with isolate 1 (Fig. 3A). Similar results were obtained when using isolate 2 (Fig. S4A). This effect resulted from inhibition of bacterial dissemination and replication, as the numbers of CFU in both blood and liver were significantly decreased by the PR73 treatment (Fig. 3B): while control animals had, on average, 1010 CFU/ml and 107 CFU/mg in their blood and liver, respectively, minihepcidin-treated animals had only 102 CFU/ml or 102 CFU/mg in their blood and liver, respectively. Next, we asked whether minihepcidin treatment could be beneficial if it was started even later than 3 h p.i. Since most of the mice became moribund by 16 h p.i., we decided to administer the first minihepcidin dose at 10 h p.i. However, at this time point the mice were already very sick and minihepcidin treatment was not beneficial (Fig. S4B). These results suggest that minihepcidin treatment prevents E. coli sepsis-associated mortality in a model of hereditary hemochromatosis, but only if it is given early enough after infection.
FIG 3.
Minihepcidin prevents E. coli sepsis-associated mortality in a model of hereditary hemochromatosis. Naturally iron-loaded HKO mice were infected i.p. with 104 CFU of E. coli (isolate 1) per mouse and treated with solvent (SO) or 100 nmol minihepcidin (MH) at +3 and +24 h p.i. Survival was monitored (A), and in two repeat experiments, tissues were collected at 16 h p.i. to assess the bacterial burden in the blood and liver (B). Dashed line, limit of detection.
Effects of iron in tissue and the plasma transferrin and nontransferrin compartments on E. coli infection.
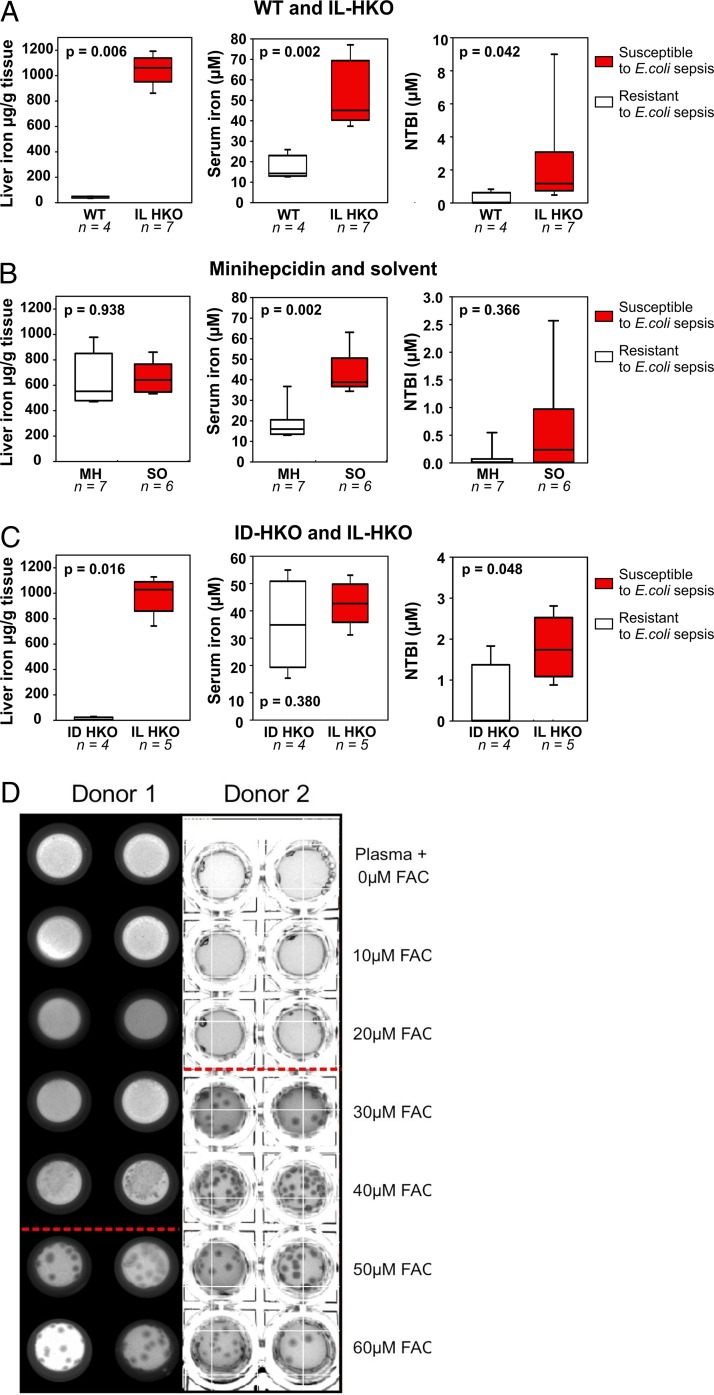
Having shown that iron overload promotes susceptibility to E. coli infection and that endogenous hepcidin or exogenous minihepcidin is protective, we next asked which iron pool may be critical in promoting infection. We analyzed tissue iron (liver), extracellular iron (serum), and a specific extracellular iron species (non-transferrin-bound iron [NTBI]) in groups of mice that were susceptible to or protected from infection (Fig. 4).
FIG 4.
Non-transferrin-bound iron (NTBI) rather than total iron promotes susceptibility to E. coli sepsis. (A to C) Liver iron, serum iron, and non-transferrin-bound iron measurements for WT (white boxes) and IL-HKO (red boxes) mice (A), IL-HKO mice treated i.p. either with a single dose of solvent (red boxes) or 100 nmol minihepcidin (white boxes) at 14 h prior to tissue collection (B), or IL-HKO (red boxes) and ID-HKO (white boxes) mice (C). All mice were infected i.p. with 104 CFU of E. coli (isolate 1) per mouse, and tissues were collected at 14 to 16 h postinfection (p.i.). IL, iron loaded; ID, iron depleted. (D) Human plasma was supplemented with 0 to 60 μM ferric ammonium citrate (FAC) and used to make agar plates. E. coli isolate 1 was plated and incubated for 38 to 41 h at 37°C, and the number of bacterial CFU was documented by photography. The results of two separate experiments using plasma from different donors are shown. Conditions below the red line had detectable NTBI.
Comparing those parameters between resistant WT and susceptible iron-loaded HKO mice, we found that iron-loaded HKO mice had high liver iron, serum iron, as well as NTBI levels (Fig. 4A), confirming that iron overload promotes susceptibility to infection.
To selectively deplete extracellular iron, we treated HKO mice with the minihepcidin PR73, a potent hepcidin agonist, at a dose that prevents sepsis-related disease signs and mortality. As expected, a single minihepcidin dose did not change liver iron levels but caused a significant drop in the serum iron and NTBI concentrations (Fig. 4B), suggesting the importance of extracellular rather than total body iron in promoting E. coli infection.
Lastly, we compared the iron distribution in iron-depleted HKO and iron-loaded HKO mice. As expected, we found tissue iron levels to be higher in iron-loaded than iron-depleted HKO mice (Fig. 4C). As HKO mice lack the ability to lower their serum iron concentrations until their stores are completely iron depleted, serum iron levels were comparable between the two groups and only NTBI levels were different. The generation of NTBI is not reflected solely in the serum iron concentration but is also dependent on the kinetics of iron efflux from macrophages, hepatocytes, and enterocytes and the ability of the liver to rapidly clear NTBI. The differences in cellular iron content and, therefore, efflux likely account for the difference in NTBI concentrations between the infection-susceptible, iron-loaded group and the infection-resistant, iron-depleted group. Altogether, the protective effects of iron depletion and minihepcidin treatment indicate that it is not primarily the tissue iron level or the serum iron level but the NTBI level that promotes susceptibility to E. coli sepsis. However, since we cannot completely uncouple the increased serum iron concentration from the increased NTBI concentration, we cannot exclude the possibility that both the transferrin-bound iron (Tf-Fe) and NTBI compartments promote E. coli virulence in this model.
To determine whether NTBI promotes E. coli growth in human plasma, we supplemented the plasma with increasing concentrations of iron. We added 0 to 60 μM ferric ammonium citrate (FAC), an iron species similar to the predominant component of NTBI (36), in order to gradually saturate transferrin, thus increasing the concentration of transferrin-bound iron (Tf-Fe) and eventually leading to the formation of NTBI. The plasma was solidified by the addition of agar, and E. coli (isolate 1) was plated on the plasma agar. We observed bacterial growth only under conditions where NTBI was present (Fig. 4D and Table S2). When testing the other 9 isolates, we observed two types of behavior (Table S3): similarly to isolate 1, five out of the nine isolates grew only under conditions where NTBI was detectable. The other four isolates likewise failed to grow within 22 h of incubation in plasma when iron was not supplemented but did start growing already at lower FAC concentrations where we could not yet detect NTBI with our methodology. We hypothesize that the plasma did not reach equilibrium with the added FAC, so that a small amount of NTBI was present and was sufficient to advance bacterial growth for these isolates. The obtained results are heterogeneous, reflecting the expected heterogeneity of clinical isolates. However, for all isolates the additional iron accelerated bacterial colony formation within 24 h of incubation. Further incubation would not be relevant for our model since all of the mice died within 24 h of infection. Several siderophore systems are known to exist in E. coli (37), so it is likely that these bacteria can use diverse sources of iron both in our in vitro assay and in vivo. However, we hypothesize that in the presence of NTBI the bacteria can initiate rapid growth, thus overwhelming host defense. The ability of hepcidin to clear NTBI from the circulation and extracellular fluid may delay bacterial growth, and this delay could be an important determinant of infection outcome.
DISCUSSION
During infection and inflammation, the plasma concentration of hepcidin is greatly increased, leading to the sequestration of iron in macrophages and enterocytes with consequent hypoferremia (4). We previously showed that this mechanism of innate immunity limits extracellular iron availability and inhibits the growth of the siderophilic pathogens Vibrio vulnificus (23) and Yersinia enterocolitica (22) in vitro and in mice. However, hepcidin-induced hypoferremia is a highly conserved response to various infections of mammals, raising the question of whether it acts against a set of bacteria broader than these relatively rare classical siderophilic pathogens. We therefore compared the outcomes after infection of HKO mice and WT mice with 10 different clinical E. coli isolates. To our surprise, with most isolates, hepcidin-deficient mice rapidly developed overwhelming sepsis and suffered 100% mortality within 24 h of infection at inoculum doses where 0% of wild-type mice with normal iron metabolism died (Table 1). Thus, hepcidin is essential for protection against E. coli infection-associated mortality. The dramatic difference in survival was observed for 8 out of the 10 clinical isolates, while the remaining 2 isolates showed lower virulence in mice, not causing significant mortality in either WT or HKO mice. These results suggest that hepcidin is an important component of the innate immune response against a range of pathogens wider than has heretofore been appreciated. Using iron depletion experiments, we showed that the protective effect of hepcidin is dependent on its effects on iron distribution rather than a direct antimicrobial effect. The hepcidin analogue PR73 completely protected the highly susceptible HKO mice with only two doses, given at 3 h and 24 h postinfection.
Hepcidin controls extracellular iron levels by occluding and degrading the iron transporter ferroportin. Inflammation resulting from administration of different pathogen-associated molecular patterns (PAMPs) in mice was reported to decrease ferroportin expression independently of hepcidin (35). In our hepcidin-deficient mice, however, this mechanism was not sufficient to alter plasma iron levels or protect mice from sepsis, indicating that in infection in vivo, hepcidin plays an essential and dominant role in controlling iron availability to pathogens.
Iron supplementation has been linked to increased infection rates in some human trials (13, 14). As a result, medical guidelines recommend avoiding i.v. iron in infected patients, although without strong evidence supporting causality (38). In our model of E. coli infection, i.v. iron administration dramatically increased sepsis and mortality (Fig. 2), thus supporting the concern about iron supplementation in patients with infections. Moreover, parenteral iron administration may be linked to systemic oxidative stress-induced mortality in experimental murine sepsis models with heat-killed E. coli (39). We cannot exclude the possibility that increased oxidative stress also contributes to mortality in our models of E. coli sepsis.
We suggest that assessing the infection status of patients should be an important consideration in the timing of iron therapy. Furthermore, as with the classical siderophilic bacteria (22), the iron species that played an important role in increased susceptibility to sepsis with clinical isolates of E. coli was NTBI. Apparently, NTBI, not tightly bound to chaperone proteins, is more readily available to pathogens and may allow them to acquire iron faster or with a lower energy expenditure, thus allowing faster growth. We cannot, however, exclude the possibility that other iron species also contribute to susceptibility to infection. E. coli has not been considered a classic siderophilic pathogen because, unlike Y. enterocolitica and V. vulnificus, its disease-causing ability has not been clearly linked to iron availability. Furthermore, most E. coli isolates produce siderophores (40) and should be able to access iron from multiple sources. However, siderophore production incurs a high metabolic cost, and secreted siderophores are readily pirated by niche competitors. Many bacteria do not produce siderophores constitutively but induce production in response to environmental clues. The initial survival of bacteria entering the host may depend on the rates of bacterial proliferation relative to the rates of bacterial clearance by host defense mechanisms. We hypothesize that if NTBI is present during early phases of infection, the bacteria may rapidly replicate, overwhelming local defense mechanisms and causing severe infection and mortality like that which we saw in HKO mice and i.v. iron-injected WT mice (Table 1 and Fig. 1). In the absence of NTBI, the bacteria depend on the induction of more complex iron acquisition systems that require additional metabolic resources, slowing the initial proliferation of bacteria (8).
Our current understanding of the role of iron in infections is still limited, and future studies may find that the disease-causing ability of many more bacterial species is enhanced by genetic or iatrogenic iron excess. In these settings, the manipulation of iron metabolism by hepcidin agonists early in the course of the disease could be an effective adjunctive treatment.
MATERIALS AND METHODS
Bacterial preparation.
Escherichia coli blood culture isolates collected in 2015 from hospitalized pediatric patients were kindly provided by the Pediatric Infectious Disease Division at UCLA, with brief patient histories being provided in Table 1. A 35% glycerol stock was prepared for each of the isolates, aliquoted, and stored at −80°C. For infection experiments, an aliquot of the stock was thawed, and 5 μl was inoculated in 5 ml Luria-Bertani broth (LB; Becton Dickinson [BD]) and cultured overnight (ON) at 37°C (18 to 22 h). An ON culture was recultured (100 μl of the ON culture in 5 ml fresh LB) for 3 h at 37°C in order to reach exponential phase and then centrifuged at 7,500 relative centrifugal force for 5 min and resuspended in sterile phosphate-buffered saline (PBS). The bacteria were diluted to the desired concentration using the optical density at 600 nm (OD600) as an indicator of the bacterial concentration (an OD600 of 1 is equivalent to 1 × 109 CFU/ml) (41).
Animal models.
All animal experiments performed for this study were approved by the UCLA Office of Animal Research Oversight. Wild-type (WT) mice (The Jackson Laboratory) and hepcidin-1 knockout (HKO) mice (42), both on the C57BL/6 mouse background, were used for the infection experiments. For survival experiments comparing HKO to WT mice, we used either WT and HKO littermates or WT mice purchased from The Jackson Laboratory and acclimated to our vivarium for at least 2 weeks, with no difference in outcomes. At arrival to the vivarium, the bedding was exchanged between purchased WT mice and resident HKO mice to partially account for differences in the microbiome. The animals were fed ad libitum a standard diet (iron concentration, 185 ppm). In order to achieve iron depletion of HKO mice, the animals were fed an iron-deficient diet (4 ppm Fe; Envigo Teklad) for 5 to 6 weeks prior to the experiment. For some experiments, WT mice were injected intravenously (i.v.) with iron sucrose or iron dextran: the animals were anesthetized using isoflurane (Clipper) and then injected i.v. in the retroorbital plexus using a 28-gauge insulin syringe. The dose used was 2 or 4 mg iron sucrose (Venofer; American Regent Inc.) per mouse in a 100- or 200-μl volume, respectively, or 2 mg iron dextran (Sigma) in 100 μl per mouse. Iron administration was performed 4 h prior to infection.
Mice were infected via intraperitoneal (i.p.) injection with different doses of E. coli. Animal well-being and behavior were monitored closely, and mice were euthanized by isoflurane (Clipper) injection when they showed symptoms of imminent mortality (decreased activity, weight loss, lack of grooming).
Preparation of minihepcidin.
Minihepcidin PR73 was synthesized as previously described (23) using standard solid-phase 9-fluorenylmethoxy carbonyl chemistry. Lyophilized minihepcidin was dissolved in 70% ethanol (Gold Shield Chemical Co.) together with the solvent DSPE-020 CN (NOF Corporation). The solution was dried in a SpeedVac apparatus to decrease the ethanol content. The dried product was stored at 4°C until needed for experiments, when it was resuspended in sterile injection water (Abbot Laboratories). i.p. injections were administered at 100 nmol PR73 in 100 μl per mouse. Control animals were injected with solvent only (100 μl i.p.).
Assessment of bacterial burden.
Blood and liver were collected at 13 to 18 h postinfection for bacterial burden measurement. Blood was collected through cardiac puncture and mixed with heparin to prevent coagulation, and serial 10-fold dilutions with sterile PBS were prepared. The whole liver was collected and homogenized, and 50 μl of the homogenate was weighed and mixed with 200 μl sterile water. The resulting mixture was used for serial 10-fold dilutions with sterile PBS. For assessment of the numbers of CFU in both blood and liver, 5 μl from each dilution was plated on LB 1.5% agar plates in duplicate. The plates were incubated at 37°C for 15 to 16 h, and the number of colonies was counted using a dissecting microscope.
Measuring nonheme iron.
The whole liver was dissected and homogenized, and 75 μl was weighed. Then, 1,125 μl protein precipitation solution (0.53 N HCl [Fisher] and 5.3% trichloroacetic acid [Fisher]) was added and the mixture was incubated at 100°C for 1 h. The samples were centrifuged, and the supernatant was analyzed for the iron concentration using the colorimetric Iron SL assay (Sekisui Diagnostics) following the manufacturer's instructions. An iron AA standard (Ricca Chemical Company) was used in serial dilutions to generate a standard curve and determine the iron concentration in the samples. For serum iron measurements, blood was collected through cardiac puncture and centrifuged in serum separator tubes (BD) to obtain serum samples. Their iron concentration was measured using the colorimetric Iron SL assay (Sekisui Diagnostics) following the manufacturer's instructions.
Gene expression assessment.
The TRIzol reagent (Life Technologies) was used to isolate RNA from snap-frozen liver samples following the instructions in the manufacturer's manual. Then, cDNA was synthesized using an iScript cDNA synthesis kit (Bio-Rad) following the manufacturer's protocol. Sso Advance SYBR green supermix (Bio-Rad) and a CFX96 Touch real-time PCR detection system (Bio-Rad) were used to perform quantitative real-time PCR for genes for the following proteins: Saa1 (forward primer, AGTCTGGGCTGCTGAGAAAA; reverse primer, ATGTCTGTTGGCTTCCTGTG), Hprt (forward primer, CTGGTTAAGCAGTACAGCCCCAA; reverse primer, CAGGAGGTCCTTTTCACCAGC), and Hamp (forward primer, TTGCGATACCAATGCAGAAGA; reverse primer, GATGTGGCTCTAGGCTATGTT). The gene for Hprt was used as the housekeeping gene, and the results in the figures are shown as the change in the threshold cycle (CT) value (ΔCT), which is equal to the CT for Hprt minus the CT for the test gene.
Hepcidin ELISA.
The serum hepcidin protein concentration was measured by performing an enzyme-linked immunosorbent assay (ELISA) as previously described (23) with the following reagents: Ab2B10 (capture) and Ab2H4-horseradish peroxidase (detection) antibodies, kindly provided by Amgen, and synthetic mouse hepcidin-25 (standard curves with concentrations ranging from 400 to 3.2 pg/ml).
NTBI assay.
The non-transferrin-bound iron (NTBI) concentration was measured as previously described (22) by a protocol adapted from that of Esposito et al. (43). All samples were frozen after collection and stored at −80°C. Once thawed, the samples were measured in triplicate at 5 μl/well and never reused.
Bacterial growth in human plasma agar.
Human plasma (anonymous leftover plasma that is institutional review board exempt) was inactivated by incubation at 56°C for 30 min and centrifuged at 9,200 × g to remove precipitated protein. Then, 0 μM to 60 μM ferric ammonium citrate (FAC; Sigma) was added and the plasma was incubated ON at 37°C to allow the iron to bind transferrin. On the following day, a few drops of hot 12% agar were added to the warm plasma and the mixture was quickly pipetted into a 96-well plate. E. coli isolates were cultured as described above and diluted to a concentration of 104 CFU/ml, and 5 μl was plated in each well in duplicate. The bacteria were incubated for up to 42 h at 37°C, and the bacterial growth was photographed.
Statistics.
Statistical analysis was performed using the SigmaPlot program. Survival was analyzed using Kaplan-Meier survival curves and the log-rank test. For the rest of the data, Student's t test was used for normally distributed data and the Mann-Whitney rank-sum test was used for data that were not normally distributed. Box plots with error bars show the median and 10th, 25th, 75th, and 90th percentiles.
Supplementary Material
ACKNOWLEDGMENTS
We thank Victoria Gabayan for maintaining the hepcidin knockout mouse colony.
The research was supported by the following grants: R01DK107309 (to E.N.) and R01DK065029 (to T.G.).
E.N. and T.G. are consultants for and shareholders of Intrinsic LifeSciences and Silarus Therapeutics and consultants for La Jolla Pharmaceutical Company and Keryx Pharmaceuticals. E.N. is a consultant for Protagonist Therapeutics. T.G. is a consultant for Fresenius-Vifor and Akebia.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00253-18.
REFERENCES
- 1.Ganz T, Nemeth E. 2006. Regulation of iron acquisition and iron distribution in mammals. Biochim Biophys Acta 1763:690–699. doi: 10.1016/j.bbamcr.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 2.Ganz T, Nemeth E. 2015. Iron homeostasis in host defence and inflammation. Nat Rev Immunol 15:500–510. doi: 10.1038/nri3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park CH, Valore EV, Waring AJ, Ganz T. 2001. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J Biol Chem 276:7806–7810. doi: 10.1074/jbc.M008922200. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez R, Jung CL, Gabayan V, Deng JC, Ganz T, Nemeth E, Bulut Y. 2014. Hepcidin induction by pathogens and pathogen-derived molecules is strongly dependent on interleukin-6. Infect Immun 82:745–752. doi: 10.1128/IAI.00983-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barber MF, Elde NC. 2014. Escape from bacterial iron piracy through rapid evolution of transferrin. Science 346:1362–1366. doi: 10.1126/science.1259329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wandersman C, Delepelaire P. 2004. Bacterial iron sources: from siderophores to hemophores. Annu Rev Microbiol 58:611–647. doi: 10.1146/annurev.micro.58.030603.123811. [DOI] [PubMed] [Google Scholar]
- 7.Miethke M, Marahiel MA. 2007. Siderophore-based iron acquisition and pathogen control. Microbiol Mol Biol Rev 71:38. doi: 10.1128/MMBR.00012-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lv H, Hung CS, Henderson JP. 2014. Metabolomic analysis of siderophore cheater mutants reveals metabolic costs of expression in uropathogenic Escherichia coli. J Proteome Res 13:1397–1404. doi: 10.1021/pr4009749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drakesmith H, Prentice AM. 2012. Hepcidin and the iron-infection axis. Science 338:768–772. doi: 10.1126/science.1224577. [DOI] [PubMed] [Google Scholar]
- 10.Michels K, Nemeth E, Ganz T, Mehrad B. 2015. Hepcidin and host defense against infectious diseases. PLoS Pathog 11:e1004998. doi: 10.1371/journal.ppat.1004998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vento S, Cainelli F, Cesario F. 2006. Infections and thalassaemia. Lancet Infect Dis 6:226–233. doi: 10.1016/S1473-3099(06)70437-6. [DOI] [PubMed] [Google Scholar]
- 12.Prestia K, Bandyopadhyay S, Slate A, Francis RO, Francis KP, Spitalnik SL, Fidock DA, Brittenham GM, Hod EA. 2014. Transfusion of stored blood impairs host defenses against Gram-negative pathogens in mice. Transfusion 54:2842–2851. doi: 10.1111/trf.12712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sazawal S, Black RE, Ramsan M, Chwaya HM, Stoltzfus RJ, Dutta A, Dhingra U, Kabole I, Deb S, Othman MK, Kabole FM. 2006. Effects of routine prophylactic supplementation with iron and folic acid on admission to hospital and mortality in preschool children in a high malaria transmission setting: community-based, randomised, placebo-controlled trial. Lancet 367:133–143. doi: 10.1016/S0140-6736(06)67962-2. [DOI] [PubMed] [Google Scholar]
- 14.Zlotkin S, Newton S, Aimone AM, Azindow I, Amenga-Etego S, Tchum K, Mahama E, Thorpe KE, Owusu-Agyei S. 2013. Effect of iron fortification on malaria incidence in infants and young children in Ghana: a randomized trial. JAMA 310:9. doi: 10.1001/jama.2013.277129. [DOI] [PubMed] [Google Scholar]
- 15.Soofi S, Cousens S, Iqbal SP, Akhund T, Khan J, Ahmed I, Zaidi AK, Bhutta ZA. 2013. Effect of provision of daily zinc and iron with several micronutrients on growth and morbidity among young children in Pakistan: a cluster-randomised trial. Lancet 382:29–40. doi: 10.1016/S0140-6736(13)60437-7. [DOI] [PubMed] [Google Scholar]
- 16.Manno D, Siame J, Larke N, Baisley K, Kasonka L, Filteau S. 2011. Effect of multiple micronutrient-fortified food on mild morbidity and clinical symptoms in Zambian infants: results from a randomised controlled trial. Eur J Clin Nutr 65:1163–1166. doi: 10.1038/ejcn.2011.103. [DOI] [PubMed] [Google Scholar]
- 17.Khan F, Fisher MA, Khakoo RA. 2007. Association of hemochromatosis with infectious diseases: expanding spectrum. Int J Infect Dis 11:6. [DOI] [PubMed] [Google Scholar]
- 18.Gerhard GS, Levin KA, Price GJ, Wojnar MM, Chorney MJ, Belchis DA. 2001. Vibrio vulnificus septicemia in a patient with the hemochromatosis HFE C282Y mutation. Arch Pathol Lab Med 125:1107–1109. [DOI] [PubMed] [Google Scholar]
- 19.Vadillo M, Corbella X, Pac V, Fernandez-Viladrich P, Pujol R. 1994. Multiple liver abscesses due to Yersinia enterocolitica discloses primary hemochromatosis: three cases reports and review. Clin Infect Dis 18:938–941. doi: 10.1093/clinids/18.6.938. [DOI] [PubMed] [Google Scholar]
- 20.Manso C, Rivas I, Peraire J, Vidal F, Richart C. 1997. Fatal Listeria meningitis, endocarditis and pericarditis in a patient with haemochromatosis. Scand J Infect Dis 29:308–309. doi: 10.3109/00365549709019049. [DOI] [PubMed] [Google Scholar]
- 21.Barry D, Reeve AW. 1977. Increased incidence of gram-negative neonatal sepsis with intramuscular iron administration. Pediatrics 60:908–912. [PubMed] [Google Scholar]
- 22.Stefanova D, Raychev A, Arezes J, Ruchala P, Gabayan V, Skurnik M, Dillon BJ, Horwitz MA, Ganz T, Bulut Y, Nemeth E. 2017. Endogenous hepcidin and its agonist mediate resistance to selected infections by clearing non-transferrin-bound iron. Blood 130:245–257. doi: 10.1182/blood-2017-03-772715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arezes J, Jung G, Gabayan V, Valore E, Ruchala P, Gulig PA, Ganz T, Nemeth E, Bulut Y. 2015. Hepcidin-induced hypoferremia is a critical host defense mechanism against the siderophilic bacterium Vibrio vulnificus. Cell Host Microbe 17:47–57. doi: 10.1016/j.chom.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Michels K, Zhang Z, Bettina AM, Cagnina RE, Stefanova D, Burdick MD, Vaulont S, Nemeth E, Ganz T, Mehrad B. 2017. Hepcidin-mediated iron sequestration protects against bacterial dissemination during pneumonia. JCI Insight 2:e92002. doi: 10.1172/jci.insight.92002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brissot P, Ropert M, Le LC, Loreal O. 2012. Non-transferrin bound iron: a key role in iron overload and iron toxicity. Biochim Biophys Acta 1820:403–410. doi: 10.1016/j.bbagen.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 26.Hirano K, Morinobu T, Kim H, Hiroi M, Ban R, Ogawa S, Ogihara H, Tamai H, Ogihara T. 2001. Blood transfusion increases radical promoting non-transferrin bound iron in preterm infants. Arch Dis Child Fetal Neonatal Ed 84:F188–F193. doi: 10.1136/fn.84.3.F188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tenaillon O, Skurnik D, Picard B, Denamur E. 2010. The population genetics of commensal Escherichia coli. Nat Rev Microbiol 8:207–217. doi: 10.1038/nrmicro2298. [DOI] [PubMed] [Google Scholar]
- 28.Kaper J, Nataro JP, Mobley HLT. 2004. Pathogenic Escherichia coli. Nat Rev Microbiol 2:123–140. doi: 10.1038/nrmicro818. [DOI] [PubMed] [Google Scholar]
- 29.Angus D, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. 2001. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Novosad S, Sapiano MRP, Grigg C, Lake J, Robyn M, Dumyati G, Felsen C, Blog D, Dufort E, Zansky S, Wiedeman K, Avery L, Dantes RB, Jernigan JA, Magill SS, Fiore A, Epstein L. 2016. Vital signs: epidemiology of sepsis: prevalence of health care factors and opportunities for prevention. Morb Mortal Wkly Rep 65:864–869. doi: 10.15585/mmwr.mm6533e1. [DOI] [PubMed] [Google Scholar]
- 31.Christopher GW. 1985. Escherichia coli bacteremia, meningitis, and hemochromatosis. Arch Intern Med 145:1908. [PubMed] [Google Scholar]
- 32.Sydow M, Crozier TA, Schauer A, Burchardi H. 1993. Septic shock with acute abdomen in idiopathic hemochromatosis. Anasthesiol Intensivmed Notfallmed Schmerzther 28:258–260. doi: 10.1055/s-2007-998921. [DOI] [PubMed] [Google Scholar]
- 33.Pai AB. 2017. Complexity of intravenous iron nanoparticle formulations: implications for bioequivalence evaluation. Ann N Y Acad Sci 1407:17–25. doi: 10.1111/nyas.13461. [DOI] [PubMed] [Google Scholar]
- 34.Shander A, Sazama K. 2010. Clinical consequences of iron overload from chronic red blood cell transfusions, its diagnosis, and its management by chelation therapy. Transfusion 50:1144–1155. doi: 10.1111/j.1537-2995.2009.02551.x. [DOI] [PubMed] [Google Scholar]
- 35.Guida C, Altamura S, Klein FA, Galy B, Boutros M, Ulmer AJ, Hentze MW, Muckenthaler MU. 2015. A novel inflammatory pathway mediating rapid hepcidin-independent hypoferremia. Blood 125:2265–2275. doi: 10.1182/blood-2014-08-595256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hider RC, Silva AM, Podinovskaia M, Ma Y. 2010. Monitoring the efficiency of iron chelation therapy: the potential of nontransferrin-bound iron. Ann N Y Acad Sci 1202:94–99. doi: 10.1111/j.1749-6632.2010.05573.x. [DOI] [PubMed] [Google Scholar]
- 37.Ellermann M, Arthur JC. 2017. Siderophore-mediated iron acquisition and modulation of host-bacterial interactions. Free Radic Biol Med 105:68–78. doi: 10.1016/j.freeradbiomed.2016.10.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Drüeke TB, Parfrey PS. 2012. Summary of the KDIGO guideline on anemia and comment: reading between the (guide)line(s). Kidney Int 82:952–960. doi: 10.1038/ki.2012.270. [DOI] [PubMed] [Google Scholar]
- 39.Zager R, Johnson AC, Hanson SY. 2004. Parenteral iron therapy exacerbates experimental sepsis. Kidney Int 65:2108–2112. [DOI] [PubMed] [Google Scholar]
- 40.Martin P, Tronnet S, Garcie C, Oswald E. 2017. Interplay between siderophores and colibactin genotoxin in Escherichia coli. IUBMB Life 69:435–441. doi: 10.1002/iub.1612. [DOI] [PubMed] [Google Scholar]
- 41.Myers J, Curtis BS, Curtis WR. 2013. Improving accuracy of cell and chromophore concentration measurements using optical density. BMC Biophys 6:4. doi: 10.1186/2046-1682-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lesbordes-Brion JC, Viatte L, Bennoun M, Lou DQ, Ramey G, Houbron C, Hamard G, Kahn A, Vaulont S. 2006. Targeted disruption of the hepcidin 1 gene results in severe hemochromatosis. Blood 108:1402–1405. doi: 10.1182/blood-2006-02-003376. [DOI] [PubMed] [Google Scholar]
- 43.Esposito B, Breuer W, Sirankapracha P, Pootrakul P, Hershko C, Cabantchik ZI. 2003. Labile plasma iron in iron overload: redox activity and susceptibility to chelation. Blood 102:2670–2677. doi: 10.1182/blood-2003-03-0807. [DOI] [PubMed] [Google Scholar]
- 44.Kato GJ, Steinberg MH, Gladwin MT. 2017. Intravascular hemolysis and the pathophysiology of sickle cell disease. J Clin Invest 127:750–760. doi: 10.1172/JCI89741. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.