ABSTRACT
The keystone periodontal pathogen Porphyromonas gingivalis produces phosphorylated dihydroceramide lipids (sphingolipids) such as phosphoethanolamine dihydroceramide (PE DHC) and phosphoglycerol dihydroceramide (PG DHC) lipids. Phosphorylated DHCs (PDHCs) from P. gingivalis can affect a number of mammalian cellular functions, such as potentiation of prostaglandin secretion from gingival fibroblasts, promotion of RANKL-induced osteoclastogenesis, promotion of apoptosis, and enhancement of autoimmunity. In P. gingivalis, these lipids affect anchoring of surface polysaccharides, resistance to oxidative stress, and presentation of surface polysaccharides (anionic polysaccharides and K-antigen capsule). In addition to phosphorylated dihydroceramide lipids, serine dipeptide lipids of P. gingivalis are implicated in alveolar bone loss in chronic periodontitis through interference with osteoblast differentiation and function and promotion of osteoclast activity. As a prerequisite for designation as bacterial virulence factors, bacterial sphingolipids and serine dipeptide lipids are recovered in gingival/periodontal tissues, tooth calculus, human blood, vascular tissues, and brain. In addition to P. gingivalis, other bacteria of the genera Bacteroides, Parabacteroides, Porphyromonas, Tannerella, and Prevotella produce sphingolipids and serine dipeptide lipids. The contribution of PDHCs and serine dipeptide lipids to the pathogenesis of periodontal and extraoral diseases may be an underappreciated area in microbe-host interaction and should be more intensively investigated.
KEYWORDS: P. gingivalis, virulence factors, sphingolipids, ceramides, periodontitis, extraoral diseases
INTRODUCTION
Sphingolipids, as amphipathic molecules, demonstrate both hydrophobic and hydrophilic constituents and, as such, have long been considered little more than structural lipid components of eukaryotic cell membranes. More recent studies, however, have shown that mammalian sphingolipids such as ceramide (the simplest sphingolipid), sphingosine, and sphingosine-1-phosphate lipids are central to a number of important biological processes. Mammalian sphingolipids are known to make complexes with proteins and other cell membrane lipids, often sterols, to form so-called lipid rafts (1, 2). Lipid rafts are fluctuating nanoscale collections of sphingolipid, cholesterol, and proteins in cell membranes, constituting platforms that operate in membrane signaling and trafficking. Of note, most bacteria of the Bacteroidetes phylum and a few bacteria of the Chlorobi phylum (3) produce sphingolipids, but only members of the Bacteroidetes phylum are reported to produce novel serine dipeptide lipids (4–7). These lipid classes demonstrate amphipathic characteristics and possess important biological properties. When members of the Bacteroidetes phylum, particularly oral Bacteroidetes, contact human cells, bacterial sphingolipids and serine lipids may incorporate into the eukaryotic cell membranes, thereby potentially affecting cell function. As an example, lipid raft components are used by the periodontal pathogen Porphyromonas gingivalis (8) for entry into host epithelial cells (9, 10).
Chronic periodontitis is manifested as progressive loss of periodontal attachment and alveolar bone, leading to pathological pocket formation around the teeth. If left untreated, it can lead to loss of teeth. Chronic periodontitis affects approximately 65 million (47%) U.S. adults of 30 years and older (11). Chronic periodontitis has also been associated with extraoral diseases such as cardiovascular diseases, diabetes, preterm birth, Alzheimer's disease, rheumatoid arthritis, and pancreatic cancer (12–14). The keystone periodontal pathogen P. gingivalis produces many virulence factors, the major classes of which include lipopolysaccharide (LPS), a polysaccharide-rich capsule, gingipains, fimbriae, and peptidyl-arginine deiminase. P. gingivalis LPS is considered to be important in bone destruction in periodontitis. However, previous reports have shown that LPS of P. gingivalis is present in only minor amounts in diseased periodontal tissues from humans (15, 16). In contrast to LPS, P. gingivalis dihydroceramides (DHCs) (17, 18) and serine dipeptide lipids (19) are exposed to diseased gingival tissues at levels capable of inducing an inflammatory response (20).
Within the diseased periodontal crevice (pocket), P. gingivalis is known to directly contact and attach to sulcular epithelial cells (21) (Fig. 1), and coculture of P. gingivalis with epithelial cells reveals an ultrastructural thickening of the merged membranes as P. gingivalis is internalized (22). This cell membrane contact could deliver bacterial lipids directly to the cell membranes of host epithelial cells. Another mechanism for bacterial lipid entry into cells is demonstrated by the uptake of total lipids of P. gingivalis into human gingival fibroblasts when cells are exposed to lipid films in culture (20). Therefore, P. gingivalis lipids are likely transferred to cells of gingival tissues either by direct contact with bacteria or by chemical diffusion from lipid-contaminated surfaces of diseased teeth. Either process can lead to deposition of bacterial lipids into eukaryotic cell membranes, thereby exposing cells, including lipid rafts, to elevated levels of nonmammalian sphingolipids and serine dipeptide lipids. In contrast to periodontal Bacteroidetes, gastrointestinal Bacteroides are recovered primarily in the colon (3) but little is known about breakdown of these bacterial sphingolipids in the colon or their transport within this organ.
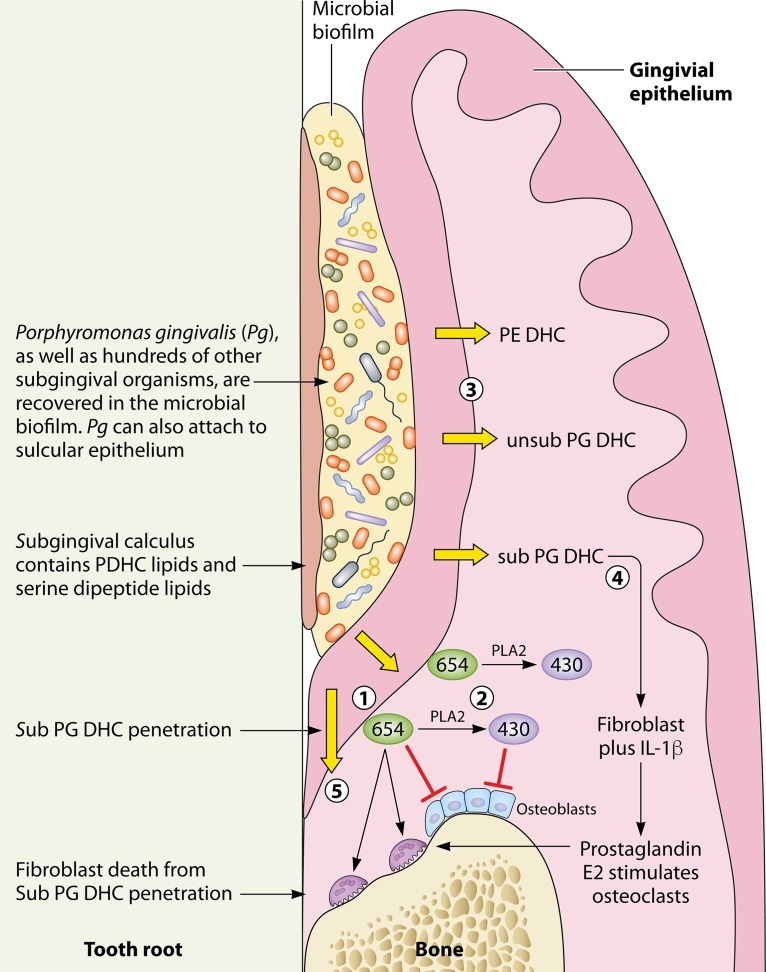
FIG 1.
Model for phosphorylated dihydroceramide and serine dipeptide lipid penetration into gingival tissues and relevant biological effects in the expression of chronic periodontitis. (1) The serine dipeptide parent lipid, lipid 654, is recovered in subgingival calculus and subgingival plaque. Lipid 654 promotes osteoclast formation from RAW cells, inhibits osteoblast differentiation and function, and is implicated in dendritic cell release of IL-6. (2) Lipid 654 can be de-esterified by mammalian phospholipase A2 (PLA2) enzymes, thus producing within gingival tissues another serine dipeptide lipid, lipid 430. Lipid 430 inhibition of osteoblast differentiation and function occurs at lower levels than with lipid 654. (3) Phosphorylated dihydroceramide lipids (PDHCs), including PE DHC, sub PG DHC, and unsub PG DHC lipids (Fig. 2), are abundant in lipid extracts of subgingival calculus but are also recovered in subgingival plaque and gingival tissues. (4) Sub PG DHC lipids promote IL-1β-mediated prostaglandin production in gingival fibroblasts and cell fusion during osteoclastogenesis of RAW cells. (5) Sub PG DHC lipids promote gingival fibroblast cell death in culture. Penetration of sub PG DHC lipids through junctional epithelium could therefore promote fibroblast cell death along the tooth root surface.
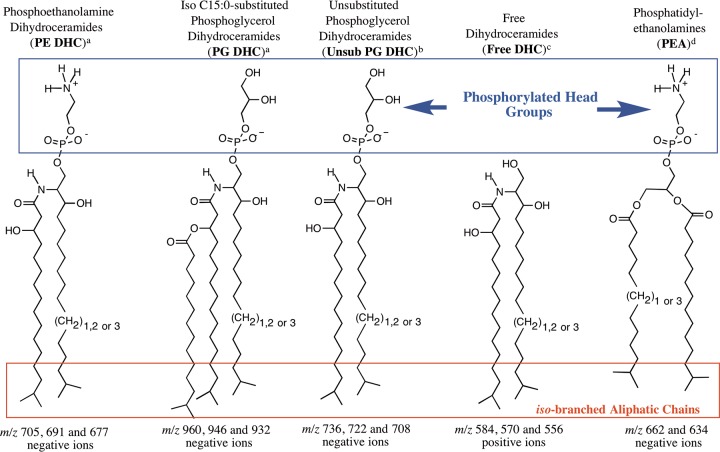
P. gingivalis produces several classes of phosphorylated dihydroceramides (PDHCs) as well as other novel complex lipids (Fig. 2 and 3), including serine dipeptide lipids. The first to be characterized were the sphingolipids, which are quite similar but not identical to mammalian sphingolipids. PDHCs can be detected, quantitated, and distinguished from eukaryotic sphingolipids using multiple-reaction monitoring (MRM)-mass spectrometry (MS) (23). The sphingoid bases in P. gingivalis DHC lipids comprise saturated aliphatic chains of 17, 18, or 19 carbons with iso-branching of the odd-numbered carbon chains (Fig. 2) (17, 18). The sphingoid base is designated sphinganine (dihydrosphingosine) or dihydroceramide (DHC) when amide linked to a fatty acid chain, which in P. gingivalis sphingolipids is usually 3-OH iso-branched C17:0 (Fig. 2) (18). From P. gingivalis lipid extracts, three major DHC species have been recognized: the free DHC, phosphoethanolamine DHC (PE DHC), and phosphoglycerol DHC (PG DHC) lipids (17, 18). The free DHC lipids are presumed to serve as precursor core structures for the synthesis of PE DHC and PG DHC lipids (Fig. 2). The iso-branched aliphatic chains of phosphoglycerol dihydroceramides and phosphatidylethanolamines (PEA) of P. gingivalis (Fig. 2) are thought to be partially responsible for the bioactivity of these lipids (24). PG DHC can be further modified with the addition of iso-branched C15:0 to the 3-OH group of the dihydroceramide core fatty acid chain (18). This lipid was later termed substituted (sub) PG DHC, but in some prior reports, the term PG DHC was used instead of sub PG DHC. However, if PG DHC lipids were not substituted with iso-C15:0 or other fatty acids, this class was specifically designated unsub PG DHC lipids in previous reports (Fig. 2).
FIG 2.
Structures of novel P. gingivalis dihydroceramide and phospholipids (17, 18, 24, 51). “(CH2)1,2 or 3” indicates that the long-chain bases vary from 17 to 19 carbons in length. The 17- and 19-carbon long-chain bases are iso-branched as shown, but the 18-carbon long-chain base exists as a straight aliphatic chain. Note the iso-branched saturated fatty acids (C15:0 and C13:0) in phosphatidylethanolamine (PEA) lipids.
FIG 3.
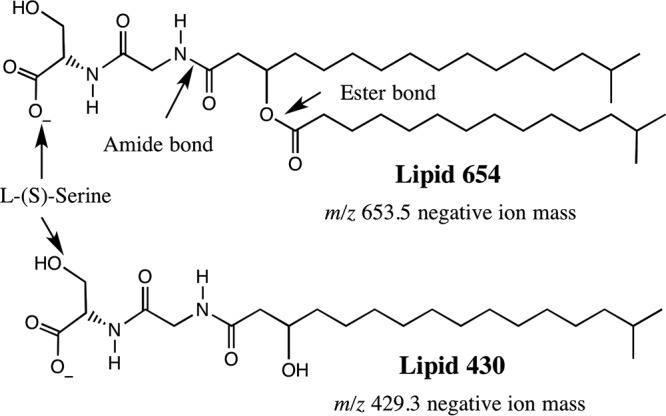
Structures of serine dipeptide lipids from P. gingivalis (7, 19). Lipid 654 was originally described in Flavobacterium meningosepticum and was termed flavolipin by Shiozaki et al. (7).
Contrasted with the structures of PDHC lipids are those of the serine dipeptide lipids of P. gingivalis, including lipid 654 and lipid 430 (Fig. 3). Lipid 654 of P. gingivalis, which is the dominant serine dipeptide lipid class of P. gingivalis, was first described in Fusobacterium meningosepticum and was termed flavolipin (7). Flavolipin was originally reported to be a Toll-like receptor 4 (TLR4) ligand (4), but recent work has shown that lipid 654 is a TLR2 ligand rather than a TLR4 ligand (19, 25). Toll-like receptors are known to recognize exogenous ligands and are reported to function within lipid rafts (26). Just as with P. gingivalis sphingolipids, serine dipeptide lipids are recovered in diseased periodontal tissues (19) as well as blood (27) and artery walls (6) of humans. Of physiological importance is that the mammalian enzyme phospholipase A2 (PLA2), typically expressed in chronic inflammatory reactions, hydrolyzed lipid 654 of P. gingivalis to another serine lipid class called lipid 430 (5, 6). Just as with lipid 654, lipid 430 also activated human embryonic kidney cells transfected with human TLR2 and also increased serum CCL2 (MCP-1) levels in wild-type mice but not TLR2 knockout mice (19). However, mouse osteoblast function and differentiation were markedly inhibited by lipid 430 regardless of TLR2 status (25). In this brief review, focus is directed toward sphingolipids and serine dipeptide lipids of P. gingivalis and their potential roles as virulence factors, particularly as they relate to bone loss in chronic periodontitis. Sphingolipids in both bacteria and fungi have previously been reviewed (28).
POTENTIATION OF PROSTAGLANDIN SECRETION FROM GINGIVAL FIBROBLASTS
It is reasonable to suspect that penetration of bacterial lipids into gingival tissues combined with chronic inflammation can promote destructive periodontitis. P. gingivalis total lipids (20), PG DHC lipids (18), and phosphatidylethanolamine (PEA) lipids (24) promoted interleukin-1β (IL-1β)-mediated secretion of inflammatory mediators (prostaglandin E2 [PGE2] and 6-keto-prostaglandin F2α) from human gingival fibroblasts and changed their cellular morphology in culture (18, 24). The potentiation of PGE2 synthesis and secretion from fibroblasts, possibly mediated through TLR2, could be an important mechanism for P. gingivalis to promote inflammatory reactions and change host responses, including osteoclast-mediated bone resorption, thereby promoting tissue breakdown in periodontal disease.
PROMOTION OF RANKL-INDUCED OSTEOCLASTOGENESIS
A possible effect of PG DHC on alveolar bone destruction, which is an essential feature of periodontitis, has been reported recently. PG DHC lipids were found to promote receptor activator of nuclear factor kappa-Β ligand (RANKL)-induced osteoclastogenesis by interacting with nonmuscle myosin IIA (Myh9) (29). The latter is an osteoclast cell fusion-regulatory cell protein localized to the cytoplasm of host cells. Myh9 elicited a signal that made Ras-related C3 botulinum toxin substrate 1 (Rac1) upregulate the expression of dendritic cell-specific transmembrane protein (DC-STAMP). The latter is known as a key osteoclast fusogen that is responsible for the cell fusion process during osteoclastogenesis. It is noteworthy that the process depended on Rac1/DC-STAMP and not on TLR2/TLR4 engagement. Thus, instead of binding to TLR2/4 expressed on the cell surface, PG DHC interacted with a cytoskeletal protein that is localized to the cytoplasm. In addition, Myh9 produced a cell signal that involved Rac1 to upregulate the expression of DC-STAMP. This key osteoclast fusogen is responsible for the process of cell fusion during osteoclastogenesis. Further, the study clearly showed that PG DHC could penetrate cell membranes of osteoclast precursors and enter the nuclei. This brought significant new insight into the process of macrophage fusion required for osteoclast formation and promotion of osteoclast-induced bone breakdown associated with the development of chronic periodontitis.
PDHC AND SERINE DIPEPTIDE LIPID INTERFERENCE WITH OSTEOBLAST DIFFERENTIATION AND OTHER CELLULAR EFFECTS
Total lipids from P. gingivalis and PE DHC and PG DHC lipids prepared free of lipid A were studied for their effects on primary calvarial osteoblast cultures from mice (30). This investigation revealed that osteoblast differentiation and fluorescent transgene expression for calvarial osteoblast differentiation (the transgene for the rat type I collagen promoter fragment pOBCol2.3GP) (31) were inhibited in a concentration-dependent manner. Osteoblast proliferation, viability, and apoptosis were not markedly affected. Simultaneously, common osteoblast differentiation genes (Runx2, ALP, OC, BSP, OPG, and DMP-1) were downregulated, whereas the RANKL, tumor necrosis factor alpha, and MMP-3 genes were upregulated. Mineral nodule formation in vitro was prevented. Total lipids and PE DHC and PG DHC lipid fractions of P. gingivalis also inhibited calvarial osteoblast gene expression and function in vivo. These lipid preparations inhibited osteoblasts through TLR2 engagement, which agrees with observations on alveolar bone loss in animals infected orally with P. gingivalis (32–34). They could therefore act as a microbial virulence factor in periodontitis by inhibiting osteoblast function and gene expression. More recent work isolated a serine dipeptide lipid fraction from P. gingivalis lipids using high-pressure liquid chromatography (HPLC) fractionation with an acidic solvent together with improved HPLC equipment capable of considerably improved chromatographic resolution. Though serine dipeptide lipids (6, 25) are minor lipid constituents of the total lipids of P. gingivalis, this approach allowed the purification of serine dipeptide lipids essentially free of contaminating phosphorylated dihydroceramides and other complex lipids. The serine dipeptide lipid class, lipid 654, has since been shown to account for the TLR2-dependent inhibition of osteoblast differentiation and function (25). It was reported that PE DHC lipids of P. gingivalis also increased secretion of IL-6 from dendritic cells in vitro (35), and this effect was mediated through engagement of TLR2. It was later determined using improved HPLC equipment that trace amounts of lipid 654 contaminating the PE DHC lipid preparation likely accounted for the TLR2-mediated effect on dendritic cells (Fig. 3) (19). The serine dipeptide lipids may also promote extraoral inflammatory diseases, e.g., cardiovascular diseases (6). This idea is supported by the fact that dihydroceramide and serine dipeptide lipids are found not only in gingival tissues but also in human blood, vascular tissue, and brain (23). Recently, it was suggested that commensal Bacteroidetes, to which P. gingivalis belongs, that reside in the oral cavity and gut contribute to the pathogenesis of TLR2-dependent atherosclerosis through deposition and metabolism of serine dipeptide lipids in artery walls (6). In addition to P. gingivalis, the common dental pulpal pathogen Porphyromonas endodontalis produces the lipid 654 class (36). The lipid 654 preparations isolated from both Porphyromonas species were shown to promote osteoclast formation from RAW 264.7 cells (36), indicating that in addition to sub PG DHC lipids, osteoclast activation is promoted by serine dipeptide lipids and that the bacterial source of lipid 654 is probably not a critical issue.
Interestingly, phosphorylated dihydroceramide and serine dipeptide lipids have been reported to contaminate free lipid A isolated from P. gingivalis LPS (37) and may be responsible, at least to some extent, for the TLR2-mediated effects of P. gingivalis LPS on osteoblasts (38). Regardless, the PG DHC and serine dipeptide lipids mediate important effects on bone cells and therefore should be regarded as virulence factors.
PROMOTION OF APOPTOSIS
Although P. gingivalis dihydroceramides promote inflammatory secretory reactions in fibroblasts, little is known about their effects on vascular cells. Zhalten et al. (39) studied the effects of extracted and purified P. gingivalis lipids on endothelial cells from the human umbilical vein. The PG DHC (sub PG DHC) lipid fraction but not the PE DHC lipid fraction of P. gingivalis initiated endothelial cell apoptosis in vitro but not necrosis. PG DHC activated caspases 3, 6, and 9. Inhibition of these caspases significantly reduced PG DHC-mediated apoptosis in endothelial cells. Sub PG DHC also induced release of apoptosis-inducing factor. Preincubation of cells with the reactive oxygen species (ROS) scavenger N-acetylcysteine reduced P. gingivalis-induced endothelial apoptosis. Apoptosis was stimulated by exogenous synthetic sphingosines that were similar to endogenous mammalian ceramides formed in endothelial and other cells (39). In addition, P. gingivalis-derived lipids were able to induce apparent apoptosis in gingival fibroblasts (18) that was similar to the apoptosis later reported in gingival fibroblasts when cocultured with P. gingivalis (40) and in chondrocytes with exposure to P. gingivalis itself (41, 42). It was proposed that lipids from P. gingivalis might induce apoptosis in joints even in the absence of live P. gingivalis (42), which is noteworthy. The authors also suggested that P. gingivalis lipids could interfere with and impair the repair process in cartilage, thereby linking P. gingivalis to rheumatoid arthritis (42–45).
EXPRESSION AND ANCHORING OF SURFACE POLYSACCHARIDES
Moye et al. (46) recently reported that deletion of the PG1780 gene in P. gingivalis strain W83 rendered this organism unable to produce dihydroceramide lipids. The PG1780 gene encodes the putative serine palmitoyltransferase enzyme of P. gingivalis. Serine palmitoyltransferase is the enzyme responsible for the first step in the synthesis of long-chain base precursors of sphingolipids. Moye et al. (46) showed that the ΔPG1780 mutant is devoid of dihydroceramide lipids, whereas dihydroceramide synthesis was restored in the complemented mutant strain (ΔPG1780/pTCOW-1780). In addition, electron micrographic evaluation of the ΔPG1780 mutant suggested that surface glycans were diminished. Further evaluation demonstrated that the ΔPG1780 mutant expressed low levels of K antigen but showed increased expression of anionic polysaccharide relative to that by the W83 parent strain. Of note, the K1 capsule null strain (ΔPG0106) showed very low expression of both K antigen and anionic polysaccharide compared with that of the ΔPG1780 mutant or parent strain. These results demonstrate that sphingolipid synthesis in P. gingivalis is associated with the expression of specific cell surface polysaccharides, although the exact role of sphingolipids in this process remains to be determined. Moye et al. (46) also demonstrated that lack of sphingolipid synthesis by P. gingivalis decreased the expression of cell-associated arginine (Arg) and lysine (Lys) gingipains (46), the “trypsin-like” proteases expressed by this organism. Bainbridge et al. (47) found that deletion of a 77-bp inverted repeat (IR) element at the 5′end of the K-antigen capsule synthesis locus of P. gingivalis changed the presentation of capsule, O-LPS, and A-LPS and reduced cell-associated Arg and Lys gingipain activity. This evidence suggests that sphingolipid synthesis may alter cell surface gingipain expression indirectly though reduced K-antigen expression. Finally, the lipid moiety responsible for covalently anchoring capsular polysaccharides to the outer membrane has been identified in only a few bacterial species (48) and includes the direct covalent attachment of capsular polysaccharides to a lyso-phosphatidylglycerol motif, as reported by Willis et al. (48). It is unknown whether capsular polysaccharides are anchored to sphingolipids in P. gingivalis cell membranes. Future research is expected to clarify this possibility.
RESISTANCE TO OXIDATIVE STRESS
Moye et al. (46) reported that the ΔPG1780 sphingolipid-deficient strain of P. gingivalis was more sensitive to oxidative stress after exposure to oxygen even for very short intervals. When the parental strain and the mutant ΔPG1780 were cultured to early exponential phase and the cultures were treated with 150 to 250 μM hydrogen peroxide or water as a control, the parental strain survived the addition of all concentrations of hydrogen peroxide. The mutant cultures treated with 200 or 250 μM H2O2 were quickly killed and did not recover after 30 h. Cultures of the mutant strain exposed to the lowest concentration of hydrogen peroxide (150 μM) initially decreased in density but recovered by the end of the experiment. This demonstrated that the ΔPG1780 mutant was much more sensitive to hydrogen peroxide than the parental strain. Accordingly, sphingolipids seem to play an important role in the resistance of P. gingivalis to oxidative stress and therefore in the survival of this bacterium. A report by An et al. (49) indicated that sphingolipids are important in Bacteroides fragilis protection from oxidative stress. However, a previous report demonstrated that B. fragilis does not produce PG DHC lipids (23), suggesting that PE DHC sphingolipids may be important in protection of B. fragilis from oxidative stress. Also, other factors can participate in the resistance of P. gingivalis to oxidative damage, such as antioxidant enzymes, DNA binding protein (Dps), the hemin layer, enzymatic removal of deleterious products caused by ROS, and response regulators (for a review, see reference 50).
ENHANCEMENT OF AUTOIMMUNITY
Phosphorylated dihydroceramides are derived from Bacteroidetes bacteria found in multiple sites in humans, such as the oral cavity, the gastrointestinal tract, and the vagina. These bacterial lipids could be tipping factors enhancing autoimmunity in humans. In a study by Nichols et al., P. gingivalis phosphorylated dihydroceramides, particularly PE DHC, significantly increased experimental allergic encephalomyelitis (EAE) in a murine model of multiple sclerosis (35). EAE was used as a model for autoimmune disease in this study. The increased autoimmune disease severity resulting from administration of P. gingivalis lipids was mediated in a TLR2-dependent manner. Though the enhanced TLR2-dependent autoimmune response was attributed to PE DHC lipids, other minor lipid classes of P. gingivalis, including the recently described serine dipeptide lipids, could account for the enhanced engagement of TLR2.
COMPARATIVE EFFECTS OF SPHINGOLIPIDS IN INFLAMMATORY DISEASES
The distribution of PDHC lipid classes was different in diseased periodontal tissues compared to tissues from healthy controls (23, 51). The primary dihydroceramide lipids of P. gingivalis recovered from diseased gingival tissue were the unsub PG DHC lipids, whereas lesser amounts of PE DHC and sub PG DHC lipids were detected. PG DHC lipids were also more abundant than PE DHC lipids on periodontally diseased teeth (51). This suggested that progression of disease could be associated with a shift in the PDHC species released from the microbiota associated with periodontitis or a shift in the transport or metabolic hydrolysis of specific PDHC lipids in diseased gingival tissues. PE DHC and PG DHCs also affected gingival fibroblasts differently, probably due to differences in their polar head groups (18). As already mentioned, sub PG DHCs caused fibroblast rounding in culture and increased the production of prostaglandin E2 with IL-1β cotreatment (18) and promoted apoptosis in endothelial cells (42) and chondrocytes (39). In contrast, the phosphatidylethanolamine (PEA) lipid fraction did not induce endothelial apoptosis in vitro (42) but did promote cell rounding in gingival fibroblasts (24). Because a homologous synthetic PEA lipid standard without iso-branched fatty acids did not cause cell rounding, it was concluded that the iso-branched aliphatic chains also contribute to the apparent cell rounding of gingival fibroblasts (24). The difference in the biological activities of PG DHC and PE DHC has been attributed to the different phosphorylated head group substitution and/or the addition of esterified iso-branched C15:0 fatty acid (18, 51). The interpretation of the relative clinical importance of dihydroceramides is additionally complicated by the fact that members of other genera in the oral and intestinal microbiota can produce PDHC lipids, such as Bacteroides, Parabacteroides, Prevotella, Tannerella, and Porphyromonas (23). However, none of these bacteria, except for T. forsythia, are considered keystone pathogens in periodontitis.
CONCLUDING REMARKS
Sphingolipids not only are structural components of mammalian membranes but also have important functions in specialized membrane domains (lipid rafts and protein complexes) and affect signaling for a number of cellular processes. Bacterial sphingolipids are similar in structure but are not identical to human sphingolipids. The importance of sphingolipids in disease has often been overshadowed by that of LPS, which in the case of P. gingivalis LPS can be contaminated with sphingolipids when prepared by published methods. P. gingivalis sphingolipids and serine dipeptide lipids produce cellular effects particularly relevant to the essential pathological features of periodontitis, including promotion of osteoclastogenesis and inhibition of osteoblast function. P. gingivalis sphingolipids also potentiate prostaglandin secretion from gingival fibroblasts and are implicated in promoting IL-6 secretion from dendritic cells. These lipids also promote apoptosis in endothelial cells, potentially contributing to vascular lesions. The latter could be important in cardiovascular disease, since P. gingivalis PDHC and serine dipeptide lipids have been detected in carotid atherosclerotic lesions. However, P. gingivalis is not the only organism responsible for production of dihydroceramide and serine dipeptide lipids, since several bacterial species and genera within members of the Bacteroidetes phylum in the oral cavity, vagina, and intestine may account for these lipids in blood, tissues, and brain. Recovery of PDHC lipids in human brain tissues could occur through transport of PDHC lipids in blood and subsequent deposition into vascular elements of neural tissues but could also result from penetration through the blood-brain barrier. Future research will evaluate these possibilities. P. gingivalis is not the sole causal agent in periodontitis either, although it is regarded as the most important keystone bacterium here. Recently discovered serine dipeptide lipids of P. gingivalis have been implicated in alveolar bone loss in chronic periodontitis and represent a new class of TLR2 ligands with structural similarity to diacylated lipopeptides. Lipid 430 is highly unusual in that it produces strong proinflammatory responses and inhibitory effects on osteoblasts and yet contains only one fatty acid acyl chain. Bacterial sphingolipids might also play a role in autoimmune disease through acute and chronic activation of the immune system, e.g., in rheumatoid arthritis and multiple sclerosis. The distribution and composition of DHCs depend on the tissue site and disease status. This implies that healthy periodontal sites may contain bacterial sphingolipids but the distribution can be different from that in diseased sites. Since these bacterial sphingolipids and serine dipeptide lipids are recovered in periodontal and other human tissues and since they possess important biological properties affecting inflammation and host responses, they should be classified as virulence factors. Research on the roles of these substances in human diseases, which are only beginning to be understood, should be intensified.
ACKNOWLEDGMENTS
F.C.N. acknowledges grants from the National Multiple Sclerosis Society and NIH grant DE021055.
REFERENCES
- 1.Lingwood D, Simons K. 2010. Lipid rafts as a membrane-organizing principle. Science 327:46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- 2.Nicolson GL. 2014. The fluid-mosaic model of membrane structure: Still relevant to understanding the structure, function and dynamics of biological membranes after more than 40 years. Biochim Biophys Acta 1838:1451–1466. doi: 10.1016/j.bbamem.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 3.Heaver SL, Johnson EL, Ley RE. 2018. Sphingolipids in host-microbial interactions. Curr Opin Microbiol 43:92–99. doi: 10.1016/j.mib.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 4.Gomi K, Kawasaki K, Kawai Y, Shiozaki M, Nishijima M. 2002. Toll-like receptor 4-md-2 complex mediates the signal transduction induced by flavolipin, an amino acid-containing lipid unique to Flavobacterium meningosepticum. J Immunol 168:2939–2943. doi: 10.4049/jimmunol.168.6.2939. [DOI] [PubMed] [Google Scholar]
- 5.Nemati R, Dietz C, Anstadt E, Clark R, Smith M, Nichols F, Yao X. 2017. Simultaneous determination of absolute configuration and quantity of lipopeptides using chiral liquid chromatography/mass spectrometry and diastereomeric internal standards. Anal Chem 89:3583–3589. doi: 10.1021/acs.analchem.6b04901. [DOI] [PubMed] [Google Scholar]
- 6.Nemati R, Dietz C, Anstadt EJ, Cervantes J, Liu Y, Dewhirst FE, Clark RB, Finegold S, Gallagher JJ, Smith MB, Yao X, Nichols FC. 2017. Deposition and hydrolysis of serine dipeptide lipids of bacteroidetes bacteria in human arteries: relationship to atherosclerosis. J Lipid Res 58:1999–2007. doi: 10.1194/jlr.M077792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shiozaki M, Degucki N, Mochizuki T, Wakabayashi T, Ishikawa T, Haruyama H, Kawai Y, Nishijima M. 1998. Revised structure of flavolipin and synthesis of its isomers. Tetrahedron Lett 39:4497–4500. doi: 10.1016/S0040-4039(98)00798-9. [DOI] [Google Scholar]
- 8.Wang M, Hajishengallis G. 2008. Lipid raft-dependent uptake, signalling and intracellular fate of Porphyromonas gingivalis in mouse macrophages. Cell Microbiol 10:2029–2042. doi: 10.1111/j.1462-5822.2008.01185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsuda K, Amano A, Umebayashi K, Inaba H, Nakagawa I, Nakanishi Y, Yoshimori T. 2005. Molecular dissection of internalization of Porphyromonas gingivalis by cells using fluorescent beads coated with bacterial membrane vesicle. Cell Struct Funct 30:81–91. doi: 10.1247/csf.30.81. [DOI] [PubMed] [Google Scholar]
- 10.Tsuda K, Furuta N, Inaba H, Kawai S, Hanada K, Yoshimori T, Amano A. 2008. Functional analysis of alpha5beta1 integrin and lipid rafts in invasion of epithelial cells by Porphyromonas gingivalis using fluorescent beads coated with bacterial membrane vesicles. Cell Struct Funct 33:123–132. doi: 10.1247/csf.08012. [DOI] [PubMed] [Google Scholar]
- 11.Eke PI, Dye BA, Wei L, Slade GD, Thornton-Evans GO, Borgnakke WS, Taylor GW, Page RC, Beck JD, Genco RJ. 2015. Update on prevalence of periodontitis in adults in the united states: Nhanes 2009 to 2012. J Periodontol 86:611–622. doi: 10.1902/jop.2015.140520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olsen I. 2015. From the Acta Prize lecture 2014: the periodontal-systemic connection seen from a microbiological standpoint. Acta Odontol Scand 73:563–568. doi: 10.3109/00016357.2015.1007480. [DOI] [PubMed] [Google Scholar]
- 13.Olsen I. 2017. Oral microbial dysbiosis precedes development of pancreatic cancer. J Oral Microbiol 9:1374148. doi: 10.1080/20002297.2017.1374148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olsen I, Singhrao SK. 2015. Can oral infection be a risk factor for Alzheimer's disease? J Oral Microbiol 7:29143. doi: 10.3402/jom.v7.29143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nichols FC. 1994. Distribution of 3-hydroxy C17:0 in subgingival plaque and gingival tissue samples: relationship to adult periodontitis. Infect Immun 62:3753–3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nichols FC, Maraj B. 1998. Relationship between hydroxy fatty acids and prostaglandin E2 in gingival tissue. Infect Immun 66:5805–5811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nichols FC. 1998. Novel ceramides recovered from Porphyromonas gingivalis: relationship to adult periodontitis. J Lipid Res 39:2360–2372. [PubMed] [Google Scholar]
- 18.Nichols FC, Riep B, Mun J, Morton MD, Bojarski MT, Dewhirst FE, Smith MB. 2004. Structures and biological activity of phosphorylated dihydroceramides of Porphyromonas gingivalis. J Lipid Res 45:2317–2330. doi: 10.1194/jlr.M400278-JLR200. [DOI] [PubMed] [Google Scholar]
- 19.Clark RB, Cervantes JL, Maciejewski MW, Farrokhi V, Nemati R, Yao X, Anstadt E, Fujiwara M, Wright KT, Riddle C, La Vake CJ, Salazar JC, Finegold S, Nichols FC. 2013. Serine lipids of Porphyromonas gingivalis are human and mouse Toll-like receptor 2 ligands. Infect Immun 81:3479–3489. doi: 10.1128/IAI.00803-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nichols FC, Levinbook H, Shnaydman M, Goldschmidt J. 2001. Prostaglandin E2 secretion from gingival fibroblasts treated with interleukin-1beta: effects of lipid extracts from Porphyromonas gingivalis or calculus. J Periodontal Res 36:142–152. doi: 10.1034/j.1600-0765.2001.360302.x. [DOI] [PubMed] [Google Scholar]
- 21.Saglie FR, Smith CT, Newman MG, Carranza FA Jr, Pertuiset JH, Cheng L, Auil E, Nisengard RJ. 1986. The presence of bacteria in the oral epithelium in periodontal disease. I. Immunohistochemical identification of bacteria. J Periodontol 57:492–500. [DOI] [PubMed] [Google Scholar]
- 22.Sandros J, Papapanou P, Dahlen G. 1993. Porphyromonas gingivalis invades oral epithelial cells in vitro. J Periodontal Res 28:219–226. doi: 10.1111/j.1600-0765.1993.tb01072.x. [DOI] [PubMed] [Google Scholar]
- 23.Nichols FC, Yao X, Bajrami B, Downes J, Finegold SM, Knee E, Gallagher JJ, Housley WJ, Clark RB. 2011. Phosphorylated dihydroceramides from common human bacteria are recovered in human tissues. PLoS One 6:e16771. doi: 10.1371/journal.pone.0016771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nichols FC, Riep B, Mun J, Morton MD, Kawai T, Dewhirst FE, Smith MB. 2006. Structures and biological activities of novel phosphatidylethanolamine lipids of Porphyromonas gingivalis. J Lipid Res 47:844–853. doi: 10.1194/jlr.M500542-JLR200. [DOI] [PubMed] [Google Scholar]
- 25.Wang YH, Nemati R, Anstadt E, Liu Y, Son Y, Zhu Q, Yao X, Clark RB, Rowe DW, Nichols FC. 2015. Serine dipeptide lipids of Porphyromonas gingivalis inhibit osteoblast differentiation: relationship to Toll-like receptor 2. Bone 81:654–661. doi: 10.1016/j.bone.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Triantafilou M, Morath S, Mackie A, Hartung T, Triantafilou K. 2004. Lateral diffusion of Toll-like receptors reveals that they are transiently confined within lipid rafts on the plasma membrane. J Cell Sci 117:4007–4014. doi: 10.1242/jcs.01270. [DOI] [PubMed] [Google Scholar]
- 27.Farrokhi V, Nemati R, Nichols FC, Yao X, Anstadt E, Fujiwara M, Grady J, Wakefield D, Castro W, Donaldson J, Clark RB. 2013. Bacterial lipodipeptide, lipid 654, is a microbiome-associated biomarker for multiple sclerosis. Clin Transl Immunol 2:e8. doi: 10.1038/cti.2013.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olsen I, Jantzen E. 2001. Sphingolipids in bacteria and fungi. Anaerobe 7:103–112. doi: 10.1006/anae.2001.0376. [DOI] [Google Scholar]
- 29.Kanzaki H, Movila A, Kayal R, Napimoga MH, Egashira K, Dewhirst F, Sasaki H, Howait M, Al-Dharrab A, Mira A, Han X, Taubman MA, Nichols FC, Kawai T. 2017. Phosphoglycerol dihydroceramide, a distinctive ceramide produced by Porphyromonas gingivalis, promotes RANKL-induced osteoclastogenesis by acting on non-muscle myosin ii-a (myh9), an osteoclast cell fusion regulatory factor. Biochim Biophys Acta 1862:452–462. doi: 10.1016/j.bbalip.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang YH, Jiang J, Zhu Q, Alanezi AZ, Clark RB, Jiang X, Rowe DW, Nichols FC. 2010. Porphyromonas gingivalis lipids inhibit osteoblastic differentiation and function. Infect Immun 78:3726–3735. doi: 10.1128/IAI.00225-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kalajzic I, Kalajzic Z, Kaliterna M, Gronowicz G, Clark SH, Lichtler AC, Rowe D. 2002. Use of type I collagen green fluorescent protein transgenes to identify subpopulations of cells at different stages of the osteoblast lineage. J Bone Miner Res 17:15–25. doi: 10.1359/jbmr.2002.17.1.15. [DOI] [PubMed] [Google Scholar]
- 32.Gibson FC III, Genco CA. 2007. Porphyromonas gingivalis mediated periodontal disease and atherosclerosis: disparate diseases with commonalities in pathogenesis through TLRs. Curr Pharm Des 13:3665–3675. doi: 10.2174/138161207783018554. [DOI] [PubMed] [Google Scholar]
- 33.Gibson FC III, Ukai T, Genco CA. 2008. Engagement of specific innate immune signaling pathways during Porphyromonas gingivalis induced chronic inflammation and atherosclerosis. Front Biosci 13:2041–2059. doi: 10.2741/2822. [DOI] [PubMed] [Google Scholar]
- 34.Papadopoulos G, Weinberg EO, Massari P, FC Gibson 3rd, Wetzler LM, Morgan EF, Genco CA. 2013. Macrophage-specific TLR2 signaling mediates pathogen-induced TNF-dependent inflammatory oral bone loss. J Immunol 190:1148–1157. doi: 10.4049/jimmunol.1202511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nichols FC, Housley WJ, O'Conor CA, Manning T, Wu S, Clark RB. 2009. Unique lipids from a common human bacterium represent a new class of Toll-like receptor 2 ligands capable of enhancing autoimmunity. Am J Pathol 175:2430–2438. doi: 10.2353/ajpath.2009.090544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mirucki CS, Abedi M, Jiang J, Zhu Q, Wang YH, Safavi KE, Clark RB, Nichols FC. 2014. Biologic activity of Porphyromonas endodontalis complex lipids. J Endod 40:1342–1348. doi: 10.1016/j.joen.2014.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nichols FC, Bajrami B, Clark RB, Housley W, Yao X. 2012. Free lipid A isolated from Porphyromonas gingivalis lipopolysaccharide is contaminated with phosphorylated dihydroceramide lipids: recovery in diseased dental samples. Infect Immun 80:860–874. doi: 10.1128/IAI.06180-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kassem A, Henning P, Lundberg P, Souza PP, Lindholm C, Lerner UH. 2015. Porphyromonas gingivalis stimulates bone resorption by enhancing RANKL (receptor activator of NF-kappaB ligand) through activation of Toll-like receptor 2 in osteoblasts. J Biol Chem 290:20147–20158. doi: 10.1074/jbc.M115.655787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zahlten J, Riep B, Nichols FC, Walter C, Schmeck B, Bernimoulin JP, Hippenstiel S. 2007. Porphyromonas gingivalis dihydroceramides induce apoptosis in endothelial cells. J Dent Res 86:635–640. doi: 10.1177/154405910708600710. [DOI] [PubMed] [Google Scholar]
- 40.Desta T, Graves DT. 2007. Fibroblast apoptosis induced by Porphyromonas gingivalis is stimulated by a gingipain and caspase-independent pathway that involves apoptosis-inducing factor. Cell Microbiol 9:2667–2675. doi: 10.1111/j.1462-5822.2007.00987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rohner E, Detert J, Kolar P, Hocke A, N′Guessan P, Matziolis G, Kanitz V, Bernimoulin JP, Kielbassa A, Burmester GR, Buttgereit F, Pischon N. 2010. Induced apoptosis of chondrocytes by Porphyromonas gingivalis as a possible pathway for cartilage loss in rheumatoid arthritis. Calcif Tissue Int 87:333–340. doi: 10.1007/s00223-010-9389-5. [DOI] [PubMed] [Google Scholar]
- 42.Rohner E, Hoff P, Matziolis G, Perka C, Riep B, Nichols FC, Kielbassa AM, Detert J, Burmester GR, Buttgereit F, Zahlten J, Pischon N. 2012. The impact of Porphyromonas gingivalis lipids on apoptosis of primary human chondrocytes. Connect Tissue Res 53:327–333. doi: 10.3109/03008207.2012.657308. [DOI] [PubMed] [Google Scholar]
- 43.de Pablo P, Chapple IL, Buckley CD, Dietrich T. 2009. Periodontitis in systemic rheumatic diseases. Nat Rev Rheumatol 5:218–224. doi: 10.1038/nrrheum.2009.28. [DOI] [PubMed] [Google Scholar]
- 44.de Pablo P, Dietrich T, McAlindon TE. 2008. Association of periodontal disease and tooth loss with rheumatoid arthritis in the US population. J Rheumatol 35:70–76. [PubMed] [Google Scholar]
- 45.Kaur S, White S, Bartold PM. 2013. Periodontal disease and rheumatoid arthritis: a systematic review. J Dent Res 92:399–408. doi: 10.1177/0022034513483142. [DOI] [PubMed] [Google Scholar]
- 46.Moye ZD, Valiuskyte K, Dewhirst FE, Nichols FC, Davey ME. 2016. Synthesis of sphingolipids impacts survival of Porphyromonas gingivalis and the presentation of surface polysaccharides. Front Microbiol 7:1919. doi: 10.3389/fmicb.2016.01919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bainbridge BW, Hirano T, Grieshaber N, Davey ME. 2015. Deletion of a 77-base-pair inverted repeat element alters the synthesis of surface polysaccharides in Porphyromonas gingivalis. J Bacteriol 197:1208–1220. doi: 10.1128/JB.02589-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Willis LM, Stupak J, Richards MR, Lowary TL, Li J, Whitfield C. 2013. Conserved glycolipid termini in capsular polysaccharides synthesized by ATP-binding cassette transporter-dependent pathways in Gram-negative pathogens. Proc Natl Acad Sci U S A 110:7868–7873. doi: 10.1073/pnas.1222317110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.An D, Na C, Bielawski J, Hannun YA, Kasper DL. 2011. Membrane sphingolipids as essential molecular signals for Bacteroides survival in the intestine. Proc Natl Acad Sci U S A 108(Suppl 1):S4666–S4671. doi: 10.1073/pnas.1001501107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Henry LG, McKenzie RM, Robles A, Fletcher HM. 2012. Oxidative stress resistance in Porphyromonas gingivalis. Future Microbiol 7:497–512. doi: 10.2217/fmb.12.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nichols FC, Rojanasomsith K. 2006. Porphyromonas gingivalis lipids and diseased dental tissues. Oral Microbiol Immunol 21:84–92. doi: 10.1111/j.1399-302X.2006.00264.x. [DOI] [PubMed] [Google Scholar]