Abstract
The impact of euthanasia methods on endocrine and metabolic parameters in rodent tissues and biological fluids is highly relevant for the accuracy and reliability of the data collected. However, few studies concerning this issue are found in the literature. We compared the effects of three euthanasia methods currently used in animal experimentation (i.e. decapitation, CO2 inhalation and pentobarbital injection) on the serum levels of corticosterone, insulin, glucose, triglycerides, cholesterol and a range of free fatty acids in rats. The corticosterone and insulin levels were not significantly affected by the euthanasia protocol used. However, euthanasia by an overdose of pentobarbital (120 mg/kg intraperitoneal injection) increased the serum levels of glucose, and decreased cholesterol, stearic and arachidonic acids levels compared with euthanasia by CO2 inhalation and decapitation. CO2 inhalation appears to increase the serum levels of triglycerides, while euthanasia by decapitation induced no individual discrepant biomarker level. We conclude that choice of the euthanasia methods is critical for the reliability of serum biomarkers and indicate the importance of selecting adequate euthanasia methods for metabolic analysis in rodents. Decapitation without anaesthesia may be the most adequate method of euthanasia when taking both animal welfare and data quality in consideration.
A number of different euthanasia protocols are used in animal research. Three common approaches are carbon dioxide (CO2) inhalation, pentobarbital injection and decapitation. To date, however, there are few empirically based studies that have investigated the extent to which different methods may influence the reliability and generalizability of results across studies. The euthanasia method can impact on data either directly, by altering the characteristics of biomarkers being measured, or indirectly, via the circumstances associated with the euthanasia technique, such as handling, site and timing of sample collection [1]. The method of euthanasia may induce various levels of stress response in the animals, which subsequently influences the levels of corticosterone or other endocrine signalling molecules, and eventually manifest in the tissues used in biomarker analysis. The 3R principles could serve as a theoretical framework when determining appropriate method for euthanasia [2]. The euthanasia method must be chosen to ensure that animals experience as little pain and distress as possible during the procedure. Moreover, the euthanasia technique should not interfere with the subsequent analysis of collected samples.
Among the euthanasia methods used in animal experimental studies, CO2 is the most commonly used agent for laboratory rodents. However, there is a growing body of evidence indicating that exposure to CO2 causes more than momentary pain and distress in rodents and other animals. Exposure to CO2 is strongly aversive to rodents, likely due to induction of dyspnoea and anxiety [3,4]. In addition, studies have shown that CO2 causes a range of neurochemical, respiratory and vascular responses [5–7]. Anaesthesia administration before killing animals is also commonly used in experimental studies, but the direct effect of anaesthetic compounds on the experimental results remains understudied. The injectable agent pentobarbital is recommended for use in euthanasia of all laboratory species, but its use requires a controlled substance licence and may not be practical for euthanasia of large number of animals. Furthermore, euthanasia methods that prolong the time to death and sample collection may interfere with the ability to use the tissues due to rapid post-mortem changes that may alter the parameters of interest [8–10]. Physical methods of euthanasia are another common and more rapid option that helps to avoid post-mortem alterations. Decapitation of conscious animals is described by the American veterinary medical association and the European Union directive on the protection of animals used for scientific purposes (2010/63/EU) as a conditionally acceptable euthanasia method in the hands of experienced personnel with properly maintained equipment [11]. Another reason why decapitation without anaesthesia is chosen over other methods is that it provides a means to obtain tissues and fluids that are not contaminated with chemicals such as gases and anaesthetics. For instance, decapitation results in significantly lower plasma glucose, insulin, and triglyceride levels compared to euthanasia by anesthesia, in rodents [12]. Some studies, however, report that decapitation may increase plasma electrolyte levels [13,14] and that both decapitation and gaseous anaesthetics may produce a significant rise in the levels of circulating catecholamines [15].
The secretion of corticosteroids and catecholamines is affected by various types of stress [16]. Corticosterone is the main glucocorticoid involved in regulation of stress response in rodents and considered as an important index of stress [17]. A physical stressor triggers a pre-programmed response that causes an increase in the release of corticosterone, which promotes lipolysis and proteolysis and stimulates gluconeogenesis [18]. These actions trigger an increase in blood glucose and free fatty acids (FFAs) [19]. The secretory response of catecholamines and norepinephrine during stress also causes lipid mobilization from adipose tissue through lipolysis in fat cells [20]. The increase in lipolytic activity following physical stress is accompanied by an elevation of circulating levels of triglycerides, FFA and glycerol [21].
Lipids have long been recognized as signalling molecules that have the capacity to trigger profound physiological responses. Fatty acids represent a class of lipids that has crucial functions in all mammalian cells, maintaining vital cellular processes at various levels [22]. FFAs are not only an essential energy source, but also regulate various cellular processes and physiological functions beyond energy metabolism [23]. In this context, elevated plasma levels of FFAs alone are sufficient to induce insulin resistance and activate pro-inflammatory responses [24]. However, the roles of fatty acids in metabolic diseases are not fully understood. It was recently suggested that exposure to a subclass of endocrine disrupting chemicals may disrupt normal development and balance of lipid metabolism, which may lead to obesity and metabolic disorders [25,26]. These diseases are a rapidly increasing global health threat; thus, the identification of new biomarkers, underlying mechanisms and novel pharmacological targets is important. For this, animal models play a central role and adequate euthanasia protocols are critical for accurate studies of serum markers and mechanisms.
Hence, the impact of euthanasia methods on endocrine and metabolic factors is an important issue that needs further attention in regard to metabolic research as well as for animal welfare. The aim of this study was to compare the effects of three commonly used euthanasia methods (i.e. decapitation, CO2 inhalation and pentobarbital injection) on endocrine and metabolic biomarkers in serum of rats.
Materials and Methods
Animals and housing
Twenty-four 5-week-old female Wistar rats (Taconic, Ejby, Denmark) were housed 4 per cage in transparent polysulfone cages (59 × 38 × 20 cm) containing wood-chip bedding and nesting material. The animals were maintained on standard pellet food (R36 Labfor; Lantmännen, Kimstad, Sweden) and water ad libitum, and were housed in a temperature- and humidity-controlled environment with a 12-hr light/dark cycle (lights on at 6 a.m.). The animal experimental protocol was approved by the Uppsala Animal Ethical Committee and was consistent with the Swedish Legislation on Animal Experimentation (Animal Welfare Act SFS1998:56) and the European Union Directive on the Protection of Animals Used for Scientific Purposes (2010/63/EU).
Effects of different euthanasia methods on metabolic biomarkers in serum
We compared the effects of the three aforementioned euthanasia methods on metabolic biomarkers in serum (n = 8 per protocol). All rats were individually handled during the week prior to testing and killed by one experienced person. The rats in the first group were killed by decapitation, using a guillotine. The rats in the second and third groups were killed by CO2 inhalation – 2 min. 30 sec., fixed time and gradually increased concentration – and an overdose of pentobarbital – 120 mg/kg intraperitoneal injection, in a volume of 1 ml/100 g of body-weight – respectively. Blood samples from all groups of animals were collected using a standardized protocol. After decapitation, 1 ml trunk blood was collected at the decapitation site and allowed to coagulate before centrifugation at 1000 × g for 10 min. and the serum was stored at −80°C until analysis.
The analysis of corticosterone and insulin was conducted using the Coat-A-Count Rat Corticosterone 125I RIA kit (Siemens Medical Solutions, Los Angeles, CA, USA) and the Mercodia Rat Insulin ELISA (Mercodia, Uppsala, Sweden) following the instructions of the manufacturer. Triglycerides, cholesterol and glucose were analysed with enzymatic colorimetric methods using an automated chemistry analyser Architect c4000 (Abbott Diagnostics, Lake Forest, IL, USA). FFAs were extracted from serum by protein precipitation and quantified by mass spectrometry. In short, 10 μl of serum was added to an equal volume of an internal standard mix (2H2-16:0, 13C16-16:1n-7, 2H2-18:0, 2H2-18:1n-9, 2H4-18:2n-6, 2H6-20:3n-6, 2H8-20:4n-6, prepared in methanol), and 80 μl of methanol. Samples were vortexed, and precipitated proteins were removed by centrifugation. Supernatants were diluted in 10 volumes of methanol in glass autosampler vials, and immediately quantified by liquid chromatography–tandem mass spectrometry as previously described [9]. Twelve FFA – myristic acid, palmitic acid, palmitoleic acid, stearic acid, oleic acid, linoleic acid, α-linolenic acid, γ-linolenic acid, dihomo-γ-linolenic acid, arachidonic acid, eicosapentaenoic acid and docosahexaenoic acid – were analysed. The analytes were separated on a Kinetex 2.6-μm core shell pentafluorophenyl column (100 × 2.1 mm, 100 Å; Phenomenex, Macclesfield, UK) using a Prominence UFLCXR system (Shimadzu, Milton Keynes, UK), and detected by ‘pseudo-molecular’ scheduled multiple reaction monitoring transition on a QTRAP 5500 hybrid triple quadrupole mass spectrometer (AB Sciex, Warrington, UK). Analyst software version 1.5.1 (AB Sciex) was used for data acquisition and analysis.
Statistical analysis
One-way ANOVA was used to compare the three different euthanasia protocols across the measured parameters. Among the biomarkers found to be different, pairwise comparison between the groups was conducted using Fischer’s LSD test. Differences were considered statistically significant at p < 0.05.
Results
No statistically significant differences in serum levels (mean ± S.E.M.) of the stress hormone corticosterone among rats were observed between the three euthanasia methods: decapitation (960 ± 197 nmol/l), CO2 inhalation (1253 ± 408 nmol/l) and pentobarbital overdose (1608 ± 270 nmol/l) (fig. 1).
Fig. 1.
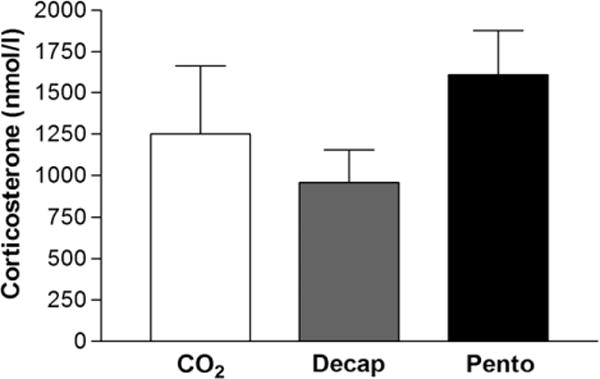
Impact of euthanasia methods on the stress hormone corticosterone levels in serum. Values represent mean ± S.E.M. There were no significant differences in corticosterone levels in the three groups (ANOVA, n = 8).
However, for the metabolic biomarkers analysed, the group killed by intraperitoneal injection of a pentobarbital overdose differed significantly from the groups that were killed by decapitation or CO2 inhalation (fig. 2). There was no statistically significant difference in serum insulin between the decapitation (0.25 ± 0.03 μg/l), CO2 inhalation (0.53 ± 0.15 μg/l) and pentobarbital overdose (0.72 ± 0.23 μg/l) euthanasia protocols. The group of animals killed by a pentobarbital overdose had significantly higher serum glucose levels (8.66 ± 0.21 nmol/l) compared with the groups killed by decapitation (7.38 ± 0.09 nmol/l, p = 0.00096) or CO2 inhalation (7.52 ± 0.34 nmol/l, p = 0.0026). This group also had significantly lower cholesterol levels (2.09 ± 0.07 nmol/l), compared with the decapitation (2.35 ± 0.07 nmol/l, p = 0.017) and CO2 (2.44 ± 0.06 nmol/l, p = 0.0020) groups. In addition, the levels of serum triglycerides were found to be higher in the group killed by CO2 inhalation (2.65 ± 0.31 nmol/l), compared with the pentobarbital (1.78 ± 0.11 nmol/l, p = 0.016) and decapitation (1.99 ± 0.24 nmol/l, p = 0.058) groups (fig. 2).
Fig. 2.
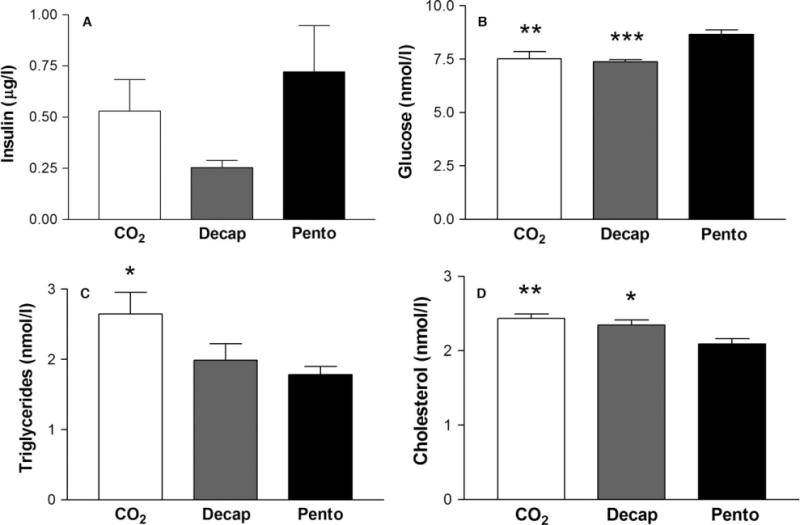
Impact of euthanasia methods on metabolic serum biomarkers. The method of euthanasia influences the levels of metabolic biomarkers in serum. Values represent mean ± S.E.M. *p < 0.05, **p < 0.01, ***p < 0.001 compared with animals killed by pentobarbital (ANOVA followed by Fischer’s LSD test; n = 8).
The analysis of FFA revealed that although the pentobarbital group had lower mean concentrations of several species, only two were statistically significant (table 1). Stearic acid was the fatty acid most affected by the euthanasia method. The group of animals killed by pentobarbital overdose had significantly lower serum stearic acid levels (16.4 ± 1.8 μM) compared with the groups killed by decapitation (26.7 ± 1.7 μM, p = 0.00016) or CO2 inhalation (23.8 ± 1.3 μM, p = 0.0034). The serum concentrations of arachidonic acid were also found to be lower in the pentobarbital group (56.8 ± 3.4 μM) compared with the decapitation (67.1 ± 1.5 μM, p = 0.023) and CO2 inhalation (66.1 ± 3.6 μM, p = 0.040) groups. No significant differences in FFA concentrations could be detected between the groups killed by CO2 or decapitation.
Table 1.
Impact of euthanasia methods on serum free fatty acids.
| Free fatty acid | CO2 inhalation | Decapitation | Pentobarbital | p-Values (ANOVA) |
|---|---|---|---|---|
| [14:0] | 31.7 ± 6.6 | 25.3 ± 2.5 | 16.0 ± 3.8 | 0.076 |
| [16:0] | 190.5 ± 31.0 | 185.2 ± 18.7 | 161.7 ± 23.5 | 0.69 |
| [16:1] | 69.4 ± 12.7 | 60.6 ± 4.5 | 53.3 ± 10.0 | 0.51 |
| [18:0] | 23.8 ± 1.3** | 26.7 ± 1.7*** | 16.4 ± 1.8 | 0.00049 |
| [18:1] | 100.6 ± 11.9 | 106.4 ± 9.2 | 107.2 ± 13.8 | 0.91 |
| [18:2] | 159.1 ± 13.2 | 163.0 ± 11.5 | 160.5 ± 17.7 | 0.98 |
| [18:3n-3] | 12.0 ± 1.5 | 12.3 ± 1.3 | 11.4 ± 1.4 | 0.89 |
| [18:3n-6] | 8.2 ± 4.4 | 8.9 ± 4.7 | 6.4 ± 3.4 | 0.91 |
| [20:3] | 6.1 ± 0.7 | 5.8 ± 0.3 | 5.4 ± 0.5 | 0.58 |
| [20:4] | 66.1 ± 3.6* | 67.1 ± 1.5* | 56.8 ± 3.4 | 0.045 |
| [20:5n-3] | 2.7 ± 0.3 | 2.6 ± 0.1 | 2.6 ± 0.3 | 0.86 |
| [22:6n-3] | 19.7 ± 1.6 | 16.9 ± 0.8 | 18.1 ± 1.5 | 0.35 |
Myristic acid (14:0), Palmitic acid (16:0), Palmitoleic acid (16:1n-7), Stearic acid (18:0), Oleic acid (18:1n-9), Linoleic acid (18:2n-6), α-Linolenic acid (18:3n-3), γ-Linolenic acid (18:3n-6), Dihomo-γ-linolenic acid (20:3n-6), Arachidonic acid (20:4n-6), Eicosapentaenoic acid (20:5n-3), Docosahexaenoic acid (22:6n-3).
Values represent mean ± S.E.M. (μM).
p < 0.05,
p < 0.01,
p < 0.001 compared with animals killed by pentobarbital (ANOVA followed by Fischer’s LSD test; n = 8).
Discussion
The present study sheds light on the effects of different euthanasia methods on the levels of serum endocrine and metabolic markers in rats. We compared three euthanasia methods currently used in the scientific research – CO2 inhalation, decapitation and intraperitoneal pentobarbital injection. Euthanasia by a pentobarbital overdose induced large alterations in the analysed metabolic biomarkers compared with CO2 inhalation and decapitation. In this group of animals, the mean glucose level was significantly higher compared with the CO2 and decapitation groups, while the mean cholesterol and stearic and arachidonic acid levels were lower, compared with the other euthanasia methods. In addition to the effects induced by the anaesthetic pentobarbital, CO2 inhalation appeared to increase the triglyceride levels in serum.
The choice of euthanasia method both affects the validity of data and is important to the humane treatment of the animals involved in the research. Inhaled gaseous agents, such as CO2 and anaesthetics including isoflurane, are considered to be an adequate euthanasia method, especially for large groups of animals and after initial sedation [11]. Nevertheless, the aversive potential of these commonly used methods has been criticized in terms of animal welfare [6,27–29]. Intraperitoneal administration of pentobarbital and other barbiturates may also cause pain, likely as a result of the high pH of the solution [30]. Although the American and European guidelines primarily recommend other euthanasia methods, research indicates that decapitation – which leads to loss of consciousness in 3–6 sec. – induces less distress in the animals and may be preferable compared with the more commonly used inhaled gaseous agents and pentobarbital injections in an animal welfare perspective [6,27]. In our experimental conditions, we also measured the lowest levels of the stress biomarker corticosterone in animals killed by decapitation – 40% and 23% lower than the groups that were killed by pentobarbital or CO2, respectively – although the differences between the euthanasia protocols did not reach statistical significance. We cannot exclude that the study is underpowered as the observed trend is in agreement with a previous study reporting that decapitation with prior administration of CO2 or pentobarbital increased corticosterone levels when compared with decapitation without anaesthesia [31]. It is well-established that serum corticosterone is elevated during experimental manipulations and can be used as a biomarker of stress in laboratory animals. However, it is also known that the basal levels of corticosterone can differ substantially from study to study and some of the disparity might be attributable to the methods of blood collection [17]. In addition, the corticosterone level fluctuates over time aggravating its use as a biomarker of stress and increasing the number of animals needed for reliable results [32,33].
To further understand the effect of the euthanasia protocol on serum biomarkers, we analysed several metabolic parameters and non-esterified fatty acids. The effect of the three euthanasia methods on the serum levels of insulin and glucose was studied as they could also be affected by other factors than typical stressors [34,35]. Anaesthesia per se may modulate glucose homeostasis by affecting pancreatic insulin release [36,37]. Interestingly, we found increased serum glucose without any observed changes in insulin levels in the group killed by a pentobarbital overdose. Increased glucose concentration without significant change in corticosterone or insulin levels could be the result of increased sympathoadrenal system activity or serum norepinephrine concentration [38,39]. Our results also showed that euthanasia with pentobarbital lowers serum cholesterol and FFA levels. Previous studies demonstrate that barbituric acid derivatives have hypolipidaemic activity. Hall and collaborators (1990) found that a barbituric analogue interferes with de novo synthesis of cholesterol and fatty acids in the early steps after 14 days of drug administration. The rat serum lipoprotein showed reduced VLDL triglycerides and HDL cholesterol contents [40]. In the present study, euthanasia by pentobarbital overdose significantly lowered stearic (31% and 39%) and arachidonic acid (14% and 15%) levels, compared with the groups that were killed by CO2 or decapitation, respectively. This is in agreement with data from previous research, which found that surgical anaesthesia with pentobarbital significantly attenuated FFA accumulation in brain during ischaemia, and the arachidonic and stearic acids are the most affected FFA [41]. In addition, our previous study demonstrated that the group killed by a pentobarbital overdose had decreased brain levels of all studied FFA (15–44%) compared with the other euthanasia protocols [9]. Barbiturates such as pentobarbital are known to mediate their pharmacological action by acting as agonist at the GABAA receptor but also altering the physiological properties of biological membranes [42]. Furthermore, pentobarbital has been shown to inhibit phospholipase activity [43], probably via reduced protein kinase A activation [44,45]. These mechanisms may explain the lower levels of serum FFA in the group of animals killed by injection of pentobarbital compared with the two other methods.
In summary, our findings clearly demonstrate that the euthanasia methods are critical for the reliability of collected serum biomarker data. An overdose of pentobarbital induced the largest alterations in the metabolic biomarkers. CO2 inhalation appears to affect only the levels of triglycerides, while euthanasia by decapitation induced no individual discrepant metabolic level, compared with the two other euthanasia methods. Decapitation without anaesthesia may be the most adequate method of euthanasia when taking both animal welfare and data quality in consideration. However, further studies of the biochemical and molecular effects of different euthanasia protocols are warranted.
Acknowledgments
The authors would like to thank Ms. Marita Berg for excellent technical assistance.
Funding
Financial support was given by the FACIAS and Carl Tryggers Foundations.
Footnotes
Disclosure Statement
The authors have nothing to disclose.
References
- 1.Dunn J, Scheving L. Plasma corticosterone levels in rats killed sequentially at the “trough” or “peak” of the adrenocortical cycle. J Endocrinol. 1971;49:347–8. doi: 10.1677/joe.0.0490347. [DOI] [PubMed] [Google Scholar]
- 2.Hubrecht RC, Kirkwood J. The UFAW Handbook on the Care and Management of Laboratory and Other Research Animals. 8th. Wiley-Blackwell; United Kingdom: 2010. p. 848. [Google Scholar]
- 3.Ziemann AE, Allen JE, Dahdaleh NS, Drebot II, Coryell MW, Wunsch AM, et al. The amygdala is a chemosensor that detects carbon dioxide and acidosis to elicit fear behavior. Cell. 2009;139:1012–21. doi: 10.1016/j.cell.2009.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Niel L, Stewart SA, Weary DM. Effect of flow rate on aversion to gradual-fill carbon dioxide exposure in rats. Appl Anim Behav Sci. 2008;109:77–84. [Google Scholar]
- 5.Woodbury DM, Rollins LT, Gardner MD, Hirschi WL, Hogan JR, Rallison ML, et al. Effects of carbon dioxide on brain excitability and electrolytes. Am J Physiol. 1958;192:79–90. doi: 10.1152/ajplegacy.1957.192.1.79. [DOI] [PubMed] [Google Scholar]
- 6.Conlee KM, Stephens ML, Rowan AN, King LA. Carbon dioxide for euthanasia: concerns regarding pain and distress, with special reference to mice and rats. Lab Anim. 2005;39:137–61. doi: 10.1258/0023677053739747. [DOI] [PubMed] [Google Scholar]
- 7.Raj ABM, Leach MC, Morton DB. Carbon dioxide for euthanasia of laboratory animals. Comp Med. 2004;54:470–1. [PubMed] [Google Scholar]
- 8.Hickman DL, Johnson SW. Evaluation of the aesthetics of physical methods of euthanasia of anesthetized rats. J Am Assoc Lab Anim Sci. 2011;50:695–701. [PMC free article] [PubMed] [Google Scholar]
- 9.Jernerén F, Söderquist M, Karlsson O. Post-sampling release of free fatty acids – effects of heat stabilization and methods of euthanasia. J Pharmacol Toxicol Methods. 2015;71:13–20. doi: 10.1016/j.vascn.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Stingl C, Soderquist M, Karlsson O, Boren M, Luider TM. Uncovering effects of ex vivo protease activity during proteomics and peptidomics sample extraction in rat brain tissue by oxygen-18 labeling. J Proteome Res. 2014;13:2807–17. doi: 10.1021/pr401232e. [DOI] [PubMed] [Google Scholar]
- 11.AVMA. American Veterinary Medical Association (AVMA) Guidelines for the Euthanasia of Animals. 2013. AVMA; 1931 N. Meacham Road, Schaumburg, IL 60173, USA: 2013. [Google Scholar]
- 12.Bhathena SJ. Comparison of effects of decapitation and anesthesia on metabolic and hormonal parameters in Sprague-Dawley rats. Life Sci. 1992;50:1649–55. doi: 10.1016/0024-3205(92)90451-t. [DOI] [PubMed] [Google Scholar]
- 13.Conahan ST, Narayan S, Vogel WH. Effect of decapitation and stress on some plasma electrolyte levels in rats. Pharmacol Biochem Behav. 1985;23:147–9. doi: 10.1016/0091-3057(85)90143-1. [DOI] [PubMed] [Google Scholar]
- 14.Traslavina RP, King EJ, Loar AS, Riedel ER, Garvey MS, Ricart-Arbona R, et al. Euthanasia by CO(2) inhalation affects potassium levels in mice. J Am Assoc Lab Anim Sci. 2010;49:316–22. [PMC free article] [PubMed] [Google Scholar]
- 15.Popper CW, Chiueh CC, Kopin IJ. Plasma catecholamine concentrations in unanesthetized rats during sleep, wakefulness, immobilization and after decapitation. J Pharmacol Exp Ther. 1977;202:144–8. [PubMed] [Google Scholar]
- 16.Thurston JH, Hauhart RE. Effect of momentary stress on brain energy metabolism in weanling mice: apparent use of lactate as cerebral metabolic fuel concomitant with a decrease in brain glucose utilization. Metab Brain Dis. 1989;4:177–86. doi: 10.1007/BF01000294. [DOI] [PubMed] [Google Scholar]
- 17.Vachon P, Moreau JP. Serum corticosterone and blood glucose in rats after two jugular vein blood sampling methods: comparison of the stress response. J Am Assoc Lab Anim Sci. 2001;40:22–4. [PubMed] [Google Scholar]
- 18.Reeder DM, Kramer KM. Stress in free-ranging mammals: integrating physiology, ecology, and natural history. J Mammal. 2005;86:225–35. [Google Scholar]
- 19.Tharp GD. The role of glucocorticoids in exercise. Med Sci Sports Exerc. 1975;7:6–11. [PubMed] [Google Scholar]
- 20.Marcus C, Ehren H, Bolme P, Arner P. Regulation of lipolysis during the neonatal period. Importance of thyrotropin. J Clin Invest. 1988;82:1793–7. doi: 10.1172/JCI113793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lonnqvist F, Wennlund A, Wahrenberg H, Arner P. Effects of mental stress on lipolysis in humans. Metabolism. 1992;41:622–30. doi: 10.1016/0026-0495(92)90054-e. [DOI] [PubMed] [Google Scholar]
- 22.Hussain G, Schmitt F, Loeffler JP, Gonzalez de Aguilar JL. Fatting the brain: a brief of recent research. Front Cell Neurosci. 2013;7:144. doi: 10.3389/fncel.2013.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wymann MP, Schneiter R. Lipid signalling in disease. Nat Rev Mol Cell Biol. 2008;9:162–76. doi: 10.1038/nrm2335. [DOI] [PubMed] [Google Scholar]
- 24.Boden G, She PX, Mozzoli M, Cheung P, Gumireddy K, Reddy P, et al. Free fatty acids produce insulin resistance and activate the proinflammatory nuclear factor-kappa B pathway in rat liver. Diabetes. 2005;54:3458–65. doi: 10.2337/diabetes.54.12.3458. [DOI] [PubMed] [Google Scholar]
- 25.Heindel JJ, Newbold R, Schug TT. Endocrine disruptors and obesity. Nat Rev Endocrinol. 2015;11:653–61. doi: 10.1038/nrendo.2015.163. [DOI] [PubMed] [Google Scholar]
- 26.Grun F, Blumberg B. Environmental obesogens: organotins and endocrine disruption via nuclear receptor signaling. Endocrinology. 2006;147:S50–5. doi: 10.1210/en.2005-1129. [DOI] [PubMed] [Google Scholar]
- 27.Valentim AM, Guedes SR, Pereira AM, Antunes LM. Euthanasia using gaseous agents in laboratory rodents. Lab Anim. 2016;50:241–53. doi: 10.1177/0023677215618618. [DOI] [PubMed] [Google Scholar]
- 28.Hawkins P, Prescott MJ, Carbone L, Dennison N, Johnson C, Makowska IJ, et al. A good death? Report of the Second Newcastle Meeting on Laboratory Animal Euthanasia. Animals (Basel) 2016;6:1–28. doi: 10.3390/ani6090050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chisholm JM, Pang DS. Assessment of carbon dioxide, carbon dioxide/oxygen, isoflurane and pentobarbital killing methods in adult female Sprague-Dawley rats. PLoS ONE. 2016;11:e0162639. doi: 10.1371/journal.pone.0162639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Svendsen O, Kok L, Lauritzen B. Nociception after intraperitoneal injection of a sodium pentobarbitone formulation with and without lidocaine in rats quantified by expression of neuronal c-fos in the spinal cord–a preliminary study. Lab Anim. 2007;41:197–203. doi: 10.1258/002367707780378140. [DOI] [PubMed] [Google Scholar]
- 31.Vahl TP, Ulrich-Lai YM, Ostrander MM, Dolgas CM, Elfers EE, Seeley RJ, et al. Comparative analysis of ACTH and corticosterone sampling methods in rats. Am J Physiol Endocrinol Metab. 2005;289:E823–8. doi: 10.1152/ajpendo.00122.2005. [DOI] [PubMed] [Google Scholar]
- 32.Calvano SE, Reynolds RW. Circadian fluctuations in plasma corticosterone, corticosterone-binding activity and total protein in male rats: possible disruption by serial blood sampling. Endocr Res. 1984;10:11–25. doi: 10.1080/07435808409046762. [DOI] [PubMed] [Google Scholar]
- 33.Lightman SL. The neuroendocrinology of stress: a never ending story. J Neuroendocrinol. 2008;20:880–4. doi: 10.1111/j.1365-2826.2008.01711.x. [DOI] [PubMed] [Google Scholar]
- 34.Winder WW, Fuller EO, Conlee RK. Adrenal hormones and liver cAMP in exercising rats – different modes of anesthesia. J Appl Physiol. 1983;55 doi: 10.1152/jappl.1983.55.5.1634. [DOI] [PubMed] [Google Scholar]
- 35.Nishiyama T, Yamashita K, Yokoyama T. Stress hormone changes in general anesthesia of long duration: isoflurane-nitrous oxide vs sevoflurane-nitrous oxide anesthesia. J Clin Anesth. 2005;17:586–91. doi: 10.1016/j.jclinane.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 36.Ewart RBL, Rusy BF, Bradfod MW. Effects of enflurane on release of insulin by pancreatic islets in vitro. Anesth Analg. 1981;60:878–84. [PubMed] [Google Scholar]
- 37.Saha JK, Xia J, Grondin JM, Engle SK, Jakubowski JA. Acute hyperglycemia induced by ketamine/xylazine anesthesia in rats: mechanism and implications for preclinical models. Exp Biol Med (Maywood) 2005;230:777–84. doi: 10.1177/153537020523001012. [DOI] [PubMed] [Google Scholar]
- 38.Diltoer M, Camu F. Glucose homeostasis and insulin secretion during isoflurane anesthesia in humans. Anesthesiology. 1988;68:880–6. doi: 10.1097/00000542-198806000-00008. [DOI] [PubMed] [Google Scholar]
- 39.Carruba MO, Bondiolotti G, Picotti GB, Catteruccia N, Da Prada M. Effects of diethyl ether, halothane, ketamine and urethane on sympathetic activity in the rat. Eur J Pharmacol. 1987;134:15–24. doi: 10.1016/0014-2999(87)90126-9. [DOI] [PubMed] [Google Scholar]
- 40.Hall IH, Patrick MA, Maguire JH. Hypolipidemic activity in rodents of phenobarbital and related derivatives. Arch Pharm. 1990;323:579–86. doi: 10.1002/ardp.19903230905. [DOI] [PubMed] [Google Scholar]
- 41.Nemoto EM, Shiu GK, Nemmer J, Bleyaert AL. Attenuation of brain free fatty acid liberation during global ischemia: a model for screening potential therapies for efficacy? J Cereb Blood Flow Metab. 1982;2:475–80. doi: 10.1038/jcbfm.1982.54. [DOI] [PubMed] [Google Scholar]
- 42.Harris RA, Schroeder F. Effects of barbiturates and ethanol on the physical properties of brain membranes. J Pharmacol Exp Ther. 1982;223:424–31. [PubMed] [Google Scholar]
- 43.Hattori T, Nishimura Y, Sakai N, Yamada H, Kameyama Y, Banno Y, et al. Inhibitory effect of pentobarbital on phospholipase C activity in ischaemic rat brain. Neurol Res. 1987;9:164–8. doi: 10.1080/01616412.1987.11739789. [DOI] [PubMed] [Google Scholar]
- 44.Dan’ura T, Kurokawa T, Yamashita A, Yanagiuchi H, Ishibashi S. Relationship between the inhibition of adenylate cyclase by pentobarbital and the functional coupling of Ns and the catalytic unit. Biochem Biophys Res Commun. 1986;140:237–42. doi: 10.1016/0006-291x(86)91081-8. [DOI] [PubMed] [Google Scholar]
- 45.Strokin M, Sergeeva M, Reiser G. Docosahexaenoic acid and arachidonic acid release in rat brain astrocytes is mediated by two separate isoforms of phospholipase A2 and is differently regulated by cyclic AMP and Ca2+ Br J Pharmacol. 2003;139:1014–22. doi: 10.1038/sj.bjp.0705326. [DOI] [PMC free article] [PubMed] [Google Scholar]


