Abstract
Cranial diabetes insipidus (CDI) is a treatable chronic condition that can potentially develop into a life-threatening medical emergency. CDI is due to the relative or absolute lack of the posterior pituitary hormone vasopressin (AVP), also known as anti-diuretic hormone. AVP deficiency results in uncontrolled diuresis. Complete deficiency can lead to polyuria exceeding 10 L/24 h. Given a functioning thirst mechanism and free access to water, patients with CDI can normally maintain adequate fluid balance through increased drinking. Desmopressin (DDAVP, a synthetic AVP analogue) reduces uncontrolled water excretion in CDI and is commonly used in treatment. Critically, loss of thirst perception (through primary pathology or reduced consciousness) or limited access to water (through non-availability, disability or inter-current illness) in a patient with CDI can lead to life-threatening dehydration. This position can be further exacerbated through the omission of DDAVP. Recent data have highlighted serious adverse events (including deaths) in patients with CDI. These adverse outcomes and deaths have occurred through a combination of lack of knowledge and treatment failures by health professionals. Here, with our guideline, we recommend treatment pathways for patients with known CDI admitted to hospital. Following these guidelines is essential for the safe management of patients with CDI.
Keywords: diabetes insipidus, pituitary, hypernatraemia, desmopressin, DDAVP, guideline
Introduction
Cranial diabetes insipidus (CDI) is due to the relative or absolute lack of the posterior pituitary hormone vasopressin (AVP), also known as anti-diuretic hormone (ADH). AVP is the major determinant of renal water resorption. AVP deficiency results in uncontrolled diuresis. Complete deficiency can lead to polyuria exceeding 10 L/24 h. Given a functioning thirst mechanism and free access to water, patients with CDI can normally maintain adequate fluid balance through increased drinking. Desmopressin (DDAVP, a synthetic AVP analogue) reduces uncontrolled water excretion in CDI and is commonly used in treatment. Critically, loss of thirst perception (through primary pathology or reduced consciousness) or limited access to water (through non-availability, disability or inter-current illness) in a patient with CDI can lead to life-threatening dehydration. This position can be further exacerbated through the omission of DDAVP. Recent data have highlighted serious adverse events (including deaths) in patients with CDI. These adverse outcomes and deaths have occurred through a combination of lack of knowledge and treatment failures by health professionals (1).
Scope of guidance
This guidance is for health care professionals with a role in the care of patients admitted to hospital, intermediate or supported care facilities. It covers the management of adult patients with established CDI. It does not cover the initial diagnosis of CDI, peri-operative management of CDI following pituitary surgery or the treatment of CDI in pregnancy. Important aspects of the decision making process during the management of inpatients with CDI are summarized in Figure 1.
Figure 1.
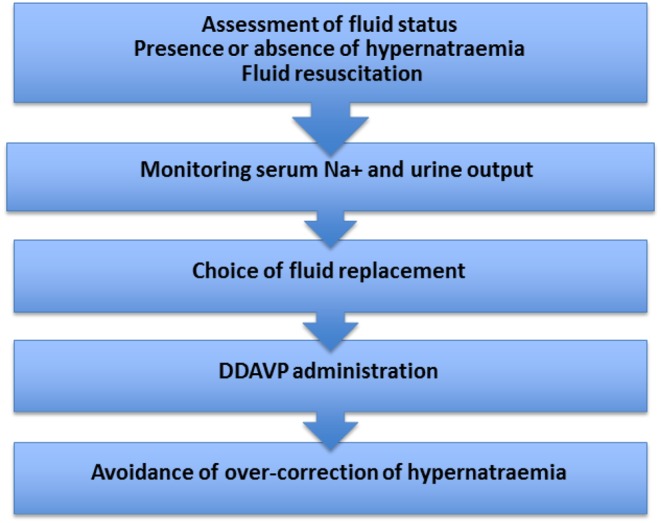
Important aspects in decision making during the management of inpatients with cranial diabetes insipidus.
Aetiology of CDI and important associated comorbidities
CDI can occur in isolation. However, CDI is commonly found in the context of wider hypopituitarism or indeed as part of a more complex neurodevelopmental or acquired neurological/post-neurosurgical clinical problem (2). Patients with CDI may thus have a range of comorbidities (sensory, motor, communication and cognitive) impacting directly and indirectly on their ability to manage fluid balance and medication independently, especially in an environment with which they are not familiar.
Patients with CDI who require hydrocortisone, prednisolone or other glucocorticoid replacement because of wider hypopituitarism require increased doses and/or parenteral administration in the acute setting during inter-current illness (3). Glucocorticoids enable renal free water excretion. If an acute inter-current illness renders a patient unable to give a history of pre-existing CDI, the problem may only become apparent to the clinician after parenteral glucocorticoid administration.
Given the importance of an intact thirst mechanism in enabling maintenance of fluid balance, patients with adipsia and CDI are particularly vulnerable. Some patients with adipsic CDI have continuous, low level, non-osmoregulated AVP production. This group of patients are thus at risk of both dehydration with hypernatraemia and dilutional hyponatraemia if fluid intake is not managed appropriately (4).
Risk assessment and risk stratification
Certain patient groups with CDI are at particular risk of rapid deterioration (Box 1).
Box 1.
Patient groups at particular risk of rapid deterioration of CDI.
| • Frail or physically unwell |
| • Acute or chronic confusion |
| • Reduced consciousness level |
| • Nil by mouth, fasting or low oral intake |
| • Unable to self-administer DDAVP |
| • Hypo- or hypernatraemia |
| • Patients with adipsia (no thirst sensation) |
| • Communication/language difficulties |
| • Patients who have multiple transfers within an institution/facility |
Desmopressin preparations
Desmopressin comes in the form of tablets, intranasal spray and solution, sublingual preparations and injectable forms (Table 1).
Table 1.
Preparations of desmopressin.
| Route of administration | Desmopressin dose |
|---|---|
| Oral or sublingual | 100–200 µg (0.1–0.2 mg) |
| Intranasal spray | 10–20 µg |
| IM or IV injection | 1–2 µg |
Organisational guidance for hospitals and other care facilities
We recommend that all patients in a hospital or care facility who have a diagnosis of CDI be identified on admission.
We recommend that the endocrinology or alternative, appropriate clinical team is alerted to and remain aware of all patients in a hospital or care facility who have a diagnosis of CDI.
We recommend that all wards or equivalent clinical areas have 24-h access to DDAVP.
We recommend that patients with CDI undergoing elective surgery are highlighted in the pre-assessment process and that colleagues in peri-operative care develop a clear plan to cover the management of CDI during their admission.
We suggest hospitals and other care facilities develop an alert system that serves to highlight all inpatients requiring on-going treatment with DDAVP to reduce harm from medication errors (including missed prescribing, incorrect prescribing and failure of dispensing/administration).
The approach to patients who are able to drink or require maintenance intravenous fluid therapy and care able to take DDAVP
We recommend that if patients are orientated, with a normal Glasgow coma scale (GCS), and an intact thirst mechanism and are able to drink, they have easy access to water by the bedside and receive DDAVP as prescribed and agreed by the clinical team.
We recommend that for those patients with normal serum sodium and who require maintenance intravenous fluid therapy, the type and volume administered should be equivalent to the daily requirements appropriate for other patients without CDI. This should be given together with the agreed, prescribed dose of DDAVP.
We recommend that in those patients requiring maintenance intravenous fluid therapy and who are taking regular DDAVP, serum sodium is monitored at least every 24 h while on intravenous fluids to avoid the occult development of hyponatraemia.
We suggest that patients able to self-administer medication are allowed to give their own DDAVP, in line with the recommended prescribed dose agreed with the clinical team.
The approach to patients with impaired consciousness or who are unable to manage own fluid intake and medication
We recommend that for those patients with normal serum sodium, the type and volume of fluid administered should be equivalent to the daily requirements appropriate for other patients without CDI. This should be given together with the agreed, prescribed dose of DDAVP.
We recommend regular assessment of fluid status, including monitoring of fluid input and urine output, together with measurement of serum sodium at least every 12 h until patients are able to manage own fluid intake or are clinically stable.
We suggest moving to oral or nasogastric water as the replacement fluid of choice as quickly as is clinically safe (including safety of swallowing) as this route provides a good buffer against rapid changes in serum sodium.
The approach to patients who are significantly unwell with inter-current illness or decompensated CDI
-
Assessment of fluid status; the presence or absence of hypernatraemia and fluid resuscitation:
We recommend the urgent clinical assessment of volume and hydration status; and measurement of serum sodium, potassium and renal function.
We recommend that patients with hypernatraemia should be managed as a medical emergency with a level 2–3 care or equivalent high dependency setting. Patients with CDI may not be polyuric at presentation if effective circulating volume status is compromised. Patients may be volume depleted even with normal sodium levels. Hypernatraemia may be classified as mild (sodium 146–149 mmol/L), moderate (sodium 150–159 mmol/L) or severe (sodium >160 mmol/L).
We recommend that in patients who are intravascular volume depleted, volume status (as shown by blood pressure and pulse rate) should be restored with 0.9% sodium chloride in water prior to consideration of free water administration.
-
Monitoring of serum sodium and urine output:
We recommend measurement of serum sodium every 4 h during fluid resuscitation, reducing to no less frequently than every 12 h until clinically and biochemically stable.
We suggest that a high urine output with low urine osmolality indicates DDAVP is required.
-
Choice of fluid replacement:
We recommend that optimising fluid replacement should take priority, followed by assessing the need for DDAVP administration.
We recommend that in patients with hypernatraemia, the type and volume of fluid replacement reflects the standard daily fluid and electrolyte requirement together with a component of the estimated fluid deficit such that correction of hypernatraemia is controlled. The sodium content of common infusates and the rate of administration will influence the change in serum sodium (Box 2).
We suggest that oral or nasogastric water is considered as soon as is clinically safe, as this route can provide a buffer against over-rapid changes in serum sodium.
-
DDAVP administration:
We recommend treatment with IV or IM DDAVP 1–2 μg in a patient with known CDI and excessive, inappropriately dilute urine output, with close observation of the clinical and biochemical response.
To avoid the development of over-rapid correction of hypernatraemia through the combination of high fluid loads together with the obligate antidiuresis of exogenous DDAVP, we suggest that further doses of DDAVP are considered when production of higher volumes of dilute (100 mosmol/kg) urine returns.
-
Avoidance of over-rapid overcorrection of hypernatraemia:
We recommend that in symptomatic patients with hypernatraemia that has developed within 48 h (acute hypernatraemia), serum sodium be corrected at a rate of 5 mmol/L in the first hour (or until symptoms improve) and is limited to 10 mmol/L per 24 h.
We recommend that in patients with no or mild symptoms, hypernatraemia be corrected at a rate not exceeding 0.5 mmol/L/h and is limited to 10 mmol/L/24 h.
-
Specialist referral for on-going management:
We recommend all patients with CDI admitted to hospital should be discussed with the endocrine team as soon as possible, to ensure optimal inpatient care and specialist follow-up thereafter.
Box 2.
Sodium content of common infusates
| • 5% dextrose in water (D5 W): 0 mmol/L |
| • 0.2% sodium chloride in 5% dextrose in water (D5 2 NS): 34 mmol/L |
| • 0.45% sodium chloride in water (0.45 NS): 77 mmol/L |
| • Ringer’s lactate solution: 130 mmol/L |
| • 0.9% sodium chloride in water (0.9 NS): 154 mmol/L |
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this guideline.
Funding
This work did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Disclaimer
The document should be considered as a guideline only. It is not intended to determine an absolute standard of medical care. The doctor concerned must make the management plan for the individual patient.
References
- 1.NHS England Patient Safety Alert. Risk of severe harm or death when desmopressin is omitted or delayed in patients with cranial diabetes insipidus. Alert reference number: NHS/PSA/W/2016/001. Redditch, UK: NHS England, 2016. (available at: https://www.england.nhs.uk/2016/02/psa-desmopressin/)
- 2.Fleseriu M, Hashim IA, Karavitaki N, Melmed S, Murad MH, Salvatori R, Samuels MH. Hormonal replacement in hypopituitarism in adults: an Endocrine Society Clinical Practice Guideline. Journal of Clinical Endocrinology and Metabolism 2016. 101 3888–3921. ( 10.1210/jc.2016-2118) [DOI] [PubMed] [Google Scholar]
- 3.Arlt W. & The Society for Endocrinology Clinical Committee. Society for Endocrinology Endocrine Emergency Guidance. Emergency management of acute adrenal insufficiency (adrenal crisis) in adult patients. Endocrine Connections 2016. 5 G1–G3. ( 10.1530/EC-16-0054) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ball S, Barth J, Levy M. & Society for Endocrinology Clinical Committee. Society for Endocrinology Endocrine Emergency Guidance. Emergency management of severe symptomatic hyponatraemia in adult patients. Endocrine Connections 2016. 5 G4–G6. ( 10.1530/EC-16-0058) [DOI] [PMC free article] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a