Abstract
Immunotherapy treatment with checkpoint inhibitors (CPI) (CTLA-4 and PD-1 inhibitors) significantly improves survival in a number of cancers. Treatment can be limited by immune-mediated adverse effects including endocrinopathies such as hypophysitis, adrenalitis, thyroiditis and diabetes mellitus. If endocrinopathies (particularly hypocortisolemia) are not recognized early, they can be fatal. The diagnosis and management of endocrinopathies can be complicated by simultaneous multi-organ immune adverse effects. Here, we present Endocrine Emergency Guidance for the acute management of the endocrine complications of checkpoint inhibitor therapy, the first specialty-specific guidance with Endocrinology, Oncology and Acute Medicine input and endorsed by the Society for Endocrinology Clinical Committee. We present algorithms for management: endocrine assessment and management of patients in the first 24 hours who present life-threateningly unwell (CTCAE grade 3–4) and the appropriate management of mild-moderately unwell patients (CTCAE grade 1–2) presenting with features compatible with an endocrinopathy. Other important considerations in relation to hypohysitis and the maintenance of glucocorticoid therapy are discussed.
Introduction
Immunotherapy treatment with checkpoint inhibitors (CPI) such as ipilimumab (CTLA-4 inhibitor), nivolumab and pembrolizumab (PD-1 inhibitors) significantly improves prognosis in a number of cancers (1, 2, 3). Combination therapy with ipilimumab and nivolumab is approved in the United Kingdom for the treatment of advanced melanoma but indications for immunotherapy, the cancers that benefit and the number of agents available are increasing. However, treatment can be limited by immune-mediated adverse effects particularly with combination treatment (3, 4, 5, 6).
Immune-mediated endocrinopathies as a consequence of treatment with checkpoint inhibitors include hypophysitis, adrenalitis, thyroiditis and diabetes mellitus (7, 8, 9, 10, 11, 12, 13, 14, 15). These can be life-threatening if not recognised and treated appropriately; deaths have been reported.
Diagnosis and management in this group can be complicated by simultaneous multi-organ immune adverse effects, e.g. presentation with colitis and hypophysitis.
Early recognition and appropriate management of these endocrinopathies is essential. Multiple, informative review articles have been published with regards to the mechanisms, incidence and screening strategies. While endocrinologists and oncologists may be familiar with the complications of CPI treatment, these patients frequently present as emergencies to those unfamiliar with these agents. This guidance has been developed as an expert consensus between endocrinologists, oncologists and an acute physician and is designed to aid the early phase of care.
This document therefore covers:
Endocrine assessment (first 24 h) of patients treated with CPI’s who present life-threateningly unwell (CTCAE (Common Terminology Criteria for Adverse Events) grade 3–4: Algorithm 1).
Appropriate management of a mild-to-moderately unwell patient presenting with clinical features compatible with an endocrinopathy (CTCAE grade 1–2: Algorithms 2 and 3).
Other important considerations; hypophysitis and maintenance glucocorticoid therapy.
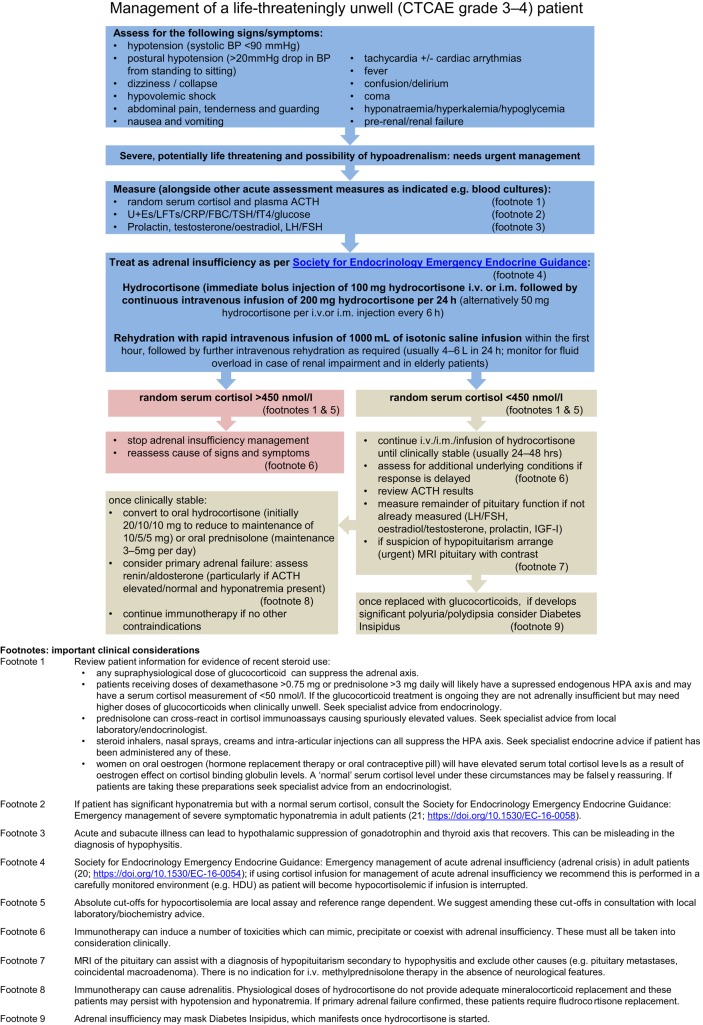
Algorithm 1.
Management of a life-threateningly unwell (CTCAE grade 3–4) patient.
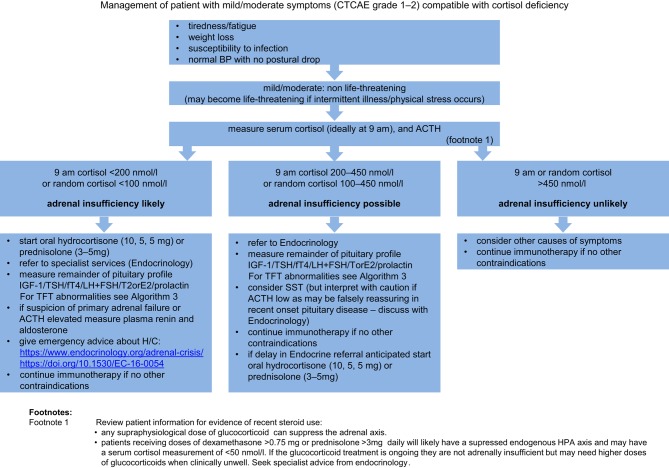
Algorithm 2.
Management of a mild/moderately unwell patient presenting with clinical features compatible with an endocrinopathy or endocrine abnormalities detecting during routine screening. CTCAE grade 1–2.
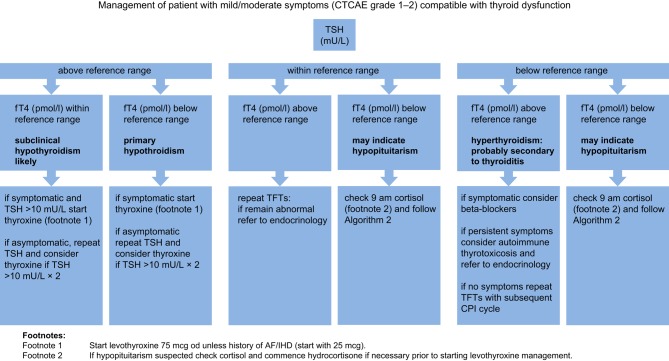
Algorithm 3.
Management of a mild/moderately unwell patient presenting with clinical features compatible with an endocrinopathy or endocrine abnormalities detecting during routine screening. CTCAE grade 1–2.
Management of a life-threateningly unwell patient (CTCAE grade 3–4)
Cortisol
Features of acute cortisol deficiency may be non-specific. Any patient receiving a CPI who presents severely unwell should be assumed to have acute cortisol deficiency until proven otherwise and treated with glucocorticoids until serum cortisol result available (20; https://doi.org/10.1530/EC-16-0054) (Algorithm 1).
In the acute setting, primary (e.g. caused by adrenalitis) and secondary (e.g. caused by hypophysitis) cortisol deficiency are treated identically.
A baseline (pre-glucocorticoid treatment) serum cortisol of >450 nmol/L excludes cortisol deficiency (for exceptions see clinical considerations in Algorithm 1), and glucocorticoid treatment can be discontinued at this point if this is the only indication. If there is any doubt about the presence of cortisol deficiency glucocorticoids should be continued and an endocrine opinion sought. It is crucial to obtain a good drug history with regards to recent glucocorticoid use to enable correct interpretation of results.
Methylprednisolone is not an appropriate treatment for acute cortisol deficiency secondary to hypophysitis or adrenalitis (16). Methylprednisolone may be beneficial for pressure effects such as optic chiasm compromise, visual field defects, cranial nerve palsies and in some cases, intractable headache. If methylprednisolone or other pharmacological dose glucocorticoids are administered for this or other non-endocrine immune complications, additional hydrocortisone is not required.
If significant polyuria, polydipsia and/or hypernatremia occurs following glucocorticoid replacement; consider the possibility of diabetes insipidus. Seek urgent specialist/endocrine input.
In view of the multiplicity of immune adverse events seen with CPI’s if there is not a significant improvement once cortisol deficiency has been corrected over the first 24 h, then additional diagnoses must also be explored.
Thyroid dysfunction
It is rare for acute CPI thyroiditis to cause a patient to be life-threateningly unwell although one potential case of ‘thyroid storm’ and one of ‘myxedema’ have been reported (17, 18) (Algorithm 3).
If severe thyrotoxicosis or thyroid storm features are present, we recommend supportive management in a critical care setting and endocrine input (19).
If myxedema secondary to hypothyroidism is suspected, specialist endocrine input should be sought. Thyroxine should never be instigated unless cortisol deficiency is excluded as it can trigger an adrenal crisis. If in doubt, treat for cortisol deficiency first.
Hyperglycemia
CPI’s can induce pancreatic failure and an insulin-deficient diabetes mellitus.
Plasma glucose should be measured in all unwell patients treated with a CPI.
Hyperglycemia should be managed as per local guidance for diabetic keto-acidosis or hyperglycemic hyperosmolar state as appropriate. Pancreatic antibodies (e.g. GAD 65) and C-peptide should be measured.
Supraphysiological doses of glucocorticoids used as therapy for immune-mediated complications can induce diabetes, potentially causing or worsening hyperglycemia and therefore monitoring of CBGs (capillary blood glucose) is essential in all patients.
If important to the oncology outcome, CPI therapy could be recommenced early after effective treatment for life-threatening endocrinopathy, with close observation.
Management of a mild/moderately unwell patient presenting with clinical features compatible with an endocrinopathy or endocrine abnormalities detected during routine screening (CTCAE grade 1–2)
Cortisol deficiency
If low serum cortisol levels (09:00 h <200 nmol/L or random cortisol <100 nmol/L) are detected in a mildly symptomatic/asymptomatic patient start oral hydrocortisone.
The serum cortisol cut-offs described in these algorithms are designed to ensure patient safety and err on initiating hydrocortisone therapy if there is any possibility of cortisol deficiency. Initiating glucocorticoid therapy on the basis of this guidance should not be taken as a definitive diagnosis of adrenal insufficiency. Referral to endocrinology services is advised in all cases.
Patients should be provided with a Steroid Emergency Card, education with regards to ‘sick day rules’, and an hydrocortisone emergency injection kit as per Society for Endocrinology guidance (20).
Immunotherapy can be continued once patient is clinically stable on appropriate endocrine replacement therapy (Algorithms 2 and 3).
Thyroid dysfunction
The most common CPI endocrinopathy is thyroiditis. In general, it starts with a transient hyperthyroid phase, which may not be symptomatic, followed by permanent subclinical or overt hypothyroidism. There have been reports of Graves’ hyperthyroidism and opthalmopathy, thyroid storm and myxedema. Thyroid function tests should be performed as per Algorithm 3.
Thyrotoxic phase of thyroiditis should be managed symptomatically with beta blockers and regular monitoring of thyroid function tests. Subsequent hypothyroidism is likely to occur and requires treatment with thyroxine (Algorithm 3 footnote 2). Pharmacological glucocorticoid therapy is not required and thioamide treatment (carbimazole/propylthiouracil) is of no value.
If there is clinical uncertainty, an iodine uptake scan should be considered (uptake reduced in thyroiditis and increased in Graves’ thyrotoxicosis). Iodinated contrast imaging may result in false-negative (sodium iodide) thyroid uptake scans. Institution-specific advice should be sought if thyroid nuclear medicine imaging is being performed after contrast administration. If thyrotoxic phase is persistent or there is evidence of thyroid eye disease, TSHRAbs should be measured and treatment with thioamides should be considered. Severe thyroid eye disease (CAS (Clinical Activity Score) >3 or evidence of optic nerve compression) should be referred urgently to ophthalmology/local thyroid eye service.
Levothyroxine should never be instigated until co-existent cortisol deficiency is excluded or treated as this can trigger an adrenal crisis.
Immunotherapy can be continued once patient is clinically stable on appropriate endocrine therapy.
Hypophysitis
Hypophysitis can present with either hormone defects or mass effect. It is an immune-mediated inflammation of the pituitary gland, the exact mechanism of this in relation to CPIs is not fully elucidated, but it almost inevitably results in permanent hypopituitarism, of one or more pituitary axis. Unlike most other causes of hypopituitarism, e.g. radiotherapy, surgery, pituitary adenoma, hypophysitis is well recognized as a cause of isolated ACTH deficiency that may present as acute cortisol deficiency and should be treated as described elsewhere in this document.
If hypophysitis is suspected clinically then a contrast-enhanced MRI pituitary scan should be performed as soon as possible.
Headache, diplopia and cranial nerve palsies (CTCAE grade 3–4) should trigger an urgent MRI scan of the pituitary; to assess the potential need for treatment with IV methylprednisolone and to distinguish intracranial metastases as a differential diagnosis.
The value of IV methylprednisolone in the treatment of autoimmune hypophysitis, of any cause, is controversial, and there is minimal evidence of reversal of hormone deficiencies in CPI-induced hypophysitis but it may be of benefit for pressure effects such as optic chiasm compromise causing visual field defects, cranial nerve palsies and in some cases, headache.
Growth hormone deficiency does not need diagnosis or treatment in the acute phase. TSH and gonadotrophin deficiencies are also potential consequences of hypophysitis (frequency may differ with different CPIs) and should be treated if persistent. Acutely unwell patients of any etiology can have a suppressed TSH (sick euthyroid syndrome) and gonadotrophin axis but acute initiation of replacement is not indicated. Referral to an endocrine service is recommended.
Maintenance steroid therapy following acute cortisol deficiency
Tapering of treatment dose glucocorticoids (hydrocortisone or prednisolone) can be started after clinical recovery (usually 48–72 h). Aim for a daily replacement dose of hydrocortisone 10 mg mane, 5 mg lunchtime and 5 mg early evening or prednisolone 3–5 mg once daily. Referral to endocrinology is recommended.
Maintenance glucocorticoid will usually be hydrocortisone but prednisolone is an acceptable alternative.
If evidence of primary adrenal insufficiency, mineralocorticoid replacement (fludrocortisone) may be needed once daily glucocorticoid dose is below 50 mg hydrocortisone/24 h or equivalent. This is not required for hypophysitis and other causes of secondary hypoadrenalism.
A short Synacthen test may be falsely reassuring if performed within 6–12 weeks of an episode of hypophysitis. Endocrinologist involvement is recommended.
Prior to discharge, patients should be provided with a Steroid Emergency Card, education with regards to ‘sick day rules’, and an hydrocortisone emergency injection kit as per Society for Endocrinology guidance (20).
The serum cortisol cut-offs described in these algorithms are designed to ensure patient safety and err on initiating hydrocortisone therapy if there is any possibility of cortisol deficiency. Initiating glucocorticoid therapy on the basis of this guidance should not be taken as a definitive diagnosis of adrenal insufficiency. Referral to endocrinology services is advised in all cases. Patients should be re-evaluated once stable to confirm diagnosis and at intermittent intervals to assess for HPA axis recovery.
Immunotherapy can be continued once patient clinically stable on appropriate endocrine replacement therapy.
Declaration of interest
CEH, TC, PC & PJT declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this work. PL has received grants, personal fees and non-financial support from Bristol Myers Squibb; personal fees and non-financial support from MSD; and personal fees from Novartis, Roche, Amgen, Nektar, Pierre Fabre, and Nera Care. AO-B is an MRC Clinical Training Fellow based at the University of Liverpool supported by the North West England Medical Research Council Fellowship Scheme in Clinical Pharmacology and Therapeutics, which is funded by the Medical Research Council (Award Ref. MR/N025989/1), Roche Pharma, Eli Lilly and Company Limited, UCB Pharma, Novartis, the University of Liverpool and the University of Manchester; and has received honoraria from BMS and MSD in providing local certified continuing education.
Funding
This work did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
References
- 1.Hodi FS, O’Day S, McDermott D, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al Improved survival with ipilimumab in patients with metastatic melanoma. New England Journal of Medicine 2010. 363 711–723. ( 10.1056/NEJMoa1003466) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ansell S, Lesokhin A, Borrello I, Halwani A, Scott EC, Gutierrez M, Schuster SJ, Millenson MM, Cattry D, Freeman GJ, et al PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. New England Journal of Medicine 2015. 372 311–319. ( 10.1056/NEJMoa1411087) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spain L, Diem S, Larkin J. Management of toxicities of immune checkpoint inhibitors. Cancer Treatment Reviews 2016. 44 51–60. ( 10.1016/j.ctrv.2016.02.001) [DOI] [PubMed] [Google Scholar]
- 4.Champiat S, Lambotte O, Barreau E, Belkhir R, Berdelou A, Carbonnel F, Cauquil C, Chanson P, Collins M, Durrbach A, et al Management of immune checkpoint blockade dysimmune toxicities: a collaborative position paper. Annals of Oncology 2016. 27 559–574. ( 10.1093/annonc/mdv623) [DOI] [PubMed] [Google Scholar]
- 5.Naidoo J, Page D, Li B, Connell LC, Schindler K, Lacouture ME, Postow MA, Wolchok JD. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Annals of Oncology 2015. 26 2375–2391. ( 10.1093/annonc/mdv383) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, et al Combined nivolumab and ipilimumab or monotherapy in metastatic melanoma. New England Journal of Medicine 2015. 373 23–34. ( 10.1056/NEJMoa1504030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Torino F, Baranbei A, Paragliola R, Marchetti P, Salvatori R, Corsello SM. Endocrine side effects of anti-cancer drugs: mAbs and pituitary dysfunction. European Journal of Endocrinology 2015. 172 195–204. ( 10.1530/EJE-14-0845) [DOI] [PubMed] [Google Scholar]
- 8.Dillard T, Yedinak C, Alumkal J, Fleseriu M. Anti-CTLA-4 antibody therapy associated autoimmune hypophysitis: serious immune related adverse events across a spectrum of cancer types. Pituitary 2010. 13 29–38. ( 10.1007/s11102-009-0193-z) [DOI] [PubMed] [Google Scholar]
- 9.Faje A, Sullivan R, Lawrence D, Tritos NA, Fadden R, Klibanski A, Nachtigall L. Ipilimumab-induced hypophysitis: a detailed longitudinal analysis in a large cohort of patients with metastatic melanoma. Journal of Clinical Endocrinology and Metabolism 2014. 99 4078–4085. ( 10.1210/jc.2014-2306) [DOI] [PubMed] [Google Scholar]
- 10.Min L, Ibrahim N. Ipilimumab-induced autoimmune adrenalitis. Lancet Diabetes and Endocrinology 2013. 1 e15 ( 10.1016/S2213-8587(13)70031-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bacanovic S, Burger J, Stolzmann P, Hafner J, Huellner MW. Ipilimumab-induced adrenalitis: a possible pitfall in 18F-FDG-PET/CT. Clinical Nuclear Medicine 2015. 40 e518–e519. ( 10.1097/RLU.0000000000000887) [DOI] [PubMed] [Google Scholar]
- 12.Min L, Vaidya A, Becker C. Thyroid autoimmunity and ophthalmopathy related to melanoma biological therapy. European Journal of Endocrinology 2011. 164 303–307. ( 10.1530/EJE-10-0833) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Orlov S, Salari F, Kashat L, Walfish PG. Induction of painless thyroiditis in patients receiving programmed death 1 receptor immunotherapy for metastatic malignancies. Journal of Clinical Endocrinology and Metabolism 2015. 100 1738–1741. ( 10.1210/jc.2014-4560) [DOI] [PubMed] [Google Scholar]
- 14.Gaudy C, Clevy C, Monestier S, Dubois N, Préau Y, Mallet S, Richard MA, Grob JJ, Valéro R, Béliard S. Anti-PD1 pembrolizumab can induce exceptional fulminant type 1 diabetes. Diabetes Care 2015. 38 e182–e183. ( 10.2337/dc15-1331) [DOI] [PubMed] [Google Scholar]
- 15.Hughes J, Vudattu N, Sznol M, Gettinger S, Kluger H, Lupsa B, Herold KC. Precipitation of autoimmune diabetes with anti-PD1 immunotherapy. Diabetes Care 2015. 38 e55–e57. ( 10.2337/dc14-2349) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Min L, Hodi F, Giobbie-Hurder A, Ott PA, Luke JJ, Donahue H, Davis M, Carroll RS, Kaiser UB. Systemic high-dose corticosteroid treatment does not improve the outcome of ipilimumab-related hypophysitis: a retrospective cohort study. Clinical Cancer Research 2015. 21 749–755. ( 10.1158/1078-0432.CCR-14-2353) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McMillen B, Dhillon MS, Yong-Yow S. A rare case of thyroid storm. BMJ Case Reports 2016. ( 10.1136/bcr-2016-214603) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khan U, Rizvi H, Sano D, Chiu J, Hadid T. Nivolumab induced myxedema crisis. Journal for ImmunoTherapy of Cancer 2017. 5 13 ( 10.1186/s40425-017-0213-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ross DS, Burch HB, Cooper DS, Greenlee MC, Laurberg P, Maia AL, Rivkees SA, Samuels M, Sosa JA, Stan MN, et al 2016 American Thyroid Association Guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid 2016. 26 1343–1421. ( 10.1089/thy.2016.0229) [DOI] [PubMed] [Google Scholar]
- 20.Arlt W.& Society for Endocrinology Clinical Committee. SOCIETY FOR ENDOCRINOLOGY EMERGENCY GUIDANCE: Emergency management of acute adrenal insufficiency (adrenal crisis) in adult patients. Endocrine Connections 2016. 5 G1–G3. ( 10.1530/EC-16-0054) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ball S, Barth J, Levy M. & Society for Endocrinology Clinical Committee. SOCIETY FOR ENDOCRINOLOGY ENDOCRINE EMERGENCY GUIDANCE: Emergency management of severe symptomatic hyponatremia in adult patients. Endocrine Connections 2016. 5 G4–G6. ( 10.1530/EC-16-0058) [DOI] [PMC free article] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a