Abstract
Cell therapy presents a promising alternative for the treatment of degenerative diseases. The main sources of adult stem cells are bone marrow, adipose tissue and peripheral blood. Within those tissues, there are cell subpopulations that share pluripotential characteristics. Nevertheless, there is insufficient data to determine which of these stem cell subtypes would have a better possibility to differentiate to a specific tissue. The objective of this research was to analyze and compare the stemness genes expression from peripheral blood and adipose tissue of plastic adherent cells, and those immune-selected by the CD133+ and CD271+ membrane markers. On all cell subpopulation groups, self-renew capacity, the membranes markers CD73, CD90 and CD105, as well as the stemness genes NANOG, OCT4, SOX2, REX1, NOTCH1 and, NESTIN expression were analyzed. Results showed that all samples presented the minimal criteria to define them as human stem cells. All cell subpopulation were capable of self-renewal. Nevertheless, the subpopulation cell types showed differences on the time needed to reach confluence. The slowest doubling times were for those cells bearing the CD133 marker from both sources. Surface markers determined by flow cytometry were positive for CD73, CD90 and, CD105, and negative for CD45. The stemness gene expression was positive in all subpopulation. However, there were significant differences in the amount and pattern of expression among them. Those differences could be advantageous in finding the best option for their application on cell therapy. Cells with high expression of OCT4 gene could be a better opportunity for neuron differentiation like CD133+ blood cells. On the other hand, lowest expression of NOTCH1 on CD271+ cells from the same source could be a better possibility for myoblast differentiation. The observed differences could be used as an advantage to find which cell type and from the different source; this represents the best option for its application on cell therapy. Experiments focused on the best response to specific differentiation, are conducted in order to confirm those possibilities.
Keywords: Blood mesenchymal stem cells, MSCs, blood CD133+ cells, blood CD271+ cells, adipose derived stem cells, ADSCs, ADSC-CD133+, ADSC-CD271+, stemness genes, NESTIN
Introduction
Mesenchymal stem cells (MSCs) comprise multiple cell types and are generally defined as undifferentiated, capable of self-renewal, and with a high proliferative capacity. These cells express the membrane markers CD73, CD90, and CD105. Of great interest is the fact that a comparative analysis of MSCs obtained from different sources such as bone marrow, umbilical cord, blood, and adipose tissue demonstrated a wide range of proliferation and differentiation capacities in vitro [1]. These events could be explained as a consequence of the presence of different cell subtypes within the initial isolated cell mass, independent of the source. In fact, there are reports indicating that the primary culprit causing the differences in proliferation and differentiation capacities toward the production of neural tissue from MSCs incubated in neuro-induction medium is this heterogeneity in cell populations [2-5]. Therefore, these findings justify a careful search for defining which stem cell subtypes are the most suitable for defined cell therapy protocols. In general, MSCs used in cell therapy have been selected based on the effects related to their capacity of trans-differentiation into specific cell lineages, neovascularization, and immune modulation, which apparently are common to all stem cells. Today, there is no consensus of which specific stem cells are better for cell therapy applications, nor about which cell sub-type should be selected for the restoration of a specific tissue. For example, in the case of a damaged heart, van der Bogt et al. [6] reported comparisons of relative efficacies defined as the recovery of cardiac ischemic insults obtained by transplantations of different cell types, and concluded that bone marrow mononuclear cells were more effective than bone marrow MSCs. Similarly, Ma et al. [7] reported that adult human stem cells were better than umbilical cord blood stem cells for treatment of myocardial ischemia. Studies on the in vivo behavior of adipose derived stem cells (ADSCs) and MSCs in the infarcted heart have demonstrated that both populations of stem cells are not prone for survival within the cardiac environment, resulting in acute donor cell death and a subsequent loss of cardiac function similar to that in control groups. However, from these bad scenarios, there was a better survival rate of MSCs [8]. Based on the reports of Davy (2013) [9], enrichment of autologous bone marrow stem cells bearing the CD34+ membrane marker could increase the efficacy of treatments in the clinical setting for this condition. Furthermore, the associated presence of CD133+ marker in these enriched subsets when used in clinical trials showed improvement in myocardial viability and local perfusion of the infarcted zone [10-15].
Therefore, the main objective of this manuscript was to analyze, under the same in vitro conditions, if the expression of genes associated with pluripotency was similar in all stem cells subtypes obtained from different sources. The results could provide some evidence to support the researcher’s decision on the cell subtype to be used in specific cell therapy protocols, looking for the best possible results.
Material and methods
Ethics statement
The study protocol was authorized by the Research and Ethics Committee of the School of Medicine Tec de Monterrey and the Research and Ethics Committee of the Hospital San Jose Tec de Monterrey (Reg. 13CI19039138).
Stem cell isolation from adipose tissue
Abdominal adipose tissue was harvested from healthy donors aged 30-40 years. Lipoaspirates were obtained under written informed consent, and the procedures were performed as previously described [6]. Briefly, the lipoaspirate were digested with 0.1% collagenase type I-S (Sigma-Aldrich) dissolved in Hank’s buffer (Gibco, Grand Island, NY). Digestion was performed on a shaker at 37°C and 250 rpm for 60 min. The enzyme was inactivated by adding an equal volume of Dulbecco’s Modified Eagle’s medium (DMEM-F12, Gibco) supplemented with 10% fetal bovine serum (FBS, Gibco). Mature adipocytes and connective tissue were separated from pellets of mononuclear cells by centrifugation at 800 g for 10 min. The debris and the dissociated tissue present in the pellets were eliminated through filtration using a 100-μm cell strainer and washing with phosphate-buffered saline (PBS). All the pellets were mixed and plated on a Ficoll-Paque gradient (GE Healthcare BioSciences, Piscataway, NJ) to reduce erythrocyte contamination. The collected mononuclear fraction was further washed with PBS and filtered through a 40-μm cell strainer to remove debris and cell clumps. The sample obtained was divided into three fractions. One fraction was seeded directly into 25-cm2 flasks containing DMEM-F12 supplemented with 10% FBS, 50 U/ml penicillin, and 50 μg/ml streptomycin (ADSCs-MSCs). When the culture of these flasks reached confluence, one sample of this fraction was processed for RNA isolation. The second fraction was used for CD271+ cell isolation by magnetic labeling beads bearing an anti-CD271 antibody (MicroBead kit, Miltenyi Biotec, Gladbach, Germany). Cells retained on the column (positive fraction: AD-CD271+) were obtained by elution and divided in two fraction, one was processed for RNA isolation and the other one seeded on into 25-cm2 flasks containing DMEM-F12 supplemented with 10% FBS, 50 U/ml penicillin, and 50 μg/ml streptomycin. The third fraction was used for CD133+ cell isolation by magnetic labeling beads bearing an anti-CD133 antibody (MicroBead kit, Miltenyi Biotec, Gladbach, Germany). Cells retained on the column (positive fraction: AD-CD133+) were also obtained by elution and divided in two fraction, one was processed for RNA isolation, and the other one seeded into 25-cm2 flasks containing DMEM-F12 supplemented with 10% FBS, 50 U/ml penicillin, and 50 μg/ml streptomycin.
Stem cell isolation from peripheral blood sample
Peripheral blood mononuclear cells from healthy donors were obtained by venipuncture and were initially enriched by Percoll density gradient centrifugation. The collected mononuclear fraction was washed three times with PBS. The sample obtained was divided into three fractions; one fraction was seeded directly into 25-cm2 flasks containing DMEM-F12 supplemented with 10% FBS, 50 U/ml penicillin, and 50 μg/ml streptomycin (PB-MSCs). When the culture reached confluence, one sample of this fraction was processed for RNA isolation. The second fraction was used for CD271+ cell isolation by magnetic labeling beads bearing an anti-CD271 antibody (MicroBead kit, Miltenyi Biotec, Gladbach, Germany) as explained above. The elution obtained cells (PB-CD271+) were divided in two fractions. One fraction was processed for RNA isolation, the other one was seeded into 25-cm2 flasks containing DMEM-F12 supplemented with 10% FBS, 50 U/ml penicillin, and 50 μg/ml streptomycin. The third fraction was used for CD133+ cell isolation by magnetic labeling beads bearing an anti-CD133 antibody (MicroBead kit, Miltenyi Biotec, Gladbach, Germany). The retained cells (PB-CD133+) after release was divided in two fractions, one fraction was processed for RNA isolation, the other one was seeded into 25-cm2 flasks containing DMEM-F12 supplemented with 10% FBS, 50 U/ml penicillin, and 50 μg/ml streptomycin.
Surface marker analysis by flow cytometry from PB and AD subpopulation cells
To analyze the expression of specific surface proteins, 3 × 105 MSCs (passage 1), CD271+ and CD133 were incubated for 30 min with 20 μl of blocking reagent and 10 μl of the following fluorochrome-conjugated monoclonal antibodies in appropriate combinations: CD34-PE, CD45-FITC, CD73-PE, CD90-FITC, CD105-PE, CD271-APC, and CD133-PE (BD Biosciences, San Diego, CA, USA). After staining, the cells were washed with MACS-BSA stock solution (Miltenyi Biotec, Teterow, Germany), centrifuged, and resuspended in MACS-BSA. The samples were evaluated by the FACSCanto II Instrument, and the data were analyzed using the FACSDiva software (BD Biosciences).
Gene expression by reverse transcription-polymerase chain reaction (RT-PCR) from PB and AD subpopulation cells
RNA was isolated according to the manufacturer’s instructions for the GenElude mammalian total RNA miniPrep kit (Sigma), and RT-PCR was performed according to the instruction manual of the One-Step RT-PCR kit (Qiagen, Crawley, UK). The selected pluripotential genes were: SOX2, NOTCH1, REX1, OCT4, and NANOG. As well as NESTIN gene to determine, proliferative state. These genes were analyzed for adherent plastic cells, or cells bearing either CD133 or CD271 membrane markers. As an internal control gene, GAPDH was used. RT-PCR products for each target gene were resolved by 2% agarose gel electrophoresis and visualized with SYBR Green (Qiagen) [diluted 1:10,000 in Tris-borate-ethylenediaminetetraacetic acid (TBE)]. The bands were observed under UV light and photographed in a UVP high-performance UV transilluminator (DigiDoc-It; Cambridge, UK) and analyzed with the GelAnalyzer program.
Statistical analysis
Statistical analyses were performed using SPSS software (v. 17.0; SPSS Inc., Chicago, IL). For each variable under study, the medians, standard deviations, and ranges were calculated. The Pearson correlation between CD133+ and CD271+ cell or MSC gene expression was considered to be significant for values of P < 0.05. Otherwise, the nonparametric Kendall’s tau b test was performed.
Results
Self-renewal
According to the protocol, cells obtained from lipoaspirate samples and peripheral blood were seeded under similar cell number and culture conditions. Results showed that all stem cell cultures obtained from AD have the capacity of self-renewal under in vitro conditions. However, the time to reach 80% confluence varied between them, with AD-MSCs mononuclear fractions being the most efficient (10-12 days). ADSCs bearing CD271+ or CD133+ antigens reached confluence at 14-18 days.
Self-renewal capacity of stem cells isolated from peripheral blood also showed differences among their subpopulations. PB-MSCs reached confluence at 10-12 days, cells bearing CD271+ reached confluence at 16-18 days, and cells bearing CD133+ reached confluence at 18-21 days. All other cell subgroups were capable of forming CFUs at the same time as the culture reached confluence.
Surface marker analysis by flow cytometry
FACS analysis of MSC fractions recovered from peripheral blood and lipoaspirate showed positive markers for CD90, CD105, CD73, CD271, CD133, and CD34. Nevertheless, after specific magnetic bead separation, samples obtained by coupling with anti-CD133 antibodies were negative to CD271 membrane marker. The opposite was true, thus making cells CD133 and CD271 mutually exclusive. None of these cells were positive for CD45 (Table 1). All the obtained fractions that showed the membrane markers were considered as multipotential markers [7].
Table 1.
Surface markers determined by FACS on samples obtained from peripheral blood (PB) and lipoaspirate (AD)
| Membrane marker | PB-MSCs | PB-CD133+ | PB-CD271+ | AD-MSCs | AD-CD133+ | AD-CD271+ |
|---|---|---|---|---|---|---|
| CD73 | +++ | +++ | +++ | +++ | +++ | +++ |
| CD90 | ++ | +++ | +++ | +++ | +++ | +++ |
| CD105 | ++ | +++ | +++ | +++ | +++ | +++ |
| CD34 | +++ | + | + | + | + | + |
| CD45 | +++ | - | - | + | - | - |
| CD133 | + | + | - | + | + | - |
| CD271 | + | - | + | + | - | ++ |
Pluripotential gene expression in samples from peripheral blood
CD133+ and CD271+ cell samples from peripheral blood showed a positive expression for all the pluripotential genes tested. Nevertheless there were significant differences between subpopulations: NANOG showed different expression levels between peripheral blood subpopulations, among which PB-MSCs showed higher expression than that observed on CD133+ and CD271+ cells. CD133+ cells showed the highest OCT4 expression. However, on PB-MSCs, OCT4 was minimally expressed when compared with CD133+ cells. The expression of REX1 on CD133+ and CD271+ cells did not show any difference, and it was not detected on PB-MSCs. NOCHT1 showed a significantly high expression on CD133+ cells and no difference between CD271+ cells and MSCs. PAX6 was detected on CD271+ cells only. Significant differences (*P ≤ 0.001) between PB-MSCs and PB-CD133 or PB-CD271 are shown on Figure 1A.
Figure 1.

Stemness genes expression on peripheral blood subpopulation (A). Upper: RT-PCR agarose gel imaging obtained from peripheral blood stem cell subpopulation. Lower: Graphic representation of the band intensity after electrophoresis of RT-PCR products for stemness gene expression. Significate difference (*P ≤ 0.001) between PB-MSCs and PB-CD133+ or PB-CD271+. (B) Stem cell pluripotential gene expression on lipoaspirate sample Upper: RT-PCR agarose gel imaging obtained from lipoaspirate stem cell subpopulations. Lower: Graphic representation of the band intensity after electrophoresis of RT-PCR products for stemness gene expression. Significative difference (*P ≤ 0.001) between AD-MSCs and AD-CD133+ or AD-CD271+.
Pluripotential gene expression in samples from lipoaspirate
MSCs as well as the subpopulations obtained from lipoaspirates expressed all the stemness genes (Figure 1B). Nevertheless, the expression of pluripotential genes was highest on CD133+ and CD271+ cells when compared with that observed on MSCs. Among those select subpopulations, there was a notorious expression variability. CD271+ cells highly express NANOG and REX1 genes, and CD133+ cells showed high expression of NOCHT1 and SOX2 genes. The other genes were expressed but without any significant difference.
Comparison stemness genes expression between CD133 and CD271 from PB and AD samples
Different expression levels were also observed in pluripotential genes on subpopulations obtained from peripheral blood compared with the expression observed from those subpopulations from lipoaspirates samples. Due to great variations for the same gene observed between donor samples, we considered significant only those with a P ≤ 0.001. NANOG expression was higher on PB-CD133+ cells and it, was also high on AD-CD271+ cells. Differential expression of OCT4 was detected in all subpopulations from both sources, being highest on PB cells. REX1, although expressed on CD133+ and CD271+ cells, showed higher levels on PB-CD133+ cells. All the subpopulations tested expressed NOTCH1 without any significant difference in its expression. No difference in the expression of SOX2 was detected between PB-CD133+ and AD-CD133+ or PB-CD271+ cells (Figure 2).
Figure 2.
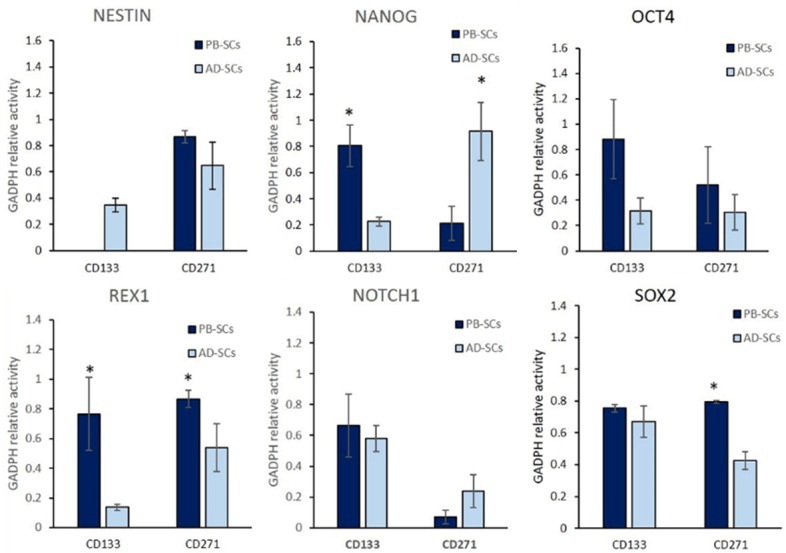
Graphic representation of band intensity after electrophoresis of RT-PCR products for stemness gene expression on subpopulations of CD133+ and CD271+ cells obtained from peripheral blood compared with the expression on those obtained from lipoaspirate (*P ≤ 0.001).
Discussion
All groups of cells studied in the present work shared the characteristics of stem cells according to Dominici et al. [16], such as self-renewal, pluripotential capacity, and expression of membrane markers such as CD73, CD90, and CD105 and, were negative for CD45. However, the expression of CD133 or CD271 were mutually excluded, which implicate two different stem cell subtypes.
Even though all cell subgroups were capable of forming CFUs at the same time as the culture reached confluence, there were important differences in the time-response to self-renew. Possibly, each group of cells has a different cell cycle status, where the response of particular cells depends on their quiescent condition. In fact, it has been suggested that CD271+ cells are the most primitive cells; however, because of the delayed self-renewed response of CD133+, they could be conceive as even more primitive. The major response for achieving confluence was observed in MSC cultures. These observations suggest a possibility of a paracrine effect between subpopulation in order to stimulate its self-renewal capacity. Despite the delay in self-renewal of cultures from AD-MSCs as well as PB-MSCs, there was a significant increase in CFUs formation in relation to time. The differences in CFU formation by cells bearing different membrane markers have already been described. A previous report comparing the capacity to form CFUs from bone marrow isolated according to two markers, MSC-CD105+ and MSC-CD271+, showed that CFU-F colonies were highest in CD271-positive fractions when compared with those bearing CD105+ and five times more than those in bone marrow MSCs [17]. Another report of CFU formation from cells bearing the antigen CD34 showed the formation of CFUs. However, the authors suggested that although this antigen belong to the precursors of the hematopoietic and stromal systems that share the expression of CD34+, it must be also expressed on non-hematopoietic cell types [18]. Reports comparing the proliferation potential of CD133+ and CD34+ populations from bone marrow and mobilized peripheral blood have demonstrated the highest CFU potential from CD133+ cells when compared with those CD34 cells obtained from peripheral blood [19]. Despite all these reports, the major CFU potential from our work was observed in MSCs from both sources, which reached confluence in less time. This could be a consequence of the paracrine relationship between the subpopulations.
All the cell subgroups studied here expressed stemness genes associated with cell self-renewal; nevertheless, there was a significant difference in the magnitude of expression not only between cell subpopulations but also between donors. Even though the group of donors were carefully selected in terms of age and health, the results showed that there are other unidentified intrinsic factors that could be responsible for the great variation observed in stemness gene expression. Furthermore, results from both sources showed a significantly high expression of REX1, NOTCH1, OCT4, and SOX2 on cells bearing CD133 and CD271 membrane markers, compared with that on MSCs. Among this subgroup of cells, OCT4 expression was highest on PB-CD133+ cells. Tondreu et al. [20] observed a similar expression of OCT4 when it was compared between MSCs and CD133+ cells obtained from umbilical cord blood, as well as between MSCs and CD133+ cells obtained from mobilized peripheral blood. This could represent an important advantage for their application in cell therapy protocols. Because OCT4 gene is well known to promote the conversion of peripheral blood cells into neural progenitor cells, as well as fibroblasts into oligodendrocytes [21-23]. Also there is a report that fibroblast can be induded to adipogenic, neurogenic and hepatogenic lineages by direct reprogramming of Oct4 [24] (Figure 3).
Figure 3.

Graphic representation that suggest the use of the best Stem Cell choice as defined by cell surface markers and source to specific tissue differentiation. The figure demonstrates that CD133+ cells obtained from blood represent the best option for differentiation protocols aimed at neurodegenerative disorders, and CD271+ cells from either source (blood or fat tissue) to protocols for muscle regeneration.
Although cells obtained from peripheral blood bearing CD133 membrane marker do not express NESTIN, the protein can be induced by cell incubation with neuro-induction medium, given a prompt response that can be detected as early as 2 h [25]. Nestin is an important cytoskeletal protein in the developing central nervous system; it is present in neural stem cells and in general is a requirement for the proliferative state [26]. Nestin is an intermediate filament protein expressed in dividing cells during the early stages of development on several tissues. Upon differentiation, nestin becomes downregulated and is replaced by tissue-specific intermediate filament proteins [27].
For the above characteristic, it has been used as a marker on cancer cells [28]. At the same time, nestin expression In addition, NESTIN expression induces the inhibition of P53 expression, the factor controlling anti-proliferative responses and cell death, this provides additional advantage by protecting them from death by apoptosis during their proliferation phase [29].
Several reports have shown the capacity of CD133+ stem cells to differentiate into preneurons [30,31]. Reports of neural progenitor cells isolated from the embryonic forebrain showed that these cells co-express SSEA4 and CD133 [32]. Spatial distribution of prominin-1 (CD133)-positive cells within germinative zones of the vertebrate brain has been determined by Jaszai et al. [33]. In addition, axon growth from the cortex to the spinal cord was significantly promoted in cultures after CD133+ cell transplantation [34]. According to Zangiacomi [35], only CD34- cells from CD133+ fraction from CB possess neuronal potential by which human cord blood (CB) CD133+ cells lose their hematopoietic signature and are converted into CB-induced neuronal-like cells (CB-iNCs) by the ectopic expression of the transcription factor SOX2 [36]. All about must be arguments to applied CD133 cells for remplace those cells lost during a neurodegenerative disease.
In vivo studies showed that CD271- mesenchymal stromal cells promoted significantly greater lymphoid engraftment than did plastic-adherent mesenchymal stromal cells when co-transplanted with CD133+ hematopoietic stem cells at a ratio of 8:1 in immunodeficient NOD/SCID-IL2Rgamma (null) mice [37]. Our results show that the expression of CD133+ or CD271+ were mutually exclusive, which implicate two different stem cell subtypes. It is also important to consider whether a mixture of cells bearing CD133 and CD271 markers could represent a synergic relationship and as consequence, a better option for engraftment and trans-differentiate during its application on stem cell protocols.
In skeletal muscle, satellite cells are residential muscle tissue stem cells. These remain in the tissue as a pool of quiescent cells responsible for maintenance, repair, and regeneration [38,39]. On those cells, the Notch-signaling pathway is involved in the activation and proliferation by maintaining the stem-cell pool in the quiescent state [40]. Then, if CD271+ cells express significate less NOCTH1, these cells could be a better option to repair damage muscle tissue (Figure 3).
REX1 was important to analyze because it is a critical factor for pluripotentiality, and maintain stem cells in an undifferentiated state [41]. On the current research, the analyzed subpopulation harvest from peripheral blood, showed highest expression of REX1, compared with those subpopulations harvest from adipose tissue. These results could indicate an advantage for their use, over cells obtained from lipoaspirates.
Another important transcription factors for pluripotentiality are NANOG and SOX2, on the subpopulation analyzed, AD-CD271+ show the less expression for SOX2. Nevertheless, NANOG was similar in AD-CD271+ to PB-CD133+.
In conclusion, all the cell subpopulations have the capacity of self-renewal, and express the stemness genes. Being PB-CD133+ apparently the group of stem cells with high stemness gene expression. Based on those differences, it is important to find out if some of the subpopulation of MSCs such as CD133+ or CD271+ cells are more prone to respond to differentiation to a specific tissue, and as consequence provide a better option for cell therapy protocols.
Acknowledgements
This work was partially funded by endowments from Instituto Tecnologico de Monterrey (cat-134) and the Zambrano-Hellion Foundation.
Disclosure of conflict of interest
None.
References
- 1.Kern S, Eichler H, Stoeve J, Klüter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 2.Kohyama J, Abe H, Shimazaki T, Koizumi A, Nakashima K, Gojo S, Taga T, Okano H, Hata J, Umezawa A. Brain from bone: efficient “meta-differentiation” of marrow stroma-derived mature osteoblasts to neurons with Noggin or a demethylating agent. Differentiation. 2001;68:235–244. doi: 10.1046/j.1432-0436.2001.680411.x. [DOI] [PubMed] [Google Scholar]
- 3.Vogel W, Grünebach F, Messam CA, Kanz L, Brugger W, Bühring HJ. Heterogeneity among human bone marrow-derived mesenchymal stem cells and neural progenitor cells. Haematologica. 2003;88:126–133. [PubMed] [Google Scholar]
- 4.Bühring HJ, Treml S, Cerabona F, de Zwart P, Kanz L, Sobiesiak M. Phenotypic characterization of distinct human bone marrow-derived MSC subsets. Ann N Y Acad Sci. 2009;1176:124–134. doi: 10.1111/j.1749-6632.2009.04564.x. [DOI] [PubMed] [Google Scholar]
- 5.Sivasubramaniyan K, Lehnen D, Ghazanfari R. Phenotypic and functional heterogeneity of human bone marrow- and amnion-derived MSC subsets. Ann N Y Acad Sci. 2012;1266:94–106. doi: 10.1111/j.1749-6632.2012.06551.x. [DOI] [PubMed] [Google Scholar]
- 6.van der Bogt KE, Sheikh AY, Schrepfer S, Hoyt G, Cao F, Ransohoff KJ, Swijnenburg RJ, Pearl J, Lee A, Fischbein M, Contag CH, Robbins RC, Wu JC. Comparison of different adult stem cell types for treatment of myocardial ischemia. Circulation. 2008;118(Suppl 6):121–129. doi: 10.1161/CIRCULATIONAHA.107.759480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma N, Ladilov Y, Moebius JM, Ong L, Piechaczek C, Dávid A, Kaminski A, Choi YH, Li W, Egger D, Stamm C, Steinhoff G. Intramyocardial delivery of human CD133+ cells in a SCID mouse cryoinjury model: bone marrow vs. cord blood-derived cells. Cardiovasc Res. 2006;71:158–169. doi: 10.1016/j.cardiores.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 8.van der Bogt KE, Schrepfer S, Yu J, Sheikh AY, Hoyt G, Govaert JA, Velotta JB, Contag CH, Robbins RC, Wu JC. Comparison of transplantation of adipose tissue- and bone marrow-derived mesenchymal stem cells in the infarcted heart. Transplantation. 2009;87:642–652. doi: 10.1097/TP.0b013e31819609d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davy P, Brienne WB, Livingston WL, Allsopp M. Hematopoietic stem cells are a critical sub-population of whole bone marrow in the treatment of myocardial infarction. Stem Cell Discovery. 2013;3:117–126. [Google Scholar]
- 10.Ahmadi H, Baharvand H, Ashtiani SK, Soleimani M, Sadeghian H, Ardekani JM, Mehrjerdi NZ, Kouhkan A, Namiri M, Madani-Civi M, Fattahi F, Shahverdi A, Dizaji AV. Safety analysis and improved cardiac function following local autologous transplantation of CD133(+) enriched bone marrow cells after myocardial infarction. Curr Neurovasc Res. 2007;4:153–160. doi: 10.2174/156720207781387141. [DOI] [PubMed] [Google Scholar]
- 11.Kovacic JC, Macdonald P, Feneley MP, Muller DW, Freund J, Dodds A, Milliken S, Tao H, Itescu S, Moore J, Ma D, Graham RM. Safety and efficacy of consecutive cycles of granulocyte-colony stimulating factor, and an intracoronary CD133+ cell infusion in patients with chronic refractory ischemic heart disease: the G-CSF in angina patients with IHD to stimulate neovascularization (GAIN I) trial. Am Heart J. 2008;156:954–963. doi: 10.1016/j.ahj.2008.04.034. [DOI] [PubMed] [Google Scholar]
- 12.Flores-Ramírez R, Uribe-Longoria A, Rangel-Fuentes MM, Gutiérrez-Fajardo P, Salazar-Riojas R, Cervantes-García D, Treviño-Ortiz JH, Benavides-Chereti GJ, Espinosa-Oliveros LP, Limón-Rodríguez RH, Monreal-Puente R, González-Treviño JL, Rojas-Martínez A. Intracoronary infusion of CD133+ endothelial progenitor cells improves heart function and quality of life in patients with chronic post-infarct heart insufficiency. Cardiovasc Revasc Med. 2010;11:72–78. doi: 10.1016/j.carrev.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 13.Yerebakan C, Kaminski A, Westphal B, Donndorf P, Glass A, Liebold A, Stamm C, Steinhoff G. Impact of preoperative left ventricular function and time from infarction on the long-term benefits after intramyocardial CD133(+) bone marrow stem cell transplant. J Thorac Cardiovasc Surg. 2011;142:1530–1539. doi: 10.1016/j.jtcvs.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Babin-Ebell J, Sievers HH, Charitos EI, Klein HM, Jung F, Hellberg AK, Depping R, Sier HA, Marxsen J, Stoelting S, Kraatz EG, Wagner KF. Transmyocardial laser revascularization combined with intramyocardial endothelial progenitor cell transplantation in patients with intractable ischemic heart disease ineligible for conventional revascularization: preliminary results in a highly selected small patient cohort. Thorac Cardiovasc Surg. 2010;58:11–16. doi: 10.1055/s-0029-1186199. [DOI] [PubMed] [Google Scholar]
- 15.Goussetis E, Manginas A, Koutelou M, Peristeri I, Theodosaki M, Kollaros N, Leontiadis E, Theodorakos A, Paterakis G, Karatasakis G, Cokkinos DV, Graphakos S. Intracoronary infusion of CD133+ and CD133-CD34+ selected autologous bone marrow progenitor cells in patients with chronic ischemic cardiomyopathy: cell isolation, adherence to the infarcted area, and body distribution. Stem Cells. 2006;24:2279–2283. doi: 10.1634/stemcells.2005-0589. [DOI] [PubMed] [Google Scholar]
- 16.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop DJ, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 17.Jarocha D, Lukasiewicz E, Majka M. Adventage of mesenchymal stem cells (MSC) expansion directly from purified bone marrow CD105+ and CD271+ cells. Folia Histochem Cytobiol. 2008;46:307–314. doi: 10.2478/v10042-008-0046-z. [DOI] [PubMed] [Google Scholar]
- 18.Simmons PJ, Torok-Storb B. CD34 expression by stromal precursors in normal human adult bone marrow. Blood. 1991;78:2848–2853. [PubMed] [Google Scholar]
- 19.Koutna I, Peterkova M, Simara P, Stejskal S, Tesarova L, Kozubek M. Proliferation and differentiation potential of CD133+ and CD34+ populations from the bone marrow and mobilized peripheral blood. Ann Hematol. 2011;90:127–137. doi: 10.1007/s00277-010-1058-2. [DOI] [PubMed] [Google Scholar]
- 20.Tondreau T, Meuleman N, Delforge A, Dejeneffe M, Leroy R, Massy M, Mortier C, Bron D, Lagneaux L. Mesenchymal stem cells derived from CD133-positive cells in mobilized peripheral blood and cord blood: proliferation, Oct4 expression, and plasticity. Stem Cells. 2005;23:1105–1112. doi: 10.1634/stemcells.2004-0330. [DOI] [PubMed] [Google Scholar]
- 21.Mitchell RR, Szabo E, Benoit YD, Case DT, Mechael R, Alamilla J, Lee JH, Fiebig-Comyn A, Gillespie DC, Bhatia M. Activation of neural cell fate programs toward direct conversion of adult human fibroblasts into tri-potent neural progenitors using OCT-4. Stem Cells Dev. 2014;23:1937–1946. doi: 10.1089/scd.2014.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee JH, Mitchell RR, McNicol JD, Shapovalova Z, Laronde S, Tanasijevic B, Milsom C, Casado F, Fiebig-Comyn A, Collins TJ, Singh KK, Bhatia M. Single transcription factor conversion of human blood fate to NPCs with CNS and PNS developmental capacity. Cell Rep. 2015;11:1367–1376. doi: 10.1016/j.celrep.2015.04.056. [DOI] [PubMed] [Google Scholar]
- 23.Kim JB, Lee H, Araúzo-Bravo MJ, Hwang K, Nam D, Park MR, Zaehres H, Park KI, Lee SJ. Oct4-induced oligodendrocyte progenitor cells enhance functional recovery in spinal cord injury model. EMBO J. 2015;34:2971–2983. doi: 10.15252/embj.201592652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu W, Jin YQ, Gao Z. Directly reprogramming fibroblasts into adipogenic, neurogenic and hepatogenic differentiation lineages by defined factors. Exp Ther Med. 2017;13:2685–2690. doi: 10.3892/etm.2017.4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gonzalez-Garza MT, Martinez HR, Caro-Osorio E, Cruz-Vega DE, Hernández-Torre M, Moreno-Cuevas JE. Differentiation of CD133+ stem cells from amyotrophic lateral sclerosis patients into preneuron cells. Stem Cells Transl Med. 2013;2:129–135. doi: 10.5966/sctm.2012-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng L, Jin Z, Liu L, Yan Y, Li T, Zhu X, Jing N. Characterization and promoter analysis of the mouse nestin gene. FEBS Lett. 2004;565:195–202. doi: 10.1016/j.febslet.2004.03.097. [DOI] [PubMed] [Google Scholar]
- 27.Michalczyk K, Ziman M. Nestin structure and predicted function in cellular cytoskeletal organisation. Histol Histopathol. 2005;20:665–671. doi: 10.14670/HH-20.665. [DOI] [PubMed] [Google Scholar]
- 28.Neradil J, Veselska R. Nestin as a marker of cancer stem cells. Cancer Sci. 2015;106:803–811. doi: 10.1111/cas.12691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tschaharganeh DF, Xue W, Calvisi DF, Evert M, Michurina TV, Dow LE, Banito A, Katz SF, Kastenhuber ER, Weissmueller S, Huang CH, Lechel A, Andersen JB, Capper D, Zender L, Longerich T, Enikolopov G, Lowe SW. p53-dependent Nestin regulation links tumor suppression to cellular plasticity in liver cancer. Cell. 2014;158:579–592. doi: 10.1016/j.cell.2014.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuçi S, Kuçi Z, Schmid S, Seitz G, Müller I, Dufke A, Leimig T, Murti G, Jurecic R, Schumm M, Lang P, Bruchelt G, Bader P, Klingebiel T, Niethammer D, Handgretinger R. Efficient in vitro generation of adult multipotent cells from mobilized peripheral blood CD133+ cells. Cell Prolif. 2008;41:12–27. doi: 10.1111/j.1365-2184.2007.00502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peh GS, Lang RJ, Pera MF, Hawes SM. CD133 expression by neural progenitors derived from human embryonic stem cells and its use for their prospective isolation. Stem Cells Dev. 2009;18:269–282. doi: 10.1089/scd.2008.0124. [DOI] [PubMed] [Google Scholar]
- 32.Barraud P, Stott S, Møllgård K, Parmar M, Björklund A. In vitro characterization of a human neural progenitor cell coexpressing SSEA4 and CD133. J Neurosci Res. 2007;85:250–259. doi: 10.1002/jnr.21116. [DOI] [PubMed] [Google Scholar]
- 33.Jászai J, Graupner S, Tanaka EM, Funk RH, Huttner WB, Brand M, Corbeil D. Spatial distribution of prominin-1 (CD133)-positive cells within germinative zones of the vertebrate Brain. PLoS One. 2013;8:e63457. doi: 10.1371/journal.pone.0063457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanaka N, Kamei N, Nakamae T, Yamamoto R, Ishikawa M, Fujiwara H, Miyoshi H, Asahara T, Ochi M, Kudo Y. CD133+ cells from human umbilical cord blood reduce cortical damage and promote axonal growth in neonatal rat organ co-cultures exposed to hypoxia. Int J Dev Neurosci. 2010;28:581–587. doi: 10.1016/j.ijdevneu.2010.07.232. [DOI] [PubMed] [Google Scholar]
- 35.Zangiacomi V, Balon N, Maddens S, Lapierre V, Tiberghien P, Schlichter R, Versaux-Botteri C, Deschaseaux F. Cord blood-derived neurons are originated from CD133+/CD34 stem/progenitor cells in a cell-to-cell contact dependent manner. Stem Cells Dev. 2008;17:1005–1016. doi: 10.1089/scd.2007.0248. [DOI] [PubMed] [Google Scholar]
- 36.Giorgetti A, Marchetto MC, Li M, Yu D, Fazzina R, Mu Y, Adamo A, Paramonov I, Cardoso JC, Monasterio MB, Bardy C, Cassiani-Ingoni R, Liu GH, Gage FH, Izpisua Belmonte JC. Cord blood-derived neuronal cells by ectopic expression of Sox2 and c-Myc. Proc Natl Acad Sci U S A. 2012;109:12556–12561. doi: 10.1073/pnas.1209523109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuçi S, Kuçi Z, Kreyenberg H, Deak E, Pütsch K, Huenecke S, Amara C, Koller S, Rettinger E, Grez M, Koehl U, Latifi-Pupovci H, Henschler R, Tonn T, von Laer D, Klingebiel T, Bader P. CD271 antigen defines a subset of multipotent stromal cells with immunosuppressive and lymphohematopoietic engraftment-promoting properties. Haematologica. 2010;95:651–659. doi: 10.3324/haematol.2009.015065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yin H, Price F, Rudnicki MA. Satellite cells and the muscle stem cell niche. Physiol Rev. 2013;93:23–67. doi: 10.1152/physrev.00043.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Relaix F, Zammit PS. Satellite cells are essential for skeletal muscle regeneration: the cell on the edge returns centre stage. Development. 2012;139:2845–2856. doi: 10.1242/dev.069088. [DOI] [PubMed] [Google Scholar]
- 40.Fujimaki S, Seko D, Kitajima Y, Yoshioka K, Tsuchiya Y, Masuda S, Ono Y. Notch1 and Notch2 coordinately regulate stem cell function in the quiescent and activated states of muscle satellite cells. Stem Cells. 2018;36:278–285. doi: 10.1002/stem.2743. [DOI] [PubMed] [Google Scholar]
- 41.Son MY, Choi H, Han YM, Cho YS. Unveiling the critical role of REX1 in the regulation of human stem cell pluripotency. Stem Cells. 2013;31:2374–2387. doi: 10.1002/stem.1509. [DOI] [PubMed] [Google Scholar]


