Abstract
Introduction
Non-invasive screening of carboxyhemoglobin saturation (SpCO) in the emergency department to detect occult exposure is increasingly common. The SpCO threshold to consider exposure in smokers is up to 9%. The literature supporting this cutoff is inadequate, and the impact of active smoking on SpCO saturation remains unclear. The primary objective was to characterize baseline SpCO in a cohort of smokers outdoors. Secondary objectives were to explore the impact of active smoking on SpCO and to compare SpCO between smokers and non-smokers.
Methods
This was a prospective cohort pilot study in two outdoor urban public areas in the USA, in a convenience sample of adult smokers. SpCO saturations were assessed non-invasively before, during, and 2 min after cigarette smoking with pulse CO-oximetry. Analyses included descriptive statistics, correlations, and a generalized estimating equation model.
Results
Eighty-five smokers had mean baseline SpCO of 2.7% (SD 2.6) and peak of 3.1% (SD 2.9), while 15 controls had SpCO 1.3% (SD 1.3). This was a significant difference. Time since last cigarette was associated with baseline SpCO, and active smoking increased mean SpCO. There was correlation among individual smokers’ SpCO levels before, during, and 2 min after smoking, indicating smokers tended to maintain their baseline SpCO level.
Conclusions
This study is the first to measure SpCO during active smoking in an uncontrolled environment. It suggests 80% of smokers have SpCO ≤ 5%, but potentially lends support for the current 9% as a threshold, depending on clinical context.
Keywords: Carbon monoxide, SpCO, Carboxyhemoglobin, Smoking, Cigarette
Introduction
Carbon monoxide (CO) is an odorless, tasteless, and colorless toxic gas [1]. CO poisoning not due to fire is responsible for approximately 15,000 annual emergency department visits in the USA, and when including fire exposures, it may account for closer to 50,000 visits annually [2, 3]. CO toxicity is difficult to diagnose as the symptoms are non-specific, including headache, nausea, weakness, and altered mental status, and patients often do not report a history of exposure [4]. Despite these challenges, it is critical for prehospital and emergency care providers to rapidly diagnose CO poisoning in order to initiate treatment with supplemental oxygen, or in severe cases hyperbaric oxygen [5]. Moreover, CO is an environmental toxin so for every patient with an elevated concentration, others may be exposed to the source and at risk for toxicity [3].
For diagnosis of CO exposure, emergency clinicians historically relied on invasive blood testing, until the development of multi-wave pulse CO-oximetry. This has allowed rapid non-invasive point-of-care screening for carboxyhemoglobin to become feasible [6–8]. These fingertip devices use transmission of light at different wavelengths during pulsatile blood flow to estimate carboxyhemoglobin saturation (SpCO) [9–11].
Previous studies have validated the accuracy of SpCO from pulse CO-oximetry compared with carboxyhemoglobin measured with blood gas analysis; finding in emergency department patients, SpCO has an overall 2.99% bias and 3.27% precision [12, 13]. Emergency department screening for elevated SpCO with pulse CO-oximetry has been shown to detect unsuspected CO exposures [14].
Appropriate interpretation of CO-oximetry testing relies upon establishing an accurate threshold level above which exposure should be suspected. There is currently no standardized cutoff, and the SpCO cutoff among non-smokers is commonly reported as < 2–3%. While a number of studies have suggested a majority of smokers have SpCO below 9%, current published guidelines by the Centers for Disease Control and Prevention continue to recommend a threshold of 9% [15]. A review article has stated SpCO in smokers may reach 15%; however, this was not found in the original cited study [16, 17]. In a large study evaluating the utility of pulse CO-oximetry, patients who self-reported as smokers had higher mean SpCO values, 5.2 versus 2.9% [14]. The literature supporting a baseline SpCO threshold among smokers is inadequate secondary to significant heterogeneity regarding smoking populations, testing conditions and timing, and testing methodology, resulting in a lack of clarity.
It is not clear when carboxyhemoglobin increases in temporal relation to last cigarette smoked, or if the level returns to normal after cessation of smoking. In two previous controlled studies, smokers had an increase in carboxyhemoglobin from approximately 1.6 to 5.3–7.8%, but this was after smoking 5–8 cigarettes [18, 19]. A separate controlled study found heavy smokers had a more significant increase from baseline than light smokers, with mean peak SpCO ≤ 6.1% at 20 min [20]. A small controlled study of smokers found a mean peak increase in COHgb of 1.6% measured 1–2 min after smoking, and it took approximately 2.5 h to return to baseline [21]. In another controlled study, subjects smoked five cigarettes sequentially over 1 h, and COHgb increased by a mean of 3.3% [22]. It is possible that CO kinetics are different among smokers and non-smokers; however, one retrospective study found no impact of smoking on COHgb half-life [23]. No prior studies have investigated the temporal relationship between smoking and carboxyhemoglobin in an uncontrolled setting.
The primary objective of this study was to characterize SpCO in a cohort of smokers encountered in uncontrolled public spaces. Secondary objectives were to explore the impact of active smoking on SpCO and to compare SpCO between smokers and non-smokers.
Methods
This was a prospective cohort pilot study, which consecutively enrolled a convenience sample of adult volunteer smokers in warm months between 2012 and 2014. Enrollment occurred during day and evening times, in two outdoor urban public areas in the Northeastern United States. The study was exempted by the hospital Institutional Review Board for obtaining informed consent.
Subjects in the smoker group were approached while actively smoking or just prior to lighting a cigarette from their usual brand of cigarettes. “Smoker” was defined as anyone smoking in a public area. Smokers were excluded if they were encountered indoors or were under age 18.
Carboxyhemoglobin saturation was monitored during the assessment period using a Masimo Rad 57 CO-oximeter (Masimo Inc., Irvine, CA) with a Masimo reusable adult fingertip sensor applied to a non-specific finger, according to the manufacturer instructions. The same device and probe were used for all subjects. Masimo Inc. does not recommend any type of calibration during the lifetime of their probe and CO-oximeter, and this model has been used in a number of other studies.
Upon enrollment, serial assessments of SpCO and oxyhemoglobin saturation (SpO2) were measured by CO-oximetry before, during, and serially at 2, 4, and 6 min after the subject smoked a cigarette in their usual manner. One reading was recorded per time point. During smoking, SpCO was measured either at 2 min into smoking, or if already actively smoking at the time of enrollment, a measurement was made at the time of enrollment. There was variation in duration to smoke one cigarette, but this variation was not recorded.
Additional data collected for potential relation to SpCO included age, sex, cardiopulmonary disease, number of years smoking, number of cigarettes smoked daily, number of cigarettes smoked on the day of measurement, and time since last cigarette.
Subjects in the control group were non-smokers who were similarly approached in an outdoor public area, and were excluded if under age 18 or if they reported a history of smoking.
Data Analysis
Descriptive statistics were calculated for age, SpCO measured at each time point, and smoking history. Spearman correlation coefficients were calculated between smoking time points, and between baseline SpCO and potential related factors including age, years smoking, cigarettes already smoked on the day of measurement, and time since last cigarette. Given SpCO measurements are reported as discrete values, Spearman correlations were utilized to determine the relationship between variables.
To determine the difference in baseline SpCO between smokers and non-smokers, a Poisson distributed linear model was fit for baseline SpCO as the outcome measure, with age, sex, and an indicator of smoking as predictors. For evaluation of SpCO while smoking, a generalized estimating equation (GEE) model was used with a Poisson distribution. Individuals were fit as clusters with a compound symmetric correlation structure using SpCO as the outcome variable, with age, sex, years smoking, cigarettes already smoked on the day of measurement, time since last cigarette, and measurement time point as predictors. A linear trend was fit for SpCO pre-, during, and 2 min post-cigarette. Additional time points after the 2-min measurement were completed by only 20 subjects and thus excluded from the model. All analyses were conducted using SAS 9.4 (SAS Cary, NC) and R (R Core Team 2017).
Results
A total of 92 smokers were approached for enrollment, 7 declined to participate, and 85 were enrolled. A total of 15 non-smoker volunteers were approached for enrollment as controls, and all participated. Self-reported demographic data including age, sex, and smoking habits are tabulated in Table 1. The mean age of smoking participants was 31.5 years (SD 11.1; range 19–65), mean number of years smoking was 12.8 (SD 10.4; range 2–52), mean number of cigarettes smoked daily was 12.8 (SD 9.1; range 0–40), mean number of cigarettes smoked thus far on the day of measurement was 5.6 (SD 4.8; range 0–22), and the mean number of minutes elapsed since the last cigarette smoked was 236 min (SD 451; range 0–1440). The mean age of non-smoking participants was 23.5 years (SD 10.3; range 18–56).
Table 1.
Subject characteristics
| Smokers (n = 85) Mean, (SD) or % |
Controls (n = 15) Mean, (SD) or % |
|
|---|---|---|
| Women | 16, 19% | 5, 33% |
| Age, [years] | 31.5 (11.1) | 23.5 (10.3) |
| Years Smoking | 12.8 (10.4) | 0 |
| Cigarettes per day | 12.8 (9.1) | 0 |
| Cigarettes on day of measurement | 5.7 (4.8) | 0 |
| Time since last cigarette, [min] | 236 (451) | 0 |
| SpCO baseline | n = 42: 2.7 (2.6) | 1.3 (0.5) |
SpCO measurements were obtained pre-cigarette from 49% of the smokers, during smoking from 100%, and post-cigarette from 91%. SpO2 for each group is reported in Table 2. There were 42 smokers with pre-cigarette SpCO measurements, all of whom also had during and post-cigarette measurements. Mean pre-cigarette SpCO was 2.7% (SD 2.6; range 0–12; 95%CI 1.9–3.5). Mean SpCO obtained from 15 non-smoker controls was 1.3% (SD 1.3; range 1–2; 95%CI 1.0–1.5).
Table 2.
SpCO saturation during smoking
| Patients included (n = 85) | SpCO Mean (SD) |
SpO2 Mean (SD) |
|---|---|---|
| Pre-cigarette (n = 42) | 2.7 (2.6) | 98.3 (1.4) |
| During cigarette (n = 85) | 2.7 (2.5) | 97.9 (2.1) |
| Post-cigarette (n = 78) | 3.1 (2.9) | 97.8 (1.9) |
Spearman correlations indicated a moderate relationship between baseline SpCO and time since last cigarette (− 0.39; p < 0.01), indicating that more recent smoking was associated with higher baseline SpCO. There was no significant correlation in this cohort between pre-cigarette SpCO and age, years smoking, number of cigarettes smoked daily, or number of cigarettes smoked on the day of measurement (Table 3).
Table 3.
SpCO correlations with smoking habits and demographics
| Relationship with baseline SpCO | Correlation coefficient (ρ) | p value |
|---|---|---|
| Age | 0.04 | 0.82 |
| Years smoking | − 0.06 | 0.71 |
| Cigarettes smoked daily | 0.19 | 0.24 |
| Cigarettes smoked on day of measurement | 0.21 | 0.19 |
| Time since last cigarette | − 0.39 | 0.01 |
There was little variation in mean SpCO before, during, or after smoking, with 2.7% (SD 2.6; range 0–12), 2.7% (SD 2.5; range 0–12), and 3.1% (SD 2.9; range 0–12), respectively. Spearman correlations between pre-, during, and 2 min post-cigarette SpCO were all significant (0.72–0.78; p < 0.0001) (Table 4). These relationships support the use of a GEE model to estimate the trend over time fitting within subject correlation.
Table 4.
SpCO correlations in smokers between time points
| Tested relationship of SpCO | Correlation coefficient (ρ) | p value |
|---|---|---|
| Pre-cigarette and during smoking (n = 42) | 0.77 | < 0.0001 |
| During smoking and post-cigarette (n = 78) | 0.72 | < 0.0001 |
| Pre-cigarette and post-cigarette (n = 42) | 0.78 | < 0.0001 |
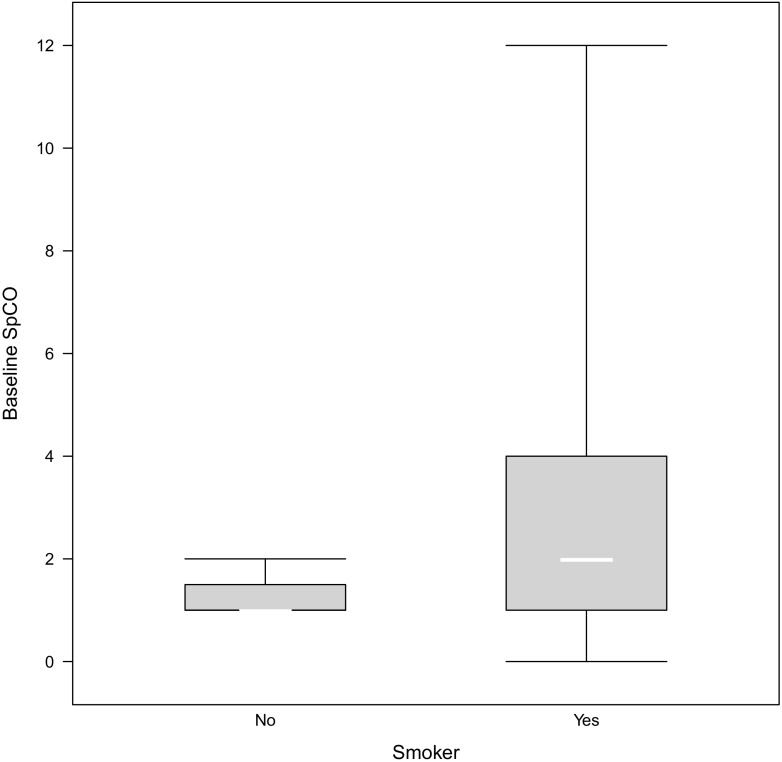
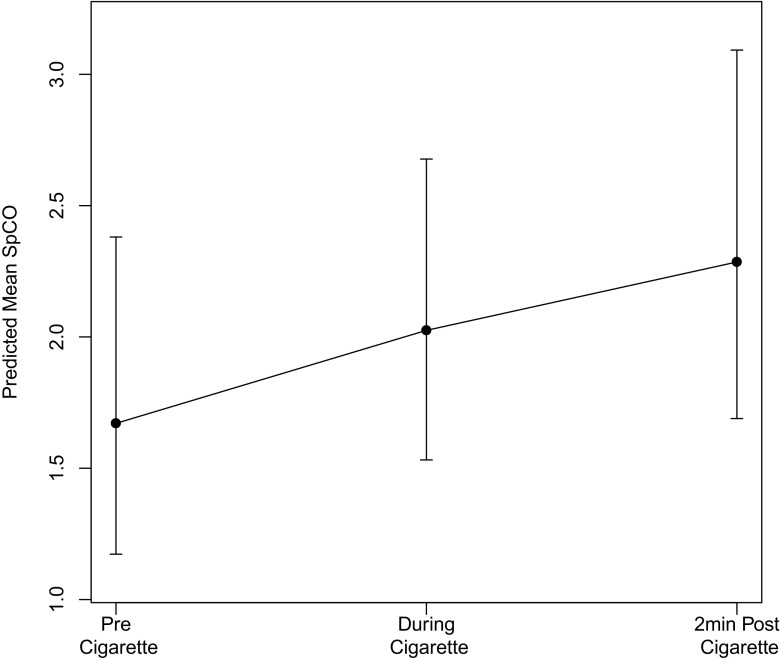
Results from the Poisson linear model indicated a significant difference between baseline SpCO of smokers and non-smokers (Fig. 1). The estimated incident risk ratio (IRR) was 1.98 (95%CI 1.20–3.26; p 0.007) in smokers compared to non-smokers for a unit increase in SpCO. The GEE model indicated a significant increase in SpCO from baseline to 2 min post-cigarette with an IRR of 1.37 (95%CI 1.13–1.66; p < 0.001) (Fig. 2). Additionally, baseline SpCO was significantly associated with time since last cigarette with an IRR of 0.93 per 60 min (95%CI 0.87–0.99; p 0.047). Testing relationships of baseline SpCO with age and smoking habits found significance only for time since last cigarette, with both Spearman correlation and GEE modeling.
Fig. 1.
Boxplot of baseline SpCO in smokers and non-smokers
Fig. 2.
SpCO trend over time due to smoking from GEE model
Discussion
This prospective pilot study’s cohort of outdoor cigarette smokers had a significant but relatively small difference in baseline SpCO compared with non-smoker controls, 2.7% versus 1.3%, respectively. This difference in means was supported by the Poisson linear model IRR of 1.98, indicating that smokers had baseline SpCO approximately two-fold higher than in non-smokers. The difference in variance between smokers and non-smokers was similarly noted in a large 1982 national study of blood carbon monoxide concentrations [24].
Smoking subjects’ mean peak SpCO of 3.1% at 2 min after smoking is lower than the 9% often used as a cutoff in smokers. This study was not designed to discriminate a specific cutoff but found 80% of subjects had SpCO ≤ 5%, and 96% had SpCO ≤ 9%. This study potentially supports the use of 9% as an SpCO threshold among smokers, depending on clinical context. There was a wide range of SpCO levels among smokers. No clear reason was identified for elevated SpCO in the subgroup of 16 smokers with SpCO > 5%. Future studies are needed to elucidate factors associated with high elevations of SpCO encountered in a subgroup of subjects. Among non-smokers, the threshold for strong suspicion of CO exposure is typically considerably lower at 2–5% [12, 13, 25].
In this study, we found a significant increase in SpCO during smoking with an IRR of 1.37, demonstrating SpCO increases between time points. Although individual time point estimates have overlapping confidence intervals, the overall slope estimate was positive with a confidence interval not overlapping one. This increase was consistent with several controlled studies which found increased SpCO after smoking [18–20]. The strong correlations between pre-, during, and post-cigarette SpCO indicate that smokers who started with high baseline SpCO maintained elevated levels at 2 min, and those who started with low levels maintained lower levels.
There was no significant correlation between pre-cigarette SpCO and age, years smoking, cigarettes smoked daily, or cigarettes already smoked on the day of measurement. Although Roth et al. found the number of cigarettes smoked daily was an independent predictor of SpCO, in this cohort, it was not associated [13]. In this study, more recent smoking was moderately associated with higher baseline SpCO. This relationship was expected, though it is noteworthy that the two variables were only moderately correlated. Other factors unaccounted for in this study may exist that influence baseline SpCO, including occupational or environmental exposure, or the outdoor environment. The moderate relationship may also be secondary to low inhaled doses of CO, which was not standardized or measured. We suggest smoking habits continue to be assessed clinically when considering CO exposure.
Sex was accounted for in the GEE model to reduce variability in the model; however, directly comparing baseline SpCO between males and females in this study was not meaningful; of the 42 subjects in whom pre-smoking SpCO was measured, only 6 were female.
Self-reported history of cardiopulmonary disease was present in five subjects (supraventricular tachycardia, hypertension, pulmonary hypertension, asthma, and unknown lung disease). The mean age of these five patients was 23 years, and the mean post-cigarette SpCO was 5.2%. These were excluded from analyses given the limited number of data points. There is suggestion SpCO may be elevated in asthma and chronic obstructive pulmonary disease, but literature remains unclear on which conditions are associated with elevated carboxyhemoglobin [26, 27]. We do not expect a relationship between SpCO and hypertension or supraventricular tachycardia. The prevalence of self-reported cardiopulmonary disease in this study is lower than would typically be expected from a smoking population; this may be related to the smokers’ mean age of 31 years.
Limitations
This study enrolled a cohort of smokers in an outdoor environment. Volunteers were disproportionally male and young and may not be representative of all smokers. Although it was our goal to enroll typical smokers, subjects may have represented an overall healthier group given they were ambulatory and otherwise well, in contrast to those encountered in a home, institutional, or healthcare setting. This study monitored a patient smoking a single cigarette. Patients who serially smoke multiple cigarettes may reach an SpCO level higher than those found in this cohort. Self-reported smoking habits may have been inaccurate. The sample size may have been underpowered to detect associations between some of the variables. We were unable to meaningfully compare males and females, given females were significantly underrepresented in the convenience sample. Finally, although the study design intended to capture pre-smoking data and an extended observation period after smoking, logistically, it was often not feasible to identify smokers in public prior to initiation of smoking, and frequently they would not remain for prolonged observation. However, once identified, all smokers allowed a during and post-cigarette measurement at 2 min.
Conclusions
With increased non-invasive screening for CO, it is imperative to establish thresholds above which CO exposure is to be suspected, both in the patient presenting for care but also for those remaining in a potentially toxic environment. This is the first study to measure SpCO during active smoking in an uncontrolled environment. Of the tested variables in smokers, only time since last cigarette was associated with baseline SpCO. There was a small but significant increase in SpCO with smoking: smokers had baseline SpCO approximately two-fold higher than non-smokers. Only 20% of ambulatory smokers had SpCO > 5%, and only 4% had SpCO > 9%. These findings suggest most smokers will have SpCO < 5%, but potentially lends support for the currently referenced threshold of 9%, depending on clinical context.
Sources of Funding
None.
Compliance with Ethical Standards
Conflict of Interest
Dr. Suner has received an unrestricted research grant from Masimo Inc. for a separate research. Drs. Schimmel, George, Schwarz, Yousif, and Hack have no potential conflicts of interest.
Footnotes
Part of these data were presented as an oral presentation at the 2015 American College of Emergency Physicians (ACEP) Annual Scientific Assembly in Boston, MA, and some data were presented as a platform presentation and poster presentation at the 2017 American College of Medical Toxicology (ACMT) Annual Scientific Meeting in San Juan, Puerto Rico.
References
- 1.Ruth-Sahd LA, Zulkosky K, Fetter ME. Carbon monoxide poisoning: case studies and review. Crit Care Nurse. 2011;30(6):303–314. doi: 10.1097/DCC.0b013e31822fb017. [DOI] [PubMed] [Google Scholar]
- 2.Hampson NB, Weaver LK. Carbon monoxide poisoning: a new incidence for an old disease. Undersea Hyperb Med. 2007;34(3):163–168. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention Carbon monoxide exposures—United States, 2000-2009. MMWR Morb Mortal Wkly Rep. 2011;60:1014–1017. [PubMed] [Google Scholar]
- 4.Ernst A, Zibrak JD. Carbon monoxide poisoning. N Engl J Med. 1998;339(22):1603–1608. doi: 10.1056/NEJM199811263392206. [DOI] [PubMed] [Google Scholar]
- 5.Weaver LK. Clinical practice: carbon monoxide poisoning. N Engl J Med. 2009;369:1217–1225. doi: 10.1056/NEJMcp0808891. [DOI] [PubMed] [Google Scholar]
- 6.Nikkanen H, Skolnik A. Diagnosis and management of carbon monoxide poisoning in the emergency department. Emerg Med Pract. 2011;13(2):1–14. [PubMed] [Google Scholar]
- 7.Bledsoe BE, Nowicki K, Creel JH, et al. Use of pulse CO-oximetry as a screening and monitoring tool in mass carbon monoxide poisoning. Prehosp Emerg Care. 2010;14(1):131–133. doi: 10.3109/10903120903349853. [DOI] [PubMed] [Google Scholar]
- 8.Shamir MY, Aharon A, Todd S. The current status of continuous noninvasive measurement of total, carboxy, and methemoglobin concentration. Anesth Analg. 2012;114(5):972–978. doi: 10.1213/ANE.0b013e318233041a. [DOI] [PubMed] [Google Scholar]
- 9.Piatkowski A, Ulrich D, Grieb G, Pallua N. A new tool for the early diagnosis of carbon monoxide intoxication. Inhal Toxicol. 2009;21(13):1144–1147. doi: 10.3109/08958370902839754. [DOI] [PubMed] [Google Scholar]
- 10.Roth D, Hubmann N, Havel C, Herkner H, Schreiber W, Laggner A. Victim of carbon monoxide poisoning identified by carbon monoxide oximetry. J Emerg Med. 2011;40(6):640–642. doi: 10.1016/j.jemermed.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 11.Suner S, McMurdy J. Masimo Rad-57 pulse CO-oximeter for noninvasive carboxyhemoglobin measurement. J Expert Rev Med Devices. 2009;6(2):125–130. doi: 10.1586/17434440.6.2.125. [DOI] [PubMed] [Google Scholar]
- 12.Barker SJ, Curry J, Redford D, Morgan S. Measurement of carboxyhemoglobin and methemoglobin by pulse oximetry: a human volunteer study. Anesthesiology. 2006;105(5):892–897. doi: 10.1097/00000542-200611000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Roth D, Herkner H, Schreiber W, Hubmann N, Gamper G, Laggner AN, Havel C. Accuracy of noninvasive multiwave pulse oximetry compared with carboxyhemoglobin from blood gas analysis in unselected emergency department patient. Ann Emerg Med. 2011;58(1):74–79. doi: 10.1016/j.annemergmed.2010.12.024. [DOI] [PubMed] [Google Scholar]
- 14.Suner S, Partridge R, Sucov A, Valente J, Chee K, Hughes A, Jay G. Non-invasive pulse CO-oximetry screening in the emergency department identifies occult carbon monoxide toxicity. J Emerg Med. 2008;34(4):441–450. doi: 10.1016/j.jemermed.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. Clinical guidance for carbon monoxide (CO) poisoning after a disasters 2014. Available at: http://emergency.cdc.gov/disasters/co_guidance.asp.
- 16.Smollin C, Olson K. Carbon monoxide poisoning (acute). Systematic review 2103. BMJ Clin Evid 2010. [PMC free article] [PubMed]
- 17.Stewart RD, Baretta ED, Platte LR, Stewart EB, Kalbfleisch JH, van Yserloo B, Rimm AA. Carboxyhemoglobin levels in American blood donors. JAMA. 1974;229(9):1187–1195. doi: 10.1001/jama.1974.03230470029019. [DOI] [PubMed] [Google Scholar]
- 18.Aronow WS, Rokaw SN. Carboxyhemoglobin caused by smoking nonnicotine cigarettes. Effects in angina pectoris. Circulation. 1971;44(5):782–788. doi: 10.1161/01.CIR.44.5.782. [DOI] [PubMed] [Google Scholar]
- 19.Aronow WS. Effect of non-nicotine cigarettes and carbon monoxide on angina. Circulation. 1980;61(2):262–265. doi: 10.1161/01.CIR.61.2.262. [DOI] [PubMed] [Google Scholar]
- 20.Sokolova-Djokic L, Milosevic S, Skrbic R, et al. Pulse carboxyhemoglobin-oximetry and cigarette smoking. J BUON. 2011;16(1):170–173. [PubMed] [Google Scholar]
- 21.Russell MA. Blood carboxyhaemoglobin changes during tobacco smoking. Postgrad Med J. 1973;49(576):684–687. doi: 10.1136/pgmj.49.576.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jay GD, Tetz DJ, Hartigan CF, Lane LL, Aghababian RV. Portable hyperbaric oxygen therapy in the emergency department with the modified Gamow bag. Ann Emerg Med. 1995;26(6):707–711. doi: 10.1016/S0196-0644(95)70042-0. [DOI] [PubMed] [Google Scholar]
- 23.Weaver LK, Howe S, Hopkins R, Chan KJ. Carboxyhemoglobin half-life in carbon monoxide-poisoned patients treated with 100% oxygen at atmospheric pressure. Chest. 2000;117(3):801–808. doi: 10.1378/chest.117.3.801. [DOI] [PubMed] [Google Scholar]
- 24.Radford EP, Drizd TA. Blood carbon monoxide levels in persons 3–74 years of age: United States, 1976–80 US Dept of Health and Human Services; Advance Data 76; March 17, 1982, Hyattsville, MD (1982) [US Dept of Health and Human Services publication PHS 82–1250].
- 25.Plante T, Harris D, Savitt J, Akhlaghi F, Monti J, Jay GD. Carboxyhemoglobin monitored by bedside continuous CO-oximetry. J Trauma. 2007;63(5):1187–1190. doi: 10.1097/01.ta.0000242769.04246.5d. [DOI] [PubMed] [Google Scholar]
- 26.Naples R, Laskowski D, McCarthy K, Mattox E, Comhair SAA, Erzurum SC. Carboxyhemoglobin and methemoglobin in asthma. Lung. 2015;193(2):183–187. doi: 10.1007/s00408-015-9686-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yasuda H, Yamaya M, Nakayama K, Ebihara S, Sasaki T, Okinaga S, Inoue D, Asada M, Nemoto M, Sasaki H. Increased arterial carboxyhemoglobin concentrations in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171(11):1246–1251. doi: 10.1164/rccm.200407-914OC. [DOI] [PubMed] [Google Scholar]