Abstract
About 20% of the general population is contact-sensitized to common haptens such as fragrances, preservatives, and metals. Many also develop allergic contact dermatitis (ACD), the clinical manifestation of contact sensitization. ACD represents a common health issue and is also one of the most important occupational diseases. Although this inflammatory skin disease is mediated predominantly by memory T lymphocytes recognizing low-molecular-weight chemicals after skin contact, the innate immune system also plays an important role. Along that line, the presence of irritants may increase the risk of ACD and therefore ACD is often seen in the context of irritant contact dermatitis. In this review article, we discuss recent progress in basic research that has dramatically increased our understanding of the pathomechanisms of ACD and provides a basis for the development of novel diagnostic and therapeutic measures. Current methods for diagnosis as well as treatment options of ACD are also discussed.
Keywords: contact dermatitis, chemical, skin, treatment
Introduction
Allergic contact dermatitis (ACD) is an inflammatory skin disease that affects about 20% of the adult general population and is also an important occupational skin disease 1– 3. A recent study showed that 27% of the general population from five European countries had contact allergy (that is, sensitization to at least one contact allergen of the European baseline series) 4. A large proportion of these individuals are at risk of developing ACD following exposure to everyday products. Among the occupational diseases, 40% are skin-related 5. Contact dermatitis (both irritant and allergic) accounts for about 90% of these. Collectively, these epidemiological data demonstrate the importance of ACD as a challenge to human health. Therefore, basic and clinical research is needed to understand the pathomechanisms of ACD and to develop better strategies for diagnosis and treatment.
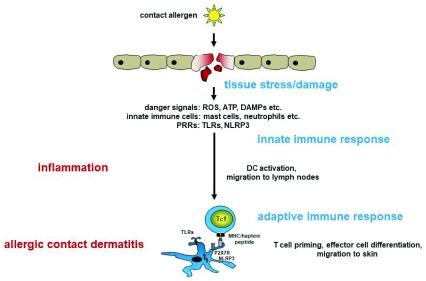
ACD is mediated by T cells recognizing low-molecular-weight organic chemicals or metal ions in the context of major histocompatibility complex (MHC) molecules 6. These usually electrophilic chemicals penetrate the skin and react with extracellular and cellular proteins. Their protein reactivity is mandatory and underlies their unusual ability to trigger innate immune as well as T-cell responses 7– 9. Activation of the innate immune system is a prerequisite for the activation and skin migration of contact allergen-specific T cells. The first skin contact with allergens initiates the activation of skin cells, most importantly the epidermal Langerhans cells and dermal dendritic cells (DCs), which subsequently migrate to the local lymph nodes and present the contact allergen(s) to naïve T cells. Contact allergen-specific T cells then proliferate and differentiate to effector T cells that enter the blood circulation. Repeated skin contact with the same contact allergen then results in the recruitment of these T cells into the skin and the elicitation of the clinical reaction of ACD ( Figure 1). The response is limited and downregulated by regulatory T and B cells, natural killer T (NKT) cells, and further cell types 9. Here, we review recent progress in basic research aimed at understanding the cellular and molecular mechanisms underlying the innate immune responses as well as the pathogenic T-cell response and its regulation. Moreover, we will give an overview of the current status of the management of ACD in the clinic.
Figure 1. Sensitization phase of allergic contact dermatitis.
Contact allergens penetrate the skin and cause tissue stress and damage. Reactive oxygen species (ROS) are formed, ATP is released from stressed cells, and damage-associated molecular patterns (DAMPs) are formed/released from cells. DAMPs then trigger activation of the innate immune system via Toll-like receptors (TLRs) and NOD-like receptor pyrin containing 3 (NLRP3). This results in skin inflammation and consequently activation of dendritic cells (DCs) and migration to the skin-draining lymph nodes. DCs present contact allergens to naïve T cells, leading to their activation and effector cell differentiation. This concludes the sensitization phase. In the elicitation phase, repeated skin contact with the same contact allergen induces inflammation, and T cells are recruited into the inflamed skin, where they exert their effector functions, leading to clinical symptoms of allergic contact dermatitis. MHC, major histocompatibility complex; PRR, pattern recognition receptor.
Protein reactivity of contact allergens
The central role of the protein modification by contact allergens is underlined by the fact that two of the three validated in vitro assays for the identification of contact allergens are based on protein reactivity. The Direct Peptide Reactivity Assay—Organisation for Economic Co-operation and Development (OECD) test guideline TG 442C—detects the depletion of model peptides containing modifiable lysine or cysteine residues. Electrophilic chemicals binding covalently to the ε-amino group of lysine or the thiol (SH) group of cysteine are classified as potential contact allergens. Similarly, covalent modification of cysteine residues in the cytosolic protein Keap1 and the subsequent release and DNA binding of the transcription factor Nrf2 lead to the activation of luciferase in HaCaT keratinocytes in the ARE-Nrf2 Luciferase Test Method (OECD test guideline TG 442D) 10. The Keap1/Nrf2 pathway is central in the antioxidant phase 2 response.
One of the most challenging research questions is the identification of (a) the target proteins that, upon hapten modification, lead to activation of the innate immune system and cellular stress responses and (b) the natural T-cell epitopes generated after protein modification. Until now, the few known physiologically relevant protein targets of contact allergens have been identified in cell lines. Recent studies addressing these questions in vivo have identified keratins in mouse skin after topical application of bromobimanes 11 and the macrophage migration inhibition factor (MIF) in skin-draining lymph nodes after topical application of tetramethylrhodamine isothiocyanate (TRITC) 12 as protein targets for hapten modification. It is still not known whether these hapten modifications alter the function of the proteins or generate T-cell epitopes. In the case of MIF, an N-terminal proline modification was detected, but potential effects on the function of MIF were not investigated.
Role of mast cells and neutrophils in contact hypersensitivity
Skin inflammation is essential in the sensitization and elicitation phase of ACD. Its initiation requires the close cooperation of different cell types, which together orchestrate this complex response ( Figure 1). It was recently demonstrated that mast cells are important innate effector cells in murine contact hypersensitivity (CHS), the mouse model for ACD 13. CHS was significantly reduced in mast cell-deficient or -depleted mice. The reasons for that were the lack of neutrophil extravasation into inflamed skin initiated by the localization of mast cells in proximity of blood vessels and by secretion of the pre-formed and de novo synthesized neutrophil-attracting chemokines CXCL1/CXCL2 as shown in a lipopolysaccharide-induced peritonitis model. Interestingly, macrophages were required for deeper neutrophil migration into the tissue 14. Granule release and de novo chemokine synthesis were Toll-like receptor 4 (TLR4)-dependent. DC emigration from the skin was compromised by the selective absence of mast cells or neutrophils, as was T-cell recruitment to the skin 13– 15.
A very interesting study in a model of chronic CHS showed that mast cells can also limit CHS 16. Using mast cell-deficient Sash or mast cell-depleted Mcpt5-Cre diphtheria toxin receptor (DTR) mice, the authors showed a significant increase of the ear swelling reaction in mast cell-deficient mice which was dependent on CD8 + tissue-resident memory T (T RM) cells. The effect correlated with elevated levels of interleukin (IL)-15 needed for cutaneous T RM cells. Thus, in this chronic CHS model, mast cells limited the CD8 + T RM cell response by degrading IL-15 via proteases such as chymase and carboxypeptidase A.
Role of tissue-resident T cells and γδ T cells in contact hypersensitivity
In general, T RM cells form local memory in tissues and are responsible for rapid and strong reactions upon re-exposure to a contact allergen. It was shown that T RM cells are generated from the same naïve precursor as central memory T (T CM) cells in the skin-draining lymph nodes. They seed the contact allergen-exposed skin sites, reside there, and produce a rapid allergic response upon re-exposure 17. Schmidt et al. identified IL-17- and interferon-gamma (IFN-γ)-producing CD8 + T RM cells in the 2,4-dinitrofluorobenzene (DNFB) CHS mouse model 18. The generation of local memory due to T RM cells was shown for both mice and nickel-allergic humans.
It was recently reported in the CHS model that dendritic epidermal T cells (DETCs), which are not found in human skin, rapidly produced IL-17 in response to contact allergens 19. However, how these cells were activated was unclear. Nielsen et al. revealed that the activating natural killer (NK) receptor NKG2D is involved in their activation 20. NKG2D was found to be expressed on most DETCs and in human CLA + γδ T cells. Mouse keratinocytes upregulated various stress-induced NKG2D ligands when exposed to contact allergens in vitro. Moreover, in the mouse system, the NKG2D ligand Mult1 was upregulated by contact allergens in the skin. Experiments with anti-NKG2D blocking antibodies showed a partial block in the activation of DETCs. This study suggests a contact allergen-induced interaction of epidermal γδ T cells with keratinocytes which results in an IL-1β-driven and NKG2D/NKG2DL-dependent T-cell activation and production of IFN-γ and IL-17. This may be part of the early antigen non-specific innate inflammatory response to contact allergens. Jiang et al. recently reported a role for dermal γδ T cells in promoting CHS by IL-17-dependent neutrophil recruitment 17. They identified a population of γδ T cells which had characteristics of tissue-resident cells with low re-circulation potential. These dermal γδ T cells produced IL-17 and IL-22. Importantly, DNFB-induced CHS was significantly reduced in mice selectively lacking dermal γδ T cells but not DETCs. The authors showed that this was due to reduced neutrophil recruitment. This study underlines the important role of neutrophils in CHS 15.
Much progress has been made in the field of metal allergies. Since nickel and cobalt were identified as the first contact allergens able to directly activate a pattern recognition receptor (that is, human TLR4 by inducing its dimerization and signaling 21– 23), it was shown that nickel also activates the NLRP3 (NOD-like receptor pyrin containing 3) inflammasome 24. Likewise, chromium VI, but not chromium III, compounds activate the NLRP3 inflammasome 25. However, chromium fails to provide a priming signal, such as TLR4 activation in the case of nickel, which is needed for inflammasome activation. This was shown in vitro, and the findings are reminiscent of contact allergens such as 2,4,6-trinitrofluorobenzene (TNCB) and oxazolone which also fail to do that in vitro 26, 27. In that case, a tissue-derived priming signal was generated by induction of hyaluronic acid breakdown 27. Thus, it may well be that chromium also has to induce a tissue-derived priming signal.
Recently, TLR3 was shown to modulate CHS responses in mice. Both irritant contact dermatitis (ICD) (croton oil) as well as ACD (TNCB) were reduced in the absence of TLR3 in knockout (KO) mice and increased in TLR3-overexpressing mice 28. For ACD to TNCB, a role for TLR3 was confined to the elicitation phase. The mechanism of TLR3 activation was not identified, but the authors speculated on the release of self-RNA from necrotic skin cells. It must also be considered that TLR3 ligands may derive from the bacterial skin flora. Experiments in germ-free mice would be needed to clarify that. Up to now, CHS experiments in germ-free mice have indicated that the innate immune system activation can be triggered by contact allergens in the absence of a microbial flora 26, 27.
In general, the data published up to now for organic chemical allergens and metal allergens in humans and mice reveal a common mechanistic innate immune response pathway. Contact allergens generate tissue-derived activators of TLRs or are themselves direct activators. Following TLR activation, pro-inflammatory mediators, among them pro-IL-1β, a central cytokine in ACD, are produced. The NLRP3 inflammasome is then activated by different means depending on the contact allergen. Oxidative stress that promotes inflammation is induced ( Figure 1). In general, TLR triggering and inflammasome activation are essential steps in the innate immune response and the mechanisms underlying their activation present as variations of a common theme. It remains to be determined how general this theme is given the high number and physicochemical diversity of the more than 4,000 contact allergens known today.
Skin inflammation can also be induced by irritant chemicals such as detergents like sodium dodecyl sulfate (SDS). This is due in part to a damaging effect on the skin barrier. Therefore, a combination of irritants and contact allergens as often found in cosmetics, household products, and workplace materials can facilitate sensitization due to the amplification of skin inflammation resulting in, for example, the augmentation of DC activation 29. Moreover, combinations of contact allergens show similar augmentation due to additive or synergistic effects of the so-called irritant effects of contact allergens (that is, their ability to activate the innate immune system) 9, 30– 32.
Genomics and proteomics
The identification of biomarkers would be very helpful in improving diagnostics and treatment of ACD 33. Dhingra et al. performed gene array studies using skin samples from patch test biopsies 34. Besides identifying 149 genes that were differentially expressed in all contact allergen-treated samples, they identified a significant number of genes that were regulated in a contact allergen-specific manner. This study nicely shows that the clinical appearance of ACD can be very similar for different contact allergens but that the underlying immune responses can be very different as highlighted here for the polarization of the T-cell response.
Quaranta et al. compared different forms of eczema (atopic and non-atopic) regarding gene expression profiles 35. They made use of an interesting study population: patients with both psoriasis and eczema. In addition, some of these patients developed ACD when tested with nickel. This study allowed an inter-individual comparison of different types of eczema which could be differentiated on the basis of their characteristic gene expression. Comparing naturally occurring eczema with nickel-induced eczema revealed that 33 genes were commonly regulated but that 172 genes were exclusively regulated in induced and 28 exclusively in naturally occurring eczema. Pathway analysis revealed marked differences in genes regulating epithelial differentiation, extracellular matrix, cell–cell adhesion, and the acute immune response. Examples for ACD-specific genes were genes for T-cell attraction ( CXCL9, 10, and 11) that had already been described as discriminators for ACD 36, 37, LCE1 and LCE2 family genes, and HAS3, EPSTI1, ICAM-1, CXCL8, IL-1β, and AIM2. Interestingly, detailed data analysis led the authors to claim a two-gene classifier ( NOS2 and CCL27) for the distinction between psoriasis and atopic dermatitis 38.
Besides analysis of gene expression, proteomics are being used to identify contact allergen-induced changes in protein profiles. Using matrix-assisted laser desorption/ionization-mass spectrometry analysis, Jakob et al. showed that Ni 2+ treatment of human CD14 + monocytes isolated from peripheral blood mononuclear cells significantly altered the expression of 56 protein species 39. Further analysis revealed the induction of proteins associated with apoptotic cell death at concentrations of around 250 µM and above, concentrations which did not affect T-cell viability. Interestingly, Schmidt et al. had previously shown a sensitization of human umbilical vein endothelial cells for TRAIL (tumor necrosis factor-related apoptosis-inducing ligand)-induced cell death by nickel 40.
Mussotter et al. compared the protein profiles of bone marrow-derived dendritic cells (BMDCs) from C57BL/6 wild-type and Nrf2-deficient mice 41. More than 50 proteins were upregulated upon BMDC treatment with cinnamic aldehyde and more than 30 upon 2,4-dinitrochlorobenzene (DNCB) treatment. Notably, almost all of these proteins were not upregulated in BMDCs from Nrf2-deficient mice. Nrf2-dependent proteins were associated with oxidative stress, cell survival/death, proteostasis, and other signaling pathways. In addition, metabolic reprogramming by contact sensitizers was revealed (for example, by upregulation of many glycolytic enzymes). This approach allows identification of Nrf2-dependent and -independent proteins and differentiation between contact sensitizer- and irritant-specific effects.
More recent profiling studies of patients with ACD used serum 42 samples from stratum corneum after tape stripping 43, skin biopsies 44, 45, or monocytes 36. Analyses were carried out by protein or gene arrays and mass spectrometry. Owing to the diversity of the samples, the different contact allergens used, and the different methods used, it is impossible to compare the data and search for common biomarkers. Nevertheless, inflammation and skin barrier-related genes and proteins are, not surprisingly, differentially regulated in all studies. The big challenge is the identification of biomarker profiles that allow differentiation between ACD and ICD and between ACD and other forms of eczema.
More such genomic and proteomic studies are needed to understand how contact sensitizers work on a mechanistic basis. Novel biomarkers can be identified that may aid in the improvement of ACD diagnostics, identification of novel drug targets, and in vitro assay development for contact allergen identification and replacement of animal testing.
Immune regulation and tolerance to contact allergens
Regulatory T cells (Tregs) are central regulators of the extent and duration of CHS responses. The important role of Tregs in CHS has been underlined by a recent study using CD103-deficient KO mice 46. The integrin αE (CD103) is expressed on subsets of DCs and on Tregs localizing to epithelia. CHS to oxazolone or DNCB was significantly increased in the KO mice. The authors then found that the level of Foxp3 was significantly reduced in CD4 +CD25 + T cells and that Tregs from the KO mice were unable to suppress CHS. This study shows that CD103 has a role in the regulation of Foxp3 expression in addition to its function of retention of Tregs in inflamed skin.
Tregs express the ectonucleotidases CD73 and CD39, which degrade ATP to tolerogenic adenosine 47. It has been shown that one consequence of this is the retention of CD8 + effector T cells in the lymph nodes. Mahnke et al. showed that CD8 + T cells shed CD62L in order to leave the lymph node, a process required for effector cell migration to inflamed skin in CHS 48. ATP is released from skin cells following contact allergen application 49. In T cells, ATP acts on P2X7R to upregulate ADAM17 which sheds CD62L from the T-cell surface. Moreover, CD73-dependent ATP degradation seems to limit the extent of DC emigration from skin and eventually the magnitude of CHS 50.
Interestingly, similar to observations in viral infections 51, 52, IL-10-producing CD8 + T cells can be detected in the late elicitation phase of CHS 53. Although in respiratory syncytial virus lung infection an autocrine regulatory role of these effector cells has been shown 52, in the CHS model this could not be confirmed. Nevertheless, one may speculate that the CD8 + CHS effector T cells start to express IL-10 later in the elicitation phase and contribute to the limitation of the extent and the downregulation of the CHS response.
The previously reported roles of NKT cells and regulatory B cells (Bregs) in CHS were supported by Fjelbye et al. 54. These authors showed increased CHS responses in CD1d-deficient mice. This was mostly due to a decrease of IL-10 and a predominant reduction in IL-10 + Bregs in the spleen and peritoneal cavity. These data strongly suggest a regulation of Bregs by CD1d-restricted NKT cells, which have been identified as an important regulatory cell type in CHS 55.
Tolerance to contact allergens can be induced experimentally by different means. Low zone tolerance (LZT) is induced by the repeated epicutaneous application of doses of contact allergens 100- to 1,000-fold lower than the dose used for sensitization. LZT involves IL-10-producing Foxp3 + Tregs which induce tolerogenic CD8 +CD11c + DCs and is most likely caused by the presentation of contact allergen to T cells in the absence of skin inflammation 56. Another way of tolerance induction is oral tolerance. Hacini-Rachinel et al. showed that oral tolerance to DNFB given to mice by gavage was dependent on TLR4 expression on hematopoietic cells 57. They demonstrated that TLR4 is necessary to induce the mobilization of tolerogenic CD103 +CD11c + lamina propria DCs to migrate to the local lymph nodes and prime Foxp3 + Tregs. Complexes of IL-2/anti-IL-2 antibodies have been shown to enhance IL-2 effects and are considered to treat inflammatory diseases on the basis of their expansion of Tregs. El Beidaq et al. demonstrated that tolerance to CHS mediated by TNCB can be enforced by injection of an IL-2/anti-IL-2 antibody reagent, IL-2/JES6-1 58. This treatment, when given before or even after sensitization, established a state of tolerance by CTLA4 +Foxp3 + Treg expansion that was still evident upon re-challenge with contact allergen even 3 weeks after the last injection. IL-10 and transforming growth factor-beta (TGF-β) levels were increased while neutrophil and CD8 + T-cell infiltration of the skin were reduced. This study is encouraging, since it shows that a longer-lasting re-establishment of tolerance to contact allergens is possible. The issue of establishing directed, antigen-specific tolerance needs to be solved. However, in a double-blinded, placebo-controlled study, Di Gioacchino et al. demonstrated the re-establishment of tolerance to nickel by oral hyposensitization 59. A total of 113 patch test-positive patients with systemic nickel syndrome due to nickel-containing foods were given nickel orally for 1 year. Re-introduction of nickel-rich food revealed significant improvement of gastrointestinal symptoms. Skin manifestations also improved, but significance was not reached. On the other end of the spectrum, oral or systemic exposure to contact allergens in sensitized individuals may result in systemic contact dermatitis, a skin reaction characterized by flexural and inverse involvement of eczema. This may occur following high-dose nickel exposure in very nickel-sensitized individuals but also following oral exposure to sesquiterpene lactone allergens (for example, chamomile in tea) in plant-allergic individuals. Other examples include systemic contact dermatitis reactions in patients with an allergy to Myroxylon pereirae resin (balsam of Peru) who eat citrus fruits.
Diagnostics in clinical allergic contact dermatitis
From the clinical point of view, ACD has hardly any specific appearance, although vesicular morphology is frequent and ACD as opposed to ICD characteristically spreads and generalizes if the allergen is not removed. In general, ACD results in an eczematous reaction of the skin, although non-eczematous reactions such as lichenoid reactions and implant failure have also been reported in contact-allergic individuals. This lack of highly specific clinical characteristics indicates the need and benefit of reproducible diagnostic procedures. Since the beginning of clinical contact allergy diagnosis with Jadassohn in 1895, epicutaneous application of suspected contact allergens has been the diagnostic method of choice. The general principle is unchanged since then: contact allergens are dissolved in adequate vehicles such as water, alcohol, and petrolatum, allowing the allergens to penetrate out of the preparation into the epidermis. For most allergens, the highest non-irritating allergen concentrations are optimal 60. Classic exceptions are glucocorticosteroids: too high a concentration causes immunosuppression rather than eliciting allergic reactions, although characteristic dermatitis is formed in a ring surrounding the chamber which contains the glucocorticosteroid. For glucocorticosteroids, optimal test concentrations are established on the basis of a more sophisticated balance of sufficient allergen to elicit the allergic reaction and too little to suppress it 61. All test preparations for contact allergens are optimized for epicutaneous application, mostly under occlusion, for 48 hours. Patient products may also be applied directly (for example, a piece of a glove or shoe, a metallic disc, nail lacquer, or scrapping from spectacle frames in a chamber). Allergen preparations are kept in place by using adhesive materials with small allergen chambers. The chambers are made from either aluminum or polypropylene and in some products with an inlay from filter paper. The total amount of allergen preparation for one chamber depends on the chamber size. For most of the commercial patch test chambers, 15–20 μL of allergen preparation suffices. Application of too high a volume can result in spoiling out of the test chamber and false-positive patch test reactions. Some allergens are volatile and might evaporate from the patch test material (for example, fragrance chemicals) 62. This can occur during the preparation of the patch tests for one patient. Hence, test materials should be prepared directly before application onto the skin. The evaporation process starts for highly volatile allergens directly with manufacturing the test materials. For these allergens, transport and storage in fridges or freezers (especially for isocyanate allergens) are advisable.
In most patients, patch tests are applied onto the back of the patient. After 48 hours of application, patches are removed and test sites are read in accordance with the current guideline 63. The readings have to be performed at at least two different time points. Common reading schedules are 48 hours, 72 hours, and 6–7 days. But reading schedules skipping the first reading and including a 96-hour reading are practiced 64. The reading of test readings is independent from interpretation of the results, which should be performed subsequently 65. Exposure analysis is important before clinical relevance of positive test reactions is decided. Positive test reactions are grouped into current, past, and unknown clinical relevance on the basis of allergen exposure, patient history, and the clinical pattern.
Current and future treatment options
In the case of clinically relevant ACD, the disease resolves if the patient avoids future skin exposure to the culprit allergen. Thorough information about sources of allergen exposure is crucial, as is instruction about how to read ingredient labels and use spot tests for metal ion release. However, time from avoidance of allergen exposure to complete resolution can take months in the case of severe disease but in milder cases may occur within a few weeks. Topical application of emollients along with anti-inflammatory agents (for example, calcineurin inhibitors and corticosteroids) is first-line treatment and typically will rapidly heal the lesions. However, ACD often occurs in the context of other eczematous conditions, including ICD or atopic dermatitis. In these cases, patients may have a more chronic disease and resolution cannot be obtained through allergen avoidance alone. Here, ultraviolet irradiation or systemic immunosuppressants such as methotrexate, cyclosporine, and azathioprine may be necessary to control inflammation. This is often the case in patients with chronic hand eczema. Systemic corticosteroids should generally be avoided in patients with chronic dermatitis because of the risk of severe complication such as osteoporosis and type 2 diabetes but may be very useful in patients with acute severe ACD following exposure to, for example, poison ivy or para-phenylenediamine (PPD). So far, no biologics have been proven to be useful in the treatment of ACD, although only case series have been performed 66. Based on the successful experience of patch testing patients who receive various biologics and immunosuppressants, none of these seems to really suppress ACD and therefore they are not suitable for treating ACD per se 67. So far, oral tolerance induction has not been successful, but a randomized Italian study showed that an improvement of gastrointestinal symptoms following oral nickel exposure by food and a significant increase in patch test negativity may be obtained 59. At the moment, it is impossible to envision any drugs that may directly inhibit ACD responses in affected patients, but it is possible that the innate immune system needs to be targeted at an early stage to prevent the cascade of reactions in chronic disease 9.
Given the complexity of ACD, a multi-layered strategy for treatment seems necessary. Interference with mechanisms of inflammation, enforcement of immune regulation, and reduction, removal, or suppression of effector and memory T cells, including T RM cells, are areas to be exploited in the future. Based on our increasing understanding of the pathomechanisms, combination therapies may have to be developed for acute and hard-to-treat chronic ACD. The ongoing search for biomarkers will hopefully lead to the identification of profiles suitable for modern molecular diagnostics. New in vitro assays should be suitable to identify contact allergens for hazard identification 10. Here, assays that allow potency assessment of contact allergens are urgently needed.
The ongoing basic and clinical research as well as in vitro testing for contact allergen identification, the recognition of the impact of contact dermatitis, and the continuing education and training to raise awareness for prevention and for improvement of workplaces 5 will result in avoidance of hazardous chemicals and improved management of this important skin disease.
Abbreviations
ACD, allergic contact dermatitis; BMDC, bone marrow-derived dendritic cell; Breg, regulatory B cell; CHS, contact hypersensitivity; DC, dendritic cell; DETC, dendritic epidermal T cell; DNCB, 2,4-dinitrochlorobenzene; DNFB, 2,4-dinitrofluorobenzene; ICD, irritant contact dermatitis; IFN-γ, interferon-gamma; IL, interleukin; KO, knockout; LZT, low zone tolerance; MIF, macrophage migration inhibition factor; NK, natural killer; NKT, natural killer T; NLRP3, NOD-like receptor pyrin containing 3; OECD, Organisation for Economic Co-operation and Development; TLR, Toll-like receptor; TNCB, 2,4,6-trinitrofluorobenzene; Treg, regulatory T cell; T RM, tissue-resident memory T
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Bruce Brod, Department of Dermatology, University of Pennsylvania, Philadelphia, PA, USA
Donald V. Belsito, Department of Dermatology, Columbia University Medical Center, New York, NY, USA
Ann-Therese Karlberg, Dermatochemistry and Skin Allergy, Department of Chemistry and Molecular Biology, University of Gothenburg, Gothenburg, Sweden
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 1; referees: 3 approved]
References
- 1. Peiser M, Tralau T, Heidler J, et al. : Allergic contact dermatitis: epidemiology, molecular mechanisms, in vitro methods and regulatory aspects. Current knowledge assembled at an international workshop at BfR, Germany. Cell Mol Life Sci. 2012;69(5):763–81. 10.1007/s00018-011-0846-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ring J: Occupational skin disease - a major health problem in Europe. J Eur Acad Dermatol Venereol. 2017;31(6):919–20. 10.1111/jdv.14307 [DOI] [PubMed] [Google Scholar]
- 3. Thyssen JP, Linneberg A, Menné T, et al. : The epidemiology of contact allergy in the general population--prevalence and main findings. Contact Dermatitis. 2007;57(5):287–99. 10.1111/j.1600-0536.2007.01220.x [DOI] [PubMed] [Google Scholar]
- 4. Diepgen TL, Ofenloch RF, Bruze M, et al. : Prevalence of contact allergy in the general population in different European regions. Br J Dermatol. 2016;174(2):319–29. 10.1111/bjd.14167 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 5. Alfonso JH, Bauer A, Bensefa-Colas L, et al. : Minimum standards on prevention, diagnosis and treatment of occupational and work-related skin diseases in Europe - position paper of the COST Action StanDerm (TD 1206). J Eur Acad Dermatol Venereol. 2017;31Suppl 4:31–43. 10.1111/jdv.14319 [DOI] [PubMed] [Google Scholar]
- 6. Martin SF, Esser PR, Schmucker S, et al. : T-cell recognition of chemicals, protein allergens and drugs: towards the development of in vitro assays. Cell Mol Life Sci. 2010;67(24):4171–84. 10.1007/s00018-010-0495-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kaplan DH, Igyártó BZ, Gaspari AA: Early immune events in the induction of allergic contact dermatitis. Nat Rev Immunol. 2012;12(2):114–24. 10.1038/nri3150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Martin SF: Contact dermatitis: from pathomechanisms to immunotoxicology. Exp Dermatol. 2012;21(5):382–9. 10.1111/j.1600-0625.2012.01471.x [DOI] [PubMed] [Google Scholar]
- 9. Martin SF: New concepts in cutaneous allergy. Contact Dermatitis. 2015;72(1):2–10. 10.1111/cod.12311 [DOI] [PubMed] [Google Scholar]
- 10. Ezendam J, Braakhuis HM, Vandebriel RJ: State of the art in non-animal approaches for skin sensitization testing: from individual test methods towards testing strategies. Arch Toxicol. 2016;90(12):2861–83. 10.1007/s00204-016-1842-4 [DOI] [PubMed] [Google Scholar]
- 11. Simonsson C, Andersson SI, Stenfeldt A, et al. : Caged fluorescent haptens reveal the generation of cryptic epitopes in allergic contact dermatitis. J Invest Dermatol. 2011;131(7):1486–93. 10.1038/jid.2010.422 [DOI] [PubMed] [Google Scholar]
- 12. Karlsson I, Samuelsson K, Simonsson C, et al. : The Fate of a Hapten - From the Skin to Modification of Macrophage Migration Inhibitory Factor (MIF) in Lymph Nodes. Sci Rep. 2018;8(1): 2895. 10.1038/s41598-018-21327-8 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 13. Dudeck A, Dudeck J, Scholten J, et al. : Mast cells are key promoters of contact allergy that mediate the adjuvant effects of haptens. Immunity. 2011;34(6):973–84. 10.1016/j.immuni.2011.03.028 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 14. De Filippo K, Dudeck A, Hasenberg M, et al. : Mast cell and macrophage chemokines CXCL1/CXCL2 control the early stage of neutrophil recruitment during tissue inflammation. Blood. 2013;121(24):4930–7. 10.1182/blood-2013-02-486217 [DOI] [PubMed] [Google Scholar]
- 15. Weber FC, Németh T, Csepregi JZ, et al. : Neutrophils are required for both the sensitization and elicitation phase of contact hypersensitivity. J Exp Med. 2015;212(1):15–22. 10.1084/jem.20130062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gimenez-Rivera VA, Siebenhaar F, Zimmermann C, et al. : Mast Cells Limit the Exacerbation of Chronic Allergic Contact Dermatitis in Response to Repeated Allergen Exposure. J Immunol. 2016;197(11):4240–6. 10.4049/jimmunol.1600236 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 17. Jiang X, Park CO, Geddes Sweeney J, et al. : Dermal γδ T Cells Do Not Freely Re-Circulate Out of Skin and Produce IL-17 to Promote Neutrophil Infiltration during Primary Contact Hypersensitivity. PLoS One. 2017;12(1):e0169397. 10.1371/journal.pone.0169397 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 18. Schmidt JD, Ahlström MG, Johansen JD, et al. : Rapid allergen-induced interleukin-17 and interferon-γ secretion by skin-resident memory CD8+ T cells. Contact Dermatitis. 2017;76(4):218–27. 10.1111/cod.12715 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 19. Nielsen MM, Lovato P, MacLeod AS, et al. : IL-1β-dependent activation of dendritic epidermal T cells in contact hypersensitivity. J Immunol. 2014;192(7):2975–83. 10.4049/jimmunol.1301689 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 20. Nielsen MM, Dyring-Andersen B, Schmidt JD, et al. : NKG2D-dependent activation of dendritic epidermal T cells in contact hypersensitivity. J Invest Dermatol. 2015;135(5):1311–9. 10.1038/jid.2015.23 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 21. Schmidt M, Raghavan B, Müller V, et al. : Crucial role for human Toll-like receptor 4 in the development of contact allergy to nickel. Nat Immunol. 2010;11(9):814–9. 10.1038/ni.1919 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 22. Raghavan B, Martin SF, Esser PR, et al. : Metal allergens nickel and cobalt facilitate TLR4 homodimerization independently of MD2. EMBO Rep. 2012;13(12):1109–15. 10.1038/embor.2012.155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rachmawati D, Bontkes HJ, Verstege MI, et al. : Transition metal sensing by Toll-like receptor-4: next to nickel, cobalt and palladium are potent human dendritic cell stimulators. Contact Dermatitis. 2013;68(6):331–8. 10.1111/cod.12042 [DOI] [PubMed] [Google Scholar]
- 24. Li X, Zhong F: Nickel induces interleukin-1β secretion via the NLRP3-ASC-caspase-1 pathway. Inflammation. 2014;37(2):457–66. 10.1007/s10753-013-9759-z [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 25. Adam C, Wohlfarth J, Haußmann M, et al. : Allergy-Inducing Chromium Compounds Trigger Potent Innate Immune Stimulation Via ROS-Dependent Inflammasome Activation. J Invest Dermatol. 2017;137(2):367–76. 10.1016/j.jid.2016.10.003 [DOI] [PubMed] [Google Scholar]
- 26. Martin SF, Dudda JC, Bachtanian E, et al. : Toll-like receptor and IL-12 signaling control susceptibility to contact hypersensitivity. J Exp Med. 2008;205(9):2151–62. 10.1084/jem.20070509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Esser PR, Wölfle U, Dürr C, et al. : Contact sensitizers induce skin inflammation via ROS production and hyaluronic acid degradation. PLoS One. 2012;7(7):e41340. 10.1371/journal.pone.0041340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nakamura N, Tamagawa-Mineoka R, Ueta M, et al. : Toll-like receptor 3 increases allergic and irritant contact dermatitis. J Invest Dermatol. 2015;135(2):411–7. 10.1038/jid.2014.402 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 29. Pedersen LK, Johansen JD, Held E, et al. : Augmentation of skin response by exposure to a combination of allergens and irritants - a review. Contact Dermatitis. 2004;50(5):265–73. 10.1111/j.0105-1873.2004.00342.x [DOI] [PubMed] [Google Scholar]
- 30. Martin SF: Adaptation in the innate immune system and heterologous innate immunity. Cell Mol Life Sci. 2014;71(21):4115–30. 10.1007/s00018-014-1676-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bonefeld CM, Nielsen MM, Rubin IM, et al. : Enhanced sensitization and elicitation responses caused by mixtures of common fragrance allergens. Contact Dermatitis. 2011;65(6):336–42. 10.1111/j.1600-0536.2011.01945.x [DOI] [PubMed] [Google Scholar]
- 32. Bonefeld CM, Geisler C, Gimenéz-Arnau E, et al. : Immunological, chemical and clinical aspects of exposure to mixtures of contact allergens. Contact Dermatitis. 2017;77(3):133–42. 10.1111/cod.12847 [DOI] [PubMed] [Google Scholar]
- 33. Koppes SA, Engebretsen KA, Agner T, et al. : Current knowledge on biomarkers for contact sensitization and allergic contact dermatitis. Contact Dermatitis. 2017;77(1):1–16. 10.1111/cod.12789 [DOI] [PubMed] [Google Scholar]
- 34. Dhingra N, Shemer A, Correa da Rosa J, et al. : Molecular profiling of contact dermatitis skin identifies allergen-dependent differences in immune response. J Allergy Clin Immunol. 2014;134(2):362–72. 10.1016/j.jaci.2014.03.009 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 35. Quaranta M, Knapp B, Garzorz N, et al. : Intraindividual genome expression analysis reveals a specific molecular signature of psoriasis and eczema. Sci Transl Med. 2014;6(244):244ra90. 10.1126/scitranslmed.3008946 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 36. Flier J, Boorsma DM, Bruynzeel DP, et al. : The CXCR3 activating chemokines IP-10, Mig, and IP-9 are expressed in allergic but not in irritant patch test reactions. J Invest Dermatol. 1999;113(4):574–8. 10.1046/j.1523-1747.1999.00730.x [DOI] [PubMed] [Google Scholar]
- 37. Meller S, Lauerma AI, Kopp FM, et al. : Chemokine responses distinguish chemical-induced allergic from irritant skin inflammation: memory T cells make the difference. J Allergy Clin Immunol. 2007;119(6):1470–80. 10.1016/j.jaci.2006.12.654 [DOI] [PubMed] [Google Scholar]
- 38. Garzorz-Stark N, Krause L, Lauffer F, et al. : A novel molecular disease classifier for psoriasis and eczema. Exp Dermatol. 2016;25(10):767–74. 10.1111/exd.13077 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 39. Jakob A, Mussotter F, Ohnesorge S, et al. : Immunoproteomic identification and characterization of Ni 2+-regulated proteins implicates Ni 2+ in the induction of monocyte cell death. Cell Death Dis. 2017;8(3):e2684. 10.1038/cddis.2017.112 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 40. Schmidt M, Hupe M, Endres N, et al. : The contact allergen nickel sensitizes primary human endothelial cells and keratinocytes to TRAIL-mediated apoptosis. J Cell Mol Med. 2010;14(6B):1760–76. 10.1111/j.1582-4934.2009.00823.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mussotter F, Tomm JM, El Ali Z, et al. : Proteomics analysis of dendritic cell activation by contact allergens reveals possible biomarkers regulated by Nrf2. Toxicol Appl Pharmacol. 2016;313:170–9. 10.1016/j.taap.2016.11.001 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 42. Zinkevičienė A, Kainov D, Lastauskienė E, et al. : Serum Biomarkers of Allergic Contact Dermatitis: A Pilot Study. Int Arch Allergy Immunol. 2015;168(3):161–4. 10.1159/000442749 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 43. Koppes SA, Ljubojevic Hadzavdic S, Jakasa I, et al. : Stratum corneum profiles of inflammatory mediators in patch test reactions to common contact allergens and sodium lauryl sulfate. Br J Dermatol. 2017;176(6):1533–40. 10.1111/bjd.15163 [DOI] [PubMed] [Google Scholar]
- 44. Mose KF, Burton M, Thomassen M, et al. : The gene expression and immunohistochemical time-course of diphenylcyclopropenone-induced contact allergy in healthy humans following repeated epicutaneous challenges. Exp Dermatol. 2017;26(10):926–33. 10.1111/exd.13345 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 45. Molin S, Merl J, Dietrich KA, et al. : The hand eczema proteome: imbalance of epidermal barrier proteins. Br J Dermatol. 2015;172(4):994–1001. 10.1111/bjd.13418 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 46. Braun A, Dewert N, Brunnert F, et al. : Integrin α E(CD103) Is Involved in Regulatory T-Cell Function in Allergic Contact Hypersensitivity. J Invest Dermatol. 2015;135(12):2982–91. 10.1038/jid.2015.287 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 47. Klein M, Bopp T: Cyclic AMP Represents a Crucial Component of Treg Cell-Mediated Immune Regulation. Front Immunol. 2016;7:315. 10.3389/fimmu.2016.00315 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 48. Mahnke K, Useliene J, Ring S, et al. : Down-Regulation of CD62L Shedding in T Cells by CD39 + Regulatory T Cells Leads to Defective Sensitization in Contact Hypersensitivity Reactions. J Invest Dermatol. 2017;137(1):106–14. 10.1016/j.jid.2016.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 49. Weber FC, Esser PR, Müller T, et al. : Lack of the purinergic receptor P2X 7 results in resistance to contact hypersensitivity. J Exp Med. 2010;207(12):2609–19. 10.1084/jem.20092489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Neuberger A, Ring S, Silva-Vilches C, et al. : Expression of CD73 slows down migration of skin dendritic cells, affecting the sensitization phase of contact hypersensitivity reactions in mice. J Dermatol Sci. 2017;87(3):292–9. 10.1016/j.jdermsci.2017.07.002 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 51. Sun J, Madan R, Karp CL, et al. : Effector T cells control lung inflammation during acute influenza virus infection by producing IL-10. Nat Med. 2009;15(3):277–84. 10.1038/nm.1929 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 52. Sun J, Cardani A, Sharma AK, et al. : Autocrine regulation of pulmonary inflammation by effector T-cell derived IL-10 during infection with respiratory syncytial virus. PLoS Pathog. 2011;7(8):e1002173. 10.1371/journal.ppat.1002173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dolch A, Kunz S, Dorn B, et al. : Contact allergens induce CD8 + T cell-derived interleukin 10 that appears dispensable for regulation of contact hypersensitivity. Exp Dermatol. 2017;26(5):449–51. 10.1111/exd.13237 [DOI] [PubMed] [Google Scholar]
- 54. Fjelbye J, Antvorskov JC, Buschard K, et al. : CD1d knockout mice exhibit aggravated contact hypersensitivity responses due to reduced interleukin-10 production predominantly by regulatory B cells. Exp Dermatol. 2015;24(11):853–6. 10.1111/exd.12792 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 55. Gomez de Agüero M, Vocanson M, Hacini-Rachinel F, et al. : Langerhans cells protect from allergic contact dermatitis in mice by tolerizing CD8 + T cells and activating Foxp3 + regulatory T cells. J Clin Invest. 2012;122(5):1700–11. 10.1172/JCI59725 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 56. Luckey U, Schmidt T, Pfender N, et al. : Crosstalk of regulatory T cells and tolerogenic dendritic cells prevents contact allergy in subjects with low zone tolerance. J Allergy Clin Immunol. 2012;130(3):781–797.e11. 10.1016/j.jaci.2012.06.022 [DOI] [PubMed] [Google Scholar]
- 57. Hacini-Rachinel F, Gomez de Agüero M, Kanjarawi R, et al. : Intestinal dendritic cell licensing through Toll-like receptor 4 is required for oral tolerance in allergic contact dermatitis. J Allergy Clin Immunol. 2018;141(1):163–70. 10.1016/j.jaci.2017.02.022 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 58. El Beidaq A, Link CW, Hofmann K, et al. : In Vivo Expansion of Endogenous Regulatory T Cell Populations Induces Long-Term Suppression of Contact Hypersensitivity. J Immunol. 2016;197(5):1567–76. 10.4049/jimmunol.1600508 [DOI] [PubMed] [Google Scholar]
- 59. Di Gioacchino M, Ricciardi L, De Pità O, et al. : Nickel oral hyposensitization in patients with systemic nickel allergy syndrome. Ann Med. 2014;46(1):31–7. 10.3109/07853890.2013.861158 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 60. Christoffers WA, Blömeke B, Coenraads PJ, et al. : The optimal patch test concentration for ascaridole as a sensitizing component of tea tree oil. Contact Dermatitis. 2014;71(3):129–37. 10.1111/cod.12199 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 61. Chaudhry HM, Drage LA, El-Azhary RA, et al. : Delayed Patch-Test Reading After 5 Days: An Update From the Mayo Clinic Contact Dermatitis Group. Dermatitis. 2017;28(4):253–60. 10.1097/DER.0000000000000297 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 62. Joy NM, Rice KR, Atwater AR: Stability of patch test allergens. Dermatitis. 2013;24(5):227–36. 10.1097/DER.0b013e3182a0a19d [DOI] [PubMed] [Google Scholar]
- 63. Johansen JD, Aalto-Korte K, Agner T, et al. : European Society of Contact Dermatitis guideline for diagnostic patch testing - recommendations on best practice. Contact Dermatitis. 2015;73(4):195–221. 10.1111/cod.12432 [DOI] [PubMed] [Google Scholar]
- 64. Engfeldt M, Hagvall L, Isaksson M, et al. : Patch testing with hydroxyisohexyl 3-cyclohexene carboxaldehyde (HICC) - a multicentre study of the Swedish Contact Dermatitis Research Group. Contact Dermatitis. 2017;76(1):34–9. 10.1111/cod.12699 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 65. Uter W, Bruze M, Rustemeyer T, et al. : Re "International survey on skin patch test procedures, attitudes and interpretation" L.K. Tanno et al., WAOJ (2016) 9:8. World Allergy Organ J. 2017;10(1):18. 10.1186/s40413-017-0149-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kim N, Notik S, Gottlieb AB, et al. : Patch test results in psoriasis patients on biologics. Dermatitis. 2014;25(4):182–90. 10.1097/DER.0000000000000056 [DOI] [PubMed] [Google Scholar]
- 67. Wee JS, White JM, McFadden JP, et al. : Patch testing in patients treated with systemic immunosuppression and cytokine inhibitors. Contact Dermatitis. 2010;62(3):165–9. 10.1111/j.1600-0536.2009.01695.x [DOI] [PubMed] [Google Scholar]