Abstract
With the exception of infant growth, there are no well-defined parameters describing normal human lactation. This represents a major gap in the continuum of care that does not exist for other major organs. Biological normality occurs naturally and is characterized by well-integrated function. We have proposed a definition that highlights four key elements that describe parameters for biological normality: comfort, milk supply, infant health, and maternal health. Notwithstanding the current limitations, published data have been collated to provide preliminary markers for the initiation of lactation and to describe objective tests once lactation is established. Reference limits have been calculated for maternal markers of secretory activation, including progesterone in maternal blood and total protein, lactose, sodium, and citrate in maternal milk. Objective measurements for established lactation, including 3-hourly pumping and 24-hour milk production, together with pre-feed to post-feed milk fat changes (a useful indicator of the available milk removed by the infant) have been outlined. Considered together with the parameters describing normal function, this information provides a preliminary objective framework for the assessment of human lactation.
Keywords: Lactation, Breastfeeding, Reference ranges, Reference limits, Normal function, Maternal, Breastmilk
Introduction
The rationale for writing a review is usually based on bringing together recent major advances and published discussion in a particular area of research. Unfortunately, basic research into the physiology and biochemistry of the lactating human mammary gland is limited, and there have been no major advances toward the assessment of its normal function in recent times. As a result, the lactating mammary gland is a poor cousin when compared with other major organs such as the heart, brain, liver, lungs, and kidneys. These all have an array of objective tests available to assess function. This review examines the evidence available toward the development of methods for the objective assessment of lactation. Discussion will be limited to the maternal aspects of lactation physiology and biochemistry during the period of exclusive breastfeeding of the infant (that is, the period of time from birth and during the period of exclusive breastfeeding).
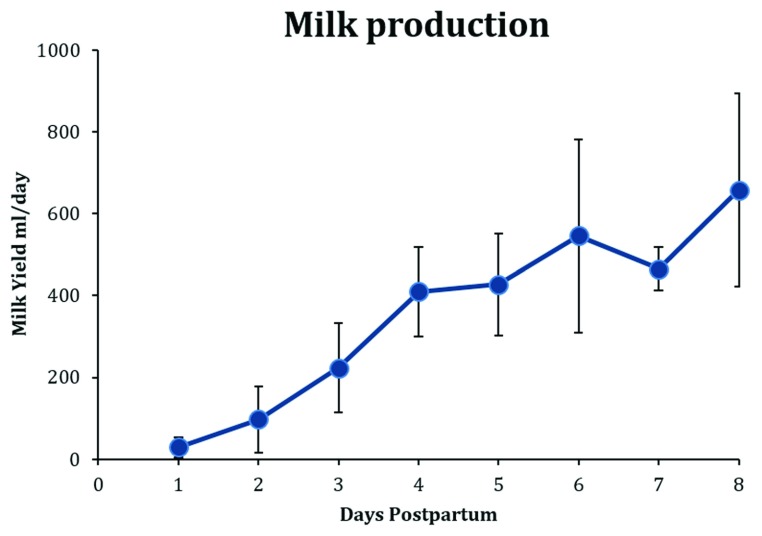
The mean milk production of lactating women by 8 days postpartum ( Figure 1) is 650 mL/24 hours, and from 1 to 6 months of lactation the mean range for exclusively breastfed infants is between 750 and 800 mL/24 hours 1. Therefore, for mothers exclusively breastfeeding their babies, it can be calculated that the energy output in human milk accounts for about 20% to 30% of the maternal resting energy requirement 2. This large energy commitment clearly demonstrates the evolutionary importance of lactation for human survival. The World Health Organization (WHO) recommends exclusive breastfeeding for the first 6 months of an infant’s life followed by the addition of nutritionally adequate and safe complementary foods while breastfeeding continues for up to 2 years of age or beyond 3. In Australia, there is a high rate of lactation initiation (96%). However, these figures decline rapidly after birth, and only 15.4% of infants are exclusively breastfed at 5 months of age 4. Thus, in Australia, as in many other high-income countries, almost all mothers elect to breastfeed, but many encounter difficulties that affect their ability to continue.
Figure 1. Daily values for milk production in the first 8 days postpartum.
Figure shows weighted means and standard deviations collated from published values. Data sources: 5– 14.
Vital to the assessment of function of major organs is the objective measurement of various aspects of their physiology and biochemistry and the comparison of these values with reference ranges. An objective measurement of normal function can then form the scientific basis for identification of potential abnormality (function falling outside the normal range), diagnosis of disease, and the evaluation of treatments. Unfortunately, there are no routine clinical tests available for the assessment of normal function for lactation.
Normal human lactation
In order to identify objective measurements for normal lactation function, it is first necessary to define normal lactation. Here, “normality” is considered in the biological sense, namely that normal function occurs naturally and not as a result of any disease, treatment, or genetic abnormality. Normal function does not require medical intervention or support. Thus, the following definition for lactation during the first 6 months after a term birth is proposed.
Normal human lactation
is comfortable for both the mother and infant
provides adequate milk for the infant’s optimal growth and development
requires coordinated maternal and infant adaptation that is facilitated by good maternal and infant health.
Reference ranges and reference limits
In the context of biological function, a reference range describes a range of values for a physiological measurement in a healthy person. A reference range for a particular measurement is the interval between which 95% of values for a reference group taken from the general population fall. Values outside the upper and lower limits of a reference range are not necessarily abnormal but can be considered to be indicators of possible pathology. A reference range is derived from a normal curve based on measurements for at least 120 healthy individuals. A reference limit describes cases where only one side of the range is of interest 15.
The above definition of normal human lactation defines the inclusive and exclusive parameters for studies aimed at determining reference ranges or reference limits (or both) for the assessment of human lactation. With the exception of the growth rate of breastfed infants (WHO growth charts 16), there are no studies that meet these criteria. Most substrates, metabolites, and hormones associated with human lactation change markedly according to the period of lactation, time of day, time of breastfeeding, duration of the breastfeed, and the degree of fullness of the breast. Thus, these factors need to be standardized for the development of normal ranges across the course of lactation. These deficiencies notwithstanding, it is nevertheless important to review the literature and construct approximate objective parameters for human lactation.
This review concentrates on areas where even roughly defined reference ranges could greatly assist in the understanding of lactation function (and consequently dysfunction). In this context, maternal parameters associated with secretory activation 17 and established lactation will be highlighted. In addition to this restriction to a consideration of maternal factors in the first instance, a pragmatic approach was taken to emphasize methods that could be implemented now (for example, blood progesterone and milk sodium) and then methods that could be readily developed in the future for clinical use (for example, milk lactose and milk production). There are many other factors that potentially could be used to assist clinical diagnosis, including certain proteins (α-lactalbumin), carbohydrates (oligosaccharides), lipids (medium-chain fatty acids), and enzymes (aurora kinase-A), but there is not enough evidence in human lactation to provide potential diagnosis and treatment protocols.
Lactation cycle
The breast reaches a mature functional state only during lactation 18. The lactation cycle begins with conception. Pregnancy induces ductal proliferation and subsequent lobular alveolar development in the mammary gland. Alveolar development leads to secretory differentiation 17 with the maturation of lactocytes and the production of unique milk components. Delivery of the placenta triggers secretory activation and the transition to copious milk secretion. Sustained milk synthesis requires the continuation of efficient and regular milk removal by the infant in the context of normal function or by breast pump or hand expression if required. The lactation cycle is completed when the breast returns to quiescence following weaning of the infant.
Secretory differentiation and secretory activation
Lactation is established in two phases. First, secretory differentiation is observed as the breast develops the capacity to synthesize unique milk products in colostrum, including lactose, casein, α-lactalbumin, and lactoferrin. The second phase, secretory activation, begins around 60 hours (range of 24–72 hours) after birth and is triggered by delivery of the placenta 19, 20.
Secretory differentiation
There is little information on variation in breast growth and function during pregnancy. Breast volume generally increases after conception, but there is considerable variation between mothers 21. Importantly, this variation does not appear to influence the potential for milk synthesis postpartum 5, 19, 22.
Secretory differentiation occurs from about 20 weeks of pregnancy and requires the action of a lactogenic complex of hormones. The increasing concentration of prolactin in maternal blood is related to the increased excretion of lactose in urine, and the increase in the concentration of human placental lactogen is related to breast growth. During this phase, the tight junctions between lactocytes are leaky, allowing milk constituents to pass into the blood. Lactose cannot be metabolized once it enters the blood vascular system and is excreted in the urine. Therefore, the 24-hour output of lactose in urine provides a measure of the synthesis of lactose in the mammary glands. Coupling the 24-hour output of lactose in urine with the measurement of lactose in colostrum provides an estimate of the rate of synthesis of colostrum during pregnancy.
These measurements confirm that the rate of colostrum synthesis at this time is low (about 30 mL/24 hours) 17, 23.
Secretory activation
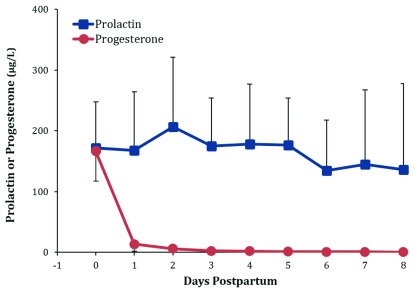
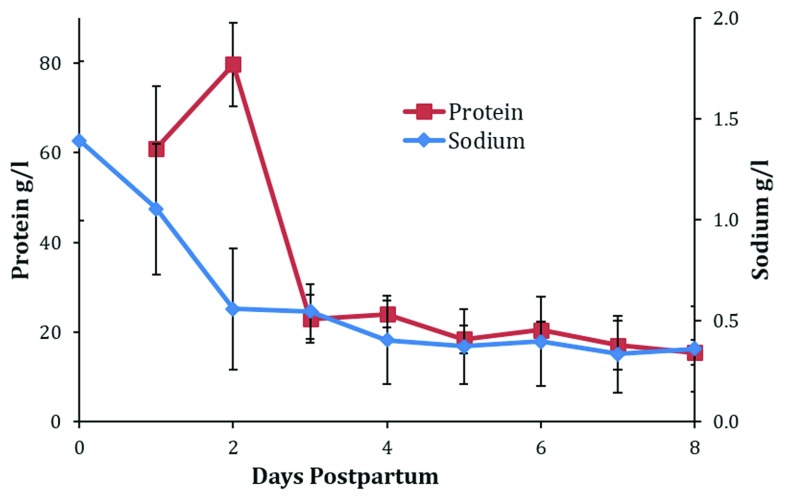
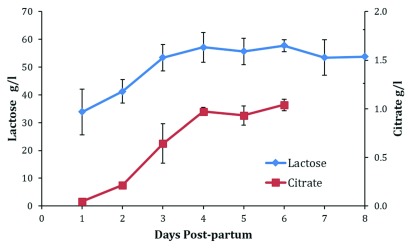
Secretory activation is a process manifest by the initiation of copious milk secretion ( Figure 1). The increase in milk secretion is accompanied by many metabolic changes. The most documented of these changes are decreases in blood progesterone ( Figure 2), milk protein, and sodium ( Figure 3) and increases in milk lactose and citrate ( Figure 4).
Figure 2. Daily concentrations of serum progesterone and prolactin in the first 8 days postpartum.
Figure shows weighted means and standard deviations collated from published values. Data sources: 24– 31.
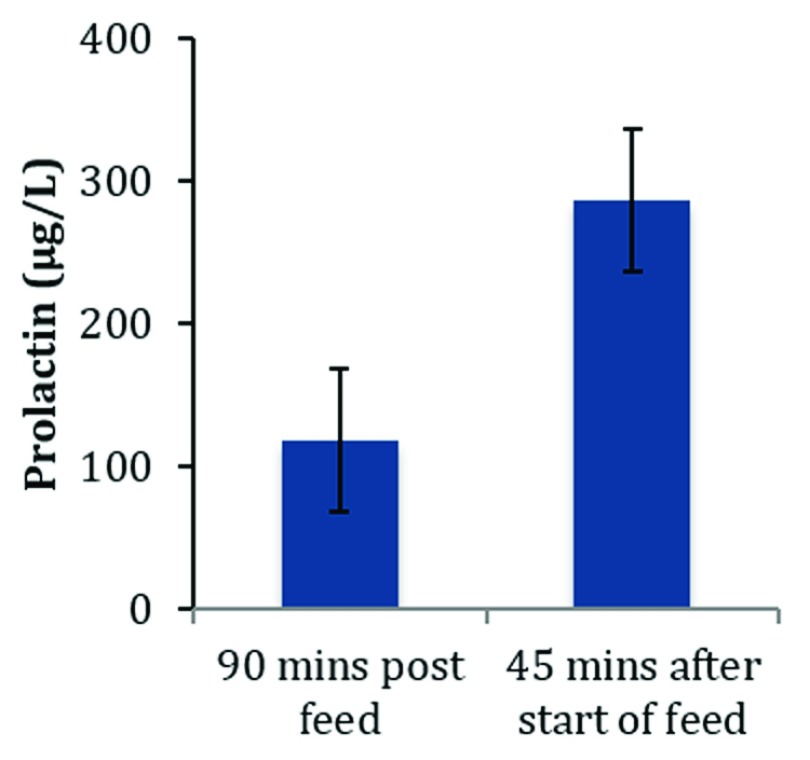
Figure 3. Serum prolactin levels 90 minutes post-feed and 45 minutes after beginning of feed at 1 month postpartum.
Data source: 32.
Figure 4. Daily breastmilk concentrations of total protein and sodium in the first 8 days postpartum.
Figure shows weighted means and standard deviations collated from published values. Data sources: 7, 36– 50.
Colostrum and the first 3 days
Colostrum is available to the infant for the first 60 hours (range of 24–72 hours) after birth 19, 20. The volume ingested by healthy newborns in the first 24 hours of life is small and mirrors synthesis (29 mL ± 24, mean ± standard deviation [SD]) ( Figure 1). Research interest in colostrum is limited for two reasons: first, from the generalized lack of research into human lactation (see above) and, second, by its appearance and small volume. More than 300 years ago, Cadogan observed that, “When a child is first born, there seems to be no provision at all made for it: for mother’s milk seldom comes in ’till the third day: so that according to nature, a child would be left a day and a half or two days, without food; to me a very sufficient proof that it wants none” 33. Furthermore, Dettwyler reported that, “In historical times, and even today, babies in some societies are denied colostrum, with all its beneficial properties, in the belief that it is a poisonous substance dangerous for the newborn” 34.
In contrast to historical beliefs, evidence is now emerging that the first 3 days postpartum are critically important for the establishment of lactation. In a study of pump-dependent mothers who had delivered preterm, Meier et al. compared milk expression by using the standard suction pattern on a breast pump with using a pattern that mimicked the infant sucking 35. The pattern was applied from birth to 3 days postpartum and then a standard pump was used up to 14 days postpartum. This treatment resulted in a 60% increase in milk synthesis from 6 to 14 days postpartum compared with the standard pumping pattern. Morton et al. studied pump-dependent mothers who had delivered preterm and combined hand massage with electric pumping in the immediate postpartum period and found that this intervention increased milk synthesis at 2 weeks compared with the use of a standard breast pump from birth 51. Additionally, a study in the Democratic Republic of the Congo reported that 14% of mothers provided with standard care and 16% provided with the Ten Steps to Successful Breastfeeding program were exclusively breastfeeding at 6 months postpartum but that 45% of mothers provided with only steps 1 to 9 were exclusively breastfeeding at 6 months 52, 53. These findings show that subtle interventions in the early postpartum period can induce profound outcomes during established lactation.
Progesterone withdrawal
Kuhn first demonstrated that the withdrawal of progesterone was related to the ovary switching from progesterone to 20αOH-progesterone synthesis 54. The resulting fall in progesterone was shown to be the “lactogenic trigger” in the rat 54. Subsequently, in all mammals studied, progesterone withdrawal has been found to be the trigger for secretory activation. In women, progesterone is synthesized in the placenta and falls precipitously by more than an order of magnitude after the delivery of the placenta ( Figure 2). Consequently, colostrum synthesis continues until about 24 to 72 hours after birth when the process of secretory activation initiates the transition of colostrum into mature breastmilk. Thus, if viable fragments of placenta are retained after delivery, secretory activation will be either fully or partly inhibited.
Assays for progesterone concentration in blood are readily available. In addition, laboratory assays for progesterone concentrations in milk are available, and an in-home assay for urinary metabolites of progesterone has been developed 55. Despite the availability of these assays, progesterone is never measured when assessing the initiation of lactation in women who are at risk of or appear to have impaired secretory activation.
Given what is known about progesterone withdrawal triggering secretory activation, it can be hypothesized that elevated blood progesterone is a sensitive indicator of retained viable placental fragments. Furthermore, it is possible that the inhibitory effects of elevated blood progesterone on secretory activation are reduced by the administration of mifepristone (RU486), a progesterone antagonist. However, more research is required before these possibilities can be adopted clinically.
Prolactin
At secretory activation, elevated concentrations of prolactin are observed during the period of progesterone withdrawal ( Figure 2). Plasma prolactin is high early in lactation and progressively decreases, but levels at 6 months of lactation are still higher than those reported for non-lactating women 32. The role of prolactin during the initiation of lactation is complex 18. The concentrations reported for prolactin during the early postpartum period are highly variable and difficult to interpret. This can be attributed to the secretion of prolactin varying in response to the suckling stimulus, and peak values are observed about 45 minutes after the infant latches to the breast ( Figure 3). Levels then decrease to about half the peak levels by the next breastfeed. Prolactin also shows a circadian variation 56 and increases at mealtimes. No studies have monitored prolactin levels in relation to the timing of breastfeeds, meals, and time of day, and this probably accounts for the large variation in reported concentrations of prolactin during secretory activation. Prolactin is present as different isoforms and undergoes considerable post-translational modifications, including glycosylation, phosphorylation, proteolytic cleavage, and polymerization. These modifications influence the function of this complex hormone. It is clear that prolactin is required for successful secretory activation 57, but it probably does not play a rate-limiting role.
The threshold concentration of prolactin (reference limit for normality) needed for normal secretory activation is unknown. This information is urgently required to ensure that mothers with normal concentrations of prolactin are not subjected to unwarranted medication with peripherally selective dopamine D 2 receptor antagonist activity to treat low milk supply.
Closure of tight junctions and the effect on milk composition
Closure of the tight junctions between lactocytes is related to the withdrawal of progesterone. At this time, the concentration of sodium in the mammary secretion rapidly decreases ( Figure 5) as milk production increases ( Figure 1) and provides a simple objective assessment of the progress of secretory activation ( Figure 4) 58. Indeed, sodium-sensitive electrodes that permit the measurement of sodium using only a few drops of breastmilk are available (Na + LAQUA twin electrode, Horiba Scientific, Kyoto, Japan).
Figure 5. Daily breastmilk concentrations of lactose to day 8 and citrate to day 6 and citrate in the first 8 days postpartum.
Figure shows weighted means and standard deviations collated from published values. Data sources: 7, 39, 42, 43, 45, 47– 49, 65– 70.
Milk volume and the concentrations of sodium, lactose, citrate, and total protein all appear to reach stable levels by 8 days postpartum ( Figure 1, Figure 4, and Figure 5).
Decreases in sodium and total protein and increases in lactose and citrate in the mammary secretion can be used to monitor secretory activation 5, 59 ( Figure 4 and Figure 5). Collation of the published values for the changes in progesterone, sodium, total protein, lactose, and citrate enables the calculation of weighted daily means. From these means and SDs, it is possible to calculate reference limits for normality ( Table 1). It follows that the mean plus two SDs for progesterone in maternal blood and sodium and total protein in milk as well as the mean minus two SDs for lactose and citrate in milk provide reference limits that could be considered normal. Reference limits for days 3 and 6 are presented in Table 1. However, it must be recognized that these are tentative values because very few studies have determined the changes in blood progesterone and milk composition in the same mothers and, more importantly, most studies do not provide an objective assessment of the success of the mothers in establishing normal lactation.
Table 1. Reference limits at 3 and 6 days postpartum *.
| 3 days postpartum | 6 days postpartum | |
|---|---|---|
| Maternal serum | Maternal serum | |
| Progesterone, μg/L | <7.1 | <2.2 |
| Milk | Milk | |
| Total protein, g/L | <34.1 | <24.3 |
| Sodium, g/L | <0.81 | <0.84 |
| Lactose, g/L | >43.4 | >53.6 |
| Citrate, g/L | >0.24 | >0.92 |
*Expressed as weighted means ± two standard deviations from the mean for maternal serum concentrations for progesterone and milk concentrations for sodium, total protein, lactose, and citrate
As stated, milk sodium can be measured to track the progress of secretory activation. In the future, timed urinary lactose measurements after birth would also be useful. High levels of urinary lactose combined with low levels of milk sodium would indicate that lactose synthesis (milk synthesis) is occurring but the milk is not being removed from the mammary gland; that is, there is a “baby” problem rather than a “mother” problem.
Citrate has been claimed to be the harbinger of lactation 60, but the metabolic stimulus driving the increase of this metabolite is not known. Citrate still provides a very good marker for secretory activation ( Figure 5). It is not measured routinely in clinical pathology laboratories and therefore is not available at this time for the assessment of secretory activation.
Milk synthesis and milk production
It is important to define the difference between milk synthesis and milk production measurements. Whereas milk synthesis is a measure of the maternal capacity to synthesize milk, milk production is a measure of the infant’s ability to remove milk from the mother’s breasts ( Figure 1). Daily progressive measurements of both of these parameters would be very useful in the assessment of lactation 6, 7. Twenty-four-hour measurements of milk production by weighing the infant immediately before and after each breastfeed to determine the milk intake is extremely difficult to carry out in the immediate postpartum period. Milk synthesis cannot currently be measured directly; however, the increase in milk citrate appears to be closely related to the mother’s ability to synthesize milk. A greater knowledge of the metabolic factors related to the increase in citrate in early postpartum milk secretion is required to confirm this possibility.
Established lactation
Once lactation is established from about 2 weeks postpartum, milk production remains relatively constant up to 6 months of lactation for infants that are exclusively breastfed 1. Milk synthesis is not limited by the capacity of the mother to synthesize milk but rather by the infant’s appetite 67. Thus, milk production in breastfeeding mothers is not a measure of the mother’s capacity to synthesize breastmilk but rather a measurement of the infant’s appetite. Furthermore, the variation in milk production reported between infants is large, ranging from about 500 to 1,000 mL/24 hours with a mean of 750–800 mL/24 hours 1. The WHO Child Growth Standards provide reference values for breastfed infants; however, nearly all studies of normal intake of exclusively breastfeeding infants precede their release. Therefore, reference values for breastmilk production require further investigation. Nevertheless, measurement of milk production is currently the best objective method available to measure normal breast function during established lactation.
Measurement of milk production
The most clinically feasible measure of breastmilk transfer to the infant is to weigh the infant immediately before and after each breastfeed over a 24-hour period 67. From a nutritional standpoint, a longer period would be desirable; however, this method is quite demanding of mother and infant and the quality of the data degrades as the collection period is extended. Alternatively, each breast can be expressed for 10 minutes every hour for three consecutive hours in a calm environment. The volume of milk expressed at the third expression multiplied by 24 also provides an estimate of 24-hour milk synthesis 67. It is possible to use this method in a clinical setting, but further validation is required. Total breastmilk transfer from both breasts over a period of 14 days can be measured by using the deuterium oxide dose-to-mother technique. This is an excellent technique for nutritional studies for determining macro- and micro-nutrient intake. However, this method is based on the dilution of the isotope over a period of 14 days and requires stable milk production over this period. Measurement of milk production is a good starting point for assessment of breast function, particularly when there are concerns that supply is inadequate.
Milk fat
Fat is the most variable component of human milk, and around 70% of this variation is due to the extent of breast fullness 68. As the breast empties, the fat content increases 68. This provides one of the few objective tests available to determine the proportion of available milk removed by the infant during a breastfeed. Mature milk from a full breast appears bluish-white because of the low fat content pre-feed. Post-feed, the milk changes in color to creamy white as the breast is drained and the fat content increases. This response is more obvious if the milk is allowed to settle. If there is not much change in the color of the milk between the pre-feed and post-feed samples, the baby has not removed much milk. Furthermore, if the pre-feed milk is bluish-white, the breast is full of milk, and if the pre-feed milk is creamy white, the breast is drained of milk. Because visual assessments of a breastfeed are very unreliable, observations of the color of the milk can provide only limited objectivity in the clinical assessment of a breastfeed. A more objective measure of breastmilk transfer was discussed previously. The change in fat content can be measured objectively with a creamatocrit centrifuge (Medela AG, Baar, Switzerland) ( Table 2) 69.
Table 2. Concentrations of fat in mature human milk 1, 19.
| Fat | Amount, g/L | Standard
deviation, g/L |
|---|---|---|
| Average | 41.1 | ± 7.8 |
| Pre-feed | 30.3 | ± 17.5 |
| Post-feed | 57.8 | ± 24.0 |
Milk ejection
Milk ejection occurs because of a neuro-hormonal reflex triggered by stimulation of the nipple areolar area, which results in the contraction of myoepithelial cells surrounding the alveoli and subsequent expulsion of milk. It is a conditioned reflex and is responsive to environmental inputs. Each woman has multiple milk ejections during a breastfeed, but most mothers sense only the first. Milk ejection can be readily measured by ultrasound imaging of the non-suckled breast 70. In mothers who do not sense milk ejection, measurement of an increase in duct diameter during a breastfeed can be used to confirm milk ejection.
Conclusions
In a whole of body comparison, the lactating human breast rivals the brain in its energy requirements and has the highest energy requirement of the organs in the reproductive cycle. Evidence-based reference ranges or limits for normal function (or both) provide a foundation for clinical diagnosis and treatment of problems encountered. Despite its metabolic importance, there is an enormous gap in our understanding of these parameters for the biochemistry and physiology of lactation. This review has shown that only approximate reference ranges can be calculated. Nevertheless, they provide a starting point.
There have been many successful health programs aimed at encouraging women to breastfeed their babies, but there is little ability to adequately monitor and support lactation initiation and establishment with objective tests. Under these conditions, it is not surprising that in high-income countries, even when most women choose to breastfeed, the rate of sustained lactation rapidly declines after birth. It seems obvious that to effectively “close the gap” more scientific evidence must be integrated with psychological and practical support for all women who want to breastfeed their infants.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Laurie A. Nommsen-Rivers, Nutritional Sciences Program, College of Allied Health Sciences, University of Cincinnati, Cincinnati, Ohio, USA
Guido E. Moro, Italian Association of Human Milk Banks (AIBLUD), Milan, Italy
Kim F. Michaelsen, Department of Nutrition, Exercise and Sports, University of Copenhagen, Copenhagen, Denmark
Shannon L. Kelleher, Department of Biomedical and Nutritional Sciences, The University of Massachusetts Lowell, Lowell, Massachusetts, USA
Funding Statement
The authors declare that no grants were involved in supporting this work. Melinda Boss gratefully acknowledges research funding from the Family Larsson-Rosenquist Foundation (PG 52000600). Hazel Gardner is supported by an unrestricted research grant from Medela AG, Switzerland (PG52085000). Peter Hartmann holds an emeritus professorial position and declares that no grants were involved in supporting his contribution to this work.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; referees: 4 approved]
References
- 1. Kent JC, Mitoulas LR, Cregan MD, et al. : Volume and frequency of breastfeedings and fat content of breast milk throughout the day. Pediatrics. 2006;117(3):e387–95. 10.1542/peds.2005-1417 [DOI] [PubMed] [Google Scholar]
- 2. Butte NF, King JC: Energy requirements during pregnancy and lactation. Public Health Nutr. 2005;8(7A):1010–27. 10.1079/PHN2005793 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 3. UNICEF, WHO: Infant and Young Child Nutrition: Global Strategy on Infant and Young Child Feeding. Geneva: World Health Organisation;2002. Reference Source [Google Scholar]
- 4. Australian Institute of Health and Welfare: Australian National Infant Feeding Survey: indicator results. Canberra: AIHW; Contract No.: Cat. no. PHE 156.2010. Reference Source [Google Scholar]
- 5. Arthur PG, Smith M, Hartmann PE: Milk lactose, citrate, and glucose as markers of lactogenesis in normal and diabetic women. J Pediatr Gastroenterol Nutr. 1989;9(4):488–96. 10.1097/00005176-198911000-00016 [DOI] [PubMed] [Google Scholar]
- 6. Neville MC, Keller R, Seacat J, et al. : Studies in human lactation: milk volumes in lactating women during the onset of lactation and full lactation. Am J Clin Nutr. 1988;48(6):1375–86. 10.1093/ajcn/48.6.1375 [DOI] [PubMed] [Google Scholar]
- 7. Saint L, Smith M, Hartmann PE: The yield and nutrient content of colostrum and milk of women from giving birth to 1 month post-partum. Br J Nutr. 1984;52(1):87–95. 10.1079/BJN19840074 [DOI] [PubMed] [Google Scholar]
- 8. Casey CE, Hambidge KM, Neville MC: Studies in human lactation: zinc, copper, manganese and chromium in human milk in the first month of lactation. Am J Clin Nutr. 1985;41(6):1193–200. 10.1093/ajcn/41.6.1193 [DOI] [PubMed] [Google Scholar]
- 9. Casey CE, Neifert MR, Seacat JM, et al. : Nutrient intake by breast-fed infants during the first five days after birth. Am J Dis Child. 1986;140(9):933–6. 10.1001/archpedi.1986.02140230103044 [DOI] [PubMed] [Google Scholar]
- 10. Dollberg S, Lahav S, Mimouni FB: A comparison of intakes of breast-fed and bottle-fed infants during the first two days of life. J Am Coll Nutr. 2001;20(3):209–11. 10.1080/07315724.2001.10719033 [DOI] [PubMed] [Google Scholar]
- 11. Evans KC, Evans RG, Royal R, et al. : Effect of caesarean section on breast milk transfer to the normal term newborn over the first week of life. Arch Dis Child Fetal Neonatal Ed. 2003;88(5):F380–2. 10.1136/fn.88.5.F380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hester SN, Hustead DS, Mackey AD, et al. : Is the macronutrient intake of formula-fed infants greater than breast-fed infants in early infancy? J Nutr Metab. 2012;2012: 891201. 10.1155/2012/891201 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 13. Roderuck C, Williams HH, Macy IG: Metabolism of women during the reproductive cycle; the utilization of thiamine during lactation. J Nutr. 1946;32(3):249–65. 10.1093/jn/32.3.249 [DOI] [PubMed] [Google Scholar]
- 14. Yamauchi Y, Yamanouchi I: Breast-feeding frequency during the first 24 hours after birth in full-term neonates. Pediatrics. 1990;86(2):171–5. [PubMed] [Google Scholar]
- 15. Katayev A, Balciza C, Seccombe DW: Establishing reference intervals for clinical laboratory test results: is there a better way? Am J Clin Pathol. 2010;133(2):180–6. 10.1309/AJCPN5BMTSF1CDYP [DOI] [PubMed] [Google Scholar]
- 16. WHO Multicentre Growth Reference Study Group: WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr Suppl. 2006;450:76–85. [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 17. Pang WW, Hartmann PE: Initiation of human lactation: secretory differentiation and secretory activation. J Mammary Gland Biol Neoplasia. 2007;12(4):211–21. 10.1007/s10911-007-9054-4 [DOI] [PubMed] [Google Scholar]
- 18. Hassiotou F, Geddes D: Anatomy of the human mammary gland: Current status of knowledge. Clin Anat. 2013;26(1):29–48. 10.1002/ca.22165 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 19. Kent JC: How breastfeeding works. J Midwifery Womens Health. 2007;52(6):564–70. 10.1016/j.jmwh.2007.04.007 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 20. Dewey KG, Nommsen-Rivers LA, Heinig MJ, et al. : Risk factors for suboptimal infant breastfeeding behavior, delayed onset of lactation, and excess neonatal weight loss. Pediatrics. 2003;112(3 Pt 1):607–19. 10.1542/peds.112.3.607 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 21. Daly SE, Kent JC, Huynh DQ, et al. : The determination of short-term breast volume changes and the rate of synthesis of human milk using computerized breast measurement. Exp Physiol. 1992;77(1):79–87. 10.1113/expphysiol.1992.sp003584 [DOI] [PubMed] [Google Scholar]
- 22. Kulski JK, Hartmann PE: Changes in human milk composition during the initiation of lactation. Aust J Exp Biol Med Sci. 1981;59(1):101–14. 10.1038/icb.1981.6 [DOI] [PubMed] [Google Scholar]
- 23. Cox DB, Kent JC, Casey TM, et al. : Breast growth and the urinary excretion of lactose during human pregnancy and early lactation: endocrine relationships. Exp Physiol. 1999;84(2):421–34. 10.1111/j.1469-445X.1999.01807.x [DOI] [PubMed] [Google Scholar]
- 24. Heidrich A, Schleyer M, Spingler H, et al. : Postpartum blues: relationship between not-protein bound steroid hormones in plasma and postpartum mood changes. J Affect Disord. 1994;30(2):93–8. 10.1016/0165-0327(94)90036-1 [DOI] [PubMed] [Google Scholar]
- 25. Klier CM, Muzik M, Dervic K, et al. : The role of estrogen and progesterone in depression after birth. J Psychiatr Res. 2007;41(3–4):273–9. 10.1016/j.jpsychires.2006.09.002 [DOI] [PubMed] [Google Scholar]
- 26. Nott PN, Franklin M, Armitage C, et al. : Hormonal changes and mood in the puerperium. Br J Psychiatry. 1976;128:379–83. 10.1192/bjp.128.4.379 [DOI] [PubMed] [Google Scholar]
- 27. O'Hara MW, Schlechte JA, Lewis DA, et al. : Controlled prospective study of postpartum mood disorders: psychological, environmental, and hormonal variables. J Abnorm Psychol. 1991;100(1):63–73. 10.1037/0021-843X.100.1.63 [DOI] [PubMed] [Google Scholar]
- 28. Abou-Saleh MT, Ghubash R, Karim L, et al. : Hormonal aspects of postpartum depression. Psychoneuroendocrinology. 1998;23(5):465–75. 10.1016/S0306-4530(98)00022-5 [DOI] [PubMed] [Google Scholar]
- 29. Turnbull AC, Patten PT, Flint AP, et al. : Significant fall in progesterone and rise in oestradiol levels in human peripheral plasma before onset of labour. Lancet. 1974;1(7848):101–3. [DOI] [PubMed] [Google Scholar]
- 30. Aono T, Shioji T, Shoda T, et al. : The initiation of human lactation and prolactin response to suckling. J Clin Endocrinol Metab. 1977;44(6):1101–6. 10.1210/jcem-44-6-1101 [DOI] [PubMed] [Google Scholar]
- 31. Rasmussen KM, Kjolhede CL: Prepregnant overweight and obesity diminish the prolactin response to suckling in the first week postpartum. Pediatrics. 2004;113(5):e465–71. 10.1542/peds.113.5.e465 [DOI] [PubMed] [Google Scholar]
- 32. Cox DB, Owens RA, Hartmann PE: Blood and milk prolactin and the rate of milk synthesis in women. Exp Physiol. 1996;81(6):1007–20. 10.1113/expphysiol.1996.sp003985 [DOI] [PubMed] [Google Scholar]
- 33. Cadogan W: An Essay Upon Nursing, and the Management of Children: From Their Birth to Three Years of Age.London: J. Roberts;1748;34 Reference Source [Google Scholar]
- 34. Dettwyler KA: A time to wean: the hominid blueprint for the natural age of weaning in modern human populations.In: Stuart-Macadam P, Dettwyler K, editors. Breastfeeding: biocultural perspectives New York: Aldine de Gruyter;1995;39–73. Reference Source [Google Scholar]
- 35. Meier PP, Engstrom JL, Janes JE, et al. : Breast pump suction patterns that mimic the human infant during breastfeeding: greater milk output in less time spent pumping for breast pump-dependent mothers with premature infants. J Perinatol. 2012;32(2):103–10. 10.1038/jp.2011.64 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 36. Gross SJ, David RJ, Bauman L, et al. : Nutritional composition of milk produced by mothers delivering preterm. J Pediatr. 1980;96(4):641–4. 10.1016/S0022-3476(80)80729-3 [DOI] [PubMed] [Google Scholar]
- 37. Hazebroek A, Hofman A: Sodium content of breast milk in the first six months after delivery. Acta Paediatr Scand. 1983;72(3):459–60. 10.1111/j.1651-2227.1983.tb09747.x [DOI] [PubMed] [Google Scholar]
- 38. Koo WW, Gupta JM: Breast milk sodium. Arch Dis Child. 1982;57(7):500–2. 10.1136/adc.57.7.500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lemons JA, Moye L, Hall D, et al. : Differences in the composition of preterm and term human milk during early lactation. Pediatr Res. 1982;16(2):113–7. 10.1203/00006450-198202000-00007 [DOI] [PubMed] [Google Scholar]
- 40. Manganaro R, Marseglia L, Mamì C, et al. : Breast milk sodium concentration, sodium intake and weight loss in breast-feeding newborn infants. Br J Nutr. 2007;97(2):344–8. 10.1017/S0007114507280572 [DOI] [PubMed] [Google Scholar]
- 41. Mastroeni SS, Okada IA, Rondó PH, et al. : Concentrations of Fe, K, Na, Ca, P, Zn and Mg in maternal colostrum and mature milk. J Trop Pediatr. 2006;52(4):272–5. 10.1093/tropej/fmk004 [DOI] [PubMed] [Google Scholar]
- 42. Morriss FH, Jr, Brewer ED, Spedale SB, et al. : Relationship of human milk pH during course of lactation to concentrations of citrate and fatty acids. Pediatrics. 1986;78(3):458–64. [PubMed] [Google Scholar]
- 43. Neville MC, Allen JC, Archer PC, et al. : Studies in human lactation: milk volume and nutrient composition during weaning and lactogenesis. Am J Clin Nutr. 1991;54(1):81–92. 10.1093/ajcn/54.1.81 [DOI] [PubMed] [Google Scholar]
- 44. Bashir S, Rashid Z, Shaheen H: Correlation of Mother Milk Sodium Levels in Term Mothers with Neonatal Hyperbilirubinemia. Pakistan Journal of Medical & Health Sciences. 2015;9(2):611–3. Reference Source [Google Scholar]
- 45. Hibberd CM, Brooke OG, Carter ND, et al. : Variation in the composition of breast milk during the first 5 weeks of lactation: implications for the feeding of preterm infants. Arch Dis Child. 1982;57(9):658–62. 10.1136/adc.57.9.658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Anderson DM, Williams FH, Merkatz RB, et al. : Length of gestation and nutritional composition of human milk. Am J Clin Nutr. 1983;37(5):810–4. 10.1093/ajcn/37.5.810 [DOI] [PubMed] [Google Scholar]
- 47. Cregan MD, de Mello TR, Kershaw D, et al. : Initiation of lactation in women after preterm delivery. Acta Obstet Gynecol Scand. 2002;81(9):870–7. 10.1034/j.1600-0412.2002.810913.x [DOI] [PubMed] [Google Scholar]
- 48. Healy DL, Rattigan S, Hartmann PE, et al. : Prolactin in human milk: correlation with lactose, total protein, and alpha-lactalbumin levels. Am J Physiol. 1980;238(1):E83–6. 10.1152/ajpendo.1980.238.1.E83 [DOI] [PubMed] [Google Scholar]
- 49. Narang AP, Bains HS, Kansal S, et al. : Serial composition of human milk in preterm and term mothers. Indian J Clin Biochem. 2006;21(1):89–94. 10.1007/BF02913072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ronayne de Ferrer PA, Baroni A, Sambucetti ME, et al. : Lactoferrin levels in term and preterm milk. J Am Coll Nutr. 2000;19(3):370–3. 10.1080/07315724.2000.10718933 [DOI] [PubMed] [Google Scholar]
- 51. Morton J, Hall JY, Wong RJ, et al. : Combining hand techniques with electric pumping increases milk production in mothers of preterm infants. J Perinatol. 2009;29(11):757–64. 10.1038/jp.2009.87 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 52. Yotebieng M, Labbok M, Soeters HM, et al. : Ten Steps to Successful Breastfeeding programme to promote early initiation and exclusive breastfeeding in DR Congo: a cluster-randomised controlled trial. Lancet Glob Health. 2015;3(9):e546–55. 10.1016/S2214-109X(15)00012-1 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 53. UNICEF: The baby-friendly hospital initiative.UNICEF;1991. Reference Source [Google Scholar]
- 54. Kuhn NJ: Progesterone withdrawal as the lactogenic trigger in the rat. J Endocrinol. 1969;44(1):39–54. 10.1677/joe.0.0440039 [DOI] [PubMed] [Google Scholar]
- 55. Brown JB, Blackwell LF, Cox RI, et al. : Chemical and homogeneous enzyme immunoassay methods for the measurement of estrogens and pregnanediol and their glucuronides in urine. Prog Clin Biol Res. 1988;285:119–38. [PubMed] [Google Scholar]
- 56. Maningat PD, Sen P, Rijnkels M, et al. : Gene expression in the human mammary epithelium during lactation: the milk fat globule transcriptome. Physiol Genomics. 2009;37(1):12–22. 10.1152/physiolgenomics.90341.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 57. Hartmann PE, Kulski JK: Changes in the composition of the mammary secretion of women after abrupt termination of breast feeding. J Physiol (Lond). 1978;275:1–11. 10.1113/jphysiol.1978.sp012173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Nguyen DA, Neville MC: Tight junction regulation in the mammary gland. J Mammary Gland Biol Neoplasia. 1998;3(3):233–46. 10.1023/A:1018707309361 [DOI] [PubMed] [Google Scholar]
- 59. Kulski JK, Hartmann PE: Milk insulin, GH and TSH: relationship to changes in milk lactose, glucose and protein during lactogenesis in women. Endocrinol Exp. 1983;17(3–4):317–26. [PubMed] [Google Scholar]
- 60. Peaker M, Linzell JL: Citrate in milk: a harbinger of lactogenesis. Nature. 1975;253(5491):464. 10.1038/253464a0 [DOI] [PubMed] [Google Scholar]
- 61. Coppa GV, Gabrielli O, Pierani P, et al. : Changes in carbohydrate composition in human milk over 4 months of lactation. Pediatrics. 1993;91(3):637–41. [PubMed] [Google Scholar]
- 62. Kent JC, Arthur PG, Retallack RW, et al. : Calcium, phosphate and citrate in human milk at initiation of lactation. J Dairy Res. 1992;59(2):161–7. 10.1017/S0022029900030405 [DOI] [PubMed] [Google Scholar]
- 63. Neubauer SH, Ferris AM, Chase CG, et al. : Delayed lactogenesis in women with insulin-dependent diabetes mellitus. Am J Clin Nutr. 1993;58(1):54–60. 10.1093/ajcn/58.1.54 [DOI] [PubMed] [Google Scholar]
- 64. Viverge D, Grimmonprez L, Cassanas G, et al. : Variations in oligosaccharides and lactose in human milk during the first week of lactation. J Pediatr Gastroenterol Nutr. 1990;11(3):361–4. 10.1097/00005176-199010000-00013 [DOI] [PubMed] [Google Scholar]
- 65. Kent JC: The roles of citrate and casein in determining the calcium concentration of the milk of women and sows.Perth, Western Australia: University of Western Australia;2000. [Google Scholar]
- 66. Dangat K, Upadhyay D, Kilari A, et al. : Altered breast milk components in preeclampsia; An in-vitro proton NMR spectroscopy study. Clin Chim Acta. 2016;463:75–83. 10.1016/j.cca.2016.10.015 [DOI] [PubMed] [Google Scholar]
- 67. Family Larsson-Rosenquist Foundation: Breastfeeding and Breast Milk - from Biochemistry to Impact. A Multidisciplinary Introduction. Stuttgart: George Thieme Verlag KG;2018. [Google Scholar]
- 68. Daly SE, Di Rosso A, Owens RA, et al. : Degree of breast emptying explains changes in the fat content, but not fatty acid composition, of human milk. Exp Physiol. 1993;78(6):741–55. 10.1113/expphysiol.1993.sp003722 [DOI] [PubMed] [Google Scholar]
- 69. Fleet I, Linzell J: Rapid method of estimating fat in very small quantities of milk. Journal of Physiology, London. 1964;175(1):P15–&. [Google Scholar]
- 70. Ramsay DT, Kent JC, Owens RA, et al. : Ultrasound imaging of milk ejection in the breast of lactating women. Pediatrics. 2004;113(2):361–7. 10.1542/peds.113.2.361 [DOI] [PubMed] [Google Scholar]