Abstract
Objective
Preterm birth (PTB) is associated with excess maternal cardiovascular disease risk. We considered that women with PTB and placental evidence of maternal malperfusion would be particularly affected.
Design
Pregnancy cohort study.
Setting
Pittsburgh Pennsylvania, US
Population
Women with PTB (n=115) and term births (n=210) evaluated 4–12 years after pregnancy.
Methods
Cardiometabolic risk markers were compared in women with prior PTB vs. term births; preeclampsia and growth restriction cases were excluded. Placental evidence of maternal vascular malperfusion (vasculopathy, infarct, advanced villous maturation, perivillous fibrin, intervillous fibrin deposition), acute infection/inflammation (chorioamnionitis, funisitis, deciduitus), and villitis of unknown etiology (chronic inflammation) was used to classify PTBs.
Main outcome measure
Carotid artery intima-media thickness (IMT), fasting lipids, blood pressure (BP) and inflammatory markers measured after delivery.
Results
Women with PTB and malperfusion lesions had higher total cholesterol (+13.5 mg/dl) and systolic BP (+4.0 mmHg) at follow up compared to women with term births, accounting for age, race, pre-pregnancy BMI, and smoking (p<0.05). Women with PTB and malperfusion accompanied by inflammatory lesions had the most atherogenic profile after pregnancy (cholesterol +18.7, Apolipoprotein B +12.7 mg/dl; all p<0.05), adjusted for pre-pregnancy features. Carotid IMT was higher in this group (+0.037 cm, p=0.031) accounting for pre-pregnancy factors; differences were attenuated after adjusting for BP and atherogenic lipids at follow up (+0.027, p=0.095).
Conclusion
PTBs with placental malperfusion were associated with an excess maternal cardiometabolic risk burden in the decade after pregnancy. The placenta may offer insight into subtypes of PTB related to maternal cardiovascular disease.
Keywords: Atherosclerosis, cardiovascular risk factors, prematurity
Introduction
Cardiovascular disease (CVD) is the leading cause of death among women in the U.S. Stagnant rates of CVD mortality in younger women have occurred alongside notable declines in men and older adults,1 suggesting that early identification of risk in women is essential. The American Heart Association’s most current guidelines indicate that a history of preterm birth may identify women in whom cardiovascular risk factor screening and management is warranted,2 although mechanisms linking these conditions are not understood.3
PTB research has traditionally focused on clinical presentation to classify subtypes. For example, spontaneous preterm labor, preterm membrane rupture or medically induced preterm deliveries are thought to have distinct pathophysiologies. This approach has limitations, as the epidemiologic outcomes, metabolic changes, and abnormalities of implantation in indicated and spontaneous PTB overlap. We have reported that using placental features to classify PTB subtypes can predict newborn health,4 and now consider that placental phenotypes may also portend maternal cardiovascular health in the decade after delivery.
Placental malperfusion has well-established associations with preeclampsia and growth restriction but also with spontaneous PTB.5–7 Indeed, ‘defective deep placentation’ is associated with a subset of each of the ‘great obstetrical syndromes,’ including PTB.8 The main pathologic features of placental malperfusion are decidual vasculopathy, villous infarcts, abruption, and accelerated villous maturation.9 Vasculopathy is the underlying cause of many other placental changes including infarcts and abruption and the prominent features are incomplete of spiral artery conversion and early atherosclerosis-like findings such as atherosis. Another common feature is accelerated villous maturation, detected as villi substantially smaller than expected for the gestational age, increased aggregates of nuclei in the syncytiotrophoblast known as syncytial knots or aberrantly narrow, elongated villi termed distal villous hypoplasia. Syncytial knots are present in modest numbers in uncomplicated pregnancies, increasing steadily throughout gestation, but excess syncytial knots may reflect chronic hypoxia-reperfusion injury.10 We considered that placental malperfusion may reflect the accumulation of maternal factors, such as hyperlipidemia and inflammation, which impair the vascular adaptations required to support a full term, healthy pregnancy. As such, placental malperfusion may also provide a window into future maternal cardiometabolic risk.
We hypothesized that women with preterm births and placental malperfusion compared to women with term, uncomplicated births would have higher blood pressure, a more atherogenic biomarker profile and higher carotid intima media thickness, a marker of subclinical atherosclerosis that predicts cardiovascular events. We further considered that signs of placental malperfusion plus additional placental lesions involving infection or inflammation may mark women with particularly high cardiometabolic risk after delivery.
Materials and Methods
The Women and Infant Study of Healthy Hearts (WISH) is a cohort study of cardiovascular factors assessed 4 to 12 years after delivery of singleton small for gestation age (SGA), preterm infants or term births. The University of Pittsburgh IRB approved all study procedures. Eligibility and recruitment results have been reported previously.11 Briefly, eligible women were randomly selected from those who gave birth between 1997 and 2002 at Magee-Womens Hospital in Pittsburgh, PA who did not have preeclampsia, pre-pregnancy hypertension or pre-existing diabetes. By design, women with these conditions were never enrolled in this study. A total of 702 women provided informed consent and were enrolled. Those with term SGA infants were excluded as the pathways involved in SGA and PTB may be distinct and the post pregnancy consequences may also be different. Six women with PTB also had SGA infants, and replication of the analysis after removing these cases did not change the results so they are included. We excluded women who reported their race/ethnicity as other than white or African American, (n=12), those with gestational diabetes (n=11) and women with triglycerides >400 mg/dl (n=2) as the estimation of LDL-cholesterol (LDL-C) is not valid in these individuals.12 We further restricted PTB cases (<37 weeks) to those with clinical placental pathology records (115/181). PTBs without pathology records were more likely to be late PTBs (mean gestational age 35.4 [SD 1.0] vs. 33.1 [2.7], p<0.0001). In the current study the comparison group was comprised of 242 women with term, uncomplicated births and no conditions warranting referral of a placenta for pathology review (79% of women with term births). Among this group, those with any self-reported preterm births were also excluded (n=32). Although no placental features were available in this group, our institutional criteria for referral to pathology were broad during this period with 45.4% of births sent for evaluation.13 Thus, placentas not referred to pathology likely represent healthy pregnancies. There were no differences in cardiovascular risk factors measured after pregnancy among women with and without placental pathology, providing assurance that bias in the availability of placental data may be small. (Supplemental Table 2).
Gestational age at delivery was based on the date of the last menstrual period (LMP) and ultrasound when available. At our hospital during this time period, 60% of deliveries had early (18–22 weeks’) dating ultrasound. When the 2 dates (LMP and ultrasound) differed by less than 2 weeks, gestational age was based on LMP; when they differed by more than 2 weeks dating was based on ultrasound. Pre-pregnancy BMI (self-reported at the first prenatal visit) and smoking status were abstracted from the medical record. For 37 women with missing pre-pregnancy BMI (n=2 term births; n=35 preterm births) we used multiple imputation to estimate pre-pregnancy BMI based on weight at first prenatal visit, delivery weight, age, race, height, smoking, and cardiometabolic factors measured at the WISH follow up visit (BMI, systolic and diastolic blood pressure, total cholesterol, HDL-C and triglycerides).14–16 By design, women with preeclampsia and term SGA infants (birth weight <10th percentile) were excluded, and the medically indicated preterm births in our study were related to placenta previa, abruption and other fetal or maternal conditions. Cases of gestational hypertension (blood pressure above 140/90 mmHg without proteinuria, n=5) were included as results were unchanged after their exclusion.
Presence of placental lesions was abstracted from the clinical pathology reports of women with PTB and categorized according to proposed schemas as placental malperfusion (vasculopathy, infarct, accelerated villous maturation, perivillous fibrin deposition, intervillous fibrin deposition), intrauterine infection/inflammation (III, acute chorioamnionitis, acute funisitis, acute vasculitis, acute deciduitis), villitis of unknown etiology (a marker of chronic inflammation), fetal thrombosis, or chorangiosis.13, 17, 18 A detailed description of placental lesion definitions is provided in Supplemental Table 1.19–22 During this period, placental pathologists prepared all reports following a standardized protocol using a uniform reporting approach and identical diagnostic criteria. As these placentas were examined for clinical indications, a limited amount of clinical history was available to the pathologist. Data abstraction was overseen by two pathologists (WTP and LNN).
To investigate the utility of the clinical pathology reports, a small validation study was performed (n=56 spontaneous PTBs, 19 medically indicated PTBs, and 50 term births). The diagnosis of malperfusion and infection/inflammation on the clinical pathology reports was compared to a review of the slides by a single pathologist blinded to all clinical information except gestational age (WTP). Among PTBs there was excellent agreement (82% overall agreement, kappa=0.74) for infection/inflammation, and fair agreement (62% overall agreement, kappa=0.22) for malperfusion lesions. Of note, the clinical report tended to under-report cases of malperfusion in both spontaneous (34% vs. 54%, respectively) and indicated PTBs (47% vs. 79%). Overall agreement was excellent for the malperfusion lesions among term births (82%) but kappa was fair (0.26), likely due to the relatively rare occurrence of this feature where the clinical pathology records indicated 4% of term, uncomplicated births had evidence of malperfusion.
At a research visit occurring 4 to 12 years after delivery (WISH follow up visit), B-mode ultrasound images of the right and left distal common carotid artery, carotid bulb, and the first centimeter of the internal carotid artery were obtained in diastole. Using semi-automated edge-detection software (Artery Measurement System, Gothenburg, Sweden), the lumen-intima and media-adventitia interfaces were identified and measured across 1 cm segments of the near and far walls of the common carotid artery and the far wall of the bulb and internal carotid artery. Reproducibility of IMT measures was excellent with an intraclass correlation coefficient between sonographers of 0.87, and between readers of 0.92.
Fasting blood samples were collected at the same study visit. Total cholesterol, HDL-C, and triglycerides were measured using standard enzymatic procedures and the coefficient of variation (CV) ranged from 1.3% to 6.5%. LDL-C was evaluated using the Friedewald calculation.12 Apolipoprotein B (ApoB) was analyzed using a variation of the Boehringer Mannheim turbidimetric procedure and the CV was 9.8%. High sensitivity C-reactive protein (hsCRP) was measured using reagents obtained from Carolina Liquid Chemicals (Brea, CA) with CV of 5.5%. IL-6 (pg/ml) was measured using Quantikine HS ELISA kit from R&D Systems, Inc. (Minneapolis, MN). The intra- and inter-assay variabilities were 7.4% and 7.8%, respectively.
Blood pressure was evaluated following a research protocol using appropriately sized cuffs based on arm measurements and a manual sphygmomanometer. Body mass index (BMI, kg/m2) was calculated from measured height and weight. Waist circumference was assessed with a tape measure at the umbilicus. Women completed a structured interview to assess reproductive and medical history, demographics, and lifestyle characteristics. Women reported the outcomes of all pregnancies before and following the index birth including gestational age and birth weight. Women also reported the first day of the last menstrual period, and days from menses to the study visit for pre-menopausal women were calculated because some markers may change during the menstrual cycle.23, 24 Menopause was defined as having no menstrual periods during the previous 12 months; surgical removal of both ovaries; or age greater than 55 accompanied by use of estrogen, hormone therapy, or a hysterectomy. Physical activity was reported using the Paffenbarger Physical Activity Questionnaire 25 and analyzed as total hours of physical activity expenditure per week (MET hours/week).
Characteristics of women with PTB according to presence or absence of placental malperfusion lesions were compared to those of women with term births using chi-square tests for categorical variables and ANOVA for continuous variables. The Wilcoxon rank sum test was used instead of the t-test if the normality assumption did not hold, based on quantile-quantile plots, histograms, and the Shapiro-Wilk test for normality. Blood pressure and biomarkers were evaluated as continuous outcomes according to presence of placental malperfusion. We then evaluated women with malperfusion plus other lesions, malperfusion only and no malperfusion lesions, compared to women with term births. Group differences were compared using linear regression, adjusted for pre-pregnancy features (age, race, pre-pregnancy BMI, smoking in pregnancy) and the years between pregnancy and cardiovascular measurement. Differences in carotid IMT were first adjusted for these pre-pregnancy features, and then subsequently adjusted for cardiovascular risk factors (blood pressure, atherogenic lipids, current smoking) that may be intermediates linking pregnancy to subclinical atherosclerosis. Lipid components were, as expected, highly correlated and therefore we tested models adjusted for each (rather than all) and present results for apolipoprotein B as that is most atherogenic lipid component measured. Estimates that separately accounted for triglycerides and total cholesterol were similar. Results were replicated after removing post-menopausal women, those with gestational hypertension and those who delivered post-term (at or after 41 completed weeks’). All tests were 2-sided with statistical significance set at p-value<0.05. We did not adjust for multiple comparisons given the modest sample studied, and results should be interpreted with caution. Analyses were performed with SAS (version 9.4, SAS Institute, Inc, Cary NC) and R (version 3.3, R Foundation for Statistical Computing, Vienna, Austria).
Results
In 115 women with PTB, 48 (42%) had evidence of placental malperfusion and 67 (58%) did not. Women with malperfusion lesions tended to be modestly older compared to women with term births, and those with PTBs with or without malperfusion had lower household income and higher rates of smoking after pregnancy compared to women with term births (Supplemental Table 3). Inflammation/infection affected 25.2% of PTBs (n=30) and these were similarly distributed among cases with or without malperfusion. Villitis of unknown etiology was relatively rare, but all cases co-occurred with malperfusion lesions. The majority of PTBs were spontaneous regardless of evidence of malperfusion.
Women with PTB and accelerated villous maturation or infection/inflammation delivered the earliest and smallest infants (Table 1). Those with malperfusion lesions (accelerated villous maturation, infarcts, or perivillous fibrin), in general, had higher blood pressure, lipids and thicker carotid IMTs 4 to 12 years after delivery compared to women with term births. Women with PTB and villitis had particularly high triglycerides and thicker IMTs, and women with infection/inflammation or perivillous fibrin had higher IL-6 concentrations in the years after pregnancy. The more atherogenic profile of women with PTB and placental evidence of malperfusion (higher SBP and total cholesterol) persisted after adjustment for age, race, pre-pregnancy BMI, smoking and years from delivery to the cardiovascular screening visit (Table 2).
Table 1.
Pregnancy and maternal features at cardiovascular screening visit 4 to 12 years after delivery, according to placental lesions
| Pregnancy | Cardiovascular screening visit | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||||
| n | pre-BMI | Gestation† | Birthweight† | TC | LDL-C | Trig | HDL-C | Apo-B | CRP | IL-6 | SBP | IMT | |
|
|
|
||||||||||||
| Term | 210 | 24.4 | 39.4 | 3318 | 185.6 | 108.6 | 99.9 | 57.1 | 83.1 | 2.04 | 1.36 | 106.7 | 0.565 |
| Malperfusion | |||||||||||||
| Accelerated villous maturation | 37 | 22.8 | 32.5 | 1999 | 205.1* | 120.8* | 113.0* | 60.3 | 93.2* | 1.37 | 1.08 | 110.9* | 0.602* |
| Infarct | 14 | 24.4 | 35.1 | 2382 | 206.9* | 122.5* | 113.0* | 59.2 | 96.8 | 2.00 | 1.68 | 114.1 | 0.590 |
| Perivillous fibrin† | 10 | 26.1 | 33.1 | 2352 | 202.3 | 127.0 | 136.0* | 47.7 | 99.1 | 2.98 | 3.10* | 118.2 | 0.639* |
| Intrauterine infection/inflammation | 30 | 23.6 | 31.3 | 1760 | 197.7 | 118.1 | 93.5 | 55.9 | 92.3 | 1.86 | 1.77* | 110.0 | 0.593 |
| Villitis of unknown origin | 6 | 21.3 | 33.2 | 2037 | 199.0 | 113.0 | 157.0* | 54.7 | 88.0 | 2.45 | 1.09 | 113.4 | 0.693* |
| Fetal thrombosis | 16 | 23.4 | 34.1 | 2125 | 202.3 | 122.9 | 99.0 | 60.2 | 85.5 | 1.32 | 1.31 | 112.8 | 0.596 |
| Chorangiosis | 9 | 22.7 | 33.9 | 2249 | 214.2 | 134.6 | 126.0 | 52.7 | 106.1* | 1.70 | 0.90 | 108.0 | 0.617 |
all comparisons with term births significant (p <0.0001);
comparison with term births significant (p <0.05);
SBP, systolic blood pressure; TC, total cholesterol; Trig, triglycerides; Apo-B, apolipoprotein-B; CRP, high sensitivity C-reactive protein; IL-6, Interleukin-6; IMT, intima medial thickness; There were too few cases of vasculopathy (n=1), fibrin deposition (n=1) or no lesions (n=2) to evaluate
Table 2.
Mean differences in maternal cardiovascular markers among women with prior preterm birth with (+) and without (−) placental evidence of malperfusion, compared to women with term births, adjusted for pre-pregnancy features and interval from pregnancy to cardiovascular screening visit
| Preterm, + Malperfusion (n=48) | Preterm, - Malperfusion (n=67) | |||
|---|---|---|---|---|
| difference | p* | difference | p* | |
| Cholesterol (mg/dl) | 13.5 (6.1) | 0.035 | 8.0 (5.2) | 0.141 |
| LDL-C (mg/dl) | 9.1 (5.4) | 0101 | 9.4 (4.6) | 0.052 |
| Triglycerides (mg/dl) | 16.2 (9.2) | 0.092 | 10.7 (8.0) | 0.191 |
| HDL-C (mg/dl) | 1.3 (2.1) | 0.548 | −0.3 (1.8) | 0.112 |
| Apolipoprotein-B (mg/dl) | 8.0 (4.0) | 0.060 | 5.5 (3.5) | 0.129 |
| Systolic BP (mmHg) | 4.0 (1.7) | 0.028 | 1.3 (1.5) | 0.386 |
| Diastolic BP (mmHg) | 2.0 (1.3) | 0.146 | 0.9 (1.2) | 0.459 |
| IMT (mm) | 0.014 (.01) | 0.231 | .012 (.01) | 0.214 |
Groups are compared to women with term births, adjusted for race, age at delivery, pre-pregnancy BMI, smoking during pregnancy and years between pregnancy and cardiovascular screening visit
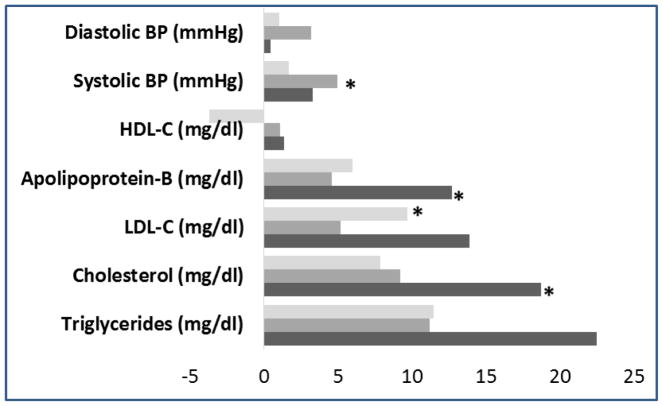
Atherogenic lipid fractions were higher in the group with malperfusion plus other lesions, compared to control women after accounting for pre-pregnancy factors (n=21; Figure 1). In particular, significant differences were noted for total cholesterol and apolipoprotien-B. In contrast, blood pressure was elevated in the group with malperfusion PTBs and no other lesions present and LDL-c was higher in the PTB group without malperfusion lesions.
Figure 1.
Mean differences in maternal lipids and blood pressure measured 4–12 years after delivery, according to prior preterm birth with placental malperfusion and other lesions (malperfusion and other lesions, black bars [n=21]; malperfusion only, medium gray bars [n=27]; no malperfusion, light gray bars [n=67]). Women with term births are the referent, and estimates are adjusted for age at delivery, race, pre-pregnancy BMI, smoking, and interval between delivery and cardiovascular screening visit. Differences noted with (*) are statistically significant (p<0.05) compared to women with term births. Other lesions include infection/inflammation (n=30), villitis (n=6), fetal thrombosis (n=20), chorangiosis (n=9). Women with PTBs and no lesions (n=2) excluded.
The group with malperfusion plus other lesions had thicker IMTs compared to women with term births (difference 0.060, p=0.002; Table 3), and this difference remained robust in models adjusted for pre-pregnancy covariates (difference +0.038, p=0.025). This was attenuated to no longer significant after accounting for traditional cardiovascular risk factors that likely emerged after delivery and may link pregnancy to later subclinical atherosclerosis (higher blood pressure and apolipoprotein B; difference +0.028, p=0.086). The malperfusion-only group and those without malperfsion lesions did not have higher IMTs. Results were unchanged after removing post-menopausal women (n=32), after removing women with gestational hypertension (n=5), and after removing women who delivered after 41 completed weeks (n=3). Although numbers were small, race specific analysis suggested that the carotid IMT differences in women with PTB and placental malperfusion plus other lesions were detectable in both white and black women (Supplementary Table 4).
Table 3.
Mean difference in maternal carotid intimal media thickness, according to prior preterm birth with placental evidence of malperfusion and other lesions
| Preterm < 37 weeks (n=21) | Preterm < 37 weeks (n=27) | Preterm < 37 weeks (n=67) | ||||
|---|---|---|---|---|---|---|
| + + |
Malperfusion Other lesions† |
+ − |
Malperfusion Other lesions† |
− + |
Malperfusion Other lesions† |
|
|
| ||||||
| difference (se) | p* | difference (se) | p* | difference (se) | p* | |
| Unadjusted | 0.060 (.02) | 0.002 | 0.013 (.02) | 0.442 | 0.020 (.01) | 0.081 |
| Model 1, adjusted for pre-pregnancy features and time (race, age, pre-pregnancy BMI, smoking, and years to study visit) | 0.037 (.02) | 0.031 | −0.003 (.02) | 0.839 | 0.015 (.01) | 0.126 |
| Model 2, additionally adjusted for intermediates (SBP, apolipoprotein B, smoking) | 0.027 (.02) | 0.095 | −0.009 (.01) | 0.541 | 0.009 (.01) | 0.376 |
Referent for all models is women with term births. Intermediates (systolic blood pressure [SBP], smoking, and apolipoprotein B [mg/dl]) measured at the cardiovascular screening visit 4–12 years after delivery
Other lesions include AIUI (n=30), villitis (n=6), fetal thrombosis (n=20), chorangiosis (n=9); Women with preterm birth and no lesions excluded (n=1)
Discussion
Main Findings
Our results suggest that classifying PTBs according to placental pathology may reveal subgroups of women with a higher cardiovascular risk factor burden in the decade after pregnancy. We have previously reported that women with a prior PTB have a more atherogenic profile after pregnancy compared to those with term births, but results were modest and comparisons imprecise.26 In contrast, characterizing PTBs according to placental findings identified groups with a particularly elevated atherogenic profile. The women in our sample with PTB and placental malperfusion plus other lesion types (18% of PTBs evaluated) had higher atherogenic lipids and thicker carotid IMTs after delivery that were independent of pre-pregnancy covariates.
Our results are consistent with the possibility that placental malperfusion may be the consequential, unifying feature that links adverse pregnancy outcomes such as PTB to long term maternal cardiovascular risk. Malperfusion alone, however, may not be sufficient to mark those at highest risk. Atherosclerotic vascular disease is a chronic inflammatory, fibroproliferative disease of large and medium-sized arteries fueled by lipids,27 and placental malperfusion combined with other pathologies may reflect a similar process. We and others have demonstrated that women who subsequently deliver preterm have higher atherogenic lipids and excess inflammation during pregnancy compared to those who deliver at term.28–32 We have recently reported that PTBs with co-occurrence of malperfusion and infection/inflammation were associated with smaller infant size, earlier delivery and excess neonatal intraventricular hemorrhage.4 Our current results suggest that placentas with evidence of multiple pathological processes may also mark women susceptible to a higher cardiometabolic burden detectable within the decade after pregnancy. Our results were robust after removing the 5 women with gestational hypertension and therefore women with malperfusion preterm births were normotensive during pregnancy and have higher blood pressure in the decade after delivery. These data support the possibility that measures of traditional cardiovascular risk factors during pregnancy may not fully capture risk that can be detected in the placenta.
Strength and Limitations
Consideration of study limitations is warranted. We could not observe overt CVD events among these relatively young, healthy women although higher carotid IMT is an early stage of arterial injury and atherosclerosis that is predictive of later CVD events.33–35 By design, women with preeclampsia were not enrolled in our study and future studies should include this group as they may likely be the most severely affected. We also had a larger portion of early preterm births, due to the requirement to have placental evaluations. Thus, our results are likely generalizable to Black and White women with early preterm births. We were unable to evaluate the maternal sequelae related to specific lesion types due to small numbers. Certain lesions, however, may be linked to a particularly adverse maternal profile (as was suggested in our data) and larger studies should examine this possibility. The placental pathology results utilized were derived from standard clinical reviews and these may have varying reproducibility. Indeed, while overall agreement between blinded research review and clinical reports for malperfusion lesions was moderate, agreement was fair according to the kappa statistic which accounts for the agreement expected by chance. Although there are limitations regarding the kappa statistic,36 current efforts to standardize placenta pathology criteria designed to improve inter-rater reliability will help to improve this in the future.37 In addition, the most severe lesions, such as vasculopthay, villitis and acute chorioamnionitis are highly reproducible.38–40 We were unable to explore how pregnancy blood pressure features, even within the normotensive range, might impact occurrence of malperfusion lesions and later life sequelae. In addition, we were unable to examine the pathology that may be present in uncomplicated, term births and thus our results may be an underestimate of the true associations. Prior evidence suggests that malperfusion affects as few as 4% of term uncomplicated births,5 and our validation study reported this same percentage of affected term placentas. We also were unable to evaluate the profile of women before pregnancy, and in the years between delivery and our one time exam. We were unable to examine preterm birth recurrence, as numbers were small and placental features for other births were not available. Strengths of our study include a robust set of cardiometabolic endpoints, the use of placental pathology to classify PTBs, and the large number of Black women (26%) as PTB disproportionately affects Black women in the U.S.
Interpretation
There are very few studies of PTB subtypes and maternal cardiometabolic risk factors after delivery and none, to our knowledge, have related placental findings to later maternal health. The Avon Longitudinal Study of Parents and Children reported that women with PTB have higher blood pressure 18 years after delivery that was largely explained by hypertension during pregnancy.41 Perng, et al have reported similar results in a U.S. cohort.42 We have previously demonstrated that women with PTBs have higher blood pressure across 20 years of follow up due to hypertension in pregnancy in the years proximal to pregnancy but after 10 years of follow up, blood pressure increased more rapidly in women with a prior normotensive preterm compared to term birth.43 In our previous report of the current cohort, women with early (<34 weeks) or medically indicated PTB had a more atherogenic lipid profile measured in the decade after delivery.26 Our current results raise the possibility that classifying PTB by placental characteristics may help identify underlying etiologies that cross clinical presentation subtypes.
Adequate placentation requires invasion of the fetal trophoblast into the maternal decidua, a process that is modulated by maternal factors.44 Malperfusion, therefore, may reflect both maternal and fetal abnormalities, and future molecular work that can distinguish each component may provide further mechanistic insight.
Conclusion
Our findings suggest that women with PTBs with placental malperfusion, absent of preeclampsia or growth restriction, have higher blood pressure, higher atherogenic lipids and more subclinical atherosclerosis compared to women with term, uncomplicated pregnancies. Those with comorbid placental pathologies, in particular those with malperfusion and acute or chronic inflammation, had the most atherogenic profile in the decade after pregnancy. The placenta may offer unique insight into how PTB can portend maternal cardiovascular disease.
Supplementary Material
Acknowledgments
Funding: The work was supported by grant number RFA-67-16, Maternal Lipids and Placental Function, provided by the Commonwealth of Pennsylvania Department of Health, and the National Institutes of Health grant number R01HL076532, Fetal growth restriction and maternal cardiovascular risk.
Footnotes
Contribution to Authorship:
JMC initiated study, conducted all analysis, and wrote the paper. SER and MFM provided scientific input and contributed to the writing. RBN oversaw the original study and contributed to the manuscript writing. LNN conducted the placental pathology record abstraction. JMY and HH contributed to the statistical methods and analysis. WTP reviewed the pathology records, guided the analysis of the pathology results and contributed to the manuscript writing. All authors approved the final version of the submitted manuscript.
Details of Ethics Approval:
The study was approved by the University of Pittsburgh Institutional Review Board on December 23, 2014 (# PRO14120130).
Disclosure of Interests: The authors report no conflict of interest. The ICMJE disclosure forms are available as online supporting information.
References
- 1.Wilmot KA, O’Flaherty M, Capewell S, Ford ES, Vaccarino V. Coronary Heart Disease Mortality Declines in the United States From 1979 Through 2011: Evidence for Stagnation in Young Adults, Especially Women. Circulation. 2015 Sep 15;132(11):997–1002. doi: 10.1161/CIRCULATIONAHA.115.015293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mosca L, Benjamin EJ, Berra K, Bezanson JL, Dolor RJ, Lloyd-Jones DM, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women - 2011 update: a guideline from the American Heart Association. J Am Coll Cardiology. 2011 Mar 22;57(12):1404–23. doi: 10.1016/j.jacc.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robbins CL, Hutchings Y, Dietz PM, Kuklina EV, Callaghan WM. History of preterm birth and subsequent cardiovascular disease: a systematic review. Am J Obstet Gynecol. 2014;210(4):285–97. doi: 10.1016/j.ajog.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Catov JM, Scifres CM, Caritis SN, Bertolet M, Larkin J, Parks WT. Neonatal outcomes following preterm birth classified according to placental features. Am J Obstet Gynecol. 2017 Jan 05; doi: 10.1016/j.ajog.2016.12.022. [DOI] [PubMed] [Google Scholar]
- 5.Germain AM, Carvajal J, Sanchez M, Valenzuela GJ, Tsunekawa H, Chuaqui B. Preterm labor: placental pathology and clinical correlation. Obstet Gynecol. 1999 Aug;94(2):284–9. doi: 10.1016/s0029-7844(99)00324-5. [DOI] [PubMed] [Google Scholar]
- 6.Kelly R, Holzman C, Senagore P, Wang J, Tian Y, Rahbar MH, et al. Placental vascular pathology findings and pathways to preterm delivery. Am J Epidemiol. 2009 Jul 15;170(2):148–58. doi: 10.1093/aje/kwp131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim YM, Bujold E, Chaiworapongsa T, Gomez R, Yoon BH, Thaler HT, et al. Failure of physiologic transformation of the spiral arteries in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2003 Oct;189(4):1063–9. doi: 10.1067/s0002-9378(03)00838-x. [DOI] [PubMed] [Google Scholar]
- 8.Brosens I, Pijnenborg R, Vercruysse L, Romero R. The “Great Obstetrical Syndromes” are associated with disorders of deep placentation. Am J Obstet Gynecol. 2011;204(3):193–201. doi: 10.1016/j.ajog.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baergen R. Manual of Benirschke and Kaufmann’s Pathology of the Human Placenta. New York, NY: Springer; 2005. [Google Scholar]
- 10.Heazell AEP, Moll SJ, Jones CJP, Baker PN, Crocker IP. Formation of Syncytial Knots is Increased by Hyperoxia, Hypoxia and Reactive Oxygen Species. Placenta. 2007;28(Supplement 0):S33–S40. doi: 10.1016/j.placenta.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Catov JM, Dodge R, Yamal JM, Roberts JM, Piller LB, Ness RB. Prior preterm or small-for-gestational-age birth related to maternal metabolic syndrome. Obstet Gynecol. 2011 Feb;117(2 Pt 1):225–32. doi: 10.1097/AOG.0b013e3182075626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedewald W, Levy R, Fredrickson D. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clinical Chemistry. 1972;18:499–502. [PubMed] [Google Scholar]
- 13.Catov JM, Peng Y, Scifres CM, Parks WT. Placental pathology measures: Can they be rapidly and reliably integrated into large-scale perinatal studies? Placenta. 2015 Jun;36(6):687–92. doi: 10.1016/j.placenta.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Rubin D. Inference and missing data (with discussion) Biometrika. 1976;63:581–92. [Google Scholar]
- 15.Yuan Y. Multiple Imputation for Missing Data: concepts and New Development. SAS Institute, Inc; [Google Scholar]
- 16.Sterne JAC, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009 doi: 10.1136/bmj.b2393. 2009-06-29 00:00:00;338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roescher AM, Timmer A, Erwich JJHM, Bos AF. Placental Pathology, Perinatal Death, Neonatal Outcome, and Neurological Development: A Systematic Review. Plos One. 2014 Feb 25;9(2) doi: 10.1371/journal.pone.0089419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao YJ, Zhang HJ, Li CX, Wu T, Shen XM, Zhang J. Selecting placental measures that have clinical implications in child development and diseases. Placenta. 2014;35(3):178–87. doi: 10.1016/j.placenta.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 19.Katzman PJ. Chronic inflammatory lesions of the placenta. Semin Perinatol. 2015 Feb;39(1):20–6. doi: 10.1053/j.semperi.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Redline R, Faye-Petersen O, Heller D, Qureshi F, Savell V, Vogler C. Amniotic Infection Syndrome: Nosology and Reproducibility of Placental Reaction Patterns. Pediatric an Developmental Pathology. 2003;6:435–48. doi: 10.1007/s10024-003-7070-y. [DOI] [PubMed] [Google Scholar]
- 21.Redline RW, Ariel I, Baergen RN, Desa DJ, Kraus FT, Roberts DJ, et al. Fetal vascular obstructive lesions: nosology and reproducibility of placental reaction patterns. Pediatric and developmental pathology: the official journal of the Society for Pediatric Pathology and the Paediatric Pathology Society. 2004 Sep-Oct;7(5):443–52. doi: 10.1007/s10024-004-2020-x. [DOI] [PubMed] [Google Scholar]
- 22.Redline RW, Boyd T, Campbell V, Hyde S, Kaplan C, Khong TY, et al. Maternal vascular underperfusion: nosology and reproducibility of placental reaction patterns. Pediatric and developmental pathology: the official journal of the Society for Pediatric Pathology and the Paediatric Pathology Society. 2004 May-Jun;7(3):237–49. doi: 10.1007/s10024-003-8083-2. [DOI] [PubMed] [Google Scholar]
- 23.Bingley CA, Gitau R, Lovegrove JA. Impact of Menstrual Cycle Phase on Insulin Sensitivity Measures and Fasting Lipids. Horm Metab Res. 2008;40(12):901–6. doi: 10.1055/s-0028-1082081. [DOI] [PubMed] [Google Scholar]
- 24.Valdes CT, Elkind-Hirsch KE. Intravenous glucose tolerance test-derived insulin sensitivity changes during the menstrual cycle. J Clin Endocrinol Metab. 1991;72:642–6. doi: 10.1210/jcem-72-3-642. [DOI] [PubMed] [Google Scholar]
- 25.Sesso HD, Paffenbarger RS, Ha T, Lee IM. Physical Activity and Cardiovascular Disease Risk in Middle-aged and Older Women. Am J Epidemiol. 1999 Aug 15;150(4):408–16. doi: 10.1093/oxfordjournals.aje.a010020. [DOI] [PubMed] [Google Scholar]
- 26.Catov JM, Dodge R, Barinas-Mitchell E, Sutton-Tyrrell K, Yamal JM, Piller LB, et al. Prior preterm birth and maternal subclinical cardiovascular disease 4 to 12 years after pregnancy. J Womens Health (Larchmt) 2013 Oct;22(10):835–43. doi: 10.1089/jwh.2013.4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Falk E. Pathogenesis of Atherosclerosis. J Am College Cardiology. 2006;47(8, Supplement 1):C7–C12. doi: 10.1016/j.jacc.2005.09.068. [DOI] [PubMed] [Google Scholar]
- 28.Catov JM, Bodnar LM, Kip KE, Hubel C, Ness RB, Harger G, et al. Early pregnancy lipid concentrations and spontaneous preterm birth. Am J Obstet Gynecol. 2007 Dec;197(6):610e1–7. doi: 10.1016/j.ajog.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 29.Catov JM, Bodnar LM, Ness RB, Barron SJ, Roberts JM. Inflammation and dyslipidemia related to risk of spontaneous preterm birth. Am J Epidemiol. 2007 Dec 1;166(11):1312–9. doi: 10.1093/aje/kwm273. [DOI] [PubMed] [Google Scholar]
- 30.Mudd LM, Holzman CB, Catov JM, Senagore PK, Evans RW. Maternal lipids at mid-pregnancy and the risk of preterm delivery. Acta Obstet Gynecol Scand. 2012 Jun;91(6):726–35. doi: 10.1111/j.1600-0412.2012.01391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pitiphat W, Gillman MW, Joshipura KJ, Williams PL, Douglass CW, Rich-Edwards JW. Plasma C-Reactive Protein in Early Pregnancy and Preterm Delivery. Am J Epidemiol. 2005 Dec 1;162(11):1108–13. doi: 10.1093/aje/kwi323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Romero R, Espinoza J, Gonçalves L, Kusanovic J, Friel L, Hassan S. The Role of Inflammation and Infection in Preterm Birth. Semin Reprod Med. 2007;25(01):21–39. doi: 10.1055/s-2006-956773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li S, Chen W, Srinivasan SR, Bond MG, Tang R, Urbina EM, et al. Childhood Cardiovascular Risk Factors and Carotid Vascular Changes in Adulthood: The Bogalusa Heart Study. JAMA. 2003 Nov 5;290(17):2271–6. doi: 10.1001/jama.290.17.2271. [DOI] [PubMed] [Google Scholar]
- 34.Lorenz MW, von Kegler S, Steinmetz H, Markus HS, Sitzer M. Carotid intima-media thickening indicates a higher vascular risk across a wide age range: prospective data from the Carotid Atherosclerosis Progression Study (CAPS) Stroke. 2006 Jan;37(1):87–92. doi: 10.1161/01.STR.0000196964.24024.ea. [DOI] [PubMed] [Google Scholar]
- 35.Urbina EM, Srinivasan SR, Tang R, Bond MG, Kieltyka L, Berenson GS. Impact of multiple coronary risk factors on the intima-media thickness of different segments of carotid artery in healthy young adults (The Bogalusa Heart Study) Am J Cardiol. 2002;90(9):953–8. doi: 10.1016/s0002-9149(02)02660-7. [DOI] [PubMed] [Google Scholar]
- 36.McHugh ML. Interrater reliability: the kappa statistic. Biochem Med (Zagreb) 2012;22(3):276–82. [PMC free article] [PubMed] [Google Scholar]
- 37.Khong TY, Mooney EE, Ariel I, Balmus NC, Boyd TK, Brundler MA, et al. Sampling and Definitions of Placental Lesions: Amsterdam Placental Workshop Group Consensus Statement. Arch Pathol Lab Med. 2016 May 25; doi: 10.5858/arpa.2015-0225-CC. [DOI] [PubMed] [Google Scholar]
- 38.Beebe LA, Cowan LD, Hyde SR, Altshuler G. Methods to improve the reliability of histopathological diagnoses in the placenta. Paediatr Perinat Epidemiol. 2000 Apr;14(2):172–8. doi: 10.1046/j.1365-3016.2000.00253.x. [DOI] [PubMed] [Google Scholar]
- 39.Kramer MS, Chen MF, Roy I, Dassa C, Lamoureux J, Kahn SR, et al. Intra- and interobserver agreement and statistical clustering of placental histopathologic features relevant to preterm birth. Am J Obstet Gynecol. 2006 Dec;195(6):1674–9. doi: 10.1016/j.ajog.2006.03.095. [DOI] [PubMed] [Google Scholar]
- 40.Simmonds M, Jeffery H, Watson G, Russell P. Intraobserver and interobserver variability for the histologic diagnosis of chorioamnionitis. Am J Obstet Gynecol. 2004 Jan;190(1):152–5. doi: 10.1016/s0002-9378(03)00870-6. [DOI] [PubMed] [Google Scholar]
- 41.Fraser A, Nelson SM, Macdonald-Wallis C, Cherry L, Butler E, Sattar N, et al. Associations of Pregnancy Complications With Calculated Cardiovascular Disease Risk and Cardiovascular Risk Factors in Middle Age The Avon Longitudinal Study of Parents and Children. Circulation. 2012 Mar 20;125(11):1367–80. doi: 10.1161/CIRCULATIONAHA.111.044784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perng W, Stuart J, Rifas-Shiman SL, Rich-Edwards JW, Stuebe A, Oken E. Preterm birth and long-term maternal cardiovascular health. Ann Epidemiol. 2014 Oct 18;25(1):40–5. doi: 10.1016/j.annepidem.2014.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Catov JM, Lewis CE, Lee M, Wellons MF, Gunderson EP. Preterm birth and future maternal blood pressure, inflammation, and intimal-medial thickness: the CARDIA study. Hypertension. 2013 Mar;61(3):641–6. doi: 10.1161/HYPERTENSIONAHA.111.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Staff AC, Dechend R, Redman CWG. Review: Preeclampsia, acute atherosis of the spiral arteries and future cardiovascular disease: Two new hypotheses. Placenta. 2013;34:S73–S8. doi: 10.1016/j.placenta.2012.11.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.