Abstract
The Peer Approaches to Lupus Self-Management program sought to address the disparate impact of systemic lupus erythematosus (SLE) on African American women through a peer mentoring intervention with aims of reducing stress, anxiety, and depression. Given the association between psychological health and immune function this study examines the relationship between patient reported outcomes (PROs) in these domains and immunologic indicators of disease activity. Twenty-three African American women with SLE served as mentees in the intervention from whom PRO measures were collected at the outset, midpoint, and end of the 12 week pilot study. Blood samples were collected pre- and post-intervention. Plasma was collected from the samples and cryopreserved for subsequent analyses. The strongest correlations were between the Generalized Anxiety Disorder measure and Th1/Th2 cytokine balance. Weaker correlations existed between depression and the Th1/Th2 cytokine balance. Assessment of fresh versus cryopreserved samples revealed that changes in Th1/Th2 cytokine balance within the intervention were generally equivalent, regardless of sample type. The PALS intervention resulted in significant improvements to anxiety and depression levels which were significantly associated with positive changes in Th1/Th2 cytokine balance indicating a possible underlying mechanism of action. The nature of this relationship warrants further study.
Keywords: Cytokines, Behavioral intervention, African American, Women, Systemic Lupus Erythematosus
1. Introduction
Systemic Lupus Erythematosus (SLE or lupus) is a chronic autoimmune disease with acute periodic flare-ups of symptoms impacting any organ system and resulting in potentially life-threatening complications [1,2]. Other significant complications of treatment include weight gain, osteoporosis, osteonecrosis, accelerated atherosclerosis, and retinal damage [1–3]. Symptoms and side effects and complications can lead to significant functional and emotional challenges [4–7]. Patients often experience a high degree of psychological symptoms, including anxiety, depression, mood disorders, and decreased health-related quality of life [8–20].
Lupus disproportionately affects women and nonwhites [21,22]. Racial and ethnic minorities, the poor, and those lacking medical insurance and education are at highest risk for the prevalence, morbidity, and mortality associated with lupus [21,23–25]. Thus, the Peer Approaches to Lupus Self-Management (PALS) program was developed for African American women, as a culturally tailored peer mentoring intervention to help address the disparate impact SLE can have on this vulnerable population. In studies of predominantly low income and minority populations, peer mentors have been shown to help support healthy behaviors along with improved medication adherence and blood glucose monitoring in people with diabetes [26–39]. Additionally, there is some evidence that peer mentoring has also led to improvements in positive affect, sleep, social coping, and perception of bodily pain in rheumatic conditions [40,41]. Thus SLE is an optimal condition within which to test the effectiveness of the peer mentoring approach due to its high burden in African American women. To determine the effectiveness of the PALS intervention on the mentees, validated patient reported outcomes (PROs) were assessed.
Humoral autoimmunity in SLE is typically characterized as a Th2 dominant disease, likely in part due to reduced function or activity of regulatory T cell components [42]. Previous studies have assessed the relationships between stress, social support, and anxiety with immune function [43]. These studies have shown that high levels of psychological stress (including anxiety, worry and depression) are associated with altered regulatory T cell numbers and an altered Th1/Th2 balance (decreased IFNg and/or increased IL4) [44–46]. There is also evidence in scientific literature that links chronic stress and SLE disease activity [43]. The consensus of these studies has determined that long term stress, low levels of social support, and high levels of anxiety can lead to immune dysregulation and reduced function [47,48]. While the action of social support is not fully understood, it has been shown that stress and anxiety can alter the expression of genes vital in immune response [47,48]. Several Given the connection between stress and immune function, the suspected mechanism of action for this particular behavioral intervention is that it reduces stress and other associated psychosocial factors and in turn, positively impacts immune function. For this reason, there may be a relationship between the PROs associated with anxiety, worry, and depression and immunologic evidence of disease risk/activity. To ensure measurement of several relevant outcomes, PRO’s assessed in the present study included disease self-management, measured with the Patient Activation Measure (PAM); health related quality of life (HRQOL), measured by the Lupus Quality of Life (LUP-QOL) questionnaire; and self-reported disease activity, measured with the Systemic Lupus Activity Questionnaire (SLAQ); along with standardized measures of stress (PSS), anxiety (GAD-7), and depression (PHQ-9). Determining an association of a particular PRO with biological processes might suggest that particular outcome is worth studying and using as an endpoint to refine the intervention and monitor its effect in patients.
The hypothesis is that such interventions will change behavior, which will lead to changes in health. However, there is a gap in the literature regarding whether there is an actual biologic mechanism (i.e., physiological changes to the immune expression of Th1/Th2 cytokine balance) that might lead to change in disease condition as well. Therefore, the primary aim of this study was to determine the effect of the PALS peer mentoring intervention on T cell immune function the relationship between PROs and immune profiles associated with disease activity as determined by blood Th1/Th2 cytokine imbalance to suggest such a mechanism. The second aim of this study was to validate our collection, storage, and analysis protocols in order to inform other trials as to the value and limitations of using cryopreserved mononuclear cell samples to determine these profiles.
2. Materials and methods
2.1. Intervention
The Peer Approaches to Lupus Self-Management (PALS) study was a single arm, pre-post pilot in which African American women with lupus were recruited from the Medical University of South Carolina (MUSC) P60 Multidisciplinary Clinical Research Center (MCRC) longitudinal cohort who consented to contact about research and through physician referral. The peer mentoring intervention (patients were matched with peer mentors who were considered competent in the management of their condition to provide modeling and reinforcement to participants) occurred by telephone for approximately 60 min every week for 12 weeks. Weekly content was adapted from the six modules of the Chronic Disease Self-Management Program (CDMP), Arthritis Self-Management Program (ASMP), and Systemic Lupus Erythematosus Self-Help (SLESH) Course [49–53], and further tailored to African American women with six added sessions based on cultural issues reported as important to African Americans in earlier research conducted by the principle investigator [54–56].
2.2. Participants
All participants were selected from a longitudinal observational web-based SLE database at the Medical University of South Carolina (MUSC), and these patients are seen on a regular basis in the MUSC lupus clinics. All participants meet at least four components of the 1997 American College of Rheumatology (ACR) revised criteria for SLE and have disease activity information available, as well as quality of life measures obtained in the database questionnaire [57]. This study was approved by the Medical University of South Carolina’s IRB and written informed consent was obtained from all participants prior to study enrollment. As part of the informed consent process, participants agree to future re-contact regarding other research studies.
2.3. Data collection
Since this was a pilot investigation to obtain preliminary results that could inform development of a larger study, our goal was to assess changes in SLE patients; not differences between patients and controls. Therefore, we adopted a pre/post study design within which participants served as their own control. Primary outcomes of the study included PROs as well as Th1/Th2 balance in peripheral blood mononuclear cells. Study questionnaires were carefully chosen based on available evidence and in order to measure key elements of the study aims. The primary method of data collection was face to face interview. Mentees were assessed during study visits at baseline (0 weeks), mid-intervention (6 weeks post-enrollment), and immediately following the intervention (12 weeks post-enrollment) for PROs. Blood collection was achieved by in-person lab visits at the baseline and post intervention time points. The MUSC REDCap system was used for data management. REDCap (Research Electronic Data Capture) is a secure, web-based application designed exclusively to support data capture for research studies [58]. Quality of life was measured using the Lupus Quality of Life measure (LUP-QOL) which incorporates the Medical Outcomes Study (MOS) Short Form 36 Health Survey (SF-36) and the Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F). The questionnaire includes questions pertaining to physical function, role function, social function, mental health, health perception and pain [59,60].
Patient reported depression was assessed using the Patient Health Questionnaire (PHQ)-9 which is a brief questionnaire that scores each of the 9 DSM-IV criteria for depression as “0” (not at all) to “3” (nearly every day). PHQ-9 score >or =10 have a sensitivity of 88% and a specificity of 88% for major depression. PHQ-9 scores of 5, 10, 15, and 20 represent mild, moderate, moderately severe, and severe depression, respectively [61,62].
Anxiety and General Anxiety Disorder (GAD) was assessed using the 7-item anxiety scale (GAD-7). This is a valid and efficient tool for screening for GAD and assessing its severity in clinical practice and research. Response options are “not at all”, “several days”, “more than half the days”, and “nearly every day”, scored as 0, 1, 2, and 3, respectively [63].
The perceived stress scale (PSS) was used to determine patient reported stress levels. It is a 4-item scale that assesses the degree to which the respondent finds situations stressful [64,65]. Responses range from “0” (never) to “4” (very often) and questions ask about the frequency of feelings related to events in the previous month. The Cronbach alpha value is 0.69 and scores are highly correlated with stress, depression and anxiety.
Self-management was measured using the Patient Activation Measure (PAM) [66,67]. The PAM assesses an individual’s knowledge, skill, and confidence for managing their health and healthcare. The PAM survey measures patients on a 0–100 scale and can segment patients into one of four activation levels along an empirically derived continuum, including “Believes Active Role Important”, “Confidence and Knowledge to Take Action”, “Taking Action”, and “Staying the Course under Stress”. Each activation level reveals insight into an array of health-related characteristics, including attitudes, motivators, behaviors, and outcomes. We attempted to obtain records of patient compliance from electronic medical records (EMRs) or the clinical data warehouse (CDW), for further validation of treatment engagement/adherence, if such data was available.
Lastly for patient reported outcomes, disease activity was assessed. The Systemic Lupus Activity Measure (SLAM) is a physician-rated, 31-item instrument that assesses symptoms and objective findings in the month prior to evaluation, in nine organ systems and seven laboratory items [109–111] [68–70]. Disease activity was assessed using the Systemic Lupus Activity Questionnaire (SLAQ) [70], which is based on items from the SLAM that could be self-reported. It asks a single Patient Global Assessment (PGA) question about presence and severity of lupus activity over the past month, questions on 24 specific symptoms of disease activity and a single Numerical Rating Scale (NRS) asking the patient to rate disease activity on a scale of 0–10 over the past three months with the 0 anchored by ‘no activity’ and 10 anchored by ‘most activity’. Likert responses with four response categories (no problem, mild, moderate, severe) are used for the PGA and specific symptom questions.
Given the significance of stress effects on immune function, analysis of blood immune-phenotype and T cell activation for intracellular cytokine detection was achieved by flow cytometry, within the singlet cell gate, after labeling with commercial monoclonal antibodies using methodology previously described [71]. Immune profiles associated with disease activity were determined by Th1/Th2 cytokine balance in peripheral blood mononuclear cells (PBMC). Blood samples were collected according to procedural guidelines of the MCRC (P60AR062755) at MUSC, with special instructions to the phlebotomist for venous blood to be collected from subjects within the same two-hour span of the day to control for diurnal variation and to allow the blood to completely fill each tube (the tubes were made with a vacuum that displaced exactly 10 ml of blood when the blood flow stops). The tubes were then gently inverted 10 times immediately after collection to prevent clotting. The tubes were stored and shipped at room temperature. If refrigerated, the cells would not be viable for the procedures. Two tubes per participant were collected at each time point; inverted adequately to prevent clotting; labeled and packaged for room temperature shipping via FedEx or UPS; and shipped to the University of Mississippi the day of collection. Once samples were received the following morning, peripheral blood mononuclear cells were isolated, the amount of PBMC needed for the fresh flow analysis used and the remaining PBMC were cryopreserved at −80 °C as previously described [72]. After enrollment completion, and freezing of all samples, cryopreserved samples were thawed for batch flow analysis. Cryopreserved samples have been reported to have the same relative T cell profile as fresh [73].
2.4. Analysis of cytokine expression in peripheral blood mononuclear cells (PBMC)
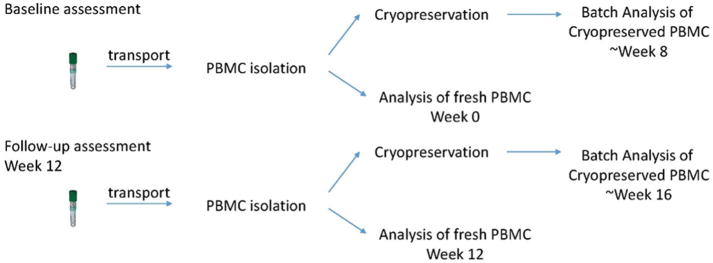
For analysis of blood Th1/Th2 cytokine balance, mentored lupus patients had blood drawn within the same two-hour time span before and after mentoring. Fresh samples were analyzed within 24 h of collection. Cryopreserved pre and post intervention samples were batched until all were received, at each time point (see Fig. 1). Therefore, the maximum length of time that pre-intervention samples were cryopreserved was 83 days (range = 56–83 days) and the maximum length of time that post-intervention samples were cryopreserved was 34 days (range = 18–34 days). The basic immunophenotyping panel for analysis of circulating cell populations included the following markers: CD3 (mature T cells), CD4 (T helper/inducer), CD8 (T suppressor/cytotoxic), CD25 (IL-2 receptor), FoxP3 (T regulator), IL10 (T reg suppressor), IFNγ (T helper 1), IL4 (T helper 2), and TGFβ1 (Treg suppressor). For the separate detection of intracellular cytokines, isolated PBMC were stimulated with phorbol myristate acetate (PMA), Ionomycin and monensin for 5 h at 37 °C and 5% CO2. Paraformaldehyde-fixed cells were stained for cytokine markers (interferon γ, IFNγ, and IL4) and surface markers (CD3 and CD8). Cells were analyzed on a Beckman Coulter Cytomics FC 500 flow cytometer, with gating and analysis performed as described [44,74]. Isotype-matched control antibodies defined negative staining. Th1 cells were defined as CD3+, CD8−, IFNγ+, IL4−. Th2 cells were defined as CD3+, CD8−, IFNγ−, IL4+. Treg cells were defined as CD4+, CD25high, FoxP3+ [73]. The ratio of Th1 to Th2 was the primary endpoint of this analysis. Finally, the bivariate relationship between fresh and cryopreserved values and patient reported disease activity and quality of life outcomes were determined to assess how well these particular markers of immunological balance predicted patient reported outcomes. Since this was a pilot investigation to obtain preliminary results that could inform development of a larger study, examining both fresh and cryopreserved samples was in line with study goals to explore both assay variability and association with outcomes to inform future research.
Fig. 1.
Timeline of sample collection and analysis.
2.5. Statistical analysis
Descriptive statistics are presented as frequencies (percentages) for patient demographics and as means (standard deviations) for cytokine measures. To assess cytokine differences between fresh and cryopreserved and between time points, repeated measures mixed-effects regression model analysis, in which the cytokine measure was the dependent variable, time and group (fresh/cryopreserved) and their interaction were the independent variables, was performed. We controlled for recovery (cells recovered from cryopreserved aliquot) and viability (live, intact, thawed cells) by including them as covariates. Post-hoc comparisons were made based on the least square means using t-tests. Similarly, repeated measures analyses were performed to assess the relationships between cytokines Th1/Th2 and Treg with patient reported disease activity and quality of life outcomes. A backward stepwise approach was used to identify a parsimonious model. A model was considered final when either both cytokines were removed or all remaining variables were found statistically significant. Statistical significance was assessed at α = 0.05. (Given that this is a pilot study, no correction for multiple comparisons was considered.) All analyses were performed using SAS v9.4.
3. Results
Our study had a total of 23 subjects, the majority were 35 or older (N = 19; 82.6%), married (N = 21; 91.3%); and college educated (N = 14; 63.6%) (See Table 1).
Table 1.
Patient demographics are presented as N (%).
| Variable | Mentees (N = 23) | |
|---|---|---|
| Age | <25 | 2 (8.7%) |
| 25–34 | 2 (8.7%) | |
| 35–44 | 8 (34.8%) | |
| 45–54 | 5 (21.7%) | |
| 55–64 | 1 (4.4%) | |
| >65 | 5 (21.7%) | |
| Married | Married | 2 (8.7%) |
| Other | 21 (91.3%) | |
| Education | 3 (13.7%) | |
| High school grad | 2 (9.1%) | |
| Some college | 3 (13.7%) | |
| College grad | 14 (63.6%) | |
| Income | <$15,000 | 5 (21.7%) |
| $15,000–$34,999 | 6 (26.1%) | |
| $35,000–$64,999 | 5 (21.7%) | |
| >or = $65,000 | 2 (8.7%) | |
| Other/did not want to respond | 5 (21.7%) |
Means and standard deviations for fresh and cryopreserved values taken at both time points are presented in Table 2. Comparisons of fresh and cryopreserved samples showed differences in only one cytokine at baseline, namely Th1 (Table 3), which had a greater cryopreserved value than fresh value. At the follow-up visit, all cytokines but Th1/Th2 and Th1 showed a significant difference. For Th2, Th17, and Treg, the cryopreserved samples had a larger concentration than fresh samples. For Tr1, the cryopreserved samples had a smaller concentration than fresh samples. (Table 3). Cell recovery was significantly reduced at follow-up compared with baseline (Table 2).
Table 2.
Cytokine and sample condition mean (standard deviation) is presented for both time points and fresh and cryopreserved samples.
| Cytokine | Baseline | Follow-up | ||
|---|---|---|---|---|
|
|
|
|||
| Fresh | Cryopreserved | Fresh | Cryopreserved | |
| Th1/Th2 | 12.49 (13.00) | 17.28 (13.54) | 7.46 (6.34) | 5.57 (18.44) |
| Th1 | 14.83 (10.29) | 21.45 (7.56) | 18.20 (8.90) | 13.34 (7.10) |
| Th2 | 1.67 (0.99) | 1.93 (1.32) | 3.63 (2.07) | 7.09 (11.27) |
| Th17 | 0.84 (0.51) | 0.83 (0.43) | 0.78 (0.52) | 1.50 (1.05) |
| Tr1 | 7.03 (5.56) | 7.93 (3.34) | 7.95 (4.43) | 2.01 (1.28) |
| Treg | 5.58 (2.72) | 8.59 (6.51) | 5.87 (3.45) | 21.11 (10.83) |
| Condition | Baseline | Follow-up | ||
| Viability | 90.85 (4.88) | 92.00 (5.03) | ||
| Recovery* | 75.95 (15.36) | 39.79 (32.42) | ||
Comparison of sample condition recovery by time point was significant (Diff = 36.16 (19.23); p < 0.0001).
Table 3.
All fresh vs cryopreserved cytokine differences are represented as least-square mean estimates and standard errors. All test results are performed using estimates from a repeated measures mixed-effects linear regression.
| Estimate (Fresh-Cryopreserved) | Standard Error | p-value | ||
|---|---|---|---|---|
| Baseline | Th1/Th2 | −4.79 | 4.44 | 0.29 |
| Th1 | −6.61 | 2.80 | 0.0301 | |
| Th2 | −0.25 | 0.97 | 0.79 | |
| Th17 | 0.01 | 0.22 | 0.95 | |
| Tr1 | −0.90 | 1.27 | 0.48 | |
| Treg | −3.01 | 2.17 | 0.18 | |
| Follow-up | Th1/Th2 | 1.89 | 4.44 | 0.67 |
| Th1 | 4.86 | 2.80 | 0.10 | |
| Th2 | −7.64 | 0.97 | <0.0001 | |
| Th17 | −0.71 | 0.22 | 0.0045 | |
| Tr1 | 5.94 | 1.27 | 0.0002 | |
| Treg | −15.24 | 2.17 | <0.0001 |
Bold: p < 0.05; Bold and italicized: p < 0.05/14 = 0.0017.
When comparing fresh and cryopreserved cytokine levels across time points, we saw a significant increase for cytokine Th2 for fresh samples (Table 4). For cryopreserved samples, a significant change was seen in all cytokines except Th1/Th2 balance, but changes were not in a consistent direction. We detected a significant decrease in concentration for cytokines Th1 and Tr1 and a significant increase in concentration for cytokines Th2, Th17, and Treg (Table 4).
Table 4.
All cytokine differences over time are represented as least-square mean estimates and standard errors. All test results are performed using estimates from a repeated measures mixed-effects linear regression controlling for reliability and viability.
| Estimate (Post-Pre) | Standard Error | p-value | ||
|---|---|---|---|---|
| Fresh | Th1/Th2 | −4.00 | 5.36 | 0.46 |
| Th1 | 2.28 | 3.38 | 0.50 | |
| Th2 | 2.70 | 1.17 | 0.0336 | |
| Th17 | −0.14 | 0.26 | 0.60 | |
| Tr1 | −0.88 | 1.53 | 0.57 | |
| Treg | −1.10 | 2.62 | 0.67 | |
| Cryopreserved | Th1/Th2 | −10.69 | 5.36 | 0.06 |
| Th1 | −9.19 | 3.38 | 0.0142 | |
| Th2 | 10.08 | 1.17 | <0.0001 | |
| Th17 | 0.58 | 0.26 | 0.0405 | |
| Tr1 | −7.73 | 1.53 | <0.0001 | |
| Treg | 11.11 | 2.62 | 0.0005 |
Bold: p < 0.05; Bold and italicized: p < 0.05/14 = 0.0017.
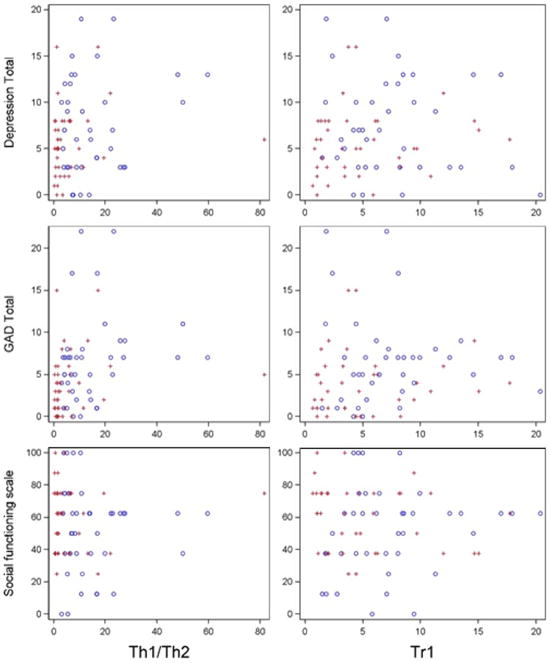
Of the PROs investigated, Th1/Th2 was significantly associated with GAD total (β = 0.09, p = 0.028) and had a moderately significant (at 10% level) association with depression total (β = 0.07, p = 0.076) (see Fig. 2). There were no significant associations observed between cytokines and disease activity, and Treg was not found to be associated with any of the investigated PROs. There were no observed differences in associations between cytokine level and PRO, according to whether the cytokine level was based on a fresh or cryopreserved sample.
Fig. 2.
Cytokine (balances) by PROs by time point (blue = T1, red = T2) Data presented are raw values and do not reflect the result of statistical modeling that controls for repeated measures or recovery and viability. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
Using common data elements pooled from participants in the PALS program we were able to assess the correlations between PROs and immunologic evidence of disease activity. In our study, the strongest correlations were between GAD and Th1/Th2 cytokine balance. Weaker correlations existed between depression and the Th1/Th2 cytokine balance. Observed levels of correlation may be due to the known actions that stress, anxiety, and depression can have on the expression of genes related to immune function [48]. These findings are consistent with other investigations that have observed that stress and anxiety can alter the expression of genes vital in immune response [47,48].
In our assessment of the comparability of fresh and cryopreserved samples, we observed that changes in Th1/Th2 cytokine balance with the intervention were generally equivalent, regardless of sample type (fresh or cryopreserved). This is consistent with earlier work [73]. While cell sorting has different recoveries and cell balance profiles compared to simply quantifying what is in the peripheral blood, a previous study assessing variability of Th1 and Th2 cytokines in PBMC of recent burn victims in fresh versus frozen samples showed no significant variation based on duration of cryopreservation and concluded samples could be frozen with minimal variation for up to one year. Overall, differences in values from baseline to follow-up were greater in cryopreserved samples.
Overall, the cryopreservation process appears to have resulted in notable changes to the immunologic profiles of the blood samples. However, at baseline these differences were less than follow-up time point fluctuations. For example, at baseline, changes were observed in Th1 only, whereas at the post-intervention time point variations were detected in the majority of the immunologic markers assessed. Potential explanations for these observations could have been related to the climatic conditions around collection and shipment. Baseline samples were drawn in the winter, while post-intervention samples were drawn in the summer. However, none of the samples were received “warm” when they reached UMMC; the cool packs remained functional and both groups were processed identically once received by the lab. There were also significant differences in self-reported disease activity, depression, and anxiety between the two time points, which could explain these differences. Additionally, different phlebotomists collected blood samples at the two time points, so it is possible that collection methods such as filling of tubes or immediate inversion were not identical and affected resulting measures by reducing cell recovery. This could suggest the need to further standardize and potentially monitor sample collection methods for future studies, to ensure consistency and reliability. However, cell recovery is always reduced with cryopreserved cells because not all cells in the PBMC fraction freeze well. This could be a cause for the trends observed in our cells and is why data from cryopreserved and fresh samples are not commonly mixed. The high viability of the cryopreserved preps is a technical check that confirms that the reduced recovery numbers was not due to a toxic event that killed off multiple cells. Cell viability is more important to cell integrity (quality check after cryopreservation) than the amount of cells recovered, and flow cytometry assays for the current study only required 500,000 cells for analysis.
While the findings of this study are notable, it is important to consider its limitations. We had a small sample size and there was no normal control group in this study. However, this study was designed as a feasibility and pilot study. The goal was to obtain initial data for planning and implementing a larger scale study. As such, results from this preliminary pilot study are not meant to be generalized. The potential of the peer mentoring intervention to normalize a patient or bring them to the level of a control will be explored in a larger study.
Additionally, cytokine levels were compared with patient reported outcomes rather than clinical or laboratory-verified end-points, which limits the validity of our findings. The possibility of bias is introduced by participants answering an extensive battery of questionnaires. All of their responses may not have accurately reflected their condition as a result of trying to complete instruments quickly. Another limitation is that blood samples were not cryopreserved before shipment to UMMC, rather they were cryopreserved upon their arrival. This doesn’t allow the study to address the question for comparisons of a sample shipped overnight then analyzed the next day as fresh versus one cryopreserved shortly after being drawn then thawed and assayed later. This study needs to be repeated to determine whether samples can be reliably shipped fresh for subsequent cryopreservation or must be cryopreserved on site shortly after being collected.
In terms of our timeline, multiple analysis points introduced inter-assay variability with both fresh and cryopreserved samples (reduced if the instrument was calibrated to fluorescent beads each time). This could have made the comparison of one sample to the next over time less precise than the comparison if pre and post intervention samples were analyzed on the same day. However, there is data from non-SLE participants that shows the relative stability and reproducibility of cytokine profiles in fresh vs. cryopreserved PBMC [73].
Additionally, we were unable to discuss where the cytokine values of PALS participants fell with respect to other populations and/or normal or ideal ranges due to the lack of published “normal ranges” for most of the markers under study. However, members of our study team have published work that addresses this in the context of various SNPs as biomarkers and Th1/Th2 cytokine balance data, and there are many published case control-type studies that show various baseline data but this is highly variable because of study population differences and technical differences in anti-body sources, instruments and even times of the day [75].
The PALS intervention resulted in significant improvements in depression and anxiety for mentees and these variables were also significantly associated with improved cytokine balance [76]. This gives biologic validity to an intervention that is also known to affect behavior that in turn affects outcomes. Such findings have significant implications for the treatment of SLE and other chronic autoimmune diseases by suggesting that improving depression and anxiety is a mechanism by which this type of intervention improves underlying biological indicators of immune function, and may need to be targeted areas of intervention for this population.
The information gleaned from this study has been incorporated into the design of a randomized, controlled study comparing the ‘peer mentoring program’ with an ‘attention’ control group to determine if similar effects are present in a larger sample size that will included matched control subjects.
Acknowledgments
Funding
This project was supported by the South Carolina Clinical & Translational Research (SCTR) Institute, with an academic home at the Medical University of South Carolina CTSA, NIH/NCATS Grant No. UL1 TR001450. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or NCATS. The funding source played no role in study design, data collection, analysis, interpretation of results, manuscript writing, or decision to publish.
We would like to thank the research participants for their contribution to this study. We also recognize the important role of the other members of the research team for their contribution to this project (Vernessa Nelson, Hetlena Johnson, and Denise Montgomery).
Abbreviations
- SLE
Systemic Lupus Erythematosus
- PALS
Peer Approaches to Lupus Self-Management
- PROs
patient reported outcomes
- MCRC
Multidisciplinary Clinical Research Center
- MUSC
Medical University of South Carolina
- CDMP
Chronic Disease Self-Management Program
- ASMP
Arthritis Self-Management Program
- SLESH
Systemic Lupus Erythematosus Self-Help
- ACR
American College of Rheumatology
- LUP-QOL
Lupus Quality of Life measure
- MOS
Medical Outcomes Study
- SF-36
Short Form 36 Health Survey
- FACIT-F
Functional Assessment of Chronic Illness Therapy-Fatigue
- PHQ
Patient Health Questionnaire
- GAD
General Anxiety Disorder
- PSS
perceived stress scale
- PAM
Patient Activation Measure
- EMRs
electronic medical records
- CDW
clinical data warehouse
- SLAM
Systemic Lupus Activity Measure
- SLAQ
Systemic Lupus Activity Questionnaire
- PGA
Patient Global Assessment
- NRS
Numerical Rating Scale
- PMA
phorbol myristate acetate
Footnotes
Conflicts of interest
None to declare.
Author roles
EMW was the principal investigator and LE, GM, and JO were senior co-investigators. GM specifically oversaw blood collection and analysis protocol. TF was involved in intervention development, implementation, evaluation, data analysis and manuscript writing. MH was involved in data analysis and manuscript writing, and VR provided statistical oversight. All authors read and approved the final version for publication.
References
- 1.Rahman A, Isenberg D. Systemic lupus erythematosus. N Engl J Med. 2008;358:929. doi: 10.1056/NEJMra071297. [DOI] [PubMed] [Google Scholar]
- 2.Pons-Estel G, Alarcón G, Scofield L, Reinlib L, Cooper G. Understanding the epidemiology and progression of systemic lupus erythematosus. Semin Arthritis Rheumatol. 2010;39:257. doi: 10.1016/j.semarthrit.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alparslan G, Kapucu S. The changes and difficulties experienced by patients using steroids. J Ren Care. 2010;36:81. doi: 10.1111/j.1755-6686.2010.00143.x. [DOI] [PubMed] [Google Scholar]
- 4.Greco C, Rudy T, Manzi S. Adaptation to chronic pain in systemic lupus erythematosus: applicability of the multidimensional pain inventory. Pain Med. 2003;4:39. doi: 10.1046/j.1526-4637.2003.03001.x. [DOI] [PubMed] [Google Scholar]
- 5.Daleboudt G, Broadbent E, McQueen F, Kaptein A. The impact of illness perceptions on sexual functioning in patients with systemic lupus erythematosus. J Psychosom Res. 2013;74:260. doi: 10.1016/j.jpsychores.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Cornwell C, Schmitt M. Perceived health status, self-esteem and body image in women with rheumatoid arthritis or systemic lupus erythematosus. Res Nurs Health. 1990;13:99. doi: 10.1002/nur.4770130206. [DOI] [PubMed] [Google Scholar]
- 7.Hochberg M, Sutton J. Physical disability and psychosocial dysfunction in systemic lupus erythematosus. J Rheumatol. 1988;15:959. [PubMed] [Google Scholar]
- 8.Kulczycka L, Sysa-Jędrzejowska A, Robak E. The influence of clinical manifestations and treatment on satisfaction with life together with positive and negative emotions in systemic lupus erythematosus patients. Acta Dermatovenerol Croat. 2011;19:6. [PubMed] [Google Scholar]
- 9.Bachen Chesney. Criswell: prevalence of mood and anxiety disorders in women with systemic lupus erythematosus. Arthritis Rheumatol. 2009;61:822. doi: 10.1002/art.24519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Danoff-Burg S, Friedberg F. Unmet needs of patients with systemic lupus erythematosus. Behav Med. 2009;35:5. doi: 10.3200/BMED.35.1.5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jarpa E, Babul M, Calderón J, et al. Common mental disorders and psychological distress in systemic lupus erythematosus are not associated with disease activity. Lupus. 2011;20:58. doi: 10.1177/0961203310381773. [DOI] [PubMed] [Google Scholar]
- 12.Jolly M. How does quality of life of patients with systemic lupus erythematosus compare with that of other common chronic illnesses? J Rheumatol. 2005;32:1706. [PubMed] [Google Scholar]
- 13.Seawell A, Danoff-Burg S. Psychosocial research on systemic lupus erythematosus: a literature review. Lupus. 2004;13:891. doi: 10.1191/0961203304lu1083rr. [DOI] [PubMed] [Google Scholar]
- 14.Beckerman N. Living with lupus: a qualitative report. Soc Work Health Care. 2011;50:330. doi: 10.1080/00981389.2011.554302. [DOI] [PubMed] [Google Scholar]
- 15.Da Costa D, Dobkin P, Fitzcharles M, Fortin P, Beaulieu A, Zummer M, et al. Determinants of health status in fibromyalgia: a comparative study with systemic lupus erythematosus. J Rheumatol. 2000;27:365. [PubMed] [Google Scholar]
- 16.Kozora E, Ellison M, Waxmonsky J, Wamboldt F, Patterson T. Major life stress, coping styles, and social support in relation to psychological distress in patients with systemic lupus erthematosus. Lupus. 2005;14:363. doi: 10.1191/0961203305lu2094oa. [DOI] [PubMed] [Google Scholar]
- 17.McElhone K, Abbott J, Teh LS. A review of health related quality of life in systemic lupus erythematosus. Lupus. 2006;15:633. doi: 10.1177/0961203306071710. [DOI] [PubMed] [Google Scholar]
- 18.Moses N, Wiggers J, Nicholas C, Cockburn J. Prevalence and correlates of perceived unmet needs of people with systemic lupus erythematosus. Patient Educ Couns. 2005;57:30. doi: 10.1016/j.pec.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 19.Philip E, Lindner H, Lederman L. Relationship of illness perceptions with depression among individuals diagnosed with lupus. Depress Anxiety. 2009;26:575. doi: 10.1002/da.20451. [DOI] [PubMed] [Google Scholar]
- 20.Sehlo M, Bahlas S. Perceived illness stigma is associated with depression in female patients with systemic lupus erythematosus. J Psychosom Res. 2013;74:248. doi: 10.1016/j.jpsychores.2012.09.023. [DOI] [PubMed] [Google Scholar]
- 21.Fernández M, Alarcón G, Calvo-Alén J, et al. A multiethnic, multicenter cohort of patients with systemic lupus erythematosus (SLE) as a model for the study of ethnic disparities in SLE. Arthritis Rheumatol. 2007;57:576. doi: 10.1002/art.22672. [DOI] [PubMed] [Google Scholar]
- 22.Lau C, Yin G, Mok M. Ethnic and geographical differences in systemic lupus erythematosus: an overview. Lupus. 2007;15:715. doi: 10.1177/0961203306072311. [DOI] [PubMed] [Google Scholar]
- 23.Yazdany J, Gillis J, Trupin L, et al. Association of socioeconomic and demographic factors with utilization of rheumatology subspecialty care in systemic lupus erythematosus. Arthritis Rheumatol. 2007;57:593. doi: 10.1002/art.22674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bae S, Hashimoto H, Karlson E, Liang M, Daltroy L. Variable effects of social support by race, economic status, and disease activity in systemic lupus erythematosus. J Rheumatol. 2001;28:1245. [PubMed] [Google Scholar]
- 25.Ow M, Ho P, Thumboo J, Wee H. Factors associated with health services utilization in patients with systemic lupus erythematosus: a systematic review. Clin Exp Rheumatol. 2010;28:892. [PubMed] [Google Scholar]
- 26.Cully J, Mignogna J, Stanley M, Davila J, Wear J, Amico K, et al. Development and pilot testing of a standardized training program for a patient-mentoring intervention to increase adherence to outpatient HIV care. AIDS Patient Care STDS. 2012;26:165. doi: 10.1089/apc.2011.0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Futterman D, Shea J, Besser M, Stafford S, Desmond K, Comulada W, et al. Mamekhaya: a pilot study combining a cognitive-behavioral intervention and mentor mothers with PMTCT services in South Africa. AIDS Care. 2010;22:1093. doi: 10.1080/09540121003600352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jerson B, D’Urso C, Arnon R, Miloh T, Iyer K, Kerkar N, et al. Adolescent transplant recipients as peer mentors: a program to improve self-management and health-related quality of life. Pediatr Transplant. 2013;17:612. doi: 10.1111/petr.12127. [DOI] [PubMed] [Google Scholar]
- 29.Richter L, Rotheram-Borus M, Van Heerden A, Stein A, Tomlinson M, Harwood J, et al. Pregnant women living with HIV (WLH) supported at clinics by peer WLH: a cluster randomized controlled trial. AIDS Behav. 2014;18:706. doi: 10.1007/s10461-014-0694-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rotheram-Borus M, Richter L, van Heerden A, van Rooyen H, Tomlinson M, Harwood J, et al. A cluster randomized controlled trial evaluating the efficacy of peer mentors to support South African women living with HIV and their infants. PLoS One. 2014;9:e84867. doi: 10.1371/journal.pone.0084867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tracy K, Burton M, Nich C, Rounsaville B. Utilizing peer mentorship to engage high recidivism substance-abusing patients in treatment. Am J Drug Alcohol Abuse. 2011;37:525. doi: 10.3109/00952990.2011.600385. [DOI] [PubMed] [Google Scholar]
- 32.Dorgo S, Robinson K, Bader J. The effectiveness of a peer-mentored older adult fitness program on perceived physical, mental, and social function. J Am Acad Nurse Pract. 2009;21:116. doi: 10.1111/j.1745-7599.2008.00393.x. [DOI] [PubMed] [Google Scholar]
- 33.Eskicioglu P, Halas J, Senechal M, Wood L, McKay E, Villeneuve S, et al. Peer mentoring for type 2 diabetes prevention in first nations children. Pediatrics. 2014;133:e1624. doi: 10.1542/peds.2013-2621. [DOI] [PubMed] [Google Scholar]
- 34.Spencer R, Bower J, Kirk S, Hancock Friesen C. Peer mentoring is associated with positive change in physical activity and aerobic fitness of grades 4, 5, and 6 students in the heart healthy kids program. Health Promot Pract. 2014;15:803. doi: 10.1177/1524839914530402. [DOI] [PubMed] [Google Scholar]
- 35.Thomas R, Lorenzetti D, Spragins W. Systematic review of mentoring to prevent or reduce tobacco use by adolescents. Acad Pediatr. 2013;13:300. doi: 10.1016/j.acap.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 36.Knox L, Huff J, Graham D, Henry M, Bracho A, Henderson C, et al. What peer mentoring adds to already good patient care: implementing the Carpeta Roja peer mentoring program in a well-resourced health care system. Ann Fam Med. 2015;13:S59. doi: 10.1370/afm.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Philis-Tsimikas A, Fortmann A, Lleva-Ocana L, Walker C, Gallo L. Peerled diabetes education programs in high-risk Mexican Americans improve glycemic control compared with standard approaches: a Project Dulce promotora randomized trial. Diabetes Care. 2011;34:1926. doi: 10.2337/dc10-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sazlina S, Browning C, Yasin S. Effectiveness of personalized feedback alone or combined with peer support to improve physical activity in sedentary older malays with type 2 diabetes: a randomized controlled trial. Front Public Health. 2015;3:178. doi: 10.3389/fpubh.2015.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woodbury M, Botros M, Kuhnke J, Greene J. Evaluation of a peer-led self-management education programme PEP talk: diabetes, healthy feet and you. Int Wound J. 2013;10:703. doi: 10.1111/iwj.12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goldenberg D, Payne L, Hayes L, Zeltzer L, Tsao J. Peer mentorship teaches social tools for pain self-management: a case study. J Pain Manage. 2013;6:61. [PMC free article] [PubMed] [Google Scholar]
- 41.Sandhu S, Veinot P, Embuldeniya G, Brooks S, Sale J, Huang S. Peer-to-peer mentoring for individuals with early inflammatory arthritis: feasibility pilot. BMJ Open. 2013;3 doi: 10.1136/bmjopen-2012-002267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dreyfus D. Autoimmune disease: a role for new anti-viral therapies. Autoimmun Rev. 2011;11:88. doi: 10.1016/j.autrev.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 43.Frieri M. Neuroimmunology and inflammation: implications for therapy of allergic and autoimmune diseases. Ann Allergy Asthma Immunol. 2003;90:34. doi: 10.1016/s1081-1206(10)61658-4. [DOI] [PubMed] [Google Scholar]
- 44.Marshall G. The adverse effects of psychological stress on immunoregulatory balance: applications to human inflammatory diseases. Immunol Allergy Clin North Am. 2011;31:133. doi: 10.1016/j.iac.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davis M, Zautra A, Yonger J, Motivala S, Attrep J, Irwin M. Chronic stress and regulation of cellular markers of inflammation in rheumatoid arthritis: implications for fatigue. Brain Behav Immun. 2008;22:24. doi: 10.1016/j.bbi.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ziang L, Del Ben K, Rehn K, Marshall G. Effects of acute stress-induced immunomodulation on TH1/TH2 cytokine and catecholamine receptor expression in human peripheral blood cells. Neuropsychobiology. 2012;65:12. doi: 10.1159/000328160. [DOI] [PubMed] [Google Scholar]
- 47.Dhabhar F. Effects of stress on immune function: the good, the bad, and the beautiful. Immunol Res. 2014;58:193. doi: 10.1007/s12026-014-8517-0. [DOI] [PubMed] [Google Scholar]
- 48.Wingo A, Gibson G. Blood gene expression profiles suggest altered immune function associated with symptoms of generalized anxiety disorder. Brain Behav Immun. 2015;43:184. doi: 10.1016/j.bbi.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lorig K, Holman H. arthritis self-management studies: a twelve-year review. Health Educ Q. 1993;20:17. doi: 10.1177/109019819302000104. [DOI] [PubMed] [Google Scholar]
- 50.Lorig K, Mazonson P, Holman H. Evidence suggesting that health education for self-management in patients with chronic arthritis has sustained health benefits while reducing health care costs. Arthrit Rheumatol. 1993;36:437. doi: 10.1002/art.1780360403. [DOI] [PubMed] [Google Scholar]
- 51.Lorig K, Ritter P, Plant K. A disease-specific self-help program compared with a generalized chronic disease self-help program for arthritis patients. Arthrit Rheumatol. 2005;53:950. doi: 10.1002/art.21604. [DOI] [PubMed] [Google Scholar]
- 52.Lorig K, Sobel D, Stewart A. Evidence that a chronic disease self-management program can improve health status while reducing utilization and costs: a randomized trial. Med Care. 1999;37:5. doi: 10.1097/00005650-199901000-00003. [DOI] [PubMed] [Google Scholar]
- 53.Arthritis Foundation. The Systemic Lupus Erythematosus Self-Help Course: Program guidelines and procedures manual. Atlanta, GA: 1987. [Google Scholar]
- 54.Williams E, Bruner L, Penfield M, Kamen D, Oates J. Stress and Depression in Relation to Functional Health Behaviors in African American Patients with Systemic Lupus Erythematosus. Rheumatology: Current Reports. doi: 10.4172/2161-1149.S4-005. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Williams E, Kamen D, Penfield M, Oates J. Stress intervention and disease in african american lupus patients: the balancing lupus experiences with stress strategies (BLESS) study. Health. 2014;6:71. doi: 10.4236/health.2014.61011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Williams E, Penfield M, Kamen D, Oates J. An intervention to reduce psychosocial and biological indicators of stress in African American lupus patients: the balancing lupus experiences with stress strategies study. Open J Prev Med. 2014;4:22. doi: 10.4236/ojpm.2014.41005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hochberg M. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus (letter) Arthrit Rheumatol. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 58.Harris P, Taylor R, Thielke R, Payne J, Gonzalez N, Conde J. Research electronic data capture (REDCap) - A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Webster K, Cella D, Yost K. The Functional Assessment of Chronic Illness Therapy (FACIT) measurement system: properties, application, and interpretation, Health Qual. Life Outcomes. 2003;1 doi: 10.1186/1477-7525-1-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Toloza S, Jolly M, Alarcón G. Quality-of-Life measurements in multiethnic patients with systemic lupus erythematosus: cross-cultural issues. Curr Rheumatol Rep. 2010;12:237. doi: 10.1007/s11926-010-0110-5. [DOI] [PubMed] [Google Scholar]
- 61.Kroenke K, Spitzer R. The PHQ-9: a new depression and diagnostic severity measure. Psychiatr Ann. 2002;32:509. [Google Scholar]
- 62.Kroenke K, Spitzer R, Williams J. The PHQ-9. J Gen Intern Med. 2001;16:606. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Spitzer R, Kroenke K, Williams J, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166 doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 64.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385. [PubMed] [Google Scholar]
- 65.Cohen S, Williamson G. Perceived stress in a probability sample of the United States. In: Oskamp S, editor. The Social Psychology of Health. Newbury Park, CA: Sage; 1988. [Google Scholar]
- 66.Hibbard J, Mahoney E, Stockard J, Tusler M. Development and testing of a short form of the patient activation measure. Health Serv Res. 2005;40:1918. doi: 10.1111/j.1475-6773.2005.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hibbard J, Stockard J, Mahoney E, Tusler M. Development of the Patient Activation Measure (PAM): conceptualizing and measuring activation in patients and consumers. Health Serv Res. 2004;39:1005. doi: 10.1111/j.1475-6773.2004.00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liang M, Socher S, Larson M, Schur P. Reliability and validity of six systems for the clinical assessment of disease activity in systemic lupus erythematosus. Arthrit Rheumatol. 1989;32:1107. doi: 10.1002/anr.1780320909. [DOI] [PubMed] [Google Scholar]
- 69.Bae S, Koh HK, Chang DK, Kim MH, Part JK, Kim SY. Reliability and validity of systemic lupus activity measure-revised (SLAM-R) for measuring clinical disease activity in systemic lupus erythematosus. Lupus. 2002;10:405. doi: 10.1191/096120301678646146. [DOI] [PubMed] [Google Scholar]
- 70.Karlson E, Daltroy L, Rivest C, et al. Validation of a systemic lupus activity questionnaire (SLAQ) for population studies. Lupus. 2003;12:280. doi: 10.1191/0961203303lu332oa. [DOI] [PubMed] [Google Scholar]
- 71.Griswold M, Marshall G. Variability in laboratory immune parameters is associated with stress hormone receptor polymorphisms. Neuroimmunomodulation. 2012;19:220. doi: 10.1159/000334711. [DOI] [PubMed] [Google Scholar]
- 72.Marshall G, Agarwal S, Lloyd C, Cohen L, Henninger E, Morris G. Cytokine dysregulation associated with exam stress in healthy medical students. Brain Behav Immunol. 1998;12:297. doi: 10.1006/brbi.1998.0537. [DOI] [PubMed] [Google Scholar]
- 73.Kilani R, Delehanty M, Shankowsky H, Ghahary A, Scott P, Tredget E. Fluorescent-activated cell-sorting analysis of intracellular interferon-gamma and interleukin-4 in fresh and cryopreserved human peripheral blood T-helper cells. Wound Repair Regen. 2005;13:441. doi: 10.1111/j.1067-1927.2005.130412.x. [DOI] [PubMed] [Google Scholar]
- 74.Marshall G. Neuroendocrine mechanisms of immune dysregulation: applications to allergy and asthma. Ann Allergy Asthma Immunol. 2004;93:11. doi: 10.1016/s1081-1206(10)61482-2. [DOI] [PubMed] [Google Scholar]
- 75.Rehm K, Xiang L, Elci O, Griswold M, Marshall G. Variability in laboratory immune parameters is associated with stress hormone receptor polymorphisms. Neuroimmunomodulation. 2012;19:220. doi: 10.1159/000334711. [DOI] [PubMed] [Google Scholar]
- 76.Williams E, Voronca D, Hyer M, Rameshkrishnan V, Faith T, Gebregziabher M, et al. Peer-to-peer mentoring for African American women with lupus: A feasibility pilot. Arthritis Care & Research. 2017 doi: 10.1002/acr.23412. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]