Abstract
The ions Na+, K+, Ca2+, Mg2+, Cl−, SO42−, and HCO3−/CO32− (referred to here as “major ions”) are present in all fresh waters and are physiologically required by aquatic organisms, but can increase to harmful levels from a variety of anthropogenic activities. It is also known that the toxicities of major ion salts can vary depending on the concentrations of other ions, and understanding these relationships is key to establishing appropriate environmental limits. In this paper we present a series of experiments with Ceriodaphnia dubia to evaluate the acute toxicity of twelve major ion salts and to determine how toxicity of these salts varies as a function of background water chemistry. All salts except CaSO4 and CaCO3 were acutely toxic below saturation, with the lowest LC50s found for K salts. All ten salts that showed toxicity also showed some degree of reduced toxicity as the ionic content of the background water increased. Experiments that independently varied Ca:Mg ratio, Na:K ratio, Cl:SO4 ratio, and alkalinity/pH demonstrated that Ca concentration was the primary factor influencing the toxicities of Na and Mg salts, while the toxicities of K salts were primarily influenced by the concentration of Na. These experiments also indicated multiple mechanisms of toxicity and suggested important aspects of dosimetry: the toxicities of K, Mg, and Ca salts were best related to the chemical activity of the cation, while the toxicities of Na salts also reflected an influence of the anions and were well correlated with osmolarity. Understanding these relationships between major ion toxicity and background water chemistry should aid in the development of sensible risk assessment and regulatory standards.
Keywords: Aquatic toxicology, Major ions, Ceriodaphnia dubia, Toxicity mechanisms, Dose-response modeling
INTRODUCTION
Inorganic ions generally present at the highest concentrations in freshwaters are Na+, K+, Ca2+, Mg2+, Cl−, SO42−, and HCO3−/CO32− (referred to as “major ions” herein) and are used to describe the basic chemistry of natural waters [1]. All have physiological roles and are actively regulated by aquatic organisms [2], but can also cause toxicity when present in sufficient excess [3]. Concentrations in natural waters are governed by a variety of atmospheric, geochemical, and biological processes [1], but these natural concentrations can be greatly increased by a wide variety of anthropogenic influences, such as mineral mining, oil and gas extraction, irrigation, road de-icing, water softening, and wastewaters from various industrial processes.
A variety of studies have shown or implicated major ions as causes of aquatic toxicity in surface waters, with sources such as oil/gas production [4,5], irrigation return flows [6,7], mining [8,9], road salt [10], and industrial wastewater [11]. In fact, toxicity identification studies on industrial and municipal effluents have shown major ions to be among the more common causes of effluent toxicity [12]. Field studies in Appalachian streams have also found associations between changes in macrobenthic communities and increased major ion concentrations from mining activities [13–15].
Understanding the aquatic hazards posed by increased major ion concentrations presents a number of challenges. For one, concentrations of major ions cannot be manipulated individually, because charge balance demands that increased concentrations of any ion be offset by equal and opposite charge from other ions, making it more difficult to infer the effects of individual ions. Second, the relative concentrations of major ions vary widely across watersheds and anthropogenic inputs, and such differences are known to influence aquatic toxicity. For example, based on total salt concentration, a 1:1 mixture (by mass) of NaCl and CaCl2 has substantially lower acute toxicity to Ceriodaphnia dubia than either salt alone [3], indicating that toxicity of this salt mixture is not simply additive. Third, the toxicity of a single salt can vary based on the characteristics of the water to which it is added, such as water hardness [16–20] and, more specifically, Ca [21]. While relationships between water hardness and the toxicity of various other chemicals are often attributed, explicitly or implicitly, to the Ca and Mg ions that comprise most hardness, more detailed studies sometimes show that the concentrations of other ions co-varying with hardness are playing important roles. For example, though “hardness” was long reported to influence toxicity of metals such as copper, later research demonstrated more detailed roles of specific ions and this enhanced understanding was incorporated into a more refined toxicity model, the Biotic Ligand Model [22].
In previous work, Mount et al. [3] approached the toxicity of major ion mixtures by developing a multivariate regression model based on a large number of acute toxicity tests conducted with many different combinations of major ion salts. The resulting models predict the survival of 3 test species, cladocerans Ceriodaphnia dubia and Daphnia magna, and the fathead minnow (Pimephales promelas) based on concentrations of the 7 major ions. While this model represented a step forward in addressing the complexities of evaluating ion mixtures and showed effectiveness as a predictive tool [5,23], there are important aspects of major ion toxicity that were not fully addressed. Notable among these was that all of the ion solutions tested were created by adding ions to a single base water. This issue underlies the failure of the model to represent some influences of background water chemistry on ion toxicity, such as the reduction of NaCl and Na2SO4 toxicity afforded by adding hardness within ranges common to natural waters [16–21]. Other aspects of the interactions among the ions (e.g., independent versus additive toxicity) also are incompletely addressed by this regression model.
This is the first of 3 papers that establish a better foundation for predicting the acute toxicity of elevated major ion concentrations to Ceriodaphnia dubia. This paper describes a comprehensive study of the influence of background water chemistry that extends earlier work on hardness effects (16-21) to more water chemistry factors and to more major ion salts. Toxicities of major ion salts were evaluated using a wide range of dilution waters; some mimicked natural waters, while others were designed specifically to isolate different components of background water chemistry to better understand their roles in influencing major ion toxicity and thereby determine what is important to risk assessment of ions in natural systems.
These experiments also allowed preliminary consideration of exposure metrics that more effectively describe major ion toxicity to C. dubia than total salt concentration. For example, are there different toxicity mechanisms among the salts that need to be addressed? Should the toxicity of a salt be related to one of the ions or both, and how should concentrations of multiple ions be combined? Should reductions in toxicity due to formation of complexes between ions and to the general effects of high ion concentrations on chemical reactivity be addressed?
The second paper will present results of mixture tests with pairs of salts to more rigorously address the preceding questions. The third paper will address how the information from the first 2 papers can be incorporated into a mathematical model applicable to any ion mixture and will test the predictions of that model for more complex mixtures relevant to field exposures.
The acute toxicity to C. dubia was selected as the endpoint for these efforts because this is a widely distributed organism with considerable sensitivity to ions, and for which it was practical to conduct the large number of tests needed to adequately address the multiple factors and interactions of interest. The knowledge gained from the present study with C. dubia supports more informed testing and model development for other endpoints and species, which are now underway and will be the subject of additional publications. When combined with the efforts of other investigators, this body of information will support better assessment of the risks of major ions to aquatic communities.
METHODS AND MATERIALS
Test water composition and study design
Twenty-six experiments were conducted on the acute toxicity of individual major ion salts to C. dubia. Each experiment consisted of 3 to 8 simultaneous toxicity tests with different combinations of dilution waters and toxicants, for a total of 149 median lethal concentration (LC50) determinations. Test waters were developed from de-ionized water (DIW), sand filtered and UV-treated Lake Superior water (LSW), or a combination of the two. DIW was produced from a Millipore® Super-Q system (Millipore Corporation) configured as specified by [24]. LSW was obtained from an intake located offshore from our laboratory at 46.840° N, 92.004° W; typical hardness and alkalinity are 47 and 43 mg/L as CaCO3, respectively; conductivity 104 μS/cm; pH circa 7.5. The full ionic composition of LSW is provided in Table 1.
Table 1.
Composition of dilution waters; DIW denotes deionized water; parentheses denote estimated geometric average ion concentrations at designated hardness for selected U.S. waters
| Dilution Water Description | Abbreviation | Base Water | Na mg/L | K mg/L | Ca mg/L | Mg mg/L | Cl mg/L | SO4 mg/L | Alkalinity mg CaCO3/L | Hardness mg CaCO3/L |
|---|---|---|---|---|---|---|---|---|---|---|
| Lake Superior Water | LSW | 1.62 | 0.60 | 14.0 | 2.92 | 1.50 | 3.40 | 43.0 | 47.0 | |
| Amended Lake Superior Water | ALSW | LSW | 6.48 | 1.51 | 14.6 | 4.09 | 7.66 | 14.9 | 43.0 | 53.3 |
| 1/3 Strength ALSW | 1/3x ALSW | 1/3x LSW | 2.16 (4.77) |
0.50 (1.18) |
4.87 (4.87) |
1.36 (1.36) |
2.55 (5.04) |
4.97 (7.88) |
14.3 (14.4) |
17.8 |
| 3x Strength ALSW | 3x ALSW | LSW | 19.4 (16.7) |
4.52 (2.74) |
43.8 (43.9) |
12.3 (12.3) |
23.0 (19.0) |
44.7 (46.0) |
129 (125) |
160 |
| Moderately Hard Reconstituted Water | MHRW | DIW | 26.3 | 2.10 | 14.0 | 12.0 | 1.90 | 81.0 | 57.2 | 84.4 |
| 1/3 Strength MHRW | 1/3x MHRW | DIW | 8.75 | 0.70 | 4.65 | 4.01 | 0.63 | 27.0 | 19.1 | 28.1 |
| 1/8 Strength MHRW | 1/8x MHRW | DIW | 3.28 | 0.26 | 1.75 | 1.50 | 0.24 | 10.1 | 7.2 | 10.6 |
| ALSW with High Ca:Mg Ratio | High Ca:Mg | 0.214x LSW | 6.48 | 1.51 | 19.0 | 1.40 | 7.66 | 14.9 | 43.0 | 53.3 |
| ALSW with Low Ca:Mg Ratio | Low Ca:Mg | 0.214x LSW | 6.48 | 1.51 | 3.00 | 11.1 | 7.66 | 14.9 | 43.0 | 53.3 |
| ALSW with High Cl:SO4 Ratio | High Cl:SO4 | LSW | 6.48 | 1.51 | 14.6 | 4.09 | 14.3 | 3.40 | 43.0 | 53.3 |
| ALSW with Low Cl:SO4 Ratio | Low Cl:SO4 | LSW | 6.48 | 1.51 | 14.6 | 4.09 | 1.50 | 20.7 | 43.0 | 53.3 |
| ALSW with High Na:K Ratio | High Na:K | 1/33x LSW | 7.65 | 0.20 | 14.6 | 4.09 | 7.66 | 14.9 | 43.0 | 53.3 |
| ALSW with Low Na:K Ratio | Low Na:K | 1/33x LSW | 1.89 | 10.0 | 14.6 | 4.09 | 7.66 | 14.9 | 43.0 | 53.3 |
| ALSW with High Alkalinity | High Alk | 0.233x LSW | 28.3 | 1.51 | 14.6 | 4.09 | 7.66 | 14.9 | 90.0 | 53.3 |
| ALSW with Low Alkalinity | Low Alk | 0.233x LSW | 28.3 | 1.51 | 14.6 | 4.09 | 7.66 | 14.9 | 10.0 | 53.3 |
| ALSW with Varying Na | LSW | 1.62 3.00 10.0 30.0 100 300 |
1.51 | 14.6 | 4.09 | 1.50 3.62 14.4 45.3 153 460 |
14.9 | 43.0 | 53.3 |
Some test waters were based on the commonly used reconstituted water formulas given by the U.S. Environmental Protection Agency (USEPA) [24], and originally proposed by Marking and Dawson [25], specifically the formula for “moderately hard reconstituted water” (MHRW), which was also used by Mount et al. [3]. While commonly used, MHRW (and other reconstituted waters based on this formula) has a chemistry (Table 1) that is atypical for surface waters of the U.S.: the Ca:Mg ratio is low, the Cl:SO4 ratio is very low, and the Na concentration is extremely high relative to hardness. The high Na concentration is a consequence of using NaHCO3 to impart alkalinity; in nature, alkalinity generally comes from dissolution of carbonate minerals of Ca and Mg, but these have solubilities and dissolution rates that are inconveniently low for water preparation in the laboratory. The low Ca:Mg and Cl:SO4 ratios in MHRW are not a practical requirement, and the reason for their original selection is not clear.
To design a dilution water with chemistry more like natural waters, we analyzed data from the U.S. Geological Survey national stream water-quality monitoring networks [26]. Data records were obtained for 425 sites in operation from 1983 to 1992. Records (n=32895) were used that had 1) measurements for all major ions; 2) a charge imbalance of no more than 10%; 3) a hardness ≥10 and ≤400 mg/L as CaCO3; and 4) pH no lower than 6.0. These data were subject to regression analyses using Sigmaplot (v11.0, Systat Software) to establish relationships between log(hardness) and the logarithms of various other ion concentrations and ratios. A formula was then developed for an “amended Lake Superior water” (ALSW, Table 1) consistent with these relationships. Salts were added to LSW to increase hardness to the estimated geometric average (53.3 mg CaCO3/L) in the USGS data for the alkalinity of LSW (43.0 mg CaCO3/L), and to increase other ions to average values for this hardness. Benefits of modifying LSW to create this test dilution water, rather than creating a completely synthetic water from DIW, are that it provides alkalinity without having to dissolve carbonate salts or add excessive Na, and that it will contain trace constituents from the source water that make the water more realistic and possibly beneficial to the test organisms.
This study was organized into twelve sets of 1-4 experiments addressing different aspects of dilution water chemistry or experimental procedure. These sets of experiments and the toxicants that were tested are summarized in Table 2 and the chemistries of the various dilution waters are provided in Table 1. The purpose and design of each experimental set were as follows:
Table 2.
Major salts tested within each set of experiments; GL denotes gluconate
| Experimental Set Description | NaCl | Na2SO4 | NaHCO3 | KCl | K2SO4 | KHCO3 | MgCl2 | MgSO4 | MgCO3 | CaCl2 | NaGL | MgGL | CaGL | Mannitol | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1/3x ALSW Culture vs 1x ALSW Culture, Tested
in 1/3x ALSW 1/3x MHRW Culture vs 1x MHRW Culture, Tested in 1/3x MHRW |
Xa | X | ||||||||||||
| 2 | 1/8x MHRW Culture vs 1x MHRW Culture, Tested in 1/8x MHRW | Xb | X | X | X | X | X | X | |||||||
| 3 | MHRW vs ALSW | X | X | X | X | X | X | X | X | X | X | ||||
| 4 | 1/3x ALSW vs 3x ALSW | Xc | Xc | X | X | X | X | X | X | X | |||||
| 5 | Low Ca:Mg vs High Ca:Mg | X | X | X | X | X | X | ||||||||
| 6 | Low Cl:SO4 vs High ClSO4 | X | X | X | X | X | X | ||||||||
| 7 | Low Na:K vs High Na:K | X | X | X | X | X | X | ||||||||
| 8 | Low Alk vs High Alk | X | X | X | X | X | |||||||||
| 9 | ALSW with Low vs Ambient vs High pH | X | X | ||||||||||||
| 10 | ALSW with Varying Na (0.07-13 mM) | X | |||||||||||||
| 11 | CaCO3 Precipitation Effects, Aged Dilution Water vs Not Aged, 1x ALSW vs 3x ALSW | X | X | ||||||||||||
| 12 | Organic Salts and Mannitol, 1x ALSW only | X | X | X | X | X |
Organisms from both ALSW cultures also tested in 1x ALSW
Organisms from both cultures also tested in 1x MHRW
Duplicate tests conducted which also included 1x ALSW
Sets 1 and 2: Effects of acclimation to dilution water
Some previous studies of the effect of dilution water on major ion toxicity to C. dubia used organisms that were cultured in the dilution water prior to testing [16–20]. This was not done routinely in the present study because the logistics involved in culturing organisms in so many different dilution waters (Table 1) were prohibitive. However, because a preliminary comparison of NaCl toxicity in MHRW and MHRW diluted by a factor of 3 (1/3x MHRW) showed much less of an impact of this dilution than the 1x, 1/2x, 1/4x, and 1/8x MHRW series tested by Elphick et al. [19], we conducted experiments to determine whether observed salt toxicity in waters more dilute than MHRW or ALSW varied significantly depending on whether the organisms were cultured in these dilute test waters or in the standard strength water formulations. Initially, organisms from our primary culture (in 1x MHRW) were used to create secondary cultures in 1x MHRW, 1/3x MHRW, 1x ALSW, and 1/3x ALSW, which were maintained a minimum of two generations before being used in tests. The toxicities of NaCl and MgCl2 in 1/3x MHRW and 1/3x ALSW were simultaneously tested using the cultures from both the dilute water and the corresponding full strength water (Table 2, Set 1). For NaCl, tests were also conducted in 1x ALSW using both the 1x ALSW and 1/3x ALSW cultures. To further explore this issue, we conducted additional acclimation studies (Table 2, Set 2) comparing the toxicity of several salts in 1/8x MHRW using C. dubia cultured in MHRW and in 1/8x MHRW (the same composition as the 10 and 80 hardness waters in the Elphick et al. [19] study). For NaCl, tests were also conducted in 1x MHRW using both cultures.
Set 3: Salt toxicity in MHRW versus ALSW
Because ALSW was developed in response to concerns about the composition of MHRW and because much existing ion toxicity information was conducted in MHRW or similar waters, comparative toxicities in these 2 waters were of interest. Therefore, a set of experiments (Table 2, Set 3) compared the toxicity of all major ion salts in MHRW and ALSW, except for CaSO4 and CaCO3, for which preliminary experiments showed no toxicity at saturation in these waters.
Set 4: Salt toxicity in different strengths of ALSW
Natural waters vary widely in total ion concentration, the 10th and 90th percentiles in our analysis of USGS data being approximately 1.5 and 16 meq/L for the sum of major cations and anions. To determine the effect of varying overall ion concentrations, another set of experiments (Table 2, Set 4) compared toxicities of 9 salts in dilution waters that had one-third and three times the ion concentrations of ALSW (1/3x ALSW and 3x ALSW; Table 1). We note that, in natural waters, average concentrations of the various ions do not vary exactly in proportion with total ion concentrations, so 1/3x ALSW and 3x ALSW do not match our estimated field water averages at the same hardnesses, which are also provided in Table 1 for comparison purposes. We elected not to match the field chemistry so that all ions would be in the same proportions across the 1/3x, 1x, 3x ALSW series, but the ion ratios are still well within the ranges observed in the USGS data.
The results of these tests were combined with separate data for 1x ALSW (from Set 3) to compare across a 1/3x, 1x, and 3x ALSW dilution water series; for NaCl and Na2SO4 an additional experiment simultaneously tested this entire series to verify results. Preparation of 1/3x ALSW was simply by dilution of ALSW with DIW. Creating the 3x ALSW chemistry required dissolution of CaCO3 to provide higher alkalinity while maintaining the desired cation ratios, in addition to adding other more soluble salts. This was accomplished by adding the appropriate amounts of the various salts to LSW in a large graduated cylinder, and bubbling CO2 gas through the solution until pH stabilized near 5.0 and all salts were dissolved. The solution was then aerated with ambient air until pH was approximately 7.8.
Sets 5-7: Effects of modifying specific ion ratios
Most previous work on the effects of dilution water chemistry on major ion toxicity to daphnids has focused on water hardness [16–20] as a generalized parameter without parsing the influence of other ions that co-varied with the hardness, although Davies and Hall [21] did identify Ca as being more important than Mg for the effects of hardness on Na2SO4 toxicity. Regarding other ions in dilution water, Soucek [17] reported an ameliorative effect of low concentrations of added NaCl on the toxicity of Na2SO4 to Hyalella azteca (attributed to the chloride requirements for this species), but no such effect on C. dubia. At higher NaCl concentrations, there was an exacerbation of Na2SO4 toxicity for both species, attributed to additive toxicity of the two salts. Soucek et al. [18] also reported a small increase in NaCl toxicity upon the addition of Na2SO4, again attributed to additive toxicity of the two salts.
To more completely evaluate the influence of particular dilution water characteristics on the toxicity of major ion salts, we tested selected salts in ALSW modified to provide different ratios of certain ion pairs, based on the tails of the distributions of these ratios in our analysis of USGS data. Waters with high Ca:Mg (13.6 by mass) and low Ca:Mg (0.27) ratios (Table 2, Set 5) were created by changing the salts added to LSW to achieve the target ratios without altering concentrations of any other ions in the water (Table 1). Similar approaches were used to create waters with high and low Cl:SO4 ratios (4.2 and 0.072 by mass) and high and low Na:K ratios (38 and 0.19 by mass) (Table 2, Sets 6 and 7). Some of these waters required that LSW be initially diluted with DIW to lower the concentrations of a target ion to its lowest value, followed by addition of salts to match the ALSW chemistry except for the ion pair being manipulated (Table 1). Where required, the CO2-aided dissolution of CaCO3 was used to restore alkalinity to waters based on diluted LSW.
Set 8 and 9: Effects of manipulating alkalinity and pH
The effects of pH and alkalinity were evaluated in two ways. First, the alkalinity of ALSW was reduced to 10 mg CaCO3/L and raised to 90 mg CaCO3/L (resulting, respectively, in pHs of 7.3-7.5 and 8.1-8.3) (Table 2, Set 8). Alkalinity was raised simply by adding NaHCO3, whereas it was lowered by diluting LSW and adding salts to restore all cations and Cl to their same values as in the high alkalinity water, with SO4 replacing the alkalinity (Table 1). Second, pH was reduced to approximately 6.8 and raised to approximately 8.5 (Table 2, Set 9) without altering alkalinity or other ions by bubbling the ALSW used in test solution preparation with air containing 1% CO2 and 0% CO2, respectively, and enclosing test vessels in sealed chambers containing these same CO2 concentrations [27].
Set 10: Effect of sodium on potassium toxicity
Based on the results of the tests contrasting MHRW and ALSW (Set 3), we hypothesized that the toxicity of K salts was dependent on the Na concentration in the dilution water. To evaluate this hypothesis, the toxicity of KCl was tested in 6 different dilution waters with Na ranging from 1.6 mg/L to 300 mg/L (Table 2, Set 10). Test waters were prepared by modifying the formulation of ALSW to have no added Na and Cl, then adding NaCl to achieve the desired levels of Na (Table 1).
Set 11: Effects of time-dependent calcium precipitation
Testing of the toxicity of NaHCO3 and MgCO3 resulted in oversaturation of CaCO3 and possible loss of Ca from solution, which would affect toxicity given the established ameliorative effect of Ca. Although Ca was monitored in these tests, the sampling was too limited to precisely determine the Ca concentration to associate with the LC50. Therefore, an additional experiment was conducted to more thoroughly characterize the dependence of any Ca loss on time and test salt concentration. This was a 2×2×2 factorial experiment, the 3 factors being test salt (NaHCO3, MgCO3), dilution water (1x ALSW, 3x ALSW), and solution age (freshly prepared vs. aged for 48 h before introducing test organisms) (Table 2, Set 11). Extra replicate test cups were prepared to allow sampling of Ca at multiple times and treatment concentrations. This experiment provided not only NaHCO3 and MgCO3 LC50s from better monitored tests, but also data with which to better estimate Ca concentrations at the LC50 in other CO3/HCO3 salt tests.
Set 12: Toxicities of gluconate salts and mannitol
As noted earlier, charge balance requires including both cations and anions in any testing of ion toxicity, making it more difficult to infer the relative toxicities of the individual ions. In addition, results of other experiments in the present study led us to hypothesize that the toxicity of some salts might be related to their effect on the osmolarity of the test solution and thus on the osmotic gradient the organism experiences. To provide more information on the role of the individual cations, we tested the toxicity of the gluconate salts of Na, Ca, and Mg (Table 2, Set 12). Gluconate is an organic ion that is not expected to be absorbed appreciably, and thus should act only through its effect on osmotic potential or on charge gradients, providing an informative contrast to Cl, SO4, and HCO3/CO3. This experiment also evaluated the toxicity of mannitol, a sugar alcohol also not expected to be appreciably absorbed, enabling us to manipulate the external osmolarity without adding any ions. NaCl was also included as a reference major ion salt.
Toxicity test procedures
Salts for all twelve combinations of the 4 major cations and 3 major anions; gluconate salts of Na, Ca, and Mg; and mannitol were obtained from Sigma-Aldrich Chemical Company (Sigma-Aldrich Chemical Co.) or Fisher Scientific (Thermo Fisher Scientific). All chemicals were ACS reagent grade or better, with the exception of MgCO3 which was specified as meeting USP requirements. The certificate of analysis for this salt was used to determine the ratio of MgCO3 to total salt weight for computing nominal MgCO3 concentrations. This certificate of analysis also specified the Ca content to be 0.73% of the Mg content, enough to appreciably affect the background Ca, and this was used to adjust the nominal Ca background concentration for tests on the toxicity of MgCO3.
Test organisms were <24 h old Ceriodaphnia dubia obtained from in-house cultures. Most test organisms were cultured in MHRW, but organisms for testing the effects of acclimation were also cultured in the dilution waters for the tests (for a least 2 wk prior to the tests), and some tests late in the present study were conducted with organisms cultured in ALSW after a switch of our culture to this water. The culture water for each toxicity test is indicated in Appendix I. General culture procedures followed those described by USEPA [24].
Static 48-h toxicity tests were conducted in 30-ml plastic cups (Berry Plastics Corporation) filled with 10 ml test solution and held in polystyrene boards with holes sized to the cups. These boards were floated in a temperature-controlled water bath with a glass sheet covering all test cups. Because preliminary studies indicated that the response curves for major ions salts were quite steep, we used closely spaced exposure concentrations, with each test concentration being approximately 80% of the next higher. Test solutions were prepared by combining the applicable salt and dilution water to achieve the highest test concentration (100%). From this, 2 additional solutions, 80% and 64% were prepared by dilution of the first; then, each of these 3 solutions was serially diluted by 0.5x to produce a total of 9 to 12 exposure concentrations, plus a dilution water control. The highest salt concentration in each test varied according to its expected toxicity (based on a combination of preliminary testing, any preceding experiments, and literature values), but all tests used the same relative dilution spacing. Most experiments were structured to compare toxicity under 2 related conditions (e.g., high and low Ca:Mg); tests were assigned to experiments such that both conditions would be tested simultaneously for a particular salt, using the same preparation of dilution water and the same cohort of test organisms.
All salts except CaCO3, MgCO3, and CaSO4 could be easily dissolved at concentrations high enough to cause mortality. For CaCO3 and MgCO3, the CO2 procedure in the description of Set 4 was used to dissolve the salts. After adjusting to pH 7.8, MgCO3 solutions so prepared were stable for 48 h at lethal concentrations, thereby allowing for testing as for other salts. However, a 15 mM (1500 mg/L) CaCO3 solution dissolved in ALSW using CO2 showed substantial precipitation and settling once the pH was raised, with the total concentration being only 6.5 mM at test start, and dropping to 2.4 mM (2.2 mM dissolved) after 24 h, and to 1.2 mM after 48 h, with no mortality observed. For CaSO4, consistent with the findings of Mount et al. [3], a saturated solution at 16.2 mM (2200 mg CaSO4/L) was not acutely toxic to C. dubia in MHRW or ALSW. Based on these results, no further testing was conducted with CaSO4 or CaCO3.
Test cups were usually prepared in duplicate, but in a few tests with CO3/HCO3 salts more replicates were used to provide test solution for more monitoring of alkalinity or calcium than in other tests. Each cup received food in the form of 100 μl of a 50:50 mixture of YCT (a mixture of yeast, cereal leaves, and trout chow [24]) and algae (Pseudokirchneriella subcapitata at 3.5 × 107 cells/ml). Food was added based on previous work indicating that it had minimal effect on toxicity of major ions [3] and to avoid possible starvation stress. Test temperature was 25 ± 1 C with fluorescent lighting on a 16:8 h (light:dark) photoperiod. Five C. dubia neonates were added to each cup, and survival was determined after 24 and 48 h of exposure; death was defined as no visible movement after gentle prodding and at least 10 s of observation.
Exposure monitoring
Temperature was monitored daily in test cups from each simultaneous test, and continuously in the surrounding water bath. Hardness and alkalinity of dilution waters in every test were measured by titration [28]. For tests with MgCO3 and NaHCO3, alkalinity was also measured in the highest test concentration. Conductivity was used to verify that dilution of the salt solutions was done properly, and was measured (Model 2052, Amber Science, Inc.) in each treatment at the start of the exposure, in every treatment that had 100% mortality after 24 h, and in all remaining treatments at 48 h. pH was measured (Beckman Coulter PH150 with A57198 probe; Beckman Coulter, Inc.) at the start of each test in the highest concentration and control (in all concentrations for tests with CO3/HCO3 salts), in every concentration that had 100% mortality at 24 h, and in all remaining treatments at 48 h. Dissolved oxygen was measured (Model #58, Yellow Springs Instruments) in the highest concentration and control at the start of the test, in the highest and lowest concentration with 100% mortality at 24 h, and in two arbitrarily selected treatments at 48 h.
Because of the large number of exposure treatments (circa 1500), it was not practical to measure major ion concentrations in every treatment. Instead, the analytical sampling program was structured with two primary purposes: 1) to verify that the dilution waters and highest concentrations were prepared properly and 2) to verify the exposure concentrations near the LC50. Accordingly, analytical samples were collected at the start of exposure from each dilution water and from the highest concentration of each salt in each dilution water. In addition, analytical samples were collected from each exposure series after 24 h in the lowest concentration with 100% mortality, and at 48 h from the concentration nearest the LC50. Beyond this routine sampling program, some tests included additional sampling from intermediate treatments at the beginning and end of exposure to verify that exposure concentrations were stable over the course of the exposure. Every dilution water was analyzed for all 4 cations plus Cl and SO4. Because cation analysis was more time-efficient than anion analysis, exposure concentrations of the salt tested were verified by analyzing for its cation. For tests of the toxicity of CO3/HCO3 salts, which caused oversaturation of CaCO3 even at the low Ca concentrations in dilution water, Ca concentrations were also measured. In the Ca precipitation experiment with NaHCO3 and MgCO3 (Set 11), extra test chambers were used to monitor Ca at 0, 24, and 48 h at the 25%, 50%, and 100% treatment concentrations, in addition to the cation samples normally taken.
For cation analysis, filtered (0.45 μm nylon syringe filter, Grainger) and unfiltered samples were collected in early experiments, but these analyses consistently showed no significant difference between the two, so later cation samples were not filtered, except for tests with NaHCO3 and MgCO3 in which CaCO3 precipitation was a concern. Samples for cation analysis were acidified by adding 0.2% (v/v) concentrated HNO3 and held at room temperature; for tests with NaHCO3 and MgCO3, this amount of acid was increased by an amount calculated to neutralize the extra alkalinity. Cation measurements were made using an Agilent 240 FS flame atomic absorption spectrophotometer (Agilent Technologies), calibrated with a blank and a series of 5 standards, and verified with a quality control standard and an independent calibration standard at both the beginning and end of the run.
Cl and SO4 concentrations were determined on samples filtered into a polypropylene centrifuge tube, stored under refrigeration and analyzed within 28 d. Quantifications were made using a Dionex DX600 Ion Chromatograph with an AS50 autosampler, an LC25 chromatography oven, ED50 electrochemical detector and GP50 gradient pump (Thermo Fisher Scientific). A typical instrument run included a blank, a series of 8 standards, and a blank spike and a quality control standard analyzed at both the beginning and the end of the run. Thirteen percent of all ion analyses were run in duplicate; the average relative percent difference was 1.6% (maximum 8%; standard deviation 1.6%).
Across all test solutions (i.e., samples other than controls) of the ten major ion salts for which LC50s were determined, comparison of measured and nominal cation concentrations averaged 98.7% with a standard deviation of 4.9% (n = 329). Individual results ranged from 83 to 116% of nominal, and 95% fell between 90% and 110% of nominal. Conductivity measurements on all treatments confirmed that the intended gradient of exposure existed among samples not sampled for cation analysis. Based on these results, and the complexity of estimating concentrations in test solutions that were not directly measured, nominal exposure concentrations were used for calculating LC50 values.
Analysis of dilution waters yielded a similarly high average agreement between the measured cation concentrations and nominal values based on past reported measurements (99.3%; n = 313), but a higher standard deviation of 10.2%. 97% of values fell between 80% and 120% of nominal and 79% of samples fell between 90% and 110% of nominal. This higher variability is associated with the much lower ion concentrations in control waters, which can inflate percentage error values; in the more dilute waters, K in particular was at very low concentrations, sometimes lower than can be confidently quantified by the methods used (e.g., <0.5 mg/L). Wherever measured concentrations deviated more than 20% from nominal (n=8), all data from that test were reviewed and in each instance, the information suggested the large deviation was likely spurious (e.g., concentrations of all other ions measured in the same water were close to nominal). Accordingly, ion concentrations in dilution water were also assumed to be equal to nominal in the data analyses, except for Ca concentrations in tests on the toxicity of CO3/HCO3 salts, in which case Ca was based on measured concentrations. Because these Ca concentrations varied with time and added salt concentration, this involved interpolation of Ca measurements that is further discussed in the supplemental material
Data analysis
As noted above, concentration response curves were extremely steep, so that even with the closely spaced exposure concentrations (0.8x), it was common for there to be only one (or no) exposure treatment with partial survival less than that attributable to background (control) mortality. For such tests, continuous concentration-effect models cannot be fit to provide point estimates for LC50s. Consequently, a tiered approach was used, based on the type of calculation the data would support. For each test, a background mortality range was first defined as all treatments up to the highest concentration at which the fraction of mortality was no greater than at any lower concentration. The number of treatments with partial mortality (i.e., above this background mortality range and below the lowest treatment with complete mortality) was then determined and used to select the type of analysis.
For tests with at least 2 partial mortalities that increased with concentration, a tolerance distribution analysis was conducted, using a 3-parameter model that included a background survival parameter and assumed a log-normal distribution for the lethal concentrations. Parameters were estimated by maximum likelihood analysis using custom software written with Intel Professional Fortran Composer XE 2011 (Intel Corporation). Of the 149 LC50s reported here, 82 supported calculation of such “probit LC50s”. 95% confidence limits were calculated using the likelihood ratio method [29]. Statistical significance of LC50 differences between treatments within an experiment was assessed based on these confidence limits not overlapping, so that such differences have a significance level of at least 95%.
For tests with insufficient partial mortalities for this probit LC50 analysis, the same likelihood ratio method was used to calculate confidence limits for the LC50 (such confidence limits can be assigned even when a unique point estimate for the LC50 cannot be calculated). For these tests, we assigned the geometric mean of these confidence limits to be the point estimate for the LC50 (“midpoint LC50s”). For cases in which there were no partial mortalities, these confidence limits are the bracketing concentrations (upper end of the background mortality range and the lowest treatment with complete mortality) and the LC50 is equivalent to linear interpolation of survival versus log concentration between these 2 concentrations. To test the performance of this methodology when tolerance distribution assumptions are met, it was applied to simulated data sets generated based on the range of observed parameters, which demonstrated the bias for LC50 estimation to be <1% and the confidence limits to equal or exceed 95%.
These LC50 calculations were based on the nominal concentration of added salt, without consideration of background ion concentrations, because different ion ratios in the dilution water and the added salt make it impossible to express total ion concentrations as an equivalent concentration of salt. Initial comparisons of how dilution water differences affect salt toxicity are also on the basis of added salt for the same reason. Background concentrations of the component ions of the test salt were <5% in all but 6 tests and never >10%; these had negligible impact on comparisons based on added salt except as noted in the Results. However, for further analyses of the aggregated data, exposure metrics included both background and added ions.
Although test preparation and chemical analysis were based on weight of the salts and the various ions, this is not a good basis for comparing the relative toxicity of the individual salts and ions, because the toxic action of these ions should be related to their molarity, not their mass. As such, salt and ion concentrations were converted to mM, which is the primary basis for toxicity comparisons of the added salts. In addition, evaluation of the dosimetry at these elevated ion concentrations should consider formation of complexes between the various cations and anions, which would affect their chemical reactivities and thus their toxicities. It should also consider that the high ionic strength of these solutions will also reduce the reactivity of the ions, as represented in lower activity coefficients, and that exposure metrics thus would be best expressed as chemical activity rather than molarity. To this end, the ion composition at each LC50 was analyzed using the chemical speciation program Visual MINTEQ (version 3.0, www2.lwr.kth.se/English/Oursoftware/vminteq) to estimate chemical speciation and the chemical activity of each chemical species. The osmolarity of each LC50 solution was also calculated based on these MINTEQ activity estimates using the method described by Robinson and Stokes [30].
RESULTS AND DISCUSSION
For each test (n=149), added-salt LC50 values with confidence limits are provided in Appendix I as both mg/L and mM, along with total ion concentrations and osmolarities at these LC50s. The pH associated with each LC50 at the end of exposure is also provided based on measurements in treatments bracketing the LC50. All reported tests had conductivity measurements that varied across treatments in a manner consistent with the intended exposure concentrations, and LC50s directly based on these conductivities are also provided in Appendix I. Dissolved oxygen was always above 7.5 mg/L, and temperature was always within 1°C of 25°C. Measurements made at the beginning and end of experiments indicated that evapoconcentration during the experiment was low (<5%). Over the background mortality range, as defined in Data analysis, survival was ≥90% in all toxicity tests and averaged 98% overall.
Effects of acclimation to dilution water
Figure 1 compares LC50s for C. dubia cultured in dilute solutions to those from our standard culture waters. Culturing organisms in 1/3x ALSW or 1/3x MHRW had no significant effect on the toxicities of NaCl and MgCl2, either in test waters at these dilutions or (for NaCl only) in 1x ALSW, the LC50 estimates differing by 20% or less. Culturing organisms in 1/8x MHRW also had no effect on the toxicities of NaCl, NaHCO3, and CaCl2, the LC50s differing by no more than 10%. However, for Na2SO4, KCl, MgCl2, and MgSO4, LC50s for 1/8x MHRW test water are 20-45% lower for organisms cultured in 1/8x MHRW than those cultured in 1x MHRW, these differences being statistically significant except for MgCl2, for which the LC50 is uncertain due to highly variable responses in this dilute test water.
Figure 1.
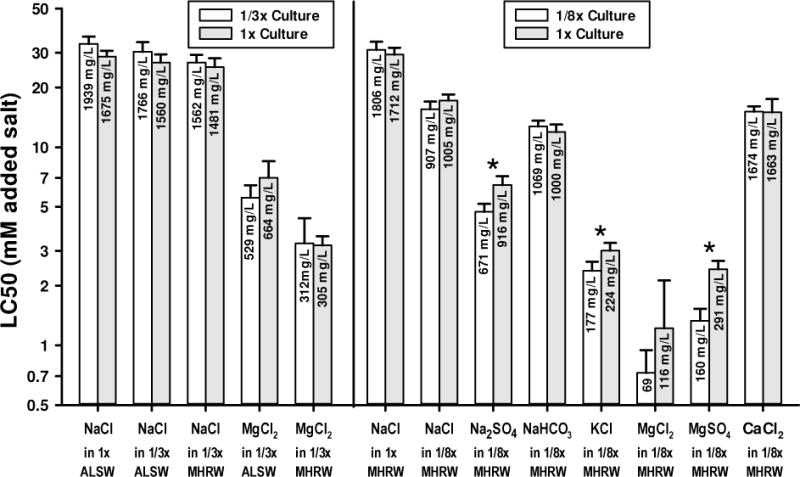
Effects of culturing and testing organisms in different strengths of amended Lake Superior water (ALSW) and moderately hard reconstituted water (MHRW) on 48-h LC50s for selected major ion salts to C. dubia. Error bars denote upper 95% confidence limits and asterisks denoted where confidence limits do not overlap.
Thus, “acclimation” to 1/8x MHRW resulted in C. dubia being more, rather than less, sensitive to some salts when tested in this dilute solution. Although both we and Elphick et al. [19] established successful cultures in this water, based on our experience with C. dubia, such a dilute solution appears to be stressful, one indication being the greater variability of organism response to salt toxicity. It is not clear whether this should be considered an artificial stress created by manipulating organisms long adapted to laboratory waters with higher ion concentrations, or a natural consequence for a species not adapted to and not endemic to such dilute waters.
These acclimation experiments were prompted by the work of Elphick et al. [19], who acclimated cultures to dilutions waters (for 2 generations or more) and found that, relative to 1x MHRW, the 7-d NaCl LC50 for C. dubia decreased by 2.1-fold in 1/2x MHRW, 3.6-fold in 1/4x MHRW, and 8.4-fold in 1/8x MHRW. In contrast, for organisms both cultured and tested in the waters, we found only an 9% decrease in the NaCl LC50 between 1x MHRW and 1/3x MHRW and only a 1.9-fold decrease between MHRW and 1/8x MHRW (Figure 1). The lack of culture water effect on our LC50s for NaCl suggests that these differences between the 2 studies were due to other factors, such as the longer duration in Elphick et al. [19] or a variability associated with culturing and/or testing C. dubia in such dilute waters. More recent communication with J. Elphick has indicated that they have observed variability in NaCl LC50s for C. dubia in very dilute test waters.
Overall, we concluded that the absence of dilution water-specific culturing was not a substantial factor for most of our experiments, but that data for the 1/8x MHRW waters should be interpreted with caution. The results presented hereafter will be only for organisms from our standard culture waters and will consider LC50s for the most dilute test waters to be more useful for qualitative insights into ion toxicity rather than establishing quantitative relationships. Any quantitative uncertainties of LC50s for such dilute waters are probably of limited practical concern because few surface waters will likely experience elevations of a single salt to toxic levels while other ions remain at such low levels.
Our culture experiments only addressed more dilute waters than our standard culture water, because discrepancies of our results from that of Elphick et al. [19] were noted for those waters. Although we did not evaluate effects of culturing at higher ion concentrations, the relationship of our NaCl LC50s to Ca concentration shows good agreement with that of Soucek et al. [18] and Elphick et al. [19] who did culture organisms in their different test waters. Also, Soucek and Kennedy [16] reported only small and statistically nonsignificant differences among Na2SO4 LC50s in MHRW for C. dubia cultured in MHRW versus MHRW with elevated Na2SO4 levels. This suggests that acclimation to higher ion concentrations may not be a significant factor, but more study would be needed to reach a definitive conclusion.
Salt toxicity in MHRW versus ALSW
Figure 2 compares LC50s for ten major ion salts tested in both ALSW and MHRW. For NaCl, MgCl2, CaCl2, Na2SO4, MgSO4, and MgCO3, LC50 differences are <15% and not statistically significant. For NaHCO3, the LC50 is 20% lower for MHRW and is statistically significant, but this test involved significant precipitation of Ca and this precipitation was more pronounced in the MHRW test (Appendix I). Given previous studies on the effect of hardness on salt toxicity [16–21], such a Ca difference would contribute to the observed LC50 difference. The fact that MHRW provides a higher background Na concentration and alkalinity would also contribute to the difference in LC50s expressed in terms of added NaHCO3.
Figure 2.
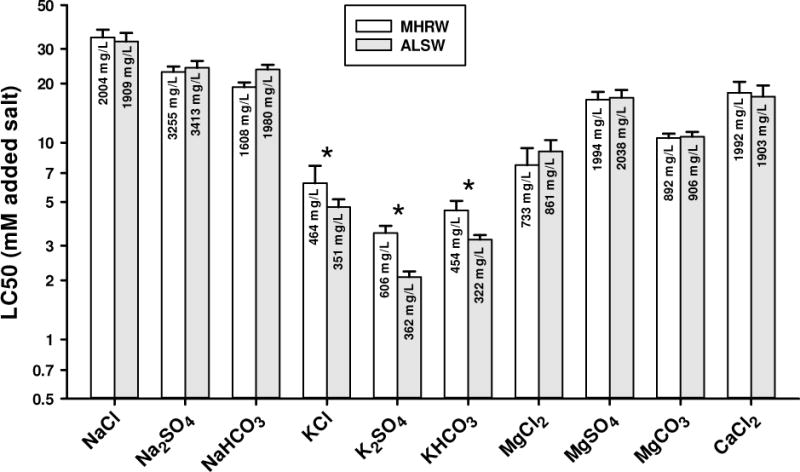
48-h LC50s for major ion salts to C. dubia in moderately hard reconstituted water (MHRW) and amended Lake Superior water (ALSW). Error bars denote upper 95% confidence limits and asterisks denoted where confidence limits do not overlap.
Only for the 3 K salts are there clear, substantial differences between the 2 dilution waters. The LC50s are 32-67% higher for MHRW than for ALSW and these differences were all statistically significant. Notable compositional differences between these waters (Table 1) include 4-fold higher Na in MHRW (due to the use of NaHCO3 to add alkalinity), 3-fold higher Mg in MHRW (but similar Ca), 5-fold higher SO4, and 3-fold lower Cl. The higher Na for MHRW is of particular interest because Na and K are linked physiologically through the central role of Na-K ATPases in ion regulation and other key cellular processes [2]. Additional information regarding this issue is provided in Effects of sodium on potassium toxicity.
Comparing the LC50s in Figure 2 across salts also supports some inferences regarding the contributions of individual ions to toxicity. On a total molarity basis, the Na salts are significantly less toxic than salts of other cations with the same anion. NaCl is also significantly less toxic than the other Na salts on a total molarity basis. For Na2SO4, the greater toxicity could be in part due to it containing 2, instead of 1, Na atoms, in addition to any anion effects. For NaHCO3, the greater toxicity should be in part due to lower Ca concentrations (Appendix I) from the CaCO3 precipitation induced by this salt. These differences among Na salts will be further addressed below in Effects of calcium on the toxicities of sodium salts.
The K salts are much more toxic than the corresponding salts with other cations, especially Na (Figure 2). The ratio of the Na salt LC50 to the K salt LC50 ranges across the different anions from 4.2 to 6.6 for MHRW and from 5.8 to 9.1 for ALSW, with smaller ratios for MHRW being due to the aforementioned dilution water effect on K toxicity. These large ratios indicate that K is the principal source of toxicity for these salts, because the anion concentrations at the LC50s for K salts are in all cases a small fraction of those present at the LC50s for the Na salts. The LC50 (mM) for K2SO4 is approximately half that of KCl, consistent with toxicity being due to the molarity of K rather than the molarity of the salt. Factors controlling K salt toxicity are explored further below in Effects of sodium on potassium toxicity.
Although less toxic than the K salts in these test waters, the Mg salts are also significantly more toxic than their corresponding Na salts (ratios of 1.4 to 4.4, Figure 2), suggesting that, like K, Mg is also more important to the toxicity of these salts than are the anions. On a total molarity basis, MgSO4 was approximately 2-fold less toxic than MgCl2. Given the same Mg stoichiometry in each salt, this suggests that the toxicity of Mg is differentially modified by the anions, perhaps by SO4 complexing Mg and thus reducing its toxicity. Unlike for NaHCO3, there was no Ca precipitation in the tests with MgCO3 (Appendix I; and see supplemental material for more information on Ca precipitation relationships), and MgCO3 was only slightly less toxic than MgCl2. The relationship of these toxicity differences among Mg salts to ion speciation and activity are discussed later in Effects of calcium on the toxicities of magnesium salts.
For Ca, only the Cl salt was acutely toxic below its solubility. On a total molarity basis, CaCl2 is more toxic (by 1.9 fold) than NaCl (Figure 2); however, because CaCl2 has twice as many chlorides as NaCl, this result by itself provides no clear evidence regarding relative ion toxicities.
Salt toxicity in different strengths of ALSW
Figure 3 shows the effect of varying all ions in dilution water by comparing LC50s for 1/3x, 1x, and 3x ALSW. These comparisons were developed by combining data from simultaneous tests in 1/3x and 3x ALSW with the 1x ALSW data generated separately (Figure 2). NaCl and Na2SO4 were also evaluated in an additional experiment with all 3 waters tested simultaneously (marked “dup” on Figure 3); this was done to determine if combining data across experiments might influence conclusions. For the 3x ALSW water, ions in the dilution water equaled between 3 and 8% of the added salt LC50, so that any amelioration of toxicity in this water would be slightly underestimated when based just on the added salt.
Figure 3.
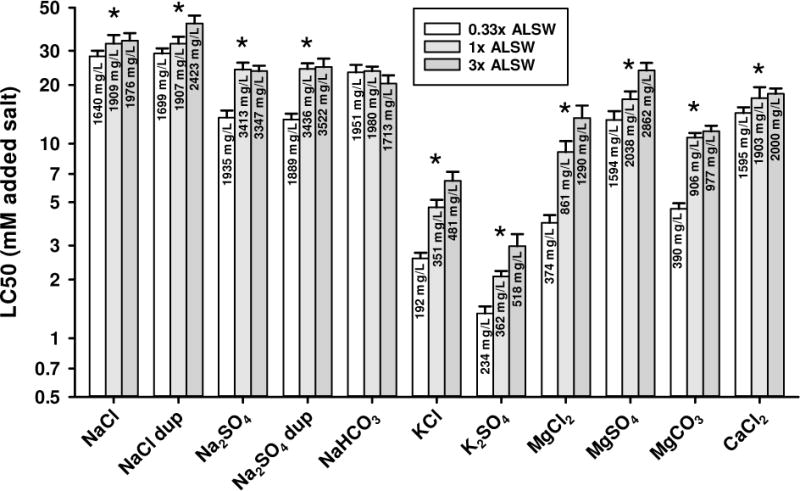
48-h LC50s for selected major ion salts to C. dubia in different strengths of amended Lake Superior water (ALSW). Error bars denote upper 95% confidence limits and asterisks denoted where confidence limits do not overlap for 1/3x and 3x ALSW.
For the K salts, there is a 2.3- to 2.5-fold increase in LC50s from 1/3x to 3x ALSW (Figure 3), with the LC50 for 1x ALSW being near the midpoint of these ranges. Because all ions varied proportionately between these dilution waters, the specific factors responsible for these differences are not directly identifiable. However, the decreased toxicity from 1x to 3x ALSW (entailing a 3-fold change in both Na and hardness), is similar to the change between ALSW and MHRW (Figure 2), between which Na differs 4-fold but hardness is just 1.5-fold different and Ca is virtually constant, suggesting that Na is more important than other cations to these toxicity differences.
For the Mg salts, there are substantial increases in the LC50s from 1/3x to 3x ALSW, totaling 3.4-, 2.5-, and 1.8-fold for MgCl2, MgCO3, and MgSO4, respectively (Figure 3). For MgCO3, the lack of difference between 1x and 3x ALSW is associated with substantial precipitation of CaCO3 in 3x ALSW (Appendix I). For MgCl2 and MgSO4, the LC50 increases between 1x and 3x ALSW are similar to the corresponding K salts, but because these Mg salts show no LC50 difference between ALSW and MHRW whereas the K salts do, the underlying reasons must be different. These effects of dilution water strength should reflect the effects of the anions on the speciation of Mg and Ca; whether such consideration of speciation accounts well for LC50 differences among dilution waters, and among the salts, is addressed later.
For the Na salts, LC50s show responses to ALSW strength that are different from each other and from the K and Mg salts (Figure 3). The LC50 for Na2SO4 increases by 1.8-fold from 1/3x to 3x ALSW in both replicate tests, similar to MgSO4 and K2SO4, but the increase is all between 1/3x to 1x ALSW. The LC50 for NaCl increases much less from 1/3x to 3x ALSW (1.2- and 1.4-fold in the different replicates). For NaHCO3, there is a non-significant but slight decrease of the LC50 from 1/3x to 3x ALSW. This is again associated with precipitation of Ca, this precipitation being more extensive in the higher ALSW strengths, leading to Ca concentrations actually being similar across these waters (Appendix I) despite the original formulations being 9-fold different. Possible reasons for these LC50 differences across Na salts, including chemical speciation and the ameliorative effects of Ca, are further addressed below in Effects of calcium on the toxicities of sodium salts.
For CaCl2, there is an increase of only 1.2-fold in the LC50 from 1/3x to 3x ALSW of marginal statistical significance. This small dependence of Ca toxicity on ALSW strength, compared to the large dependence of Mg toxicity, results in the toxicity of MgCl2 being very similar to CaCl2 in 3x ALSW (a factor of 1.3 difference) and very different in 1/3x ALSW (a factor of 3.7 difference). To the extent that the toxicity of MgCl2, and other salts, depend on Ca, judging relative potencies is best done at high, but non-toxic, Ca concentrations because any adverse consequences of low Ca cannot, by definition, affect Ca toxicity. Thus, Ca and Mg should be considered to have similar intrinsic toxicities.
Effects of manipulating specific ion ratios
To evaluate the factors behind the responses in the 1/3x versus 3x ALSW and MHRW versus ALSW, 3 sets of experiments were conducted in which the Ca:Mg, Cl:SO4, and Na:K ratios were manipulated while other ions remained constant. The LC50s for MgCl2, Na2SO4, and MgSO4 are at least 2-fold greater at high Ca:Mg than low (Figure 4). Because total hardness and all other ion concentrations were the same in high Ca:Mg and low Ca:Mg waters, these differences are presumably due to Ca, rather than overall hardness. These same salts have large LC50 differences in the 1/3x versus 3x ALSW comparisons (Figure 3), for which Ca also changed substantially (in concert with other ions), but small LC50 differences for the MHRW versus ALSW comparisons (Figure 2), for which Ca was essentially the same but other ions varied markedly. Overall, this suggests that Ca is the important factor in differences among these various dilution waters for these salts. NaCl and NaHCO3 have smaller (20-30%) increases in the LC50s at high Ca:Mg (Figure 4), but the magnitude of these effects are also consistent with the results shown in Figures 2 and 3 and their associated Ca concentrations (Appendix I). In contrast, although KCl has a higher (15%) LC50 at high Ca:Mg, this is not statistically significant and is much smaller than the LC50 differences for both the MHRW and ALSW comparison (Figure 2) and the 1/3x to 3x ALSW comparison (Figure 3) for this salt. This reinforces the earlier suggestion of the importance of Na to K salt toxicity.
Figure 4.
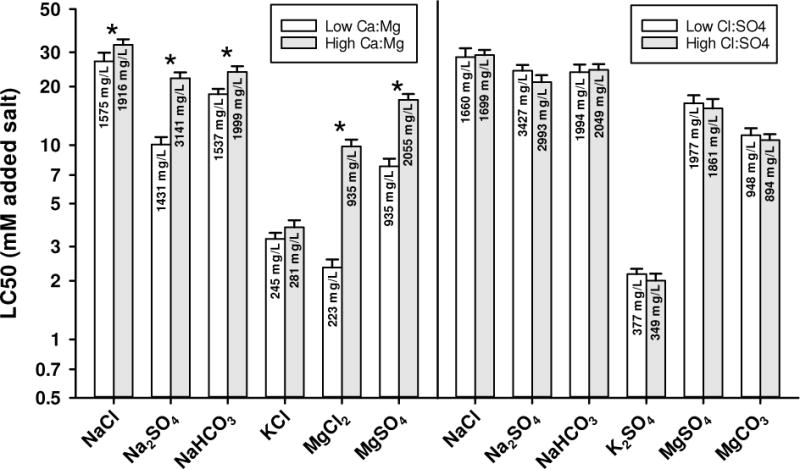
48-h LC50s for selected major ion salts to C. dubia in amended Lake Superior water (ALSW) modified to have different ratios of Ca to Mg and different ratios of Cl to SO4. Error bars denote upper 95% confidence limits and asterisks denoted where confidence limits do not overlap.
When the Cl:SO4 ratio was altered (Figure 4), no significant differences in LC50s (<15%) exist for any of the tested salts (Table 2), indicating these anions are not important characteristics of dilution water for major ion toxicity to C. dubia. When the Na:K ratio was altered (Appendix I), there also are no significant effects on the LC50s of the tested salts. No K salts were included in Na:K ratio studies because the effect of Na on K salt toxicity was evaluated separately.
Effects of manipulating alkalinity and pH
Two sets of toxicity tests evaluated the effects of dilution water alkalinity and pH on salt toxicity (Figure 5). In the first set, alkalinity was decreased (by dilution, then restoring other ions) and increased (by NaHCO3 addition) relative to 1x ALSW water, resulting in pHs of approximately 7.4 and 8.3 in the low and high alkalinity treatments, respectively, compared to a typical pH of approximately 7.9 in ALSW. The effects of these combined changes in alkalinity and pH on LC50s are <10% and not statistically significant for NaCl, Na2SO4, and MgSO4, but are significant for MgCl2 and KCl. For MgCl2, increasing the alkalinity decreased the LC50 by 1.4-fold. This test was repeated, yielding virtually identical results (Appendix I). We initially speculated that complexation by the higher HCO3/CO3 decreased Ca activity and thus increased toxicity. However, to maintain a constant Na concentration in these tests, the low alkalinity water had higher SO4, which also complexes Ca. As a result, the calculated Ca activity in the high alkalinity treatment is actually higher than in the low alkalinity treatment, so it would not explain the observed LC50 shift. We similarly have no explanation for the lower LC50 (20%) for KCl at high alkalinity. One area of needed future work is direct ion activity measurements to verify speciation model calculations before further exploring the cause of these effects.
Figure 5.
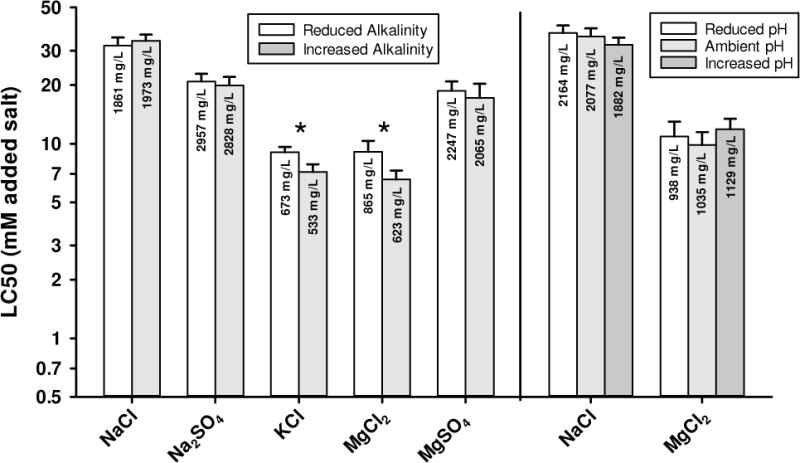
48-h LC50s for selected major ion salts to C. dubia in amended Lake Superior water (ALSW) modified to have different alkalinities and pH, or different pH with alkalinity unchanged. Error bars denote upper 95% confidence limits and asterisks denoted where confidence limits do not overlap.
In the second set of tests, pH was manipulated by CO2 partial pressure, leaving alkalinity and all other ions constant as pH varied. For this experiment, pH had no effect on NaCl toxicity over the pH range 6.75 to 8.20 or on MgCl2 toxicity over the pH range 6.75 to 8.50 (Figure 5). (The high pH treatment for the NaCl tests had lower pH than for the MgCl2 tests due to an air leak in the chamber for the low CO2 treatment, so that the range of tested pHs was less than intended.) This insensitivity of toxicity to pH manipulation suggests that something other than pH itself is responsible for the effects of altered alkalinity on MgCl2 toxicity noted in the previous experiment. However, although these tests at different pHs and alkalinities indicate limited or no effect of these factors on the toxicity of SO4 and Cl salts, they do not encompass the much higher pHs and buffering levels of the tests with HCO3/CO3 salts. Therefore, pH might still have a role in the relative toxicity of those salts.
Effects of sodium on potassium toxicity
To evaluate the suspected effect of Na on the toxicity of K salts, 6 dilution waters were prepared, each having the composition of ALSW except that the Na+ concentrations were varied to be 0.070 (background in LSW), 0.13, 0.43, 1.3, 4.3, and 13 mM (1.62, 3, 10, 30, 100, and 300 mg/L), added as NaCl. NaCl was chosen so that the anion was the same as for the toxicant, KCl. The LC50 of KCl differs consistently and substantially across these dilution waters (Figure 6), from a low of 2.05 mM (153 mg/L) at the lowest Na to a high of 10.1 mM (752 mg /L) at the highest Na. The magnitudes of these changes are consistent with the behavior observed for KCl in the MHRW versus ALSW and 1/3x versus 3x ALSW experiments (Figures 2 and 3 and also included in in Figure 6).
Figure 6.
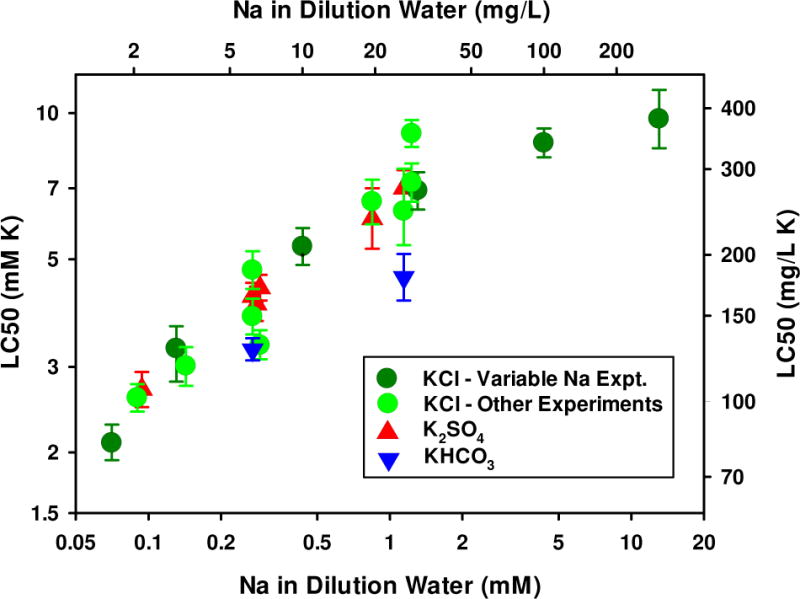
Effect of Na on 48-h LC50s for K salts to C. dubia. Error bars denote 95% confidence limits.
Mount et al. [3] and our initial experiments (Figure 2) demonstrated that K is the most toxic of the major ions to C. dubia and as a result, the effects of K seem to greatly exceed any effect of the different anions. This is demonstrated in Figure 6, which shows agreement of other K salts and dilution waters with the experiment regarding KCl toxicity across different NaCl levels. Note that by compiling data for tests conducted across a variety of dilution water compositions, the correlation of Na with other major ions that might exist in a single experiment is reduced, strengthening the case for Na being the primary factor influencing toxicity of K salts. There might be some lesser effects of other factors, as evidenced by slightly lower LC50s (expressed as K) for KHCO3 than for KCl and K2SO4 (Figure 2, Figure 6). As noted previously, the carbonate tests entailed much higher pH and buffering for which effects are uncertain. However, any such effects appear to be of less consequence than the primary effect of Na on K toxicity.
One implication of the Na-dependence of K toxicity demonstrated here is that the high toxicity of K in typical test waters with low Na may be of limited field relevance because K would be unlikely to be present at toxic concentrations when Na is so low. For example, the K-dominated effluent evaluated by Jop and Askew [11] had approximately 1.5 mM Na, which would put the expected acute LC50 for K at 10 mM or higher (Figure 6). This is similar to the toxicity of Mg salts, and only 2- to 3-fold more toxic than Na salts.
Effects of calcium on the toxicities of sodium salts
Aside from the effect of Na on the toxicity of K, Ca is the only other ion in our study which exerted a substantial influence on the acute toxicity of major ion salts to C. dubia. Several studies have shown hardness to influence major ion toxicity to C. dubia [16–20], though the way in which hardness was manipulated differed across studies. Experiments by Soucek et al. [16–18] varied hardness by adding CaCl2+MgCl2 (for NaCl toxicity tests) or CaSO4+MgSO4 (for Na2SO4 toxicity tests) in fixed ratios and keeping the remaining ions constant, while Elphick et al. [19–20] varied all ions proportionately (as in the 1/8x, 1/3x, and 1x MHRW waters used here). In our experiments, the combination of manipulating all ions simultaneously in some tests and only specific ion pairs in others (at constant hardness), allowed us to conclude that the “hardness effect” on the toxicity of Na salts is primarily an effect of Ca, rather than total hardness or the other ions that co-vary with hardness. The same conclusion was reached by Davies and Hall [21], who manipulated both total hardness and Ca:Mg ratio to show the dominance of Ca in determining toxicity of Na2SO4 to Daphnia magna.
Figure 7 shows the relationship of LC50s to Ca for all our Na salt toxicity tests using organisms cultured in 1x ALSW or MHRW. Each panel considers different exposure metrics for how LC50 and Ca are expressed. As a comparative performance measure for these different metrics, residual standard deviations of the logLC50s (logRSD) around the mean trend of logLC50 with calcium are provided. Mean data trends were calculated by least-square regression of logLC50 vs logCa using Sigmaplot with a model for an exponential rise to a maximum. This is not intended to provide a definitive model for the relationship of toxicity to calcium, but rather only an empirical description of the mean data trend suitable for calculating the logRSD.
Figure 7.
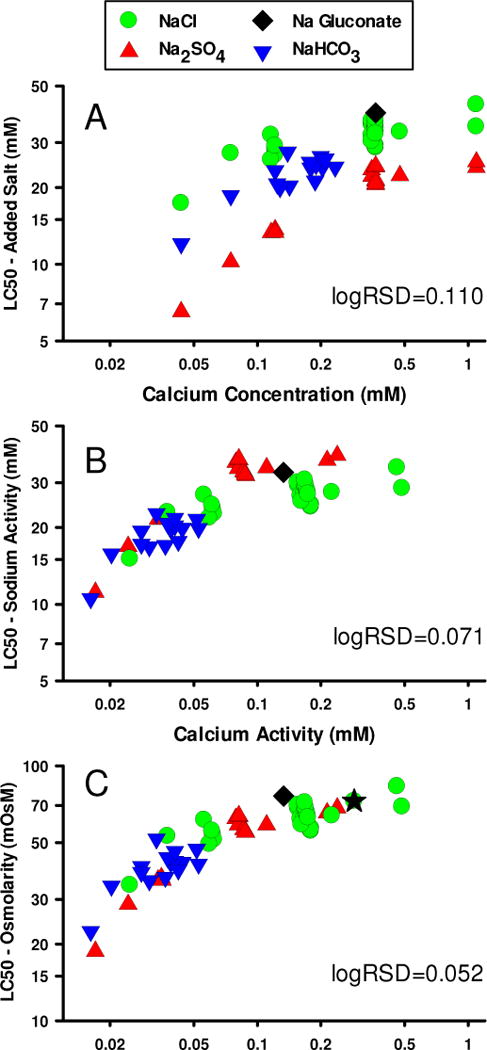
Effect of Ca on 48-h LC50s for Na salts to C. dubia using different exposure metrics. In Panel A, the metrics are LC50 as concentration of added salt and Ca concentration. In Panel B, the metrics are Na and Ca activities. In Panel C, the LC50 metric is changed to osmolarity.
In Figure 7A, LC50s are plotted as mM of added salt and Ca is plotted as total concentration (measured Ca for NaHCO3 tests, nominal for others). Although the 3 inorganic salts have a similar and substantial (2- to 3-fold) dependence on Ca, there is an apparent difference in the relative toxicities of the salts, with LC50s being ordered NaCl ≈ NaGluconate > NaHCO3 > Na2SO4. The logRSD is 0.110, for which ±2 standard deviations (±2SD) corresponds to nearly a 3-fold range in LC50 at a fixed Ca concentrations, indicative of the substantial variability among the different salts.
Because chemical reactions are governed by chemical activity rather than concentration, the relative toxicities of salts and their relationships to Ca should be based on chemical activities rather than total concentrations as in Figure 7A. Activities will differ from total concentrations due to formation of chemical complexes as well as reduced activity coefficients associated with high ion concentrations (especially for the divalent Ca ion), and these factors will differ among the salts and dilution waters. Another issue for comparing toxicities across these salts is that, per mole of salt, Na2SO4 provides twice as many Na ions and 1.5 times as many total ions as do the other salts. To address these issues, in Figure 7B, Ca is plotted as Ca activity and the LC50s are based on the activity of Na. This results in much closer agreement among the salts, with the logRSD reduced to 0.071 and the ±2SD range for LC50 reduced to less than 2-fold. NaCl and NaHCO3 now show similar toxicity, because complexation of Ca by CO3 and HCO3 in the NaHCO3 exposures causes Ca activity to be lower than that in NaCl exposures at the same total Ca concentration. Accounting for Na2SO4 having twice the Na atoms of other Na salts has also made its LC50s more similar to the other salts, but Na2SO4 now appears to be less, rather than more, toxic than NaCl, based on the repeated tests with Na2SO4 and NaCl in 1x ALSW and other waters with similar Ca concentration (i.e., the cluster of red triangles near 0.1 mM Ca activity and green circles near 0.2 mM Ca activity). This continued discrepancy between Na2SO4 and NaCl suggests that the anions play some role in determining toxicity beyond their effects on cation speciation.
A simple metric for expressing the aggregate effect of Na and anions is osmolarity, which reflects the combined, unweighted influence of all dissolved species. Figure 7C provides LC50s on the basis of calculated osmolarity, resulting in excellent agreement among all the tests of Na salt toxicity. The LC50s for the different salts now overlap within experimental variability; the logRSD is reduced to 0.052 and the ±2SD range to 1.6-fold. Further support for using osmolarity as an exposure metric comes from the test conducted with mannitol (black star in Figure 7C); whose LC50 is not significantly different than those of the Na salts even though its only expected effect would be on osmolarity. While the case for osmolarity here is simply correlative and does not establish osmotic potential as the primary stressor, an exposure metric such as osmolarity that includes multiple ions in a non-specific manner is indicated by these data.
Effects of calcium on magnesium toxicity
Figure 8 examines the relationship of LC50s to Ca for all our Mg salt toxicity tests using organisms cultured in 1x ALSW or MHRW. The panels address different exposure metrics parallel to those examined in Figure 7 for Na salts, and also include logRSD as a performance measure for these different expressions of exposure.
Figure 8.
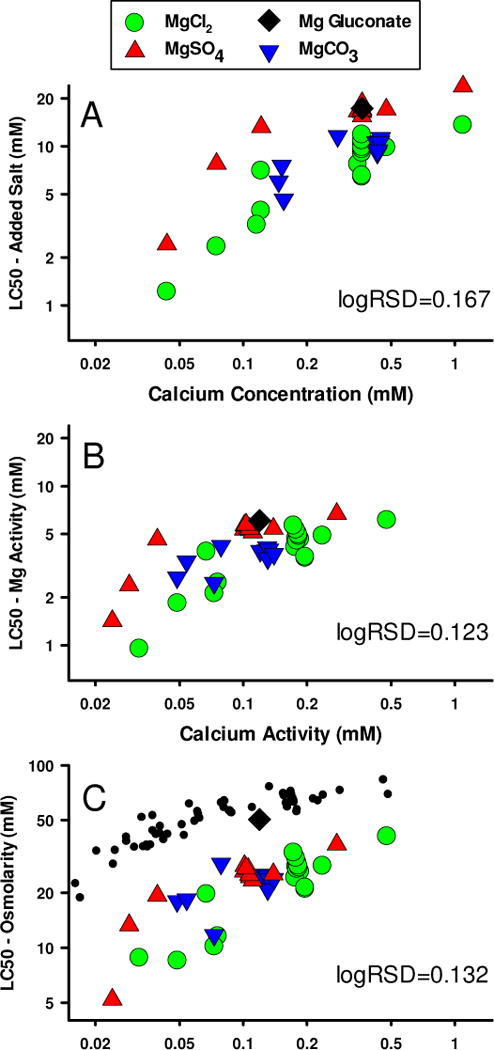
Effect of Ca on 48-h LC50s for Mg salts to C. dubia using different exposure metrics. In Panel A, the metrics are LC50 as concentration of added salt and Ca concentration. In Panel B, the metrics are Mg and Ca activities. In Panel C, the LC50 metric is changed to osmolarity and black dots provide comparison to Na salt data from Figure 7C.
In Figure 8A, LC50s are plotted as mM of added salt and Ca is plotted as total concentration (measured Ca as appropriate for MgCO3 tests, nominal for others). There is an even greater effect of Ca (approximately a 10-fold LC50 range over the 0.05-1.0 mM Ca range) for Mg salts than for Na salts (note scale change from Figure 7). On the basis of total added salt concentrations, the salts have different apparent toxicities, with MgSO4 and Mg gluconate being approximately 2- to 3-fold less toxic than MgCl2 and MgCO3. These differences make the logRSD (0.167) even larger than for Na salts (Figure 7A), corresponding to a ±2SD range of almost 5-fold at a fixed Ca concentration.
Expressing exposure on the basis of chemical activities of Mg and Ca (Figure 8B) reduces the residual variability and the differences between the salts, due to the different degrees of complexation of both Ca and Mg by the different anions. The total range of the LC50s is also less because of a concentration-dependence for complexation and activity coefficients. However, the closer agreement among the data is mainly for Ca activity >0.05 mM; at lower Ca activity, there remains considerable disparity between MgSO4 and MgCl2. As noted earlier, these tests in very dilute test water raised some concerns about variable response and stresses associated with low ions, which might involve effects other than from Mg and Ca. Thus, although basing LC50s on Mg activity does provide a better metric than total Mg concentration, it does not account for all factors of significance. For all the data, the logRSD is reduced to 0.123, but if only the data at Ca activity > 0.05 mM are considered the logRSD is 0.102 and the ±2SD range is then 2.5-fold, half of what is was for LC50s based on total added salt concentration.
Although osmolarity provides a possible unifying exposure metric for Na salts (Figure 7C), it is not a useful metric for Mg salt toxicity (Figure 8C). Using osmolarity to express Mg salt toxicity did not improve, but rather increased the logRSD of the data. More importantly, the osmolarities at the LC50 for Mg salts are 2- to 4-fold lower than those for Na salts at the same Ca activity. This indicates specific action of Mg and is incompatible with using a non-specific, total ion metric such as osmolarity for the toxicity of Mg salts.
Our results indicate a strong effect of Ca on MgSO4 and MgCl2 toxicity – nearly a ten-fold change in LC50s from the Ca concentration in 1/8x MHRW (0.044 mM) to that in 1x MHRW (0.35 mM), with Figure 1 indicating even lower LC50s at 1/8x MHRW if organisms are cultured in this water. This is in contrast to results from van Dam et al. (2010) for an Australian cladoceran (Moinodaphnia macleayi) collected from and cultured in very low ionic strength water (Ca <0.02 mM). In 6-7 d reproductions tests, the MgSO4 EC50 (reproduction) for M. macleayi was 2.6 mM in the culture water, but only 2-fold higher (5.0 mM) when CaSO4 was added to increase the Ca concentration by roughly 20-fold, to 0.34 mM. While there are several possible explanations for the difference in Ca effect for the 2 species, the van Dam (2010) study does reinforce concerns that species like C. dubia tested at very low ionic strength may not respond the same way as species (or strains) naturally adapted to low ionic strength water.
The toxicity of calcium
As noted earlier, due to solubility constraints, only limited testing of Ca salts could be conducted, and conclusions about any specific Ca toxicity could not be made based on simple comparisons of the toxicities of CaCl2 and NaCl in our standard test waters. However, there are some indications that the toxicity of CaCl2 is dominated by the cation, as are the toxicities of K and Mg salts. First, any comparison of the toxicity of CaCl2 to NaCl should be made for NaCl tests in the dilution waters with the highest Ca. Otherwise, the comparison is confounded by the exacerbation of Na toxicity by low Ca. For the CaCl2 tests (Appendix I), osmolarities at the LC50s average 45 mOsM (range 39-52), well below the average osmolarity of 76 mOsM (range 69-83) for the NaCl tests in 3x ALSW. Unless the Cl from these 2 salts acts differently, this indicates some Ca-specific toxicity beyond the toxicity exerted by Na and the various anions (Figure 7). Second, in ALSW, the calculated Ca activity is 7.5 mM for CaCl2 and 8.1 mM for Ca gluconate; such similarity would not be expected if these disparate anions were contributing to toxicity. However, the conclusion that there is some specific toxicity of Ca is tentative and will be further addressed in the next paper in this series.
Relationship of toxicity to conductivity
Figure 9 replots the data from Figures 7 and 8 with LC50s based on conductivity. Given the results already presented for the toxicity of different salts, it is not surprising that these conductivity LC50s also show a strong dependence of toxicity on Ca and substantial variation across the different salts. The total range of conductivity LC50s is around 10-fold, with up to a 5-fold range among salts at the same Ca concentration. This indicates that assessment approaches using conductivity as an exposure metric should have a restricted scope of applicability regarding relative ion concentrations, as is the case for the conductivity benchmark of Cormier et al. [14,15].
Figure 9.
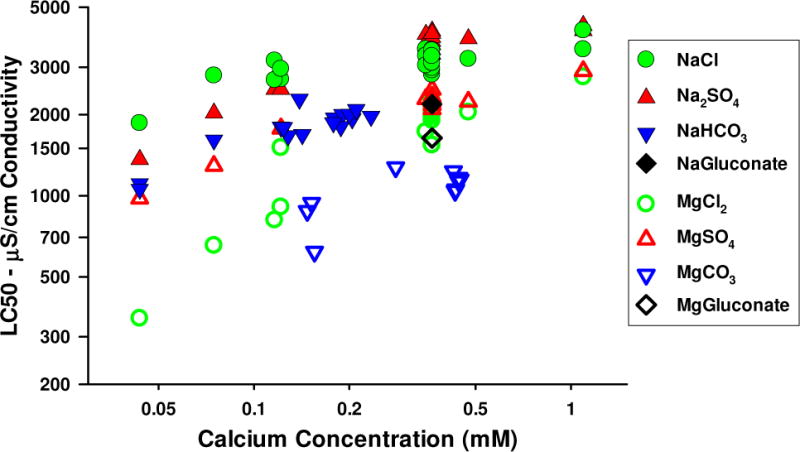
48-h LC50s of Na and Mg salts expressed as conductivity.
SUMMARY AND IMPLICATIONS
In the present study, the key influences of background water chemistry on the acute toxicity of major ions salts to C. dubia were the effect of Na on toxicity of K salts and the effects of Ca on the toxicity of Na and Mg salts. Although the practical implications of the Na effect on K toxicity are limited due to the unlikelihood of K-dominated exposures, this result does mean that, when K exposures are of concern [11], effects concentrations based on typical laboratory tests with low Na concentrations would overestimate risk. Regarding the Ca effects, although a hardness dependence of toxicity was already known for some Na and Mg salts [16–20] and Davies and Hall [21] implicated Ca as the specific factor for Na2SO4 toxicity, our results more thoroughly establish a role of Ca for a variety of salts. Predicting salt toxicity across waters will thus be more accurate if done on the basis of Ca rather than hardness, but the benefits of accuracy must be weighed against the availability of Ca versus hardness data. Uncertainties associated with using hardness as a surrogate for Ca can be addressed based on the regional variability of the Ca:Mg ratio, the resultant uncertainty on Ca, and the consequent uncertainty on toxicity estimated from Ca (e.g., Figures 7 and 8); in many cases, the resultant uncertainty should be limited.
An important tool in addressing the toxicity of ion mixtures has been the model of Mount et al. [3] and part of the purpose of the current work was to address some limitations of that model. The results here do indicate that not addressing the hardness (or Ca specifically) of the background water can introduce uncertainty into this model, and also raise questions regarding how joint toxicity across the different ions was addressed by Mount et al. [3]. However, any quantitative evaluation of that model relative to our results must wait until an alternative model based on our work is presented in a later paper.
In addition to describing the dependence of major ion salt toxicities on background water chemistry, the results of the present study allowed some inferences about mechanisms and some conclusions about how salt toxicity dosimetry should be expressed. At least 3 separate mechanisms were apparent. First, a K-related mechanism is indicated by the high toxicities of K salts, the dependence of this toxicity on Na concentration, and its good correlation to K concentration. Second, a Mg-related mechanism is indicated based on the greater toxicity of Mg salts than Na salts and the correlation of Mg salt toxicity to Mg activity. Third, a mechanism related to multiple ions is indicated, based 1) on Na salts being less toxic than salts of other cations; 2) on anions affecting the toxicity of Na salts; 3) on the good correlation of this toxicity to osmolarity; and 4) on the agreement of toxicity from Na gluconate and mannitol with that of inorganic Na salts when expressed as osmolarity. Ca salt toxicity also likely represents a mechanism different from that of Na salts because of the greater toxicity of CaCl2 compared to Na salts tested at high but non-toxic Ca concentrations; however, this might be the same mechanism as Mg, which has similar toxicity to Ca when tested in waters with higher Ca background.
Regarding dosimetry, rigorously understanding and describing salt toxicity requires examining toxicity on the basis of chemical activities and consideration of various ion interactions. The cation-related mechanisms would be expected to be best related to cation activities, and the multiple ion mechanism associated with Na salts requires some measure, such as osmolarity, which addresses multiple chemical species. These mechanistic and dosimetry conclusions are preliminary and will be further addressed in the subsequent papers mentioned in the Introduction, but it is evident that regulations based solely on a single ion may lack robustness from failing to address the relative toxicities and interactions of multiple ions.
One limitation of the results presented here is that they just concern the toxicities of single salts, with other ions being at low to moderate background concentrations reported for U.S. waters. Most field situations would involve enrichment of more ions – to well above ambient levels, if not to toxic levels. For example, it would not be expected that Mg would be enriched to toxic levels while Ca stays at background levels, and the high toxicity of K salts depends on very low Na concentrations, which would be an unusual exposure scenario. The exposure metrics noted here would be expected to apply to more complex mixtures (e.g., all ions contributing to osmolarity), but there is a need to better define the scope of applicability of the relationships discussed here. Their extension to more complex mixtures will be the subject of the subsequent papers mentioned earlier.
Although a complete model for the acute toxicity of major ions to C. dubia will be the subject of a subsequent paper, Figures 6–8 make it clear that this will be complicated, involving chemical speciation calculations, multiple toxicity mechanisms, and interactions among 7 toxicants. Any practical application will require simplifications, but we feel that the detail provided here and in later papers provides a starting point for determining what simplifications are possible and how they should be structured. Another application issue concerns the relevance of acute ion toxicity for C. dubia to the assessment of an entire aquatic community, especially in low-ion, lotic systems. Again as noted earlier, the toxicity endpoint in this paper is the starting point for work that will address more endpoints and species. However, the relationships developed for C. dubia will be directly relevant to many aquatic systems and will also have value in developing and interpreting major ion toxicity data for other species.
Supplementary Material
Acknowledgments
T.D. Dawson supervised the culture of test organisms used in this research and provided input to the experimental design and execution. We thank D.J. Soucek and J.R.F Elphick for technical consultation and for providing additional details regarding their work. We also wish to thank C.M. Wood for the suggestion to test the toxicities of mannitol and gluconate salts. The views expressed in this paper are those of the authors and do not necessarily reflect the views or policies of the U.S. Environmental Protection Agency. Mention of trade names or commercial products does not constitute endorsement or recommendation for use.
Appendix I
Results of tests on the acute toxicity of major ion salts, gluconate salts, and mannitol to Ceriodaphnia dubia in various tests waters; see text for designations and formulations for test and culture waters; horizontal lines delineate experiments and experimental sets. For LC50s, “Probit” denotes exposureeffects curve calculation by probit method; “Midpoint” denotes geometric mean of confidence limits; parentheses denote confidence limits; see text for methodology. Except where noted, LC50s and component concentrations are nominal, and expected to be within +/−10% based on analysis of subset of test solutions
| Set # | Test Chemical | Culture Water | Test Water | LC50 Method | LC50 mg/L (≥95% CL) | LC50 mM (≥95% CL) | Na mM | K mM | Ca mM | Mg mM | Cl mM | SO4 mM | Alk meq/L | pH | LC50 uS/cm | Osmol mOsM |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | NaCl | MHRW | 0.33x MHRW | Midpoint | 1860 (1740-1990) | 31.8 (29.8-34.0) | 32.2 | 0.02 | 0.116 | 0.16 | 31.8 | 0.28 | 0.38 | 7.40 | 3170 | 61.4 |
| NaCl | MHRW | MHRW | Probit | 2060 (2020-2490) | 35.3 (34.5-42.6) | 36.5 | 0.05 | 0.348 | 0.50 | 35.4 | 0.84 | 1.14 | 7.90 | 3500 | 70.1 | |
| Na2SO4 | MHRW | 0.33x MHRW | Midpoint | 1860 (1730-2010) | 13.1 (12.2-14.2) | 26.6 | 0.02 | 0.116 | 0.16 | 0.02 | 13.4 | 0.38 | 7.60 | 2480 | 35.7 | |
| Na2SO4 | MHRW | MHRW | Probit | 3060 (2810-3350) | 21.5 (19.8-23.6) | 44.2 | 0.05 | 0.348 | 0.50 | 0.05 | 22.4 | 1.14 | 8.00 | 3680 | 58.7 | |
|
| ||||||||||||||||
| NaCl | ALSW | ALSW | Midpoint | 1680 (1560-1790) | 28.7 (26.8-30.7) | 28.9 | 0.04 | 0.364 | 0.17 | 28.9 | 0.16 | 0.86 | 7.95 | 2980 | 56.1 | |
| NaCl | ALSW | 0.33x ALSW | Probit | 1560 (1420-1720) | 26.7 (24.4-29.4) | 26.8 | 0.01 | 0.121 | 0.06 | 26.8 | 0.05 | 0.29 | 7.55 | 2700 | 51.3 | |
| NaCl | 0.33x ALSW | 0.33x ALSW | Probit | 1770 (1580-1980) | 30.2 (27.0-33.9) | 30.3 | 0.01 | 0.121 | 0.06 | 30.3 | 0.05 | 0.29 | 7.55 | 3030 | 57.9 | |
| NaCl | 0.33x ALSW | ALSW | Probit | 1940 (1780-2110) | 33.2 (30.5-36.2) | 33.5 | 0.04 | 0.364 | 0.17 | 33.4 | 0.16 | 0.86 | 7.90 | 3180 | 64.5 | |
| NaCl | MHRW | MHRW | Probit | 1810 (1680-1950) | 31.0 (28.8-33.4) | 32.1 | 0.05 | 0.348 | 0.50 | 31.0 | 0.84 | 1.14 | 8.00 | 3310 | 62.0 | |
| NaCl | 0.33x MHRW | 0.33x MHRW | Midpoint | 1640 (1530-1760) | 28.1 (26.2-30.1) | 28.4 | 0.02 | 0.116 | 0.16 | 28.1 | 0.28 | 0.38 | 7.60 | 2990 | 54.4 | |
|
| ||||||||||||||||
| NaCl | 0.33x MHRW | 0.33x MHRW | Probit | 1560 (1440-1710) | 26.7 (24.6-29.2) | 27.1 | 0.02 | 0.116 | 0.16 | 26.8 | 0.28 | 0.38 | 7.70 | 2770 | 51.9 | |
| NaCl | MHRW | 0.33x MHRW | Probit | 1480 (1340-1640) | 25.3 (23.0-28.1) | 25.7 | 0.02 | 0.116 | 0.16 | 25.4 | 0.28 | 0.38 | 7.70 | 2690 | 49.3 | |
| MgCl2 | 0.33x ALSW | 0.33x ALSW | Probit | 529 (457-612) | 5.56 (4.80-6.43) | 0.09 | 0.01 | 0.121 | 5.61 | 11.2 | 0.05 | 0.29 | 7.45 | 1230 | 15.9 | |
| MgCl2 | ALSW | 0.33x ALSW | Probit | 667 (556-810) | 7.01 (5.84-8.51) | 0.09 | 0.01 | 0.121 | 7.06 | 14.1 | 0.05 | 0.29 | 7.45 | 1510 | 19.7 | |
| MgCl2 | MHRW | 0.33x MHRW | Probit | 305 (271-338) | 3.20 (2.85-3.55) | 0.38 | 0.02 | 0.116 | 3.37 | 6.42 | 0.28 | 0.38 | 7.55 | 813 | 10.2 | |
| MgCl2 | 0.33x MHRW | 0.33x MHRW | Probit | 312 (192-417) | 3.28 (2.02-4.38) | 0.38 | 0.02 | 0.116 | 3.44 | 6.57 | 0.28 | 0.38 | 7.60 | 835 | 10.3 | |
|
| ||||||||||||||||
| 2 | NaCl | 0.125x MHRW | 0.125x MHRW | Midpoint | 907 (828-993) | 15.5 (14.2-17.0) | 15.7 | 0.01 | 0.044 | 0.06 | 15.5 | 0.11 | 0.14 | 7.45 | 1750 | 31.0 |
| NaCl | 0.125x MHRW | MHRW | Midpoint | 1810 (1640-1990) | 30.9 (28.1-34.0) | 32.0 | 0.05 | 0.348 | 0.50 | 31.0 | 0.84 | 1.14 | 8.05 | 3260 | 61.9 | |
| NaCl | MHRW | 0.125x MHRW | Midpoint | 1010 (940-1080) | 17.2 (16.1-18.4) | 17.3 | 0.01 | 0.044 | 0.06 | 17.2 | 0.11 | 0.14 | 7.35 | 1860 | 34.1 | |
| NaCl | MHRW | MHRW | Probit | 1710 (1580-1850) | 29.3 (27.1-31.7) | 30.4 | 0.05 | 0.348 | 0.50 | 29.4 | 0.84 | 1.14 | 8.05 | 3040 | 58.9 | |
| MgCl2 | 0.125x MHRW | 0.125x MHRW | Probit | 69 (53-90) | 0.72 (0.56-0.95) | 0.14 | 0.01 | 0.044 | 0.79 | 1.46 | 0.11 | 0.14 | 7.25 | 229 | 3.3 | |
| MgCl2 | MHRW | 0.125x MHRW | Probit | 116 (94-202) | 1.22 (0.99-2.12) | 0.14 | 0.01 | 0.044 | 1.28 | 2.44 | 0.11 | 0.14 | 7.25 | 351 | 8.8 | |
|
| ||||||||||||||||
| Na2SO4 | 0.125x MHRW | 0.125x MHRW | Midpoint | 671 (613-734) | 4.72 (4.32-5.17) | 9.59 | 0.01 | 0.044 | 0.06 | 0.01 | 4.83 | 0.14 | 7.40 | 1070 | 14.2 | |
| Na2SO4 | MHRW | 0.125x MHRW | Midpoint | 916 (829-1012) | 6.45 (5.84-7.12) | 13.0 | 0.01 | 0.044 | 0.06 | 0.01 | 6.55 | 0.14 | 7.20 | 1360 | 18.8 | |
| MgSO4 | 0.125x MHRW | 0.125x MHRW | Probit | 160 (137-184) | 1.33 (1.14-1.53) | 0.14 | 0.01 | 0.044 | 1.39 | 0.01 | 1.43 | 0.14 | 7.05 | 630 | 3.6 | |
| MgSO4 | MHRW | 0.125x MHRW | Probit | 291 (264-321) | 2.42 (2.19-2.67) | 0.14 | 0.01 | 0.044 | 2.48 | 0.01 | 2.52 | 0.14 | 7.20 | 978 | 5.2 | |
| KCl | 0.125x MHRW | 0.125x MHRW | Probit | 177 (159-197) | 2.37 (2.13-2.64) | 0.14 | 2.38 | 0.044 | 0.06 | 2.38 | 0.11 | 0.14 | 7.40 | 389 | 5.9 | |
| KCl | MHRW | 0.125x MHRW | Midpoint | 224 (204-245) | 3.0 (2.74-3.29) | 0.14 | 3.01 | 0.044 | 0.06 | 3.01 | 0.11 | 0.14 | 7.40 | 474 | 7.1 | |
|
| ||||||||||||||||
| NaHCO3 | 0.125x MHRW | 0.125x MHRW | Midpoint | 1070 (1000-1140) | 12.7 (11.9-13.6) | 12.9 | 0.01 | 0.044 | 0.06 | 0.01 | 0.11 | 12.9 | 9.20 | 1110 | 23.8 | |
| NaHCO3 | MHRW | 0.125x MHRW | Midpoint | 1000 (920-1090) | 11.9 (10.9-13.0) | 12.0 | 0.01 | 0.044 | 0.06 | 0.01 | 0.11 | 12.0 | 9.15 | 1060 | 22.4 | |
| CaCl2 | 0.125x MHRW | 0.125x MHRW | Midpoint | 1670 (1570-1780) | 15.1 (14.2-16.1) | 0.14 | 0.01 | 15.1 | 0.06 | 30.2 | 0.11 | 0.14 | 6.90 | 2930 | 41.2 | |
| CaCl2 | MHRW | 0.125x MHRW | Probit | 1660 (1620-1940) | 15.0 (14.6-17.5) | 0.14 | 0.01 | 15.0 | 0.06 | 30.0 | 0.11 | 0.14 | 6.95 | 2920 | 40.9 | |
|
| ||||||||||||||||
| 3 | KCl | MHRW | ALSW | Probit | 351 (321-384) | 4.71 (4.31-5.15) | 0.28 | 4.75 | 0.364 | 0.17 | 4.92 | 0.16 | 0.86 | 7.95 | 781 | 11.1 |
| KCl | MHRW | MHRW | Probit | 464 (395-568) | 6.22 (5.30-7.62) | 1.14 | 6.28 | 0.348 | 0.50 | 6.28 | 0.84 | 1.14 | 8.10 | 1140 | 15.7 | |
| CaCl2 | MHRW | ALSW | Probit | 1900 (1690-2170) | 17.2 (15.2-19.5) | 0.28 | 0.04 | 17.5 | 0.17 | 34.5 | 0.16 | 0.86 | 7.90 | 3220 | 46.9 | |
| CaCl2 | MHRW | MHRW | Probit | 1990 (1760-2260) | 18.0 (15.9-20.4) | 1.14 | 0.05 | 18.3 | 0.50 | 36.0 | 0.84 | 1.14 | 7.95 | 3250 | 50.3 | |
| MgCl2 | MHRW | ALSW | Probit | 861 (759-981) | 9.04 (7.97-10.3) | 0.28 | 0.04 | 0.364 | 9.21 | 18.3 | 0.16 | 0.86 | 7.85 | 1900 | 26.1 | |
| MgCl2 | MHRW | MHRW | Probit | 733 (615-895) | 7.70 (6.46-9.40) | 1.14 | 0.05 | 0.348 | 8.19 | 15.4 | 0.84 | 1.14 | 7.95 | 1730 | 24.2 | |
|
| ||||||||||||||||
| K2SO4 | MHRW | ALSW | Midpoint | 362 (339-386) | 2.08 (1.95-2.22) | 0.28 | 4.19 | 0.364 | 0.17 | 0.22 | 2.23 | 0.86 | 7.90 | 690 | 7.7 | |
| K2SO4 | MHRW | MHRW | Midpoint | 606 (557-659) | 3.48 (3.20-3.78) | 1.14 | 7.01 | 0.348 | 0.50 | 0.05 | 4.32 | 1.14 | 8.05 | 1150 | 13.2 | |
| Na2SO4 | MHRW | ALSW | Midpoint | 3410 (3150-3700) | 24.0 (22.2-26.0) | 48.3 | 0.04 | 0.364 | 0.17 | 0.22 | 24.18 | 0.86 | 7.90 | 4080 | 63.4 | |
| Na2SO4 | MHRW | MHRW | Midpoint | 3260 (3060-3460) | 22.9 (21.5-24.4) | 47.0 | 0.05 | 0.348 | 0.50 | 0.05 | 23.76 | 1.14 | 8.00 | 3970 | 62.2 | |
| MgSO4 | MHRW | ALSW | Probit | 2040 (1880-2230) | 16.9 (15.6-18.5) | 0.28 | 0.04 | 0.364 | 17.1 | 0.22 | 17.10 | 0.86 | 7.85 | 2240 | 25.2 | |
| MgSO4 | MHRW | MHRW | Probit | 1990 (1830-2180) | 16.6 (15.2-18.1) | 1.14 | 0.05 | 0.348 | 17.1 | 0.05 | 17.4 | 1.14 | 8.00 | 2280 | 26.3 | |
|
| ||||||||||||||||
| KHCO3 | MHRW | ALSW | Probit | 322 (306-340) | 3.22 (3.06-3.40) | 0.28 | 3.25 | 0.364 | 0.17 | 0.22 | 0.16 | 4.08 | 8.70 | 487 | 8.0 | |
| KHCO3 | MHRW | MHRW | Probit | 454 (407-507) | 4.53 (4.07-5.06) | 1.14 | 4.59 | 0.348 | 0.50 | 0.05 | 0.84 | 5.68 | 8.85 | 844 | 13.3 | |
| NaHCO3 | MHRW | ALSW | Midpoint | 1980 (1880-2090) | 23.6 (22.3-24.9) | 23.8 | 0.04 | 0.187a | 0.17 | 0.22 | 0.16 | 24.4 | 9.35 | 1940 | 41.7 | |
| NaHCO3 | MHRW | MHRW | Probit | 1610 (1440-1700) | 19.1 (17.2-20.3) | 20.3 | 0.05 | 0.141a | 0.50 | 0.05 | 0.84 | 20.3 | 9.25 | 1680 | 36.6 | |
|
| ||||||||||||||||
| NaCl | MHRW | ALSW | Probit | 1910 (1730-2110) | 32.7 (29.6-36.1) | 33.0 | 0.04 | 0.364 | 0.17 | 32.9 | 0.16 | 0.86 | 8.00 | 3110 | 63.6 | |
| NaCl | MHRW | MHRW | Midpoint | 2000 (1830-2190) | 34.3 (31.4-37.5) | 35.4 | 0.05 | 0.348 | 0.50 | 34.4 | 0.84 | 1.14 | 8.10 | 3470 | 68.1 | |
| MgCO3 | MHRW | ALSW | Midpoint | 906 (859-956) | 10.8 (10.2-11.3) | 0.28 | 0.04 | 0.443 | 10.9 | 0.22 | 0.16 | 22.4 | 9.25 | 1160 | 23.2 | |
| MgCO3 | MHRW | MHRW | Midpoint | 892 (848-938) | 10.6 (10.1-11.1) | 1.14 | 0.05 | 0.426 | 11.1 | 0.05 | 0.84 | 22.3 | 9.25 | 1230 | 25.2 | |
|
| ||||||||||||||||
| 4 | NaHCO3 | MHRW | 0.33x ALSW | Midpoint | 1950 (1780-2130) | 23.2 (21.2-25.4) | 23.3 | 0.01 | 0.121 | 0.06 | 0.07 | 0.05 | 23.5 | 9.30 | 1800 | 40.3 |
| NaHCO3 | MHRW | 3.0x ALSW | Probit | 1710 (1560-1890) | 20.4 (18.6-22.5) | 21.2 | 0.12 | 0.231a | 0.50 | 0.65 | 0.47 | 23.0 | 9.35 | 1800 | 39.1 | |
| NaCl | MHRW | 0.33x ALSW | Midpoint | 1640 (1530-1760) | 28.1 (26.2-30.0) | 28.2 | 0.01 | 0.121 | 0.06 | 28.1 | 0.05 | 0.29 | 7.40 | 2700 | 53.9 | |
| NaCl | MHRW | 3.0x ALSW | Probit | 1980 (1770-2160) | 33.8 (30.2-36.9) | 34.7 | 0.12 | 1.093 | 0.50 | 34.5 | 0.47 | 2.58 | 8.30 | 3490 | 69.1 | |
| Na2SO4 | MHRW | 0.33x ALSW | Probit | 1940 (1770-2100) | 13.6 (12.5-14.8) | 27.3 | 0.01 | 0.121 | 0.06 | 0.07 | 13.7 | 0.29 | 7.55 | 2480 | 36.5 | |
| Na2SO4 | MHRW | 3.0x ALSW | Midpoint | 3350 (3140-3570) | 23.6 (22.1-25.2) | 23.8 | 0.12 | 1.093 | 0.50 | 0.65 | 0.47 | 2.58 | 8.45 | 4090 | 65.4 | |
|
| ||||||||||||||||
| KCl | MHRW | 0.33x ALSW | Midpoint | 192 (180-205) | 2.58 (2.41-2.75) | 0.09 | 2.59 | 0.121 | 0.06 | 2.65 | 0.05 | 0.29 | 7.65 | 430 | 5.7 | |
| KCl | MHRW | 3.0x ALSW | Probit | 481 (432-534) | 6.45 (5.79-7.16) | 0.85 | 6.57 | 1.093 | 0.50 | 7.10 | 0.47 | 2.58 | 8.45 | 1220 | 18.1 | |
| MgSO4 | MHRW | 0.33x ALSW | Midpoint | 1590 (1440-1770) | 13.2 (11.9-14.7) | 0.09 | 0.01 | 0.121 | 13.3 | 0.07 | 13.3 | 0.29 | 7.45 | 1780 | 19.4 | |
| MgSO4 | MHRW | 3.0x ALSW | Midpoint | 2860 (2620-3130) | 23.8 (21.7-26.0) | 0.85 | 0.12 | 1.093 | 24.3 | 0.65 | 24.2 | 2.58 | 8.35 | 2910 | 36.9 | |
| MgCl2 | MHRW | 0.33x ALSW | Probit | 374 (344-409) | 3.93 (3.61-4.30) | 0.09 | 0.01 | 0.121 | 3.98 | 7.93 | 0.05 | 0.29 | 7.25 | 910 | 11.6 | |
| MgCl2 | MHRW | 3.0x ALSW | Probit | 1290 (1160-1490) | 13.6 (12.2-15.7) | 0.85 | 0.12 | 1.093 | 14.0 | 27.8 | 0.47 | 2.58 | 8.35 | 2760 | 40.8 | |
|
| ||||||||||||||||
| CaCl2 | ALSW | 0.3x ALSW | Midpoint | 1600 (1490-1710) | 14.4 (13.4-15.4) | 0.09 | 0.01 | 14.5 | 0.06 | 28.8 | 0.05 | 0.29 | 7.35 | 2870 | 38.7 | |
| CaCl2 | ALSW | 3.0x ALSW | Midpoint | 2000 (1870-2130) | 18.0 (16.9-19.2) | 0.85 | 0.12 | 19.1 | 0.50 | 36.7 | 0.47 | 2.58 | 7.95 | 3510 | 52.4 | |
| K2SO4 | ALSW | 0.3x ALSW | Probit | 234 (215-254) | 1.34 (1.23-1.46) | 0.09 | 2.70 | 0.121 | 0.06 | 0.07 | 1.39 | 0.29 | 7.40 | 426 | 4.5 | |
| K2SO4 | ALSW | 3.0x ALSW | Midpoint | 518 (449-598) | 2.97 (2.58-3.43) | 0.85 | 6.06 | 1.093 | 0.50 | 0.65 | 3.44 | 2.58 | 8.50 | 1120 | 13.7 | |
| MgCO3 | ALSW | 0.3x ALSW | Midpoint | 390 (365-417) | 4.63 (4.33-4.95) | 0.09 | 0.01 | 0.155 | 4.68 | 0.07 | 0.05 | 9.54 | 8.95 | 619 | 11.8 | |
| MgCO3 | ALSW | 3.0x ALSW | Midpoint | 977 (916-1041) | 11.6 (10.9-12.4) | 0.85 | 0.12 | 0.318a | 12.1 | 0.65 | 0.47 | 25.8 | 9.15 | 1280 | 28.9 | |
| NaCl | ALSW | 0.33x ALSW | Midpoint | 1700 (1600-1800) | 29.1 (27.4-30.9) | 29.2 | 0.01 | 0.121 | 0.06 | 29.1 | 0.05 | 0.29 | 7.50 | 2960 | 55.7 | |
| NaCl | ALSW | ALSW | Probit | 1910 (1760-2070) | 32.6 (30.1-35.4) | 32.9 | 0.04 | 0.364 | 0.17 | 32.8 | 0.16 | 0.86 | 7.90 | 3320 | 63.5 | |
| NaCl | ALSW | 3.0x ALSW | Midpoint | 2420 (2210-2660) | 41.5 (37.8-45.5) | 42.3 | 0.12 | 1.093 | 0.50 | 42.1 | 0.47 | 2.58 | 8.40 | 4110 | 83.1 | |
| Na2SO4 | ALSW | 0.33x ALSW | Midpoint | 1890 (1760-2020) | 13.3 (12.4-14.2) | 26.7 | 0.01 | 0.121 | 0.06 | 0.07 | 13.4 | 0.29 | 7.55 | 2480 | 35.7 | |
| Na2SO4 | ALSW | ALSW | Midpoint | 3440 (3220-3670) | 24.2 (22.6-25.8) | 48.7 | 0.04 | 0.364 | 0.17 | 0.22 | 24.4 | 0.86 | 7.95 | 4000 | 63.8 | |
| Na2SO4 | ALSW | 3.0x ALSW | Probit | 3520 (3200-3880) | 24.8 (22.5-27.3) | 50.4 | 0.12 | 1.093 | 0.50 | 0.65 | 25.3 | 2.58 | 8.50 | 4280 | 68.5 | |
|
| ||||||||||||||||
| 5 | NaCl | MHRW | Low Ca:Mg | Midpoint | 1580 (1420-1740) | 27.0 (24.3-29.8) | 27.2 | 0.04 | 0.075 | 0.46 | 27.2 | 0.15 | 0.86 | 7.90 | 2790 | 53.0 |
| NaCl | MHRW | HighCa:Mg | Midpoint | 1920 (1790-2050) | 32.8 (30.7-35.0) | 33.1 | 0.04 | 0.475 | 0.06 | 33.0 | 0.15 | 0.86 | 7.90 | 3218 | 63.8 | |
| NaHCO3 | MHRW | Low Ca:Mg | Midpoint | 1540 (1440-1640) | 18.3 (17.2-19.5) | 18.6 | 0.04 | 0.075 | 0.46 | 0.22 | 0.15 | 19.2 | 9.25 | 1611 | 33.8 | |
| NaHCO3 | MHRW | HighCa:Mg | Midpoint | 2000 (1870-2140) | 23.8 (22.2-25.5) | 24.1 | 0.04 | 0.200a | 0.06 | 0.22 | 0.15 | 24.6 | 9.30 | 1970 | 41.2 | |
| Na2SO4 | MHRW | Low Ca:Mg | Probit | 1430 (1340-1560) | 10.1 (9.41-11.0) | 20.4 | 0.04 | 0.075 | 0.46 | 0.22 | 10.2 | 0.86 | 7.95 | 2027 | 28.6 | |
| Na2SO4 | MHRW | HighCa:Mg | Midpoint | 3140 (2940-3360) | 22.1 (20.7-23.6) | 44.5 | 0.04 | 0.475 | 0.06 | 0.22 | 22.3 | 0.86 | 8.00 | 3822 | 58.7 | |
|
| ||||||||||||||||
| KCl | MHRW | Low Ca:Mg | Midpoint | 245 (229-263) | 3.29 (3.07-3.53) | 0.28 | 3.32 | 0.075 | 0.46 | 3.50 | 0.15 | 0.86 | 7.95 | 626 | 8.4 | |
| KCl | MHRW | HighCa:Mg | Midpoint | 281 (258-306) | 3.77 (3.46-4.10) | 0.28 | 3.81 | 0.475 | 0.06 | 3.99 | 0.15 | 0.86 | 7.95 | 663 | 9.3 | |
| MgSO4 | MHRW | Low Ca:Mg | Probit | 935 (857-1020) | 7.77 (7.12-8.52) | 0.28 | 0.04 | 0.075 | 8.23 | 0.22 | 7.92 | 0.86 | 7.90 | 1292 | 13.3 | |
| MgSO4 | MHRW | HighCa:Mg | Midpoint | 2060 (1920-2200) | 17.1 (15.9-18.3) | 0.28 | 0.04 | 0.475 | 17.1 | 0.22 | 17.2 | 0.86 | 7.95 | 2232 | 25.4 | |
| MgCl2 | MHRW | Low Ca:Mg | Probit | 223 (203-245) | 2.34 (2.13-2.57) | 0.28 | 0.04 | 0.075 | 2.80 | 4.90 | 0.15 | 0.86 | 7.90 | 654 | 8.5 | |
| MgCl2 | MHRW | HighCa:Mg | Probit | 935 (865-1015) | 9.82 (9.09-10.7) | 0.28 | 0.04 | 0.475 | 9.88 | 19.9 | 0.15 | 0.86 | 7.90 | 2041 | 28.1 | |
|
| ||||||||||||||||
| 6 | Na2SO4 | MHRW | Low Cl:SO4 | Midpoint | 3430 (3200-3670) | 24.1 (22.5-25.8) | 48.5 | 0.04 | 0.365 | 0.17 | 0.04 | 24.3 | 0.86 | 8.00 | 4001 | 63.5 |
| Na2SO4 | MHRW | HighCl:SO4 | Probit | 2990 (2770-3250) | 21.1 (19.5-22.9) | 42.4 | 0.04 | 0.365 | 0.17 | 0.40 | 21.1 | 0.86 | 7.95 | 3632 | 56.2 | |
| K2SO4 | MHRW | Low Cl:SO4 | Midpoint | 377 (355-401) | 2.16 (2.04-2.30) | 0.28 | 4.37 | 0.365 | 0.17 | 0.04 | 2.38 | 0.86 | 8.00 | 710 | 7.8 | |
| K2SO4 | MHRW | HighCl:SO4 | Midpoint | 349 (322-379) | 2.00 (1.85-2.17) | 0.28 | 4.04 | 0.365 | 0.17 | 0.40 | 2.04 | 0.86 | 8.00 | 669 | 7.6 | |
| MgSO4 | MHRW | Low Cl:SO4 | Midpoint | 1980 (1800-2170) | 16.4 (15.0-18.0) | 0.28 | 0.04 | 0.365 | 16.6 | 0.04 | 16.6 | 0.86 | 7.90 | 2172 | 24.4 | |
| MgSO4 | MHRW | HighCl:SO4 | Probit | 1860 (1670-2080) | 15.5 (13.9-17.3) | 0.28 | 0.04 | 0.365 | 15.6 | 0.40 | 15.5 | 0.86 | 7.90 | 2097 | 23.4 | |
|
| ||||||||||||||||
| NaHCO3 | MHRW | Low Cl:SO4 | Probit | 1990 (1830-2180) | 23.7 (21.8-26.0) | 24.0 | 0.04 | 0.190a | 0.17 | 0.04 | 0.22 | 24.6 | 9.35 | 1927 | 41.9 | |
| NaHCO3 | MHRW | HighCl:SO4 | Midpoint | 2050 (1910-2200) | 24.4 (22.8-26.1) | 24.7 | 0.04 | 0.166a | 0.17 | 0.40 | 0.04 | 25.2 | 9.35 | 2003 | 42.2 | |
| NaCl | MHRW | Low Cl:SO4 | Probit | 1660 (1500-1840) | 28.4 (25.7-31.5) | 28.7 | 0.04 | 0.365 | 0.17 | 28.4 | 0.22 | 0.86 | 7.95 | 2816 | 55.5 | |
| NaCl | MHRW | HighCl:SO4 | Midpoint | 1700 (1600-1800) | 29.1 (27.4-30.9) | 29.4 | 0.04 | 0.365 | 0.17 | 29.5 | 0.04 | 0.86 | 7.90 | 2921 | 57.0 | |
| MgCO3 | MHRW | Low Cl:SO4 | Probit | 948 (874-1030) | 11.2 (10.4-12.2) | 0.28 | 0.04 | 0.447 | 11.4 | 0.04 | 0.22 | 23.4 | 9.20 | 1149 | 24.7 | |
| MgCO3 | MHRW | HighCl:SO4 | Midpoint | 894 (836-957) | 10.6 (9.92-11.4) | 0.28 | 0.04 | 0.442 | 10.8 | 0.40 | 0.04 | 22.1 | 9.20 | 1131 | 23.8 | |
|
| ||||||||||||||||
| 7 | NaCl | ALSW | Low K ALSW | Midpoint | 1920 (1800-2040) | 32.8 (30.9-35.0) | 33.2 | 0.01 | 0.364 | 0.17 | 33. | 0.16 | 0.86 | 7.85 | 3110 | 63.9 |
| NaCl | ALSW | High K ALSW | Probit | 1980 (1830-2160) | 34.0 (31.3-37.0) | 34.0 | 0.26 | 0.364 | 0.17 | 34.1 | 0.16 | 0.86 | 7.85 | 3216 | 66.0 | |
| Na2SO4 | ALSW | Low K ALSW | Probit | 2920 (2650-3230) | 20.6 (18.7-22.8) | 41.5 | 0.01 | 0.364 | 0.17 | 0.22 | 20.8 | 0.86 | 7.90 | 3351 | 54.9 | |
| Na2SO4 | ALSW | High K ALSW | Probit | 3420 (3140-3730) | 24.0 (22.1-26.3) | 48.2 | 0.26 | 0.364 | 0.17 | 0.22 | 24.2 | 0.86 | 7.85 | 3858 | 63.5 | |
| NaHCO3 | ALSW | Low K ALSW | Midpoint | 2090 (1960-2220) | 24.8 (23.3-26.5) | 25.2 | 0.01 | 0.139a | 0.17 | 0.22 | 0.16 | 25.7 | 9.35 | 1867 | 43.8 | |
| NaHCO3 | ALSW | High K ALSW | Midpoint | 2220 (2010-2450) | 26.4 (23.9-29.1) | 26.5 | 0.26 | 0.192a | 0.17 | 0.22 | 0.16 | 27.2 | 9.35 | 2007 | 46.2 | |
|
| ||||||||||||||||
| CaCl2 | ALSW | Low K ALSW | Probit | 1870 (1690-2050) | 16.9 (15.2-18.5) | 0.33 | 0.01 | 17.2 | 0.17 | 34.0 | 0.16 | 0.86 | 7.90 | 3168 | 46.2 | |
| CaCl2 | ALSW | High K ALSW | Probit | 1710 (1550-1880) | 15.4 (14.0-17.0) | 0.08 | 0.26 | 15.8 | 0.17 | 31.1 | 0.16 | 0.86 | 7.85 | 2942 | 42.5 | |
| MgCl2 | ALSW | Low K ALSW | Probit | 970 (853-1103) | 10.2 (8.96-11.6) | 0.33 | 0.01 | 0.364 | 10.4 | 20.6 | 0.16 | 0.86 | 7.85 | 2022 | 29.1 | |
| MgCl2 | ALSW | High K ALSW | Probit | 912 (794-1050) | 9.58 (8.34-11.0) | 0.08 | 0.26 | 0.364 | 9.75 | 19.4 | 0.16 | 0.86 | 7.90 | 1968 | 27.5 | |
| MgSO4 | ALSW | Low K ALSW | Probit | 2030 (1790-2300) | 16.8 (14.9-19.1) | 0.33 | 0.01 | 0.364 | 17.0 | 0.22 | 17.0 | 0.86 | 7.95 | 2232 | 25.1 | |
| MgSO4 | ALSW | High K ALSW | Probit | 2240 (1980-2470) | 18.6 (16.5-20.5) | 0.08 | 0.26 | 0.364 | 18.8 | 0.22 | 18.8 | 0.86 | 8.00 | 2329 | 27.3 | |
|
| ||||||||||||||||
| 8 | KCl | MHRW | Low Alk ALSW | Midpoint | 673 (632-718) | 9.03 (8.48-9.63) | 1.23 | 9.07 | 0.364 | 0.17 | 9.24 | 0.95 | 0.20 | 7.45 | 1433 | 20.2 |
| KCl | MHRW | High Alk ALSW | Probit | 533 (487-584) | 7.15 (6.53-7.83) | 1.23 | 7.19 | 0.364 | 0.17 | 7.37 | 0.16 | 1.80 | 8.30 | 1188 | 17.6 | |
| NaCl | MHRW | Low Alk ALSW | Probit | 1860 (1690-2050) | 31.8 (28.9-35.1) | 33.1 | 0.04 | 0.364 | 0.17 | 32.1 | 0.95 | 0.20 | 7.40 | 3316 | 62.9 | |
| NaCl | MHRW | High Alk ALSW | Probit | 1970 (1790-2120) | 33.8 (30.6-36.3) | 35.0 | 0.04 | 0.364 | 0.17 | 34.0 | 0.16 | 1.80 | 8.25 | 3474 | 67.3 | |
| MgCl2 | MHRW | Low Alk ALSW | Probit | 899 (784-1008) | 9.44 (8.23-10.6) | 1.23 | 0.04 | 0.364 | 9.61 | 19.1 | 0.95 | 0.20 | 7.30 | 2076 | 27.9 | |
| MgCl2 | MHRW | High Alk ALSW | Midpoint | 611 (571-654) | 6.42 (6.00-6.87) | 1.23 | 0.04 | 0.364 | 6.59 | 13.0 | 0.16 | 1.80 | 8.25 | 1542 | 21.0 | |
|
| ||||||||||||||||
| Na2SO4 | MHRW | Low Alk ALSW | Probit | 2960 (2700-3240) | 20.8 (19.0-22.8) | 42.9 | 0.04 | 0.364 | 0.17 | 0.22 | 21.8 | 0.20 | 7.45 | 3736 | 56.3 | |
| Na2SO4 | MHRW | High Alk ALSW | Probit | 2830 (2560-3130) | 19.9 (18.0-22.0) | 41.0 | 0.04 | 0.364 | 0.17 | 0.22 | 20.1 | 1.80 | 8.25 | 3481 | 54.9 | |
| MgSO4 | MHRW | Low Alk ALSW | Midpoint | 2250 (2010-2510) | 18.7 (16.7-20.8) | 1.23 | 0.04 | 0.364 | 18.8 | 0.22 | 19.6 | 0.20 | 7.30 | 2475 | 28.2 | |
| MgSO4 | MHRW | High Alk ALSW | Probit | 2060 (1850-2450) | 17.2 (15.4-20.3) | 1.23 | 0.04 | 0.364 | 17.3 | 0.22 | 17.3 | 1.80 | 8.20 | 2263 | 27.1 | |
| MgCl2 | MHRW | Low Alk ALSW | Probit | 865 (754-985) | 9.09 (7.92-10.4) | 1.23 | 0.04 | 0.364 | 9.25 | 18.4 | 0.95 | 0.20 | 7.25 | 1986 | 27.0 | |
| MgCl2 | MHRW | High Alk ALSW | Probit | 623 (559-692) | 6.54 (5.87-7.27) | 1.23 | 0.04 | 0.364 | 6.71 | 13.3 | 0.16 | 1.80 | 8.10 | 1546 | 21.4 | |
|
| ||||||||||||||||
| 9 | NaCl | ALSW | ALSW | Probit | 2080 (1890-2280) | 35.5 (32.3-39.1) | 35.8 | 0.04 | 0.364 | 0.17 | 35.8 | 0.16 | 0.86 | 7.85 | 3342 | 68.9 |
| NaCl | ALSW | Low pH ALSW | Midpoint | 2160 (1980-2370) | 37.0 (33.9-40.5) | 37.3 | 0.04 | 0.364 | 0.17 | 37.2 | 0.16 | 0.86 | 6.75 | 3459 | 71.9 | |
| NaCl | ALSW | High pH ALSW | Probit | 1880 (1730-2050) | 32.2 (29.7-35.0) | 32.5 | 0.04 | 0.364 | 0.17 | 32.4 | 0.16 | 0.86 | 8.20 | 3116 | 62.7 | |
| MgCl2 | ALSW | ALSW | Probit | 938 (841-1090) | 9.85 (8.83-11.4) | 0.28 | 0.04 | 0.364 | 10.0 | 19.9 | 0.16 | 0.86 | 7.85 | 2045 | 28.2 | |
| MgCl2 | ALSW | Low pH ALSW | Probit | 1040 (894-1230) | 10.9 (9.39-13.0) | 0.28 | 0.04 | 0.364 | 11.0 | 22.0 | 0.16 | 0.86 | 6.75 | 2057 | 31.0 | |
| MgCl2 | ALSW | High pH ALSW | Probit | 1130 (1040-1280) | 11.9 (10.9-13.4) | 0.28 | 0.04 | 0.364 | 12.0 | 23.9 | 0.16 | 0.86 | 8.50 | 2257 | 33.2 | |
|
| ||||||||||||||||
| 10 | KCl | MHRW | 1.6 Na ALSW | Probit | 153 (141-167) | 2.05 (1.89-2.24) | 0.07 | 2.09 | 0.364 | 0.17 | 2.09 | 0.16 | 0.86 | 7.95 | 422 | 5.6 |
| KCl | MHRW | 3 Na ALSW | Probit | 241 (206-268) | 3.23 (2.76-3.59) | 0.13 | 3.27 | 0.364 | 0.17 | 3.34 | 0.16 | 0.86 | 8.00 | 599 | 8.0 | |
| KCl | MHRW | 10 Na ALSW | Midpoint | 393 (360-429) | 5.27 (4.83-5.75) | 0.43 | 5.31 | 0.364 | 0.17 | 5.68 | 0.16 | 0.86 | 7.95 | 872 | 12.5 | |
| KCl | MHRW | 30 Na ALSW | Midpoint | 513 (469-560) | 6.88 (6.29-7.51) | 1.30 | 6.92 | 0.364 | 0.17 | 8.16 | 0.16 | 0.86 | 7.95 | 1193 | 17.3 | |
| KCl | MHRW | 100 Na ALSW | Midpoint | 644 (602-690) | 8.64 (8.08-9.26) | 4.35 | 8.68 | 0.364 | 0.17 | 13.0 | 0.16 | 0.86 | 7.95 | 1656 | 26.4 | |
| KCl | MHRW | 300 Na ALSW | Probit | 752 (641-1360) | 10.1 (8.60-18.2) | 13.0 | 10.13 | 0.364 | 0.17 | 23.1 | 0.16 | 0.86 | 7.90 | 2616 | 45.3 | |
|
| ||||||||||||||||
| 11 | NaHCO3 | ALSW | ALSW Aged | Midpoint | 1660 (1531-1790) | 19.7 (18.2-21.3) | 20.0 | 0.04 | 0.134b | 0.17 | 0.22 | 0.16 | 20.6 | 9.35 | 1670 | 35.5 |
| NaHCO3 | ALSW | 3.0x ALSW Aged | Midpoint | 1670 (1530-1810) | 19.8 (18.3-21.5) | 20.7 | 0.12 | 0.126b | 0.50 | 0.65 | 0.47 | 22.4 | 9.35 | 1793 | 38.2 | |
| NaHCO3 | ALSW | ALSW Fresh | Probit | 2150 (1990-2320) | 25.6 (23.7-27.6) | 25.9 | 0.04 | 0.207b | 0.17 | 0.22 | 0.16 | 26.4 | 9.15 | 2087 | 47.2 | |
| NaHCO3 | ALSW | 3.0x ALSW Fresh | Midpoint | 2240 (2080-2420) | 26.7 (24.8-28.8) | 27.5 | 0.12 | 0.139b | 0.50 | 0.65 | 0.47 | 29.3 | 9.10 | 2284 | 51.7 | |
| MgCO3 | ALSW | ALSW Aged | Probit | 767 (699-843) | 9.10 (8.29-10.0) | 0.28 | 0.04 | 0.431 | 9.27 | 0.22 | 0.16 | 19.0 | 9.30 | 1043 | 20.9 | |
| MgCO3 | ALSW | 3.0x ALSW Aged | Probit | 507 (467-551) | 6.01 (5.54-6.54) | 0.85 | 0.12 | 0.147b | 6.52 | 0.65 | 0.47 | 14.6 | 9.30 | 879 | 18.0 | |
| MgCO3 | ALSW | ALSW Fresh | Midpoint | 792 (736-853) | 9.39 (8.73-10.1) | 0.28 | 0.04 | 0.433 | 9.56 | 0.22 | 0.16 | 19.6 | 9.15 | 1054 | 23.8 | |
| MgCO3 | ALSW | 3.0x ALSW Fresh | Probit | 635 (529-752) | 7.53 (6.27-8.92) | 0.85 | 0.12 | 0.152b | 8.04 | 0.65 | 0.47 | 17.6 | 9.00 | 941 | 18.4 | |
|
| ||||||||||||||||
| 12 | NaCl | ALSW | ALSW | Midpoint | 1950 (1840-2070) | 33.4 (31.5-35.5) | 33.7 | 0.04 | 0.364 | 0.17 | 33.6 | 0.16 | 0.86 | 7.85 | 3296 | 65.0 |
| Mannitol | ALSW | ALSW | Probit | 73.7 (66.2-82.1) | 0.28 | 0.04 | 0.364 | 0.17 | 0.22 | 0.16 | 0.86 | 7.75 | 75.7 | |||
| NaGluconate | ALSW | ALSW | Midpoint | 39.2 (36.8-42.0) | 39.5 | 0.04 | 0.364 | 0.17 | 0.22 | 0.16 | 0.86 | 7.85 | 2188 | 76.1 | ||
| CaGluconate | ALSW | ALSW | Probit | 32.9 (29.9-36.2) | 0.28 | 0.04 | 33.3 | 0.17 | 0.22 | 0.16 | 0.86 | 7.80 | 2074 | 84.6 | ||
| MgGluconate | ALSW | ALSW | Probit | 17.1 (14.3-18.7) | 0.28 | 0.04 | 0.364 | 17.3 | 0.22 | 0.16 | 0.86 | 7.80 | 1638 | 50.6 | ||
Ca concentrations are an extrapolation of Ca measurements to LC50 based on model for time dependence of Ca as a function of alkalinity; see Supplemental Material for more information
Ca concentrations are a weighted average of measurements and 24 and 48 hours that bracket the LC50
Footnotes
SUPPLEMENTAL DATA
Data analysis and Figure S1: The concentration- and time- dependence of calcium concentrations in tests of the acute toxicity of NaHCO3 and MgCO3 to Ceriodaphnia dubia
References
- 1.Drever JI. The Geochemistry of Natural Waters. Prentice-Hall; Englewood Cliffs, CA, USA: 1988. [Google Scholar]
- 2.Bradley T. Animal Osmoregulation. Oxford University Press; New York NY, USA: 2009. [Google Scholar]
- 3.Mount DR, Gulley DD, Hockett JR, Garrison TD, Evans JM. Statistical models to predict the toxicity of major ions to Ceriodaphnia dubia, Daphnia magna and Pimephales promelas (fathead minnows) Environ Toxicol Chem. 1997;16:2009–2019. [Google Scholar]
- 4.Boulter AM, Lamming FN, Faraq AM, Bergman HL. Environmental effects of saline oil-field discharges on surface waters. Environ Toxicol Chem. 1992;11:1187–1195. [Google Scholar]
- 5.Mount DR, Drottar KR, Gulley DD, Fillo JP, O’Neill PE. Use of laboratory toxicity data for evaluating the environmental acceptability of produced water discharge to surface waters. In: Ray JP, Engelhart FR, editors. Produced Water–Technological/Environmental Issues and Solutions. Plenum Publishing Corporation; New York, NY, USA: 1993. pp. 175–182. [Google Scholar]
- 6.Ingersoll CG, Dwyer FJ, Burch SA, Nelson MK, Buckler DR, Hunn JB. The use of freshwater and saltwater animals to distinguish between the toxic effects of salinity and contaminants in irrigation drain water. Environ Toxicol Chem. 1992;11:503–511. [Google Scholar]
- 7.Dickerson, KK, Hubert WA, Bergman HL. Toxicity assessment of water from lakes and wetlands receiving irrigation drainwater. Environ Toxicol Chem. 1996;15:1097–1101. [Google Scholar]
- 8.Chapman PM, Bailey H, Canaria E. Toxicity of total dissolved solids associated with two mine effluents to chironomid larvae and early life stages of rainbow trout. Environ Toxicol Chem. 2000;19:210–214. [Google Scholar]
- 9.Kennedy AJ, Cherry DS, Currie RJ. Field and laboratory assessment of a coal procesing effluent in the Leading Creek watershed, Meigs County, Ohio. Arch Environ Contam Toxicol. 2003;44:324–331. doi: 10.1007/s00244-002-2062-x. [DOI] [PubMed] [Google Scholar]
- 10.Findlay SEG, Kelly VR. Emerging indirect and long-term road salt effects on ecosystems. Ann NY Acad Sci. 2011;1223:58–68. doi: 10.1111/j.1749-6632.2010.05942.x. [DOI] [PubMed] [Google Scholar]
- 11.Jop KM, Askew AM. Toxicity identification evaluation using a short-term chronic test with Ceriodaphnia dubia. Bull Environ Contam Toxicol. 1994;53:91–97. doi: 10.1007/BF00205144. [DOI] [PubMed] [Google Scholar]
- 12.Ankley GT, Hockett JR, Mount DI, Mount DR. Early evolution of the Toxicity Identification Evaluation process: Contributions from the United States Environmental Protection Agency effluent testing program. In: Brack W, editor. Effects Directed Analysis of Complex Environmental Contamination. Springer Publishing; New York, NY, USA: 2011. [Google Scholar]
- 13.Pond GJ, Passmore ME, Borsuk FA, Reynolds L, Rose CJ. Downstream effects of mountaintop coal mining: Comparing biological conditions using family- and genus-level macroinvertebrate bioassessment tools. J N Am Benthol Soc. 2008;27:717–737. [Google Scholar]
- 14.Cormier SM, Suter GW., II A method for deriving water-quality benchmarks using field data. Environ Toxicol Chem. 2013;32:255–262. doi: 10.1002/etc.2057. [DOI] [PubMed] [Google Scholar]
- 15.Cormier SM, Suter GW, II, Zheng L. Derivation of a benchmark for freshwater ionic strength. Environ Toxicol Chem. 2013;32:263–271. doi: 10.1002/etc.2064. [DOI] [PubMed] [Google Scholar]
- 16.Soucek DJ, Kennedy AJ. Effects of hardness, chloride, and acclimation on the acute toxicity of sulfate to freshwater invertebrates. Environ Toxicol Chem. 2005;24:1204–1210. doi: 10.1897/04-142.1. [DOI] [PubMed] [Google Scholar]
- 17.Soucek DJ. Comparison of hardness-and chloride-regulated acute effects of sodium sulfate on two freshwater crustaceans. Environ Toxicol Chem. 2007;26:773–779. doi: 10.1897/06-229r.1. [DOI] [PubMed] [Google Scholar]
- 18.Soucek DJ, Linton TK, Tarr CD, Dickinson A, Wickramanayake N, Delos CG, Cruz LA. Influence of water hardness and sulfate on the acute toxicity of chloride to sensitive freshwater invertebrates. Environ Toxicol Chem. 2011;30:930–938. doi: 10.1002/etc.454. [DOI] [PubMed] [Google Scholar]
- 19.Elphick JR, Bergh KD, Bailey HC. Chronic toxicity of chloride to freshwater species: Effects of hardness and implications for water quality guidelines. Environ Toxicol Chem. 2011a;30:239–246. doi: 10.1002/etc.365. [DOI] [PubMed] [Google Scholar]
- 20.Elphick JR, Davies M, Gilron G, Canaria EC, Lo B, Bailey HC. An aquatic toxicological evaluation of sulfate: The case for considering hardness as a modifying factor in setting water quality guidelines. Environ Toxicol Chem. 2011b;30:247–253. doi: 10.1002/etc.363. [DOI] [PubMed] [Google Scholar]
- 21.Davies TD, Hall KJ. Importance of calcium in modifying the acute toxicity of sodium sulphate to Hyalella azteca and Daphnia magna. Environ Toxicol Chem. 2007;26:1243–1247. doi: 10.1897/06-510r.1. [DOI] [PubMed] [Google Scholar]
- 22.Di Toro DM, Allen HE, Bergman HL, Meyer JS, Paquin PR, Santore RC. Biotic ligand model of the acute toxicity of metals. 1. Technical basis. Environ Toxicol Chem. 2001;20:2383–2396. [PubMed] [Google Scholar]
- 23.Tietge JE, Hockett JR, Evans JM. Major ion toxicity of six produced waters to three freshwater species: application of ion toxicity models and TIE procedures. Environ Toxicol Chem. 1997;16:2002–2008. [Google Scholar]
- 24.U.S. Environmental Protection Agency. Short-term methods for estimating the chronic toxicity of effluents and receiving waters to freshwater organisms EPA/821/R-02/013. U.S. Environmental Protection Agency; Washington, DC, USA: 2002. [Google Scholar]
- 25.Marking LL, Dawson VK. Invest Fish Contr No. 48. U. S. Fish and Wildlife Service; Washington, DC: 1973. Toxicity of quinaldine sulfate to fish. [Google Scholar]
- 26.Alexander RB, Ludtke AS, Fitzgerald KK, Shertz TL. Report 96-337 U.S. Geological Survey. Reston, VA, USA: 1996. Data from selected U.S. Geological Survey national stream water-quality monitoring networks (WQN) on CD-ROM. [Google Scholar]
- 27.Mount DR, Mount DI. A simple method of pH control for static and static-renewal aquatic toxicity tests. Environ Toxicol Chem. 1992;11:609–614. [Google Scholar]
- 28.American Public Health Association, American Water Works Association, and the Water Environment Federation. Standard Methods for the Examination of Water and Wastewater. 20th. American Public Health Association; Washington, D.C, USA: 1998. [Google Scholar]
- 29.Williams DA. Interval estimation of the median lethal dose. Biometrics. 1986;42:641–645. [PubMed] [Google Scholar]
- 30.Robinson RA, Stokes RH. Electrolyte solutions. Academic Press; London, England: 1959. [Google Scholar]
- 31.van Dam RA, Hogan AC, McCullough CD, Houston MA, Humphrey CL, Harford AJ. Aquatic toxicity of magnesium sulfate, and the influence of calcium, in very low ionic concentration water. Environ Toxicol Chem. 2010;29:410–421. doi: 10.1002/etc.56. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


