Abstract
Objectives
Describe axial elongation using 14-year longitudinal data in a large, ethnically diverse group of myopic children, estimate age and axial length (AL) at stabilization and evaluate associations between the progression and stabilization of AL and myopia.
Methods
AL was measured by A-scan ultrasonography annually. AL data were fit with individual polynomial functions and curve-based parameters (AL at stabilization and age at stabilization when annual rate of axial elongation ≤0.06 mm) were estimated. For myopia progression, non-cycloplegic spherical equivalent refractions were fit with Gompertz functions.
Results
Four hundred thirty-one participants, with AL and myopia data fit successfully, were classified into four cohorts: Younger (n=30); Older (n=334); AL Stabilized at Baseline (n=19); AL Not Stabilized (n=48). At AL stabilization, participants in the Younger and Older Cohorts, mean (sd) age and AL were 16.3 (2.4) years and 25.2 (0.9) mm, respectively. No associations were found between age at AL stabilization and ethnicity, sex, or number of myopic parents. At stabilization, sex and number of myopic parents (both p< 0.003), but not ethnicity, were significantly associated with AL. AL and myopia progression curves were highly correlated overall (all r>0.77, p< 0.0001). However, unlike AL, the amount of myopia did not differ significantly between males and females.
Conclusions
In the majority of participants, AL increased rapidly at younger ages and then slowed and stabilized. The close association between growth and stabilization of AL and myopia is consistent with the suggestion that axial elongation is the primary ocular component in myopia progression and stabilization.
Keywords: axial length, myopia, refractive error, children’s vision
INTRODUCTION
Refractive error occurs when there is a mismatch between the location of the focal plane, produced by the cornea and the crystalline lens, and the location of the retina, which is controlled by the axial length of the eye. In the early postnatal period, refractive error is common in both humans and animals. Most children’s eyes are hyperopic during this period; the axial length is too short for the eye’s optics.1–4 During postnatal development, an emmetropization mechanism modulates axial growth of the eye, so that the retina comes to lie at or near the focal plane, as evidenced by animal studies.5–10 This process typically occurs in children before two years of age.2,3,11 During early childhood, refractive error remains low in most children even though the axial length increases by several millimeters,12–16 suggesting that the emmetropization mechanism continues to actively match eye growth to the optical power of the eye.
Despite the presence of an emmetropization mechanism, some children’s eyes gradually elongate beyond the point of emmetropia, so that the retina comes to be located behind the focal plane, producing myopia. Juvenile-onset myopia typically develops between six and 12 years of age.17–21 Why the eyes of these children grow more than is needed to maintain emmetropia is not fully understood. Genetic factors can be involved,22,23 as may factors related to the visual environment such as inaccurate accommodation to near targets, which produces hyperopic defocus,24,25 peripheral defocus26 or limited time outdoors.27–29 In many children, the amount of myopia increases for several years during the adolescent period before myopia stabilization occurs.30,31
Of considerable interest is the role of axial elongation in progression and stabilization of juvenile-onset myopia. Animal studies have concluded that induced myopia is primarily due to increases in axial length over and above normal growth.8,32,33 Only a few studies have directly compared myopia progression and axial elongation in children, and most have evaluated the relationship over a limited period of time.12,13,15,34–38 The consistent finding has been a high correlation between the progression of myopia and increases in axial length, particularly in the early phases of more rapid progression and eye growth. For instance, the COMET study36 found a high correlation between increased axial length and myopia progression for males and females (R2=0.90 and 0.87, respectively) over a three-year period. However, most previous studies have been limited by short follow-up periods at younger ages or high losses to follow-up for the full duration of the follow-up period, limiting their ability to characterize the full time course of axial growth and stabilization and the degree to which it parallels myopia progression and its stabilization.
The previous COMET study30 evaluated myopia progression over 11 years of visits, but not axial elongation. No study has followed the course of axial elongation and myopia progression in individual children until and beyond the time when myopia has stabilized. This information can be useful for understanding the mechanisms that influence the course of myopia. It also may be helpful in assessing whether changes in axial length can serve as a surrogate measure for myopia progression in clinical trials where refractive changes cannot be assessed independently, such as those evaluating the success of orthokeratology as a myopia treatment.
The present report describes results from the COMET cohort. COMET began as a clinical trial evaluating two types of spectacle lenses for myopia control. After five years, COMET became a longitudinal observational study that examined myopia progression and stabilization and also measured axial length over 14-years in a large, ethnically diverse group of children. The current study aimed to: 1) describe the course of axial elongation, 2) estimate the age and axial length at stabilization, 3) evaluate associations between parameters at axial length stabilization with baseline age, sex, ethnicity, treatment and number of myopic parents, and 4) evaluate associations between the progression and stabilization of axial length and myopia.
MATERIALS AND METHODS
Study Population
Details of the COMET study cohort, design, recruitment and baseline characteristics have been described previously.39–41 The COMET and protocols conform to the tenets of the Declaration of Helsinki. The research protocols were reviewed and approved by the institutional review boards of each participating institution. Informed consent(parents) and assent(children) were obtained. In brief, 469 children were recruited from the following four clinical sites: University of Alabama at Birmingham School of Optometry, Birmingham, Alabama; New England College of Optometry, Boston, Massachusetts; University of Houston College of Optometry, Houston, Texas; and Salus University Pennsylvania College of Optometry, Philadelphia, Pennsylvania. Inclusion criteria were: ages 6 to <12 years old, spherical equivalent between −1.25 and −4.50 D in each eye, astigmatism ≤1.50 D in either eye, anisometropia ≤1.00 D SER, birth weight ≥1250 grams, and visual acuity with distance correction of 0.20 LogMAR (20/32) or better. At baseline, all participants were randomly assigned to wear either progressive addition lenses (PALs), Varilux comfort with +2.0 D add, or single vision lenses (SVLs) during all waking hours and followed for three years, with the clinical trial results reported at that time.41 The children wore their originally assigned lenses for an additional two years, after which they could wear any type of spectacle or contact lenses, in consultation with their study optometrist. The study then became an observational longitudinal study, with annual visits continuing for up to 14 years.
Inclusion in this current study required at least 6 years of follow-up and seven measures of axial length. Therefore, twenty-nine participants with less than 6 years of follow-up and one participant who developed keratoconus during the study were excluded from all analyses. Of the remaining 439 COMET participants who completed at least six years, 84% (n=367) completed their 14-year visits. Of the 367 completing 14-year visits, 74% (n=272) of participants had all 14 follow-up visits, 17% (n=63) missed only one visit, 5% (20) missed 2 visits, 3% (n=11) missed 3 visits, and only 0.3% (n=1) missed four visits. No participant missed more than four visits. Four participants underwent refractive surgery, two before the 11-year visit, one before the 12-year visit, and one before the 14- year visit; therefore, their data after refractive surgery were omitted from the analyses.
Procedures
At each annual visit, axial length was measured by Sonomed A-2500 (New York, USA) ultrasonography. At least three but ideally five consecutive measurements were taken for each eye and poor quality A-scans (e.g. poor component definition, flattening anterior chamber or incomplete corneal touch) were deleted.42 Axial length values were then averaged over three to five measurements. At baseline and each subsequent visit over the 14-year follow up period the percentage of participants having five measurements for each eye was 94% (441/469) or higher. Refractive error (sphere, cylinder, and axis) was measured by non-cycloplegic and cycloplegic autorefraction using two drops of 1% tropicamide in both eyes using a Nidek ARK 700 autorefractor (Nidek, Gamagori, Japan). Cycloplegic autorefraction, the main clinical trial outcome measure, was measured annually. Non-cycloplegic autorefraction was measured semi-annually for the first four and half years and annually thereafter. Five sequential non-cycloplegic autorefraction measurements were taken in each eye. Non-cycloplegic measurements, on average, were more myopic than the cycloplegic autorefraction at baseline by 0.19 D (0.22)39 and by 0.25 D (0.23) throughout the 14 years of follow-up. Non-cycloplegic refractions were used for analyses in this study because more data points were available for curve fitting. Spherical equivalent refraction (SER) values were calculated and the five values were averaged for analyses. Because of the high correlation between right and left eyes for both axial length and non-cycloplegic refraction measurements at baseline39 and at follow-up41, right eye data were used for these analyses.
Curve Fitting for Axial Elongation (Fractional Polynomial Function)
The fractional polynomial function was used to model longitudinal axial elongation Because the fractional polynomial function has the flexibility to fit a larger variety of curves43 and was successfully used to model nonlinear axial length data in a cohort of 1775 Asian children aged 6 to 10 years.15 The Gompertz function, which was used to model myopia progression and stabilization in COMET,30 could not be applied to axial length data because it requires imputing values for onset.
For these analyses, axial length data of each participant were fit with an individual polynomial function. Curve-based parameters (i.e., age and axial length at stabilization) then were estimated for each participant based on the individual function. Mean squared error (averaged predicted values from the functions – observed values) was used to evaluate the quality of the model fits.
Using y to represent the axial length value at each visit, a general form of the fractional polynomial function was specified as:
where a and b are unknown polynomial coefficients to be estimated, and p1 and p2 are two exponents for the power transformations of age. The values p1 and p2 were selected from the candidate set {−2, −1, −0.5, 0, 0.5, 1, 2}.43 For each combination of p1 and p2 values selected, a fractional polynomial model was fit to the axial length data and a total of 28 models were tested for each participant. The combination of p1 and p2 values that provided the best model fitting indices (i.e., Akaike’s Information Criterion and Bayesian Information Criterion) was selected for the final analysis. The axial length data of 97.4% of participants had p1=-2 and p2=-1 as the best fitting functions. Therefore the final model, y = intercept + a × (age)−2+ b × (age)−1 was used for all analyses.
Definition of Outcomes (Age and Axial Length at Axial Length Stabilization)
The age at which axial length stabilized, a primary outcome of interest, was determined using the annual rate of change of axial length (i.e. first derivative of the fractional polynomial function). The age at axial length stabilization for each participant was defined as the age at which the annual rate of axial elongation was less than a specified cut-off point. This cut-off point was derived from the average within-subject variability for five repeated measures of axial length values at each visit (i.e. measurement error). Among COMET participants, the average within-subject variability of five repeated measures was 0.06 mm at baseline42 and ranged from 0.04 mm to 0.05 mm for all follow-up visits (years 1 – 14). Since within-subject variability can be treated as the measurement error, axial elongation less than the within-subject variability/measurement error was considered as axial length stabilization. Therefore, the largest within-subject variability value of 0.06 mm was used as our criterion to define the age at axial length stabilization for each participant. Once this age was established, the axial length value at axial length stabilization was estimated from the fractional polynomial function. In addition, axial length at any age could be estimated from the function.
Axial length stabilized at baseline
A group of participants was observed to have axial elongation rates less than 0.06 mm/year during the entire fourteen-year follow-up period. Since participants in this group appeared to have reached stable axial length before they enrolled in the study, their ages at axial length stabilization could not be determined by the fractional polynomial function and they were not included in the analyses for parameters of axial length stabilization.
Axial length not stabilized before the last study visit
Participants whose annual change rate of axial elongation was higher than 0.06 mm/year at all study visits were classified as “axial length not stabilized”. Since parameters of axial length stabilization for these participants were not available, they were excluded from all analyses related to axial length stabilization.
Baseline age and axial elongation status groups
Based on the foregoing, the axial elongation data were classified into four cohorts: 1) children with baseline age of 6−<8 years and axial elongation after baseline (Younger cohort), 2) children with baseline age ≥8 years and axial elongation after baseline (Older cohort), 3) children with axial length stabilized at baseline (Axial Length Stabilized at Baseline cohort) and, 4) children with axial elongation not stabilized before the last study visit (Axial Length Not Stabilized cohort). Younger and Older cohorts were examined separately because our study of myopia stabilization30 found differences between younger and older children.
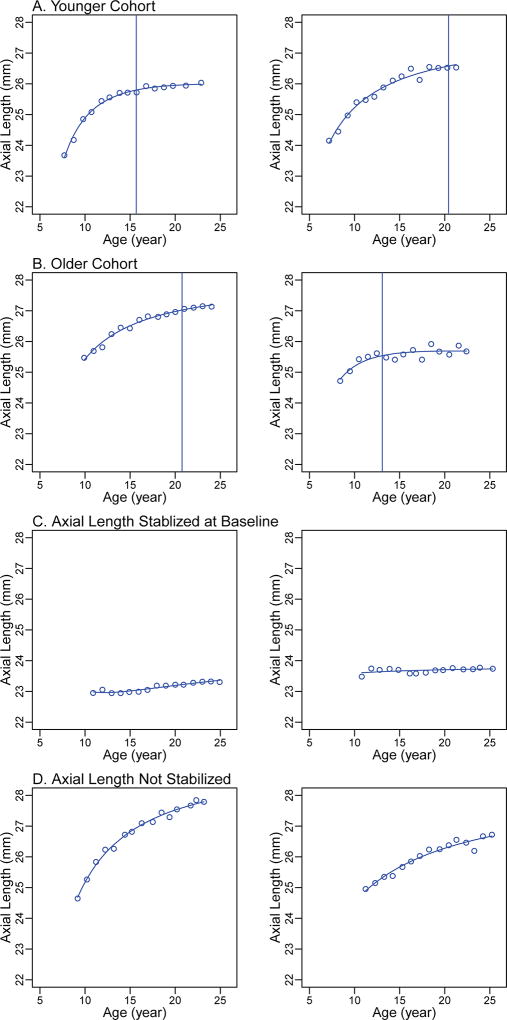
Two examples, within the 5th (low error) and 95th (higher error) percentiles of mean square error, from each of these four groups are shown in Figure 1. These examples include observed axial length values (circles) at each visit, the fitted fractional polynomial curves, and the estimated ages at stabilization (vertical lines).
Figure 1.
Individual curve examples. (A) Younger cohort: children with baseline age of 6-<8 years and axial elongation after baseline; (B) Older cohort: children with baseline age ≥8 years and axial elongation after baseline; (C) Axial Length Stabilized at Baseline cohort: the annual change rates of axial elongation between all visits < 0.06mm/year; (D) Axial Length Not Stabilized cohort: the annual change rates of axial elongation at all visits > 0.06mm/year. Circles indicate the observed axial length; vertical lines indicate the age of axial length stabilization.
Myopia Curve Fitting (Gompertz function)
In addition to examining axial length stabilization, an aim of this study was to evaluate the association between axial elongation and myopia progression. To evaluate the myopia progression in each participant, the spherical equivalent refractions of the right eye at each visit were fit with a Gompertz function based on 14 years of data using the “NLIN” procedure of SAS. Myopia stabilization was defined as the estimated SER within 0.50 D of the asymptote of the Gompertz function. This was the same procedure used in the previous COMET analysis of myopia stabilization, based on 11-year, rather than 14-year data.30 All individual curves were evaluated for non-convergence and poor model fit to ensure a valid comparison between axial elongation and myopia progression; non-converged and poor fit curves were excluded from analyses as described below. Also, from the fitted function the amount of myopia at any age could be calculated.
Statistical Analysis
Baseline characteristics were summarized across all participants and the four cohorts using means and standard deviations for continuous variables and frequencies and percentages for categorical variables. Relationships among baseline characteristics and the four axial elongation cohorts were evaluated using ANOVA or Chi-square tests. Curve-based parameters of age and axial length at stabilization were summarized using means and standard deviations and were compared between the Younger and Older cohorts using two-sample t tests. Correlations between age and axial length at stabilization were evaluated using Pearson’s correlations. The associations between baseline characteristics and age and axial length at stabilization were evaluated independently in univariate ANOVA models and jointly in multivariable regression models. To evaluate the correlations between axial elongation and myopia progression curves, average curves for the four cohorts and for males and females were created using averaged polynomial coefficients. Within each cohort, the overall relationship between the axial length and myopia curves was evaluated by semi-partial correlations based on linear mixed models, i.e. a weighted correlation between axial length and myopia at all visits. The overall correlation was tested for significance using a post-hoc F-test. Linear mixed models were also used to compare average axial length curves between males and females. Myopia curves were compared between males and females similarly. Associations between the axial length curve-based parameters (e.g. age and axial length at axial length stabilization) and the Gompertz curve- based parameters (e.g. age and amount of myopia at stabilization) were assessed using scatter plots and Pearson correlations.
All analyses were performed using SAS v 9.3 (the SAS institute, Cary, NC). For all linear mixed models, the “MIXED” procedure of SAS was used. P-values <0.05 were considered statistically significant.
RESULTS
Cohort characteristics
Longitudinal axial length data from the right eyes of 439 participants were fit successfully with fractional polynomial functions (mean square error, mean (SD): 0.013 (0.014) mm2, maximum=0.15 mm2). Longitudinal refractive data (mean spherical equivalent) of the same 439 participants were also fit with Gompertz curves; eight curves were excluded due to non-convergence after 2000 iterations (n=3) or poor fits (mean square error >0.4) (n=5). Analyses presented in this report were based on the remaining 431 participants with both axial elongation curves and Gompertz curve fits. As shown in Table 1 (overall column), this group at baseline was on average (mean (SD)) 9.81 (1.31) years old and ethnically diverse, with 27% reporting their ethnicity as African-American, 8% as Asian, 15% Hispanic, 5% Mixed and 46% White; 53% were female. In a subset of 225 participants with parental refraction data, 37% had two myopic parents, 47% had one myopic parent and 16% had neither parent with myopia (Table 1, overall column). Of the 431 participants with valid axial length and Gompertz curves, 212 and 219 were originally assigned to the PAL and SVL treatment groups, respectively. No significant difference was found for age or axial length at stabilization, or associated factors, between the two treatment groups (data not shown). Therefore, data from the two groups were combined for all analyses.
TABLE 1.
Characteristics of COMET Participants with Axial Length Curves (N = 431).
| Characteristic | Overall | Younger Cohort |
Older Cohort |
Axial Stabilized at Baseline |
Axial Not Stabilized |
P Valuea |
|---|---|---|---|---|---|---|
| n = 431 | n = 30 | n = 334 | n = 19 | n=48 | ||
|
| ||||||
| Baseline Age | ||||||
| Mean (SD) | 9.81 (1.31) | 7.41 (0.51) | 10.06 (1.06) | 11.05 (0.85) | 9.08 (1.41) | <0.0001 |
| n (%) | n (%) | n (%) | n (%) | n (%) | ||
|
|
||||||
| Ethnicity | 0.69 | |||||
| African-American | 115 (27) | 10 (33) | 88 (26) | 9 (47) | 8 (17) | |
| Asian | 33 (8) | 2 (7) | 26 (8) | 1 (5) | 4 (8) | |
| Hispanic | 63 (15) | 3 (10) | 49 (15) | 1 (5) | 10 (21) | |
| Mixed | 22 (5) | 1 (3) | 17 (5) | 1 (5) | 3 (6) | |
| White | 198 (46) | 14 (47) | 154 (46) | 7 (37) | 23 (48) | |
| Sex | 0.69 | |||||
| Male | 202 (47) | 11 (37) | 158 (47) | 9 (47) | 24 (50) | |
| Female | 229 (53) | 19 (63) | 176 (53) | 10 (53) | 24 (50) | |
| Number of Myopic parentsb | 0.47 | |||||
| None | 35 (16) | 2 (17) | 29 (16) | 0 (0) | 4 (15) | |
| 1 | 106 (47) | 4 (33) | 87 (49) | 3 (33) | 12 (44) | |
| 2 | 84 (37) | 6 (50) | 61 (34) | 6 (67) | 11 (41) | |
Younger cohort: children with baseline age of 6–7 years and axial elongation after baseline.
Older cohort: children with baseline age ≥8 years and axial elongation after baseline.
Axial Length Stabilized at Baseline cohort: the annual change rates of axial elongation at all visits < 0.06mm/year.
Axial Length Not Stabilized cohort: the annual change rates of axial elongation at all visits > 0.06mm/year.
based on ANOVA/Chi-square tests comparing means/proportions across four groups for baseline age, ethnicity, sex, treatment group and the number of myopic parents.
Based on a subset of participants with parental myopia data (n=225).
The group of 431 participants was divided into the four cohorts described previously: 1) Younger cohort (n=30), 2) Older cohort (n=303), 3) Axial Length Stabilized at Baseline cohort (n=19), and 4) Axial Length Not Stabilized cohort (n=48). As shown in Table 1, ethnicity, sex and number of myopic parents did not vary among the four cohorts. However, baseline ages varied significantly between cohorts (all pairwise comparisons, p<0.0001). As expected, participants in the Younger cohort, were the youngest (7.41 (0.51) years) and those in the Axial Length Stabilized at Baseline cohort were the oldest (11.05 (0.85) years).
Axial Elongation
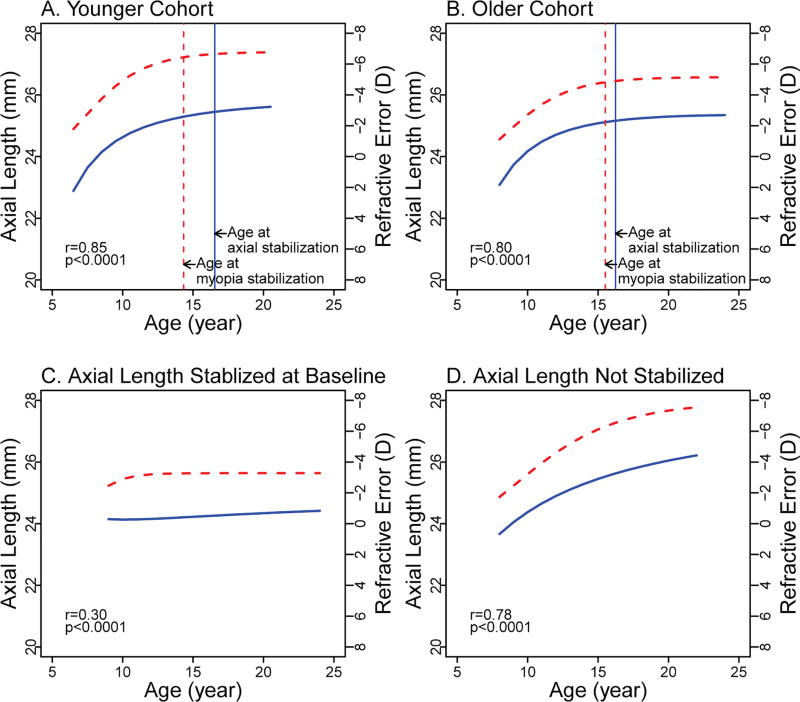
In the Younger and Older cohorts, axial elongation demonstrated a growth pattern of rapid increase followed by slowed elongation and stabilization (Figure 2 A & B). From ages 8 to 11 years (the earliest age both cohorts could be compared), the 3-year increase in axial length in the Younger cohort was 0.9 (0.3) mm. This was slightly, but not significantly larger in the Older cohort (1.10 (0.3) mm), (p=0.07). In contrast, over a later three-year span of ages (13 to 16 years), the increase was slower (p<0.001) and similar in the Younger cohort (0.3 (0.1) mm) and the Older cohort (0.3 (0.1) mm) (p=0.74), approximately 1/3 of the earlier increase. As expected, in the Axial Length Stabilized at Baseline cohort, axial length, by definition, showed very slight elongation (<0.06 mm/year) throughout the study (Figure 2C). In the Axial Length Not Stabilized cohort, axial length increased 1.0 (0.4) mm from 8 to 11 years, similar to both the Younger and Older cohorts. From 13 to 16 years axial length in this group increased 0.5 (0.1) mm (approximately half of the earlier increase) and did not stabilize before the last study visit (Figure 2D).
Figure 2.
Axial elongation and myopia progression by analysis cohort. Axial length curves are represented by blue solid curves and myopia progression curves are represented by red dashed curves. Vertical solid blue lines indicate the age of myopia stabilization; vertical dashed red lines indicate the age at myopia stabilization. r: semi-partial correlation between axial length and myopia curves based on linear mixed models. (A) Younger cohort: children with baseline age of 6-<8 years and axial elongation after baseline; (B) Older cohort: children with baseline age ≥8 years and axial elongation after baseline; (C) Axial Length Stabilized at Baseline cohort: the annual change rates of axial elongation at all visits < 0.06mm/year; (D) Axial Length Not Stabilized cohort: the annual change rates of axial elongation at all visits > 0.06mm/year.
Age and Axial Length at Stabilization in the Younger and Older Cohorts
At stabilization, the average age of the 364 participants in the combined Younger and Older cohorts was 16.3 (2.4) years and axial length was 25.2 (0.9) mm (Table 2). Age and axial length at stabilization were not significantly different between the two cohorts (Table 2). In analyses evaluating the relationship between age and axial length at stabilization, older age of axial length stabilization was moderately correlated with longer axial length at stabilization overall, and for each of these cohorts separately (correlations ranging from 0.44 to 0.48, p values <0.01) (Table 2).
TABLE 2.
Curve-Based Estimates of Age and Axial Length at Stabilization and their Correlations Overall and by Baseline Age and Axial Elongation Status Group (N=364)a.
| Overall n = 364 |
Younger Cohort n = 30 |
Older Cohort n = 334 |
||
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | p valueb |
|
| Age at Stabilization (years) | 16.3 (2.4) | 16.5 (2.7) | 16.2 (2.4) | 0.56 |
| Axial Length at Stabilization (mm) | 25.2 (0.9) | 25.5 (0.9) | 25.2 (0.9) | 0.11 |
| Correlation between Age and Axial Length at Stabilization | 0.44 | 0.48 | 0.44 | |
| Pearson’s Correlation(p value) | <0.0001 | 0.008 | <0.0001 | N/A |
The “Axial Length Stabilized at Baseline” cohort (n=19) and the “Axial Not Stabilized” cohort (n=48) were not included since parameters of axial stabilization were not available for these two groups.
Based on two-sample t-tests comparing means across three cohorts for the age and axial length at axial elongation stabilization.
Factors Associated with Age and Axial Length at Stabilization
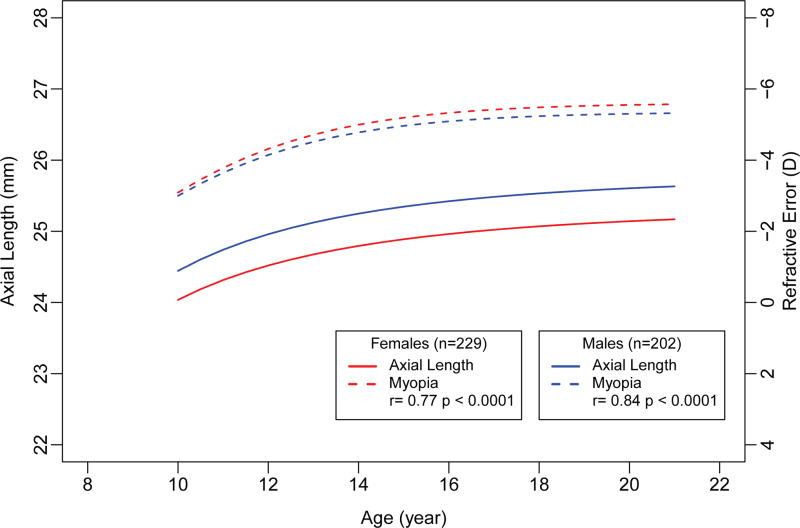
In the Younger and Older cohorts, no associations were found between the age at which axial length stabilized and ethnicity, sex, or number of myopic parents (Table 3). However, axial length at stabilization (Table 4) was significantly associated with sex and number of myopic parents, but not ethnicity. Male eyes (25.45 [0.06] mm) were significantly longer on average than female eyes (25.02 [0.07] mm) (adjusted difference 0.44 mm, p<0.0001). Male eyes were 0.50 mm longer on average than female eyes (p <0.0001) at baseline and at all follow-up visits (p<0.0001) (Figure 3). However, the rate of axial elongation by sex was similar (p=0.12).
TABLE 3.
Age (Years) at Axial Elongation Stabilization and Associated Factors (N = 364)a.
| Unadjustedb | Adjustedcd | |||||
|---|---|---|---|---|---|---|
| Participant Characteristic |
n | Age (y) Mean [SE] |
Difference (y) vs Reference Group (95% CI) P Value |
Overall P Value |
Difference (y) vs. Reference Group (95% CI) P Value |
Overall p Value |
| Ethnicityc | 0.86 | 0.86 | ||||
| African American | 98 | 16.29 [0.24] | Reference | Reference | ||
| Asian | 28 | 16.12 [0.45] | −0.17 (−1.18, 0.84) | −0.17 (−1.18, 0.84) | ||
| 0.74 | 0.74 | |||||
| Hispanic | 52 | 15.96 [0.22] | −0.33 (−1.14, 0.48) | −0.31 (−1.12, 0.50) | ||
| 0.42 | 0.46 | |||||
| Mixed | 18 | 16.27 [0.64] | −0.02 (−1.23, 1.19) | −0.02 (−1.23, 1.19) | ||
| 0.98 | 0.98 | |||||
| White | 168 | 16.38 [0.20] | 0.09 (−0.51, 0.69) | 0.10 (−0.50, 0.71) | ||
| 0.77 | 0.74 | |||||
| Sexc | 0.58 | 0.59 | ||||
| Male | 169 | 16.19 [0.17] | Reference | Reference | ||
| Female | 195 | 16.33 [0.18] | 0.14 (−0.35, 0.63) | 0.13 (−0.36, 0.64) | ||
| 0.58 | 0.59 | |||||
| Number of Myopic parentsd | 0.48 | 0.43 | ||||
| None | 31 | 15.91 [0.38] | Reference | Reference | ||
| 1 | 91 | 16.36 [0.26] | 0.45 (−0.54, 1.44) | 0.37 (−0.66, 1.40) | ||
| 0.64 | 0.40 | |||||
| 2 | 67 | 16.54 [0.31] | 0.64 (−0.40, 1.67) | 0.71 (−0.39, 7.80) | ||
| 0.23 | 0.21 | |||||
The “Axial Length Stabilized at Baseline” cohort (n=19) and the “Axial Length Not Stabilized” cohort (n=48) were not included since parameters of axial stabilization were not available for these two groups.
Based on univariable ANOVA tests.
Adjusted estimates were based on a multivariable model with ethnicity and sex as covariates.
Based on the subset with parental myopia data (n = 225). Adjusted estimates were based on a multivariable model with ethnicity, sex and number of myopic parents as covariates.
TABLE 4.
Axial Length (mm) at Axial Elongation Stabilization and Associated Factors (N = 364)a.
| Unadjustedb | Adjustedcd | |||||
|---|---|---|---|---|---|---|
| Participant Characteristic |
n | Axial Length (mm) Mean [SE] |
Difference vs Reference Group (95% CI) p Value |
Overall p Value |
Difference vs Reference Group (95% CI) P Value |
Overall p Value |
| Ethnicityc | 0.34 | 0.20 | ||||
| African-American | 98 | 25.17 [0.09] | Reference | Reference | ||
| Asian | 28 | 25.47 [0.15] | 0.30 (−0.08, 0.69) | 0.32 (−0.05, 0.69) | ||
| 0.12 | 0.09 | |||||
| Hispanic | 52 | 25.30 [0.11] | 0.13 (−0.18, 0.44) | 0.06 (−0.24, 0.36) | ||
| 0.42 | 0.71 | |||||
| Mixed | 18 | 25.46 [0.17] | 0.29 (−0.17, 0.75) | 0.29 (−0.15, 0.74) | ||
| 0.22 | 0.20 | |||||
| White | 168 | 25.17 [0.07] | 0.00 (−0.23, 0.23) | 0.05 (−0.27, 0.18) | ||
| 0.98 | 0.68 | |||||
| Sexc | <0.0001 | <0.0001 | ||||
| Male | 169 | 25.45 [0.06] | Reference | Reference | ||
| Female | 195 | 25.02 [0.07] | −0.43 (−0.61, −0.24) | −0.44 (−0.63, −0.26) | ||
| <0.0001 | <0.0001 | |||||
| Number of Myopic parentsd | 0.08 | 0.003 | ||||
| None | 31 | 25.05 [0.14] | Reference | Reference | ||
| 1 | 91 | 25.20 [0.10] | 0.14 (−0.23, 0.52) | 0.24 (−0.14, 0.62) | ||
| 0.08 | 0.18 | |||||
| 2 | 67 | 25.45 [0.11] | 0.40 (0.01, 0.79) | 0.44 (0.03, 0.84) | ||
| 0.05 | 0.03 | |||||
The “Axial Length Stabilized at Baseline” cohort (n=19) and the “Axial Not Stabilized” cohort (n=48) were not included since parameters of axial stabilization were not available for these two groups.
Based on univariable ANOVA tests.
Adjusted estimates were based on a multivariable model with ethnicity and sex as covariates.
Based on the subset with parental myopia data (n = 225). Adjusted estimates were based on a multivariable model with ethnicity, sex and number of myopic parents as covariates.
Figure 3.
Axial elongation and myopia progression by age for males and females (n=431). The axial length and myopia curves were truncated at ages 10 and 21 years to include data from both the Younger and Older cohorts) at each age. The Younger cohort did not have data after age 21 years and the Older cohort did not have data before age 8 years. r: semi-partial correlations between the axial length and myopia curves were based on linear mixed models and performed separately for males and females.
In the subset of participants with data on the number of myopic parents (Table 4), axial length at stabilization varied by number of myopic parents (p=0.003), with the longest eyes seen in participants with two myopic parents (18% of participants). In paired comparisons among the three myopic parent categories, axial length at stabilization differed only between participants with two myopic parents versus no myopic parents (9% of participants) (adjusted difference 0.44 mm, p=0.03).
Comparison of Axial Elongation and Myopia Progression
For all four cohorts, myopia progression paralleled axial elongation with a high correlation between the myopia and axial length curves (Figure 2). In the Younger and Older cohorts, progression and stabilization of myopia generally paralleled the pattern of initial rapid increase in axial length followed by slowed elongation and stabilization. Semi-partial correlations based on linear mixed models comparing the axial length and myopia curves for the four cohorts were Younger cohort, 0.85; Older cohort, 0.80; Axial Length Stabilized at Baseline, 0.30; and Axial Length Not Stabilized, 0.78 (p values <0.0001). The correlations were similar across all ages. The low correlation for the Axial Length Stabilized at Baseline was expected because there was little change in both myopia and axial length over time.
Myopia progression curves were similar for males and females (p=0.74) (Figure 3). However, unlike axial length, the amount of myopia did not differ between males and females at any visit (p>0.29 over all visits). Moreover, the correlations between the axial length and myopia curves were similar (male r=0.77 and female r=0.84, both p<0.0001).
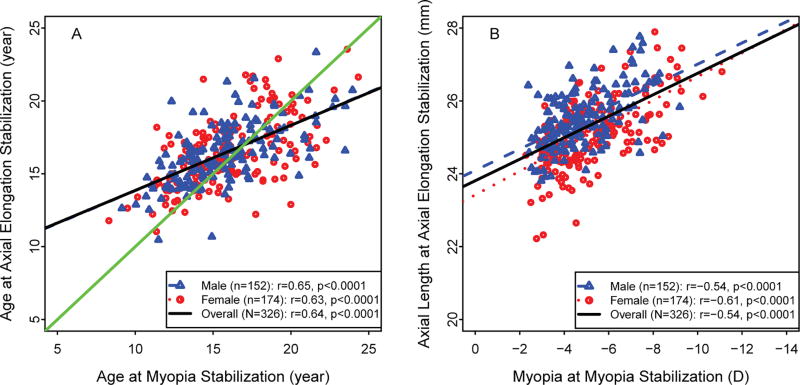
Because their ages at axial stabilization could not be determined, participants in the Axial Length Stabilized at Baseline and Axial Length Not Stabilized cohorts were not included in the following discussion. In the Younger and Older cohorts, the age at which axial length and myopia each stabilized was positively correlated overall (i.e. older axial length stabilization age was associated with older myopia stabilization age (r=0.64, p<0.0001) and by sex (male r=0.65 and female r=0.63, both p<0.0001) (Figure 4A). Similar results were observed for axial length and amount of myopia at stabilization (Figure 4B). At stabilization, longer axial length was associated with more myopia overall (r=0.54, p<0.0001) and for each sex (male r=0.54 and female r=0.61, both p<0.0001).
Figure 4.
Relationship between axial length stabilization and myopia stabilization for the Younger and Older cohorts (n = 364). (A) Relationship between age at axial length stabilization and age at myopia stabilization and (B) relationship between axial length at stabilization and myopia at stabilization. Correlations (r) and p values were based on Pearson’s correlations. Data from the Axial Length Stabilized at Baseline cohort (n=19) and the Axial Length Not Stabilized cohort (n=48) were not included because axial length stabilization could not be estimated.
In the Younger and Older cohorts, age at stabilization of axial length and of myopia could be compared. Axial length stabilized at an older age than did myopia in both the Younger cohort (axial length 16.5 (2.7) years and myopia 14.3 (3.4) years, p<0.0001) (Figure 2A) and the Older cohort (axial length 16.2 (2.4) years and myopia 15.5 (3.6) years, p<0.0001) (Figure 2B), though the age difference of 0.7 years was smaller for the Older cohort.
DISCUSSION
This 14-year longitudinal study in a large, ethnically diverse group of myopic participants from COMET found three patterns of axial length change over time. On average, most (84.4%) participants (the Younger and Older cohorts) showed similar high rates of axial elongation during the first three years after baseline, followed by slower axial elongation between ages 13 and 16 years and eventual stabilization of axial length at 16.3 (2.4) years. A few (4.4%) participants (the Axial Length Stabilized at Baseline cohort) had a negligible change in axial length. In the Axial Length Not Stabilized cohort (11.2% of participants), axial elongation initially was similar to the Younger and Older cohorts, but slowed less over time and had not stabilized by the end of 14 years of observation.
Stabilization of Axial Length
An important innovation of this study was providing a criterion for axial length stabilization. Unlike the Gompertz function, used to estimate myopia progression and stabilization here and previously,30,31 the individual polynomial functions did not provide an asymptote defining axial length stabilization. We used a definition based on the variability encountered in making A-scan ultrasound measures across the 14 years of the study. When annual increases in an individual function remained smaller than measurement variability (0.06 mm), the axial length had effectively stabilized.
Importantly, this was an objective criterion based solely on axial length measurements, allowing us to characterize the progression and stabilization of axial length and measure age and axial length at stabilization. In addition, we could then examine factors associated with these two parameters. Furthermore, because we independently characterized myopia progression and stabilization in the same participants we could compare, as independent variables, axial elongation and stabilization with myopia progression and stabilization.
Two other studies13,15 provided axial length curves in progressing myopic children at ages overlapping with the ages in our study. Generally, the curves are similar, but differences in subject groupings make it difficult to compare in detail the curves across these studies. For instance, we separately plotted curves for the Younger and Older cohorts and for males vs. females, which was not done in these studies. Wong et al.15 used a comparable nonlinear fractional polynomial function. Interestingly, their “persistent myopia” curve (616 Singaporean children) is almost identical to our Younger cohort and covers approximately the same ages (6–11 years), though the COMET Younger cohort has data until age 20 years. In the Jones et al.13 study, the calculated curve for axial length in the 247 “myopes” as a function of age was more linear (less curved) than the curves of our Younger, Older, and Axial Length Not Stabilized cohorts. The slope of their curve, showing increased axial length over time, was similar to the present study, but the axial length values (24.5 mm at age 14 years vs. 25.5 mm in the present study) were lower, possibly because the values were derived from emmetropic data and applied to the myopic group. Neither previous studies provided estimates of the age of axial length stabilization or axial length at stabilization.
Age of Axial Length Stabilization
In the myopic participants in COMET, the range of ages at which axial length stabilized was wide and varied across the four cohorts. Average age at stabilization of the 364 participants was 16.3 years in the combined Younger and Older cohorts and older age at stabilization was moderately correlated with longer axial length at stabilization. Furthermore, the age at axial length stabilization did not differ significantly across ethnicity, sex, or number of myopic parents (Table 3). Mean age at axial length stabilization was similar for males (mean [SE] 16.19 [0.17] years) and females (16.33 [0.18] years), suggesting that the factors controlling the cessation of eye growth are independent of sex. Age at axial length stabilization in these cohorts also did not vary based on the number of myopic parents. In the 19 participants in the Axial Length Stabilized at Baseline cohort, axial length stabilized at a mean age of 11 years. In the 48 participants from the Axial Length Not Stabilized cohort, axial length was still increasing at a mean age of about 23 years; thus, these data were not included in the above analyses.
Axial Length when Stabilized
The axial length at stabilization was significantly associated with sex (males had longer eyes) (Table 4) but not ethnicity. The difference in eye length in males and females occurred at an early age. At baseline, male eyes were longer than female eyes by 0.44 mm.39 This difference was sustained from baseline to stabilization, so that males had longer eyes than females at all visits. However, the rate of axial elongation from baseline to stabilization did not vary by sex. The similar axial elongation rate in males and females was seen initially in COMET between baseline and the 3-year visit (non-significant difference of 0.02 mm in axial elongation).36 Comparable rates of axial elongation in males and females after two years of follow-up also were found in a clinical trial evaluating orthokeratology compared to spectacles to slow myopia progression in 128 Chinese children between 7–14 years of age.44
Axial length at stabilization also was significantly longer in participants with two (18% of participants) versus no (9% of participants) myopic parents, suggesting a role for genetics and/or a shared environment. However, there was not a significant difference in axial length between participants with one vs. two myopic parents, suggesting that having any myopic parent could promote longer axial length.
Progression of Axial Length and Myopia
Using longitudinal data, our study evaluated the correlation between axial elongation and myopia progression. This is the first study to follow and compare longitudinal data on both parameters over a long period of time in a large, ethnically-diverse group of myopes. For all four cohorts, there was a strong parallel between axial and myopia longitudinal curves (Figure 2). Participants in the Younger and Older cohorts had a parallel, rapid increase in both axial length and myopia, followed by slowing and stabilization in both. The small number of participants whose axial length had stabilized when they entered the study showed very limited myopia progression. The myopia in the group whose axial elongation did not stabilize after 14 years also showed a parallel progression with axial elongation.
The correlation (r=0.87) between axial elongation and myopia progression from baseline to axial length stabilization in the present study was very similar to the correlation (r= 0.88) between myopia elongation and myopia progression we reported in the COMET cohort after 3 years of follow-up.36 To the extent their data allowed, other longitudinal studies13,15,34 noted a correspondence between axial elongation and myopia progression. However, those studies did not directly compare the shapes of the axial elongation and myopia progression curves in the same individuals, nor could they follow both axial length and myopia to stabilization as done in the present study. Two studies with much shorter follow-up of 2.5 – 3 years in children at earlier stages of myopia found significant correlations between axial elongation and myopia progression of 0.6437 and 0.7745, respectively.
The strong association between axial elongation and myopia progression is further supported by our finding that in 74% (14/19) of participants whose axial length did not change during 14 years of follow-up, their myopia remained unchanged.
Stabilization of Axial Length and Myopia
Our study also found that although axial length and myopia stabilized at generally similar ages (Figure 2) axial length tended to stabilize slightly later than myopia. In the Younger cohort, axial length stabilized at 16.5 (2.7) years and myopia stabilized at 14.3 (3.4) years. In the Older cohort, axial length stabilized at 16.2 (2.4) years and myopia stabilized at 15.5 (3.6) years. For participants in the Axial Length Not Stabilized cohort, the predicted age of myopia stabilization from the Gompertz function was older (21.5 (6.0) years), significantly older than the myopia stabilization age of the Younger and Older cohorts (p<0.0001).
These ages of myopia stabilization in the Younger and the Older cohorts differ from those reported previously30 for the “6-<8” year group (15.54 (3.64)) and “Overall” group 15.61 (4.17)). The reason is that, in the present study, cohort classification was based on axial length rather than myopia measurements, therefore the groups were not identical to the previous study. In addition, participants in the Axial Length Not Stabilized cohort, which had a much older age of myopia stabilization, were considered separately in the present study, but not previously.
Another difference from the previous study is that no association was found between age or amount of myopia at myopia stabilization and ethnicity. These different results likely occurred because African Americans were over-represented in the Axial Length Stabilized at Baseline cohort (Table 1), which was not examined separately in the earlier report.
Although age of stabilization of axial length and of myopia were generally similar, there was a tendency, in both the Younger and Older cohorts, for the age at axial length stabilization to be older than the age at which myopia stabilized. This raises the possibility that the factors producing myopic axial growth are added to those that produce normal eye growth. For instance, normal axial elongation occurs without myopia in children who remain emmetropic throughout childhood.46 Perhaps the factors that produce myopia-related axial elongation are added to genetically controlled normal elongation. To the extent that myopiagenic axial elongation is separate from normal axial growth, they need not stabilize at the same age. The “excess” elongation that produces myopia could dissipate before the end of normal growth and some axial elongation could continue afterwards without an increase in myopia, similar to the situation in emmetropic children. Of course, our determination of stabilization of axial length and of myopia was based on fixed criteria: <0.06 mm/year for axial length and within 0.5 D of the asymptote of the Gompertz curve for myopia, so using different criteria could have altered the observed relationship.
The parallel progression and stabilization of axial length and myopia in the present study contrast strongly with the absence of a parallel between myopia progression and measures of the optical components of the eye: small change in corneal power and unrelated changes in lens thickness. In a large (over 400) subset of COMET participants, corneal power decreased by less than 0.25 D over 14 years.47 In an analysis of the COMET cohort followed over 11 years, lens thickness was found to decrease and then increase similarly in all participants, whether their myopia progressed or not, and the minimum lens thickness was not correlated with the myopia after 11 years of follow up.48 These results: a parallel between axial elongation and myopia and a lack of parallel with the two optical ocular components, support axial length as the ocular component whose change is a primary determinant of refractive error.49,50
As shown in the COMET baseline paper,39 girls have significantly shorter axial lengths than boys (23.92 mm vs 24.36 mm, p<0.0001) but significantly steeper corneas (horizontal meridian 44.0 D in girls vs 43.5 D in boys, p<0.001). This combination resulted in a similar amount of myopia for male and female at baseline.
Clinical implications
Because of the strong association between axial elongation and myopia progression found in this study, changes in axial length may serve as a surrogate measure for myopia progression in clinical trials where refractive changes cannot be assessed independently, e.g., evaluating orthokeratology as a myopia treatment. If axial length is used as the primary outcome measurement, it would be helpful to relate it to myopia, for example, when talking to patients and their parents. Using these longitudinal data as a start, growth charts could be generated separately for boys and girls showing expected changes in myopia and the risk for high myopia, given a child’s age, sex, and axial length. They also could be used to evaluate the efficacy of a particular treatment.
Strengths and Limitations
A main strength of COMET was the large, ethnically diverse cohort, with well-characterized longitudinal axial length and myopia measurements collected over 14 years by trained and certified investigators. Retention in the cohort was excellent with 78% (367) of participants followed for 14 years and 94% of the cohort having at least 6 years of follow-up. A strength of the present study is that individual fractional polynomial curves were fit to each participant’s axial length data, providing individual estimates of age and axial length at stabilization. Further, we devised a criterion, based on measurement variability, to determine when axial length stabilization occurred, providing an estimate independent of myopia stabilization. This permitted us to examine the correlation between axial length stabilization parameters and myopia stabilization parameters based on Gompertz functions for each participant whose data met our criteria for inclusion in this analysis. Thus, the values for average age and average axial length at stabilization for the COMET cohort are reliable estimates that should be generalizable to a larger group of myopic children and young adults.
Because all COMET participants were myopic at the start of the study, we were unable to examine axial length and refractive error before myopia onset and during the earliest stage of myopia and axial length progression. Despite these limitations, this study provided valuable insight into the time-course of axial elongation and myopia progression and their stabilization.
CONCLUSIONS
This longitudinal study described the course of axial elongation from the early fast elongation stage through its slowing and stabilization using 14-year longitudinal data in a large, ethnically diverse group of myopic children, estimated the age and axial length at stabilization and evaluated associations between the progression and stabilization of axial length and myopia. This approach allowed us not only to characterize the course of axial elongation and stabilization and associated factors, but also to compare the axial changes with myopia progression in the same participants over many years. The high correlation between these two parameters supports a major role for axial elongation in the progression and stabilization of myopia. These results, a parallel between axial elongation and myopia and a lack of parallel with the two optical ocular components, support axial length as the ocular component whose change is a primary determinant of refractive error.49,50
Acknowledgments
This work was supported by NEI/NIH grants EY11740, 11805, 11756, 11754, 11752, and 11755.
Abbreviations
- COMET
the Correction of Myopia Evaluation Trial
- SD
standard deviation
- Younger cohort
children with baseline age of 6-<8 years and axial elongation after baseline
- Older cohort
children with baseline age ≥8 years and axial elongation after baseline
- Axial Length Stabilized at Baseline cohort
children with axial length stabilized at baseline
- Axial Length Not Stabilized cohort
children with axial elongation not stabilized before the last study visit
APPENDIX
COMET Study Group
Study Chair’s Office. New England College of Optometry, Boston, Massachusetts: Jane Gwiazda (Study Chair/Principal Investigator); Thomas Norton (Consultant); Li Deng (Biostatistician, 6/10–8/16); Kenneth Grice (Study Coordinator 9/96–7/ 99); Christine Fortunato (Study Coordinator 8/99–9/00); Cara Weber (Study Coordinator 10/ 00–8/03); Alexandra Beale (Study Coordinator 11/03–7/05); David Kern (Study Coordinator 8/05–8/08); Sally Bittinger (Study Coordinator 8/08–4/11); Debanjali Ghosh (Study Coordinator 5/11–7/13); Rosemarie Smith (Study Coordinator 8/13-present); Rosanna Pacella (Research Assistant 10/96–10/98). Changes in Lens Thickness in Myopic ! 2016 Taylor & Francis Group, LLC
Coordinating Center. Department of Preventive Medicine, Stony Brook University Health Sciences Center, Stony Brook, New York: Leslie Hyman (Principal Investigator); M. Cristina Leske (Co–Principal Investigator until 9/03); Mohamed Hussein (Co-investigator /Biostatistician until 10/03); Li Ming Dong (Co-Investigator /Biostatistician 12/03–5/10); Melissa Fazzari (Co-Investigator /Biostatistician 5/ 11–4/12); Wei Hou (Co-investigator /Biostatistician 10/12–present); Lynette Dias (Study Coordinator 6/ 98–present); Rachel Harrison (Study Coordinator 4/ 97–3/98); Wen Zhu (Senior Programmer until 12/06); Elinor Schoenfeld (Epidemiologist until 9/05); Qinghua Zhang (Data Analyst 04/06–present); Ying Wang (Data Analyst 1/00–12/05); Ahmed Yassin (Data Analyst 1/98–1/99); Elissa Schnall (Assistant Study Coordinator 11/97–11/98); Cristi Rau (Assistant Study Coordinator 2/99–11/00); Jennifer Thomas (Assistant Study Coordinator 12/00–04/04); Marcela Wasserman (Assistant Study Coordinator 05/ 04–07/06); Yi-Ju Chen (Assistant Study Coordinator 10/06–1/08); Sakeena Ahmed (Assistant Study Coordinator 1/09–6/11); Leanne Merill (Assistant Study Coordinator 10/11–8/13); Lauretta Passanant (Project Assistant 2/98–12/04); Maria Rodriguez (Project Assistant 10/00–6/13); Allison Schmertz (Project Assistant 1/98–12/98); Ann Park (Project Assistant 1/99–4/00); Phyllis Neuschwender (Administrative Assistant until 11/99); Geeta Veeraraghavan (Administrative Assistant 12/99–4/01); Angela Santomarco (Administrative Assistant 7/0l–8/04); Laura Sisti (Administrative Assistant 4/05–10/06); Lydia Seib (Administrative Assistant 6/07–present). National Eye Institute, Bethesda, Maryland: Donald Everett (Project Officer).
Clinical Centers
University of Alabama at Birmingham School of Optometry, Birmingham, Alabama: Wendy Marsh-Tootle (Principal Investigator); Katherine Weise (Optometrist 9/98– present); Marcela Frazier (Optometrist 1/10–present); Catherine Baldwin (Primary Optician and Clinic Coordinator 10/98–6/13); Carey Dillard (Clinic Coordinator and Optician10/09–6/13); Kristine Becker (Ophthalmic Consultant 7/99–3/03); James Raley (Optician 9/97–4/99); Angela Rawden (Back-up Optician 10/97–9/98); Nicholas Harris (Clinic Coordinator 3/98–9/99); Trana Mars (Back-up Clinic Coordinator 10/97–3/03); Robert Rutstein (Consulting Optometrist until 8/03).
New England College of Optometry, Boston, Massachusetts: Daniel Kurtz (Principal Investigator until 6/07); Erik Weissberg (Optometrist 6/99–present; Principal Investigator since 6/07); Bruce Moore (Optometrist until 6/99); Elise Harb (Optometrist 8/08–present); Robert Owens (Primary Optician until 6/13); Sheila Martin (Clinic Coordinator until 9/98); Joanne Bolden (Coordinator 10/98–9/03); Justin Smith (Clinic Coordinator 1/01–8/08); David Kern (Clinic Coordinator 8/05–8/08); Sally Bittinger (Position 8/08–4/11); Debanjali Ghosh (Clinic Coordinator 5/11–8/13); Benny Jaramillo (Back-up Optician 3/00–6/03); Stacy Hamlett (Back-up Optician 6/98– 5/00); Laura Vasilakos (Back-up Optician 2/02–12/05); Sarah Gladstone (Back-up Optician 6/04–3/07); Chris Owens (Optician 6/06–9/09; Patricia Kowalski (Consulting Optometrist until 6/01); Jennifer Hazelwood (Consulting Optometrist, 7/01–8/03).
University of Houston College of Optometry, Houston, Texas: Ruth Manny (Principal Investigator); Connie Crossnoe (Optometrist until 5/03); Karen Fern (Consulting Optometrist until 8/03; Optometrist since 9/03); Heather Anderson (Optometrist 1/10-present); Sheila Deatherage (Optician until 3/07); Charles Dudonis (Optician until 1/07); Sally Henry (Clinic Coordinator until 8/98); Jennifer McLeod (Clinic Coordinator 9/98–8/04; 2/07–5/08); Mamie Batres (Clinic Coordinator 8/04–1/06); Julio Quiralte (Back-up Coordinator 1/98–7/05); Giselle Garza (Clinic Coordinator 8/05–1/07); Gabynely Solis (Clinic Coordinator 3/07–8/11); Joan Do (Clinic Coordinator 4/12–8/13); Andy Ketcham (Optician 6/07–9/11).
Pennsylvania College of Optometry, Philadelphia, Pennsylvania: Mitchell Scheiman (Principal Investigator); Kathleen Zinzer (Optometrist until 4/04); Karen Pollack (Clinic Coordinator 11/03–6/13); Timothy Lancaster (Optician until 6/99); Theresa Elliott (Optician until 8/01); Mark Bernhardt (Optician 6/99–5/00); Daniel Ferrara (Optician 7/00–7/01); Jeff Miles (Optician 8/01–12/04); Scott Wilkins (Optician 9/01–8/03); Renee Wilkins (Optician 01/02–8/03); Jennifer Nicole Lynch (Optician & Back-up Coordinator 10/03–9/05); Dawn D’Antonio (Optician 2/05–5/08); Lindsey Lear (Optician 5/06–1/08); Sandy Dang (Optician 1/08–2/10); Charles Sporer (Optician 3/10–10/11); Mary Jameson (Optician 10/11–6/13); Abby Grossman (Clinic Coordinator 8/01–11/03); Mariel Torres (Clinic Coordinator 7/97–6/00); Heather Jones (Clinic Coordinator 8/00–7/01); Melissa Madigan-Carr (Coordinator 7/01–3/03); Theresa Sanogo (Backup Coordinator 7/99–3/03); JoAnn Bailey (Consulting Optometrist until 8/03).
Data and Safety Monitoring Committee: Robert Hardy (Chair); Argye Hillis; Donald Mutti; Richard Stone; Sr. Carol Taylor.
Footnotes
DECLARATION OF INTEREST
The authors report no commercial relationships or conflicts of interest.
References
- 1.Cook RC, Glasscock RE. Refractive and ocular findings in the newborn. American Journal of Ophthalmology. 1951;34:1407–13. doi: 10.1016/0002-9394(51)90481-3. [DOI] [PubMed] [Google Scholar]
- 2.Mutti DO, Mitchell GL, Jones LA, Friedman NE, Frane SL, Lin WK, et al. Axial growth and changes in lenticular and corneal power during emmetropization in infants. Invest Ophthalmol Vis Sci. 2005;46(9):3074–80. doi: 10.1167/iovs.04-1040. [DOI] [PubMed] [Google Scholar]
- 3.Mayer DL, Hansen RM, Moore BD, Kim S, Fulton AB. Cycloplegic refractions in healthy children aged 1 through 48 months. Arch Ophthalmol. 2001;119(11):1625–8. doi: 10.1001/archopht.119.11.1625. [DOI] [PubMed] [Google Scholar]
- 4.Chen J, Xie A, Hou L, Su Y, Lu F, Thorn F. Cycloplegic and non-cycloplegic refractions of Chinese neonatal infants. Invest Ophthalmol Vis Sci. 2011;52(5):2456–61. doi: 10.1167/iovs.10-5441. [DOI] [PubMed] [Google Scholar]
- 5.Norton TT. Animal models of myopia: Learning how vision controls the size of the eye. ILAR J. 1999;40(2):59–77. doi: 10.1093/ilar.40.2.59. [DOI] [PubMed] [Google Scholar]
- 6.Schaeffel F, Feldkaemper M. Animal models in myopia research. Clin Exp Optom. 2015;98(6):507–17. doi: 10.1111/cxo.12312. [DOI] [PubMed] [Google Scholar]
- 7.Smith EL, III, Hung LF, Arumugam B. Visual regulation of refractive development: insights from animal studies. Eye (Lond) 2014;28(2):180–8. doi: 10.1038/eye.2013.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wallman J, Winawer J. Homeostasis of eye growth and the question of myopia. Neuron. 2004;43(4):447–68. doi: 10.1016/j.neuron.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 9.Wildsoet CF. Active emmetropization - evidence for its existence and ramifications for clinical practice. Ophthalmic & Physiological Optics. 1997;17(4):279–90. [PubMed] [Google Scholar]
- 10.Bradley DV, Fernandes A, Lynn M, Tigges M, Boothe RG. Emmetropization in the rhesus monkey (Macaca mulatta): birth to young adulthood. Invest Ophthalmol Vis Sci. 1999;40(1):214–29. [PubMed] [Google Scholar]
- 11.Gwiazda J, Thorn F, Bauer J, Held R. Emmetropization and the progression of manifest refraction in children followed from infancy to puberty. Clinical Vision Science. 1993;8:337–44. [Google Scholar]
- 12.Fledelius HC, Christensen AC. Reappraisal of the human ocular growth curve in fetal life, infancy, and early childhood. British Journal of Ophthalmology. 1996;80:918–21. doi: 10.1136/bjo.80.10.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones LA, Mitchell GL, Mutti DO, Hayes JR, Moeschberger ML, Zadnik K. Comparison of Ocular Component Growth Curves among Refractive Error Groups in Children. Invest Ophthalmol Vis Sci. 2005;46(7):2317–27. doi: 10.1167/iovs.04-0945. [DOI] [PubMed] [Google Scholar]
- 14.Larsen JS. The sagittal growth of the eye. IV. Ultrasonic measurement of the axial length of the eye from birth to puberty. Acta Ophthalmol (Copenh) 1971;49(6):873–86. doi: 10.1111/j.1755-3768.1971.tb05939.x. [DOI] [PubMed] [Google Scholar]
- 15.Wong HB, Machin D, Tan SB, Wong TY, Saw SM. Ocular Component Growth Curves among Singaporean Children with Different Refractive Error Status. Investigative Ophthalmology & Visual Science. 2010;51(3):1341–7. doi: 10.1167/iovs.09-3431. [DOI] [PubMed] [Google Scholar]
- 16.Sorsby A, Benjamin B, Sheridan M, Stone J, Leary GA. Refraction and its components during the growth of the eye from the age of three. Medical Research Council Special Report Series. 1961;301:1–67. [PubMed] [Google Scholar]
- 17.Kleinstein RN, Sinnott LT, Jones-Jordan LA, Sims J, Zadnik K. New cases of myopia in children. Arch Ophthalmol. 2012;130(10):1274–9. doi: 10.1001/archophthalmol.2012.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edwards MH. The development of myopia in Hong Kong children between the ages of 7 and 12 years: a five-year longitudinal study. Ophthalmic Physiol Opt. 1999;19(4):286–94. doi: 10.1046/j.1475-1313.1999.00445.x. [DOI] [PubMed] [Google Scholar]
- 19.Saw SM, Tong L, Chua WH, Chia KS, Koh D, Tan DT, et al. Incidence and progression of myopia in Singaporean school children. Invest Ophthalmol Vis Sci. 2005;46(1):51–7. doi: 10.1167/iovs.04-0565. [DOI] [PubMed] [Google Scholar]
- 20.Zhao J, Mao J, Luo R, Li F, Munoz SR, Ellwein LB. The progression of refractive error in school-age children: Shunyi district, China. Am J Ophthalmol. 2002;134(5):735–43. doi: 10.1016/s0002-9394(02)01689-6. [DOI] [PubMed] [Google Scholar]
- 21.French AN, Ashby RS, Morgan IG, Rose KA. Time outdoors and the prevention of myopia. Exp Eye Res. 2013;114:58–68. doi: 10.1016/j.exer.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 22.Hammond CJ, Snieder H, Gilbert CE, Spector TD. Genes and environment in refractive error: the twin eye study. Invest Ophthalmol Vis Sci. 2001;42(6):1232–6. [PubMed] [Google Scholar]
- 23.Hawthorne FA, Young TL. Genetic contributions to myopic refractive error: Insights from human studies and supporting evidence from animal models. Exp Eye Res. 2013;114:141–9. doi: 10.1016/j.exer.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 24.Gwiazda J, Thorn F, Bauer J, Held R. Myopic children show insufficient accommodative response to blur. Invest Ophthalmol Vis Sci. 1993;34(3):690–4. [PubMed] [Google Scholar]
- 25.Goss DA. Clinical accommodation and heterophoria findings preceding juvenile onset of myopia. Optometry and Vision Science. 1991;68:110–6. doi: 10.1097/00006324-199102000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Smith EL., III Prentice award lecture 2010: a case for peripheral optical treatment strategies for myopia. Optom Vis Sci. 2011;88(9):1029–44. doi: 10.1097/OPX.0b013e3182279cfa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rose KA, Morgan IG, Ip J, Kifley A, Huynh S, Smith W, et al. Outdoor activity reduces the prevalence of myopia in children. Ophthalmology. 2008;115(8):1279–85. doi: 10.1016/j.ophtha.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 28.Jones LA, Sinnott LT, Mutti DO, Mitchell GL, Moeschberger ML, Zadnik K. Parental history of myopia, sports and outdoor activities, and future myopia. Invest Ophthalmol Vis Sci. 2007;48(8):3524–32. doi: 10.1167/iovs.06-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deng L, Gwiazda J, Thorn F. Children’s refractions and visual activities in the school year and summer. Optom Vis Sci. 2010;87(6):406–13. doi: 10.1097/OPX.0b013e3181da8a85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.The_COMET_Group. Myopia Stabilization and Associated Factors Among Participants in the Correction of Myopia Evaluation Trial (COMET) Investigative Ophthalmology & Visual Science. 2013;54(13):7871–84. doi: 10.1167/iovs.13-12403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thorn F, Gwiazda J, Held R. Myopia progression is specified by a double exponential growth function. Optom Vis Sci. 2005;82(4):286–97. doi: 10.1097/01.opx.0000159370.66540.34. [DOI] [PubMed] [Google Scholar]
- 32.Smith EL, III, Hung LF. The role of optical defocus in regulating refractive development in infant monkeys. Vision Research. 1999;39(8):1415–35. doi: 10.1016/s0042-6989(98)00229-6. [DOI] [PubMed] [Google Scholar]
- 33.Marsh-Tootle WL, Norton TT. Refractive and structural measures of lid-suture myopia in tree shrew. Investigative Ophthalmology and Visual Science. 1989;30:2245–57. [PubMed] [Google Scholar]
- 34.Mutti DO, Hayes JR, Mitchell GL, Jones LA, Moeschberger ML, Cotter SA, et al. Refractive error, axial length, and relative peripheral refractive error before and after the onset of myopia. Invest Ophthalmol Vis Sci. 2007;48(6):2510–9. doi: 10.1167/iovs.06-0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lam CS, Edwards M, Millodot M, Goh WS. A 2-year longitudinal study of myopia progression and optical component changes among Hong Kong schoolchildren. Optom Vis Sci. 1999;76(6):370–80. doi: 10.1097/00006324-199906000-00016. [DOI] [PubMed] [Google Scholar]
- 36.Hyman L, Gwiazda J, Hussein M, Norton TT, Wang Y, Marsh-Tootle W, et al. Relationship of age, sex, and ethnicity with myopia progression and axial elongation in the correction of myopia evaluation trial. Arch Ophthalmol. 2005;123(7):977–87. doi: 10.1001/archopht.123.7.977. [DOI] [PubMed] [Google Scholar]
- 37.Huang CY, Hou CH, Lin KK, Lee JS, Yang ML. Relationship of lifestyle and body stature growth with the development of myopia and axial length elongation in Taiwanese elementary school children. Indian J Ophthalmol. 2014;62(8):865–9. doi: 10.4103/0301-4738.141047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fledelius HC, Goldschmidt E. Oculometry findings in high myopia at adult age: considerations based on oculometric follow-up data over 28 years in a cohort-based Danish high-myopia series. Acta Ophthalmol. 2010;88(4):472–8. doi: 10.1111/j.1755-3768.2008.01472.x. [DOI] [PubMed] [Google Scholar]
- 39.Gwiazda J, Marsh-Tootle WL, Hyman L, Hussein M, Norton TT. Baseline refractive and ocular component measures of children enrolled in the correction of myopia evaluation trial (COMET) Invest Ophthalmol Vis Sci. 2002;43(2):314–21. [PubMed] [Google Scholar]
- 40.Hyman L, Gwiazda J, Marsh-Tootle WL, Norton TT, Hussein M. The Correction of Myopia Evaluation Trial (COMET): design and general baseline characteristics. Control Clin Trials. 2001;22(5):573–92. doi: 10.1016/s0197-2456(01)00156-8. [DOI] [PubMed] [Google Scholar]
- 41.Gwiazda J, Hyman L, Hussein M, Everett D, Norton TT, Kurtz D, et al. A randomized clinical trial of progressive addition lenses versus single vision lenses on the progression of myopia in children. Invest Ophthalmol Vis Sci. 2003;44(4):1492–500. doi: 10.1167/iovs.02-0816. [DOI] [PubMed] [Google Scholar]
- 42.Kurtz D, Manny R, Hussein M, Group CS. Variability of the ocular component measurements in children using A-scan ultrasonography. Optom Vis Sci. 2004;81(1):35–43. doi: 10.1097/00006324-200401000-00008. [DOI] [PubMed] [Google Scholar]
- 43.Royston PAD. Regression using fractional polynomials of continuous covariates: parsimonious modeling (with discussion) Appl Stat. 1994;43:429–67. [Google Scholar]
- 44.Zhu MJ, Feng HY, He XG, Zou HD, Zhu JF. The control effect of orthokeratology on axial length elongation in Chinese children with myopia. BMC Ophthalmol. 2014;14:141. doi: 10.1186/1471-2415-14-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fulk GW, Cyert LA, Parker DE. A randomized trial of the effect of single-vision vs. bifocal lenses on myopia progression in children with esophoria. Optom Vis Sci. 2000;77(8):395–401. doi: 10.1097/00006324-200008000-00006. [DOI] [PubMed] [Google Scholar]
- 46.Siegwart JT, Norton TT. Perspective: How might emmetropization & genetic factors produce myopia in normal eyes? Optom Vis Sci. 2011;88:365–372. doi: 10.1097/OPX.0b013e31820b053d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scheiman M, Gwiazda J, Zhang Q, Deng L, Fern K, Manny RE, et al. Longitudinal changes in corneal curvature and its relationship to axial length in the Correction of Myopia Evaluation Trial (COMET) cohort. J Optom. 2016;9(1):13–21. doi: 10.1016/j.optom.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gwiazda J, Norton TT, Hou W, Hyman L, Manny R. Longitudinal Changes in Lens Thickness in Myopic Children Enrolled in the Correction of Myopia Evaluation Trial (COMET) Curr Eye Res. 2016;41(4):492–500. doi: 10.3109/02713683.2015.1034372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Curtin BJ. The Myopias: Basic Science and Clinical Management. Philadelphia: Harper & Row; 1985. p. 1985. [Google Scholar]
- 50.van Alphen GWHM. On emmetropia and ametropia. Optica Acta (Lond) 1961;142(Suppl):1–92. [PubMed] [Google Scholar]