Abstract
Background
The prefrontal cortex (PFC) plays a critical role in regulating emotional behaviors, and dysfunction of PFC-dependent networks has been broadly implicated in mediating stress-induced behavioral disorders including major depressive disorder (MDD).
Methods
Here we acquire multi-circuit in vivo activity from eight cortical and limbic brain regions as mice are subjected to the tail suspension test (TST) and an open field test (OFT). We use a linear decoder to determine whether cellular responses across each of the cortical and limbic areas signal movement during the TST and OFT. We then perform repeat behavioral testing to identify which brain areas show cellular adaptations that signal the increase in immobility induced by repeat TST exposure.
Results
The increase in immobility observed during repeat TST exposure is linked to a selective functional upregulation of cellular activity in infralimbic cortex (IL) and medial dorsal thalamic (Thal), and an increase in the spatiotemporal dynamic interaction between these structures. Inducing this spatiotemporal dynamic using “closed-loop” optogenetic stimulation is sufficient to increase movement in the TST in stress-naïve mice, while stimulating above the carrier frequency of this circuit suppressed movement. This demonstrates that the adaptations in IL-Thal circuitry observed after stress reflect a compensatory mechanism whereby the brain drives neural systems to counterbalance stress effects.
Conclusion
Our findings provide evidence that targeting endogenous spatiotemporal dynamics is a potential therapeutic approach for treating stress-induced behavioral disorders, and that dynamics are a critical axis of manipulation for causal optogenetic studies.
Keywords: infralimbic cortex, thalamus, synchrony, limbic, neural network, depression
Introduction
Major depressive disorder (MDD) is the leading cause of disability in the world. Despite the heterogeneous nature of the disorder, multiple studies support hyperactivity and/or network-hyperconnectivity involving subgenual cingulate cortex (Brodmann Area 25) as key neurophysiological alterations in MDD (1–3). Though these neural ‘biomarkers’ have been exploited to guide the development of deep brain stimulation and transcranial magnetic stimulation into viable MDD therapeutics (4, 5), we hypothesize that the spatiotemporal dynamics are a key feature associated with subgenual cingulate cortex-dependent network pathology in MDD. Knowledge of these pathological dynamics would be particularly important because they potentially could be controlled to optimize brain stimulation based therapies.
In this study, we use a data driven strategy identify the brain regions in mice that showed neural processing adaptations in response to repeat tail suspension stress. Using machine learning, we found that repeat tail suspension stress induced hyper-reactivity in IL, which is the rodent anatomical equivalent of subgenual cingulate cortex in humans based on anatomical connections (6). We then developed a closed-loop stimulation system based on the precise spatiotemporal alterations observed in IL-dependent long range circuitry after stress exposure. In particular, we monitored the activity within the circuit and controlled activity in Thal in a way that produced a specific set of spatiotemporal dynamics within the whole circuit. Closed-loop stimulation increased TST activity in stress naïve mice, while two standard fixed frequency stimulation patterns either failed to show effects or suppressed movement. We suggest that spatiotemporal dynamics that are endogenously activated in healthy animals to compensate for stress pathology can by harnessed and exploited to optimize brain stimulation based treatments.
The tail suspension test (TST) is a classic assay used to probe the impact of antidepressant therapeutics on the behavioral response of mice to a challenging experience (7, 8). In this assay, mice are subjected to an inescapable stressor in which they are suspended upside-down by their tail. The test induces a robust stress response (9), and the time animals spend immobile relative to the time they spend engaging in escape actions is interpreted as an indicator of their behavioral response to the uncontrollable stressor (7). Critically, prior exposure to stress diminishes behavioral responses during the TST (10). Thus, the TST assays an animal’s behavioral adaptation in response to prior stress exposure, and the test in and of itself induces a strong stress response. We exploited these two features of the test for our experiments. First, we measured behavioral and neurophysiological activity continuously during the TST (Fig. 1A). We defined the strength of the neural responses during an initial TST session in naïve mice. We then repeated testing on the subsequent day. This approach diminished their behavioral responses on the TST. Overall, this experimental strategy allowed us to monitor neural responses in the same animals when they were stress-naïve, and again after exposure to stress (in this case, the stress induced by the first TST session) using a single behavioral assay that is responsive to stress. We then uncovered the spatiotemporal dynamics across the specific brain areas that showed neural adaptions during the repeat test. Finally, we also performed control behavioral experiments using repeating testing in an open field.
Figure 1. Distributed cortico-limbic neuronal activity signals escape actions.
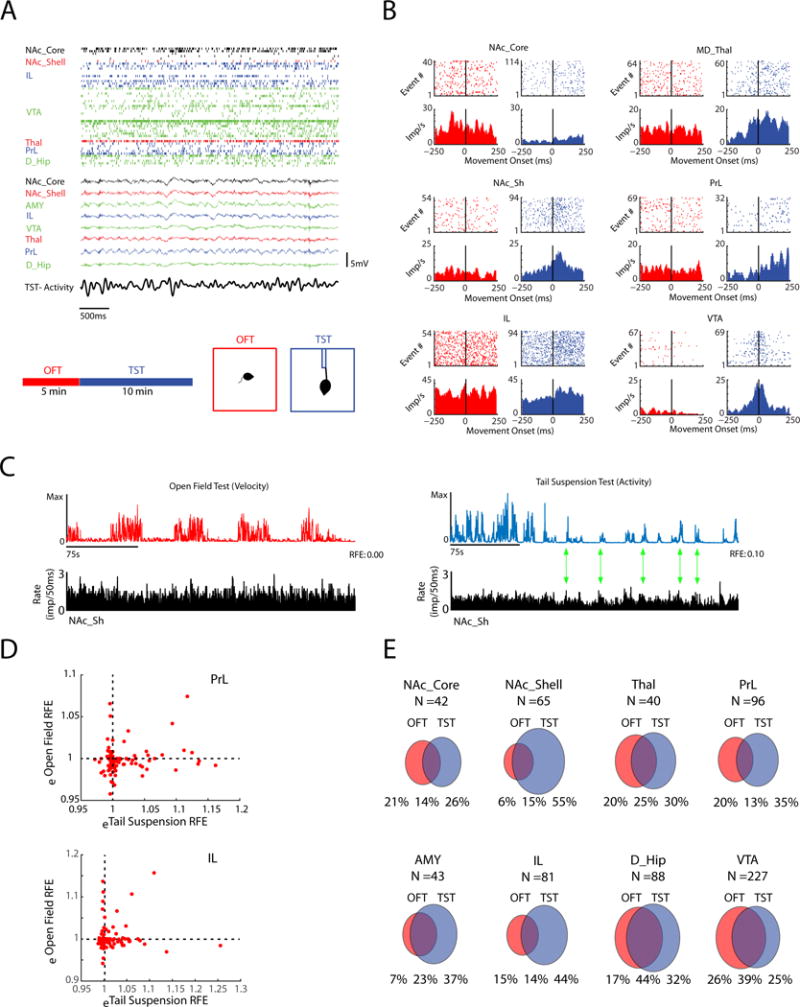
A) Raster plot showing unit and local field potential (LFP) activity acquired concurrently from cortical and limbic brain areas during the tail suspension test (TST; top). Schematic of behavioral recording sessions (bottom). B) Raster plot and peri-event time histograms showing examples of neuronal firing time-locked to movement onset in the open field test (OFT; red) and TST (blue). Histograms show average unit impulses per ms (imp/ms). C) Example of firing rate histogram of a NAc_Shell neuron (bottom) relative to the movement activity trace (top) in the OFT (left) and TST (right). Note that for this neuron, high TST movement was observed during periods of high cell activity (green arrows highlight peak to peak correlations). D) Example plots showing the relationship between spiking and behavioral activity for each of the PrL and IL neuron measured using a metric based on the reduction in fractional error (RFE). Units that showed eRFE values greater than 1 (RFE >0) explained signal action during each behavioral test. E) Venn diagrams quantify the portion of neurons in each brain area that signal action during each behavioral test. All data shown is from WT mice.
Materials and Methods
Animal Care and Use
Clock-Δ19 mice were created by N-ethyl-N-nitrosurea mutagenesis and produce a dominant-negative CLOCK protein as previously described (11). Mice used for TST recording experiments were bred on from heterozygouss (Clock Δ19/+) breeding pairs on a BALB/CJ and C57BL/6J mixed strain background, backcrossed > 8 generations onto a BALB/CJ strain background. Male Clock-Δ19 (ClockΔ19/ClockΔ19) and WT (+/+) littermate controls were used for all electrophysiological recording experiments presented in this study. Inbred BALB/cJ male mice (strain: 000651) purchased from the Jackson Labs were used for optogenetic stimulation and nCLASP experiments. Mice were housed three-five/cage on a 12-hour light/dark cycle, and maintained in a humidity- and temperature-controlled room with water and food available ad libitum. Behavioral and electrophysiological experiments were conducted during the light cycle (Zeitgeber time: 4–12). All studies were conducted with approved protocols from the Duke University Institutional Animal Care and Use Committees and were in accordance with the NIH guidelines for the Care and Use of Laboratory Animals.
Behavioral testing
Headstages were connected without anesthesia, and animals were habituated to the recording room for 90 minutes prior to testing. All behavioral testing was conducted under low illumination conditions (1–2 lux). Mice were initially placed in a 17.5in × 17.5in × 11.75in (L×W×H) chamber for five minutes of open field testing. The location of the animals was acquired in real time using NeuroMotive (Blackrock Microsystems, Inc., Salt Lake City, UT). Mice were then transferred to a tail suspension test (TST) apparatus (Med Associates, St. Albans, VT. MED-TSS-MS) that was modified to allow for continuous acquisition of animal motion. Mice were suspended 1cm from the tip of their tail for ten minutes. The activity trace was digitized at 2000Hz and stored in real time with our neurophysiological recording data. Open field and TST neurophysiological data were acquired during a single testing session, and the behavior testing session was repeated the next day. The quality of video tracking was confirmed offline using NeuroMotive.
Neurophysiological Data acquisition
Neurophysiological recordings were performed during the open field and TST. Neuronal activity was sampled at 30kHz, highpass filtered at 500Hz, sorted online, and stored using the CerePlex Direct acquisition system (Blackrock Microsystems Inc., UT). Neuronal data were referenced online against a wire within the same brain area that did not exhibit a signal to noise ratio greater than three to one. At the end of the recording, cells were sorted again using an offline sorting algorithm (Plexon Inc., TX) to confirm the quality of the recorded cells. Local field potentials (LFPs) were bandpass filtered at 0.5–250Hz and stored at 1000Hz. All neurophysiological recordings were referenced to a ground wire connected to both ground screws. Video recordings were acquired in real time using NeuroMotive, and synchronized with neurophysiological data.
Results
Broad cortical and limbic firing signaled TST-related behavior during the initial TST session. We used an unbiased approach to perform in vivo neurophysiological recordings of action potentials and local field potentials (LFPs). Specifically, animals were implanted in IL and seven additional brain regions that mediate emotional behavior including prelimbic cortex (PrL), nucleus accumbens (NAc-core and shell), amygdala (AMY; basolateral amygdala and central amygdala), Thal, dorsal hippocampus (D_Hip), and ventral tegmental area (VTA). Recordings were obtained while animals were subjected to a TST, and each recording session was immediately preceded by open field testing (OFT) run in low lighting conditions so as to be non-stressful. This enabled us to distinguish movement-related neuronal responses in the TST from those observed in a non-stressful test (Fig. 1A). The location of each mouse was recorded continuously during the OFT using video tracking, and behavioral activity was measured continuously during the TST using an accelerometer. When we performed event triggered averaging analysis relative to movement onset/offset, we found a wide array of neural responses. For example, we found cells that fired with movement onset, cells that fired prior to movement, and cells that fired after movement was initiated (see Fig. 1B, see also Supplemental Figure S1 for examples). To quantify the relationship between cellular firing and the complex patterns of movements measured during the two behavioral tests, we employed a linear decoder. This decoder allowed us to model the extent to which any specific pattern of behavior observed during a task condition (e.g. movement onset, acceleration, velocity, deceleration, immobility, etc.) was related to the spiking of each neuron. This approach quantified movement relative to neuronal spiking (rather than neuronal spiking relative to movement, Fig. 1B; see also Supplemental Figure S4), by creating a model based on the behavioral profile observed in the one-second window surrounding neuronal firing. This model returned an error metric, based on the reduction in fractional error (RFE) (12), that indicated how much behavioral variance was explained by the spiking of each neuron (Fig. 1C). If any type of movement observed during a test condition was related to the firing of a neuron, the model returned a RFE value greater than zero (Fig. 1D). On the other hand, if neuronal firing was not related to any type of movement observed during a test condition, the RFE returned a value less than or equal to zero. Using the decoder, we identified neurons from each cortical and limbic brain area that signaled movement during the TST, OFT, or both tests during the initial session (Fig. 1D). Several areas including VTA and D_Hip showed neurons that encoded movement generated during both tests. Other areas including IL, NAc_Shell, and AMY showed stronger bias towards signaling TST movement compared to movement in the OFT (Fig. 1E). Thus as expected, since the OFT and TST are very different contexts, many neurons exhibited firing that was related to the specific patterns of movement elicited during each test condition (8, 13).
Strikingly, when we trained additional linear decoders on data acquired during the TST session on the second day and compared them to the first TST session, we found that the brain-wide population of neurons showed an increase in their ability to signal TST-movement, as evidenced by an overall increase in the RFE values obtained for the neurons’ models (P<0.001, Kstat=0.11 using Kolmogorov-Smirnov test; N=647 and 620 neurons during session 1 and 2, respectively; Fig. 2A). As expected, animals exhibited higher immobility during the second testing session as well (p=0.002 using paired t-test; tstat1,13= 3.8; N=14; Fig. 2C left). In contrast, no differences were observed in open field movement-related signaling (P=0.23, Kstat=0.06 using the Kolmogorov-Smirnov test; Fig. 2B) or in the distance traveled in the open field between sessions (p=0.74 using paired t-test; tstat1,13= 0.3 Fig. 2C right). Thus, both the neural and behavioral adaptations that occurred with repeat testing were specific to the stressful assay.
Figure 2. Infralimbic cortex and thalamic responses increase during repeat TST testing.

A–B) Histogram of test related neuronal firing for all neurons recorded during repeated a) TST and b) OFT testing. Test related firing was quantified using the RFE of our spike-behavioral models (*P<0.05 for comparisons across days using Kolmogorov-Smirnov test; N=682 and 655 units for Day 1 and Day 2, respectively). C) Immobility time and distance traveled during repeat TST and OFT testing in WT mice (**P<0.01 using paired t-test; N=14 for WT mice). D) Immobility time and distance traveled during repeat TST and OFT testing in Clock-Δ19 mice (#P<0.05 for genotype x session effect of TST immobility using Mixed model ANOVA; N=14 WT mice and 13 mutant mice; P>0.05 using paired t-test for immobility time and distance travelled; N=13 for Clock-Δ19). No statistical difference in the variance of the immobility change was observed across the two populations of mice (F=0.66, P=0.47 using two sample F-test for equal variances). E) Area-specific expansion of TST-related movement function in WT mice (**P<0.05 using G-test of independence with Williams correction). F) No area-specific expansion of TST-related movement function was observed in Clock-Δ19 mice (P>0.05 for all comparisons using G-test of independence with Williams correction).
After finding that repeat exposure to the TST increased test-specific movement-related neuronal responses, we tested whether these neural changes were specific to any brain regions. We found that only IL and Thal showed significant increases in the portion of TST-responsive neurons that showed an RFE greater than zero (P<0.05 using G-test with Williams correction; Fig. 2E). Thus, our unbiased approach suggested that the changes in behavior that occurred with repeat testing were selectively associated to changes in IL and Thal population activity. This increase in the portion of TST-responsive neurons suggested either new IL/Thal neurons were added to the ensemble that encoded the TST-movements observed during the first session, and/or additional IL-Thal neurons signaled a new type of TST-movement that emerged during the second session.
To determine whether the IL and Thal hyper-reactivity observed in normal animals during the second TST session were behaviorally relevant and not simply a reflection of repeated exposure to the same TST assay, we performed our recording protocol in a genetic mouse line (Clock-Δ19 mice) that we have previously shown to exhibit resilience to multiple distinct assays of behavioral challenge including the forced swim test and learned helplessness (14–16). As expected, Clock-Δ19 mice were resilient to the adaptation induced by repeated TST exposure in the full group of normal animals (P=0.017; F1,25=6.51 for session × genotype interaction using two-way ANOVA, tstat1,12=0.2; P=0.83 for post-hoc testing using a paired t-test within Clock-Δ19 genotype; see Fig. 2D). No differences in TST immobility were observed across genotype within the first testing session (P=0.75, tstat1,25=0.3 for post-hoc testing across genotype using unpaired t-test). Thus, Clock-Δ19 mice were resilient to the behavioral adaptation induced by repeat TST exposure. Critically, no changes in IL or Thal TST-activity (or any of the other brain areas) were observed in the Clock-Δ19 mice (Fig. 2F). Thus, repeat TST testing failed to induce IL or Thal hyper-reactivity in a mouse model of stress resilience. This suggests that the reorganization of IL and Thal that occurred with repeat tail suspension testing was linked to the susceptibility of the WT animals to the initial exposure.
After determining that only IL and Thal showed changes in TST-related firing during repeat testing, we set out to test whether repeat testing also altered neurophysiological interactions between these structures. Cross-frequency phase coupling (CFPC) analysis has been shown to signal emotional responses across cortico-limbic circuitry (17, 18). Thus, we calculated CFPC between IL and Thal LFP activity. We limited our analysis to LFP segments selected from intervals when animals were immobile on the TST in order to ensure that difference in coupling observed across testing sessions did not simply reflect differences in the total movement (19). Using this approach, we found that the phase of 3–7Hz activity in IL coupled to the amplitude of low-gamma activity (30–70Hz) in the Thal (Fig. 3A–B). Critically, CFPC between these structures increased across testing sessions (t13 = 3.3, P = 0.0059 using paired t-test; Fig. 3B), similar to the cellular and behavioral changes described above. The strength of IL-Thal CFPC exhibited by individual animals was directly related to their immobility time during both testing sessions (P<0.05 for both comparisons using linear regression; Fig. 3C), and this relationship became stronger during the second session (F1,24= 5.63; P=0.026 using analysis of covariance). Thus, the behavioral relevance of this circuit increased with repeated testing. Directionality analysis showed that 3–7Hz LFP activity in the IL temporally preceded oscillatory activity in Thal, providing evidence that IL-Thal CFPC reflected directional activity in the IL-Thal pathway (Fig. 3D). No differences in low frequency (2–12Hz) power within IL or Thal, or coherence between the two structures were observed across testing sessions (F11,286=0.68, 0.63, and 0.08; P = 0.58, 0.53, 0.94, for comparison of cortical power, thalamic power, and IL-Thal coherence, respectively using repeated measures [RM] ANOVA, Fig. 3E). However, we found an increase in IL and Thal gamma power and coherence between across two testing sessions(t13=5.68, 3.53, and 4.28; P = 7.5×10−5, 0.004, 9×10−4, for comparison of cortical power, thalamic power, and IL-Thal coherence, respectively using repeated measures [RM] ANOVA, Fig. 3F). Thus, the adaptations in IL-Thal function that resulted from TST exposure were specific to the spatiotemporal dynamics involving gamma oscillatory across these brain areas.
Figure 3. Limbic reorganization is specific to stress-induced behavioral adaptation.
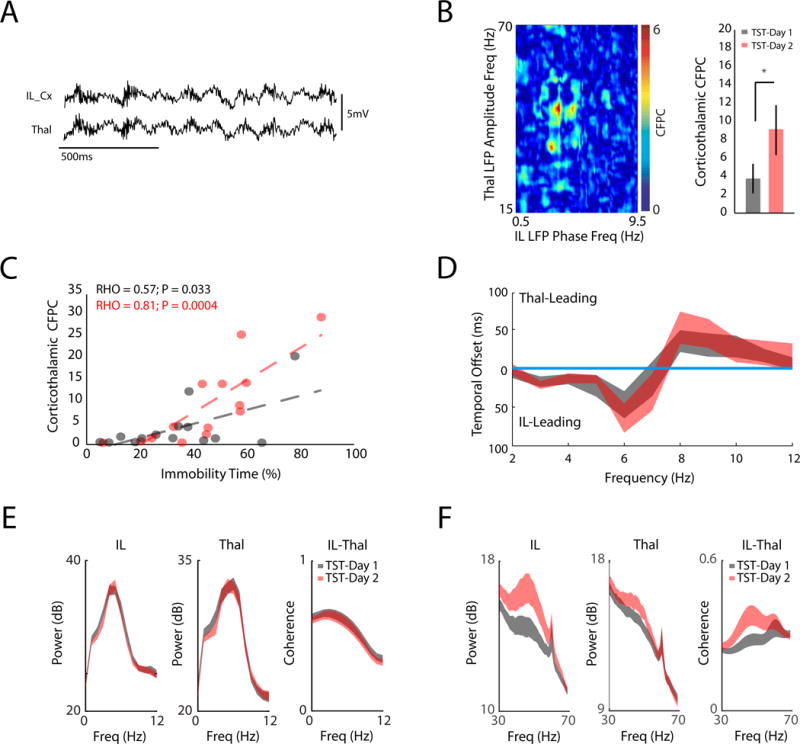
A) IL and Thal LFP traces (top). B) Image shows coupling between the phase of IL oscillations in frequencies ranging from 0.5–9.5Hz, and the amplitude of Thal oscillations ranging from 15–70Hz (left). CFPC was quantified between IL 3–7Hz oscillations and Thal 30–70Hz oscillations across TST testing sessions (P<0.05 using paired t-test; N=14 mice; right). C) IL-Thal CFPC was directly correlated with the immobility time observed across animals (P<0.05 using linear regression; bottom). This relationship increased across testing sessions (P<0.05 using analysis of covariance). D) Temporal offsets at which IL and Thal oscillations optimally phase synchronized at each frequency (figure shows 95% confidence interval observed across animals for both testing days; N=14, top). Frequencies that showed significant lag did not overlap with zero offset. E) IL and Thal 2–12Hz power, and IL-Thal 2–12Hz coherence across testing sessions (P>0.05 for all three measures across testing sessions using RMANOVA; data shown as mean±SEM). F) IL and Thal gamma power, and IL-Thal gamma coherence across testing sessions (P<0.05 for all three measures across testing sessions using paired t-test; data shown as mean±SEM).
After demonstrating that the IL and Thal cellular adaptations induced by stress were marked by changes in the spatiotemporal dynamics across IL-Thal circuitry, we set out to test whether the patterns we observed in IL-Thal circuitry were causally related to behavior. In order to accomplish this, we developed a new approach to manipulate spatiotemporal dynamics across PFC-dependent networks. Specifically, we injected a trans-synaptic wheat-germ agglutinin-tagged Cre recombinanse (WGA-Cre-mCherry)(20) into the PFC (prefrontal cortex; IL and PrL) of mice naïve to behavioral challenge. Infection with WGA-Cre results in high Cre expression in PFC neurons and modest Cre expression in the other neurons in the brain that form synaptic connections with PFC (both efferent and afferent synaptic connections). We then infected Thal with a floxed- ultra-fast Channel Rhodopsin-2 variant (ChETA) (21) (Fig. 4A; see also Supplemental Fig. S5). This strategy ultimately resulted in ChETA expression in cortical neurons that projected to thalamus, thalamic neurons that received input from PFC, and thalamic neurons that sent efferents to PFC. Finally, we invented a neural Closed Loop Actuator for Synchronizing Phase (nCLASP) to stimulate this subset of PFC axonal terminals and Thal neurons with gamma bursts timed to on-going oscillatory activity in cortex (Fig. 4B). nCLASP allowed us to drive bursts of Thal gamma activity that were phase-coupled to 3–7Hz oscillations in cortex, paralleling the CFPC physiological parameters of the IL-Thal circuit we measured during the TST. We calibrated this nCLASP system to deliver gamma bursts (3 successive 5ms light pulses with an inter-pulse interval of 15ms) initiated at the rising phase of IL 3–7Hz oscillatory cycles (Fig. 4B). Experimental animals were stimulated with blue light to activate ChETA and control animals were stimulated with yellow light which does not activate the opsin (Fig. 4B). Behavioral and neural responses were monitored while these animals were subjected to the TST. Stimulation with blue, but not yellow, light evoked Thal gamma activity (Fig. 4B; tstat1,13= 1.2 and 4.8; P=0.24 and 0.0003, for comparisons of IL and Thal evoked potential amplitude, respectively, using paired unpaired t-test; N=7–8 mice/group)
Figure 4. Properly timed IL-Thal stimulation increases resilience to behavioral challenge.

A) Schematic of protocol for IL-Thal stimulation (top), viral infection strategy (middle), and histological images (bottom). EYFP expression was present in layer V/IV PFC neurons and their apical dendrites, PFC axon terminals in Thal, and the soma of Thal neurons (bottom). B) Detailed schematic for nCLASP system (top). This protocol was used to deliver light pulses to Thal at the trough of IL 3–7Hz oscillations (bottom). Histogram shows the IL phase distribution at which gamma pulses were initiated during a TST session. Phase coupling of gamma burst light pulses was quantified using the Rayleigh test where Z = −log(P) (bottom left). IL and Thal evoked activity during nCLASP stimulation. C) Effects of nCLASP stimulation on OFT and TST-behavior (P<0.01 for comparison of blue and yellow light stimulation groups using RMANOVA for TST-behavior; red bars on the x-xais highlight time points where P<0.05 for post-hoc testing using unpaired t-test; data shown as mean±SEM). Mean TST-activity was quantified as mVolts/gram mouse. D–E) Effects of two open loop stimulation protocols on OFT and TST-behavior (**P<0.01, *P<0.05 for comparison of total activity across groups using students t-test; N=6–8 mice/group.
Activation of the IL-Thal circuit using nCLASP rendered the stress-naïve mice more resilient to the behavioral adaptation that occurred within a TST session. Specifically, mice stimulated with blue light exhibited less immobility and higher activity profiles compared to the animals stimulated with yellow light (F9,117=3.89, P = 0.008 for light effect on immobility time; F9,117=2.73, P = 0.009 for light effect on mean activity; comparisons of blue and yellow light groups were made using repeated measures ANOVA; P<0.05, tstat1,13=3.3 for comparison of full test immobility time using students t-test; Fig. 4C). Importantly, no differences in gross locomotion were observed when animals were stimulated in the open field (tstat11=−0.59; P=0.57 using two-tailed Students t-test), showing that stimulation of the IL-Thal pathway induced a behavioral effect that was specific to the TST paradigm. This was consistent with our findings that IL neurons preferentially signal behavior in the TST (compared to the open field; see Fig. 1D). Furthermore, IL-Thal stimulation had no impact on the immobility time or mean activity observed during the first four minutes of the TST (Fig. 4C), demonstrating that this stimulation approach did not simply induce hyperactivity. Rather, stimulation of the IL-Thal pathway using nCLASP diminished the behavioral adaptation that occurred across the 10 minute TST session.
Next, we tested two additional open-loop patterns in a new group of stress naïve mice. Our first open-loop stimulation protocol was designed to deliver the same number of light pulses as nCLASP, but in a manner not linked to oscillatory activity in the cortex or local gamma oscillations (constant 14.05Hz, 5ms pulse width; see Fig. 4D). IL-Thal stimulation with blue light reduced TST escape behavior during testing (tstat10=−2.24 and 2.27; P=0.049 and 0.047 for full test immobility time and activity, respectively, using two-tailed Students t-test), though this stimulation had no impact on OFT-behavior (t10=1.5 and P=0.316 using two-tailed students t-test; see Fig. 4D). Thus, IL-Thal circuit stimulation using nCLASP induced escape behaviors, while IL-Thal stimulation using open loop stimulation at 14.05Hz suppressed escape behaviors. Notably, the carrier frequency used for our first open-loop stimulation protocol was three times as fast as the carrier frequency of IL to Thal input (14.05Hz compared to 2–7Hz). Our second open-loop control stimulation was designed to deliver gamma bursts consisting of three light pulses at 4.68 Hz (14.05Hz/3; see Fig. 4E), which was similar to the frequency administered during nCLASP stimulation but, importantly, was not timed to IL activity. Stimulation with this open-loop pattern had no effect on TST behavior (tstat10=0.13 and 0.45; P=0.899 and 0.659 for full test immobility time and activity, respectively, using two-tailed Students t-test) or OFT behavior (t9=−0.57 and P=0.582 using Student’s t-test; see Fig. 4E). Thus, gamma burst stimulation of the IL-Thal pathway had to be timed to the endogenous IL oscillations in order induce movement, and carrier stimulation frequencies above the normal input frequency of the pathway (2–7Hz) suppressed it.
Discussion
The TST is widely used as a preclinical model of major depressive disorder due to the assay’s sensitivity to acute treatment with clinically relevant antidepressants. Additional testing is typically performed in an open field to clarify whether pharmacological/genetics manipulations induce TST activity due to their specific antidepressant-like effects or because they more generally induce hyperactivity. The TST is classically performed during a single 6 minute session in mice (7). However, to exploit the observation that the TST induces a robust stress response (9), we performed neural recordings during two TST sessions on successive days. This approach allowed us to concurrently dissect both the neural circuits responsible for TST escape action under normal conditions and the behaviorally relevant neural adaptions induced by repeated stress exposure.
Using a linear decoder, we found that neurons in all the cortical and limbic regions we probed exhibited firing linked to TST-movement. Furthermore, many cells in these areas signaled movement in TST but not the OFT. Thus, either a different set of movements were induced by the two tests, or a different population of cells encoded the overlapping set of movements induced by the two distinct tests. In both instances, the cellular responses we discovered clearly demonstrate that the OFT and TST exploit distinct neural processes reflected across limbic circuity. The differential sensitivity of TST and OFT-related movement to acute antidepressant treatment may be due to these non-overlapping neuronal responses. Importantly, while immobility during the TST by no means recapitulates the range of symptoms observed across major depressive disorder (22), our findings suggest that the TST can indeed be used to effectively probe the function of widely distributed brain networks implicated in MDD. Normal TST behavior reflects normal function in these brain networks, and altered activity in the brain areas that compose the TST network can be reflected by behavioral dysfunction in the TST vs. the OFT. Additional experiments will be needed to clarify the exact nature of the information that is encoded by these TST-movement related neurons.
Repeated TST-stress exposure selectively induced changes in IL and Thal firing. Based on the spatiotemporal dynamics observed between IL and Thal, our findings showed that IL-Thal CFPC was directly correlated with TST-immobility during the initial testing session. Paradoxically, the correlation slope between IL-Thal CFPC and TST-immobility increased during the repeat TST session (see Fig. 3C), such that the amount of immobility exhibited by mice during the second session was lower than expected based on their IL-Thal CFPC. Consistent with the observations that direct PFC stimulation induces an anti-depressant like effect in multiple MDD-related test paradigms (6, 23), these findings suggested that IL-Thal CFPC may reflect a neurophysiological process that induces TST movement. When we used our nCLASP system to recapitulate the spatiotemporal dynamic we identified in IL-Thal circuitry, our findings confirmed that IL-Thal CFPC induces TST-related movement. Importantly, though we found that gamma power increased across the IL-Thal circuit with repeat TST exposure, open loop gamma stimulation of the circuit had no behavioral effect. Taken together, these results indicate that IL-Thal CFPC likely reflects a compensatory circuit (i.e. circuit adaptations that occur in an attempt to return neural systems to ‘homeostasis’). This circuit is activated in response to uncontrollable stress, and disruption of the spatiotemporal dynamic observed in this circuit using an open loop stimulation above the circuit carrier frequency (‘circuit jamming’) renders animals less tolerant to stress. Critically, additional experiments will be needed to dissect the primary circuit changes that promote increases in TST immobility in response to stress.
It remains unknown whether the IL-Thal compensatory circuit we found diminishes the subjective stress experience or activates behavior that counters the behavioral stress-response; nevertheless, it holds promise as a target for further research into its therapeutic potential. For example, the spatiotemporal dynamic patterns we identified in the IL-Thal circuit may serve as a preclinical biomarker of stress induced behavioral dysfunction that can be exploited for therapeutic development. Future experiments that probe the impact of antidepressant treatment on IL-Thal CFPC are warranted. These experiments may also prove useful in dissecting the ‘primary’ circuit adaptations that result from stress exposure.
Conclusion
Overall our nCLASP stimulation system induced escape behaviors in naïve mice during the TST, while stimulation using a standard open-loop protocol that delivered an equivalent number of light pulses with a different carrier frequency tended to have the opposing effect (circuit jamming). This finding provides clear evidence that the neural state timing at which stimulation is delivered plays a critical role in determining the impact of cellular activation on behavior. This principle has particularly profound implications for interpreting the link between the activity of specific cell types and behavior, and for optimizing DBS based therapies. Critically, these findings also raise the provocative hypothesis that stress-induced behavioral disorders may result from altered neural timing across widely distributed circuits.
Supplementary Material
Acknowledgments
We would like to thank K. Deisseroth for generously proving access to viral tools, L. Jiang-Xie and F. Wang for generating the ChR2 confocal microscopy image; H. Mayberg, M. M. Halassa, I. Chou, and S. Lisberger for helpful comments on this manuscript. This work was supported by funding from IMHRO/One Mind RSA, NIMH grant R01MH099192, and a generous contribution from Kerima L. Collier to KD; NSF grant DGF1106401 to MTV. A special thanks to Freeman Hrabowski, Robert and Jane Meyerhoff, and the Meyerhoff Scholarship Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors report no biomedical financial interests or potential conflicts of interest.
DC modeled spike-behavioral activity relationships, and analyzed neuronal data; LKD performed viral surgeries, and optogenetic stimulation experiments; NMG jointly developed methods for closed loop optogenetic stimulation with KD; MTV jointly conceived analytical methods for LFP analysis, interpreted data, and wrote the paper with KD; MS integrated and synchronized the TST apparatus with neurophysiological recording system, and analyzed continuous TST behavioral measurements; CB scored TST behavioral activity from video recordings. RH performed ChR2 histological confirmations and helped to write the paper; JW constructed recording and stimulation electrodes, performed implantation surgeries, and assistant with ChR2 histological confirmations; CM provided mice and helped to write the paper; LC provided oversight for spike-behavioral activity modeling and analysis. SK performed implantation surgeries and behavioral experiments for in vivo recording TST studies, and confirmed implantation sites histologically; SDM performed viral and electrode surgeries with LKD for optogenetic stimulation experiments, and performed ChR2 histological confirmations; KD conceived in vivo TST and optogenetic experiments, analyzed spike-behavioral activity data, performed LFP analysis, developed closed-loop stimulation methods with NG, and wrote the paper with MTV, with input from LKD, SS, DC, RH, CM, DD, LC, and SDM.
References
- 1.Drevets WC, Price JL, Simpson JR, Jr, Todd RD, Reich T, Vannier M, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 2.Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- 3.Sheline YI, Price JL, Yan Z, Mintun MA. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc Natl Acad Sci U S A. 2010;107:11020–11025. doi: 10.1073/pnas.1000446107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 5.Fox MD, Buckner RL, White MP, Greicius MD, Pascual-Leone A. Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biol Psychiatry. 2012;72:595–603. doi: 10.1016/j.biopsych.2012.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamani C, Diwan M, Macedo CE, Brandao ML, Shumake J, Gonzalez-Lima F, et al. Antidepressant-like effects of medial prefrontal cortex deep brain stimulation in rats. Biol Psychiatry. 2010;67:117–124. doi: 10.1016/j.biopsych.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 7.Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berl) 1985;85:367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- 8.Tye KM, Mirzabekov JJ, Warden MR, Ferenczi EA, Tsai HC, Finkelstein J, et al. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature. 2012 doi: 10.1038/nature11740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ide S, Sora I, Ikeda K, Minami M, Uhl GR, Ishihara K. Reduced emotional and corticosterone responses to stress in mu-opioid receptor knockout mice. Neuropharmacology. 2010;58:241–247. doi: 10.1016/j.neuropharm.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iniguez SD, Aubry A, Riggs LM, Alipio JB, Zanca RM, Flores-Ramirez FJ, et al. Social defeat stress induces depression-like behavior and alters spine morphology in the hippocampus of adolescent male C57BL/6 mice. Neurobiol Stress. 2016;5:54–64. doi: 10.1016/j.ynstr.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.King DP, Zhao Y, Sangoram AM, Wilsbacher LD, Tanaka M, Antoch MP, et al. Positional cloning of the mouse circadian clock gene. Cell. 1997;89:641–653. doi: 10.1016/s0092-8674(00)80245-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carlson D, Schaich Borg J, Dzirasa K, Carin L. On the relations of LFPs & Neural Spike Trains. Advances in Neural Information Processing Systems. 2014;27 [Google Scholar]
- 13.Warden MR, Selimbeyoglu A, Mirzabekov JJ, Lo M, Thompson KR, Kim SY, et al. A prefrontal cortex-brainstem neuronal projection that controls response to behavioural challenge. Nature. 2012;492:428–432. doi: 10.1038/nature11617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roybal K, Theobold D, Graham A, DiNieri JA, Russo SJ, Krishnan V, et al. Mania-like behavior induced by disruption of CLOCK. Proc Natl Acad Sci U S A. 2007;104:6406–6411. doi: 10.1073/pnas.0609625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dzirasa K, McGarity DL, Bhattacharya A, Kumar S, Takahashi JS, Dunson D, et al. Impaired Limbic Gamma Oscillatory Synchrony during Anxiety-Related Behavior in a Genetic Mouse Model of Bipolar Mania. J Neurosci. 2011;31:6449–6456. doi: 10.1523/JNEUROSCI.6144-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sidor MM, Spencer SM, Dzirasa K, Parekh PK, Tye KM, Warden MR, et al. Daytime spikes in dopaminergic activity drive rapid mood-cycling in mice. Mol Psychiatry. 2015;5 doi: 10.1038/mp.2014.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Likhtik E, Stujenske JM, Topiwala MA, Harris AZ, Gordon JA. Prefrontal entrainment of amygdala activity signals safety in learned fear and innate anxiety. Nat Neurosci. 2014;17:106–113. doi: 10.1038/nn.3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dzirasa K, Ramsey AJ, Takahashi DY, Stapleton J, Potes JM, Williams JK, et al. Hyperdopaminergia and NMDA receptor hypofunction disrupt neural phase signaling. J Neurosci. 2009;29:8215–8224. doi: 10.1523/JNEUROSCI.1773-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dzirasa K, Coque L, Sidor MM, Kumar S, Dancy EA, Takahashi JS, et al. Lithium ameliorates nucleus accumbens phase signaling dysfunction in a genetic mouse model of mania. J Neurosci. 2010;30:16314–16323. doi: 10.1523/JNEUROSCI.4289-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gradinaru V, Zhang F, Ramakrishnan C, Mattis J, Prakash R, Diester I, et al. Molecular and cellular approaches for diversifying and extending optogenetics. Cell. 2010;141:154–165. doi: 10.1016/j.cell.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gunaydin LA, Yizhar O, Berndt A, Sohal VS, Deisseroth K, Hegemann P. Ultrafast optogenetic control. Nat Neurosci. 2010;13:387–392. doi: 10.1038/nn.2495. [DOI] [PubMed] [Google Scholar]
- 22.Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci. 2010;13:1161–1169. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hultman R, Mague SD, Li Q, Katz BM, Michel N, Lin L, et al. Dysregulation of Prefrontal Cortex-Mediated Slow-Evolving Limbic Dynamics Drives Stress-Induced Emotional Pathology. Neuron. 2016;91:439–452. doi: 10.1016/j.neuron.2016.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


