Abstract
Oncolytic virus (OV) therapy is potentially a game-changing cancer treatment that has garnered significant interest due to its versatility and multi-modal approaches towards tumor eradication. In the field of cancer immunotherapy, the immunological phenotype of the tumor microenvironment (TME) is an important determinant of disease prognosis and therapeutic success. There is accumulating data that OVs are capable of dramatically altering the TME immune landscape, leading to improved antitumor activity alone or in combination with assorted immune modulators. Herein, we review how OVs disrupt the immunosuppressive TME and can be used strategically to create a “pro-immune” microenvironment that enables and promotes potent, long-lasting host antitumor immune responses.
Keywords: Tumor microenvironment, Oncolytic viruses, Cancer immunotherapies, Combination therapies
Highlights
-
•
A tumor is a complex ecosystem that comprises diverse cellular populations that can impact the efficacy of immunotherapies
-
•
Oncolytic viruses can turn “immunologically cold” tumors into favorable environments for antitumor immune cell attack
-
•
Oncolytic viruses can be used in combination approaches with other cancer therapies to successfully combat tumors
1. Introduction
The wealth of insight into intra- and inter-tumoral heterogeneity and the supportive tumor microenvironment (TME) is shedding light on cancer survival mechanisms that contribute to treatment resistance and relapse. Broadly, as the host attempts to eradicate the tumor by generating an antitumor immune response, the tumor prompts a counteractive response by recruiting immunosuppressive immune cells and other TME components to build a physical and immunological fortress against attack. Current therapeutic efforts are directed towards harnessing principles of immunology to stimulate tumor-specific T-cell responses.
Oncolytic or “cancer-killing” viruses (OVs) are a class of self-replicating immunotherapeutic agents that present substantial potential to supplement the oncologist's cancer-fighting arsenal. In this review, we discuss a number of recent discoveries that demonstrate how OVs alone or in combination with other anticancer drugs act not only as direct tumor-killing weapons but also hold the capacity to promote in situ vaccination against the whole tumor. Indeed, compared to other immunotherapies that require patient-specific tumor-associated antigen (TAA) identification, OVs potently induce the release of the full range of TAAs into an inflammatory environment via tumor lysis and contribute to the establishment of tumor-specific T-cell immunity.
2. The Tumor Microenvironment
The original paradigm that tumors are a mass of proliferating cancer cells has now shifted to an in-depth understanding of tumors as complex entities. In addition to cancer cells, tumors harbour a variety of other cell types, including vascular endothelial cells (ECs), cancer-associated fibroblasts (CAFs) and various resident or migratory immune cell subsets [e.g. T cells, dendritic cells (DCs), Natural-Killer cells (NKs)]. Together, these various cell populations and the extracellular matrix that glues them together create an organized and dynamic community known as the tumor ecosystem or TME. It is now well established that the reciprocal crosstalk and cooperative interactions between cancer cells and these other cell types promote tumorigenesis and further sustain tumor growth, proliferation, and invasion [1]. For instance, certain biomolecules secreted by the immune cells within the TME can be critical to several of the established “cancer hallmarks” [2].
Although these general concepts apply to a wide range of tumors, individual tumors are unique ecosystems and are heterogeneous in the cellular composition of the TME within and between patients [1,2]. The TME's immune phenotypes are generally classified in three broad categories: immune desert, immune-excluded, and inflamed [3]. Inflamed tumors typically contain both cytokine-secreting CD4+ and cytotoxic CD8+ T cells and myeloid cells [4]. Unlike inflamed tumors, “immunologically cold” tumors contain less immune cells or cell subsets associated with immune suppression including regulatory T cells (Treg), myeloid-derived suppressor cells (MDSCs) and M2 macrophages. Whereas immune desert tumors are generally characterized by a very low number or even an absence of immune cell infiltrates, immune-excluded tumors contain immune cells that remain stuck in the surrounding stroma, thus unable to colonize the TME to exert their antitumor functions [5].
3. The Tumor Immune Microenvironment Shapes The Response to Anticancer Therapies
The exciting yet still limited success of immunotherapies to date highlights the need to better understand the unique characteristics of individual tumors for the rational design of treatment plans. For instance, identifying the type of immune landscape may predict therapeutic effectiveness of certain immunotherapies like immune checkpoint blockade [3]. In the case of non-inflamed tumors, there is a need for novel therapeutic strategies that change the TME landscape into an inflamed phenotype to promote the priming of antitumor immune responses [6].
Accumulating evidence indicates that type I interferons (IFNα/β) are crucial in the establishment of antitumor responses. In addition to their antiviral and antitumor properties, type I IFNs stimulate diverse immune cell subsets within the TME (e.g. the cytotoxic activity of NK and CD8+ T cells, the secretion of pro-inflammatory cytokines by macrophages, and the cross-presentation activity of mature DCs) [7]. It has been described that the efficacy of many chemotherapies, radiotherapies, immunotherapies, and targeted anticancer agents depends upon major contribution of type I IFNs [7]. However, systemic administration of type I IFNs often has undesirable side-effects and as a result it has become of strong interest in the field of cancer therapy to select for therapeutic modalities that specifically induce type I IFNs expression in the TME. Recently, two studies have demonstrated that DNA methyltransferases inhibitors upregulate the expression of cytosolic dsRNAs derived from endogenous retroviral elements that subsequently activate viral sensors to induce type I and III IFN signaling associated with antitumor effects [8,9]. Alternatively, agonists of viral nucleic acid cellular sensors, such as RIG-I, STING or TLR3, elicit the production of type I IFNs and therefore promote tumor cell death and antitumor immunity [7]. In the following review, we argue that rather than using a viral mimetic, it is preferable to use a multi-functional replicating virus that directly attacks cancer cells while heating up the TME to stimulate antitumor immune responses.
4. Oncolytic Immunotherapy “Wakes Up” Tumors in an “Immunological Coma”
During their transformation, cancer cells acquire defects in numerous signaling pathways that simultaneously impinge on cellular growth control and innate antiviral defense systems [10]. As a result, many cancers are susceptible to a range of oncolytic virus therapeutics, a class of naturally occurring or genetically modified viruses that selectively replicate within and kill tumor cells without harming healthy tissues. The most advanced of these is Talimogene laherparepvec (T-Vec, Imlygic®), an engineered Herpes Simplex Virus (HSV) that was recently approved for the treatment of unresectable melanoma by the FDA and EMA [11]. Currently, numerous OV candidates are under extensive study, with several in late phases of clinical investigation (e.g. NCT02562755, NCT02879760, NCT02192775, NCT02364713). Due to space restrictions, we apologize that we cannot discuss all important pre-clinical and clinical studies that have been or are currently being studied in the context of various OV platforms. Additional information can be found in the following review article [12].
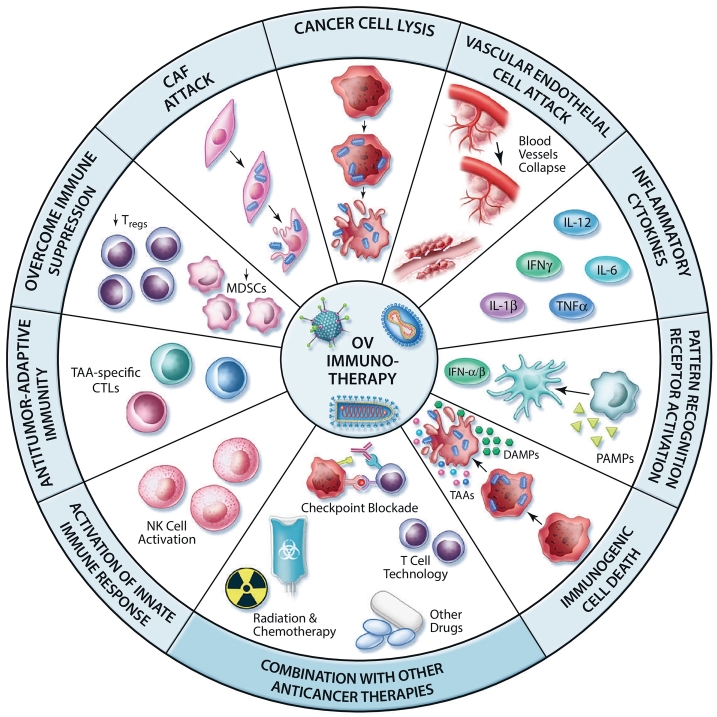
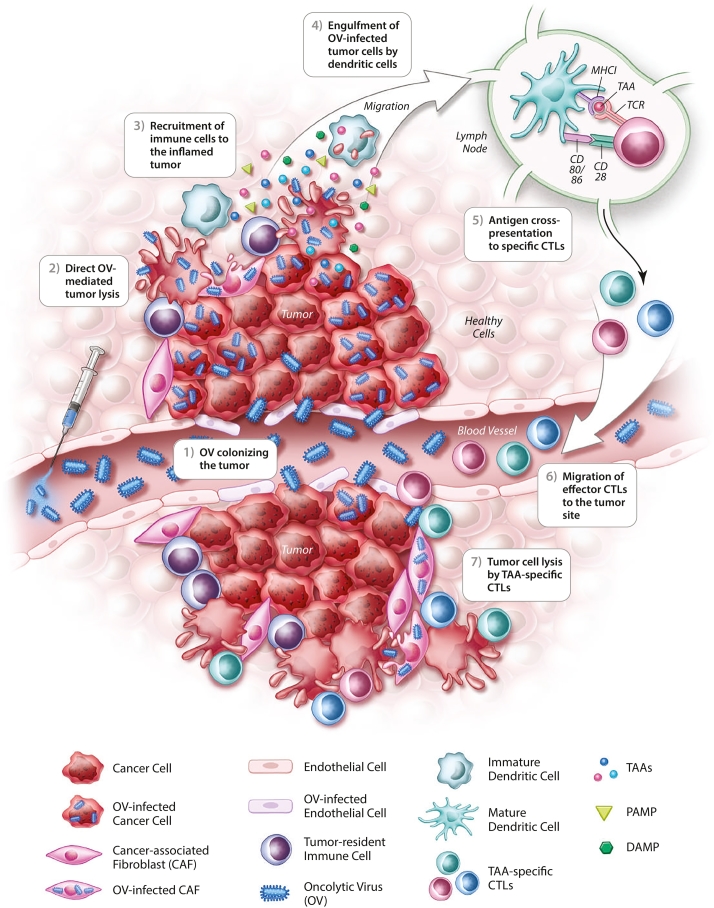
Several studies have highlighted the crucial role of tumor-specific T cells in OV-mediated therapeutic efficacy. For instance, it has been shown that the intratumoral injection of reovirus or vesicular stomatitis virus (VSV) potently primes adaptive antitumor immune responses playing a key role in primary and metastatic tumors regression [13,14]. The question remains, how are OVs able to reverse TME immune suppression and facilitate T cell recognition of tumor antigens? Although originally designed or selected to be cytolytic agents, it is now clear that OVs have pleotropic impacts on the TME (Fig. 1). While awakening of the immune system within the TME is initiated through OV-mediated cell killing, this is just the first of several events that ultimately culminate in the induction of a robust and long-lasting antitumor immune response [15]. One critical early event is the triggering of immunogenic cell death (ICD) of OV-infected cancer cells (Fig. 1, Fig. 3) [12]. ICD is characterized in part by the expression and/or release of damage-associated molecular patterns (DAMPs) (i.e. ecto-calreticulin, ATP, and HMGB1) which attract and activate DCs in the TME [16]. In addition, pathogen-associated molecular patterns (PAMPs) in the tumor milieu are recognized by specific pathogen recognition receptors (PRRs) expressed by innate immune cells (Fig. 1, Fig. 3). For example, it has been shown that the dsRNA genome of reovirus directly activates DCs through protein kinase receptor (PKR) signaling leading to the secretion of pro-inflammatory cytokines (e.g. IFN-α, IL-12, TNF-α and IL-6) [17]. Upon exposure to oncolytic MeV, two subsets of human blood DCs [plasmacytoid DCs (pDCs) and a subset of myeloid DCs] secrete IFN-α following activation of RIG-I-like receptors (RLRs) and/or Toll-like receptors (TLRs). These MeV-stimulated DCs acquire cytotoxic functions through the IFN-dependent expression of TNF-related apoptosis-inducing ligand (TRAIL) [18]. Thus, the combination of PAMPs, DAMPs, and pro-inflammatory cytokines induced by OVs in the TME results in a massive recruitment and appropriate activation of immune cells, notably DCs that are key orchestrators of innate and adaptive antitumor immunity (Fig. 3). Once at the tumor site, DCs engulf OV-infected cancer cells and capture TAAs for cross-presentation to specific T cells (Fig. 3). It has been shown that phagocytosis of MeV-infected cells by either human monocyte-derived DCs (Mo-DCs) or pDCs result in the maturation of these cells and cross-presentation of TAAs to naïve CD8+ T cells [19, 20, 21]. The role of MeV-mediated ICD in inducing this response is vital: a lack of immunogenicity of uninfected or UV-irradiated cancer cells leave DCs in an immature phenotype thus failing to prime tumor-specific T cells [21]. The authors also emphasize that activated CD8+ T cells secrete IFN-γ and kill cancer cells expressing the specific TAAs, leaving irrelevant target cells unharmed. Other studies have shown T cell cross-priming by DCs following parvovirus and reovirus-induced cancer cell oncolysis [22,23]. Once tumor-specific T cells have been primed in lymph nodes, they enter the blood stream to reach the inflamed tumor site where they exert their cytotoxic functions against remaining cancer cells displaying TAAs (Fig. 3). A recent study demonstrated that cancer cell infection by different OVs induces the intercellular transfer of a TAA released in the TME, allowing TAA-loaded cancer cells to be recognized by specific effector CD4+ T cells [24].
Fig. 1.
Oncolytic viruses in the tumor microenvironment. In addition to their cancer cell lysing activity, OVs can target several other components of the TME including CAFs and ECs. Moreover, OV infection causes the release of inflammatory cytokines, DAMPs, PAMPs and TAAs in the tumor milieu. This results in the recruitment and maturation of innate immune cells which can cross-present TAAs to CD8+ T cells, thus generating populations of TAA-specific CTLs. The generation of an antitumor immune response mediated by OV infection also counteracts the immune suppression associated to Tregs and MDSCs. Thus, OV infection engages both the innate and adaptive arms of the immune system which act together to destroy the tumor mass and generate potent antitumor immune responses. These properties of OV-infected tumors can be harnessed to rationally combine therapies that enhance OV replication and the immune response they generate.
Fig. 3.
Oncolytic virus invasion of the tumor serves as in situ vaccination. After colonization of the tumor, OVs mediate direct lysis of cancer cells as well as components of the TME (e.g. CAFs and ECs). The release of danger signals and pro-inflammatory cytokines in the TME by cancer cells undergoing ICD after OV infection, as well as the OV itself, attract and activate immune cells. Among them, DCs will engulf OV-infected tumor cells and migrate to the lymph node, where the antigen cross-presentation to specific CTLs will take place. The TAA-specific CTLs then enter the blood stream to reach the tumor, where they can exert their cytotoxic activity against cancer cells displaying specific TAAs. OV therapy thus induces in situ vaccination leading to specific antitumor immune responses that act in concert with the virus to eradicate the tumor.
5. In Situ Vaccination Induced by Viral ONCOLYSIS
OV infection of the tumor induces in situ vaccination against crucial TAAs through their release in the inflamed TME and their uptake by DCs [15]. While many cancer immunotherapies depend on the identification and targeting of single TAAs shared by cancer patients, OVs vaccinate against the entire tumor TAA repertoire. OV-induced oncolysis and the subsequent epitope spreading in the tumor milieu serves as a personalized immunotherapeutic approach. A recent study elegantly described that adenovirus-induced tumor oncolysis elicits specific T cell responses to a panel of putative neoepitopes whereas other tested therapies failed to trigger such tumor-specific responses [25]. Therefore, OVs have the potential to convert immunologically inert tumors into highly immune-reactive ones (Fig. 2). In line with the above-mentioned preclinical observations, recent clinical trials have indicated a role of OV-induced antitumor immunity in the observed efficacy of this treatment. T cells specific for ovarian cancer antigens have been found in the blood of patients undergoing treatment with MeV [26]. In patients with advanced melanoma treated with T-Vec, T cells recognizing the melanoma MART-1 antigen were detected in the TME of injected and non-injected lesions [27], suggesting that local injection of T-Vec induces a potent systemic antitumor immunity in patients. A recent study further substantiated the importance of tumor-specific T cells in HSV therapeutic responses [28].
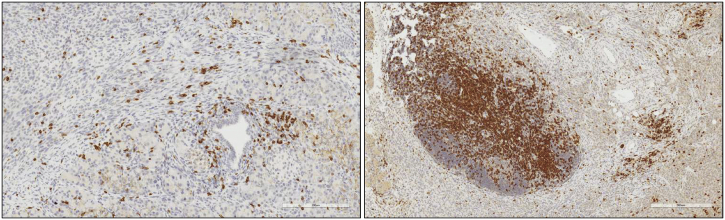
Fig. 2.
Warming up immunologically “cold” tumors with OVs. PanCO2 tumor-bearing mice treated with an infected cell vaccine (ICV) (right) show higher intratumoral CD3+ cells in the TME in comparison to its untreated counterpart (left) (large mass of staining is a peritoneal lymph node). ICV technology harnesses the immune activation properties of both OVs and tumor cell vaccines to maximize therapeutic benefit. Following treatment, the infiltration of activated T cells in the local TME allows previously immunologically ignorant or immunosuppressed tumors to enact a potent antitumor immune response towards tumor destruction. Scale bar: 300 μm.
OVs have been armed with immunostimulatory cytokines or chemokines, such as IL-12, IFN-α/β/γ, RANTES or GM-CSF (granulocyte-macrophage colony-stimulating factor) [12,29] to promote the trafficking of immune cells to the TME and the priming of antitumor responses. GM-CSF, a known DC recruiter and activator, is the most common OV-encoded cytokine and is notably encoded by T-Vec [27].
6. Oncolytic Viruses Attack The Tumor Biostructure
Oncolytic viruses also target tumor stromal cells, including CAFs, ECs and pericytes (Fig. 1), thus aiding the disruption of complex tumor structures. A study examining OV-infected pancreatic cancer cell crosstalk with surrounding CAFs revealed that cancer cell-secreted TGF-β renders CAFs sensitive to OV infection [30]. OVs can also contribute to the reduction of fibrosis within the TME. Indeed, oncolytic VSV has previously been shown to infect hepatic stellate cells (HSCs), thus resulting in improved liver staging in hepatic tumor-bearing rats [31]. Furthermore, OVs can infect and replicate within ECs of the tumor vasculature, a process mediated through the suppression of antiviral mechanisms by EC-secreted vascular endothelial growth factor (VEGF) [32]. Thus, OV-targeting of stromal cells can increase the permissiveness of the TME for immune infiltration.
7. Combination Therapies Involving Oncolytic Viruses
The heterogeneous nature of cancer highlights the need for combination approaches towards tumor eradication. OVs can be combined with drugs or biologics that potentiate OV cytolysis within the TME and/or enhance immune-mediated tumor destruction.
7.1. Generating a Receptive TME for OV Replication with Chemical Agents
The process of adapting wild-type viruses for safe use in clinical settings ultimately results in a significant loss of virulence. While defects in type I IFN signaling leave cancer cells vulnerable to OV infection, only a fraction of tumors have these anomalies and the intratumoral IFN status is further complicated by the heterogeneous nature of tumors [15,33]. RNA interference (RNAi) knockdown technology and antibody blockade strategies offer some hope in rectifying these concerns. However, combination approaches with small molecule drugs that easily diffuse into tissue and rapidly elicit their effects, allows for tight control of timing and duration. Therefore, small molecule drugs can be harnessed to synthetically alter the TME to one that is more susceptible to OV growth, oncolysis, and OV-induced antitumor immune responses (Fig. 1).
Histone deacetylase (HDAC) activity is classically associated with transcriptional repression. Chang et al. show that it is also essential in inducing cellular antiviral programs. In fact, HDAC inhibitors (HDACis) have been used to block IFN-stimulated gene expression and provide a virus-permissive environment for VSV infection [34,35]. They have also been shown to selectively induce apoptosis in cancer cells and augment the transcription of OV-delivered transgenes [36,37]. Pre-treatment with particular HDACis can re-sensitize cancers that are resistant to infection in a variety of in vitro and in vivo tumor models including ovarian cancer and sarcomas [38].
FDA-approved small molecules with demonstrated OV-potentiating activity are already available on the market. Numerous OV-chemotherapy combinations have been studied at the pre-clinical and clinical stages and have demonstrated success [39]. The JAK1/2 inhibitor ruxolitinib, overcoming resistance to OVs like VSV [40], can be found on the clinical shelf for the treatment of intermediate or high-risk myelofibrosis. Valproic acid, prescribed for the treatment of epilepsy, contains HDACi activity and has shown positive outcomes in combinations with HSV [41]. The possibilities for chemically-driven combination approaches can be vastly expanded by high throughput studies that aim to identify new small molecules that augment OV growth. This approach was used to identify a new class of pyrrole-based potentiators of tumor-specific OV killing with enhanced stability and reduced electrophilicity [42]. Diallo et al. have uncovered “virus sensitizer molecules” that dampen the cellular innate response by disrupting IFN-induced antiviral responses [35]. Numerous studies have demonstrated synergy between the pro-apoptotic microtubule-destabilizing agents (MDAs) and OV for cancer treatment [43,44]. This cooperative killing is achieved by various mechanisms including mitotic slippage and bystander killing [43].
7.2. Oncolytic Virus Combinations with Immune Modulators
Other groups have focused their efforts in combining OVs with drugs that potentiate their immune-stimulatory properties. Smac mimetic compounds (SMCs), antagonizing inhibitor of apoptosis proteins (IAPs), not only induce apoptosis of cancer cells but also effectively boost cancer-vaccine-induced T-cell activity [45]. Pre-clinical studies have also shown synergistic activity in OV/chemotherapeutics combination approaches [46]. Cisplatin, for instance, increases antigen-specific CD8+ T-cell mediated antitumor immunity [47]. Combining MG1 with Paclitaxel, a chemotherapeutic drug demonstrated to increase MHC I and upregulate antigen processing activity, shows encouraging results in murine breast cancer models [48]. Alternatively, depletion of the immunosuppressive cells MDSCs in the TME with the use of some chemotherapies (e.g. gemcitabine, sunitinib, 5-FU, docetaxel or retinoic acid) improved tumor clearance when combined with OVs [49].
It is now widely accepted that both the induction of antitumor responses and the downregulation of immunosuppressive mechanisms are required to achieve durable therapeutic outcomes. T cells express molecules like programmed cell death-1 (PD-1) or cytotoxic T-lymphocyte antigen-4 (CTLA-4) that play a role in immune regulation. However, tumor cells achieve immune evasion by hijacking these mechanisms to impede antitumor specific T-cell attack [6].
The development of monoclonal antibodies targeting these immune checkpoint receptors, either PD-1 (nivolumab and pembrolizumab) or CTLA-4 (ipilimumab), has revolutionized the field of anticancer treatment. They have shown impressive results in the treatment of metastatic melanoma and anti-PD-1 received FDA approval for other tumor types [50,51]. However, recent pre-clinical and clinical studies underscore the role of pre-existing antitumor T cells in the TME as a key parameter for favorable clinical responses to immune checkpoint blockade [50]. This evidence led to the hypothesis that co-administration of checkpoint blockade antibodies and OVs could synergistically fight tumors (Fig. 1). As previously mentioned, OVs can inflame the tumor niche, enable strong T cell infiltration and subsequently allow checkpoint inhibitors to exert their maximal therapeutic potential. The latter might disrupt the immune inhibitory interaction between cancer cells and antitumor effector T cells, restoring T cell cytotoxicity and tumor rejection.
Several pre-clinical studies have supported the use of CTLA-4 or PD-1 blocking antibodies in combination with either oncolytic Newcastle disease virus (NDV) [52], vaccinia virus (VACV) [53], VSV [54, 55, 56], reovirus [57] or Maraba virus [58]. Interestingly, the NDV/CTLA-4 blockade combination therapy controlled both local and distant tumors, and induced memory immune responses protecting against tumor challenge. Even though emerging data provide evidence of significant synergy between OVs and immune checkpoint inhibitors to achieve antitumor therapeutic efficacy, several important future considerations arose from these studies. Notably, the importance of the timing and the route of administration of checkpoint inhibitors have been highlighted [59]. For instance, it would be optimal to systemically administer the anti-CTLA-4 antibody to target CTLA-4 in the lymph node where it can impact the early phase of the antitumor immune response, and it might be more efficacious to deliver anti-PD-1 antibodies directly in the tumor [60].
The combination of oncolytic immunotherapy and checkpoint inhibitors has also been evaluated in the clinic. A phase II study combining T-Vec and ipilimumab shows objective responses in patients with advanced melanoma treated with the combination modality, compared to ipilimumab alone or to historical data obtained with T-Vec alone [61]. The authors highlight a decrease in non-injected visceral lesion burden in addition to the injected ones, suggesting a systemic antitumor effect induced by the combination treatment. Another recent study from Ribas and colleagues reported the promising results of a phase Ib trial testing the combination of T-Vec with pembrolizumab in patients with advanced melanoma [62]. Although this phase Ib trial has been performed on a small cohort of patients (n = 21), the important work on sequential biopsies obtained from treated patients suggests that T-Vec induced immune changes in the TME, which became conducive to the therapeutic action of the anti-PD-1 agent. A randomized phase III trial is currently ongoing (NCT02263508) and the results could be important in elucidating the complex interaction between OV and immune checkpoint inhibition.
7.3. Improving Adoptive-Cell Transfer Therapy for Solid Tumors with Oncolytic Virus
Development of adoptive-cell transfer (ACT) immunotherapy has been on the rise for the last decade and holds great promise as an effective cancer treatment. Despite initial success in melanoma patients, there is a need to reduce associated toxicity and extend its applicability to other cancer types. Originally based on the ex vivo expansion and reinfusion of patient's own tumor-reactive T cells, T cells can now be redirected towards cancer cells through the expression of a TAA-specific T cell receptor (TCR), or a CAR (chimeric antigen receptor), a fusion of a single-chain variable fragment (scFv) specific for a TAA and TCR intracellular signaling domains [63]. CAR T cells are particularly attractive because of their ability to recognize a TAA in a non-MHC-restricted manner, which enables their use for a broad variety of patients. So far, CAR T cells have shown promising clinical efficacy against hematological cancers, resulting in a recent FDA approval of CAR T cells directed against CD19 for the treatment of B-cell malignancies [64]. However, the success of ACT has been limited in solid tumors [65]. This can be attributed to poor trafficking of the transferred T cells into the TME, poor persistence and expansion as well as impaired activity in the immunosuppressive TME. However, it is becoming increasingly evident that OVs can dismantle these obstacles and act synergistically with ACT immunotherapy by converting the hostile TME into an immunostimulatory region for optimal T cell migration and antitumor activity (Fig. 1) [66]. It has been notably demonstrated that TAA (either ovalbumin or gp100)-encoding VSV can act as an adjuvant for adoptively transferred specific T cells [67]. This combination results in long-term cure of established melanoma tumors in mice and is associated with potent antitumor efficacy of T cells both in the injected tumor and distant tumor sites. Another study demonstrated that tumor resistance to T cell transfer in a melanoma mouse model can be overcome by the infection of cancer cells by an oncolytic adenovirus. Indeed, costimulatory signals induced on DCs results in an optimal activation of the tumor-specific T cells within the TME [68]. OV bioengineering offers a plethora of opportunities to enhance their synergy with T cell therapies and these approaches are thoroughly discussed in a recent review [66]. One noteworthy example is the expression of pro-T cells cytokines and chemokines via OV vectors to attract tumor-specific CAR T cells and promote their survival within the TME. Overall, these recombinant OV platforms show better control of solid tumors [69,70].
A novel strategy has been developed to induce robust activation and expansion of CAR T cells, involving dual-specific T cells co-expressing a virus-specific TCR and a tumor-specific CAR. The TCR can either be endogenously expressed (e.g. specific for Epstein-Barr virus or Cytomegalovirus) [71, 72, 73], or be genetically engineered to react against a strong immunogen, such as influenza virus [74]. The latter approach induces superior activation and proliferation of T cells following immunization, with the CAR domain providing a means for tumor targeting. Recently, a group demonstrated that a therapeutic regimen of ACT incorporating vaccination induces durable complete remission of a variety of large solid tumors [75]. The authors generated dual-specific T cells expressing both a CAR specific for Her2 and a TCR specific for gp100, where gp100 is the immunogen. Using an oncolytic vaccinia virus encoding gp100, they obtain an extensive proliferation of the infused dual-specific T cells that subsequently traffic to the tumor to exert their cytotoxicity against cancer cells.
By disrupting the TME and changing its immune landscape, there is encouraging evidence for OV-mediated improved ACT immunotherapy efficacy in solid tumors. Even if some mechanisms of action remain to be elucidated, the sustained efforts to improve these two exciting biotherapies should lead to an optimal combination that will ultimately benefit patients.
8. Concluding Remarks
Through several pre-clinical and clinical studies, OVs have proven that they can efficiently dismantle the tumor fortress by attacking several of its pillars. Indeed, by targeting the cancer cells and diverse components of the TME, OVs disorganize the tumor ecosystem. The inflammatory milieu resulting from OV therapy induces in situ vaccination and the efficient priming of antitumor responses, thus waking up the patient's own T cell arsenal. However, joining forces with other therapies has the potential to lead a more powerful offensive. By counteracting immunosuppression and rendering the TME more permissive to OV infection, the tumor is left vulnerable to the combined efforts of the OV, combinatorial drug and the host's immune attack. Ultimately, the outlook of OV therapy is extremely promising. Its multi-modal antitumor functionality, efficacy in a wide range of tumors and potentiating activity with existing therapies will quickly make OV therapy a widespread anticancer therapy of choice in the oncologist's toolbox.
9. Key Outstanding Questions
-
•
What is the best timing, route of administration and dosing for OV in combination with other immunotherapies or chemical agents in order to achieve the optimal therapeutic response in patients, without triggering toxicity (e.g. cytokine storm)?
-
•
Given the diversity of TME immune landscapes, would a sole combination therapy be suitable for all tumor types?
-
•
Considering the variety of OVs and the multitude of possible genetic modifications, what innovative OV platforms can be developed to polarize the TME favorably for combination therapies to exert their maximal therapeutic potential?
10. Search Strategy and Selection Criteria
Data for this review were identified by searches of PubMed, and references from relevant articles using the search terms “oncolytic viruses”, “tumor microenvironment”, “immunogenic cell death”, “in situ vaccination”, “chemical combinations with oncolytic viruses”, “combination therapies with oncolytic virus therapy”, “oncolytic virus and immune checkpoint blockade”, “adoptive cell transfer”, and “chimeric antigen receptor T cell therapy”.
Author's Contributions
Conceptualization: C.A, J.C·B and C.S.I.; Literature search: C.A., A.S., and M.E.W.; Writing and formatting: C.A., A.S., and M.E.W.; Figures: C.A., A.S., and C.S.I.; Review and editing: C.A., A.S., M.E. W., G.U., J.C.B., and C.S.I. All authors approved the final draft for submission.
Funding
This work was funded by grants from the Terry Fox Research Foundation (2015-0889) and the Canadian Institutes of Health Research (153187) to G.U., J.C.B. and C.S.I. J.C.B. is also supported by the contributions from the Ontario Institute for Cancer Research and the Ottawa Regional Cancer Foundation. M.E.W. is funded by scholarships from the Canadian Institutes of Health Research Frederick Banting and Charles Best Master's Award. The funders did not play a role in manuscript design, data collection, data analysis, interpretation nor writing of the manuscript.
Acknowledgments
Acknowledgements
We would like to thank Dr. Rebecca Auer, Christiano Tanese de Souza and Katherine Baxter from the Auer lab for providing the immunohistochemistry images for Fig. 2.
Conflict of Interest
We declare that Dr. John Bell has interest in Turnstone Biologics, which develops the oncolytic Maraba virus as an OV platform. The other authors declare no competing interests.
References
- 1.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D., Coussens L.M. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 3.Chen D.S., Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541:321–330. doi: 10.1038/nature21349. [DOI] [PubMed] [Google Scholar]
- 4.Gajewski T.F., Schreiber H., Fu Y.X. Innate and adaptive immune cells in the tumor microenvironment. Nat. Immunol. 2013;14:1014–1022. doi: 10.1038/ni.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joyce J.A., Fearon D.T. T cell exclusion, immune privilege, and the tumor microenvironment. Science. 2015;348:74–80. doi: 10.1126/science.aaa6204. [DOI] [PubMed] [Google Scholar]
- 6.Pardoll D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zitvogel L., Galluzzi L., Kepp O., Smyth M.J., Kroemer G. Type I interferons in anticancer immunity. Nat. Rev. Immunol. 2015;15:405–414. doi: 10.1038/nri3845. [DOI] [PubMed] [Google Scholar]
- 8.Chiappinelli K.B., Strissel P.L., Desrichard A., Li H., Henke C., Akman B. Inhibiting DNA methylation causes an interferon response in Cancer via dsRNA including endogenous retroviruses. Cell. 2015;162:974–986. doi: 10.1016/j.cell.2015.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roulois D., Loo Yau H., Singhania R., Wang Y., Danesh A., Shen S.Y. DNA-demethylating agents target colorectal cancer cells by inducing viral mimicry by endogenous transcripts. Cell. 2015;162:961–973. doi: 10.1016/j.cell.2015.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pikor L.A., Bell J.C., Diallo J.S. Oncolytic viruses: exploiting Cancer's deal with the devil. Trends Cancer. 2015;1:266–277. doi: 10.1016/j.trecan.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 11.Rehman H., Silk A.W., Kane M.P., Kaufman H.L. Into the clinic: Talimogene laherparepvec (T-VEC), a first-in-class intratumoral oncolytic viral therapy. J. Immunother. Cancer. 2016;4:53. doi: 10.1186/s40425-016-0158-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo Z.S., Liu Z., Kowalsky S., Feist M., Kalinski P., Lu B. Oncolytic immunotherapy: conceptual evolution, current strategies, and future perspectives. Front. Immunol. 2017;8:555. doi: 10.3389/fimmu.2017.00555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diaz R.M., Galivo F., Kottke T., Wongthida P., Qiao J., Thompson J. Oncolytic immunovirotherapy for melanoma using vesicular stomatitis virus. Cancer Res. 2007;67:2840–2848. doi: 10.1158/0008-5472.CAN-06-3974. [DOI] [PubMed] [Google Scholar]
- 14.Prestwich R.J., Ilett E.J., Errington F., Diaz R.M., Steele L.P., Kottke T. Immune-mediated antitumor activity of reovirus is required for therapy and is independent of direct viral oncolysis and replication. Clin. Cancer Res. 2009;15:4374–4381. doi: 10.1158/1078-0432.CCR-09-0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lichty B.D., Breitbach C.J., Stojdl D.F., Bell J.C. Going viral with cancer immunotherapy. Nat. Rev. Cancer. 2014;14:559–567. doi: 10.1038/nrc3770. [DOI] [PubMed] [Google Scholar]
- 16.Kroemer G., Galluzzi L., Kepp O., Zitvogel L. Immunogenic cell death in cancer therapy. Annu. Rev. Immunol. 2013;31:51–72. doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- 17.Errington F., Steele L., Prestwich R., Harrington K.J., Pandha H.S., Vidal L. Reovirus activates human dendritic cells to promote innate antitumor immunity. J. Immunol. 2008;180:6018–6026. doi: 10.4049/jimmunol.180.9.6018. [DOI] [PubMed] [Google Scholar]
- 18.Achard C., Guillerme J.B., Bruni D., Boisgerault N., Combredet C., Tangy F. Oncolytic measles virus induces tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-mediated cytotoxicity by human myeloid and plasmacytoid dendritic cells. Oncoimmunology. 2017;6 doi: 10.1080/2162402X.2016.1261240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donnelly O.G., Errington-Mais F., Steele L., Hadac E., Jennings V., Scott K. Measles virus causes immunogenic cell death in human melanoma. Gene Ther. 2013;20:7–15. doi: 10.1038/gt.2011.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gauvrit A., Brandler S., Sapede-Peroz C., Boisgerault N., Tangy F., Gregoire M. Measles virus induces oncolysis of mesothelioma cells and allows dendritic cells to cross-prime tumor-specific CD8 response. Cancer Res. 2008;68:4882–4892. doi: 10.1158/0008-5472.CAN-07-6265. [DOI] [PubMed] [Google Scholar]
- 21.Guillerme J.B., Boisgerault N., Roulois D., Menager J., Combredet C., Tangy F. Measles virus vaccine-infected tumor cells induce tumor antigen cross-presentation by human plasmacytoid dendritic cells. Clin. Cancer Res. 2013;19:1147–1158. doi: 10.1158/1078-0432.CCR-12-2733. [DOI] [PubMed] [Google Scholar]
- 22.Moehler M.H., Zeidler M., Wilsberg V., Cornelis J.J., Woelfel T., Rommelaere J. Parvovirus H-1-induced tumor cell death enhances human immune response in vitro via increased phagocytosis, maturation, and cross-presentation by dendritic cells. Hum. Gene Ther. 2005;16:996–1005. doi: 10.1089/hum.2005.16.996. [DOI] [PubMed] [Google Scholar]
- 23.Prestwich R.J., Errington F., Ilett E.J., Morgan R.S., Scott K.J., Kottke T. Tumor infection by oncolytic reovirus primes adaptive antitumor immunity. Clin. Cancer Res. 2008;14:7358–7366. doi: 10.1158/1078-0432.CCR-08-0831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Delaunay T., Violland M., Boisgerault N., Dutoit S., Vignard V., Munz C. Oncolytic viruses sensitize human tumor cells for NY-ESO-1 tumor antigen recognition by CD4+ effector T cells. Oncoimmunology. 2018;7 doi: 10.1080/2162402X.2017.1407897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woller N., Gurlevik E., Fleischmann-Mundt B., Schumacher A., Knocke S., Kloos A.M. Viral infection of tumors overcomes resistance to PD-1-immunotherapy by broadening neoantigenome-directed T-cell responses. Mol. Ther. 2015;23:1630–1640. doi: 10.1038/mt.2015.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galanis E., Atherton P.J., Maurer M.J., Knutson K.L., Dowdy S.C., Cliby W.A. Oncolytic measles virus expressing the sodium iodide symporter to treat drug-resistant ovarian cancer. Cancer Res. 2015;75:22–30. doi: 10.1158/0008-5472.CAN-14-2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaufman H.L., Kim D.W., Deraffele G., Mitcham J., Coffin R.S., Kim-Schulze S. Local and distant immunity induced by intralesional vaccination with an oncolytic herpes virus encoding GM-CSF in patients with stage IIIc and IV melanoma. Ann. Surg. Oncol. 2010;17:718–730. doi: 10.1245/s10434-009-0809-6. [DOI] [PubMed] [Google Scholar]
- 28.Moesta A.K., Cooke K., Piasecki J., Mitchell P., Rottman J.B., Fitzgerald K. Local delivery of OncoVEX(mGM-CSF) generates systemic antitumor immune responses enhanced by cytotoxic T-lymphocyte-associated protein blockade. Clin. Cancer Res. 2017;23:6190–6202. doi: 10.1158/1078-0432.CCR-17-0681. [DOI] [PubMed] [Google Scholar]
- 29.Bastin D., Walsh S.R., Al Saigh M., Wan Y. Capitalizing on Cancer specific replication: oncolytic viruses as a versatile platform for the enhancement of cancer immunotherapy strategies. Biomedicine. 2016:4. doi: 10.3390/biomedicines4030021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ilkow C.S., Marguerie M., Batenchuk C., Mayer J., Ben Neriah D., Cousineau S. Reciprocal cellular cross-talk within the tumor microenvironment promotes oncolytic virus activity. Nat. Med. 2015;21:530–536. doi: 10.1038/nm.3848. [DOI] [PubMed] [Google Scholar]
- 31.Altomonte, J., Marozin, S., DE Toni, E. N., Rizzani, A., Esposito, I., Steiger, K., Feuchtinger, A., Hellerbrand, C., Schmid, R. M. & Ebert, O. 2013. Antifibrotic properties of transarterial oncolytic VSV therapy for hepatocellular carcinoma in rats with thioacetamide-induced liver fibrosis. Mol. Ther., 21, 2032–42. [DOI] [PMC free article] [PubMed]
- 32.Arulanandam, R., Batenchuk, C., Angarita, F. A., Ottolino-Perry, K., Cousineau, S., Mottashed, A., Burgess, E., Falls, T. J., Silva, N., Tsang, J., Howe, G. A., Bourgeois-Daigneault, M. C., Conrad, D. P., Daneshmand, M., Breitbach, C. J., Kirn, D. H., Raptis, L., Sad, S., Atkins, H., Huh, M. S., Diallo, J. S., Lichty, B. D., Ilkow, C. S., LE Boeuf, F., Addison, C. L., Mccart, J. A. & Bell, J. C. 2015a. VEGF-mediated induction of PRD1-BF1/Blimp1 expression sensitizes tumor vasculature to oncolytic virus infection. Cancer Cell, 28, 210–24. [DOI] [PubMed]
- 33.Stojdl D.F., Lichty B., Knowles S., Marius R., Atkins H., Sonenberg N. Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat. Med. 2000;6:821–825. doi: 10.1038/77558. [DOI] [PubMed] [Google Scholar]
- 34.Chang H.M., Paulson M., Holko M., Rice C.M., Williams B.R., Marie I. Induction of interferon-stimulated gene expression and antiviral responses require protein deacetylase activity. Proc. Natl. Acad. Sci. U. S. A. 2004;101:9578–9583. doi: 10.1073/pnas.0400567101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Diallo, J. S., LE Boeuf, F., Lai, F., Cox, J., Vaha-Koskela, M., Abdelbary, H., Mactavish, H., Waite, K., Falls, T., Wang, J., Brown, R., Blanchard, J. E., Brown, E. D., Kirn, D. H., Hiscott, J., Atkins, H., Lichty, B. D. & Bell, J. C. 2010. A high-throughput pharmacoviral approach identifies novel oncolytic virus sensitizers. Mol. Ther., 18, 1123–9. [DOI] [PMC free article] [PubMed]
- 36.Fouladi M. Histone deacetylase inhibitors in cancer therapy. Cancer Investig. 2006;24:521–527. doi: 10.1080/07357900600814979. [DOI] [PubMed] [Google Scholar]
- 37.Yamano T., Ura K., Morishita R., Nakajima H., Monden M., Kaneda Y. Amplification of transgene expression in vitro and in vivo using a novel inhibitor of histone deacetylase. Mol. Ther. 2000;1:574–580. doi: 10.1006/mthe.2000.0074. [DOI] [PubMed] [Google Scholar]
- 38.Nguyen T.L., Abdelbary H., Arguello M., Breitbach C., Leveille S., Diallo J.S. Chemical targeting of the innate antiviral response by histone deacetylase inhibitors renders refractory cancers sensitive to viral oncolysis. Proc. Natl. Acad. Sci. U. S. A. 2008;105:14981–14986. doi: 10.1073/pnas.0803988105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wennier S.T., Liu J., Mcfadden G. Bugs and drugs: oncolytic virotherapy in combination with chemotherapy. Curr. Pharm. Biotechnol. 2012;13:1817–1833. doi: 10.2174/138920112800958850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Escobar-Zarate D., Liu Y.P., Suksanpaisan L., Russell S.J., Peng K.W. Overcoming cancer cell resistance to VSV oncolysis with JAK1/2 inhibitors. Cancer Gene Ther. 2013;20:582–589. doi: 10.1038/cgt.2013.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Otsuki A., Patel A., Kasai K., Suzuki M., Kurozumi K., Antonio Chiocca E. Histone deacetylase inhibitors augment antitumor efficacy of herpes-based oncolytic viruses. Mol. Ther. 2008;16:1546–1555. doi: 10.1038/mt.2008.155. [DOI] [PubMed] [Google Scholar]
- 42.Dornan M.H., Krishnan R., Macklin A.M., Selman M., El Sayes N., Son H.H. First-in-class small molecule potentiators of cancer virotherapy. Sci. Rep. 2016;6:26786. doi: 10.1038/srep26786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arulanandam, R., Batenchuk, C., Varette, O., Zakaria, C., Garcia, V., Forbes, N. E., Davis, C., Krishnan, R., Karmacharya, R., Cox, J., Sinha, A., Babawy, A., Waite, K., Weinstein, E., Falls, T., Chen, A., Hamill, J., DE Silva, N., Conrad, D. P., Atkins, H., Garson, K., Ilkow, C., Kaern, M., Vanderhyden, B., Sonenberg, N., Alain, T., Boeuf, F., Bell, J. C. & Diallo, J. S. 2015b. Microtubule disruption synergizes with oncolytic virotherapy by inhibiting interferon translation and potentiating bystander killing. Nat. Commun., 6, 6410. [DOI] [PubMed]
- 44.Ingemarsdotter C.K., Poddar S., Mercier S., Patzel V., Lever A.M.L. Expression of herpes simplex virus thymidine kinase/ganciclovir by RNA trans-splicing induces selective killing of HIV-producing cells. Mol. Ther. Nucleic Acids. 2017;7:140–154. doi: 10.1016/j.omtn.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dougan M., Dougan S., Slisz J., Firestone B., Vanneman M., Draganov D. IAP inhibitors enhance co-stimulation to promote tumor immunity. J. Exp. Med. 2010;207:2195–2206. doi: 10.1084/jem.20101123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nounamo B., Liem J., Cannon M., Liu J. Myxoma virus optimizes cisplatin for the treatment of ovarian cancer in vitro and in a syngeneic murine dissemination model. Mol. Ther. Oncolytics. 2017;6:90–99. doi: 10.1016/j.omto.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tseng C.W., Hung C.F., Alvarez R.D., Trimble C., Huh W.K., Kim D. Pretreatment with cisplatin enhances E7-specific CD8+ T-cell-mediated antitumor immunity induced by DNA vaccination. Clin. Cancer Res. 2008;14:3185–3192. doi: 10.1158/1078-0432.CCR-08-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bourgeois-Daigneault M.C., St-Germain L.E., Roy D.G., Pelin A., Aitken A.S., Arulanandam R. Combination of Paclitaxel and MG1 oncolytic virus as a successful strategy for breast cancer treatment. Breast Cancer Res. 2016;18:83. doi: 10.1186/s13058-016-0744-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nguyen A., Ho L., Wan Y. Chemotherapy and oncolytic virotherapy: advanced tactics in the war against cancer. Front. Oncol. 2014;4:145. doi: 10.3389/fonc.2014.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sharma P., Allison J.P. The future of immune checkpoint therapy. Science. 2015;348:56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 51.Wolchok J.D., Chiarion-Sileni V., Gonzalez R., Rutkowski P., Grob J.J., Cowey C.L. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N. Engl. J. Med. 2017;377:1345–1356. doi: 10.1056/NEJMoa1709684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zamarin D., Holmgaard R.B., Subudhi S.K., Park J.S., Mansour M., Palese P. Localeized oncolytic virotherapy overcomes systemic tumor resistance to immune checkpoint blockade immunotherapy. Sci. Transl. Med. 2014;6:226ra32. doi: 10.1126/scitranslmed.3008095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rojas J.J., Sampath P., Hou W., Thorne S.H. Defining effective combinations of immune checkpoint blockade and oncolytic virotherapy. Clin. Cancer Res. 2015;21:5543–5551. doi: 10.1158/1078-0432.CCR-14-2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cockle J.V., Rajani K., Zaidi S., Kottke T., Thompson J., Diaz R.M. Combination viroimmunotherapy with checkpoint inhibition to treat glioma, based on location-specific tumor profiling. Neuro-Oncology. 2016;18:518–527. doi: 10.1093/neuonc/nov173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gao Y., Whitaker-Dowling P., Griffin J.A., Barmada M.A., Bergman I. Recombinant vesicular stomatitis virus targeted to Her2/neu combined with anti-CTLA4 antibody eliminates implanted mammary tumors. Cancer Gene Ther. 2009;16:44–52. doi: 10.1038/cgt.2008.55. [DOI] [PubMed] [Google Scholar]
- 56.Shen W., Patnaik M.M., Ruiz A., Russell S.J., Peng K.W. Immunovirotherapy with vesicular stomatitis virus and PD-L1 blockade enhances therapeutic outcome in murine acute myeloid leukemia. Blood. 2016;127:1449–1458. doi: 10.1182/blood-2015-06-652503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rajani K., Parrish C., Kottke T., Thompson J., Zaidi S., Ilett L. Combination therapy with Reovirus and anti-PD-1 blockade controls tumor growth through innate and adaptive immune responses. Mol. Ther. 2016;24:166–174. doi: 10.1038/mt.2015.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bourgeois-Daigneault M.C., Roy D.G., Aitken A.S., El Sayes N., Martin N.T., Varette O. Neoadjuvant oncolytic virotherapy before surgery sensitizes triple-negative breast cancer to immune checkpoint therapy. Sci. Transl. Med. 2018:10. doi: 10.1126/scitranslmed.aao1641. [DOI] [PubMed] [Google Scholar]
- 59.Rajani K.R., Vile R.G. Harnessing the power of onco-immunotherapy with checkpoint inhibitors. Viruses. 2015;7:5889–5901. doi: 10.3390/v7112914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Engeland C.E., Grossardt C., Veinalde R., Bossow S., Lutz D., Kaufmann J.K. CTLA-4 and PD-L1 checkpoint blockade enhances oncolytic measles virus therapy. Mol. Ther. 2014;22:1949–1959. doi: 10.1038/mt.2014.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chesney J., Puzanov I., Collichio F., Singh P., Milhem M.M., Glaspy J. Randomized, open-label phase ii study evaluating the efficacy and safety of talimogene laherparepvec in combination with ipilimumab versus ipilimumab alone in patients with advanced, unresectable melanoma. J. Clin. Oncol. 2017 doi: 10.1200/JCO.2017.73.7379. (JCO2017737379) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ribas A., Dummer R., Puzanov I., Vanderwalde A., Andtbacka R.H.I., Michielin O. Oncolytic virotherapy promotes intratumoral T cell infiltration and improves anti-PD-1 immunotherapy. Cell. 2017;170(1109–1119) doi: 10.1016/j.cell.2017.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Houot R., Schultz L.M., Marabelle A., Kohrt H. T-cell-based immunotherapy: adoptive cell transfer and checkpoint inhibition. Cancer Immunol. Res. 2015;3:1115–1122. doi: 10.1158/2326-6066.CIR-15-0190. [DOI] [PubMed] [Google Scholar]
- 64.Lichtman E.I., Dotti G. Chimeric antigen receptor T-cells for B-cell malignancies. Transl. Res. 2017;187:59–82. doi: 10.1016/j.trsl.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 65.Newick K., O'brien S., Moon E., Albelda S.M. CAR T cell therapy for solid tumors. Annu. Rev. Med. 2017;68:139–152. doi: 10.1146/annurev-med-062315-120245. [DOI] [PubMed] [Google Scholar]
- 66.Ajina A., Maher J. Prospects for combined use of oncolytic viruses and CAR T-cells. J. Immunother. Cancer. 2017;5:90. doi: 10.1186/s40425-017-0294-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rommelfanger D.M., Wongthida P., Diaz R.M., Kaluza K.M., Thompson J.M., Kottke T.J. Systemic combination virotherapy for melanoma with tumor antigen-expressing vesicular stomatitis virus and adoptive T-cell transfer. Cancer Res. 2012;72:4753–4764. doi: 10.1158/0008-5472.CAN-12-0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tahtinen S., Gronberg-Vaha-Koskela S., Lumen D., Merisalo-Soikkeli M., Siurala M., Airaksinen A.J. Adenovirus improves the efficacy of adoptive T-cell therapy by recruiting immune cells to and promoting their activity at the tumor. Cancer Immunol. Res. 2015;3:915–925. doi: 10.1158/2326-6066.CIR-14-0220-T. [DOI] [PubMed] [Google Scholar]
- 69.Nishio N., Diaconu I., Liu H., Cerullo V., Caruana I., Hoyos V. Armed oncolytic virus enhances immune functions of chimeric antigen receptor-modified T cells in solid tumors. Cancer Res. 2014;74:5195–5205. doi: 10.1158/0008-5472.CAN-14-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rosewell Shaw A., Porter C.E., Watanabe N., Tanoue K., Sikora A., Gottschalk S. Adenovirotherapy delivering cytokine and checkpoint inhibitor augments CAR T cells against metastatic head and neck Cancer. Mol. Ther. 2017;25:2440–2451. doi: 10.1016/j.ymthe.2017.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cruz C.R., Micklethwaite K.P., Savoldo B., Ramos C.A., Lam S., Ku S. Infusion of donor-derived CD19-redirected virus-specific T cells for B-cell malignancies relapsed after allogeneic stem cell transplant: a phase 1 study. Blood. 2013;122:2965–2973. doi: 10.1182/blood-2013-06-506741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Louis C.U., Savoldo B., Dotti G., Pule M., Yvon E., Myers G.D. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood. 2011;118:6050–6056. doi: 10.1182/blood-2011-05-354449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pule M.A., Savoldo B., Myers G.D., Rossig C., Russell H.V., Dotti G. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat. Med. 2008;14:1264–1270. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Murphy A., Westwood J.A., Brown L.E., Teng M.W., Moeller M., Xu Y. Antitumor activity of dual-specific T cells and influenza virus. Cancer Gene Ther. 2007;14:499–508. doi: 10.1038/sj.cgt.7701034. [DOI] [PubMed] [Google Scholar]
- 75.Slaney C.Y., Von Scheidt B., Davenport A.J., Beavis P.A., Westwood J.A., Mardiana S. Dual-specific chimeric antigen receptor T cells and an indirect vaccine eradicate a variety of large solid tumors in an immunocompetent, self-antigen setting. Clin. Cancer Res. 2017;23:2478–2490. doi: 10.1158/1078-0432.CCR-16-1860. [DOI] [PMC free article] [PubMed] [Google Scholar]