Abstract
Transgenic Brassica napus plants overexpressing AtNHX1, a vacuolar Na+/H+ antiport from Arabidopsis thaliana, were able to grow, flower, and produce seeds in the presence of 200 mM sodium chloride. Although the transgenic plants grown in high salinity accumulated sodium up to 6% of their dry weight, growth of the these plants was only marginally affected by the high salt concentration. Moreover, seed yields and the seed oil quality were not affected by the high salinity of the soil. Our results demonstrate the potential use of these transgenic plants for agricultural use in saline soils. Our findings, showing that the modification of a single trait significantly improved the salinity tolerance of this crop plant, suggest that with a combination of breeding and transgenic plants it could be possible to produce salt-tolerant crops with far fewer target traits than had been anticipated.
Agricultural productivity is severely affected by soil salinity, and the damaging effects of salt accumulation in agricultural soils have influenced ancient and modern civilizations. The detrimental effects of salt on plants are a consequence of both a water deficit that results from the relatively high solute concentrations in the soil and a Na+-specific stress resulting from altered K+/Na+ ratios and Na+ ion concentrations that are inimical to plants. The alteration of ion ratios in the plant is caused by the influx of Na+ through pathways that function in the acquisition of K+ (1).
Wild plants that tolerate salt and grow in saline environments have high intracellular salt levels. A major component of the osmotic adjustment in these cells is accomplished by ion uptake. The utilization of inorganic ions for osmotic adjustment would suggest that salt-tolerant plants must be able to tolerate high levels of salts within their cells. However, enzymes extracted from these plants show high sensitivity to salt (2, 3), suggesting that these plants are able to keep Na+ away from the cytosol. Plants can use three strategies for the maintenance of a low Na+ concentration: sodium exclusion, sodium compartmentation, and sodium secretion. Sodium transport out of the cell can be accomplished by the operation of plasma membrane-bound Na+/H+ antiports. Biochemical evidence for the operation of plasma membrane Na+/H+ antiports (4) and the characterization of SOS1, a putative plasma membrane Na+/H+ antiport from Arabidopsis thaliana (5), have been reported. Transport mechanisms can also actively move ions across the tonoplast into the vacuole, removing the potentially harmful ions from the cytosol. These ions, in turn, act as an osmoticum within the vacuole, which then maintain water flow into the cell (3). The presence of large, acidic-inside, tonoplast-bound vacuoles in plant cells allows the efficient compartmentation of sodium into the vacuole, through the operation of vacuolar Na+/H+ antiports (6, 7).
The overexpression of AtNHX1, a vacuolar Na+/H+ antiport from A. thaliana, in Arabidopsis (7) and tomato (8) plants allowed the transgenic plants to grow in 200 mM NaCl, suggesting the possibility of engineering crop plants with improved salt tolerance.
Salt tolerance is not exclusively associated with cellular Na+ homeostasis, but also involves adaptations to secondary effects of salinity such as oxidative damage and changes in the levels and composition of fatty acids of the major glycerolipids in roots and leaves of a wide range of plants (9). Changes in the level of fatty acid saturation/unsaturation have been reported as a response to salt stress (10, 11), and a reduction in the levels of triacylglycerols containing unsaturated fatty acids has been reported in seed oil from cotton under salt stress (12).
Here we show that transgenic Brassica napus plants overexpressing a vacuolar Na+/H+ antiport were able to grow, flower, and produce seeds in the presence of 200 mM NaCl. B. napus, commonly known as canola or rapeseed, represents one of the most important oilseed crops that is being cultivated worldwide. The sustained growth of the transgenic plants, the seed yields, and the quality of the seed oil demonstrate the potential use of these transgenic plants for agricultural use in saline soils.
Materials and Methods
Plant Material.
Seeds of B. napus cv. Westar were rinsed with running water for 2 days, surface-sterilized with a solution of 10% commercial bleach (0.525% sodium hypochlorite) and 0.1% SDS for 5 min, and washed three times with sterile distilled water. Seeds were germinated on Murashige and Skoog medium. Cotyledon explants were excised from 7-day-old seedlings. The binary Ti vector pBI121 was used for transformation (13). The GUS gene (13) of the binary vector was replaced with the AtNHX1 gene to gain the new expression construct pHZX1. The new construct was electroporated into Agrobacterium tumefaciens strain LBA4404. For cocultivation, 1 ml of pHZX1 containing LBA4404 Agrobacterium was inoculated into 15 ml LB medium containing 50 mg/liter kanamycin, 50 mg/liter rifampicin, and 200 μM acetone-syringone. The culture was incubated 1 day at room temperature under constant shaking (250 rpm) and then diluted one time with liquid Murashige and Skoog medium. The cotyledon explants were submerged in the Agrobacterium solution for 3 min, blotted on sterile paper towels, and returned to the feeder plates for 2 days of cocultivation. After cocultivation, the explants were transferred to a selective regeneration medium (14). Regenerated shoots were transferred to fresh medium biweekly. When the green shoots were 1–2 cm tall, they were separated from the calli and transferred onto rooting medium that contained modified Murashige and Skoog medium supplemented with 3.7 mM KNO3, 4.1 mM NH4NO3, 0.5 mM MgSO4, 75 mg/liter kanamycin, 200 mg/liter ampicillin, and 1 mg/liter indolebutyric acid. Under these conditions, about 98% shoots formed roots in 2 weeks. Rooted shoots were transplanted to soil, plants were grown, and seeds (T1) were collected. T1 seeds were grown on Murashige and Skoog medium plates containing 15 mg/liter kanamycin, plants were grown, and homozygous seeds (T2) were selected. For salt-tolerance experiments, wild-type and transgenic seeds (T2) overexpressing the vacuolar Na+/H+ antiport were germinated in 250-ml pots containing pro-mix BX peat moss, perlite, and Vermiculite medium (Premier Brands, New Rochelle, NY) and grown in the greenhouse. Two weeks after germination the plants were watered biweekly with a nutrient solution with low (10 mM) or high (200 mM) concentrations of NaCl. Sixty wild-type plants and 60 transgenic plants were distributed in two groups of 30 plants each, and each group was watered with a solution with low or high salinity. The nutrient solution was obtained by mixing 1.2 g/liter stock fertilizer (6–11–31, Plant-Prod, Brampton, Ontario) and 1 g/liter Ca(NO3)2. The final nutrient solution contained 15 mM N, 2 mM P, 6.5 mM K, 4 mM Ca, 2 mM Mg, 9.5 mM S micronutrients and was supplemented with 5 mM or 200 mM NaCl. Day temperature was maintained at 28 ± 2°C and night temperature was 20 ± 2°C. Relative humidity was maintained at 50 ± 10%. Plants were grown under a 14-h/10-h light/dark photoperiod. Supplemental lighting consisted of eight high-pressure sodium lamps and resulted in a total flux (sunlight and supplemental light) of ≈1,450 μmol⋅m−2⋅s−1.
Membrane Isolation and Western Blots.
Tonoplast-enriched membrane fractions were isolated from leaves of 10-week-old plants as described (8). Western blots were performed as described (8).
Leaf, Root, and Seed Chemical and Lipid Analysis.
Roots were rinsed with distilled water, leaves and roots were collected from 15 plants from each treatment, pooled in three groups, and dried at 70°C for 24 h, and the material was ground to a fine powder. Seeds were collected from the rest of the plants 3 weeks later. For the determination of soluble sugars and proline contents, 100 mg of each pool was resuspended in 2 ml of water, sonicated, and centrifuged for 10 min at 2,500 × g. Soluble sugar, proline, and protein contents were determined in the supernatant as described (6, 15, 16). Ion contents were determined by atomic absorption spectrophotometry. Lipids were extracted from 2 g of mature leaf tissue or 3 g of root tissue with chloroform/methanol (2:1, vol/vol) and purified as described (17). Lipid classes were separated by TLC on silica gel G plates containing ammonium sulfate by using acetone/benzene/water (91:30:8, vol/vol/vol) (18). The lipids were scraped from the plate and transesterified with 1 ml of 1.5 M HCl in dry methanol in a microwave oven as described (19), and the fatty acid methyl esters (FAME) were extracted from the methanolic HCl with hexane. Seed oil fatty acid compositions were determined by direct transesterification of whole seeds by using the microwave technique. The FAME were analyzed by gas-liquid chromatography using a Hewlett–Packard model 5890 gas-liquid chromatograph with a 30 m × 0.25 mm ID DB-23 capillary column (J & W Scientific, Folsom, CA) programmed from 160°C to 210°C at 3°C min−1. The FAME were estimated quantitatively by using methylpentadecanoate as an internal standard.
Results
It has been suggested that the overexpression of vacuolar Na+/H+ antiports could serve for the engineering of salt-tolerant crops (7). To assess this possibility we overexpressed AtNHX1, coding for a vacuolar Na+/H+ antiport from A. thaliana, in Brassica plants. Our assumption was that if the plants were genetically modified to have an enhanced ability to sequester Na+ in their vacuoles, the transgenic plants would be able to use salty water for cell expansion and growth. The enhanced capacity of the transgenic plants to accumulate Na+ ions inside the vacuole would avert the toxic effects of Na+ in the cell cytosol and also would maintain an osmotic balance by using Na+ ions to drive water into the cells.
A construct containing AtNHX1 was introduced into the genome of B. napus cv. Westar. Sixty-four transgenic plants were obtained, and nine homozygous lines from these transgenic plants were obtained in the T2 generation (data not shown). To assess whether the enhanced expression of the vacuolar Na+/H+ antiport would allow plants to grow in high-salt conditions, wild-type and three lines of transgenic plants (with relatively low, medium, and high levels of transgene expression) were grown in the presence of 200 mM NaCl (Fig. 1), a concentration that inhibits the growth of almost all crop plants. The overexpression of the vacuolar Na+/H+ antiport did not affect the growth of the transgenic plants because similar growth was observed when the wild-type and transgenic plants were grown in the presence of 10 mM NaCl (Table 1). The growth of the wild-type plants was severely affected by the presence of 200 mM NaCl in the growth solution, plant growth was inhibited, and the plants were severely stunted (Fig. 1). On the other hand, the transgenic plants grew, flowered, and produced seeds (Fig. 1, Table 1). The growth of the transgenic plants in 200 mM NaCl was correlated with the increased levels of AtNHX1 protein (Fig. 1). Immunoblots of membrane fractions isolated from wild-type and transgenic plants detected AtNHX1 only in the tonoplast-enriched fractions from transgenic plants, indicating the proper targeting of the Na+/H+ antiport to the tonoplast (Fig. 1).
Figure 1.
Salt tolerance of wild-type (WT) plants and transgenic Brassica plants overexpressing AtNHX1 grown in the presence of 200 mM NaCl. Wild-type and homozygous plants showing high (X1OE1), medium (X1OE2), and low (X1OE3) levels of expression were grown in the presence of 200 mM NaCl. Plants are shown after 10 weeks of growth. (Inset) Western blots of leaf tonoplast-enriched membrane fractions isolated from wild-type and transgenic plants with low, medium, and high levels of expression of AtNHX1. Blots were probed with antibodies raised against the C terminus of AtNHX1. Equal amounts of protein (20 μg) were loaded in each lane. Relative molecular masses are indicated on the left.
Table 1.
Plant and seed yield of wild-type (WT) plants grown in the presence of 10 mM NaCl and transgenic plants overexpressing AtNHX1 (OEX1) grown in the presence of 10 mM and 200 mM NaCl
| WT, 10 mM NaCl | OEX1
|
||
|---|---|---|---|
| 10 mM NaCl | 200 mM NaCl | ||
| Height, cm | 210 ± 15 | 218 ± 13 | 183 ± 17 |
| Fresh weight, kg | 1.75 ± 0.10 | 1.79 ± 0.11 | 1.63 ± 0.13 |
| Seeds per plant | 470 ± 39 | 481 ± 43 | 463 ± 35 |
Each value is the mean ± SD (n = 15).
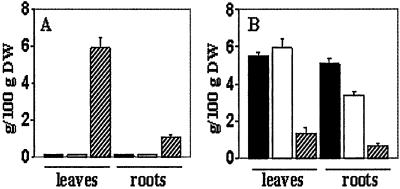
We determined the Na+, K+, soluble sugars, proline, total protein, nitrogen, and phosphorus contents of wild-type and transgenic plants grown at low (10 mM) NaCl and transgenic plants grown at high (200 mM) NaCl (Figs. 2 and 3). At low salinity, no significant differences were seen in the leaf and root Na+ content from wild-type and transgenic plants (Fig. 2). Dramatic changes were seen in transgenic plants grown at high salinity. A 70- and 9-fold increase in Na+ content was seen in the leaves and roots of these plants, respectively. The K+ content of leaves and roots of transgenic plants growing at high salinity decreased by 75% and 82%, respectively. Whereas the leaf soluble sugars content declined during growth at high salinity (Fig. 3), a 6-fold increase in proline content was seen in high-salt grown leaves. There were no significant differences in N (Fig. 3) or total P content (data not shown). It should be noted that a comparison with wild-type plants grown at high salinity was not possible because all of the wild-type plants grown in these conditions died.
Figure 2.
Na+ and K+ contents of leaves and roots from wild-type plants grown at 10 mM NaCl (filled bars) and transgenic plants (X1OE1) grown at 10 mM NaCl (empty bars) and 200 mM NaCl (hatched line bars). (A) Na+ content. (B) K+ content. Leaves and roots were collected from 15 plants from each treatment, the material was pooled in three groups, and ion contents were measured as described in Materials and Methods. Values are the mean ± SD (n = 3).
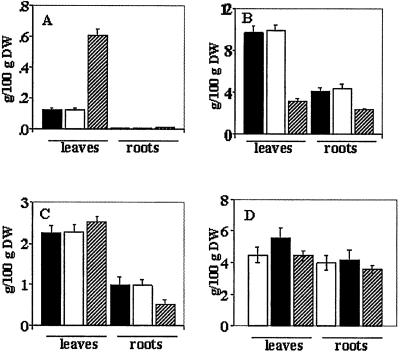
Figure 3.
Proline, soluble sugars, protein, and total nitrogen contents of leaves and roots from wild-type plants grown at 10 mM NaCl (filled bars) and transgenic plants (X1OE1) grown at 10 mM NaCl (empty bars) and 200 mM NaCl (hatched line bars). (A) Proline content. (B) Soluble sugar content. (C) Total protein content. (D) Total nitrogen content. Leaves and roots were collected from 15 plants from each treatment, the material was pooled in three groups, and contents were measured as described in Materials and Methods. Values are the mean ± SD (n = 3).
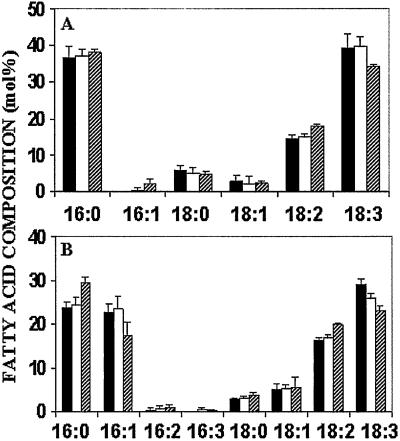
The major root and leaf lipids from wild-type plants grown at low salinity and transgenic plants grown at low and high salinity were analyzed (Table 2). No significant differences in the major chloroplastic and extraplastidic lipids were found. The fatty acid composition of the two major extraplastidic lipids, phosphatidylcholine (PC) and phosphatidylethanolamine (PE), did not differ in either the 16/18C ratio or the degree of unsaturation (not shown). Similarly, no differences were observed in the fatty acid compositions of the chloroplastic lipids digalactosyldiacylglycerol (DGDG) and mongalactosyldiacylglycerol (MGDG). Neither DGDG (synthesized predominantly through the eukaryotic pathway) nor MGDG (synthesized predominantly through the prokaryotic pathway) showed any significant difference in the 16/18C ratio or degree of unsaturation (results not shown). Some differences, however, were seen in the minor chloroplastic lipids, sulfoquinovosyldiacylglycerol (SQDG) and phosphatidylglycerol (PG) (Fig. 4).
Table 2.
Total lipid content (μmol/g fresh weight) of leaves and roots from wild-type (WT) plants grown in the presence of 10 mM NaCl and transgenic plants overexpressing AtNHX1 (OEX1) grown in the presence of 10 mM and 200 mM NaCl
| Tissue | Lipid | WT, 10 mM NaCl | OEX1
|
|
|---|---|---|---|---|
| 10 mM NaCl | 200 mM NaCl | |||
| Leaves | PC | 1.12 ± 0.54 | 1.34 ± 0.37 | 1.16 ± 0.29 |
| PE | 0.67 ± 0.25 | 0.81 ± 0.27 | 0.59 ± 0.21 | |
| SQDG | 0.40 ± 0.10 | 0.53 ± 0.11 | 0.59 ± 0.72 | |
| PG | 0.90 ± 0.07 | 0.83 ± 0.18 | 0.78 ± 0.16 | |
| DGDG | 1.64 ± 0.36 | 1.78 ± 0.29 | 1.82 ± 0.33 | |
| MGDG | 4.41 ± 0.53 | 4.32 ± 0.79 | 3.66 ± 0.75 | |
| Roots | PC | 0.84 ± 0.11 | 0.69 ± 0.06 | 0.83 ± 0.09 |
| PE | 0.69 ± 0.11 | 0.63 ± 0.06 | 0.66 ± 0.06 | |
| MQDG | 0.39 ± 0.09 | 0.56 ± 0.08 | 0.63 ± 0.05 | |
Each value is the mean ± SD (n = 5).
Figure 4.
Fatty acid composition of the minor chloroplastic lipids from wild-type plants grown at 10 mM NaCl (filled bars) and transgenic plants grown (X1OE1) at 10 mM NaCl (empty bars) and 200 mM NaCl (hatched line bars). (A) SQDG. (B) PG. Leaves were collected as leaf discs from 15 plants from each treatment, the material was pooled in three groups of 2 g each, and contents were purified and measured as described in Materials and Methods. Values are the mean ± SD (n = 5).
Although the 16/18C ratios were the same, there were differences in the degree of unsaturation of the 18C fatty acids in both SQDG and PG from transgenic plants grown in 200 mM NaCl. The ratio of palmitic acid (16:0)/trans-Δ3-hexadecenoic acid in PG from transgenic plants grown in 200 mM NaCl was significantly higher than in plants grown in 10 mM NaCl.
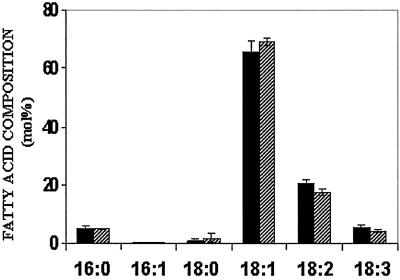
In roots, the predominant lipids are the extraplastidic phospholipids. Although the levels of MGDG, synthesized predominantly through the eukaryotic pathway in roots, are similar to those in leaves, the other plastidic lipids are found in very low quantities in roots. There were no significant differences in the fatty acid compositions of PC, PE, and MGDG from wild-type and transgenic plants grown at 10 mM NaCl or 200 mM NaCl (results not shown). Total fatty acid analyses of the seed oil did not differ significantly in seeds from wild-type plants grown in 10 mM NaCl and transgenic plants grown in 200 mM NaCl (Fig. 5). Quantitatively and qualitatively the seed oil from the transgenic plants is identical with seed oil from the wild-type plants.
Figure 5.
Fatty acid composition of seeds from wild-type plants grown in 10 mM NaCl (filled bars) and transgenic plants (X1OE1) grown in the presence of 200 mM NaCl (hatched line bars). Seeds were collected from individual plants, and batches of three seeds per plant were used for each measurement. Values are the mean ± SD (n = 5).
Discussion
Taken together, our results demonstrate the ability of the transgenic plants to use salty water for growth. Despite the high Na+ content in the leaves of the transgenic plants grown at 200 mM NaCl, these plants were able to grow, flower, and set seed. These results clearly demonstrate that the enhanced accumulation of Na+, mediated by the vacuolar Na+/H+ antiport (7, 8), allowed the transgenic plants to mitigate the toxic effects of Na+. Notably, transgenic plants grown at 200 mM NaCl produced numbers of seeds similar to those of wild-type plants grown at low salinity. Moreover, qualitative and quantitative analyses of the oil content showed no significant differences between seeds from wild-type plants grown at low salinity and transgenic plants grown at high salinity. It should be noted that although our experiments were carried out in the greenhouse, our results were obtained under growth conditions with a relatively low humidity and high light intensity. The leaf and root K+ contents of the transgenic plants grown in 200 mM NaCl were lower than those from plants grown in low salinity. Adaptation of plants to saline environments not only depends on their ability to ameliorate the toxic effects of Na+ per se, but also on their ability to overcome salt-induced impaired nutrient acquisition (20). This is of particular importance regarding K+ uptake and K+ homeostasis. Potassium concentrations in plant cells are kept under homeostatic control with cytosolic K+ concentrations in the order of 100–200 mM (21). When exposed to relatively low NaCl concentrations, Na+ ions can promote growth of many plants, in particular at low K+ concentrations in the growth medium (22). Under high-salinity conditions, Na+ ions may displace K+ from its carrier binding sites and this competition results in impaired K+ uptake and lower K+ cytosolic concentrations. Nevertheless, the growth of the transgenic plants was not significantly affected by high salinity, suggesting that K+ nutrition was not compromised in our experiments. It should be noted that we have used a high level of K+ (6.5 mM) in our solutions. It would be interesting to determine the tolerance of the transgenic Brassica plants overexpressing AtNHX1 in conditions of low K+ availability. Transgenic plants grown in 200 mM NaCl displayed a 6-fold increase in proline content compared with plants grown in low salinity. This accumulation of proline in response to high salinity is well documented. Proline contributes to osmotic adjustment (23), the protection of macromolecules during dehydration (24), and as a hydroxyl radical scavenger (25). Evidence supporting the role of proline during salt stress was obtained on the basis of salt tolerance in transgenic tobacco plants with enhanced levels of proline biosynthesis (26) and salt tolerance of Arabidopsis with suppressed levels of proline degradation (27). Moreover, a similar increase in proline content was observed in transgenic tomato plants overexpressing AtNHX1 growing at high salinity (8).
In all plant cells there are two major sites of lipid synthesis and desaturation of fatty acids. Glycerolipids derived from diacylglycerols synthesized in the extraplastidic compartments of the cell are synthesized by the eukaryotic pathway, whereas lipids derived from diacylglycerol synthesized in plastids are produced by a prokaryotic pathway (28, 29). Each compartment possesses different isoforms of glycerol-3-phosphate acyltransferase (GPAT) and lysophosphatidic acid acyltransferase (LPAT) that show differing specificity toward the fatty acid esterified to the two sn positions of the diacylglycerol. In addition, the desaturases of these diacylglycerol are specific to the specific compartment. Thus, through analyses of fatty acid composition it is possible to determine any specific effect of stress on lipid synthesis in the cell compartments. Our data suggest that the major structural lipids of the extraplastidic compartments (PC and PE) and of the chloroplasts (DGDG and MGDG) were unaffected by the overexpression of AtNHX1 and by the growth of the transgenic plants at high salinity. Only minor changes in the chloroplast lipids, SQDG and PG, were seen in transgenic plants grown in 200 mM NaCl. Little differences in the quantity of lipid or fatty acids were detected in the structural lipids of the cell. The 16/18C ratio remained similar, suggesting little effect on GPAT or LPAT activities. Further, the levels of unsaturation remained constant, indicating little or no effect on the desaturase activity. Only in the minor chloroplast lipids were changes in desaturation seen, the major difference being the 16:0/trans16:1 ratio in PG (1.7 and 1.0 in transgenic plants grown in 200 mM NaCl and plants grown in low salinity, respectively). Previous work has shown that this difference reflects a change in the light-harvesting complexes of the thylakoid membranes during the acclimation of plants to stress (30). Our results would suggest that the transgenic plants displayed little signs of stress or acclimation to high NaCl conditions. Analyses of the seed oil show no significant difference between seeds from wild-type and transgenic plants grown at low or high salinity.
Worldwide, more than 60 million hectares of irrigated land (representing 25% of the total irrigated acreage in the world) have been damaged by salt (31). Almost 20 years ago, Epstein (32) argued for the development of salt-tolerant crops with truly halophytic responses to salinity, i.e., accumulation of salt, in which the consumable part is botanically a fruit, such as grain, berries, or pomes. In these plants, Na+ ions would accumulate mainly in their leaves, and because the water transport to the fruits and seeds is mainly symplastic (33–35) much of the salt from these organs would be screened. Our results clearly support Epstein's argument (32). Recently, we have shown that when transgenic tomato plants, overexpressing AtNHX1, were grown at high salinity, salt accumulated in the leaves and not in the fruits (8). These results together with the data presented here clearly demonstrate the feasibility of generating salt-tolerant crops for agricultural use. Much of the effort toward breeding crop cultivars with improved salt tolerance assumed that salt tolerance will be achieved only after pyramiding several characteristics in a single genotype (36, 37). However, the modification of a single trait (vacuolar Na+ accumulation) significantly improved the salinity tolerance of Brassica plants. These results strongly suggest that with a combination of breeding and transgenic plants it could be possible to produce salt-tolerant crops with far fewer introduced traits than had been anticipated.
Acknowledgments
This paper is dedicated to Professor Ronald J. Poole (McGill University, Montreal, Canada) on the occasion of his retirement, in recognition of his significant contribution to plant membrane transport. This work was supported by a grant from the Natural Sciences and Engineering Research Council of Canada (E.B) and by the Will W. Lester Endowment from the University of California (E.B.).
Abbreviations
- PC
phosphatidylcholine
- PE
phosphatidylethanolamine
- DGDG
digalactosyldiacylglycerol
- MGDG
mongalactosyldiacylglycerol
- SQDG
sulfoquinovosyldiacylglycerol
- PG
phosphatidylglycerol
References
- 1.Maathuis F J M, Amtmann A. Ann Bot. 1999;84:123–133. [Google Scholar]
- 2.Flowers T J, Troke P F, Yeo A R. Annu Rev Plant Physiol. 1977;28:89–21. [Google Scholar]
- 3.Glenn E, Brown J J, Blumwald E. Crit Rev Plant Sci. 1999;18:227–255. [Google Scholar]
- 4.Blumwald E, Aharon G S, Apse P. Biochim Biophys Acta. 2000;1465:140–151. doi: 10.1016/s0005-2736(00)00135-8. [DOI] [PubMed] [Google Scholar]
- 5.Shi H, Ishitani M, Kim C, Zhu J-K. Proc Natl Acad Sci USA. 2000;97:6896–6901. doi: 10.1073/pnas.120170197. . (First Published May 23, 2000; 10.1073/pnas.120170197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blumwald E, Poole R J. Plant Physiol. 1985;78:163–167. doi: 10.1104/pp.78.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Apse M P, Aharon G S, Snedden W S, Blumwald E. Science. 1999;285:1256–1258. doi: 10.1126/science.285.5431.1256. [DOI] [PubMed] [Google Scholar]
- 8.Zhang H-X, Blumwald E. Nat Biotechnol. 2001;19:765–768. doi: 10.1038/90824. [DOI] [PubMed] [Google Scholar]
- 9.Ben Raïs L, Alpha M-J, Bahl J, Guillot-Salomon T, Dubacq J-P. Plant Physiol Biochem. 1993;31:547–557. [Google Scholar]
- 10.Wu J, Seliskar D M, Gallagher J L. Physiol Plant. 1998;102:307–317. [Google Scholar]
- 11.Yu B, Gong H, Lui Y. J Plant Nutr. 1998;21:1589–1600. [Google Scholar]
- 12.Smaoui A, Cherif A. Biochem Soc Trans. 2000;28:902–905. [PubMed] [Google Scholar]
- 13.Jefferson R A, Kavanagh T A, Bevan M W. EMBO J. 1987;6:3901–3907. doi: 10.1002/j.1460-2075.1987.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moloney M M, Walker J M, Sharma K K. Plant Cell Rep. 1989;8:238–242. doi: 10.1007/BF00778542. [DOI] [PubMed] [Google Scholar]
- 15.Dubois M, Gilles R A, Hamilton J K, Roberts P A, Smith F. Anal Chem. 1956;28:350–356. [Google Scholar]
- 16.Bates L S, Waldren R P, Teare I D. Plant Soil. 1973;39:205–207. [Google Scholar]
- 17.Williams J P, Merrilees P A. Lipids. 1970;5:367–370. doi: 10.1007/BF02532100. [DOI] [PubMed] [Google Scholar]
- 18.Khan M, Williams J P. J Chromatogr. 1977;140:79–185. doi: 10.1016/s0021-9673(00)88412-5. [DOI] [PubMed] [Google Scholar]
- 19.Khan M U, Williams J P. Lipids. 1993;28:953–955. [Google Scholar]
- 20.Marschner H. Mineral Nutrition of Higher Plants. New York: Academic; 1995. [Google Scholar]
- 21.Wyn Jones R G, Pollard A. In: Encyclopedia of Plant Physiology, New Series. Lauchli A, Bieleski R L, editors. 15B. Berlin: Springer; 1983. pp. 528–562. [Google Scholar]
- 22.Elzam O E, Epstein E. Agrochimie. 1969;13:187–195. [Google Scholar]
- 23.LeRudulier D, Strom A R, Dandekar A M, Smith L T, Valentine R C. Science. 1984;224:1064–1068. doi: 10.1126/science.224.4653.1064. [DOI] [PubMed] [Google Scholar]
- 24.Yancey P, Clark M, Hand S, Bowlus R, Somero G. Science. 1982;217:1214–1222. doi: 10.1126/science.7112124. [DOI] [PubMed] [Google Scholar]
- 25.Smirnoff N, Cumbes Q J. Phytochemistry. 1989;28:1057–1060. [Google Scholar]
- 26.Kishor P B K, Hong Z, Miao G-H, Hu C-A A, Verma D P S. Plant Physiol. 1995;108:1387–1394. doi: 10.1104/pp.108.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nanjo T, Kobayashi M, Yoshiba Y, Kakubari Y, Yamaguchi-Shinozaki K, Shinozaki K. FEBS Lett. 1999;461:205–210. doi: 10.1016/s0014-5793(99)01451-9. [DOI] [PubMed] [Google Scholar]
- 28.Browse J A, Somerville C R. Annu Rev Plant Physiol Plant Mol Biol. 1991;42:467–506. [Google Scholar]
- 29.Williams J P, Imperial V, Khan M U, Hodson J N. Biochem J. 2000;349:127–133. doi: 10.1042/0264-6021:3490127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huner N P A, Krol M, Williams J P, Maissan E, Low P S, Roberts D, Thompson J E. Plant Physiol. 1987;84:12–18. doi: 10.1104/pp.84.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ghassemi F, Jakeman A, Nix H. Salinization of Land and Water Resources: Human Causes, Extent, Management, and Case Studies. Sydney: Univ. of New South Wales Press; 1995. [Google Scholar]
- 32.Epstein E. In: Better Crops for Food, Ciba Foundation Symposium. Nugent J, O'Connor M, editors. Vol. 97. London: Pitman; 1983. pp. 61–82. [Google Scholar]
- 33.Ehret D L, Ho L C. J Exp Bot. 1986;37:1294–1302. [Google Scholar]
- 34.Lee D R A. Can J Bot. 1986;67:1898–1902. [Google Scholar]
- 35.Davies W J, Bacon M A, Thompson D S, Sobeih W, Rodriguez L G. J Exp Bot. 2000;51:1617–1626. doi: 10.1093/jexbot/51.350.1617. [DOI] [PubMed] [Google Scholar]
- 36.Yeo A R, Yeo M E, Flowers S A, Flowers T J. Theor Appl Genet. 1990;79:377–384. doi: 10.1007/BF01186082. [DOI] [PubMed] [Google Scholar]
- 37.Cuartero J, Fernandez-Muñoz R. Sci Hortic. 1999;78:83–125. [Google Scholar]