Abstract
Histone deacetylase inhibitors (HDACi) are small molecules targeting epigenetic enzymes approved for hematologic neoplasms, which have also demonstrated clinical activities in solid tumors. In our present study, we screened our internal compound library and discovered a novel HDACi, WW437, with potent anti-breast cancer ability in vitro and in vivo. WW437 significantly inhibited phosphorylated EphA2 and EphA2 expression. Further study demonstrated WW437 blocked HDACs-EphA2 signaling axis in breast cancer. In parallel, we found that EphA2 expression positively correlates with breast cancer progression; and combined use of WW437 and an EphA2 inhibitor (ALW-II-41-27) exerted more remarkable effect on breast cancer growth than either drug alone. Our findings suggested inhibition of HDACs-EphA2 signaling axis with WW437 alone or in combination with other agents may be a promising therapeutic strategy for advanced breast cancer.
Keywords: HDACs, EphA2, WW437, Breast cancer, Growth, Metastasis
Highlights
-
•
WW437 is a novel HDACi, which displays potent anticancer activity in breast cancer.
-
•
HDACs-EphA2 signaling axis represents a novel target in breast cancer.
-
•
WW437 is a promising therapeutic agent for advanced breast cancer, alone or in combination with EphA2 inhibitor.
Histone deacetylase inhibitors (HDACi) are small molecules targeting epigenetic enzymes approved for cutaneous T-cell lymphoma (CTCL), peripheral T-cell lymphoma (PTCL) and multiple myeloma (MM) treatment, which have also demonstrated clinical activities in solid tumors, including lung cancer and breast cancer. Herein we report a novel HDACi WW437, which displays potent anticancer activity in both cultured cancer cells and xenograft models. Importantly, our work reveals WW437 significantly blocked the HDACs-EphA2 signaling axis in breast cancer. WW437 exhibited significant inhibitory effects on tumor growth and metastases with little toxicity, and tumors from treated mice showed decreased EphA2 expression, suggesting that EphA2 may be a useful biomarker of response to WW437. We also found that EphA2 expression positively correlates with tumor progression in aggressive breast cancer.
1. Introduction
Histone deacetylase inhibitors (HDACis) are a series of compounds capable of inducing epigenetic changes via targeting epigenetic enzymes [1]. To date, four classes of HDACi have been discovered or developed, including short-chain fatty acids (butyrate, valproic acid), hydroxamic acids (trichostatin A, vorinostat, and pracinostat), tetrapeptides (romidepsin) and benzamidines. Among them, vorinostat and romidepsin are approved for the treatment of cutaneous T-cell lymphoma (CTCL), and belinostat has been approved for the treatment of peripheral T-cell lymphoma (PTCL) [[2], [3], [4]]. Histone deacetylase inhibitors have also demonstrated clinical activities either alone or in combination with other agents in solid tumors [5].
Eph receptor tyrosine kinase signaling regulates cancer initiation and metastatic progression through multiple mechanisms [6]. Among the distinct EphA receptors, EphA2 is an important modulator of tumor growth, angiogenesis, and metastasis [7,8]. The role of EphA2 differs in distinct tumor types. Several researches suggest EphA2 plays an oncogenic-suppressive role in cancer and deletion of EphA2 receptor tyrosine kinase leads to increased susceptibility to carcinogenesis in mouse skin [9]. However, in lung cancer, genetic and pharmacologic inhibition of EphA2 results in increased tumor cell death in vitro and decreased tumor burden in vivo [10]. EphA2 is proved to promote tumor cell migration/invasion and can be considered as a poor prognostic marker in colorectal cancer [11]. In parallel, EphA2 amplification has been found in >80% of breast cancer clinical samples [12,13]. Previous studies reported that targeting EphA2 in ERBB2-driven murine mammary tumor models resulted in inhibited tumor formation and metastatic progression [12]. Targeting EphA2 using shRNA or inhibitor intervention impairs cell cycle progression and growth in basal-like/triple-negative breast cancer [8].
Breast cancer is a serious health problem and the second leading cause of cancer-related death among women. Epigenetic changes in cancer are common and have been involved in breast cancer occurrence and development [14,15]. Several HDACis are being determined as single agents or combined with conventional therapies in clinical trials of metastatic breast cancer [16,17]. In these preclinical and clinical settings, it is necessary to develop novel HDAC inhibitors as well as investigate their exact mechanisms.
Here, we identified a novel HDACi, WW437, which demonstrates potent anti-breast tumor activity in vitro and in preclinical animal model. Mechanistically, we found WW437 significantly inhibits HDACs-EphA2 signal axis. Our results suggest that HDACs-EphA2 signaling axis may represent a novel target in breast cancer.
2. Materials and Methods
2.1. Cell Lines, Cell Culture, and Reagents
The breast cancer cell line MDA-MB-231 (MDA231), BT549 and 4 T1 were purchased from ATCC (Manassas, VA, USA). MDA-MB-231 cells were maintained in DMEM supplemented with 10% FBS and 1% penicillin/streptomycin. BT549 and 4 T1 cells were maintained in RPMI 1640 medium supplemented with 10% FBS and 1% penicillin/streptomycin. All breast cancer cells were maintained at 37 °C under a humidified 5% CO2 incubator. Mycoplasma contamination was monitored periodically.
Cell culture reagents were purchased from Invitrogen Life Technologies (Carlsbad, CA, USA). Matrigel was purchased from BD Bioscience (Pasadena, CA, USA). Antibodies against acetyl-histone H3, acetyl-histone H4, HDAC1, HDAC2, HDAC3, HDAC5, HDAC6, E-cadherin, Zeb1, Vimentin, c-Myc, p21, cleaved PARP, Sp1, EphA2, Phospho-Tyrosine (p-Tyr-1000), Acetylated-Lysine and Flag were purchased from Cell Signaling Technology Inc. (Danvers, MA, USA). Antibody against HDAC4 was purchased from Abcam (Hong Kong, China). Antibody against actin and dimethyl sulfoxide (DMSO) was obtained from Sigma-Aldrich (Sigma-Aldrich, Inc., Shanghai, China). The detailed information of the antibodies we used in our study was shown in supplementary 1. WW437 were synthesized as described in the Supplementary Information (Supplementary Fig. 1). The synthetic route of SAHA was described previously [18]. The stock solutions of compound were prepared in dimethyl sulfoxide (DMSO) at a concentration of 50 mM and stored at −80 °C. Breast cancer tissue array were obtained from Alenabio (Alenabio, Xian, Shanxi, China).
2.2. HDAC Inhibitor Activity Assay
HDAC inhibitor activity assay was executed using the HDAC inhibitor drug screening kit (BioVision, Inc.) as described previously [19]. Briefly, HDACi candidates were incubated with HDAC enzymes (HeLa nuclear extract or MDA-MB-231 cell lysates) and HDAC fluorometric substrates at 37 °C for 1 h. The lysine developer was used to stop the reaction and the fluorescence units were obtained at Ex/Em 355/460 nm.
2.3. Cell Viability Assay
Breast cancer cells were seeded in 96-well plates. After 24 h, the cells were treated with different concentrations of WW437, and the cell viability was measured by MTS assay as described previously [20].
2.4. Western Blotting
Western blot analysis was performed as previously described [21]. Cell lysates were prepared in RIPA lysis buffer containing protease and phosphatase inhibitors.
2.5. Immunofluorescent Staining
Immunofluorescent staining was conducted as previously described [20].
2.6. Colony Formation Assay
Colony formation assay was conducted as previously reported [19]. Breast cancer cells were seeded in a 6-well plate and treated with or without WW437. Culture medium was refreshed every other day. All the cells were cultured for 10 days. Then the clones were stained with 0.1% crystal violet and counted manually.
2.7. Assessment of Apoptosis
Apoptosis was assessed using the Apoptosis Detection Kit (BD Biosciences) according to the manufacture's guidelines.
2.8. Wound Healing Assay
Wound-healing migration assay was performed as described previously [22]. When tumor cells grew to full confluence, the “wounds” were created by a sterile 100 μL pipette tip. Next, fresh medium was added containing different concentrations of WW437. After 12 h incubation, cells were fixed and photographed. The migrated cells were manually quantified.
2.9. Invasion Assay
Invasion assay was performed as described previously [19]. In brief, a total of 5 × 104 cells (for MDA-MB-231 cells) or 1 × 105 cell (for 4T1 cells) in 100 μL of FBS-free medium were added in the upper chamber, and 500 μL of complete medium was added at the bottom. Indicated concentrations of WW437 were added to both chambers. After 12 h, invaded cells were stained with 0.1% crystal violet and counted manually.
2.10. Fluorescent-Gelatin Degradation Assay
This assay was performed as reported previously [23]. Briefly, Cells were seeded on coverslips (precoated with FITC-gelatin) with different concentrations of WW437, incubated for 12 h, and followed by immunofluorescence. Gelatin degradation was quantified using Image-Pro Plus 6.0 software.
2.11. Three-Dimensional on-Top Assay
Three-dimensional on-top assay was performed as previously described [21].
2.12. Animal Model
All animal care and experimental studies were performed according to the guidelines and approval of the Animal Investigation Committee of the Shanghai General Hospital, Shanghai Jiao Tong University School of Medicine. Female BALB/c mice were bred and maintained at the animal center in Shanghai General Hospital (21 °C, 55% humidity, on a 12-h light–dark cycle).
4 T1 cells (1 × 105) resuspended in 0.1 mL PBS were injected subcutaneously into the 4th abdominal mammary fat pad of BALB/c mice. On day 7, the mice were randomly divided into four groups (n = 7 per group) and received i.p. injection of WW437 (10 mg/kg/day and 30 mg/kg/day) and SAHA (30 mg/kg/day) as compared with mice injected with DMSO (control group). Tumor size was measured every one week, and tumor volume was calculated according to the formula: V = L × W2 × 0.52, where L and W refers to length and width, respectively. After 35 day treatment, all the mice were killed. Lung metastases were manually counted using a dissecting microscope by three individuals who do not have personal biases with the experiment. The lungs were fixed and prepared for H&E staining. The primary tumors were weight and then prepared for western blotting assay.
Another independent animal experiment (n = 5 per group) was performed to determine the potential toxicity of WW437 on mice.
2.13. Co-Immunoprecipitation (Co-IP)
The cells were treated with WW437 for 24 h and cell lysates were collected with cell lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% Triton ×100) plus protease inhibitor cocktail. Cell lysates (equal amount of proteins) were incubated with anti-EphA2 antibody for 12 h at 4 °C. Then the immunoprecipitated pellets were incubated with protein A/G agarose beads for 4 h, followed by three washes with wash buffer. The beads were resuspended in SDS loading buffer, and boiled for 10 min. Western blot analysis was performed using indicated antibodies.
2.14. RNA Interference
siRNA transfection was conducted with Lipofectamine 3000 (Thermo) following the manufacturer's instruction. Specific custom siRNAs were synthesized in GenePharma (Shanghai, China). 48 h after transfection, the cell lysates were collected and subjected to western blot. The sequences of siRNAs used in this study were listed in Supplementary Table 2.
2.15. Real Time PCR
RNA samples from breast cancer cells were prepared using Trizol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's protocols. Total RNA (1 μg) was converted to cDNA using oligo dT primer, then quantified mRNA levels by Real-time PCR using SYBR Green (Takara, Ohtsu, Japan). We ran samples in technical triplicates and calculated relative mRNA levels normalized to actin mRNA levels in the same samples. Primers for human EphA2 were: 5 ACTACGGCACCAACTTCCAG 3′ (forward) and 5′ GTAGAAGCCTTTGCGGGTGA 3′ (reverse). Primers for mouse EphA2 were: 5′ GCACAGGGAAAGGAAGTTGTT 3 “(forward) and 5” CATGTAGATAGGCATGTCGTCC 3′(reverse). Primers for human Actin were: 5 CATGTACGTTGCTATCCAGGC 3′ (forward) and 5′ CTCCTTAATGTCACGCACGAT 3′ (reverse). Primers for mouse Actin were: 5′ GGCTGTATTCCCCTCCATCG 3 “(forward) and 5” CCAGTTGGTAACAATGCCATGT 3′(reverse). Primers for human HDAC2 were: 5′ ATGGCGTACAGTCAAGGAGG 3′ (forward) and 5′ TGCGGATTCTATGAGGCTTCA 3′ (reverse). Primers for human HDAC4 were: 5′ GGCCCACCGGAATCTGAAC 3′ (forward) and 5′ GAACTCTGGTCAAGGGAACTG 3′ (reverse).
2.16. Chromatin Immunoprecipitation (ChIP) and ChIP-Re-ChIP Assays
ChIP assay was performed using an EZ CHIP KIT (Millpore, Millipore, Bill erica, MA, USA) according to manufacturer's protocol. Immunoprecipitated DNA was analysed by real-time PCR using primers that were specific for regions spanning the Sp1 binding sites in the promoter of EphA2. ChIP-Re-ChIP Assays was performed as described previously with some modifications [24]. Briefly, the chromatin was immunoprecipitaed in a sequential manner with the indicated antibodies. The immunoprecipitated DNA was uncross-linked and subjected to proteinase K digestion and purified. Primers for ChIP and ChIP-Re-ChIP assays were: 5′ CAAATCCTGCTTCTACTACCCG 3′ (forward) and 5′ GCTGCTGATGGTCAAAAAGAGTAT 3′ (reverse).
2.17. H&E and Immunohistochemical (IHC) Staining Analysis
H&E and IHC staining analysis were performed as previously reported [25]. Breast cancer tissue array were stained with anti-EphA2 antibody. Images were obtained with Leica microscope (Leica, DM4000b).
2.18. Statistical Analysis
The sample size was used to make sure adequate statistical power (≥ 80%) according to formal power calculation. Results are presented as mean ± SD. A Student's t-test was used to compare two groups (p < 0.05 was considered statistically significant) unless otherwise indicated. All experiments were performed at least three times except animal models.
3. Results
3.1. WW437 is a Novel HDACi
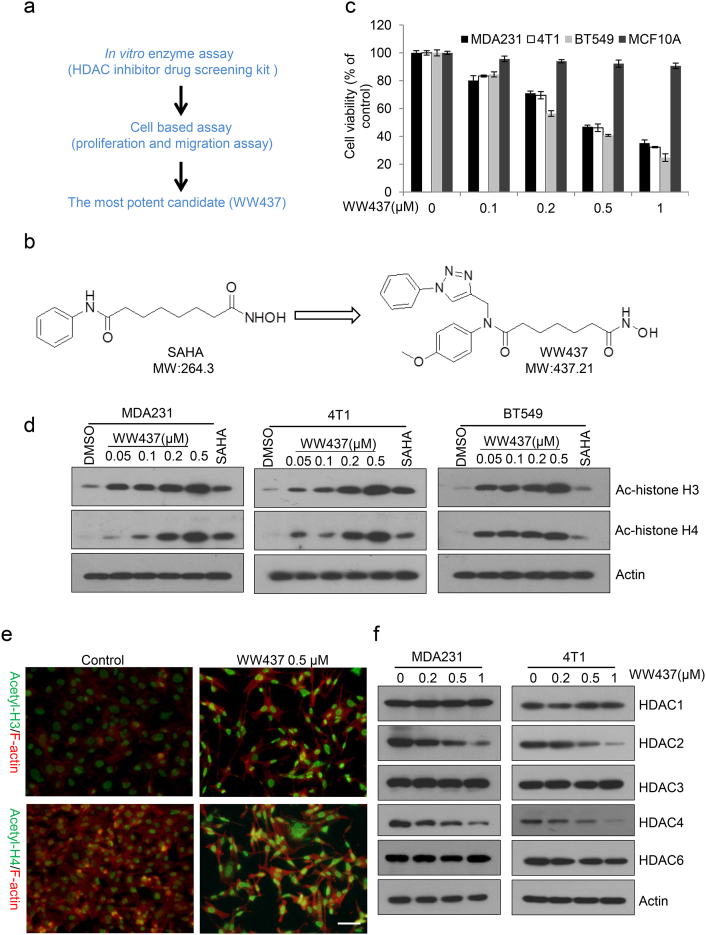
HDACs are becoming one of the most attractive cancer therapeutic targets in last decades, and a batch of novel HDACis were subsequently designed and identified based on our previous research [26]. In order to increase the cancer inhibitory activities as well as to improve potential physicochemical properties of new generation HDACis, 1, 2, 3-triazole unit, as one of well-known privileged structures in modern medicinal chemisty, was merge into our initial scaffold according to docking results. After our initial screening, a novel HDACi, WW437 (N1-hydroxy-N7-(4-methoxyphenyl)-N7- ((1-phenyl-1H-1, 2, 3-triazol-4-yl) methyl) heptanediamide), was identified in our internal compound library with the highest enzyme inhibitory activity (Fig. 1a and b). The results indicated WW437 inhibited HDAC activity (HeLa cell nuclear extracts and MDA231 cell lysates) in a dose-dependent manner (Supplementary Fig. 2a and b). The inhibitory effect of WW437 on a panel of breast cancer cell lines (MDA231, 4 T1 and BT549) and non-tumorigenic breast epithelial cell line MCF10A was next evaluated. WW437 significantly inhibited breast cancer cell proliferation and the IC50 is ranged from 0.2 μM to 0.5 μM (Fig. 1c). We also found WW437 exerted little cytotoxicity against MCF10A (Fig. 1c). This result suggested that WW437 shows specific killing effect on breast cancer cells. Our previous study indicated that the IC50 of SAHA (vorinostat), a FDA approved known HDACi belonging to hydroxamic acid, is between 2 μM and 5 μM on breast cancer cell [19]. A gold standard of HDAC inhibition is the increased expression of histone acetylation (histone H3 and histone H4) [27]. We then examined whether WW437 could induce the expression of acetylated histone H3 and histone H4, and found the protein level of acetylated histone H3 and histone H4 in breast cancer cell was upregulated as the concentration of WW437 increased (Fig. 1d and e). In addition, we also investigated the effect of WW437 on HDACs expression. As shown in Fig. 1f, WW437 led to a dose dependent decreased of HDAC2 and HDAC4 after 24 h exposure to different concentrations of compound. However, the expression of other HDACs (HDAC1, HDAC3, HDAC5 and HDAC6) remained relatively constant (Fig. 1f). We also performed PCR assay and found the mRNA level of HDAC2 and HDAC4 were reduced after WW437 treatment (Supplementary Fig. 3a and b). This suggests that WW437 may play a role in selectively down-regulating HDAC2 and HDAC4 gene transcription. Together, our results suggest that WW437 is a novel HDAC inhibitor and has a potential antitumor activity on breast cancer.
Fig. 1.
WW437 is a novel HDACi. (a) Schematic diagram of the screening strategy. (b) Chemical structure of SAHA and WW437. (c) A panel cell lines (MDA231, 4 T1, BT549 and MCF10A) were treated with indicated concentrations of WW437. After 48 h, the MTS assay was performed. The bars indicate the mean ± SD. (d) Acetylated histone H3 (Ac-H3) and Acetylated histone H4 (Ac-H4) protein level was induced by WW437 in various breast cancer cell lines. SAHA was used as a positive control (1 µM). Actin was used as loading control. (e) MDA231 cells were seeded on coverslips. Cells were exposed to WW437 for 24 h, and then fixed, and stained with the indicated antibodies. Scale bar, 40 μm. (f) Cells were treated with different concentrations of WW437 for 24 h and then whole cell lysates were collected. Western blot assays were performed using indicated antibodies.
3.2. WW437 Displays Potent Tumor Growth and Metastasis-Inhibitory Effects on Breast Cancer Cells
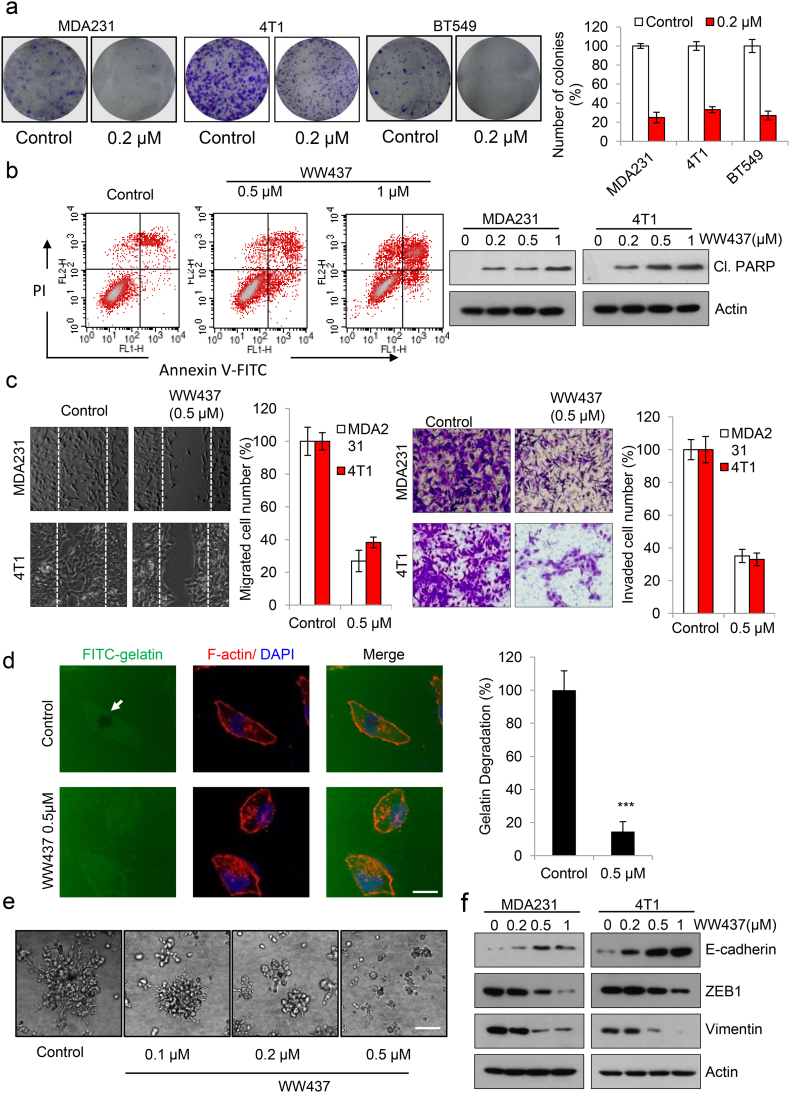
To further evaluate the potential antitumor growth effect of WW437 in vitro, we performed colony assay. As shown in Fig. 2a, WW437 significantly inhibited tumor cell colony formation at 0.2 μM. Evading apoptosis of tumor cells is a key hallmark in tumor growth [28]. Therefore, we sought to investigate whether treatment with WW437 could induce tumor cell apoptosis. We found WW437 caused a dose-dependent induction of apoptosis in MDA231 (Fig. 2b, left panel). Meanwhile, cleaved-PARP expression was significantly increased (Fig. 2b, right panel). Successful tumor cell migration and invasion is essential for tumor metastasis, and inhibition of these is a feasible strategy to suppress metastasis [29]. We then evaluated the inhibitory propensity of WW437 in tumor migration and invasion. WW437 treatment remarkably decreased migration and invasion ability in two highly malignant breast cancer cell lines tested (Fig. 2c). Proteolytic degradation of extracellular matrix (ECM) is a critical step during cell invasion that is required for tumor metastasis [30]. We next performed a fluorescent-gelatin degradation assay to assess whether WW437 inhibits tumor cells to degrade ECM. As shown in Fig. 2d, MDA231 cells potently degraded ECM in 12 h, while WW437-treated group lost this ability. Three-dimensional culture systems can be used to study cancer growth, invasion and metastasis. In a 3D culture model, WW437 significantly blocked tumor cell growth and invasion into the surrounding Matrigel (Fig. 2e). Numerous studies have suggested that epithelial-to-mesenchymal transition (EMT) contributes to dissemination of cancer cells and is pivotal for metastasis of breast cancer [31]. Previous studies reported HDAC-dependent histone deacetylation is essential for repressing expression of epithelial genes such as E-cadherin during EMT [32] and a pan-HDAC inhibitor panobinostat inhibits triple-negative breast cancer metastasis via inhibition of ZEB family [33]. We therefore examined the effect of WW437 on EMT related proteins expression. E-cadherin was significantly induced with WW437 treatment and WW437 downregulated the expression of Vimentin and ZEB1 (Fig. 2f). Together, these results indicated that WW437 displays potent tumor growth and metastasis-inhibitory effects in a dose-dependent manner on breast cancer cells.
Fig. 2.
WW437 displays potent tumor growth and metastasis-inhibitory effects on breast cancer cells. (a) WW437 significantly inhibited breast cancer cell colony formation. Breast cancer cells were seeded on six-well plates. After 12 h, cells were treated with indicated concentrations of WW437. On day 10, the number of colonies were counted. Results represent the average of three replications. The bars indicate the mean ± SD. (b) Apoptosis was assessed by Annexin V/PI staining and flow cytometry (left panel). Cleaved PARP was evaluated by western blot (right panel). (c) WW437 (0.5 μM) inhibited cell migration and invasion in two highly malignant breast cancer cell lines MDA231 and 4 T1. Left, representative images and quantification of the migration assay; right, representative images and quantification of the invasion assay. The bars indicate the mean ± SD. (d) MDA231 cells were seeded on coverslips (procoated with FITC conjugated gelatin). After treated with WW437 for 12 h, F-actin was stained with phalloidin (red) and nuclei were stained with DAPI (blue). Areas of gelatin degradation were quantified using Image-Pro Plus 6.0 software. Scale bar, 10 μm. The bars indicate the mean ± SD. Statistically significant differences (Student's t-test), ***, P < 0.001. (e) 3D culture assay. MDA231 cells were seeded onto solidified Matrigel. After 30 min, medium containing 10% Matrigel and various concentrations of WW437 were added on top of the cells. The Matrigel-medium mixture was replaced every other day. Four days later, cells were photographed. Scale bar, 40 μm. (f) The effect of WW437 on EMT related protein. Western blot analysis was performed with indicated antibodies.
3.3. WW437 Significantly Inhibited Tumor Growth and Metastasis in a Preclinical Animal Model
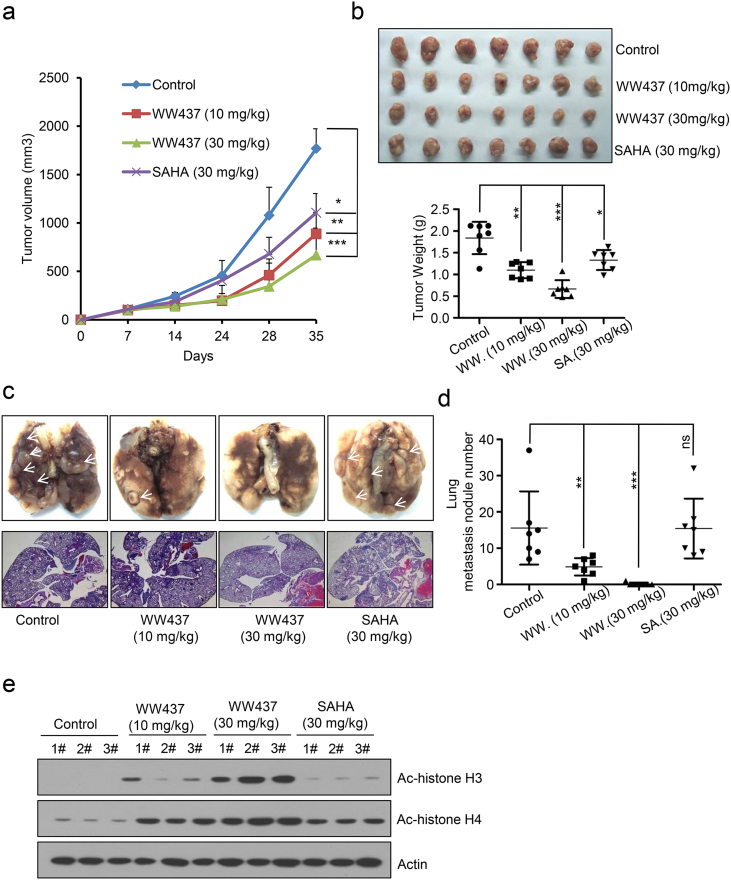
To investigate the potential antitumor effect of WW437 in vivo, we generated a spontaneous breast tumor animal model. 4 T1 cells were injected subcutaneously into the 4th abdominal mammary fat pad of BALB/c mice that were treated 7 days later by i.p. injection of DMSO (control group), WW437 (10 mg/kg/day and 30 mg/kg/day) and SAHA (30 mg/kg/day, positive control group) over 35 days. As shown in Fig. 3a and b, treatment of mice with WW437 and SAHA significantly inhibited the tumor growth compared with control group. We also found the tumors derived from WW437 (10 mg/kg/day and 30 mg/kg/day) treated group were smaller and lighter than SAHA treated group. Consistent with our in vitro results, WW437 treatment dramatically diminished breast tumor metastasis to the lungs, as indicated by the number of metastatic nodules in control and treatment groups (Fig. 3c and d). However, SAHA showed little effect on tumor metastasis in vivo (Fig. 3c and d). Notably, the increased Ac- histone H3 and Ac-histone H4 levels in primary tumor tissue of WW437 or SAHA treated group confirmed the HDAC inhibitory effect of WW437 on breast cancer in vivo (Fig. 3e). We then examined the potential toxicity of WW437 (30 mg/kg/day). Female BALB/c mice received intraperitoneal injection of WW437 or SAHA (30 mg/kg/day) for 35 days (n = 5 per group). Body weight was measured once a week. No significant changes in mice body was found after treatment (Supplementary Fig. 4). These results may imply WW437 showed more superior pharmacodynamic properties in vivo and few side effects on the mouse body at our therapeutic concentration.
Fig. 3.
WW437 significantly inhibited tumor growth and metastasis in a preclinical animal model. 4 T1 cells (1 × 105) were injected into mouse mammary fat pads. Mice were divided into 4 groups (n = 7 per group) on day 7 after tumor cell implantation. After 35 days, all mice were executed. (a) Primary tumor volume was measured every week. Statistically significant differences (Student's t-test), *P < 0.05, **P < 0.01, ***P < 0.001. (b) Primary tumors were removed and weighted. Statistically significant differences (Student's t-test), *P < 0.05, **P < 0.01, ***P < 0.001. (c, d) Lung metastasis nodules were visualized and lung metastasis nodules were counted manually. Statistically significant differences (Student's t-test), *P < 0.01, ***P < 0.001, ns: No significant. (e) Acetylated histone H3 (Ac—H3) and acetylated histone H4 (Ac-H4) protein level in primary tumor tissue were detected using western blot analysis.
3.4. WW437 Significantly Inhibited EphA2 Involved Signaling in Breast Cancer
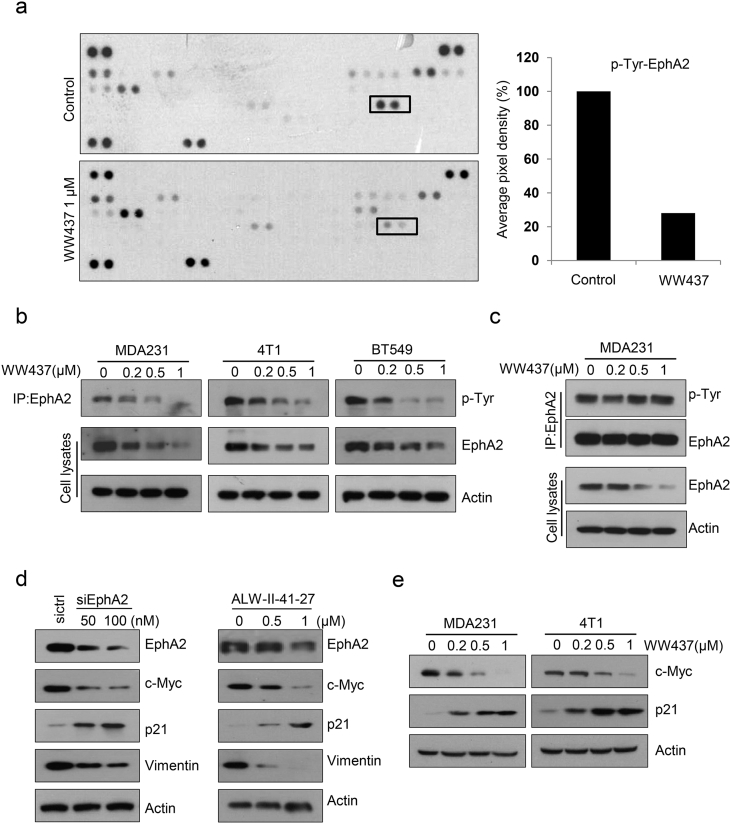
To interrogate the detailed mechanisms by which WW437 suppresses breast cancer growth and metastasis, we utilized a RTK array kit. We found WW437 significantly inhibited phosphorylation of EphA2 at 1 μM (Fig. 4a). WW437 also remarkably suppressed EphA2 expression in three different breast cancer cell lines (Fig. 4b). We wondered whether the downregulation of phosphorylated EphA2 is due to EphA2 expression decrease. A co-immunoprecipitation assay was performed and we adjusted the amount of EphA2 to equal. The phosphorylated EphA2 level hardly reduced after WW437 treatment (Fig. 4c). These results implied that WW437-mediated EphA2 activity is EphA2 expression dependent. Previous studies reported EphA2 is essential for tumor growth and metastasis [34,35]. Next, we examined the function of EphA2 in breast cancer. Knockdown of EphA2 by EphA2 siRNA markedly inhibited the growth and migration of MDA231 cells (Supplementary Fig. 5a and b). As shown in Fig. 4d, EphA2 siRNA or ALW-II-41-27 (an EphA2 inhibitor) significantly downregulated the expression of c-Myc and Vimentin and unregulated p21 expression. We found the similar results when tumor cells were exposed to WW437. To further investigated whether EphA2 is involved in WW437 induced growth arrest, we transfected pcDNA3.1-EphA2 vector to rescue WW437-induced EphA2 inhibition. EphA2 overexpression significantly impeded WW437-induced growth arrest in WW437-treated MDA231 cells (Supplementary Fig. 6a and b). In conclusion, WW437 significantly inhibited EphA2 involved signaling in breast cancer, and EphA2 may be a target of WW437.
Fig. 4.
WW437 significantly inhibited EphA2 involved signaling in breast cancer. A receptor tyrosine kinase (RTK) array kit was used to screen RTKs altered with WW437 treatment in MDA231 cell line. Cells were treated with 1 μM WW437 for 24 h, and lysates were hybridized to the array. (a) RTK array hybridization signals were shown. Black box showed RTKs significantly altered with WW437 treatment. (b) A Co-IP assay was performed to detect the p-Tyr of EphA2 and EphA2 expression. (c) EphA2 was immunoprecipiated and adjusted to equal in different groups. Western blot assays were performed using indicated antibodies. (d) MDA231 was treated with EphA2 siRNAs and EphA2 inhibitor (ALW-II-41-27). Western blot analysis was performed to detect the level of indicated proteins. (e) Western blot analysis was performed to detect the level of indicated proteins.
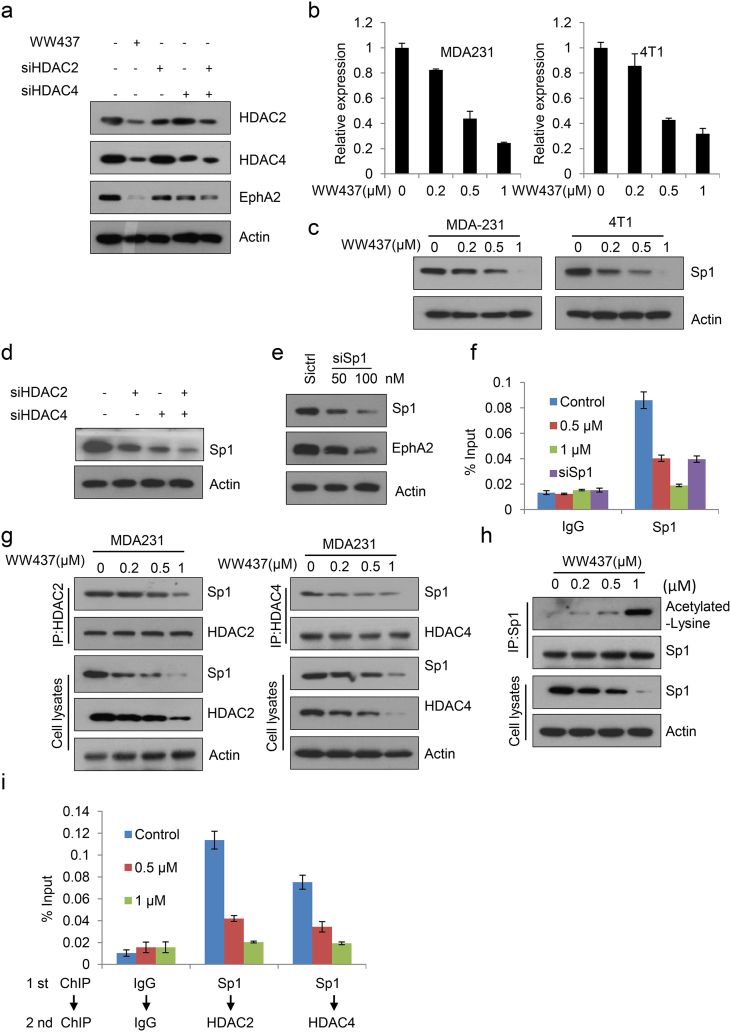
3.5. WW437 Significantly Inhibited HDAC2/4- EphA2 Axis in Breast Cancer
In Fig. 1f, we found WW437 specifically suppressed HDAC2 and HDAC4 expression. Therefore, we hypothesized that HDA2/HDAC4 is responsible for EphA2 expression. After transfection of siRNAs duplex targeting HDAC2 or HDAC4, the expression of EphA2 was decreased. When double knockdown of HDAC2 and HDAC4, EphA2 expression was inhibited remarkably (Fig. 5a). This result suggested HDAC2 and HDAC4 play a key role in sustaining EphA2 expression. The result of qPCR revealed the suppression of EphA2 mRNA in two breast cancer cell lines after WW437 treatment (Fig. 5b). These data implied that the reduced EphA2 protein expression may be, in part, caused by a decrease in its mRNA level. The transcription factor Sp1 regulates the expression of multiple genes [36,37]. We found WW437 significantly down-regulated Sp1 expression (Fig. 5c). Knockdown of HDAC2 and HDAC4 resulted in lower level of Sp1 (Fig. 5d). Next, we investigated whether Sp1 modulated EphA2 expression. As expected, depletion of Sp1 with siRNA resulted in the down-regulated EphA2 protein level (Fig. 5e). These results demonstrated Sp1 maybe a transcription factor of EphA2. To further confirm this result, we performed quantitative ChIP assays. We then determined Sp1 binding site by in silico predictions using the JASPAR database. Sequence analysis revealed “tggggatgat” at EphA2 promoter is putative Sp1 binding site and ChIP analyses indicated Sp1 was enriched at the promoter region covering the binding site and WW437 inhibited its binding to the prompter of EphA2 (Fig. 5f). Aberrant recruitment of HDAC2 or HDAC4 to promoters through interacting with Sp1 has shown to regulate gene transcription. To evaluate whether Sp1 was bound to HDAC2 or HDAC4 and what effect WW437 had on these interactions, HDAC2 and HDAC4 were immunoprecipitated and adjusted to equal amount. As shown in Fig. 5g, WW437 inhibited the binding of HDAC2 or HDAC4 with Sp1 in a dose dependent manner. When adjusted Sp1 to equal amount, we found WW437 induced Sp1 acetylation in breast cancer cells (Fig. 5h). Previous studies reported Sp1 was constitutively acetylated at lysine 703 (K703) and K703 acetylated is essential for its transcriptional activity [38]. We assume that WW437 induced Sp1 acetylation at K703. When we mutated K703 of Sp1 to alanine (A), no Sp1 acetylation was detected after WW437 treatment (Supplementary Fig. 7). Therefore, we hold that WW437 induced Sp1 acetylation at K703. The ChIP-re-ChIP assay further showed WW437 significantly impeded the recruitment of HDAC2 /Sp1 or HDAC4/ Sp1 to the EphA2 promoter (Fig. 5i). Overall, our results suggested that WW437 significantly inhibited HDACs-EphA2 axis in breast cancer.
Fig. 5.
WW437 significantly inhibited HDAC2/4-EphA2 axis in breast cancer. (a) Cells were treated with HDAC2 and EphA2 siRNAs, and then western blot analysis was performed to detect the level of indicated proteins. (b) Cells were treated with various concentrations of WW437; then qPCR was performed to detect the mRNA level of EphA2. (c) After treatment with WW437 for 24 h, western blot analysis was performed to detect Sp1 expression. (d) Cells were treated with HDAC2 and HDAC4 siRNAs, and then western blot analysis was performed to detect Sp1 expression. (e) Cells were treated with Sp1 siRNAs, and then western blot analysis was performed to detect EphA2 expression. (f) After treatment with WW437 for 24 h or Sp1 siRNAs for 48 h, ChIP assays were conducted to detect the binding capability of Sp1 to the promoter of EphA2. (g, h) MDA231 cells were treated with WW437 and nuclear extracts were prepared. The immunoprecipitation was performed using anti-HDAC2, anti-HDAC4 or Sp1 antibodies. The immunoprecipitated pellets were analysed by western blotting with indicated antibodies. (i) MDA231 cells were treated with WW437 for 24 h, ChIP-re-ChIP assays were conducted to detected the binding capability of HDAC2/Sp1 or HDAC4/Sp1 to the promoter of EphA2.
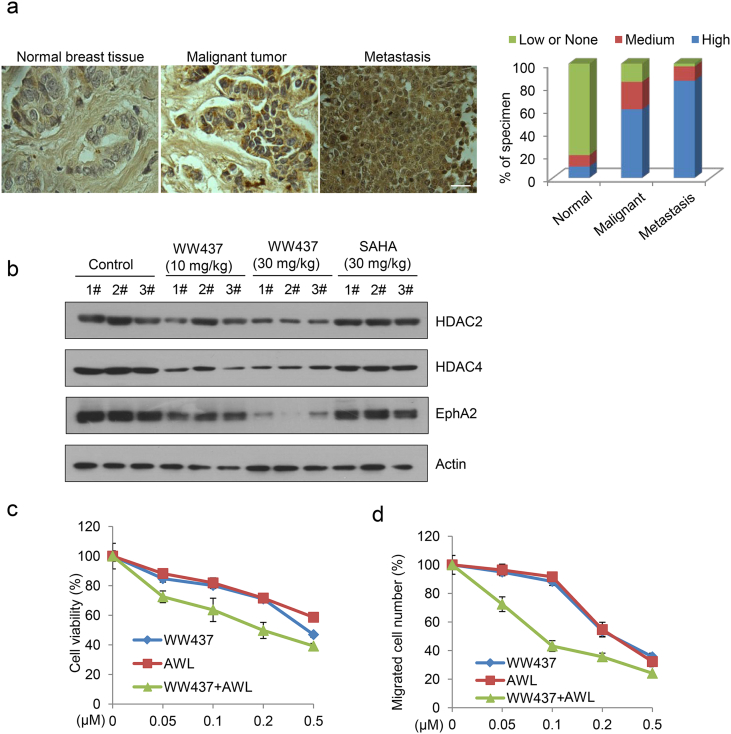
3.6. EphA2 is Correlated with Breast Tumor Progression
To further investigate the role of EphA2 in breast cancer, we performed immunohistochemical analysis. As shown in Fig. 6a, EphA2 was overexpressed in breast cancer and that high level of EphA2 was significantly involved in breast tumor metastasis. We also detected the expression of HDAC2, HDAC4 and EphA2 in primary tumors from 4T1 animal model of animal. WW437 remarkably inhibited the indicated protein expression in tumor tissue (Fig. 6b). Further, we found that combination treatment with WW437 and an EphA2 inhibitor (ALW-II-41-27) displayed significantly synergistic effect in breast cancer (Fig. 6c and d), suggesting that the combination of HDACi and EphA2 inhibitor may be a promising strategy for advanced breast cancer treatment.
Fig. 6.
EphA2 is correlated with breast tumor progression. (a) IHC staining analysis was performed to detect the expression of EphA2 in breast cancer tissue microarray. Left, representative staining for EphA2; right, the relative expression of EphA2 in different breast cancer stages (normal, malignant and metastatic). Scale bar, 20 μm. (b) Western blot analysis was performed to detect indicated protein expression in 4T1 primary tumor tissue. (c) MDA231 cells were treated with WW437 and an EphA2 inhibitor either alone or in combination. The cell viability was tested by MTS assay (48 h). (d) MDA231 cells were treated with WW437 and an EphA2 inhibitor either alone or in combination. The cell migration was tested by wound-healing migration assay. The bars indicate the mean ± SD.
4. Discussion
HDACi have shown pleiotropic anticancer activities including inhibition of tumor cell proliferation, survival, and metastasis. In the present study, we describe a novel HDACi, WW437, which displayed potent anti-breast cancer activity in vitro and in vivo. Mechanistic studies revealed HDACs-EphA2 axis was largely disrupted by WW437. Our results also indicated that WW437 combined with an EphA2 inhibitor (ALW-II-41-27) displayed significantly synergistic effect in breast cancer. Our findings suggest inhibition of HDACs-EphA2 signaling axis with WW437 may be a promising therapeutic strategy for advanced breast cancer in alone or in combination with other agents. Our studies revealed the in vitro bioactivity of WW437 was much higher (>10 fold) compared with that of SAHA. The similar result was observed in vivo. Compared to the chemical structure of SAHA, we reckon introducing 1, 2, 3-triazole into benzyl group as one part of the Cap group of WW437 provided lots of advantages in the physicochemical properties and greatly enhanced the potential biological target binding ability. 1, 2, 3-triazole is electron deficient heterocyclic pressed, with strong dipole moment [39]. Under the pH condition in the organism, 1, 2, 3-triazole can hardly be protonated, meanwhile, hydrolysis and oxidation also difficult to occur even in high temperature conditions. These qualities ensure it as a pharmacophore with excellent in vivo metabolic stability [40]. At the same time, 1, 2, 3-triazole has highly polar carbon atoms and nitrogen atoms which can be used as hydrogen bond receptors, so it has strong affinity for some biological molecular targets, and can effectively improve the water solubility of compounds[41]. Based on these features, 1, 2, 3-triazole as an important heterocyclic structure has been developed as the core structure and widely used in antiviral [42], anti-inflammatory [43], antibacterial, tuberculosis treatment drugs [44], and drug structure optimization in the field of cancer therapy. Overall, 1, 2, 3-triazole plays a key role in increasing the antitumor bioactivity of WW437.
Receptor tyrosine kinases (RTKs) are essential for cellular transformation and tumor progression [45]. The relationship between EphA2 and tumorigenicity still remains controversial. In recent year, there is a growing body of evidence, on the basis of ongoing research, that EphA2 may be important in conferring the oncogenic potential. EphA2 amplification is observed in aggressive breast cancer and associated with poor prognosis [12]. In our study, we also found a hyper- phosphorylation status of EphA2 receptor in breast cancer cell MDA231 (Fig. 4a). Moreover, previous studies have delineated that blocking EphA2 receptor activation via EphA2-Fc resulted in decreasing tumor volume in the 4 T1 animal model [46]. EphA2-deficiency impairs growth and progression in the C3-TAg transgenic breast cancer model [8]. In our 4 T1 animal model, WW437 significantly inhibited tumor growth and metastasis (Fig. 3). In WW437-treated groups, the expression of EphA2 is reduced compared to control group. These results suggested the effects of WW437 on breast cancer are EphA2 dependent.
To our knowledge, this is the first evidence that HDACs-EphA2 axis may play a key role in breast tumor, and we found WW437 significantly inhibited this axis, resulting in breast cancer growth and metastasis regression. We speculated WW437 exerted the inhibitory effect on HDACs-EphA2 axis mainly through the following ways (Supplementary Fig. 8): 1) Sp1 recruits HDACs to the promoter of targeted genes and previous studies indicated that the post-translational modifications can modulate the transcription activity of Sp1. Sp1 acetylation is associated with loss of DNA binding at promoters associated with cell cycle arrest and cell death in tumor cells [47]. Our results showed the interaction between Sp1 and HDAC2 or HDAC4 was disrupted, thus the acetylation level of Sp1 increased and the transcriptional potency decreased. 2) Our data indicated Sp1 is a transcription factor modulating EphA2 expression and WW437 significantly downregulated Sp1 expression. In addition, WW437 reduced the binding of Sp1 to the EphA2 promoter, leading to the transcriptional suppression of EphA2.
In consistent with previous research [35], we found WW437 inhibited EphA2 expression and therefore, the level of its downstream target c-Myc was decreased whereas that of p21 was increased. Moreover, genetic or pharmacologic inhibition of EphA2 markedly blocked tumor cell migration and diminished the expression of Vimentin, implying EphA2 is involved in tumor metastasis.
In summary, we demonstrated that WW437 significantly inhibited breast cancer growth and metastasis via suppressing HDACs-EphA2 axis. Our findings indicated inhibition of HDACs-EphA2 signaling axis with WW437 may be a promising therapeutic strategy for advanced breast cancer in alone or in combination with other agents. Our ongoing efforts will continue to investigate combination therapies to maximize the clinical potential of WW437.
Acknowledgments
Acknowledgements
We thank all members in Shanghai Bone Tumor Institution.
Funding Sources
This work was supported by NSFC (81502604, 81673304 and 81702328); Shanghai Science and Technology Commission (14140904000 and 15431902200), and Research Grant from Shanghai Hospital Development Center (SHDC12013107). Funding agencies played no role in the design, execution, interpretation or writing of this manuscript.
Conflict of Interest Statement
The authors declare no competing financial interests.
Author Contributions
Conception and design: T. Zhang, J. J. Li, Y.H.Chen and Z. D. Cai.
Development of methodology: T. Zhang, J. J. Li, X. J. Ma, and Y. Yang.
Acquisition of data: T. Zhang, J. J. Li, W. Sun, W. R. Jin, L. Wang, Y. He and F. F. Yang.
Analysis and interpretation of data: T. Zhang, J. J. Li, Y. H. Chen and Z. D. Cai.
Writing, review, and/or revision of the manuscript: T. Zhang, J. J. Li, Y. H. Chen and Z. D. Cai.
Administrative, technical, or material support: Z. F. Yi, Y. Q. Hua and M. Y. Liu.
Supervision: Y. H. Chen and Z. D. Cai.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2018.05.003.
Contributor Information
Tao Zhang, Email: zhangtaoabc@2008.sina.com.
Yihua Chen, Email: yhchen@bio.ecnu.edu.cn.
Zhengdong Cai, Email: caizhengdong@sjtu.edu.cn.
Appendix A. Supplementary Data
Supplementary material
References
- 1.Minucci S., Pelicci P.G. Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat. Rev. Cancer. 2006;6:38–51. doi: 10.1038/nrc1779. [DOI] [PubMed] [Google Scholar]
- 2.Chen J.S., Faller D.V., Spanjaard R.A. Short-chain fatty acid inhibitors of histone deacetylases: promising anticancer therapeutics? Curr. Cancer Drug Targets. 2003;3:219–236. doi: 10.2174/1568009033481994. [DOI] [PubMed] [Google Scholar]
- 3.Gherardini L., Sharma A., Capobianco E., Cinti C. Targeting Cancer with epi-drugs: a precision medicine perspective. Curr. Pharm. Biotechnol. 2016;17:856–865. doi: 10.2174/1381612822666160527154757. [DOI] [PubMed] [Google Scholar]
- 4.West A.C., Johnstone R.W. New and emerging HDAC inhibitors for cancer treatment. J. Clin. Invest. 2014;124:30–39. doi: 10.1172/JCI69738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Munster P., Marchion D., Bicaku E., Schmitt M., Lee J.H., Deconti R. Phase I trial of histone deacetylase inhibition by valproic acid followed by the topoisomerase II inhibitor epirubicin in advanced solid tumors: a clinical and translational study. J. Clin. Oncol. 2007;25:1979–1985. doi: 10.1200/JCO.2006.08.6165. [DOI] [PubMed] [Google Scholar]
- 6.Pasquale E.B. Eph receptors and ephrins in cancer: bidirectional signalling and beyond. Nat. Rev. Cancer. 2010;10:165–180. doi: 10.1038/nrc2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fang W.B., Brantley-Sieders D.M., Parker M.A., Reith A.D., Chen J. A kinase-dependent role for EphA2 receptor in promoting tumor growth and metastasis. Oncogene. 2005;24:7859–7868. doi: 10.1038/sj.onc.1208937. [DOI] [PubMed] [Google Scholar]
- 8.Song W., Hwang Y., Youngblood V.M., Cook R.S., Balko J.M., Chen J. Targeting EphA2 impairs cell cycle progression and growth of basal-like/triple-negative breast cancers. Oncogene. 2017;36:5620–5630. doi: 10.1038/onc.2017.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo H., Miao H., Gerber L., Singh J., Denning M.F., Gilliam A.C. Disruption of EphA2 receptor tyrosine kinase leads to increased susceptibility to carcinogenesis in mouse skin. Cancer Res. 2006;66:7050–7058. doi: 10.1158/0008-5472.CAN-06-0004. [DOI] [PubMed] [Google Scholar]
- 10.Kinch M.S., Moore M.B., Harpole D.H., Jr. Predictive value of the EphA2 receptor tyrosine kinase in lung cancer recurrence and survival. Clin. Cancer Res. 2003;9:613–618. [PubMed] [Google Scholar]
- 11.Dunne P.D., Dasgupta S., Blayney J.K., Mcart D.G., Redmond K.L., Weir J.A. EphA2 expression is a key Driver of migration and invasion and a poor prognostic marker in colorectal Cancer. Clin. Cancer Res. 2016;22:230–242. doi: 10.1158/1078-0432.CCR-15-0603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brantley-Sieders D.M., Zhuang G., Hicks D., Fang W.B., Hwang Y., Cates J.M. The receptor tyrosine kinase EphA2 promotes mammary adenocarcinoma tumorigenesis and metastatic progression in mice by amplifying ErbB2 signaling. J. Clin. Invest. 2008;118:64–78. doi: 10.1172/JCI33154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ogawa K., Pasqualini R., Lindberg R.A., Kain R., Freeman A.L., Pasquale E.B. The ephrin-A1 ligand and its receptor, EphA2, are expressed during tumor neovascularization. Oncogene. 2000;19:6043–6052. doi: 10.1038/sj.onc.1204004. [DOI] [PubMed] [Google Scholar]
- 14.Huang Y., Nayak S., Jankowitz R., Davidson N.E., Oesterreich S. Epigenetics in breast cancer: what's new? Breast Cancer Res. 2011;13:225. doi: 10.1186/bcr2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lo P.K., Sukumar S. Epigenomics and breast cancer. Pharmacogenomics. 2008;9:1879–1902. doi: 10.2217/14622416.9.12.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Munster P.N., Thurn K.T., Thomas S., Raha P., Lacevic M., Miller A. A phase II study of the histone deacetylase inhibitor vorinostat combined with tamoxifen for the treatment of patients with hormone therapy-resistant breast cancer. Br. J. Cancer. 2011;104:1828–1835. doi: 10.1038/bjc.2011.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tate C.R., Rhodes L.V., Segar H.C., Driver J.L., Pounder F.N., Burow M.E. Targeting triple-negative breast cancer cells with the histone deacetylase inhibitor panobinostat. Breast Cancer Res. 2012;14:R79. doi: 10.1186/bcr3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gediya L.K., Chopra P., Purushottamachar P., Maheshwari N., Njar V.C. A new simple and high-yield synthesis of suberoylanilide hydroxamic acid and its inhibitory effect alone or in combination with retinoids on proliferation of human prostate cancer cells. J. Med. Chem. 2005;48:5047–5051. doi: 10.1021/jm058214k. [DOI] [PubMed] [Google Scholar]
- 19.Zhang T., Chen Y., Li J., Yang F., Wu H., Dai F. Antitumor action of a novel histone deacetylase inhibitor, YF479, in breast cancer. Neoplasia. 2014;16:665–677. doi: 10.1016/j.neo.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang T., Li S., Li J., Yin F., Hua Y., Wang Z. Natural product pectolinarigenin inhibits osteosarcoma growth and metastasis via SHP-1-mediated STAT3 signaling inhibition. Cell Death Dis. 2016;7 doi: 10.1038/cddis.2016.305. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Li J., Zhang T., Yang F., He Y., Dai F., Gao D. Inhibition of breast cancer progression by a novel histone deacetylase inhibitor, LW479, by down-regulating EGFR expression. Br. J. Pharmacol. 2015;172:3817–3830. doi: 10.1111/bph.13165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang T., Li J., Dong Y., Zhai D., Lai L., Dai F. Cucurbitacin E inhibits breast tumor metastasis by suppressing cell migration and invasion. Breast Cancer Res Treat. 2012;135:445–458. doi: 10.1007/s10549-012-2175-5. [DOI] [PubMed] [Google Scholar]
- 23.Lee G.Y., Kenny P.A., Lee E.H., Bissell M.J. Three-dimensional culture models of normal and malignant breast epithelial cells. Nat. Methods. 2007;4:359–365. doi: 10.1038/nmeth1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Truax A.D., Greer S.F. ChIP and Re-ChIP assays: investigating interactions between regulatory proteins, histone modifications, and the DNA sequences to which they bind. Methods Mol. Biol. 2012;809:175–188. doi: 10.1007/978-1-61779-376-9_12. [DOI] [PubMed] [Google Scholar]
- 25.Zhang T., Li J., Yin F., Lin B., Wang Z., Xu J. Toosendanin demonstrates promising antitumor efficacy in osteosarcoma by targeting STAT3. Oncogene. 2017;36:6627–6639. doi: 10.1038/onc.2017.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang F., Zhang T., Wu H., Yang Y., Liu N., Chen A. Design and optimization of novel hydroxamate-based histone deacetylase inhibitors of bis-substituted aromatic amides bearing potent activities against tumor growth and metastasis. J. Med. Chem. 2014;57:9357–9369. doi: 10.1021/jm5012148. [DOI] [PubMed] [Google Scholar]
- 27.Richon V.M., Sandhoff T.W., Rifkind R.A., Marks P.A. Histone deacetylase inhibitor selectively induces p21WAF1 expression and gene-associated histone acetylation. Proc. Natl. Acad. Sci. U. S. A. 2000;97:10014–10019. doi: 10.1073/pnas.180316197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 29.Friedl P., Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat. Rev. Cancer. 2003;3:362–374. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- 30.Vlodavsky I., Korner G., Ishai-Michaeli R., Bashkin P., Bar-Shavit R., Fuks Z. Extracellular matrix-resident growth factors and enzymes: possible involvement in tumor metastasis and angiogenesis. Cancer Metastasis Rev. 1990;9:203–226. doi: 10.1007/BF00046361. [DOI] [PubMed] [Google Scholar]
- 31.Ma L., Teruya-Feldstein J., Weinberg R.A. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature. 2007;449:682–688. doi: 10.1038/nature06174. [DOI] [PubMed] [Google Scholar]
- 32.Singh A., Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene. 2010;29:4741–4751. doi: 10.1038/onc.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rhodes L.V., Tate C.R., Segar H.C., Burks H.E., Phamduy T.B., Hoang V. Suppression of triple-negative breast cancer metastasis by pan-DAC inhibitor panobinostat via inhibition of ZEB family of EMT master regulators. Breast Cancer Res. Treat. 2014;145:593–604. doi: 10.1007/s10549-014-2979-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brantley-Sieders D.M., Fang W.B., Hwang Y., Hicks D., Chen J. Ephrin-A1 facilitates mammary tumor metastasis through an angiogenesis-dependent mechanism mediated by EphA receptor and vascular endothelial growth factor in mice. Cancer Res. 2006;66:10315–10324. doi: 10.1158/0008-5472.CAN-06-1560. [DOI] [PubMed] [Google Scholar]
- 35.Walker-Daniels J., Coffman K., Azimi M., Rhim J.S., Bostwick D.G., Snyder P. Overexpression of the EphA2 tyrosine kinase in prostate cancer. Prostate. 1999;41:275–280. doi: 10.1002/(sici)1097-0045(19991201)41:4<275::aid-pros8>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 36.Kaczynski J., Cook T., Urrutia R. Sp1- and Kruppel-like transcription factors. Genome Biol. 2003;4:206. doi: 10.1186/gb-2003-4-2-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kingsley C., Winoto A. Cloning of GT box-binding proteins: a novel Sp1 multigene family regulating T-cell receptor gene expression. Mol. Cell. Biol. 1992;12:4251–4261. doi: 10.1128/mcb.12.10.4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hung J.J., Wang Y.T., Chang W.C. Sp1 deacetylation induced by phorbol ester recruits p300 to activate 12(S)-lipoxygenase gene transcription. Mol. Cell. Biol. 2006;26:1770–1785. doi: 10.1128/MCB.26.5.1770-1785.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bourne Y., Kolb H.C., Radic Z., Sharpless K.B., Taylor P., Marchot P. Freeze-frame inhibitor captures acetylcholinesterase in a unique conformation. Proc. Natl. Acad. Sci. U. S. A. 2004;101:1449–1454. doi: 10.1073/pnas.0308206100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chemama M., Fonvielle M., Arthur M., Valery J.M., Etheve-Quelquejeu M. Synthesis of stable aminoacyl-tRNA analogues containing triazole as a bioisoster of esters. Chemistry. 2009;15:1929–1938. doi: 10.1002/chem.200801563. [DOI] [PubMed] [Google Scholar]
- 41.Nimmo C.M., Shoichet M.S. Regenerative biomaterials that "click": simple, aqueous-based protocols for hydrogel synthesis, surface immobilization, and 3D patterning. Bioconjug. Chem. 2011;22:2199–2209. doi: 10.1021/bc200281k. [DOI] [PubMed] [Google Scholar]
- 42.He Y.W., Dong C.Z., Zhao J.Y., Ma L.L., Li Y.H., Aisa H.A. 1,2,3-Triazole-containing derivatives of rupestonic acid: click-chemical synthesis and antiviral activities against influenza viruses. Eur. J. Med. Chem. 2014;76:245–255. doi: 10.1016/j.ejmech.2014.02.029. [DOI] [PubMed] [Google Scholar]
- 43.Bengtsson C., Lindgren A.E., Uvell H., Almqvist F. Design, synthesis and evaluation of triazole functionalized ring-fused 2-pyridones as antibacterial agents. Eur. J. Med. Chem. 2012;54:637–646. doi: 10.1016/j.ejmech.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 44.Yempala T., Sridevi J.P., Yogeeswari P., Sriram D., Kantevari S. Rational design and synthesis of novel dibenzo[b,d]furan-1,2,3-triazole conjugates as potent inhibitors of Mycobacterium tuberculosis. Eur. J. Med. Chem. 2014;71:160–167. doi: 10.1016/j.ejmech.2013.10.082. [DOI] [PubMed] [Google Scholar]
- 45.Gschwind A., Fischer O.M., Ullrich A. The discovery of receptor tyrosine kinases: targets for cancer therapy. Nat. Rev. Cancer. 2004;4:361–370. doi: 10.1038/nrc1360. [DOI] [PubMed] [Google Scholar]
- 46.Brantley D.M., Cheng N., Thompson E.J., Lin Q., Brekken R.A., Thorpe P.E. Soluble Eph A receptors inhibit tumor angiogenesis and progression in vivo. Oncogene. 2002;21:7011–7026. doi: 10.1038/sj.onc.1205679. [DOI] [PubMed] [Google Scholar]
- 47.Waby J.S., Chirakkal H., Yu C., Griffiths G.J., Benson R.S., Bingle C.D. Sp1 acetylation is associated with loss of DNA binding at promoters associated with cell cycle arrest and cell death in a colon cell line. Mol. Cancer. 2010;9:275. doi: 10.1186/1476-4598-9-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material