Abstract
Background
Dengue and West Nile viruses are highly cross-reactive and have numerous parallels in geography, potential vector host (Aedes family of mosquitoes), and initial symptoms of infection. While the vast majority (> 80%) of both dengue and West Nile virus infections result in asymptomatic infections, a minority of individuals experience symptomatic infection and an even smaller proportion develop severe disease. The mechanisms by which these infections lead to severe disease in a subset of infected individuals is incompletely understood, but individual host differences including genetic factors and immune responses have been proposed. We sought to identify genetic risk factors that are associated with more severe disease outcomes for both viruses in order to shed light on possible shared mechanisms of resistance and potential therapeutic interventions.
Methods
We applied a search strategy using four major databases (Medline, PubMed, Embase, and Global Health) to find all known genetic associations identified to date with dengue or West Nile virus disease. Here we present a review of our findings and a meta-analysis of genetic variants identified.
Results
We found genetic variations that are significantly associated with infections of these viruses. In particular we found variation within the OAS1 (meta-OR = 0.83, 95% CI: 0.69–1.00) and CCR5 (meta-OR = 1.29, 95% CI: 1.08–1.53) genes is significantly associated with West Nile virus disease, while variation within MICB (meta-OR = 2.35, 95% CI: 1.68–3.29), PLCE1 (meta-OR = 0.55, 95% CI: 0.42–0.71), MBL2 (meta-OR = 1.54, 95% CI: 1.02–2.31), and IFN-γ (meta-OR = 2.48, 95% CI: 1.30–4.71), is associated with dengue disease.
Conclusions
Despite substantial heterogeneity in populations studied, genes examined, and methodology, significant associations with genetic variants were found across studies within both diseases. These gene associations suggest a key role for immune mechanisms in susceptibility to severe disease. Further research is needed to elucidate the role of these genes in disease pathogenesis and may reveal additional genetic factors associated with disease severity.
Electronic supplementary material
The online version of this article (10.1186/s12879-018-3186-6) contains supplementary material, which is available to authorized users.
Keywords: Dengue virus, West Nile virus, Disease severity, Genetic variation, Meta-analysis, Single nucleotide polymorphism
Background
Dengue (DENV) and West Nile (WNV) viruses are mosquito-borne viruses in the Flaviviridae family, which also includes other viruses such as Zika and yellow fever. These viruses can cause disease with substantial public health impact. DENV and WNV are found in similar areas of the world, can be carried by the Aedes family of mosquitoes, have similar initial stages of infections and similar symptoms of mild febrile illness, and are highly cross-reactive; however, severe disease manifests differently for these two viruses [1–3]. West Nile Virus was first identified in Uganda in 1937, has been endemic in the United States since 1999 [4], and is estimated to have infected 3 million people [5]. While the majority of infections are asymptomatic, ~ 20% of infections lead to mild febrile disease in infected individuals and 1% of infected individual experience severe, neurological disease such as meningitis and encephalitis [6]. DENV has a vastly higher disease burden, with an estimated 50 million cases and 25,000 fatalities worldwide annually [7, 8]. The majority of DENV infections can be classified as asymptomatic or mild febrile illness, with approximately < 1% progressing to Dengue Hemorrhagic Fever (DHF) or Dengue Shock Syndrome (DSS). DHF is delineated from mild DENV febrile illness by the increase in vascular permeability, while DSS has the additional development of circulatory shock [7, 8].
For both WNV and DENV, known risk factors such as immune-compromised states or advanced age are associated with susceptibility to mild and severe disease [9, 10]. The mechanisms by which an infection leads to severe disease in a subset of all infected individuals is incompletely explained. Differing immune responses to infections, including elevated cytokine responses, have been proposed [11–13] and we have recently shown that geographic location is not a driver of severity of WNV infection in a localized region [14]. In addition to similarities in the early stages of infection [15–17], both viruses induce strong immune responses including chemokines (such as IL-8) and cytokines which up-regulate inflammatory reaction (such as TNF-α, IL-1, Il-6, and IFN-β) [18–21]. Renewed interest in understanding flaviviral infection and disease susceptibility comes as climate change expands the number of individuals at risk of exposure to WNV and DENV [3, 22], and with outbreaks of related flaviviruses, most notably Zika [23, 24].
Genetic differences are additional explanations of individual susceptibility to symptomatic disease, and previous genome-wide association studies (GWAS) and candidate-gene studies have identified genetic factors associated with DENV or WNV disease pathogenesis. To assess the current state of knowledge on genetic variation associated with these flaviviral diseases, and to identify any shared features of anti-viral responses, we conducted a systematic review and meta-analysis of the published associations to date between genetic variants and development of DENV or WNV disease.
Methods
A systematic review of genetic factors and WNV or DENV disease was conducted using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (Additional file 1) [25].
Search strategy
Medline, PubMed, Embase, and Global Health databases were used to search the literature. Search terms included West Nile or DENV and genetic factors; the same set of text words was used for all databases in conjunction with subject headings that were tailored for each database. The text word search specified West Nile or Dengue in the title, a genetic term in the title or abstract, and a human-related term in the title or abstract (Table 1). A sample search strategy is included in the appendix (Additional file 2). Case-control studies which examined at least one genetic factor associated with either viral disease were included. Studies on non-human (e.g., viral, mosquito) genetics and case reports on single patients were excluded. Reports published prior to May 2017 were included in the review. An ancestry search was done of references of selected studies to collect additional potentially relevant references.
Table 1.
Text word selection for search of selected databases. Text words used for the search strategy, with one term from each column required in the title for the viral term, or in the title or abstract for the genetic and human terms
| Viral terms | Genetic Factor terms | Human-related terms |
|---|---|---|
| • West Nile • Dengue |
▪ microsatellite(s) ▪ genetic variation ▪ genetic factor(s) ▪ genetic marker(s) ▪ genetic analysis/analyses ▪ SNP(s) ▪ single nucleotide polymorphism(s) ▪ copy number variant(s) ▪ genetic predisposition ▪ genetic susceptibility ▪ disease susceptibility ▪ GWAS ▪ genome-wide association study/studies ▪ genetic association(s) ▪ genetic association study/studies ▪ candidate gene study/studies ▪ genetic predisposition to disease ▪ susceptibility to disease ▪ genetic variability ▪ gene identity |
▪ human ▪ man/men ▪ woman/women ▪ child/children ▪ teenager(s) ▪ middle-aged ▪ elderly ▪ infant(s) ▪ male(s) ▪ female(s) ▪ patient(s) ▪ participant(s) ▪ citizen(s) ▪ subject(s) ▪ case(s) ▪ control(s) |
Study selection and data extraction
Two researchers reviewed the titles and abstracts of all studies and identified potentially relevant articles within Covidence with 98.6% consistency [26]. Discrepancies were resolved through re-review and mutual consensus. Both researchers read the full text of all of the selected potentially relevant articles and identified the final reports to be included in this review. Data sets were extracted without personal identifiers and organized into literature tables. The main fields included authors, year of publication, country, sample size, case and control group definitions, genotyping method, genes and genetic variants analyzed, genotype count data when available, odds ratios (OR), and statistical analysis method.
When two or more studies examined the same variants, we used the raw genotype data to calculate ORs with 95% confidence intervals using the R package Epitools [27]. When the raw genotype data were not available within the published papers, we requested the data sets from corresponding authors of the studies. Of the six authors contacted, three shared data, two indicated they no longer had access to the data, and one did not respond by date of submission. In order to make comparisons across the different DENV phenotypes used in the studies, we compared asymptomatic DENV infections and controls with all symptomatic infections (DENV fever, DENV hemorrhagic fever, and DENV shock syndrome). Using the genotype data, we calculated ORs for each study under a dominant model, recessive model, homozygote mutant versus homozygote wild-type, and heterozygote versus homozygote wild-type. We meta-analyzed the ORs using RevMan [28]. The genetic model with the most significant meta-OR is presented here. When this model was the homozygote mutant versus homozygote wild or the heterozygote versus homozygote wild, we included both of these models for that particular single nucleotide polymorphism (SNP).
Quality assessment
We assessed the quality of each study with the Newcastle-Ottawa Quality Assessment Scale for Case-Control Studies [29], which assesses each study’s selection, comparability, and exposure ascertainment approach.
Results
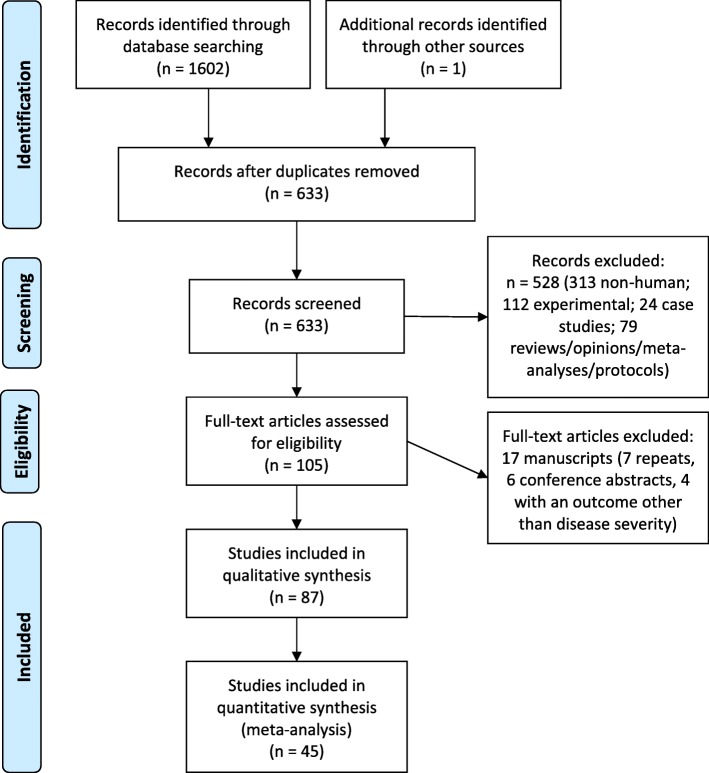
To identify all published research assessing the role of genetic variation with DENV or WNV disease, we executed the above search strategy and identified 633 published reports (Table 1, Fig. 1). Two researchers independently reviewed the titles and abstracts of these 633 papers and identified 104 papers for further full-text review in this meta-analysis (Additional file 3). One additional paper from 1987 was identified as pertinent during the ancestry search and was added to the review. The final analysis includes data from 87 of the 105 publications, following exclusion of 18 papers for cause (seven repeats, six conference abstracts, and five with an outcome other than disease severity). Reflecting the higher disease burden and longer research history of DENV virus, of these 87 papers selected, 74 studied DENV-infected populations and 13 focused on WNV.
Fig. 1.
PRISMA Flowchart of strategy to identify papers assessing genetic variation and WNV or DENV disease
HLA genetic variation associated with disease severity
Notably, 27 separate HLA alleles were examined by two or more research groups for an association with severe DENV disease (Additional file 4). Four research groups analyzed HLA alleles for an association with WNV disease (Additional file 5), however there was no overlap in the alleles studied. Although HLA variants show substantial contribution to disease outcome, significant variations in study design, data analysis platforms, data availability and presentation precluded our in-depth meta-analysis of these data.
Multiple genes are associated with severity of WNV infection
Previous reports of genetic associations with WNV disease severity focused on U.S. or Canadian populations and compared severe and non-severe infections. Overall, these studies identified 12 gene variants and significant findings include SNPs of multiple immune-related genes such as RFC1, SCN1A, and IRF3 (Table 2).
Table 2.
Genetic variation significantly associated with West Nile virus disease. We include in this table all variants studied by two or more research groups and variants found to have a significant association by one research group
| Gene | Genetic variant | Entrez Gene ID [31] | Major Allele | Minor Allele | Number of Cases | Number in Comparison Group | Country | Key Results | Included in meta-analysis |
|---|---|---|---|---|---|---|---|---|---|
| ANPEP | rs25651 | 290 | T | C | 560 severe | 950 non-severe infections | US & Canada | 0.69 odds of severe disease [59] | Noa |
| G | A | 39 severe | 61 controls | Israel | No significant association with disease [60] | ||||
| CACNA1H | rs113802594 | 8912 | A | G | 1330 severe | 919 non-severe infections | US & Canada | 8.58 odds of encephalitis [61] | No |
| CCR5 | CCR5 Δ32 | 1234 | – | Δ32 deletion | 560 severe | 950 non-Severe infections | US & Canada | No significant association with disease [59] | Yes |
| – | Δ32 deletion | 39 severe | 61 controls | Israel | No significant association with disease [60] | ||||
| – | Δ32 deletion | 422 symptomatic | 331 asymptomatic infections | US & Canada | No significant association with disease [62] | ||||
| – | Δ32 deletion | 634 infections | 422 controls | US | No significant association with disease, but significant association with more severe disease (p = 0.0016) [46] | ||||
| – | Δ32 deletion | 395 symptomatic (two cohorts of 247 and 148) | 1318 controls | US | Significantly associated with disease in two cohorts (OR = 4.4 [1.6–11.8] and OR = 9.1 [3.4–24.8]), and fatal outcomes in one cohort with OR = 13.2 [1.9–89.9] [63] | ||||
| HERC5 | rs148556308 | 51,191 | A | G | 1330 severe | 919 non-severe infections | US & Canada | Significantly associated with severe disease (p-value = 6.5 × 10− 10) [61] | No |
| IRF3 | rs2304207 | 3661 | G | C | 422 severe | 331 asymptomatic infections | US | 0.52 odds of symptomatic infection under dominant model [62] | Noa |
| G | C | 39 severe | 61 controls | Israel | No significant association with disease [60] | ||||
| MIF | rs5844572 | 4282 | 5 or 6 CATT repeats | 7 CATT repeats | 518 severe | 514 non-severe | US & Canada | 1.73 odds of encephalitis among patients with high-expression allele as compared to all other types of WNV disease [64] | No |
| MX1 | rs7280422 | 4599 | C | G | 39 severe | 61 controls | Israel | 4.05 odds of infection associated with variant allele [60] | Noa |
| C | G | 422 severe | 331 asymptomatic infections | US | 0.25 odds of symptomatic infection under a recessive model [62] | ||||
| OASL | rs3213545 | 8638 | C | T | 422 severe | 331 asymptomatic infections | US | No significant association with disease [62] | Yes |
| C | T | 39 severe | 61 controls | Israel | 1.85 (1.03–3.3) odds of infection [60] | ||||
| C | T | 33 symptomatic | 60 controls | US | Significantly associated with disease (P < 0.004) [65] | ||||
| OAS1 | rs10774671 | 4938 | A | G | 422 severe | 331 asymptomatic infections | US | No significant association with disease [62] | Yes |
| A | G | 39 severe | 61 controls | Israel | No significant association with disease [60] | ||||
| A | G | 501 seropositive | 552 controls | US | 1.6 [95% CI 1.2–2.0] odds of seroconversion [66] | ||||
| OAS1 | rs34137742 | 4938 | C | T | 422 severe | 331 asymptomatic infections | US | 9.79 [95% CI 3.60–26.61] odds of encephalitis and paralysis [62] | Yes |
| C | T | 39 severe | 61 controls | Israel | No significant association with disease [60] | ||||
| RFC1 | rs2066786 | 5981 | T | C | 560 severe | 950 non-severe infections | US & Canada | 0.68 odds of severe disease associated with minor allele [59] | Noa |
| G | A | 39 severe | 61 controls | Israel | 2.8 odds under dominant model [60] | ||||
| SCN1A | rs2298771 | 6323 | C | T | 560 severe | 950 non-severe infections | US & Canada | 1.47 odds of severe disease associated with minor allele [59] | Noa |
| A | G | 39 severe | 61 controls | Israel | No significant association with disease [60] | ||||
| TFCP2L1 | rs11122852 | 29,842 | A | T | 1330 severe | 919 non-severe infections | US & Canada | 3.57 odds of severe disease and 4.94 odds of Acute Flaccid Paralysis than controls [61] | No |
agenotype data not available for meta-analysis
OAS1 and CCR5 have significant associations with WNV disease across multiple studies
For genes with genotype count data available for ≥2 studies, we conducted a meta-analysis of genetic association to disease severity. Meta-analysis allows recognition of well-established genetic associations and identification of redundant studies for genes with null associations. We found that SNPs in MX1, OASL, OAS1, RFC1, and CCR5 were studied by multiple research groups for an association with WNV disease (Table 2). To assess the overall association of these SNPs with WNV disease, we calculated a combined OR for each gene based on the genotype counts under four different genetic models. Of these, CCR5 and OAS1 meta-ORs were significant under a dominant model, with meta-OR of 0.83 [95% CI: 0.69–1.00] and 1.29 [1.08–1.53], respectively (Fig. 2). The CCR5 meta-OR was also significant under an allelic model with a meta-OR of 1.22 [95% CI: 1.03–1.44]. The CCR5 delta 32 deletion is associated with more severe disease while the OAS1 allele G was associated with less severe disease.
Fig. 2.
Significant meta-ORs for associations between OAS1 (rs10774671) and CCR5 (Δ32) and West Nile virus disease. Genotype count data from published reports of WNV subjects were meta-analyzed using RevMan. The meta-odds ratio (OR) for more severe disease is indicated with the genetic model for each gene. For each gene, the allele or genotype is shown which is associated with asymptomatic infection and controls (blue) or severe disease (yellow) outcome
Multiple genes are associated with severity of DENV infection
Seventy-four studies have examined genetic associations with DENV disease severity and more than 30 genes have been implicated in DENV disease (Additional file 3). SNPs that were studied by only a single research group are presented in Table 3. We also include SNPs studied by multiple research groups, but for which genotype data was unavailable or the comparison groups of multiple studies could not be analyzed together.
Table 3.
Genetic variation associated with DENV disease. We include in this table all variants studied by two or more research groups and variants found to have a significant association by one research group
| Gene | Genetic Variation | Entrez Gene ID [31] | Major Allele | Minor Allele | Number of Cases | Number in Comparison Group | Country | Key Results | Meta-Analyzed |
|---|---|---|---|---|---|---|---|---|---|
| BAK1 | rs5745568 | 578 | G | T | 509 DHF/DSS | 409 DF | Thailand | 1.32 (1.09–1.60) odds of severe disease associated with G allele [67] | No |
| CCR5 | rs333 | 1234 | – | Δ32 deletion | 56 DF | 91 controls | Australia | No significant association with disease [68] | Yes |
| 88 DHF/DSS | 335 controls | Brazil | No significant association with disease [69] | ||||||
| CD209/ DCSIGN | rs480803 | 30,835 | A | G | 509 DHF/DSS | 409 DF | Thailand | No significant association with disease [67] | Yes |
| A | G | 88 DHF/DSS | 335 controls | Brazil | No significant association with disease [69] | ||||
| A | G | 112 symptomatic | 104 controls | India | No significant association with disease [70] | ||||
| A | G | 103 symptomatic | 145 asymptomatic infections | Mexico | No significant association with disease [71] | ||||
| A | G | 156 DF and 12 DHF | 72 controls | Brazil | No significant association with disease [72] | ||||
| A | G | 606 symptomatic | 696 controls | Thailand | 5.84 (2.77–12.31) odds of DHF compared to DF and 0.204 (P = 2.0 × 10− 6) odds of symptomatic infection compared to controls [73] | ||||
| A | G | 286 symptomatic | 236 asymptomatic infections | Brazil | No significant association with disease [74] | ||||
| A | G | 176 DF and 135 DH0046 | 120 controls | Taiwan | 2.36 (1.12–4.97) odds of symptomatic infection compared to controls, 3.68 (1.67–8.09) odds of DHF compared to controls, and 2.46 (1.32–4.59) odds of DHF compared to DF [75] | ||||
| CFH | rs3753394 | 3075 | C | T | 187 DHF | 121 DF | Thailand | No significant association with disease [76] | Noa |
| C | T | 87 DHF | 34 DF | Brazil | 2.53 (1.38–4.69) odds of severe disease compared to mild disease under dominant model [77] | ||||
| CLEC5A | rs1285933 | 23,601 | T | C | 88 DHF/DSS | 335 controls | Brazil | 2.25 (1.07–4.87) odds of severe disease for TT compared to CC genotype [69] | No |
| CXCL8/ IL8 | rs4973 | 3576 | T | A | 45 DHF | 108 controls | India | 0.43 (0.20–0.93) odds of severe disease [78] | No |
| DDX58 | rs3205166 | 23,586 | T | G | 120 DENV positive | 109 controls | India | 0.66 odds of disease associated with G allele for rs3205166 [79] | No |
| rs11795343 | |||||||||
| rs669260 | |||||||||
| FCγRII-α | rs1801274 | 2212 | A (H amino acid) | G (R amino acid) | 103 symptomatic | 145 asymptomatic infections | Mexico | 0.51 (0.26–0.98) odds of symptomatic infection and 0.45 (0.21–0.96) odds of severe disease compared to controls [71] | Yes |
| T (H) | C (R) | 89 DF and 33 DHF | 107 controls | India | No significant association with disease [80] | ||||
| T (H) | C (R) | 68 DF, 29 DHF/DSS | 42 asymptomatic infections | Cuba | 10.56 (2.33–54.64) odds of DHF compared to asymptomatic disease for under dominant model [81] | ||||
| T (H) | C (R) | 302 DHF | 238 controls | Vietnam | No significant association with disease [82] | ||||
| T (H) | C (R) | 40 DF, 30 DHF/DSS | 40 asymptomatic infections | Pakistan | 3.21 (1.29–7.97) odds of symptomatic disease, 2.82 (1.00–7.97) odds of DF, and 3.90 (1.13–13.07) odds of DHF/DSS over asymptomatic infection [83] | ||||
| HPA | HPA 1a/1a | Not available | 1a antigen | 1b antigen | 75 DHF | 90 DF | India | 1.93 odds (p = 0.006) of severe disease [84] | No |
| HPA 2a/2b | 2a antigen | 2b antigen | 75 DHF | 90 DF | India | 2.8 odds (p = 0.007) of severe disease [84] | No | ||
| IFN-γ | rs2430561 | 3458 | A | T | 80 symptomatic | 100 DEN-negative febrile cases and 99 healthy controls | Brazil | 2.23 (p = 0.0255) odds compared to DEN-negative and 2.37 (p = 0.0165) odds compared to controls [85] | Yes |
| A | T | 25 DHF | 41 DF | Venezuela | No significant association with disease [86] | ||||
| A | T | 43 DHF | 99 controls | Cuba | No significant association with disease [87] | ||||
| IL-1B | rs16944 | 3553 | A | G | 45 DHF | 108 controls | India | No significant association with disease [78] | Yes |
| C | T | 118 symptomatic | 80 controls | Brazil | No significant association with disease [88] | ||||
| rs1143627 | C | T | 367 secondary DHF and 74 secondary DSS | 313 secondary DF | Thailand | 3.49 (1.36–8.95) odds of DSS compared to DHF and 2.81 (1.12–7.06) odds of DSS compared to DF under dominant models [89] | No | ||
| IL-1RA | 86 base pair tandem repeat | 3557 | 4 repeats | 2 repeats | 367 secondary DHF and 74 secondary DSS | 313 secondary DF | Thailand | 1.86 (1.05–3.26) odds of DSS compared to DHF and 1.86 (1.05–3.27) odds of DSS compared to DF for the 2/4 genotype [89] | Noa |
| 1 repeat | 2–4 repeats | 280 DHF | 229 controls | Vietnam | No significant association between IL-1RA repeats and DHF [82] | ||||
| IL-6 | rs1800795 | 3569 | G | C | 25 DHF | 41 DF | Venezuela | No significant association with disease [86] | Yes |
| G | C | 43 DHF | 99 controls | Cuba | No significant association with disease [87] | ||||
| G | C | 118 symptomatic | 80 controls | Brazil | No significant association with disease [88] | ||||
| G | C | 200 DF | 309 controls | Brazil | 0.62 (0.42–0.91) odds of disease among heterozygotes compared to homozygote wild-type [90] | ||||
| IL-10 | rs1800871 | 3586 | C | T | 88 DSS | 335 controls | Brazil | No significant association with disease [69] | Yes |
| A | G | 45 DHF | 108 controls | India | No significant association with disease [78] | ||||
| C | T | 25 DHF | 41 DF | Venezuela | No significant association with disease [86] | ||||
| C | T | 43 DHF | 99 controls | Cuba | No significant association with disease [87] | ||||
| C | T | 200 DF | 309 controls | Brazil | No significant association with disease [90] | ||||
| T | C | 86 DF, 182 DHF, 14 DSS | 120 controls | Malaysia | No significant association with disease [91] | ||||
| C | T | 107 DHF | 62 controls | Sri Lanka | No significant association with disease [92] | ||||
| rs1800872 | C | A | 25 DHF | 41 DF | Venezuela | No significant association with disease [86] | Yes | ||
| C | A | 43 DHF | 99 controls | Cuba | No significant association with disease [87] | ||||
| C | A | 200 DF | 309 controls | Brazil | No significant association with disease [90] | ||||
| A | C | 86 DF, 182 DHF, 14 DSS | 120 controls | Malaysia | No significant association with disease [91] | ||||
| A | C | 107 DHF | 62 controls | Sri Lanka | No significant association with disease [92] | ||||
| rs1800896 | A | G | 25 DHF | 41 DF | Venezuela | No significant association with disease [86] | Yes | ||
| A | G | 43 DHF | 99 controls | Cuba | No significant association with disease [87] | ||||
| A | G | 200 DF | 309 controls | Brazil | No significant association with disease [90] | ||||
| A | G | 86 DF, 182 DHF, 14 DSS | 120 controls | Malaysia | No significant association with disease [91] | ||||
| A | G | 107 DHF | 62 controls | Sri Lanka | No significant association with disease [92] | ||||
| JAK1 | rs11208534 | 3716 | T | C | 50 DHF | 236 DF | Brazil | 4.20 (1.7–10.4) odds of severe disease [74] | No |
| rs2780831 | G | A | 50 DHF | 236 DF | Brazil | 2.1 (1.1–4.1) odds of severe disease [74] | No | ||
| rs310196 | T | G | 50 DHF | 236 DF | Brazil | 0.4 (0.2–0.7) odds of severe disease [74] | No | ||
| MBL2 | Exon 1 | 4153 | A | O | 110 symptomatic | 150 controls | Brazil | No significant association with disease [93] | Yes |
| A | O | 57 DHF | 104 DF | Brazil | 7.24 (1.38–38.02) odds of DHF among OO genotype compared to AA genotype [94] | ||||
| MICB | rs3132468 | 4277 | T | C | 76 DSS | 409 DF, 432 DHF | Thailand | 1.58 (1.02–2.40) odds of DSS compared to non-DSS [95] | Yes |
| T | C | 2008 DSS | 2018 controls | Vietnam | 1.34 (1.23–1.46) odds of DSS per allele [96] | ||||
| T | C | 3961 cases | 1068 controls | Vietnam | 1.42 (1.20–1.64) odds of DSS per allele [97] | ||||
| PLCE1 | rs3740360 | 51,196 | A | C | 2008 DSS | 2018 controls | Vietnam | 0.80 (0.75–0.86) odds per allele of DSS [96] | Yes |
| A | C | 3961 cases | 1068 controls | Vietnam | 0.77 (0.59–0.99) odds per allele of DSS [97] | ||||
| rs3765524 | C | T | 76 DSS | 409 DF, 432 DHF | Thailand | 1.49 (1.00–2.26) odds of DSS compared to non-DSS [95] | Yes | ||
| C | T | 2008 DSS | 2018 controls | Vietnam | 0.80 (0.75–0.86) odds per allele of DSS [96] | ||||
| RXRA | rs12339163 | 6256 | G | A | 60 DHF | 137 asymptomatic infections and controls | Cuba | 0.36 (0.17–0.77) odds of severe disease [98] | No |
| rs3118593 | A | C | 60 DHF | 137 asymptomatic infections and controls | Cuba | 0.44 (0.25–0.77) odds of severe disease [98] | |||
| rs4262378 | G | A | 60 DHF | 137 asymptomatic infections and controls | Cuba | 0.41 (0.24–0.72) odds of severe disease [98] | |||
| rs4424343 | A | G | 60 DHF | 137 asymptomatic infections and controls | Cuba | 0.43 [0.24–0.76] odds of severe disease [98] | |||
| rs62576287 | C | A | 60 DHF | 137 asymptomatic infections and controls | Cuba | 0.10 (0.01–0.83) odds of severe disease [98] | |||
| TAP1 | amino acid 333 | 6890 | Ile | Val | 90 DF, 75 DHF, 32 DSS | 100 controls | India | 2.58 (p = 0.007) odds of DHF among heterozygotes compared to DF [84] | Yes |
| Ile | Val | 107 DHF | 62 controls | Sri Lanka | No significant association with disease [92] | ||||
| TAP2 | amino acid 379 | 6891 | Val | Ile | 107 DHF | 62 controls | Sri Lanka | No significant association with disease [92] | Yes |
| Val | Ile | 90 DF, 75 DHF, 32 DSS | 100 controls | India | 2.11 (p = 0.001) odds of DHF among heterozygotes [99] | ||||
| TGFβ1 | rs1800471 | 7040 | G | C | 25 DHF | 41 DF | Venezuela | No significant association with disease [86] | |
| G | C | 200 DF | 309 controls | Brazil | No significant association with disease [90] | ||||
| rs1982073 | T | C | 25 DHF | 41 DF | Venezuela | No significant association with disease [86] | Yes | ||
| T | C | 43 DHF | 99 controls | Cuba | No significant association with disease [87] | ||||
| T | C | 200 DF | 309 controls | Brazil | No significant association with disease [90] | ||||
| TIRAP | rs8177374 | 114,609 | C | T | 33 DHF | 109 controls | India | 2.64 (1.17–5.99) odds of severe disease among heterozygotes [100] | No |
| TLR3 | rs3775291 | 7098 | C | T | 33 DHF | 87 DF | India | 0.39 (0.16–0.88) odds of severe disease associated with T allele [100] | No |
| TNF-α | rs361525 | 7124 | G | A | 86 DF, 182 DHF, 14 DSS | 120 controls | Malaysia | 4.92 (1.10–21.90) odds of DHF compared to control group for heterozygotes [91] | Yes |
| G | A | 41 DF, 32 DHF | 169 controls | Mexico | 0.19 (0.02–0.78) odds of disease with A allele [101] | ||||
| rs1800629 | G | A | 80 symptomatic | 100 DEN-negative febrile cases and 99 healthy controls | Brazil | No significant association with disease [85] | Yes | ||
| G | A | 25 DHF | 41 DF | Venezuela | 2.5 (1.47–4.13) odds of severe disease [86] | ||||
| G | A | 43 DHF | 99 controls | Cuba | 3.51 (1.77–7.00) odds of severe disease [87] | ||||
| G | A | 200 DF | 309 controls | Brazil | No significant association with disease [90] | ||||
| G | A | 86 DF, 182 DHF, 14 DSS | 120 controls | Malaysia | 0.43 (0.22–0.84) odds of DHF compared to control for heterozygotes [91] | ||||
| G | A | 107 DHF | 62 controls | Sri Lanka | 2.53 (1.10–5.83) odds of disease for GG genotype [92] | ||||
| G | A | 85 DF, 29 DHF | 110 controls | India | No significant association with disease [102] | ||||
| G | A | 19 DF, 82 DHF | 106 controls | Thailand | No significant association with disease [103] | ||||
| G | A | 85 DF, 45 DHF | 163 controls | Mexico | No significant association with disease [101] | ||||
| TLR4 | amino acids 299 and 399 | 7099 | Asp299, Thr399 | Gly299, Ile399 | 201 DHF | 179 controls | Indonesia | No significant association with disease [104] | Yes |
| Asp299, Thr399 | Gly299, Ile399 | 63 DF, 57 DHF/DSS | 200 controls | India | 2.00 (1.17–3.43) odds associated with Gly299 for DF versus controls, and 2.38 (1.16–4.85) associated with Ile399 for DF versus controls [105] | ||||
| VDR | rs731236 | 7421 | T | C | 302 DHF | 238 controls | Vietnam | Associated with more severe disease (p = 0.033) [82] | Yes |
| T | C | 83 DF, 29 DHF | 105 controls | India | No significant association with disease [106] |
a genotype data not available for meta-analysis
Significant associations with DENV disease
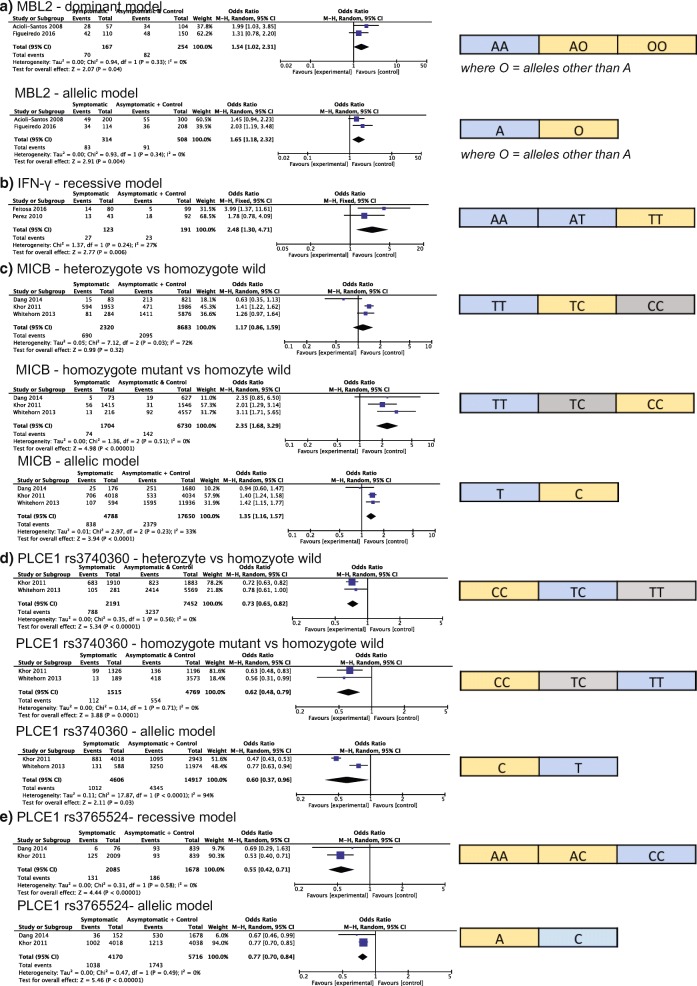
Among the DENV studies, the same variant within 17 genes was studied by two or more research groups (Table 3). Four genes had significant meta-ORs (Fig. 3). For a SNP in MBL2 (exon 1), we calculated a meta-OR of 1.54 [1.02–2.31] under a dominant model and 1.65 [1.18–2.32] under an allelic model, with alleles other than the A allele being associated with more severe disease. The T allele for SNP rs2430561 in the IFN-γ gene was associated with severe disease under a recessive model with a meta-OR of 2.48 [0.30–4.71]. For a SNP located within MICB (rs3132468), we found the CC genotype had a significantly greater association with severe disease (meta-OR 2.35 [1.68–3.29]), but the heterozygote genotype showed no significant association with disease severity as compared to the TT genotype (meta-OR = 1.17 [0.86–1.59]). For this SNP, the C allele was also found to be significantly associated with disease as compared to the T allele (meta-OR = 1.35 [1.16–1.57]). For two SNPs located within the PLCE1 gene, every model tested was significant, with the most significant meta-ORs being 0.62 [0.48–0.79] for TT genotype as compared to CC genotype for rs3740360 and 0.55 [0.42–0.71] under a recessive model for rs3765524. TNF-α (rs1800629 and rs361525) was the most studied gene, but none of the models tested provided a significant meta-OR.
Fig. 3.
Meta-analyzed genetic variation associated with DENV disease. Genotype count data from published reports of WNV subjects were meta-analyzed using RevMan. The meta-odds ratio (OR) for more severe disease is indicated with the genetic model for each gene: MBL2 (a), IFN-γ (b), MICB (c), PLCE1 (d and e). If multiple models were significant, we present the most significant model. The alleles or genotypes associated with asymptomatic infection and controls (blue) or with severe disease (yellow) outcome are shown for each gene
Quality scores
Based on the Newcastle Ottawa Scoring System, the average quality score was 5.76 (range: 3–7) for the WNV publications and 5.10 (range: 2–7) for the DENV publications (Additional file 6). We also assessed whether the study authors corrected for multiple testing, and found less than half of both WNV and DENV studies provided corrected p-values when appropriate, indicating an inflated type I error rate.
Discussion
We have examined genetic variants that show association with DENV or WNV disease severity. This analysis was undertaken to identify genetic differences that are significant drivers of susceptibility to symptomatic disease that may shed light on mechanisms of immune resistance to these viruses. Among the 87 studies examined, a wide range of genetic targets was found to be significant, with many of the genes unsurprisingly playing a key role in the immune system defense against viral infections (Additional file 7).
Despite the large number of studies, only 27 genes were studied by more than one research group for an association with either disease. Throughout these studies, several key genes rose to the forefront as the most studied and the most significant associations. Many studies focused on the HLA region of the genome, and, although inconsistencies in data presentation preclude a meta-analyze of these results, there were clear signs of the importance of this area for both diseases.
With the central role of HLA for the immune system, polymorphisms in this region have been well studied for associations with disease. The area is highly polymorphic, however, leading to difficulties for comparing the diverse range of alleles. Adding to this complexity, DENV serotypes interact differently with HLA [30]. The regions identified in this systematic review, including DRB1, DQA1, DQB1, A, B, and C, are among the most diverse regions of the HLA region [31]. A recent study examined some of these regions by supertype, and found the B44 supertype could be protective against DHF during secondary infections and that the A02 and A01/03 supertypes could be associated with more severe disease [32].
KIR genes, which are expressed on the surface of natural killer cells, also have wide genetic variability as noted with HLA genes [33]. While several KIR alleles were studied in DENV-infected populations, only one publication to date has examined KIR genotypes in West Nile virus-infected individuals, and this study had a sample size of four [34]. The results suggested a possible association; this, in conjunction with the results of the DENV research in this area and the genes’ highly polymorphic nature, could be an area that should be explored further. Infection with WNV has been shown to lead to diversification of KIR receptor expression [35]. In addition to the research outlined above, researchers have examined the association of KIR genotypes with DENV infection in vitro. Within the in vitro research, the timing of natural killer cell activation has been linked to disease severity and interactions between KIR and HLA have been suggested [36–38].
Another key non-HLA gene identified to be associated with WNV disease was OASL, which codes for an enzyme that is induced by type 1 interferon and viruses [39]. OASL was first identified to have a potentially critical role in WNV disease pathogenesis in 2002, when researchers found that mice with a truncated form of the gene were more susceptible to disease [40]. Elevated activity of the OAS genes has also been associated with more severe DENV infection in vitro [41]. This data and the significance of variation within the OAS genes for WNV outcomes highlight the importance of the interferon pathways in response to flavivirus infections and suggest a need for further in depth examination the association of genetic variability within OAS and DENV severity.
CCR5Δ32 was the only gene studied by two or more research groups for each disease. CCR5 was first identified as a co-receptor for HIV in 1996, and CCR5 deficiency, or a homozygous genotype of CCR5Δ32, was found to be protective against HIV infection [42–45]. In West Nile, CCR5 deficiency is not associated with incidence of infection, but is associated with severity of disease for infected individuals [46]. Subsequent research showed that CCR5 specifically plays a role in the ability of cortical neurons to combat West Nile virus infection of the brain [47]. In DENV, CCR5 deficiency has been linked with increased viral load and disease severity [48]. The study also found that the CCR5 receptor in macrophages is necessary for replication of DENV serotype 2, an early step in the infection process [49]. Given the similarities of these flaviviral diseases and the significant association of CCR5, the only gene looked at by research groups for both diseases, further research could be beneficial to further understanding the role of genetic variation in the development of severe flavivviral disease [50].
When we meta-analyzed the DENV studies, we found significant associations between DENV disease and genetic variation in MBL2, PLCE1, IFN-γ, and MICB. The role of many of these genes in disease pathogenesis has been characterized through in vivo and in vitro studies. MBL2, the mannose-binding lectin 2 gene, encodes a protein with a role in innate immunity and complement pathway, while PLCE1 encodes an enzyme critical to the generation of the inositol 1,4,5-triphosphate (IP3) and diacylglycerol (DAG) messengers [51]. MICB and IFN-y are both critical in the immune response, and thus variations within these genes could have strong effects on the initial response to the viral infection and the subsequent disease pathogenesis [51].
Our study is limited by several factors, most notably by the available literature. To ensure we found as many papers as possible, we constructed a search strategy that involved multiple databases, used both subject headings and text words, was not limited to English articles, and included an ancestry search [52]. Despite our focus on significant results and genes studied by at least two research groups, the wide heterogeneity among the populations studied limited our ability to interpret the meta-analyzed results. Lack of diversity in genetic studies is well-documented [53], and the absence of certain affected populations, particularly in Africa, among the identified studies further demonstrates this unmet research need [54]. The diversity of results among studies that examined the same SNP could be due to population heterogeneity, as well as to differences in study approach, including selection of control and comparison groups. Additionally, previous exposure history, DENV serotype, and WNV or DENV genotype are all factors that can affect disease severity, but were not accounted for in the included studies [4]. The number and type of genes examined varied greatly between studies, and we were limited by what genes researchers chose to sequence and include in publications. The unavailability of comparable genotype data and the incomparability of research groups across some studies preclude a more in depth analysis at present.
Conclusions
The genes found to be significantly associated with WNV or DENV disease pathogenesis varied in function, with most being linked to the immune response. As the regions of the world affected by WNV, DENV, and related viruses such as Zika, continue to expand due in part to climate change, an improved understanding of the association between genetic variation and disease severity will be valuable for all potentially affected populations [55–58]. Based on the growing incidence of these diseases, the paucity of consistency in the associations found, and the limited overlap in genetic targets studied to date, there is need for continued and deeper studies examining the role of genetic factors in WNV and DENV disease severity. In addition to conducting new studies such as whole-exome sequencing within larger population samples, further analyses could be conducted of existing data, to glean novel findings such as gene-gene or gene-environment interactions, rare and low frequency variants, and pathways of significant determinants of anti-viral resistance.
Additional files
PRISMA Checklist. This systematic review of genetic factors and WNV or dengue disease was conducted using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines outlined in this checklist. (DOC 95 kb)
Sample Search Strategy for the Embase Database. Medline, PubMed, Embase, and Global Health databases were used to search the literature. Search terms included West Nile or Dengue and genetic factors; the same set of text words was used for all databases in conjunction with subject headings that were tailored for each database. As an example, this search strategy for the Embase database is provided. These subject headings, in conjunction with the text search (Table 1), were used to find relevant literature in Embase. (DOCX 13 kb)
Included Papers. All publications included in this review are listed, with study details on authors, year of publication, country, sample size, case and control group definitions, and genotyping method. (XLSX 54 kb)
HLA Associations with DENV Disease Severity. HLA alleles studied by two or more research groups for association with DENV disease severity. (DOCX 311 kb)
HLA Associations with WNV Disease Severity. All examined targets from the analyzed publications are listed, with significant associations shown in bold. (XLSX 9 kb)
Quality Scores. The quality of each study was evaluated with the Newcastle-Ottawa Quality Assessment Scale for Case-Control Studies, which assesses each study’s selection, comparability, and exposure ascertainment approach. (XLSX 16 kb)
Extracted Data from Included Studies. All genotype data extracted from the manuscripts or collected from study authors is provided. (XLSX 54 kb)
Acknowledgements
The authors are grateful to many colleagues for valuable advice, and particularly wish to thank Ms. Xiaomei Wang for technical input, Ms. Kate Nyhan, MLS, (Yale University Cushing/Whitney Medical Library) for guidance in the development of the search strategy, and Dr. Marina Antillón for translating Spanish-language articles during the full text review.
Funding
MEC & RRM: AI 089992; SC: Self and Family Management T32 NR008346. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Abbreviations
- DENV
Dengue virus
- DHF
Dengue Hemorrhagic Fever
- DSS
Dengue Shock Syndrome
- GWAS
genome-wide association studies
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- SNP
single nucleotide polymorphism
- WNV
West Nile virus
Authors’ contributions
Conceptualization (MEC, ATD, RRM); project administration (MEC, RRM); data curation (MEC, SC); methodology (MEC, SC, ATD); formal analysis (MEC); writing – original draft preparation (MEC); writing – review and editing (MEC, SC, ATD, RRM). All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable. Data sets were extracted without personal identifiers and organized into literature tables.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s12879-018-3186-6) contains supplementary material, which is available to authorized users.
Contributor Information
Megan E. Cahill, Email: megan.cahill@yale.edu
Samantha Conley, Email: samantha.conley@yale.edu.
Andrew T. DeWan, Email: andrew.dewan@yale.edu
Ruth R. Montgomery, Email: ruth.montgomery@yale.edu
References
- 1.Papa A, Karabaxoglou D, Kansouzidou A. Acute West Nile virus neuroinvasive infections: cross-reactivity with dengue virus and tick-borne encephalitis virus. J Med Virol. 2011;83(10):1861–1865. doi: 10.1002/jmv.22180. [DOI] [PubMed] [Google Scholar]
- 2.Hua RH, Chen NS, Qin CF, Deng YQ, Ge JY, Wang XJ, Qiao ZJ, Chen WY, Wen ZY, Liu WX, et al. Identification and characterization of a virus-specific continuous B-cell epitope on the PrM/M protein of Japanese encephalitis virus: potential application in the detection of antibodies to distinguish Japanese encephalitis virus infection from West Nile virus and dengue virus infections. Virol J. 2010;7:249. doi: 10.1186/1743-422X-7-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daep CA, Munoz-Jordan JL, Eugenin EA. Flaviviruses, an expanding threat in public health: focus on dengue, West Nile, and Japanese encephalitis virus. J Neuro-Oncol. 2014;20(6):539–560. doi: 10.1007/s13365-014-0285-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gubler DJ. The continuing spread of West Nile virus in the western hemisphere. Clin Infect Dis. 2007;45(8):1039–1046. doi: 10.1086/521911. [DOI] [PubMed] [Google Scholar]
- 5.Petersen LR, Brault AC, Nasci RS. West Nile virus: review of the literature. JAMA. 2013;310(3):308–315. doi: 10.1001/jama.2013.8042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Symptoms & Treatments [http://www.cdc.gov/westnile/symptoms/index.html].
- 7.Coffey LL, Mertens E, Brehin AC, Fernandez-Garcia MD, Amara A, Despres P, Sakuntabhai A. Human genetic determinants of dengue virus susceptibility. Microbes Infect. 2009;11(2):143–156. doi: 10.1016/j.micinf.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 8.Kalayanarooj S. Clinical manifestations and Management of Dengue/DHF/DSS. Trop Med Health. 2011;39(4 Suppl):83–87. doi: 10.2149/tmh.2011-S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montgomery RR, Murray KO. Risk factors for West Nile virus infection and disease in populations and individuals. Expert Rev Anti-Infect Ther. 2015;13(3):317–325. doi: 10.1586/14787210.2015.1007043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whitehorn J, Hubbard S, Anders KL, Quyen NTH, Simmons C. Dengue pathogenesis: host factors. Dengue and Dengue Hemorrhagic Fever, 2nd Edition. 2014:214–28.
- 11.James EA, Gates TJ, LaFond RE, Yamamoto S, Ni C, Mai D, Gersuk VH, O'Brien K, Nguyen QA, Zeitner B, et al. Neuroinvasive West Nile infection elicits elevated and atypically polarized T cell responses that promote a pathogenic outcome. PLoS Pathog. 2016;12(1):e1005375. doi: 10.1371/journal.ppat.1005375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qian F, Thakar J, Yuan X, Nolan M, Murray KO, Lee WT, Wong SJ, Meng H, Fikrig E, Kleinstein SH, et al. Immune markers associated with host susceptibility to infection with West Nile virus. Viral Immunol. 2014;27(2):39–47. doi: 10.1089/vim.2013.0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yao Y, Montgomery RR. Role of immune aging in susceptibility to West Nile virus. Methods Mol Biol. 2016;1435:235–247. doi: 10.1007/978-1-4939-3670-0_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cahill ME, Yao Y, Nock D, Armstrong PM, Andreadis TG, Diuk-Wasser MA, Montgomery RR. West Nile Virus Seroprevalence, Connecticut, USA, 2000-2014. Emerg Infect Dis. 2017;23(4):708–710. doi: 10.3201/eid2304.161669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krishnan MN, Sukumaran B, Pal U, Agaisse H, Murray JL, Hodge TW, Fikrig E. Rab 5 is required for the cellular entry of dengue and West Nile viruses. J Virol. 2007;81(9):4881–4885. doi: 10.1128/JVI.02210-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campos RK, Wong B, Xie XP, Lu YF, Shi PY, Pompon J, Garcia-Blanco MA, Bradrick SS. RPLP1 and RPLP2 are essential Flavivirus host factors that promote early viral protein accumulation. J Virol. 2017;91(4) [DOI] [PMC free article] [PubMed]
- 17.Gack MU, Diamond MS. Innate immune escape by dengue and West Nile viruses. Curr Opin Virol. 2016;20:119–128. doi: 10.1016/j.coviro.2016.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chaturvedi UC, Agarwal R, Elbishbishi EA, Mustafa AS. Cytokine cascade in dengue hemorrhagic fever: implications for pathogenesis. FEMS Immunol Med Microbiol. 2000;28(3):183–188. doi: 10.1111/j.1574-695X.2000.tb01474.x. [DOI] [PubMed] [Google Scholar]
- 19.Kumar M, Verma S, Nerurkar VR. Pro-inflammatory cytokines derived from West Nile virus (WNV)-infected SK-N-SH cells mediate neuroinflammatory markers and neuronal death. J Neuroinflammation. 2010;7:73. doi: 10.1186/1742-2094-7-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rossini G, Landini MP, Gelsomino F, Sambri V, Varani S. Innate host responses to West Nile virus: implications for central nervous system immunopathology. World J Virol. 2013;2(2):49–56. doi: 10.5501/wjv.v2.i2.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sprague AH, Khalil RA. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem Pharmacol. 2009;78(6):539–552. doi: 10.1016/j.bcp.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mackenzie JS, Gubler DJ, Petersen LR. Emerging flaviviruses: the spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat Med. 2004;10(12):S98–S109. doi: 10.1038/nm1144. [DOI] [PubMed] [Google Scholar]
- 23.Hotez PJ, Murray KO. Dengue, West Nile virus, chikungunya, Zika - and now Mayaro? PLoS Negl Trop Dis. 2017;11(8): e0005462. [DOI] [PMC free article] [PubMed]
- 24.Yun SI, Lee YM. Zika virus: An emerging flavivirus. J Microbiol. 2017;55(3):204–219. doi: 10.1007/s12275-017-7063-6. [DOI] [PubMed] [Google Scholar]
- 25.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Covidence systematic review software. www.covidence.org.
- 27.Tomas J. Aragon MPF, Daniel Wollschlaeger, Adam Omidpanah: epitools: Epidemiology Tools. In.; 2017.
- 28.Review Manager (RevMan). In., 5.3 EDN Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration; 2014.
- 29.Wells G, Shea B, O’connell D, Peterson J, Welch V, Losos M, Tugwell P: The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. In.; 2000.
- 30.Gan CS, Yusof R, Othman S. Different serotypes of dengue viruses differently regulate the expression of the host cell antigen processing machinery. Acta Trop. 2015;149:8–14. doi: 10.1016/j.actatropica.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 31.Shiina T, Hosomichi K, Inoko H, Kulski JK. The HLA genomic loci map: expression, interaction, diversity and disease. J Hum Genet. 2009;54(1):15–39. doi: 10.1038/jhg.2008.5. [DOI] [PubMed] [Google Scholar]
- 32.Vejbaesya S, Thongpradit R, Kalayanarooj S, Luangtrakool K, Luangtrakool P, Gibbons RV, Srinak D, Ngammthaworn S, Apisawes K, Yoon IK, et al. HLA class I Supertype associations with clinical outcome of secondary dengue virus infections in ethnic Thais. J Infect Dis. 2015;212(6):939–947. doi: 10.1093/infdis/jiv127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uhrberg M. The KIR gene family: life in the fast lane of evolution. Eur J Immunol. 2005;35(1):10–15. doi: 10.1002/eji.200425743. [DOI] [PubMed] [Google Scholar]
- 34.Spiroski M, Milenkovic Z, Petlichkovski A, Ivanovski L, Topuzovska IK, Djulejic E. Killer cell immunoglobulin-like receptor genes in four human West Nile virus infections reported 2011 in the republic of Macedonia. Hum Immunol. 2013;74(3):389–394. doi: 10.1016/j.humimm.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 35.Strauss-Albee DM, Fukuyama J, Liang EC, Yao Y, Jarrell JA, Drake AL, Kinuthia J, Montgomery RR, John-Stewart G, Holmes S, et al. Human NK cell repertoire diversity reflects immune experience and correlates with viral susceptibility. Sci Transl Med. 2015;7(297):297ra115. doi: 10.1126/scitranslmed.aac5722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Azeredo EL, De Oliveira-Pinto LM, Zagne SM, Cerqueira DI, Nogueira RM, Kubelka CF. NK cells, displaying early activation, cytotoxicity and adhesion molecules, are associated with mild dengue disease. Clin Exp Immunol. 2006;143(2):345–356. doi: 10.1111/j.1365-2249.2006.02996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Townsley E, O'Connor G, Cosgrove C, Woda M, Co M, Thomas SJ, Kalayanarooj S, Yoon IK, Nisalak A, Srikiatkhachorn A, et al. Interaction of a dengue virus NS1-derived peptide with the inhibitory receptor KIR3DL1 on natural killer cells. Clin Exp Immunol. 2016;183(3):419–430. doi: 10.1111/cei.12722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yao Y, Strauss-Albee DM, Zhou JQ, Malawista A, Garcia MN, Murray KO, Blish CA, Montgomery RR. The natural killer cell response to West Nile virus in young and old individuals with or without a prior history of infection. PLoS One. 2017;12(2):e0172625. doi: 10.1371/journal.pone.0172625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.OASL - 2′-5′-oligoadenylate synthase-like protein. http://www.uniprot.org/uniprot/Q15646.
- 40.Mashimo T, Lucas M, Simon-Chazottes D, Frenkiel MP, Montagutelli X, Ceccaldi PE, Deubel V, Guenet JL, Despres P. A nonsense mutation in the gene encoding 2′-5′-oligoadenylate synthetase/L1 isoform is associated with West Nile virus susceptibility in laboratory mice. Proc Natl Acad Sci U S A. 2002;99(17):11311–11316. doi: 10.1073/pnas.172195399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simon-Loriere E, Lin RJ, Kalayanarooj SM, Chuansumrit A, Casademont I, Lin SY, Yu HP, Lert-Itthiporn W, Chaiyaratana W, Tangthawornchaikul N, et al. High anti-dengue virus activity of the OAS gene family is associated with increased severity of dengue. J Infect Dis. 2015;212(12):2011–2020. doi: 10.1093/infdis/jiv321. [DOI] [PubMed] [Google Scholar]
- 42.Alkhatib G, Combadiere C, Broder CC, Feng Y, Kennedy PE, Murphy PM, Berger EA. CC CKR5: a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272(5270):1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 43.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton RE, Hill CM, et al. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381(6584):661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 44.Dragic T, Litwin V, Allaway GP, Martin SR, Huang Y, Nagashima KA, Cayanan C, Maddon PJ, Koup RA, Moore JP, et al. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381(6584):667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 45.Liu R, Paxton WA, Choe S, Ceradini D, Martin SR, Horuk R, MacDonald ME, Stuhlmann H, Koup RA, Landau NR. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell. 1996;86(3):367–377. doi: 10.1016/S0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- 46.Lim JK, McDermott DH, Lisco A, Foster GA, Krysztof D, Follmann D, Stramer SL, Murphy PM. CCR5 deficiency is a risk factor for early clinical manifestations of West Nile virus infection but not for viral transmission. J Infect Dis. 2010;201(2):178–185. doi: 10.1086/649426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Durrant DM, Daniels BP, Pasieka T, Dorsey D, Klein RS. CCR5 limits cortical viral loads during West Nile virus infection of the central nervous system. J Neuroinflammation. 2015;12:233. doi: 10.1186/s12974-015-0447-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marques RE, Guabiraba R, Del Sarto JL, Rocha RF, Queiroz AL, Cisalpino D, Marques PE, Pacca CC, Fagundes CT, Menezes GB, et al. Dengue virus requires the CC-chemokine receptor CCR5 for replication and infection development. Immunology. 2015;145(4):583–596. doi: 10.1111/imm.12476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen YC, Wang SY. Activation of terminally differentiated human monocytes/macrophages by dengue virus: productive infection, hierarchical production of innate cytokines and chemokines, and the synergistic effect of lipopolysaccharide. J Virol. 2002;76(19):9877–9887. doi: 10.1128/JVI.76.19.9877-9887.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Loeb M. Genetic susceptibility to West Nile virus and dengue. Public Health Genomics. 2013;16(1–2):4–8. doi: 10.1159/000345934. [DOI] [PubMed] [Google Scholar]
- 51.Gene. https://www.ncbi.nlm.nih.gov/gene/.
- 52.Akobeng AK. Understanding systematic reviews and meta-analysis. Arch Dis Child. 2005;90(8):845–848. doi: 10.1136/adc.2004.058230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Popejoy AB, Fullerton SM. Genomics is failing on diversity. Nature. 2016;538(7624):161–164. doi: 10.1038/538161a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Were F. The dengue situation in Africa. Paediatr Int Child Health. 2012;32(Suppl 1):18–21. doi: 10.1179/2046904712Z.00000000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ebi KL, Nealon J. Dengue in a changing climate. Environ Res. 2016;151:115–123. doi: 10.1016/j.envres.2016.07.026. [DOI] [PubMed] [Google Scholar]
- 56.Paz S. Climate change impacts on West Nile virus transmission in a global context. Philos Trans R Soc Lond Ser B Biol Sci. 2015;370(1665) [DOI] [PMC free article] [PubMed]
- 57.Carlson CJ, Dougherty ER, Getz W. An ecological assessment of the pandemic threat of Zika virus. PLoS Negl Trop Dis. 2016;10(8):e0004968. doi: 10.1371/journal.pntd.0004968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lessler J, Chaisson LH, Kucirka LM, Bi Q, Grantz K, Salje H, Carcelen AC, Ott CT, Sheffield JS, Ferguson NM, et al. Assessing the global threat from Zika virus. Science. 2016;353(6300):aaf8160. doi: 10.1126/science.aaf8160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Loeb M, Eskandarian S, Rupp M, Fishman N, Gasink L, Patterson J, Bramson J, Hudson TJ, Lemire M. Genetic variants and susceptibility to neurological complications following West Nile virus infection. J Infect Dis. 2011;204(7):1031–1037. doi: 10.1093/infdis/jir493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Danial-Farran N, Eghbaria S, Schwartz N, Kra-Oz Z, Bisharat N. Genetic variants associated with susceptibility of Ashkenazi Jews to West Nile virus infection. Epidemiology & Infection. 2015;143(4):857–863. doi: 10.1017/S0950268814001290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Long D, Deng X, Singh P, Loeb M, Lauring AS, Seielstad M. Identification of genetic variants associated with susceptibility to West Nile virus neuroinvasive disease. Genes & Immunity. 2016;17(5):298–304. doi: 10.1038/gene.2016.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bigham AW, Buckingham KJ, Husain S, Emond MJ, Bofferding KM, Gildersleeve H, Rutherford A, Astakhova NM, Perelygin AA, Busch MP et al: Host genetic risk factors for West Nile virus infection and disease progression. PLoS One 2011, 6 (9) (no pagination)(e24745). [DOI] [PMC free article] [PubMed]
- 63.Glass WG, McDermott DH, Lim JK, Lekhong S, Yu SF, Frank WA, Pape J, Cheshier RC, Murphy PM. CCR5 deficiency increases risk of symptomatic West Nile virus infection. J Exp Med. 2006;203(1):35–40. doi: 10.1084/jem.20051970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Das R, Loughran K, Murchison C, Qian F, Leng L, Song Y, Montgomery RR, Loeb M, Bucala R. Association between high expression macrophage migration inhibitory factor (MIF) alleles and West Nile virus encephalitis. Cytokine. 2016;78:51–54. doi: 10.1016/j.cyto.2015.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yakub I, Lillibridge KM, Moran A, Gonzalez OY, Belmont J, Gibbs RA, Tweardy DJ. Single nucleotide polymorphisms in genes for 2′-5′-oligoadenylate synthetase and RNase L inpatients hospitalized with West Nile virus infection. J Infect Dis. 2005;192(10):1741–1748. doi: 10.1086/497340. [DOI] [PubMed] [Google Scholar]
- 66.Lim JK, Lisco A, McDermott DH, Huynh L, Ward JM, Johnson B, Johnson H, Pape J, Foster GA, Krysztof D, et al. Genetic variation in OAS1 is a risk factor for initial infection with West Nile virus in man. PLoS Pathog. 2009;5(2):e1000321. doi: 10.1371/journal.ppat.1000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dang TN, Naka I, Sa-Ngasang A, Anantapreecha S, Wichukchinda N, Sawanpanyalert P, Patarapotikul J, Tsuchiya N, Ohashi J. Association of BAK1 single nucleotide polymorphism with a risk for dengue hemorrhagic fever. BMC Medical Genetics. 2016;17(1):43. doi: 10.1186/s12881-016-0305-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brestovac B, Halicki LA, Harris RP, Sampson I, Speers DJ, Mamotte C, Williams D. Primary acute dengue and the deletion in chemokine receptor 5 (CCR5DELTA32) Microbes & Infection. 2014;16(6):518–521. doi: 10.1016/j.micinf.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 69.Xavier-Carvalho C, Gibson G, Brasil P, Ferreira RX, de Souza Santos R, Goncalves Cruz O, de Oliveira SA, de Sa Carvalho M, Pacheco AG, Kubelka CF, et al. Single nucleotide polymorphisms in candidate genes and dengue severity in children: a case-control, functional and meta-analysis study. Infection, Genetics & Evolution. 2013;20:197–205. doi: 10.1016/j.meegid.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 70.Alagarasu K, Damle IM, Bachal RV, Mulay AP, Shah PS, Dayaraj C. Association of promoter region polymorphisms of CD209 gene with clinical outcomes of dengue virus infection in western India. Infection, Genetics & Evolution. 2013;17:239–242. doi: 10.1016/j.meegid.2013.04.024. [DOI] [PubMed] [Google Scholar]
- 71.Noecker CA, Amaya-Larios IY, Galeana-Hernandez M, Ramos-Castaneda J, Martinez-Vega RA. Contrasting associations of polymorphisms in FcgammaRIIa and DC-SIGN with the clinical presentation of dengue infection in a Mexican population. Acta Trop. 2014;138:15–22. doi: 10.1016/j.actatropica.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 72.Oliveira LF, Lima CP, Azevedo Rdo S, Mendonca DS, Rodrigues SG, Carvalho VL, Pinto EV, Maia AL, Maia MH, Vasconcelos JM, et al. Polymorphism of DC-SIGN (CD209) promoter in association with clinical symptoms of dengue fever. Viral Immunol. 2014;27(5):245–249. doi: 10.1089/vim.2013.0119. [DOI] [PubMed] [Google Scholar]
- 73.Sakuntabhai A, Turbpaiboon C, Casademont I, Chuansumrit A, Lowhnoo T, Kajaste-Rudnitski A, Kalayanarooj SM, Tangnararatchakit K, Tangthawornchaikul N, Vasanawathana S, et al. A variant in the CD209 promoter is associated with severity of dengue disease. Nat Genet. 2005;37(5):507–513. doi: 10.1038/ng1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Silva LK, Blanton RE, Parrado AR, Melo PS, Morato VG, Reis EA, Dias JP, Castro JM, Vasconcelos PF, Goddard KA, et al. Dengue hemorrhagic fever is associated with polymorphisms in JAK1. Eur J Hum Genet. 2010;18(11):1221–1227. doi: 10.1038/ejhg.2010.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang L, Chen RF, Liu JW, Lee IK, Lee CP, Kuo HC, Huang SK, Yang KD. DC-SIGN (CD209) promoter −336 a/G polymorphism is associated with dengue hemorrhagic fever and correlated to DC-SIGN expression and immune augmentation. PLoS Neglected Tropical Diseases [electronic resource] 2011;5(1):e934. doi: 10.1371/journal.pntd.0000934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kraivong R, Vasanawathana S, Limpitikul W, Malasit P, Tangthawornchaikul N, Botto M, Screaton GR, Mongkolsapaya J, Pickering MC. Complement alternative pathway genetic variation and dengue infection in the Thai population. Clinical & Experimental Immunology. 2013;174(2):326–334. doi: 10.1111/cei.12184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pastor AF, Rodrigues Moura L, Neto JW, Nascimento EJ, Calzavara-Silva CE, Gomes AL, Silva AM, Cordeiro MT, Braga-Neto U, Crovella S, et al. Complement factor H gene (CFH) polymorphisms C-257T, G257A and haplotypes are associated with protection against severe dengue phenotype, possible related with high CFH expression. Hum Immunol. 2013;74(9):1225–1230. doi: 10.1016/j.humimm.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Alagarasu K, Bachal RV, Tillu H, Mulay AP, Kakade MB, Shah PS, Cecilia D. Association of combinations of interleukin-10 and pro-inflammatory cytokine gene polymorphisms with dengue hemorrhagic fever. Cytokine. 2015;74(1):130–136. doi: 10.1016/j.cyto.2015.03.021. [DOI] [PubMed] [Google Scholar]
- 79.Alagarasu K, Memane RS, Shah PS. Polymorphisms in the retinoic acid-1 like-receptor family of genes and their association with clinical outcome of dengue virus infection. Arch Virol. 2015;160(6):1555–1560. doi: 10.1007/s00705-015-2417-z. [DOI] [PubMed] [Google Scholar]
- 80.Alagarasu K, Bachal RV, Damle I, Shah PS, Cecilia D. Association of FCGR2A p.R131H and CCL2 c.-2518 A>G gene variants with thrombocytopenia in patients with dengue virus infection. Hum Immunol. 2015;76(11):819–822. doi: 10.1016/j.humimm.2015.09.042. [DOI] [PubMed] [Google Scholar]
- 81.Garcia G, Sierra B, Perez AB, Aguirre E, Rosado I, Gonzalez N, Izquierdo A, Pupo M, Danay Diaz DR, Sanchez L, et al. Asymptomatic dengue infection in a Cuban population confirms the protective role of the RR variant of the FcgammaRIIa polymorphism. American Journal of Tropical Medicine & Hygiene. 2010;82(6):1153–1156. doi: 10.4269/ajtmh.2010.09-0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Loke H, Bethell D, Phuong CX, Day N, White N, Farrar J, Hill A. Susceptibility to dengue hemorrhagic fever in Vietnam: evidence of an association with variation in the vitamin d receptor and fc gamma receptor IIa genes. American Journal of Tropical Medicine & Hygiene. 2002;67(1):102–106. doi: 10.4269/ajtmh.2002.67.102. [DOI] [PubMed] [Google Scholar]
- 83.Mohsin SN, Mahmood S, Amar A, Ghafoor F, Raza SM, Saleem M. Association of FcgammaRIIa polymorphism with clinical outcome of dengue infection: first insight from Pakistan. American Journal of Tropical Medicine & Hygiene. 2015;93(4):691–696. doi: 10.4269/ajtmh.15-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Soundravally R, Hoti SL. Immunopathogenesis of dengue hemorrhagic fever and shock syndrome: role of TAP and HPA gene polymorphism. Hum Immunol. 2007;68(12):973–979. doi: 10.1016/j.humimm.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 85.Feitosa RNM, Vallinoto ACR, Vasconcelos PFDC, Azevedo RDSDS, Azevedo VN, MacHado LFA, Lima SS, Ishak MDOG, Ishak R. Gene polymorphisms and serum levels of pro- and anti-inflammatory markers in dengue viral infections. Viral Immunol. 2016;29(7):379–388. doi: 10.1089/vim.2016.0026. [DOI] [PubMed] [Google Scholar]
- 86.Fernandez-Mestre MT, Gendzekhadze K, Rivas-Vetencourt P, Layrisse Z. TNF-alpha-308A allele, a possible severity risk factor of hemorrhagic manifestation in dengue fever patients. Tissue Antigens. 2004;64(4):469–472. doi: 10.1111/j.1399-0039.2004.00304.x. [DOI] [PubMed] [Google Scholar]
- 87.Perez AB, Sierra B, Garcia G, Aguirre E, Babel N, Alvarez M, Sanchez L, Valdes L, Volk HD, Guzman MG. Tumor necrosis factor-alpha, transforming growth factor-beta1, and interleukin-10 gene polymorphisms: implication in protection or susceptibility to dengue hemorrhagic fever. Hum Immunol. 2010;71(11):1135–1140. doi: 10.1016/j.humimm.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 88.Cansancao IF, do Carmo AP, Leite RD, Portela RD, de Sa Leitao Paiva Junior S, de Queiroz Balbino V, Rabenhorst SH. Association of genetic polymorphisms of IL1beta −511 C>T, IL1RN VNTR 86 bp, IL6–174 G>C, IL10–819 C>T and TNFalpha −308 G>A, involved in symptomatic patients with dengue in Brazil. Inflamm Res. 2016;65(11):925–932. doi: 10.1007/s00011-016-0975-5. [DOI] [PubMed] [Google Scholar]
- 89.Sa-Ngasang A, Ohashi J, Naka I, Anantapreecha S, Sawanpanyalert P, Patarapotikul J. Association of IL1B -31C/T and IL1RA variable number of an 86-bp tandem repeat with dengue shock syndrome in Thailand. J Infect Dis. 2014;210(1):138–145. doi: 10.1093/infdis/jiu042. [DOI] [PubMed] [Google Scholar]
- 90.Moreira ST, Cardoso DM, Visentainer JE, Fonzar UJV, Moliterno RA. The possible protective role of the Il6<sup>-174GC</sup> genotype in Dengue Fever. Open Tropical Medicine Journal. 2008;1:87–91. doi: 10.2174/1874315300801010087. [DOI] [Google Scholar]
- 91.Sam SS, Teoh BT, Chinna K, AbuBakar S. High producing tumor necrosis factor alpha gene alleles in protection against severe manifestations of dengue. Int J Med Sci. 2015;12(2):177–186. doi: 10.7150/ijms.8988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fernando AN, Malavige GN, Perera KL, Premawansa S, Ogg GS, De Silva AD. Polymorphisms of transporter associated with antigen presentation, tumor necrosis factor-alpha and Interleukin-10 and their implications for protection and susceptibility to severe forms of dengue fever in patients in Sri Lanka. J Global Infect Dis. 2015;7(4):157–164. doi: 10.4103/0974-777X.170501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Acioli-Santos B, Segat L, Dhalia R, Brito CA, Braga-Neto UM, Marques ET, Crovella S. MBL2 gene polymorphisms protect against development of thrombocytopenia associated with severe dengue phenotype. Hum Immunol. 2008;69(2):122–128. doi: 10.1016/j.humimm.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 94.Figueiredo GG, Cezar RD, Freire NM, Teixeira VG, Baptista P, Cordeiro M, Carmo RF, Vasconcelos LR, Moura P. Mannose-binding lectin gene (MBL2) polymorphisms related to the mannose-binding lectin low levels are associated to dengue disease severity. Hum Immunol. 2016;77(7):571–575. doi: 10.1016/j.humimm.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 95.Dang TN, Naka I, Sa-Ngasang A, Anantapreecha S, Chanama S, Wichukchinda N, Sawanpanyalert P, Patarapotikul J, Tsuchiya N, Ohashi J. A replication study confirms the association of GWAS-identified SNPs at MICB and PLCE1 in Thai patients with dengue shock syndrome. BMC Medical Genetics. 2014;15:58. doi: 10.1186/1471-2350-15-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Khor CC, Chau TN, Pang J, Davila S, Long HT, Ong RT, Dunstan SJ, Wills B, Farrar J, Van Tram T, et al. Genome-wide association study identifies susceptibility loci for dengue shock syndrome at MICB and PLCE1. Nat Genet. 2011;43(11):1139–1141. doi: 10.1038/ng.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Whitehorn J, Chau TN, Nguyet NM, Kien DT, Quyen NT, Trung DT, Pang J, Wills B, Van Vinh Chau N, Farrar J, et al. Genetic variants of MICB and PLCE1 and associations with non-severe dengue. PLoS ONE [Electronic Resource] 2013;8(3):e59067. doi: 10.1371/journal.pone.0059067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sierra B, Triska P, Soares P, Garcia G, Perez AB, Aguirre E, Oliveira M, Cavadas B, Regnault B, Alvarez M, et al. OSBPL10, RXRA and lipid metabolism confer African-ancestry protection against dengue haemorrhagic fever in admixed Cubans. PLoS Pathog. 2017;13(2):e1006220. doi: 10.1371/journal.ppat.1006220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Soundravally R, Hoti SL. Significance of transporter associated with antigen processing 2 (TAP2) gene polymorphisms in susceptibility to dengue viral infection. J Clin Immunol. 2008;28(3):256–262. doi: 10.1007/s10875-007-9154-3. [DOI] [PubMed] [Google Scholar]
- 100.Alagarasu K, Bachal RV, Memane RS, Shah PS, Cecilia D. Polymorphisms in RNA sensing toll like receptor genes and its association with clinical outcomes of dengue virus infection. Immunobiology. 2015;220(1):164–168. doi: 10.1016/j.imbio.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 101.Garcia-Trejo AR, Falcon-Lezama JA, Juarez-Palma L, Granados J, Zuniga-Ramos J, Rangel H, Barquera R, Vargas-Alarcon G, Ramos C. Tumor necrosis factor alpha promoter polymorphisms in Mexican patients with dengue fever. Acta Trop. 2011;120(1–2):67–71. doi: 10.1016/j.actatropica.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 102.Alagarasu K, Mulay AP, Singh R, Gavade VB, Shah PS, Cecilia D. Association of HLA-DRB1 and TNF genotypes with dengue hemorrhagic fever. Hum Immunol. 2013;74(5):610–617. doi: 10.1016/j.humimm.2013.01.027. [DOI] [PubMed] [Google Scholar]
- 103.Chuansumrit A, Anantasit N, Sasanakul W, Chaiyaratana W, Tangnararatchakit K, Butthep P, Chunhakan S, Yoksan S. Tumour necrosis factor gene polymorphism in dengue infection: association with risk of bleeding. Paediatrics and International Child Health. 2013;33(2):97–101. doi: 10.1179/2046905512Y.0000000049. [DOI] [PubMed] [Google Scholar]
- 104.Djamiatun K, Ferwerda B, Netea MG, van der Ven AJ, Dolmans WM, Faradz SM. Toll-like receptor 4 polymorphisms in dengue virus-infected children. American Journal of Tropical Medicine & Hygiene. 2011;85(2):352–354. doi: 10.4269/ajtmh.2011.10-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Swati S, Singh SK, Kavita K, Nikky N, Dhole TN, Rajesh K, Saba H. Analysis of TLR4 (Asp299 Gly and Thr399Ile) gene polymorphisms and mRNA level in patients with dengue infection: a case-control study. Infect Genet Evol. 2016;43:412–417. doi: 10.1016/j.meegid.2016.06.027. [DOI] [PubMed] [Google Scholar]
- 106.Alagarasu K, Honap T, Mulay AP, Bachal RV, Shah PS, Cecilia D. Association of vitamin D receptor gene polymorphisms with clinical outcomes of dengue virus infection. Hum Immunol. 2012;73(11):1194–1199. doi: 10.1016/j.humimm.2012.08.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA Checklist. This systematic review of genetic factors and WNV or dengue disease was conducted using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines outlined in this checklist. (DOC 95 kb)
Sample Search Strategy for the Embase Database. Medline, PubMed, Embase, and Global Health databases were used to search the literature. Search terms included West Nile or Dengue and genetic factors; the same set of text words was used for all databases in conjunction with subject headings that were tailored for each database. As an example, this search strategy for the Embase database is provided. These subject headings, in conjunction with the text search (Table 1), were used to find relevant literature in Embase. (DOCX 13 kb)
Included Papers. All publications included in this review are listed, with study details on authors, year of publication, country, sample size, case and control group definitions, and genotyping method. (XLSX 54 kb)
HLA Associations with DENV Disease Severity. HLA alleles studied by two or more research groups for association with DENV disease severity. (DOCX 311 kb)
HLA Associations with WNV Disease Severity. All examined targets from the analyzed publications are listed, with significant associations shown in bold. (XLSX 9 kb)
Quality Scores. The quality of each study was evaluated with the Newcastle-Ottawa Quality Assessment Scale for Case-Control Studies, which assesses each study’s selection, comparability, and exposure ascertainment approach. (XLSX 16 kb)
Extracted Data from Included Studies. All genotype data extracted from the manuscripts or collected from study authors is provided. (XLSX 54 kb)
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.