Abstract
Background: Consistent use of continuous glucose monitoring (CGM) has been associated with improved glycemic control in youth with type 1 diabetes (T1D). There are many barriers to device uptake and continued use. There is a need to understand patient-specific characteristics when considering CGM. We evaluated patterns of CGM use and associations between baseline psychosocial measures and frequency of CGM use over 1 year.
Methods: Youth with T1D (n = 120), ages 8–18 years, completed questionnaires at CGM initiation and after 6 and 12 months assessing depressive symptoms, diabetes burden, and diabetes-specific and generic quality of life (QOL).
Results: Youth (51% male and 95% white) had mean age 12.7 ± 2.7 years, diabetes duration 6.1 ± 3.6 years, and glycated hemoglobin (HbA1c) 8.0 ± 0.8%. Over 1 year, 35% of youth used CGM 6 to 7 days per week, 45% used CGM 3–5 days per week, and 20% used CGM only 0–2 days per week. Youth who used CGM 3–7 days per week over 12 months had lower HbA1c at months 9 and 12 than youth who used CGM 0–2 days per week (9 months: 7.9 ± 0.9% vs. 8.5 ± 1.1%, P = 0.006 and 12 months: 8.0 ± 0.9% vs. 8.5 ± 1.1%, P = 0.02). Those using CGM 0–2 days per week had greater endorsement of depressive symptoms and diabetes burden and reported lower QOL at baseline compared with those using CGM 3–7 days per week.
Conclusions: CGM use for 3 or more days per week over 12 months had a protective effect on HbA1c. Providers should consider addressing psychosocial parameters when initiating CGM to maximize uptake and promote continued use in youth with T1D.
Keywords: : Continuous glucose monitoring, Depressive symptoms, Diabetes burden, Quality of life, Youth, Technology
Introduction
Continuous glucose monitoring (CGM) provides opportunities for improved glycemic management in children and adolescents with type 1 diabetes (T1D) through hypoglycemia and hyperglycemia alerts, facilitation of real-time bolus adjustments in response to trend arrows, and optimization of basal and bolus insulin needs based on retrospective review of patients' CGM data.1,2 Families of children with T1D may choose to initiate CGM for a variety of reasons including hopes of optimizing glycemic control, reducing hypoglycemia, easing and facilitating sports participation, and reducing wide glycemic excursions.3 However, there are many barriers to device uptake and continued use, including cost, nuisance alarms, accuracy concerns, discomfort, and hassle of wearing devices.4 Survey data of current CGM users have shown that those who had greater confidence in using CGM data and greater satisfaction with CGM accuracy and usability had the greatest quality of life (QOL)-related benefits from CGM.5 Other psychosocial benefits include decreased diabetes distress and increased comfort around hypoglycemic excursions.6
In addition to potential psychosocial benefits, CGM has been associated with improved glycemic control when worn consistently in pediatric and adult patients with diabetes.7–10 Consistent CGM use is typically defined as 6 or more days per week,9,11–13 although one recent retrospective multicenter study of 129 children and adolescents with T1D treated with CGM found significant improvement in glycated hemoglobin (HbA1c) with mean sensor use of 13.4 days per month.3 Longitudinal use of CGM is also an important factor, and a recent study reported that frequent (≥6 days per week) CGM use for 12 months (durability) was associated with improved glycemic control compared with frequent CGM use for 6 months dropping to less frequent (<6 days per week) use at 12 months.12 Unfortunately, data from the T1D Exchange clinic registry indicated that 41% of individuals who used CGM at baseline had discontinued use within 1 year and only 5%–8% of young patients ages 6–17 years old use CGM.13,14 The high discontinuation rate and low use may have reflected earlier CGM devices with poorer performance characteristics than currently available devices. In fact, recent data suggest that with substantially improved performance of devices, CGM uptake is higher; in a recent large (n = 549) cross-sectional study, 32% of youth <7 years old were using CGM.15
To optimize diabetes care, one approach is to increase uptake of CGM in youth, given the glycemic benefits and opportunities for improved psychosocial outcomes. Knowledge of factors related to durable use and predictors related to discontinuation of CGM use may allow providers to distinguish those likely to be successful CGM users from those who need additional education and support with CGM implementation. In this study we aimed to evaluate patterns of CGM use over 1 year following CGM initiation, and to investigate predictors of consistent and durable CGM use by investigating associations between baseline demographic, diabetes, and psychosocial characteristics and frequency of CGM use over the subsequent year.
Research Design and Methods
Participants and procedures
This study was a secondary analysis of a randomized controlled trial that evaluated the effectiveness of a family-focused behavioral teamwork intervention aimed at overcoming barriers to CGM use compared to standard CGM education alone. In the primary analysis,16 there was no significant difference in CGM use or glycemic outcomes between the intervention and control groups, so groups were analyzed together in this secondary analysis.
Eligibility criteria included age 8–17 years, T1D duration ≥1 year, HbA1c 6.5%–10%, daily insulin dose ≥0.5 U/kg, blood glucose monitoring frequency ≥4 times per day, and no consistent CGM use (6+ days per week) in the past 6 months. All subjects completed a 1-week run-in period of CGM use to confirm usability. A total of 120 youth with T1D were recruited and randomized. The protocol was approved by the Institutional Review Board. Parents/youth provided written informed consent/assent before beginning any study procedures.
All participants were provided with an approved Dexcom™ CGM system. From the start of the study in October 2011 through November 2012, participants were started on the Seven Plus Dexcom CGM system at enrollment (n = 82). Beginning in November 2012 when the Dexcom G4 Platinum CGM system had been approved by the Food and Drug Administration (FDA), all newly enrolled participants were started on G4 (n = 38). Of those using the Seven Plus device, participants who had completed their 6-month visit by November 2012 continued on the Seven Plus for the remainder of the year (n = 52); those who had not yet completed their 6-month visit by November 2012 were switched to the G4 at the next visit (n = 30).
Demographic and biomedical data were collected by chart review and interview. Baseline data included sex, age, age at diagnosis, race, and family factors (household income, parental marital status, and parental education). HbA1c was measured uniformly at quarterly visits using a laboratory method (Roche Integra) standardized to the Diabetes Control and Complications Trial (reference range 4%–6%).
CGM devices were downloaded at the 3, 6, 9, and 12 month visits and captured the 4 weeks of CGM use preceding each visit. For subjects who discontinued study participation before 12 months, CGM use was counted as zero for all subsequent visits. In light of prior work indicating that consistent (frequent) and durable (longitudinal) CGM use has the greatest impact on HbA1c,12 annualized CGM use (mean CGM use over the 12 months) was calculated and subjects were grouped based on the annualized CGM use: 0–2 days per week, 3–5 days per week, and 6–7 days per week. As those using CGM 3–5 days per week and 6–7 days per week had similar glycemic outcomes, they were grouped together for additional analyses.
Youth completed previously validated psychosocial surveys at CGM initiation (baseline) and after 6 and 12 months. Depressive symptoms were assessed using Center for Epidemiologic Studies Depression Scale for Children (CES-DC).17 The CES-DC is a 20-item widely used, self-administered survey that measures cognitive, affective, and behavioral symptoms of depression in youth aged 6–17 years. For each item, youth choose one of three statements that best describes their feelings over the past week. Total scores range from 0 to 60; higher scores indicate more depressive symptoms and scores ≥15 indicate clinical elevation. Diabetes burden was assessed with Problem Areas in Diabetes survey—Pediatric version (PAID-Peds), which is a 20-item measure of diabetes burden over the past month; scores range from 0 to 100 and higher scores indicate more burden.18 Generic and diabetes-specific QOL were assessed using the Pediatric Quality of Life Inventory (PedsQL) Generic Core Scales (23 items) and Diabetes Module (28 items) that evaluate QOL over the past month; scores range from 0 to 100 and higher scores indicate better QOL.19,20
Statistical analyses
Statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC). Demographic and clinical characteristics are described as means ± standard deviations or medians and interquartile ranges for continuous variables, and as frequencies or proportions for categorical variables. Comparisons between annualized groups of CGM use and analyses of trends over time were performed with analysis of variance, paired and unpaired t-tests, and Mantel–Haenszel Chi-square tests. P-values <0.05 were considered statistically significant.
Results
Baseline demographic and diabetes characteristics
Baseline characteristics are shown in Table 1. Youth (51% male and 95% white) had a mean age of 12.7 ± 2.7 years, mean diabetes duration of 6.1 ± 3.6 years, and mean HbA1c of 8.0 ± 0.8% with 27% at target HbA1c <7.5%. The majority (84%) used insulin pumps, and mean blood glucose monitoring frequency was 6.7 ± 2.4 times per day.
Table 1.
Baseline Characteristics (n = 120)
| Age (years) | 12.7 ± 2.7 |
| Diabetes duration (years) | 6.1 ± 3.6 |
| Sex, male | 51% |
| Race, white | 95% |
| BMI z-score (SDS) | 0.51 ± 0.97 |
| Insulin regimen, pump | 84% |
| Blood glucose monitoring frequency (times per day) | 6.7 ± 2.4 |
| HbA1c (%) | 8.0 ± 0.8 |
| HbA1c at target (<7.5%) | 27% |
| Family structure, two-parent | 93% |
| Household income, >$100K per year | 52% |
| Parental education, college or above | 73% |
All results are expressed as mean ± SD or %.
BMI, body mass index; HbA1c, glycated hemoglobin; SD, standard deviation; SDS, standard deviation score.
Frequency of CGM use over time
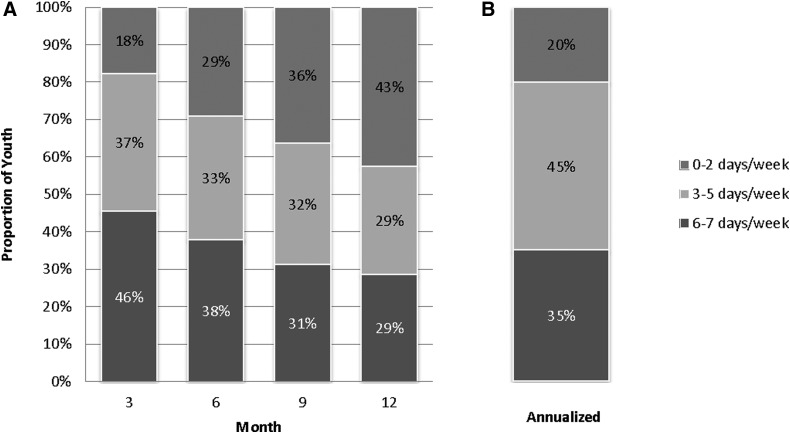
CGM use, assessed quarterly, declined over time. Distributions of CGM use in days per week at 3, 6, 9, and 12 months are shown in Figure 1. The proportion of participants using CGM 6–7 days per week decreased over time (46% at 3 months, 38% at 6 months, 31% at 9 months, and 29% at 12 months), while the proportion of youth wearing CGM 0–2 days per week increased (18% at 3 months, 29% at 6 months, 36% at 9 months, and 43% at 12 months). The majority of youth (59%) remained in the same CGM use category during the first and second 6 months of the study; 36% had a decrease in CGM use category and only 5% increased CGM use. With respect to annualized CGM use, 35% of youth (n = 42) used CGM 6–7 days per week, 45% (n = 54) used CGM 3–5 days per week, and 20% (n = 24) used CGM only 0–2 days per week. Among the three annualized CGM groups, there were no differences in baseline demographic and diabetes characteristics (Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/dia) except for a greater proportion of youth from families with a higher household income using CGM more frequently.
FIG. 1.
Frequency of CGM use by month (A), and annualized CGM use (B). CGM, continuous glucose monitoring.
Associations between CGM use and glycemic control
While baseline HbA1c for the sample was 8.0 ± 0.8% (27% at target HbA1c of <7.5%), mean HbA1c was slightly but significantly higher at 12 months (8.1 ± 0.9%, P = 0.04). The change in HbA1c was significantly related to mean annualized CGM use; those who used CGM only 0–2 days per week had the greatest deterioration in glycemic control (+0.4%, P = 0.04). Youth who used CGM 3–7 days per week over the 12 months had significantly lower HbA1c at months 9 and 12 than youth who used CGM 0–2 days per week over the 12 months (9 months: 7.9 ± 0.9% vs. 8.5 ± 1.1%, P = 0.006 and 12 months: 8.0 ± 0.9% vs. 8.5 ± 1.1%, P = 0.02) as shown in Table 2.
Table 2.
Mean Glycated Hemoglobin Over Time According to Annualized Continuous Glucose Monitoring Use
| Annualized CGM use | ||||
|---|---|---|---|---|
| 0–2 days per week (n = 24) | 3–5 days per week (n = 54) | 6–7 days per week (n = 42) | P-value, (t-test 0–2 vs. 3–7 days per week) | |
| Baseline | 8.1 ± 0.6 | 8.0 ± 0.8 | 7.9 ± 0.9 | 0.42 |
| 3 months | 8.0 ± 0.7 | 7.9 ± 0.9 | 7.7 ± 0.8 | 0.17 |
| 6 months | 8.2 ± 1.1 | 7.8 ± 0.8 | 7.8 ± 0.8 | 0.07 |
| 9 months | 8.5 ± 1.1 | 7.9 ± 1.0 | 7.8 ± 0.8 | 0.006 |
| 12 months | 8.5 ± 1.1 | 8.0 ± 1.0 | 8.0 ± 0.8 | 0.02 |
All results are expressed as mean ± SD.
Associations between baseline psychosocial characteristics and CGM use
Depressive symptoms
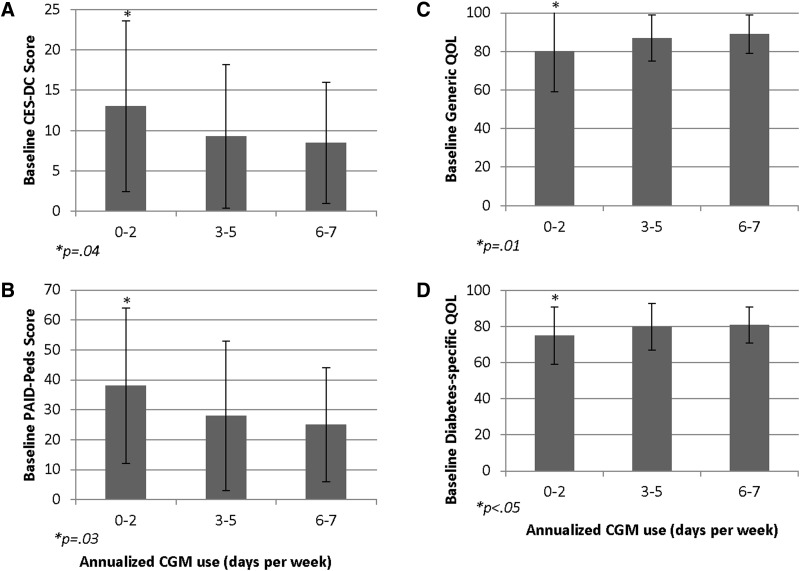
Baseline mean CES-DC score was 10 ± 9, and 22% of youth had an elevated score ≥15. Endorsement of higher baseline depressive symptoms was associated with lower annualized CGM use (r = −0.18, P < 0.05). Those using CGM infrequently (0–2 days per week) had significantly higher baseline CES-DC scores (13 ± 11) compared with those using CGM 3–7 days per week (9 ± 8, P < 0.05) (Fig. 2A). CES-DC scores did not change significantly over time (Supplementary Table S2).
FIG. 2.
Baseline depressive symptoms (A), diabetes burden (B), generic QOL (C), and diabetes-specific QOL (D) according to annualized CGM use. P-values reflect comparisons of 0–2 days per week versus 3–7 days per week. Error bars represent standard deviation. QOL, quality of life.
Burden and QOL
Those using CGM infrequently (0–2 days per week) reported significantly higher baseline diabetes burden and lower baseline generic and diabetes-specific QOL compared with those using CGM 3–7 days per week (PAID-Peds: 38 ± 26 vs. 27 ± 22, P = 0.03; generic QOL: 80 ± 21 vs. 88 ± 11, P = 0.01; and diabetes-specific QOL: 75 ± 16 vs. 81 ± 12, P < 0.05) (see Figure 2B–D). Youth report of diabetes burden and generic QOL remained stable over time while youth report of diabetes-specific QOL improved over time from baseline to 6 and 12 months (Supplementary Table S2).
Discussion
While some prior studies have shown that CGM use of 6 or more days per week is necessary for glycemic benefit,9,11–13 this study suggests potential for relative glycemic benefit (protection against deterioration in glycemic control) with CGM use of 3 or more days per week in pediatric patients with T1D. This may be promising news, particularly given that 80% of youth in this study used CGM at least 3 days per week over 1 year in this study. Indeed, it may be feasible to encourage skeptical young persons with T1D to maintain durable CGM use for at least 3 days per week.
Additionally, youth in this study were not burdened by CGM. Within any of the annualized CGM groups, psychosocial measures remained stable over time. That is to say that there was no increase in diabetes burden or depressive symptoms, and no decrease in generic or diabetes-specific QOL among those who wore CGM frequently over the year.
From a psychosocial perspective, those who used CGM only 0–2 days per week seem distinct from those who used CGM 3–7 days per week. Higher baseline depressive symptoms, higher baseline diabetes burden, and lower baseline generic and diabetes-specific QOL were associated with less frequent CGM use over 1 year. The association between depressive symptoms and CGM use was modest, and, in fact, some of the psychosocial characteristics that were measured in this study may represent overlapping constructs.21 Fisher et al.22,23 have demonstrated in adults with T1D that many of the symptoms that lead to diagnoses of depression may be more appropriately attributed to diabetes distress, and that both distress and depression may be linked to poorer diabetes management over time. In addition to an overlap between psychosocial constructs, there may be some degree of common determinants between baseline psychosocial factors and successful uptake of diabetes technology.24 Recognition of patients experiencing depressive symptoms, higher diabetes burden, and lower QOL may reflect a need for intervention before embarking on use of advanced diabetes technologies; furthermore, knowledge of baseline psychosocial factors may help clinicians predict which patients will need more ongoing support to maximize uptake and continued use.
This study has several limitations. First, as a clinical trial, the sample was a selected population willing to use CGM technology. In such a group of youth, it is unsurprising that a high proportion of participants used insulin pumps (84%) and also frequently checked their blood glucose levels (6.7 ± 2.4 times per day); likewise, the sample had a high proportion of youth (73%) with highly educated parents and most (93%) lived in two-parent households. These characteristics must be considered when extrapolating these findings to clinical populations. Further, in a clinical population, one might expect associations between psychosocial factors and CGM use to be even more pronounced. Second, the study utilized Dexcom CGM systems that were FDA-approved at the time of the study but that lacked some of the features of more recent devices (e.g., share function); more research is needed to determine whether CGM use would be improved uniformly in youth using newer devices, or whether groups with specific demographic, diabetes, or psychosocial characteristics might benefit disproportionately. Third, participants in this study were given all CGM supplies free of charge for the 12-month study period. In a clinical population, price and ability to obtain supplies might be a barrier that would reduce use. While we observed a difference in household income across groups of annualized CGM use, it is notable that there were no significant differences in family structure or parental education across groups; the difference in household income merits further investigation, and may be spurious. Finally, because 80% of participants used CGM 3–7 days per week compared with only 20% using CGM 0–2 days per week, there may have been insufficient power to find a difference between groups with regard to some baseline predictors of CGM use.
Although consistent (6 or more days per week) and durable (longitudinal) CGM use is desirable in youth with T1D, this is often difficult to achieve. In this study, a large proportion of youth exhibited moderate CGM use (3 or more days per week), and this moderate use was associated with a relative glycemic benefit, which is encouraging. Furthermore, attention to psychosocial parameters, such as depressive symptomatology and perceived burden, may offer some predictive value regarding the optimal timing to begin CGM in youth with T1D and what supplemental support/interventions would need to be in place to make for successful CGM use.
Supplementary Material
Acknowledgments
This research was supported by the National Institutes of Health under grants K12DK094721, T32DK007260, R01DK089349, P30DK036836, and K23HL125976; the Katherine Adler Astrove Youth Education Fund; the Maria Griffin Drury Pediatric Fund; and the Eleanor Chesterman Beatson Fund. The content is solely the responsibility of the authors and does not necessarily represent the official views of these organizations. The study sponsors were not involved in designing the study; collecting, analyzing, or interpreting the data; writing the article; or deciding to submit the article for publication. Portions of this article were presented as an abstract at the 77th Scientific Sessions of the American Diabetes Association, June 9–13, 2017, San Diego, CA.
Author Disclosure Statement
The authors report no potential conflicts of interest relevant to this research. None of the authors received any form of payment to produce the article.
References
- 1.Phillip M, Danne T, Shalitin S, et al. : Use of continuous glucose monitoring in children and adolescents. Pediatr Diabetes 2012;13:215–228 [DOI] [PubMed] [Google Scholar]
- 2.Tansey M, Laffel L, Cheng J, et al. : Satisfaction With continuous glucose monitoring in adults and youths With type 1 diabetes. Diabet Med 2011;28:1118–1122 [DOI] [PubMed] [Google Scholar]
- 3.Scaramuzza AE, Iafusco D, Rabbone I, et al. : Use of integrated real-time continuous glucose monitoring/insulin pump system in children and adolescents With type 1 diabetes: a 3-year follow-up study. Diabetes Technol Ther 2011;13:99–103 [DOI] [PubMed] [Google Scholar]
- 4.Tanenbaum ML, Hanes SJ, Miller KM, et al. : Diabetes device use in adults With type 1 diabetes: barriers to uptake and potential intervention targets. Diabetes Care 2017;40:181–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polonsky WH, Hessler D: What are the quality of life-related benefits and losses associated With real-time continuous glucose monitoring? A survey of current users. Diabetes Technol Ther 2013;15:295–301 [DOI] [PubMed] [Google Scholar]
- 6.Polonsky WH, Hessler D, Ruedy KJ, et al. : The impact of continuous glucose monitoring on markers of quality of life in adults With type 1 diabetes: further findings from the DIAMOND randomized clinical trial. Diabetes Care 2017;40:736–741 [DOI] [PubMed] [Google Scholar]
- 7.Deiss D, Bolinder J, Riveline JP, et al. : Improved glycemic control in poorly controlled patients With type 1 diabetes using real-time continuous glucose monitoring. Diabetes Care 2006;29:2730–2732 [DOI] [PubMed] [Google Scholar]
- 8.Pickup JC, Freeman SC, Sutton AJ: Glycaemic control in type 1 diabetes during real time continuous glucose monitoring compared With self monitoring of blood glucose: meta-analysis of randomised controlled trials using individual patient data. BMJ 2011;343:d3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giani E, Snelgrove R, Volkening LK, Laffel LM: Continuous glucose monitoring (CGM) adherence in youth With type 1 diabetes: associations With biomedical and psychosocial variables. J Diabetes Sci Technol 2017;11:476–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beck RW, Riddlesworth T, Ruedy K, et al. : Effect of continuous glucose monitoring on glycemic control in adults With type 1 diabetes using insulin injections: the DIAMOND randomized clinical trial. JAMA 2017;317:371–378 [DOI] [PubMed] [Google Scholar]
- 11.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group: Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med 2008;359:1464–1476 [DOI] [PubMed] [Google Scholar]
- 12.Chase HP, Beck RW, Xing D, et al. : Continuous glucose monitoring in youth With type 1 diabetes: 12-month follow-up of the Juvenile Diabetes Research Foundation continuous glucose monitoring randomized trial. Diabetes Technol Ther 2010;12:507–515 [DOI] [PubMed] [Google Scholar]
- 13.Wong JC, Foster NC, Maahs DM, et al. : Real-time continuous glucose monitoring among participants in the T1D exchange clinic registry. Diabetes Care 2014;37:2702–2709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller KM, Foster NC, Beck RW, et al. : Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D exchange clinic registry. Diabetes Care 2015;38:971–978 [DOI] [PubMed] [Google Scholar]
- 15.Van Name MA, Hilliard ME, Boyle CT, et al. : Nighttime is the Worst time: parental fear of hypoglycemia in young children With type 1 diabetes. Pediatr Diabetes 2018;19:114–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laffel LM, Volkening LK, McMullen WJ, et al. : Improved CGM performance predicts CGM use and outcomes in pediatric patients With T1D [Abstract]. Diabetes 2016;65:LB44 [Google Scholar]
- 17.Fendrich M, Weissman MM, Warner V: Screening for depressive disorder in children and adolescents: validating the Center for Epidemiologic Studies Depression Scale for Children. Am J Epidemiol 1990;131:538–551 [DOI] [PubMed] [Google Scholar]
- 18.Markowitz JT, Volkening LK, Butler DA, Laffel LM: Youth-perceived burden of type 1 diabetes: problem areas in diabetes survey-pediatric version (PAID-Peds). J Diabetes Sci Technol 2015;9:1080–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Varni JW, Burwinkle TM, Jacobs JR, et al. : The PedsQL in type 1 and type 2 diabetes: reliability and validity of the Pediatric Quality of Life Inventory Generic Core Scales and type 1 diabetes module. Diabetes Care 2003;26:631–637 [DOI] [PubMed] [Google Scholar]
- 20.Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care 2001;39:800–812 [DOI] [PubMed] [Google Scholar]
- 21.Hagger V, Hendrieckx C, Cameron F, et al. : Diabetes distress is more strongly associated With HbA1c than depressive symptoms in adolescents With type 1 diabetes: results from diabetes MILES youth-Australia. Pediatr Diabetes 2018. [Epub ahead of print]; doi: 10.1111/pedi.12641 [DOI] [PubMed] [Google Scholar]
- 22.Fisher L, Gonzalez JS, Polonsky WH: The confusing tale of depression and distress in patients With diabetes: a call for greater clarity and precision. Diabet Med 2014;31:764–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fisher L, Hessler DM, Polonsky WH, et al. : Prevalence of depression in type 1 diabetes and the problem of over-diagnosis. Diabet Med 2016;33:1590–1597 [DOI] [PubMed] [Google Scholar]
- 24.Hessler DM, Fisher L, Polonsky WH, et al. : Diabetes distress is linked With Worsening diabetes management over time in adults With type 1 diabetes. Diabet Med 2017;34:1228–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.