Abstract
Background: To examine trimester-specific associations among glycemic variability, fetal growth, and birthweight in pregnancies with type 1 diabetes mellitus (Type 1 DM).
Methods: In this retrospective cohort study of 41 pregnant women with Type 1 DM, we used continuous glucose monitoring (CGM) data to calculate glycemic variability (coefficient of variation of glucose) over a 7-day interval in each trimester. Clinical data, including fetal biometry, birthweight, and perinatal complications, were extracted from medical records.
Results: Women maintained good glycemic control during pregnancy, with mean HbA1c in the first, second, and third trimester 6.5%, 6.1%, and 6.4%, respectively. Sixty-three percent of infants were large for gestational age (LGA). Estimated fetal weight percentile (EFW%ile) and abdominal circumference percentile (AC%ile) increased during pregnancy, consistent with accelerated prenatal growth. Correlations between trimester-specific glycemic variability and EFW, AC, and birthweight were not statistically significant. After maternal age adjustment, glycemic variability was not associated with birthweight for any trimester (adj. β for first trimester: −38.46, 95% CI: −98.58 to 21.66; adj. β for second trimester: −12.20, 95% CI: −51.47 to 27.06; adj. β for third trimester: −26.26, 95% CI: −79.52 to 27.00).
Conclusions: The occurrence of LGA remains very high in contemporary U.S. women with Type 1 DM, despite the use of CGM and overall good glycemic control. Neither HbA1c nor glycemic variability predicted fetal overgrowth or birthweight. Since LGA is a key driver of maternal and newborn complications in pregnancies with Type 1 DM, our data emphasize the importance of investigating both glucose-dependent and glucose-independent underlying mechanisms.
Keywords: : Pregnancy, Type 1 diabetes, Continuous glucose monitoring, Fetal growth, Birthweight
Introduction
Women with Type 1 diabetes mellitus (Type 1 DM) are at high risk for obstetric and perinatal complications; excessive fetal growth is a major driver of adverse pregnancy outcomes.1,2 “Large for gestational age” (LGA) neonates, defined as birthweight >90th percentile for gestational age and sex, are at increased risk for complications both in the perinatal period and in later life, as LGA is a risk factor for future obesity and type 2 diabetes.3–5 Fetal exposure to maternal hyperglycemia is thought to be the major driver of fetal overgrowth in Type 1 DM, and the overarching goal of prenatal care in women with Type 1 DM is to achieve tight glycemic control through intensive nutritional and insulin therapy.
However, maternal glycated hemoglobin (HbA1c) may not adequately reflect fetal glycemic exposure, as it does not assess postprandial glucose rise or capture time spent above the normal glucose range. Moreover, shifts in red blood cell production during pregnancy reduce the accuracy of HbA1c as a measure of mean glucose levels.6 The emergence of continuous glucose monitoring (CGM) technology now allows for a more precise understanding of how glycemic patterns, such as mean glucose and variation in glucose levels (glycemic variability), may influence pregnancy outcomes. Law et al. recently reported that in pregnant women with Type 1 and Type 2 DM, LGA was associated with lower mean glucose and less glycemic variability in the first trimester, and higher mean glucose and more variable second and third trimester glucose levels.7 Other groups have similarly shown that glycemic variability, especially during late pregnancy, may increase risk of LGA.8,9 A recent randomized trial by Feig et al. demonstrated improved neonatal outcomes in women with Type 1 DM who used CGM during pregnancy, including a lower incidence of LGA infants and a decrease in neonatal hypoglycemia.10
It is unclear, however, to what extent CGM-derived measures of glycemic variability may predict LGA in a clinical setting. Moreover, no previous studies have examined how glycemic variability may affect fetal growth patterns. We hypothesized that higher glycemic variability, as measured by trimester-specific CGM data, would be associated with fetal overgrowth, assessed by estimated fetal weight (EFW) and fetal abdominal circumference percentiles (AC%ile) on antenatal growth ultrasounds, and with higher birthweight for gestational age.
Methods
Participants
This was a retrospective cohort study of women with Type 1 DM who used CGM and delivered between January 2012 and December 2015 at Beth Israel Deaconess Medical Center (Boston, MA). Two hundred seventy-six women with Type 1 DM delivered during this time period, of which 17% (n = 47) had both CGM and birthweight data available. All women received multispecialty care, including endocrinology, nutrition, and maternal–fetal medicine; glucose targets were between 60–99 mg/dL fasting and 100–129 mg/dL 1-h postmeal, consistent with current guidelines.11 Exclusion criteria were CGM use in a previous pregnancy (n = 3), multiple gestations (n = 1), delivered <28 weeks' gestation (n = 1), and major fetal anomalies (n = 1); for a data set of n = 41. Maternal demographic and clinical data during the index pregnancy were collected from medical records.
Continuous glucose monitoring
We reviewed CGM data from each trimester of pregnancy for ≥7 consecutive days (median 7 days, maximum 30 days). Women used a Medtronic Enlite (Medtronic-MiniMed, Northridge, CA) or a Dexcom 7 or G4 (Dexcom, Inc., San Diego, CA) CGM system. Glycemic variability was defined as the coefficient of variation (standard deviation/mean) of glucose for each data period. We chose to study coefficient of variation as a surrogate of glycemic variability because mean glucose and standard deviation were reported consistently across different CGM systems, whereas the time in range is not consistent across systems; moreover, the coefficient of variation is readily accessible to clinicians.
Fetal growth
We reviewed fetal growth ultrasounds from medical records. EFW in grams and EFW percentile (EFW%ile) for gestational age were computed using the Williams formula.12 AC percentile (AC%ile) for gestational age was computed using the Hadlock formula.13 Birthweight in grams was collected from medical records, and LGA was defined as birthweight >90%ile.14,15
Statistical analysis
Descriptive statistics were calculated for all continuous and categorical variables. We conducted bivariate analysis to evaluate gestational age by EFW%ile and AC%ile. Pearson correlation coefficients were computed to examine trimester-specific associations between glycemic variability and birthweight. A two-tailed P-value of <0.05 denoted statistical significance.
We used maternal age-adjusted linear mixed models to prospectively assess trimester-specific associations between glycemic variability in first, second, and third trimesters and subsequent EFW%ile and AC%ile. We used linear regression to evaluate the association between trimester-specific glycemic variability and birthweight adjusted for maternal age. As a secondary analysis, we also assessed trimester-specific mean HbA1c and CGM glucose levels with EFW%ile and AC%ile using Pearson correlation coefficients and age-adjusted linear mixed models. In addition, we evaluated trimester-specific mean HbA1c and CGM glucose levels and birthweight by calculating Pearson correlations and linear regression adjusting for maternal age. Analyses were conducted with R 3.3.1.
Results
Maternal and infant data
Clinical characteristics, CGM data, and pregnancy outcomes of the 41 women are displayed in Table 1. Twenty-two (54%) of women had CGM data available in the first trimester (<13 weeks), 36 (88%) in the second trimester (13–26 weeks), and 35 (85%) in the third trimester (≥27 weeks). Twelve women (29%) used a Medtronic Enlite CGM system, 2 (5%) used a Dexcom 7, and 27 (66%) used a Dexcom G4. Forty (98%) used an insulin pump and one (2%) used multiple daily injections. Mean HbA1c in each trimester was 6.5% (47 mmol/mol), 6.1% (43 mmol/mol), and 6.4% (46 mmol/mol), while mean CGM glucose levels for first, second, and third trimesters were 121.5 mg/dL (6.75 mmol/L), 130.7 mg/dL (7.26 mmol/L), and 121.9 mg/dL (6.77 mmol/L), respectively. Mean CGM glucose levels for 75th and 90th percentiles per trimester were also recorded; the 90th percentile was >140 mg/dL in all trimesters. Thirty-three women (80%) of women delivered via cesarean. Of these, 10 were repeat cesarean deliveries and 23 were primary cesarean deliveries, of which 9 were for diabetes-related indications (7 for suspected macrosomia and 2 for retinopathy). The rest were for usual obstetric indications. Sixty-three percent of infants were LGA. Mean birthweight was 4007 ± 735 g and mean birthweight percentile was 93rd ±15th (median 99th percentile interquartile range 92nd–100th). Fifteen (37%) of infants were admitted to the neonatal intensive care unit with a primary diagnosis of hypoglycemia.
Table 1.
Maternal and Infant Characteristics, Continuous Glucose Monitoring Data, and Pregnancy Outcomes
| Maternal characteristics | n = 41 |
|---|---|
| Age (years) | 32.5 ± 3.2 |
| Race | |
| White | 37 (90.2) |
| Non-white | 4 (9.8) |
| Body mass index (kg/m2) | 28.3 ± 5.6 |
| Gestational weight gain (kg) | 13.4 ± 5.5 |
| Parity | 1 ± 1 |
| Years with Type 1 DM | 21.4 ± 7.9 |
| Retinopathy | |
| Yes | 27 (66) |
| No | 14 (34) |
| Nephropathy | |
| Yes | 1 (2.4) |
| No | 40 (97.6) |
| Chronic hypertension | |
| Yes | 7 (17) |
| No | 34 (83) |
| Hemoglobin A1c,% (mmol/mol) | |
| First trimester | 6.5 ± 0.7 (47 ± 8.1) |
| Second trimester | 6.1 ± 0.5 (43 ± 5.5) |
| Third trimester | 6.4 ± 0.6 (46 ± 6.7) |
| Mean CGM glucose, mg/dL (mmol/L) | |
| First trimester | 121.5 ± 19.0 (6.75 ± 1.1) |
| Second trimester | 130.7 ± 20.7 (7.26 ± 1.2) |
| Third trimester | 121.9 ± 19.0 (6.77 ± 1.1) |
| 75th percentile CGM glucose, mg/dL (mmol/L) | |
| First trimester | 131.8 (7.31) |
| Second trimester | 143.8 (7.98) |
| Third trimester | 132.0 (7.33) |
| 90th percentile CGM glucose, mg/dL (mmol/L) | |
| First trimester | 145.7 (8.09) |
| Second trimester | 153.5 (8.52) |
| Third trimester | 144.6 (8.03) |
| Glycemic variability CGM glucose (coefficient of variation, %) | |
| First trimester | 35.5 ± 6.2 |
| Second trimester | 35.5 ± 6.6 |
| Third trimester | 33.3 ± 5.3 |
| Gestational age at delivery (weeks) | 38.0 ± 1.9 |
| Preeclampsia | |
| Yes | 10 (24) |
| No | 31 (76) |
| Cesarean delivery | |
| Yes | 33 (80) |
| No | 8 (20) |
| Infant characteristics | |
| Birthweight (g) | 4007 ± 735 |
| Birthweight percentile, mean | 93 ± 15 |
| LGA | |
| Yes | 26 (63) |
| No | 15 (37) |
| Neonatal intensive care unit admission for hypoglycemia | |
| Yes | 15 (37) |
| No | 26 (63) |
All data are n (%) or mean ± SD unless otherwise specified.
CGM, continuous glucose monitoring; LGA, large for gestational age; Type 1 DM, type 1 diabetes mellitus.
Glycemic variability and fetal growth
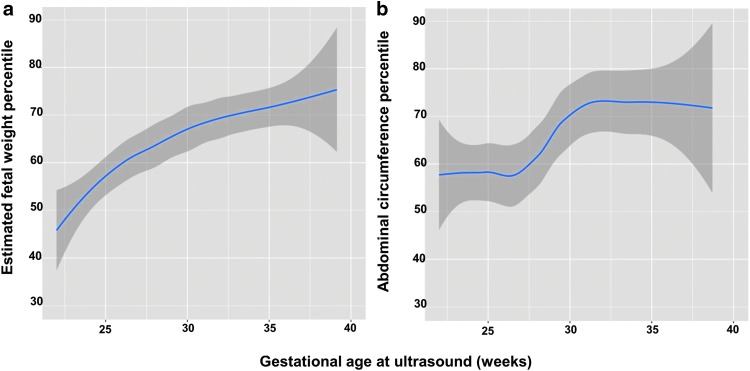
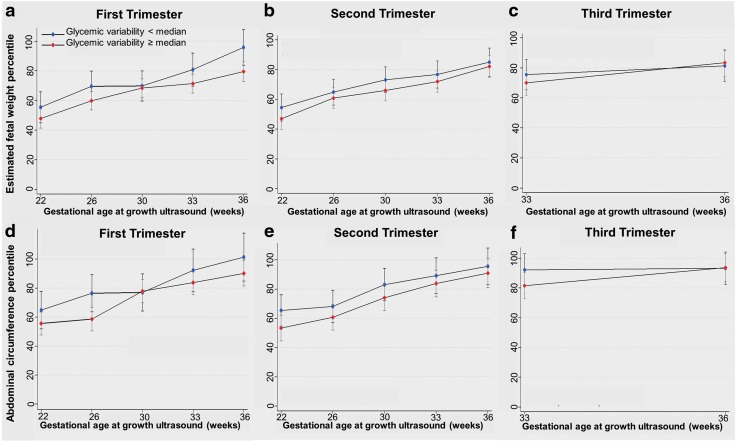
Based on analysis of serial prenatal ultrasounds, fetal growth parameters (i.e., EFW%ile and AC%ile) increased during pregnancy, consistent with accelerated prenatal growth (Fig. 1). There was no association between first, second, or third trimester maternal age-adjusted continuous glycemic variability and EFW%ile or AC%ile, which remained unchanged when glycemic variability was categorized as ≥ and < median levels (Fig. 2). In our secondary data analysis, there was no association between HbA1c and EFW%ile or AC%ile for any trimester. There was a significant association between first trimester mean CGM glucose levels and EFW%ile (P = 0.03); however, there was no association between second and third trimester mean CGM glucose levels and EFW%ile or AC%ile for any trimester.
FIG. 1.
Fetal growth parameters by gestational age. (a) Change in EFW%ile during pregnancy. (b) Change in AC%ile during pregnancy. AC%ile, abdominal circumference percentile; EFW%ile, estimated fetal weight percentile.
FIG. 2.
Trimester-specific glycemic variability and fetal growth. (a) First trimester glycemic variability and change in EFW%ile during pregnancy, (b) Second trimester glycemic variability and change in EFW%ile during pregnancy, (c) Third trimester glycemic variability and change in EFW%ile during pregnancy, (d) First trimester glycemic variability and change in AC%ile during pregnancy, (e) Second trimester glycemic variability and change in AC%ile during pregnancy, (f) Third trimester glycemic variability and change in AC%ile during pregnancy.
Glycemic variability and birthweight
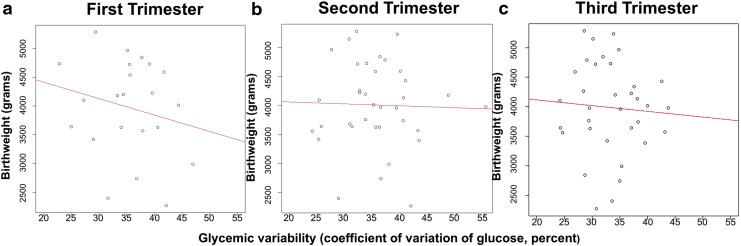
Pearson correlation coefficients between glycemic variability and birthweight per trimester were r = 0.08 (P = 0.71), r = 0.01 (P = 0.93), and r = 0.05 (P = 0.76), respectively (Fig. 3). Even after maternal age-adjustment, glycemic variability was not associated with birthweight for any trimester (adj. β for first trimester: −38.46, 95% CI: −98.58 to 21.66; adj. β for second trimester: −12.20, 95% CI: −51.47 to 27.06; adj. β for third trimester: −26.26, 95% CI: −79.52 to 27.00).
FIG. 3.
Trimester-specific glycemic variability and birthweight. (a) Association between first trimester glycemic variability and birthweight. (b) Association between second trimester glycemic variability and birthweight. (c) Association between third trimester glycemic variability and birthweight.
With respect to secondary data analyses, correlations between trimester-specific HbA1c and birthweight were not statistically significant (r = 0.05, P = 0.72; r = 0.10, P = 0.51; r = 0.78, P = 0.78). Trimester-specific mean CGM glucose levels and birthweight also showed no correlation (r = 0.37, P = 0.07; r = 0.08, P = 0.59; r = 0.19, P = 0.25) (Fig. 3). After maternal age-adjustment, no associations existed for neither trimester-specific HbA1c and birthweight nor trimester-specific CGM glucose levels and birthweight.
Discussion
We report, in a contemporary cohort of U.S. women, that the risk of high birthweight and LGA remains extremely high in pregnancies complicated by Type 1 DM, with mean birthweight >4000 g, despite overall good glycemic control, CGM use, and multispecialty care. Our findings are concerning given the strong associations between LGA and risk for perinatal complications,16 neonatal intensive care unit admission,17 and complications during later life, including obesity, insulin resistance, and diabetes3,4 Surprisingly, we did not observe trimester-specific associations among glycemic variability, mean glucose, or HbA1c and birthweight nor with prenatal ultrasound-derived measures of fetal growth, with the exception of an association between higher first trimester mean glucose and higher EFW%ile. Our findings contrast with those of Law et al.7; this difference may have arisen because we chose to use coefficient of variation as a surrogate for glycemic variability, whereas Law et al. relied on a variety of other CGM-derived measures.
To our knowledge, we are the first to examine detailed prenatal ultrasound data in pregnancies complicated by Type 1 DM. Our results suggest that fetal overgrowth may emerge earlier in pregnancy than previous reports. Surprisingly, the good glycemic control attained in our cohort did not prevent fetal growth acceleration, which highlights the importance of identifying additional strategies to prevent this complication. Accelerated prenatal growth has been reported previously in pregnancies complicated by diabetes, but not specifically in pregnancies with Type 1 DM. Landon et al. reported an accelerated fetal AC by 32 weeks' gestation in LGA infants whose mothers had diabetes during pregnancy.18 Langer et al. described two patterns of accelerated growth; “early” (EFW >90%ile at 30 weeks' gestation) and “late” (36 weeks' gestation) in 522 women with diabetes in pregnancy. However, of the 81 women with insulin-dependent diabetes, only 11 infants were LGA.19 Our observed high incidence of LGA is comparable to the CGM arm of the recently published Feig et al. randomized controlled trial; including LGA (63% vs. 53%), and neonatal intensive care unit admission (37% vs. 27%). Although the Feig et al. trial demonstrated that CGM improved neonatal outcomes, over half of infants in the CGM arm were LGA.10 Similarly, Persson et al. reported a 47% rate of LGA in a population-based cohort of 3705 infants born to mothers with Type 1 DM in Sweden,2 while a smaller study (n = 221) by Ladfors et al. reported a 50% LGA prevalence.20 Taken together, these data indicate that the prevalence of fetal overgrowth in Type 1 DM remains alarmingly high, even with modern diabetes management.
Although maternal hyperglycemia is a key pathway associated with fetal overgrowth,21–23 factors other than glycemic control may contribute to LGA. We previously reported that third trimester maternal placental growth factor levels were predictive of birthweight, independent of HbA1c.24 These data, together with the low rate of diabetes complications in our study population, point to the possibility that this contemporary group of women with Type 1 DM has better vascular function and placental blood flow than historical controls. Others have reported that Type 1 DM is associated with increased placental weight, altered placental structure, altered expression of vascular endothelial growth factor and other angiogenic factors, and changes in the placental growth hormone-insulin-like growth factor axis, which further supports the possibility that differences in placental function could contribute to macrosomia risk.25–28 Other mechanisms for fetal overgrowth may be related to the rising prevalence of obesity in the Type 1 DM population.29,30 Indeed, the mean prepregnancy body mass index (BMI) in our study population was 28.3 kg/m2, and 68% of women were overweight or obese (BMI ≥25 kg/m2) at the first prenatal visit. Obesity may cause insulin resistance,31 altered levels of adipokines (e.g., adiponectin, leptin),32–34 altered appetite regulating factors such as ghrelin,35 inflammation,36 and differences in placental nutrient transport,37 each of which is associated with fetal growth.
We recognize some limitations to our study. First, this was a clinically based observational study, in which we relied on coefficient of variation as a measure of glycemic variability, since other measures (e.g., mean amplitude of glycemic excursion (MAGE, time within target range, etc.) were not accessible from the clinical CGM data. We acknowledge that use of coefficient of variation limits our ability to directly compare our results to some other studies. However, as a metric that can be easily accessed from CGM download data in clinical settings, it is important to document the utility (or lack thereof) of coefficient of variation (CV) as predictor of LGA in Type 1 DM pregnancies. The use of clinical CGM downloads also limited our ability to examine the effects of specific glucose thresholds; this will be an important area for future study. Another limitation is that women did not use the same CGM brand, which might have affected glycemic variability measurements. Previous studies have shown that the Dexcom G4 CGM system is more accurate than the Medtronic Enlite, including in the hypoglycemic range.38,39 Our small population limited our ability to fully adjust for potential confounders, including maternal obesity; 68% of women were overweight or obese (BMI ≥25 kg/m2) at the first prenatal visit. Future studies will need to account for these factors to better assess these associations. The majority of women were white, age ≥30, and college educated, which may potentially limit generalizability to other populations. Despite these limitations, our study has several strengths, including assessment of glycemic variability in each trimester to evaluate trimester-specific associations with birthweight. Our analysis of the timing of accelerated growth across AC%ile and EFW%ile may help predict when fetal overgrowth starts to occur, which may identify important therapeutic windows for future intervention studies.
In conclusion, glycemic variability based on CGM data did not predict birthweight or fetal overgrowth. Mean birthweight and LGA prevalence were extremely high despite use of CGM technology and multispecialty care with intensive insulin management. As LGA is a key driver of maternal and newborn complications in pregnancies with Type 1 DM, our data indicate the urgency of investigating the underlying mechanisms.
Acknowledgments
This study was funded by the National Institute of Environmental Health Sciences, with the following grant numbers: T32ES007069 and R01ES026166.
Authors' Contributions
B.M.M. collected the data and wrote the article. N.N. analyzed and interpreted the data, and reviewed/edited the article. T.J.T. analyzed and interpreted the data, and reviewed/edited the article. E.I. contributed to the introduction and discussion sections of the article and reviewed/edited the article. T.T. collected the data and reviewed/edited the article. A.C. collected the data and reviewed/edited the article. C.W. collected the data and reviewed/edited the article. K.E.O. designed the study and reviewed/edited the article. F.M.B. designed the study and reviewed/edited the article. B.M.M., K.E.O., and F.M.B. are the guarantors of this work and had full access to all of the data (B.M.M. and K.E.O. to the Beth Israel Deaconess Medical Center data, and F.M.B. to the Joslin Diabetes Center data) and take responsibility for the integrity of the data and the accuracy of the data analysis.
Author Disclosure Statement
There are no reported potential conflicts of interest relevant to this article for any of the authors. No competing financial interests exist.
References
- 1.Evers IM, de Valk HW, Mol BW, et al. : Macrosomia despite good glycaemic control in Type I diabetic pregnancy; results of a nationwide study in The Netherlands. Diabetologia 2002;45:1484–1489 [DOI] [PubMed] [Google Scholar]
- 2.Persson M, Pasupathy D, Hanson U, et al. : Birth size distribution in 3,705 infants born to mothers with type 1 diabetes: a population-based study. Diabetes Care 2011;34:1145–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sørensen HT, Sabroe S, Rothman KJ, et al. : Relation between weight and length at birth and body mass index in young adulthood: cohort study. BMJ 1997;315:1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiavaroli V, Giannini C, D'Adamo E, et al. : Insulin resistance and oxidative stress in children born small and large for gestational age. Pediatrics 2009;124:695–702 [DOI] [PubMed] [Google Scholar]
- 5.Kuchlbauer V, Vogel M, Gausche R, et al. : High birth weights but not excessive weight gain prior to manifestation are related to earlier onset of diabetes in childhood: “accelerator hypothesis” revisited. Pediatr Diabetes 2014;15:428–435 [DOI] [PubMed] [Google Scholar]
- 6.Law GR, Gilthorpe MS, Secher AL, et al. : Translating HbA1c measurements into estimated average glucose values in pregnant women with diabetes. Diabetologia 2017;60:618–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Law GR, Ellison GT, Secher AL, et al. : Analysis of CGM in pregnant women with diabetes: distinct temporal patterns of glucose associated with LGA infants. Diabetes Care 2015;38:1319–1325 [DOI] [PubMed] [Google Scholar]
- 8.Gupta R, Khoury J, Altaye M, et al. : Glycemic excursions in type 1 diabetes in pregnancy: a semiparametric statistical approach to identify sensitive time points during gestation. J Diabetes Res 2017;2017:2852913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McGrath RT, Glastras SJ, Seeho SK, et al. : Association between glycemic variability, HbA1c, and large-for-gestational-age neonates in women with type 1 diabetes. Diabetes Care 2017;40:e98–e100 [DOI] [PubMed] [Google Scholar]
- 10.Feig DS, Donovan LE, Corcoy R, et al. : CONCEPTT Collaborative Group. Continuous glucose monitoring in pregnant women with type 1 diabetes (CONCEPTT): a multicentre international randomised controlled trial. Lancet 2017;pii: [Epub ahead of print]; DOI: 10.1016/S0140-673632400-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kitzmiller JL, Block JM, Brown FM, et al. : Managing preexisting diabetes for pregnancy. Diabetes Care 2008;31:1060–1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams RL, Creasy RK, Cunningham GC, et al. : Fetal growth and perinatal viability in California. Obstet Gynecol 1982;59:624–632 [PubMed] [Google Scholar]
- 13.Hadlock FP, Harrist RB, Sharman RS, et al. : Estimation of fetal weight with the use of head, body and femur measurements—a prospective study. Am J Obstet Gynecol 1985;151:333–337 [DOI] [PubMed] [Google Scholar]
- 14.WHO Child Growth Standards: 2006 World Health Organization. https://www.cdc.gov/growthcharts/who_charts.htm (accessed March1, 2018)
- 15.Oken E, Kleinman KP, Rich-Edwards J, et al. : A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr 2003;3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Das S, Irigoyen M, Patterson MB, et al. : Neonatal outcomes of macrosomic births in diabetic and non-diabetic women. Arch Dis Child Fetal Neonatal Ed 2009;94:F419–F422 [DOI] [PubMed] [Google Scholar]
- 17.Gillean JR, Coonrod DV, Russ R, et al. : Big infants in the neonatal intensive care unit. Am J Obstet Gynecol 2005;192:1948–1953 [DOI] [PubMed] [Google Scholar]
- 18.Landon MB, Mintz MC, Gabbe SG: Sonographic evaluation of fetal abdominal growth: predictor of the large-for-gestational-age infant in pregnancies complicated by diabetes mellitus. Am J Obstet Gynecol 1989;160:115–121 [DOI] [PubMed] [Google Scholar]
- 19.Langer O, Kozlowski S, Brustman L: Abnormal growth patterns in diabetes in pregnancy: a longitudinal study. Isr J Med Sci 1991;27:516–523 [PubMed] [Google Scholar]
- 20.Ladfors L, Shaat N, Wiberg N, et al. : Fetal overgrowth in women with type 1 and type 2 diabetes mellitus. PLoS One 2017;12:e0187917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pedersen J: Weight and length at birth of infants of diabetic mothers. Acta Endocrinol (Copenh) 1954;16:330–342 [DOI] [PubMed] [Google Scholar]
- 22.Landon MB, Gabbe SG, Piana R, et al. : Neonatal morbidity in pregnancy complicated by diabetes mellitus: predictive value of maternal glycemic profiles. Am J Obstet Gynecol 1987;156:1089–1095 [DOI] [PubMed] [Google Scholar]
- 23.’Jovanic-Peterson L, Peterson CM, Reed GF, et al. : Maternal postprandial glucose levels and infant birth weight: the Diabetes in Early Pregnancy Study. The National Institute of Child Health and Human Development: Diabetes in Early Pregnancy Study. Am J Obstet Gynecol 1991;164 (1 Pt 1):103–111 [DOI] [PubMed] [Google Scholar]
- 24.James-Todd T, Cohen A, Wenger J, et al. : Time-specific placental growth factor (PIGF) across pregnancy and infant birth weight in women with preexisting diabetes. Hypertens Pregnancy 2016;35:436–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nelson SM, Coan PM, Burton GJ, et al. : Placental structure in type 1 diabetes: relation to fetal insulin, leptin and IGF-1. Diabetes 2009;58:2634–2641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iciek R, Wender-Ozegowska E, Mikolajczak P, et al. : Placental vascular endothelial growth factor expression in pregnancies complicated by type 1 diabetes. J Physiol Pharmacol 2014;65:577–583 [PubMed] [Google Scholar]
- 27.Dubova EA, Pavlov KA, Esayan RM, et al. : Vascular endothelial growth factor and its receptors in the placenta of women with type 1 diabetes mellitus. Bull Exp Biol Med 2012;152:367–370 [DOI] [PubMed] [Google Scholar]
- 28.Higgins MF, Russell NE, Crossey PA, et al. : Maternal and fetal placental growth hormone and IGF axis in type 1 diabetic pregnancy. PLoS One 2012;7:e29164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Persson M, Pasupathy D, Hanson U, et al. : Pre-pregnancy body mass index and the risk of adverse outcomes in type 1 diabetic pregnancies: a population-based cohort study. BMJ Open 2012;2:e000601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nielson GL, Dethlefsen C, Møller M, et al. : Maternal glycated haemoglobin, pre-gestational weight, pregnancy weight gain and risk of large-for-gestational-age babies: a Danish cohort study of 209 singleton Type 1 diabetic pregnancies. Diabet Med 2007;24:384–387 [DOI] [PubMed] [Google Scholar]
- 31.Sandler V, Reisetter AC, Bain JR, et al. : HAPO Study Cooperative Research Group. Associations of maternal BMI and insulin resistance with the maternal metabolome and newborn outcomes. Diabetologia 2017;60:518–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lekva T, Roland MCP, Michelsen AE, et al. : Large reduction in adiponectin during pregnancy is associated with large-for-gestational-age newborns. J Clin Endocrinol Metab 2017;102:2552–2559 [DOI] [PubMed] [Google Scholar]
- 33.Lazo-de-la-Vega-Monroy ML, González-Domínguez MI, Zaina S, et al. : Leptin and its receptors in human placenta of small, adequate, and large for gestational age newborns. Horm Metab Res 2017;49:350–358 [DOI] [PubMed] [Google Scholar]
- 34.Iciek R, Wender-Ozegowska E, Zawiejska A, et al. : Placental leptin and its receptor genes expression in pregnancies complicated by type 1 diabetes. J Physiol Pharmacol 2013;64:579–585 [PubMed] [Google Scholar]
- 35.Kos K, Syn WK, Lewandowski KC, et al. : Comparison of maternal ghrelin and leptin in healthy mothers and mothers with Type 1 diabetes. Diabet Med 2008;25:1400–1405 [DOI] [PubMed] [Google Scholar]
- 36.Nelson SM, Sattar N, Freeman DJ, et al. : Inflammation and endothelial activation is evident at birth in offspring of mothers with type 1 diabetes. Diabetes 2007;56:2697–2704 [DOI] [PubMed] [Google Scholar]
- 37.Ditchfield AM, Desforges M, Mills TA, et al. : Maternal obesity is associated with a reduction in placental taurine transporter activity. Int J Obes (Lond) 2015;39:557–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matuleviciene V, Joseph JI, Andelin M, et al. : A clinical trial of the accuracy and treatment experience of the Dexcom G4 sensor (Dexcom G4 system) and Enlite sensor (guardian real-time system) tested simultaneously in ambulatory patients with type 1 diabetes. Diabetes Technol Ther 2014;16:759–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kropff J, Bruttomesso D, Doll W, et al. : Accuracy of two continuous glucose monitoring systems: a head-to-head comparison under clinical research centre and daily life conditions. Diabetes Obes Metab 2015;17:343–349 [DOI] [PMC free article] [PubMed] [Google Scholar]