Abstract
Preconception behaviors and experiences of mothers and fathers can affect future offspring. Recently, our laboratory showed that alcohol-naive offspring of parents who were exposed to repeated binge alcohol during adolescence showed altered DNA methylation patterns in the hypothalamus, a brain region involved in regulation of pubertal development, stress, and behavior. These observations have potentially far-reaching consequences for human health, as more than 4.6 million Americans under the age of 21 years report engaging in the rapid intoxication behavior of binge-pattern alcohol (EtOH) drinking. Therefore, we tested the hypothesis that offspring of binge EtOH‒exposed parents would have altered hypothalamic function manifested phenotypically as improper pubertal development, impaired socialization, and dysregulated stress response. In addition, we tested the hypothesis that parental EtOH exposure would confer adaptive protection from the negative effects of EtOH when offspring were themselves exposed to EtOH. Rats received EtOH via oral gavage once daily for 6 days at both early [postnatal day (PND) 37] and late puberty (PND 67). Animals were paired (EtOH-EtOH, vehicle-vehicle) for mating 24 hours after the last EtOH dose. After weaning, offspring were randomized to vehicle treatment to assess changes in normal development or to EtOH treatment to assess the effect of parental EtOH exposure on offspring response to this treatment. We found that offspring had smaller body weights and displayed fewer play behaviors when parents had been exposed to EtOH before conception. In addition, offspring showed a reduction in pubertal development markers that could indicate that parental preconception EtOH exposure confers maladaptive epigenetic traits in first-generation offspring.
Keywords: binge alcohol, epigenetic inheritance, preconception habits, pubertal development
We characterized alterations in offspring hypothalamic function caused by parental preconception alcohol exposure along with the effect of parental alcohol on offspring response to alcohol treatment.
Epigenetic mechanisms work together to modify gene expression throughout the body and are heritable from cell to daughter cell and from parent to offspring. In this way, parental experience can affect offspring traits independent of Mendelian genetics [1–4]. DNA methylation is a relatively stable epigenetic modification by which environmental information can be transmitted to first-generation offspring faster than evolutionary adaptation, similar to Lamarckian inheritance theories [1, 5]. DNA methylation patterns can be induced through preconception behaviors and/or experiences of parents, including diet [3], exercise [6], and drug exposure [2]. Using adolescent alcohol exposure as a model for both physiological and psychological stress, our laboratory previously showed that preconception parental binge-pattern alcohol exposure altered DNA methylation patterns in the hypothalamus of their alcohol-naive offspring, which is a region of the brain involved in regulation of pubertal development, stress regulation, and social behaviors [7]. These observations have potentially serious implications for human health, as alcohol is the most widely abused drug in the United States, with more than 4.6 million Americans under the age of 21 years engaging in binge-pattern alcohol abuse [8]. Binge-pattern alcohol drinking is distinguished from casual consumption by the large volumes of alcohol that are consumed in a short time, reaching minimum blood alcohol content (BAC) of 0.08% within 2 hours. It is known that this type of rapid consumption during adolescent development can have long-lasting effects in the brain [9–15], and our recent evidence suggests these effects may extend beyond the individual to directly affect first-generation offspring [7, 9, 16, 17].
It has been hypothesized that epigenetic marks passed to offspring can serve as adaptive aids, such that encountering similar environmental stressors or compounds would be more easily tolerated [4, 18]. If this were the case for parental binge alcohol consumption, we would expect that offspring of parents who were exposed to binge-pattern alcohol consumption would have an increased ability to metabolize alcohol, resulting in lower BAC when exposed to alcohol, and have an attenuation to the normal physiological stress response observed after alcohol consumption. Therefore, in this study we tested two parallel hypotheses: (1) offspring of binge alcohol (EtOH)-exposed parents will have altered normal development through puberty, and (2) parental preconception EtOH exposure will provide adaptive protection against the effects of EtOH exposure in offspring. Our goal was to first determine functional consequences of parental EtOH exposure in offspring as they develop through puberty and secondarily to assess how parental EtOH exposure would affect offspring when they were exposed to alcohol themselves.
1. Materials and Methods
A. Experiment 1: Baseline Characteristics of Male and Female Offspring From Parents Administered Preconception Binge-Pattern EtOH During Adolescence
A-1. Animals
Male and female Wistar rats were purchased from Charles River Laboratory (Wilmington, MA) at postnatal day (PND) 25 and were allowed to acclimate for 5 days. Animals were then handled by experimenters for 5 minutes once daily for 7 days to control for nonspecific handling stress. Animals were pair-housed within the same treatment group. Food and water were available ad libitum, and animals were kept on a 12:12 light/dark cycle, with lights on at 7:00 am and handling/treatment beginning at 10:00 am. Animal procedures were approved by the Loyola University Medical Center Institutional Animal Care and Use Committee (permit no. 2012021). All measures were taken to minimize pain and suffering.
A-2. Binge alcohol paradigm
We used an established model of repeated binge-pattern EtOH exposure (Fig. 1) during which animals were administered EtOH once per day for a defined period of time during early puberty (PND 37) and a second period during late puberty (PND 67) [10]. Briefly, animals were given food-grade EtOH (Everclear; Luxco, St Louis, MO) diluted in tap water at a dose of 3g/kg body weight (20% v/v solution) or an equal volume of vehicle (tap water) via oral gavage. This dose of EtOH is equivalent to a BAC reached by six to seven drinks in humans within a 2-hour window. EtOH was given once daily for 3 days, followed by 2 days of water only, and then another 3 days of EtOH. Control animals received equal volumes of tap water gavage each day for the entire 8-day period. Following the early puberty exposure, animals were left undisturbed until PND 67, when they underwent the same treatment.
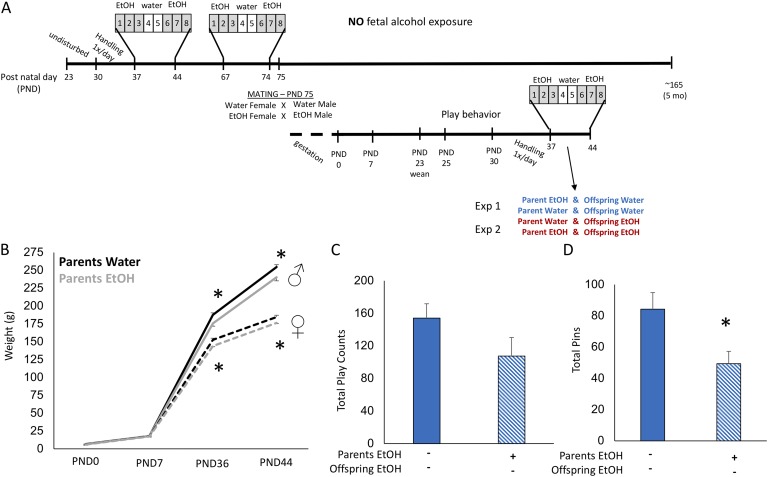
Figure 1.
Offspring of EtOH-treated parents were smaller after pubertal onset and displayed fewer play behaviors. (A) Wistar rats received EtOH exposure during early and late puberty and were pair-housed for mating 24 hours after the last dose in pairs of Water-Water or EtOH-EtOH. After normal gestation and birth, litters were culled to 10 pups per dam (five males, five females) and left undisturbed until weaning at PND 23. Offspring were then moved into same-sex group housing of five animals, all from the same parental treatment group, and home-cage play behavior was recorded daily from PNDs 25 to 30. Animals were then moved to pair-housing and randomly assigned to Experiment 1, where all offspring received water, or Experiment 2, where offspring underwent the same EtOH treatment paradigm as their parents. (B) Male and female offspring of EtOH-treated parents were smaller at PNDs 36 and 44 than offspring of water-treated parents, although there was no difference at birth or PND 7 and growth rate between PNDs 7 and 36 was the same. (C) Offspring of EtOH-treated parents displayed fewer pinning behaviors than offspring of water-treated parents. (D) There was a modest decrease in overall play behaviors exhibited by offspring of EtOH-treated parents compared with offspring of water-treated parents. Two-sample t test. *P < 0.05, mean ± SEM; n = 10 per group. Solid bars indicate parental vehicle treatment. Hatched bars indicate parental EtOH exposure.
A-3. Mating
Animals were paired (EtOH-EtOH, vehicle-vehicle) for mating 24 hours after the last EtOH dose at PND 75. After 7 days, females were moved to single-housing to properly gestate and nest, and males were returned to pair-housing with their previous cage mate. Litters were culled to 10 pups per dam in equal sex ratios within 1 hour of natural birth. Pups were left with their biological mother until weaning at PND 23. After offspring weaning, parents remained undisturbed and housed for future studies. Offspring were housed from PNDs 23 to 30 in same-sex groups of five from litters of the same treatment (i.e., parental EtOH housed together; parental vehicle housed together). Behavioral observations were made from recordings of home cage activity daily from PNDs 25 to 30.
B. Experiment 2: Effects of Adolescent EtOH Exposure in Offspring With Parental History of Preconception EtOH Consumption
B-1. Animals
Offspring of mating pairs were separated into pair-housing following play behavior analyses. Animals were handled daily from PNDs 30 to 36 and then administered the same adolescent binge EtOH paradigm described previously during early puberty (PNDs 37 to 44). All offspring were anesthetized with isoflurane and euthanized by decapitation 1 hour after the last EtOH dose at PND 44. Blood alcohol level (i.e., BAC) was measured in offspring plasma using the Alcohol Reagent Kit according to the manufacturer’s instructions (cat# A7504-150; Pointe Scientific, Canton, MI).
B-2. Tissue collection
Trunk blood was collected on ice into heparinized tubes and centrifuged at 4500 rpm for 8 minutes at 4°C, and plasma was aliquoted and stored at −20°C. Brains and testes were immediately removed and flash frozen in isopentane on dry ice. Tissue was stored at −80°C until microdissection and processed for total RNA isolation with TRIzol reagent (#15596018; Life Technologies, Carlsbad, CA) according to the manufacturer’s instructions. Briefly, brains were sectioned rostral to caudal at 200 μm using a Leica CM3050 S cryostat, and the whole hypothalamus (−0.8 to −3.8 mm relative to the bregma) was microdissected using a Palkovits brain punch tool (Stoelting Co., Wood Dale, IL) according to TheRat Brain in Stereotaxic Coordinates [19].
B-3. ELISA
Circulating concentrations of corticosterone (CORT), testosterone (T), and luteinizing hormone (LH) were measured in all animals using an ELISA assay kit according to the manufacturer’s instructions (Corticosterone Enzyme Immunoassay Kit, cat #K014-H1, lot #16CS080d; Arbor Assays, Ann Arbor, MI; Testosterone Enzyme Immunoassay Kit, cat #K032-H1, lot #17T051b; Arbor Assays; LH ELISA Kit, cat #ENZ-KIT107-0001, lot #02061708A; Enzo Life Sciences, Farmingdale, NY). For all assays, samples that fell below the limit of detection were included in the analysis with a value equal to that of the detection limit, as indicated by the dashed line in each graph.
B-4. Quantitative polymerase chain reaction (RT-qPCR)
cDNA was reverse transcribed from 1.0 µg of total RNA isolated from male offspring hypothalamus and testes using the SuperScript IV First-Strand Synthesis System (cat #18-091-200; Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. RT-qPCR of hypothalamic genes was performed to measure mRNA expression of gonadotropin-releasing hormone (GnRH), corticotrophin releasing factor (CRF), arginine vasopressin (AVP), and glucocorticoid receptor (GR). In addition, RT-qPCR was performed on cDNA prepared from testes to measure mRNA expression of the mature sperm marker genes acrosomal vesicle protein 1 (Acrv1), lactate dehydrogenase C (LHDC), and androgen-binding protein (ABP). FastStart SYBR Green Master Mix (cat #04913914001; Roche, Indianapolis, IN) was used for all RT-qPCR, adding 2 µL of cDNA and final primer concentrations of 0.25 µM for each gene. RT-qPCR data were analyzed using the ΔΔCt method, comparing with 18S RNA expression of each sample. Primer sequences used were as follows:
GnRH (F: 5′-CTGCTGACTGTGTGTTTGGAAGG, R: 5′-CCTGGCTTCCTCTTCAATCA)
CRF (F: 5′-GAGAAAGGGGAAAGGCAAAG, R: 5′-ATCAGAATCGGCTGAGGTTG)
AVP (F: 5′-GGGCAGGTAGTTCTCCTCCT, R: 5′-CACCTCTGCCTGCTACTTCC)
GR (F: 5′-CACCCATGATCCTGTCAGTG, R: 5′-AAAGCCTCCCTCTGCTAACC)
Acrv1 (F: 5′-CATGCTTCTACCGAGCACAC, R: 5′-CACCTGAAACTTGTTCGCCTG)
LHDC (F: 5′-TTTCTTAACTGCTGCGGGGT, R: 5′-TGTTGAGCCTTCACAGGTGG)
ABP (F: 5′-TCTGAGCCACTGGGTGACAG, R: 5′-CAACAACAGAAGCAGTCGGC)
18S (F: 5′-CATTCGAACGTCTGCCCTAT, R: 5′-GTTTCTCAGGCTCCCTCTCC)
B-5. Testes histology and analysis
Testes were postfixed and cryoprotected in sucrose before sectioning at 16-µm thickness on a Leica CM3050 S cryostat. Frozen sections were thaw-mounted on Superfrost Plus slides (Fisher Scientific, Chicago, IL) and dried at 37°C for 30 minutes. Tissue was stained using hematoxylin and eosin (H&E) and imaged under a light microscope, as previously described [20]. Images were analyzed by two blinded reviewers and qualitatively scored for the presence of elongated spermatids and lumen opening.
B-6. Play behavior
Juvenile social play behavior was assessed using paradigms adapted from those previously reported [21–23]. Recordings of home-cage behavior were made for 5 consecutive days at 3 hours after lights off (10 pm). For each recording day, every animal was marked with permanent marker on the back and tail within 2 hours before lights off. Recordings were made by replacing the cage lid with Plexiglass and moving the cage to a table under a night vision camera. Cages were left beneath the camera for 5-minute trials, for a total observation time of 25 minutes per cage. Play behavior recordings were scored by a blinded experimenter measuring the following criteria: (1) biting: one rat bites another; (2) chasing: one rat chases another; (3) pouncing: one rat pounces or lunges at another; (4) pinning: one rat stands over another, with its forepaws on the ventral surface of the opposing rat; and (5) boxing: both rats stand on hind legs and engage each other with forepaws. Both autogrooming and allogrooming behaviors were also scored. Counts across all observation windows were totaled per cage.
B-7. Statistics
Statistical analysis was performed using a two-sample t test for each end point, using SYSTAT Software (version 13) and a statistically significant difference indicated by P < 0.05.
2. RESULTS
A. Experiment 1: Baseline Characteristics of Male and Female Offspring From Parents Administered Preconception Binge-Pattern EtOH During Adolescence (Figs. 1–3 , Blue Graphs)
A-1. Body weight: offspring of alcohol-exposed parents had decreased body weight after puberty but not at birth
To assess the effects of preconception parental EtOH exposure on offspring outcomes, we mated males and females in adulthood after adolescent exposure to EtOH (Fig. 1A). Impregnated females were left undisturbed through normal gestation and birth. There were no statistically significant differences in the time from pair-housing to birth (indicative of gestation length), number of pups, or sex ratio of offspring between EtOH- and water-treated mating pairs. Within 1 hour of birth, all animals were weighed, and litters were culled to 10 rats per dam (five males, five females). Offspring (male or female) weights were not different at birth (PND 0) or at PND 7 between treatment groups (Fig. 1B). However, as the offspring grew through weaning and entered puberty (PND 36), the male offspring of parents who received EtOH were significantly smaller than the offspring of water-treated parents, with average weights of 176 and 188 g, respectively (Fig. 1B). This was also true for female offspring at PND 36, with average weights of 144.05 g for offspring from EtOH-treated parents and 152.75 g for offspring from water-treated parents. The offspring weights remained consistently lower for both male and female offspring, although the rate of growth, as analyzed by calculated differences in the slope of the growth curves as 4 g/d for females and 8 g/d for males, was not different from that of control counterparts (Fig. 1B).
A-2. Play behavior: juvenile play behavior was altered in offspring of alcohol-exposed parents
Offspring were scored on their home-cage play behaviors for 5 consecutive days during the prepubertal juvenile period from PNDs 25 to 30. During this time, animals were housed in groups of five, which allowed for quantification of the group play behaviors of biting, chasing, pouncing, pinning, and boxing along with normal grooming behaviors. There were no differences in the scored behaviors between the sexes; therefore, males and females were combined for statistical analysis. Our results demonstrated a significant reduction of 42% in pinning behaviors, a core component of juvenile play, in offspring whose parents were exposed to EtOH preconception (Fig. 1D). Pinning behavior in juvenile rats is considered the most reliable and high-frequency component of juvenile play, indicating disrupted juvenile social interactions in offspring of EtOH-treated parents [24]. There were no statistically significant differences in overall play counts (Fig. 1C; P = 0.10), other play behaviors, or grooming behaviors (data not shown).
A-3. Hypothalamo-pituitary-gonad axis function: pubertal markers were dysregulated by parental preconception exposure to alcohol
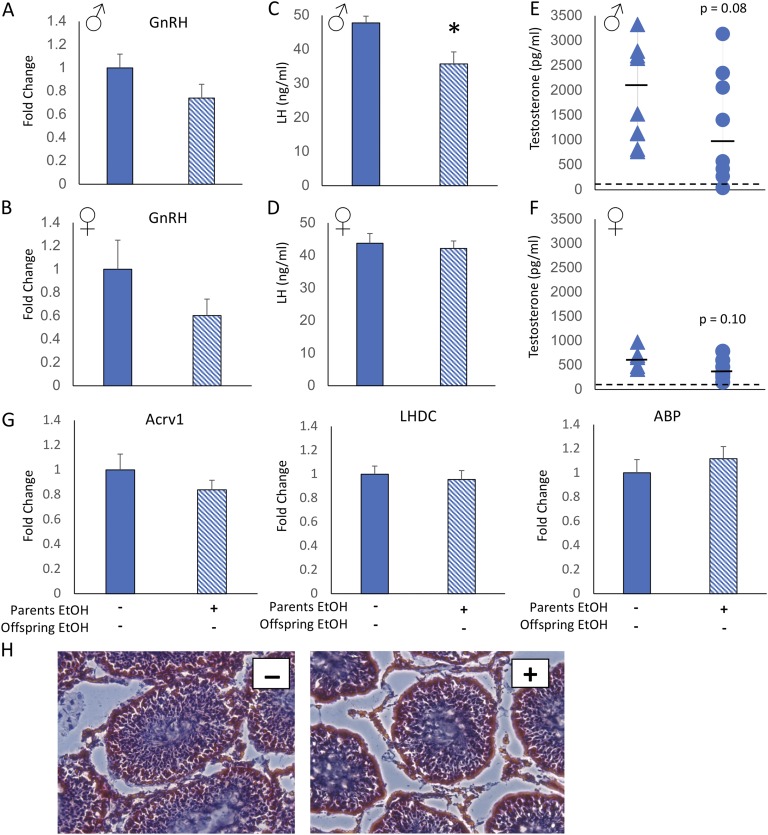
The hypothalamus not only controls the physiological stress response, but it is also central to pubertal regulation as part of the hypothalamo-pituitary-gonad (HPG) axis. Considering that both male and female offspring of parents who were given EtOH displayed stunted growth after the onset of puberty, we hypothesized that pubertal hormones would be dysregulated. First, GnRH mRNA expression was measured in the hypothalamus to determine whether there was pubertal perturbation at the level of the brain. GnRH mRNA was not statistically different in male or female offspring from parents given EtOH treatment, although there was a consistent decreased trend in both sexes (Fig. 2A and 2B). Next, we measured circulating LH (pituitary function) and T (gonadal function) from trunk blood collected at the time of euthanasia (PND 44). In female pubertal development, T is also involved in the development of the HPG axis, vaginal opening, and somatic growth [25], and we therefore measured T in both males and females. We did not measure estradiol levels because of the confounding factor of cyclicity, in that we did not sync the female estrous cycles. Hormone measurements were made using ELISA (Arbor Assays), and any samples that fell below the limit of detection were given a value of the lowest limit for statistical analysis. LH was significantly decreased in male (Fig. 2C) but not female (Fig. 2D) offspring of parents exposed preconception to EtOH compared with offspring of water-treated parents. This had potentially functionally relevant consequences as T levels were also modestly reduced [P = 0.08 for males (Fig. 2E) and P = 0.10 for females (Fig. 2F)].
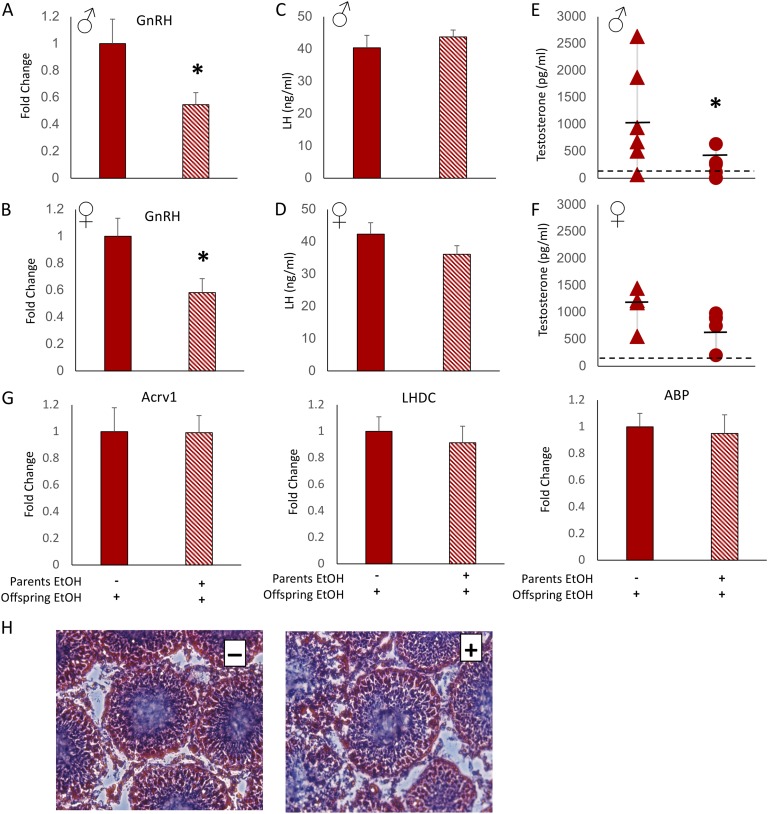
Figure 2.
Offspring of EtOH-treated parents displayed changes in baseline HPG axis parameters but not testicular development. Hypothalamic GnRH mRNA expression was decreased in (A) male and (B) female offspring of parents who were exposed to EtOH treatment before conception. Circulating LH level was decreased in (C) male but not (D) female offspring of EtOH-treated parents. Circulating T level was reduced in (E) male and (F) female offspring of EtOH-treated offspring compared with offspring of water-treated parents. (E and F) Assay limit of detection is indicated by dashed horizontal lines. (G) mRNA expression of mature sperm markers Acrv1, LHDC, and ABP was not different in the testes of male offspring of EtOH- or water-treated parents. (H) Hematoxylin and eosin staining of the testes showed no apparent difference in gross morphology or number of elongated spermatids between offspring whose parents received vehicle (left, −) or EtOH (right, +). Two-sample t test. *P < 0.05, mean ± SEM, n = 10 per group. Solid bars and triangles indicate parental vehicle treatment. Hatched bars and circles indicate parental EtOH exposure.
A-4. Testicular development: testicular development was not overtly affected by parental EtOH exposure
Spermatogenesis is dependent on high intratesticular T levels, which greatly exceed circulating concentrations and are maintained independently through local production by Leydig cells [26]. Our results demonstrated that preconception parental EtOH exposure reduced male offspring LH and to a lesser extent T, suggesting that spermatogenesis could be affected. Therefore, we measured genetic markers of mature sperm (Acrv1, LHDC, and ABP) in the testes. Our results showed no significant differences in mature sperm or ABP (Fig. 2G) between male offspring of EtOH- and water-treated parents. In addition, testes were cryosectioned and stained with H&E and scored for presence of mature (i.e., elongated) spermatids and opening of a central lumen in the seminiferous tubules. Consistent with the lack of changes in genetic markers, we also observed no gross anatomical differences in testes size, spermatid count, and luminal opening (Fig. 2H).
A-5. Hypothalamo-pituitary-adrenal (HPA) axis function: male offspring of EtOH-treated parents had lower baseline CORT levels than offspring of control animals, but expression of feedback genes was unchanged
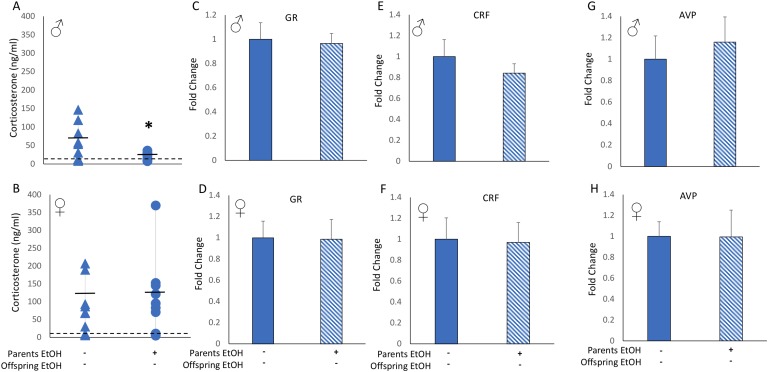
Next, we assessed baseline function of the HPA axis in offspring whose parents were treated with EtOH, as alcohol is a known potent activator of the HPA axis. We found that male offspring had a statistically significant lower circulating CORT level when parents were given EtOH preconception (Fig. 3A). In contrast, circulating CORT levels of female offspring were not dependent on parental preconception EtOH exposure (Fig. 3B). Hypothalamic mRNA expression of GR, CRF, and AVP genes, which are controlled by CORT negative feedback and mediate the HPA-axis response, were not different between offspring of EtOH-treated or control-treated parents for either male or female offspring (Fig. 3C‒3H).
Figure 3.
Offspring of EtOH-treated animals showed decreased baseline HPA axis activity. (A) Male but not (B) female offspring of EtOH-treated parents had significantly lower levels of circulating CORT than offspring of water-treated parents. Assay limit of detection is indicated by the dashed horizontal line. Hypothalamic mRNA expression of GR, CRF, and AVP was not affected by parental EtOH exposure in either (C, E, G) male or (D, F, H) female offspring. Two-sample t test. *P < 0.05, mean ± SEM, n = 10 per group. Solid bars and triangles indicate parental vehicle treatment. Hatched bars and circles indicate parental EtOH exposure.
B. Experiment 2: Effects of Adolescent EtOH Exposure in Offspring With Parental History of Preconception EtOH Consumption (Figs. 4–6 , Red Graphs)
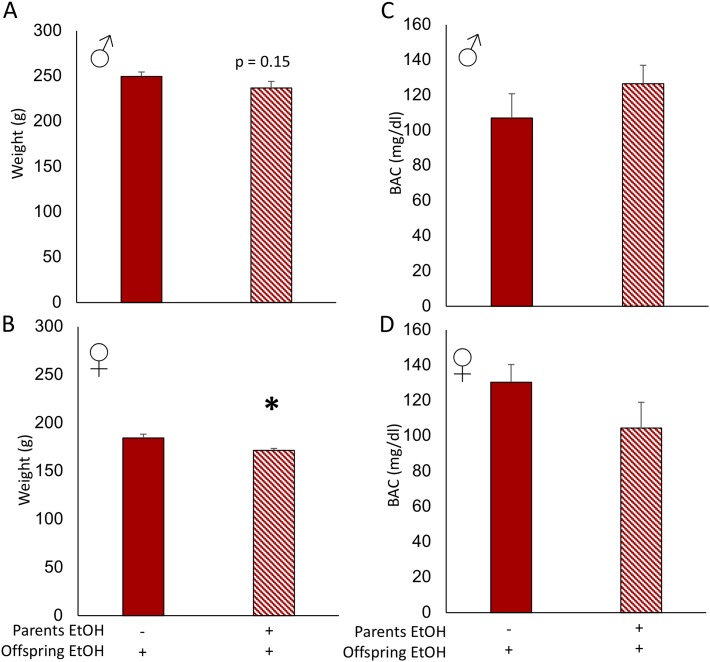
Figure 4.
Body weight decreased in female EtOH-treated offspring of EtOH-treated parents. (A) Male EtOH-treated offspring of EtOH-exposed parents did not have significantly lower body weights than EtOH-treated offspring from water-treated parents. (B) Female offspring treated with EtOH had significantly lower body weights when parents had also been treated with EtOH. There was no difference in the BAC between (C) male and (D) female offspring of EtOH-treated or vehicle-treated parents. Two-sample t test. *P < 0.05, mean ± SEM, n = 10 per group. Solid bars indicate parental vehicle treatment. Hatched bars indicate parental EtOH exposure.
In these studies, all offspring were administered the binge-pattern EtOH paradigm (see Fig. 1) during early puberty (PNDs 37 to 44) and were euthanized 1 hour after the last dose of EtOH at PND 44. The variable of interest was whether the offspring responded differently to the EtOH treatment depending on parental history of preconception binge EtOH exposure, thereby testing our hypothesis that parental EtOH exposure predisposed offspring to an adaptive response to adolescent binge-pattern EtOH (i.e., first-generation consequence).
B-1. Body weight and BAC: adolescent binge-pattern EtOH exposure significantly decreased body weight of female offspring whose parents had a history of binge drinking
Similar to what we observed with offspring body weight during normal development, adolescent binge-pattern EtOH exposure significantly decreased body weight in females but not males (Fig. 4A and 4B). Further, pursuant to our hypothesis that heritable epimutations in offspring may be adaptive aids in preparation for exposure to alcohol, we measured BACs in offspring. Blood alcohol levels were not affected by parental exposure to alcohol, with male offspring of EtOH-treated parents averaging 126.5 mg/dL compared with control counterparts averaging 107.0 mg/dL. Female offspring also showed no significant effect of parental treatment on BAC, with 130 mg/dL average for control parents and 104.4 mg/dL average for the parental-EtOH group (Fig. 4C and 4D), suggesting that the offspring were not able to metabolize EtOH differently on the basis of parental EtOH‒exposure history.
B-2. HPG axis function: effects of offspring EtOH exposure on pubertal development were exacerbated by parental preconception exposure to alcohol
Previous studies have shown that EtOH treatment during pubertal development can impair pubertal progression, dampening T and LH rises and slowing gonad maturation [27]. In our alcohol-naive offspring, we found a similarly attenuated HPG profile dependent on parental preconception EtOH treatment (see Fig. 2). In this experiment in which the offspring were treated with EtOH, we observed a more severe decrease in some of the HPG parameters, which depended on paternal history of EtOH exposure. First, there was a marked decrease in hypothalamic GnRH mRNA expression in EtOH-treated offspring when parents had been exposed to EtOH, but not water, before conception. This reduction was around 50% for both male and female offspring (Fig. 5A and 5B). However, the reduction in GnRH mRNA was not reflected in correspondingly reduced LH levels, as LH levels were unaffected by EtOH treatment in both male and female offspring (Fig. 5C and 5D). Circulating T levels were also significantly reduced in male offspring whose parents had been exposed to EtOH preconception (Fig. 5E). By contrast, EtOH exposure did not significantly affect T levels in female offspring, although there was a strong trend for decreased levels in most of the animals tested (Fig. 5F).
Figure 5.
EtOH-treated offspring of EtOH-treated parents had decreased HPG axis activity but no change in testicular development. Hypothalamic mRNA expression of GnRH was significantly decreased in both (A) male and (B) female offspring who were treated with EtOH when their parents were exposed to EtOH before conception. Circulating LH level was not different between EtOH-treated (C) male or (D) female offspring whose parents received EtOH or water treatment. T level was significantly decreased in EtOH-treated (E) male but not (F) female offspring of EtOH-treated parents compared with EtOH-treated offspring of water-treated parents. (E and F) Assay limit of detection is indicated by dashed horizontal line. (G) mRNA levels of Acrv1, LHDC, and ABP were measured in testes after offspring EtOH treatment and were not differentially expressed in male offspring of EtOH-treated or water-treated parents. (H) Hematoxylin and eosin staining of the testes revealed no apparent morphological differences or numbers of elongated spermatids between EtOH-treated offspring whose parents received water (left, −) or EtOH (right, +). Two-sample t test. *P < 0.05, mean ± SEM, n = 10 per group. Solid bars and circles indicate parental vehicle treatment. Hatched bars and triangles indicate parental EtOH exposure.
B-3. Testicular development: EtOH treatment in offspring did not affect testicular development irrespective of parental EtOH exposure
The observed reductions in HPG parameters suggested that testicular development might be further affected if offspring of EtOH-treated parents were exposed to EtOH themselves during puberty. Therefore, we measured the same mature sperm markers (Acvr1 and LHDC) and ABP in the testes and scored H&E-stained cross sections of EtOH-treated offspring testes. Our results showed that there were no differences in mRNA expression for any of the genes measured (Fig. 5G). In addition, there was no effect of offspring EtOH exposure on seminiferous tubule lumen opening or number of elongated spermatids irrespective of parental EtOH exposure (Fig. 5H).
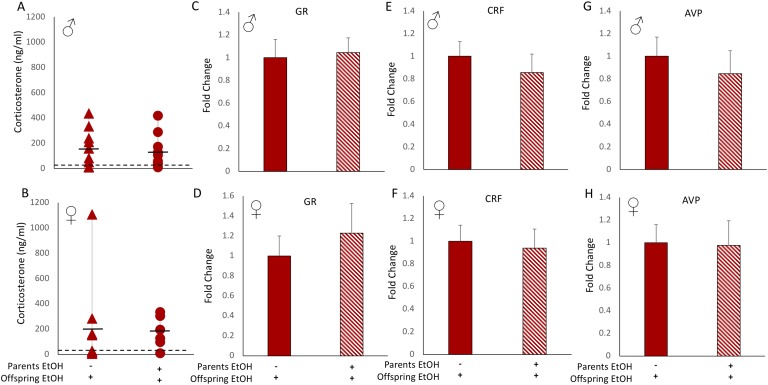
B-4. HPA axis function: offspring of EtOH-exposed parents had no adaptation in the stress response to EtOH
EtOH is a physiological stressor, as evidenced by a sharp spike in circulating CORT levels shortly after exposure. Our previous studies demonstrated that adolescent binge-pattern alcohol exposure resulted in dysfunction of the HPA axis; specifically, negative feedback in the adult brain was impaired, and CORT levels remained significantly elevated after subsequent alcohol exposure [10]. Therefore, our hypothesis was that offspring would display a resistance to this potentially harmful spike in CORT. Our results showed that EtOH induced an increase in circulating CORT levels in all our EtOH-treated offspring, but these levels were not different between offspring whose parents received EtOH and those who received water (Fig. 6A and 6B). Similarly, the hypothalamic mRNA expression of GR, CRF and AVP after EtOH treatment was not affected by preconception parental EtOH exposure for either male or female offspring (Fig. 6C‒6H).
Figure 6.
HPA axis response to EtOH exposure was not dependent on parental history of EtOH treatment. Parental EtOH exposure did not change the levels of circulating CORT in (A) male or (B) female offspring after EtOH treatment. Assay limit of detection is indicated by dashed horizontal line. mRNA expressions of GR, CRF, and AVP after EtOH treatment in offspring were unaffected by parental EtOH exposure in either (C, E, G) male or (D, F, H) female offspring. Two-sample t test. Mean ± SEM, n = 10 per group. Solid bars and triangles indicate parental vehicle treatment. Hatched bars and circles indicate parental EtOH exposure.
3. Conclusions
Taken together, our results demonstrated that parental preconception EtOH exposure did not confer any apparent adaptive phenotypic traits for the offspring, but rather had potentially maladaptive consequences on offspring growth, social interactions, and pubertal development. In these studies, we used adolescent binge-pattern EtOH consumption as a model for intergenerational inheritance of parental preconception behaviors, building on recent work demonstrating epigenetic modifications induced by a mild physiological/toxicological stressor. Adolescent binge-pattern alcohol consumption is a major public health concern, and many studies have documented the long-term negative consequences of this risky behavior for the individual [10–15, 28]. Moreover, we previously showed that the F1 generation offspring of animals administered binge-pattern EtOH during the adolescent period had numerous differentially methylated cytosine residues throughout the genome, as well as widespread alterations in gene expression (assessed at PND 7), despite never having been exposed to EtOH directly; however, the functional consequences of these epimutations conferred by the parents were unclear [7, 16]. Because these changes occurred genome wide, we chose to pursue a systems-level characterization of offspring hypothalamic function in alcohol-naive offspring as well as measure the potential adaptive and/or maladaptive consequences of this parental behavior.
T is an anabolic steroid hormone that is critical for somatic growth in males and plays a role in both males and females during early puberty [25]. Further, T is associated with increased play behaviors in juveniles of both sexes [21]. Parental preconception, adolescent, binge EtOH exposure decreased circulating T levels in both male and female offspring, although the high variability of this outbred rat strain resulted in no statistically significant differences (P = 0.08 male; P = 0.10 female). Along with the slight changes in LH concentration in circulation for male offspring and the differential GnRH expression in the hypothalamus, these may indicate a disturbance in the synchronization of puberty, during which changes in hormone balance can affect the timing of body growth and development. Although EtOH has been shown to disrupt HPG function in the individual exposed, we found HPG disruption in offspring of parents exposed to only preconception EtOH doses [27]. In conjunction with circulating hormones, we examined several markers of sperm development in the males and did not observe any changes in testes or sperm maturation, which argues that the hormone imbalances are not affecting sexual development. Intratesticular levels of T are maintained through different mechanisms than circulating concentrations [26], and the preservation of testicular development suggests that Leydig cell function may be unaffected by parental EtOH exposure. However, future experiments testing the fertility of these offspring would provide more concrete evidence of the effects of parental EtOH exposure on offspring sexual maturation.
Somatic growth from birth through puberty was assessed on the basis of prior evidence that offspring exposed to EtOH in utero tend to have lower birth weights [29, 30]. Our results revealed that parental preconception EtOH exposure did not affect offspring weight at birth or at PND 7, and body weight differences in both male and female offspring arose only after weaning from the mother. These data suggest that there was no overt malnutrition of these offspring, but a latent difference in control of body weight that was evident only later in life during the peripubertal growth spurt. Along with the decreased body weight, the offspring showed a perturbation in postweaning juvenile play behavior, with less pinning behavior in both male and female offspring of EtOH-treated parents. Decreased pinning and engagement in other bouts of play have been linked to social withdrawal and social anxiety phenotypes and represent a less-masculine/dominant display of play [21]. Therefore, these data indicate that offspring of EtOH-treated parents have disrupted juvenile social interactions, which are markers of atypical juvenile social development. The slight reduction in T, accompanied by alterations in GnRH expression, may be the cause of the reduction in body weight and play behaviors observed in these offspring.
We also measured a similar if not exacerbated effect of parental EtOH exposure on HPG function in offspring when they were given EtOH. GnRH expression in the hypothalamus was reduced by almost 50% in both males and females after EtOH exposure. In addition, T was significantly reduced in male offspring and trended toward a decrease in female offspring. These measures, as previously noted, indicate a dysfunction in the hypothalamic control of hormone balance. However, we did not see a difference in gonad development in male offspring who were exposed to EtOH themselves, again indicating that sexual maturation was progressing, but the viability of the mature sperm and functional capacity to fertilize ova was not assessed.
Our original hypothesis was that the epimutations we had previously observed in offspring after parental EtOH exposure would confer to them an adaptation to EtOH exposure themselves. Indeed, in EtOH-naive offspring, we measured a lower baseline CORT level in male offspring whose parents had been treated with alcohol, which was consistent with the phenotype that we and others previously reported in adult animals who, after exposure to adolescent EtOH, exhibited a lower baseline CORT level in adulthood [10, 12, 14, 31]. However, an alternative hypothesis is that preconception parental binge alcohol consumption leaves lasting epigenetic marks that may result in phenotypic disadvantages for the offspring, such as a heightened stress response when exposed to a similar dose of alcohol.
Previous studies from our laboratory and others have demonstrated adolescent alcohol-induced, long-term dysfunction of the HPA axis, the primary mediator of the physiological stress response, that persisted even after prolonged periods of alcohol abstinence [10]. These maladaptive long-term changes in adulthood suggest that offspring of these animals could inherit a maladaptive phenotypic response to EtOH, as EtOH is a known activator of the HPA axis. We examined blood alcohol levels, circulating CORT levels, and stress-responsive gene expression patterns in the hypothalamus and found none of these measures to be different in either alcohol-naive or alcohol-exposed offspring. Interestingly, we observed that parental EtOH exposure caused a reduction in baseline (i.e., alcohol-naive offspring) circulating CORT level; however, when offspring were treated with EtOH, there was no difference in CORT concentration between offspring depending on parental treatment. This suggests that male offspring whose parents received EtOH have an increased magnitude of CORT surge after EtOH exposure themselves, as they have a similar end concentration of CORT but started from a lower baseline, almost doubling the CORT response. Therefore, the spike in stress response may actually be greater in offspring of EtOH-treated parents, indicating a maladaptive response to EtOH. In addition, the lack of adaptive response to EtOH does not preclude a lack of adaptation from parental drinking as a whole. These offspring may be adept at handling heterotypic stressors, such as psychological stress, or they may have more efficient glucose mobilization with the same CORT surge.
A potential confounding factor in our experimental design was the rearing of the litter by the biological (i.e., EtOH-exposed) mother, which may have conferred compensatory factors for phenotypic changes in the juvenile offspring. For example, increased/decreased maternal grooming behaviors have been linked with epigenetic changes in DNA methylation and histone acetylation marks in the offspring brain [32]. Our design in this study was intended to better mimic the human societal situation in which the majority of offspring would be reared by the biological parents. In addition, cross-fostering could induce its own set of stressors and epigenetic changes that would be difficult to assess and were beyond the scope of these studies. Therefore, future studies investigating the growth and development of offspring after cross-fostering to EtOH-naive mothers, and likewise of control animals raised by EtOH-exposed mothers, would shed light on the interaction of maternal behavior with preconception EtOH exposure on these offspring.
Previous work in our laboratory demonstrated genome-wide changes in DNA methylation as well as widespread changes in gene expression patterns in the hypothalamus at PND 7, a period in rat neurodevelopment similar to that of humans at birth [33]. These changes, resulting from preconception EtOH exposure of the parents only, may underlie the moderate changes in hypothalamic function we observed in the experiments presented here. It is well known that development is accompanied by changes in gene expression and DNA methylation patterns, but future studies are needed to investigate how and when these changes may occur. The inheritance of epigenetic marks may be important in the establishment of offspring gene expression profiles, but developmental environments may provide the stimulation to ameliorate some of the inherited marks.
The data presented here show that preconception EtOH exposure of parents during puberty had consequences for offspring of smaller body size, disrupted juvenile social play, and decreased circulating T level. In addition, parental exposure to EtOH did not confer any apparent adaptive phenotypic traits for future offspring in encountering EtOH. With advances in research technology, we are now able to understand which parental preconception behaviors represent salient events for multigenerational inheritance, how they may be caused, and ultimately, what we can do to prevent their perpetuation.
Acknowledgments
Financial Support: This work was supported by the National Institutes of Health awards R01AA021517 and R01AG033605 (to T.R.P.).
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- ABP
androgen-binding protein
- Acrv1
acrosomal vesicle protein 1
- AVP
arginine vasopressin
- BAC
blood alcohol content
- CORT
corticosterone
- CRF
corticotrophin releasing factor
- EtOH
alcohol
- GnRH
gonadotropin-releasing hormone
- GR
glucocorticoid receptor
- H&E
hematoxylin and eosin
- HPA
hypothalamo-pituitary-adrenal
- HPG
hypothalamo-pituitary-gonad
- LH
luteinizing hormone
- LHDC
lactate dehydrogenase C
- PND
postnatal day
- RT-qPCR
quantitative polymerase chain reaction
- T
testosterone
References and Notes
- 1. Carone BR, Fauquier L, Habib N, Shea JM, Hart CE, Li R, Bock C, Li C, Gu H, Zamore PD, Meissner A, Weng Z, Hofmann HA, Friedman N, Rando OJ. Paternally induced transgenerational environmental reprogramming of metabolic gene expression in mammals. Cell. 2010;143(7):1084–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Minnes S, Singer L, Min MO, Wu M, Lang A, Yoon S. Effects of prenatal cocaine/polydrug exposure on substance use by age 15. Drug Alcohol Depend. 2014;134:201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Öst A, Lempradl A, Casas E, Weigert M, Tiko T, Deniz M, Pantano L, Boenisch U, Itskov PM, Stoeckius M, Ruf M, Rajewsky N, Reuter G, Iovino N, Ribeiro C, Alenius M, Heyne S, Vavouri T, Pospisilik JA. Paternal diet defines offspring chromatin state and intergenerational obesity. Cell. 2014;159(6):1352–1364. [DOI] [PubMed] [Google Scholar]
- 4. Weyrich A, Benz S, Karl S, Jeschek M, Jewgenow K, Fickel J. Paternal heat exposure causes DNA methylation and gene expression changes of Stat3 in Wild guinea pig sons. Ecol Evol. 2016;6(9):2657–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jones PA. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat Rev Genet. 2012;13(7):484–492. [DOI] [PubMed] [Google Scholar]
- 6. Denham J. Exercise and epigenetic inheritance of disease risk. Acta Physiol (Oxf). 2018;222(1):e12881. [DOI] [PubMed] [Google Scholar]
- 7. Asimes A, Torcaso A, Pinceti E, Kim CK, Zeleznik-Le NJ, Pak TR. Adolescent binge-pattern alcohol exposure alters genome-wide DNA methylation patterns in the hypothalamus of alcohol-naïve male offspring. Alcohol. 2017;60:179–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Substance Abuse and Mental Health Services Administration. Key Substance Use and Mental Health Indicators in the United States: Results From the 2016 National Survey on Drug Use and Health. Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration; 2017. HHS publication no. SMA 17-5044, NSDUH Series H-52.
- 9. Govorko D, Bekdash RA, Zhang C, Sarkar DK. Male germline transmits fetal alcohol adverse effect on hypothalamic proopiomelanocortin gene across generations. Biol Psychiatry. 2012;72(5):378–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Przybycien-Szymanska MM, Rao YS, Pak TR. Binge-pattern alcohol exposure during puberty induces sexually dimorphic changes in genes regulating the HPA axis. Am J Physiol Endocrinol Metab. 2010;298(2):E320–E328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Przybycien-Szymanska MM, Mott NN, Pak TR. Alcohol dysregulates corticotropin-releasing-hormone (CRH) promoter activity by interfering with the negative glucocorticoid response element (nGRE). PLoS One. 2011;6(10):e26647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Crews FT, Vetreno RP, Broadwater MA, Robinson DL. Adolescent alcohol exposure persistently impacts adult neurobiology and behavior. Pharmacol Rev. 2016;68(4):1074–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Trantham-Davidson H, Centanni SW, Garr SC, New NN, Mulholland PJ, Gass JT, Glover EJ, Floresco SB, Crews FT, Krishnan HR, Pandey SC, Chandler LJ. Binge-like alcohol exposure during adolescence disrupts dopaminergic neurotransmission in the adult prelimbic cortex. Neuropsychopharmacology. 2017;42(5):1024–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vetreno RP, Yaxley R, Paniagua B, Johnson GA, Crews FT. Adult rat cortical thickness changes across age and following adolescent intermittent ethanol treatment. Addict Biol. 2017;22(3):712–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Spear LP. Consequences of adolescent use of alcohol and other drugs: studies using rodent models. Neurosci Biobehav Rev. 2016;70:228–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Przybycien-Szymanska MM, Rao YS, Prins SA, Pak TR. Parental binge alcohol abuse alters F1 generation hypothalamic gene expression in the absence of direct fetal alcohol exposure. PLoS One. 2014;9(2):e89320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hill SY, Rompala G, Homanics GE, Zezza N. Cross-generational effects of alcohol dependence in humans on HRAS and TP53 methylation in offspring. Epigenomics. 2017;9(9):1189–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Skinner MK. Environmental epigenetics and a unified theory of the molecular aspects of evolution: a neo-Lamarckian concept that facilitates neo-Darwinian evolution. Genome Biol Evol. 2015;7(5):1296–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Cambridge, MA:Academic Press; 2013. [DOI] [PubMed] [Google Scholar]
- 20. Pak TR, Lynch GR, Tsai P-S. Estrogen accelerates gonadal recrudescence in photo-regressed male siberian hamsters. Endocrinology. 2002;143(10):4131–4134. [DOI] [PubMed] [Google Scholar]
- 21. Meaney MJ, McEwen BS. Testosterone implants into the amygdala during the neonatal period masculinize the social play of juvenile female rats. Brain Res. 1986;398(2):324–328. [DOI] [PubMed] [Google Scholar]
- 22. Olesen KM, Jessen HM, Auger CJ, Auger AP. Dopaminergic activation of estrogen receptors in neonatal brain alters progestin receptor expression and juvenile social play behavior. Endocrinology. 2005;146(9):3705–3712. [DOI] [PubMed] [Google Scholar]
- 23. Edelmann MN, Demers CH, Auger AP. Maternal touch moderates sex differences in juvenile social play behavior. PLoS One. 2013;8(2):e57396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Panksepp J, Siviy S, Normansell L. The psychobiology of play: theoretical and methodological perspectives. Neurosci Biobehav Rev. 1984;8(4):465–492. [DOI] [PubMed] [Google Scholar]
- 25. Zarrow MX, Naqvi RH, Denenberg VH. Androgen-induced precocious puberty in the female rat and its inhibition by hippocampal lesions. Endocrinology. 1969;84(1):14–19. [DOI] [PubMed] [Google Scholar]
- 26. Coviello AD, Bremner WJ, Matsumoto AM, Herbst KL, Amory JK, Anawalt BD, Yan X, Brown TR, Wright WW, Zirkin BR, Jarow JP. Intratesticular testosterone concentrations comparable with serum levels are not sufficient to maintain normal sperm production in men receiving a hormonal contraceptive regimen. J Androl. 2004;25(6):931–938. [DOI] [PubMed] [Google Scholar]
- 27. Rachdaoui N, Sarkar DK. Effects of alcohol on the endocrine system. Endocrinol Metab Clin North Am. 2013;42(3):593–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Torcaso A, Asimes A, Meagher M, Pak TR. Adolescent binge alcohol exposure increases risk assessment behaviors in male Wistar rats after exposure to an acute psychological stressor in adulthood. Psychoneuroendocrinology. 2017;76:154–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Carter RC, Jacobson JL, Sokol RJ, Avison MJ, Jacobson SW. Fetal alcohol-related growth restriction from birth through young adulthood and moderating effects of maternal prepregnancy weight. Alcohol Clin Exp Res. 2013;37(3):452–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sampson PD, Bookstein FL, Barr HM, Streissguth AP. Prenatal alcohol exposure, birthweight, and measures of child size from birth to age 14 years. Am J Public Health. 1994;84(9):1421–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fernandez GM, Stewart WN, Savage LM. Chronic drinking during adolescence predisposes the adult rat for continued heavy drinking: neurotrophin and behavioral adaptation after long-term, continuous ethanol exposure. PLoS One. 2016;11(3):e0149987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Weaver ICG, Champagne FA, Brown SE, Dymov S, Sharma S, Meaney MJ, Szyf M. Reversal of maternal programming of stress responses in adult offspring through methyl supplementation: altering epigenetic marking later in life. J Neurosci. 2005;25(47):11045–11054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3(1):79–83. [DOI] [PubMed] [Google Scholar]