Abstract
Statin therapy reduces cardiovascular events in patients with, or at risk of, atherosclerotic cardiovascular disease. However, statins are underutilized in patients for whom they are indicated and are frequently discontinued. Discontinuation may be the result of statin-associated muscle symptoms (SAMS), which encompass a broad spectrum of clinical phenotypes from myalgia to severe myopathy. As with many adverse drug reactions (ADRs), inter-individual variability in susceptibility to SAMS is due, at least in part, to differences in host genetics. The genetic basis for SAMS has been investigated in candidate gene studies, genome-wide association studies, and, more recently, studies of multi-omic networks, including at the transcriptome level. In this article, we provide a systematic review of the pharmacogenetic basis of SAMS, focusing on how an understanding of the genetic and molecular determinants of SAMS can be considered in a personalized approach to reduce the incidence of this ADR, optimize statin adherence, and reduce the risk for cardiovascular events.
Keywords: Statin , Pharmacogenomics , Myopathy , Statin-associated muscle symptoms , Genetics , Myalgia , Adverse drug reaction
1. Introduction
Statin therapy has been shown to reduce atherosclerotic cardiovascular events in individuals with, or at risk of, cardiovascular disease.1,2 Utilization of high-intensity statins in patients hospitalized for myocardial infarction (MI) has improved after publication of the American College of Cardiology (ACC)—American Heart Association (AHA) secondary prevention guidelines3; however, utilization of statin therapy in this population remains suboptimal.4 In high-risk primary prevention individuals, utilization of statin therapy is even lower.3
After initiation of high-intensity statin therapy in patients hospitalized for a MI, down-titration of statin or a change to a non-statin regimen is associated with a 1.5-fold increased risk of recurrent MI or hospitalization for a cardiovascular event.5 Within 1 month of a non-ST elevation MI, statin discontinuation or down-titration is associated with a higher risk of cardiovascular events and higher mortality.6
In clinical trials of participants who experience statin-associated muscle symptoms (SAMS) on two or more occasions, 57–75% do not experience muscle symptoms upon re-challenge when evaluated in a double-blind, placebo-controlled crossover trial.7–10 Thus, reliance on patient-reported symptoms, and interpretation of those symptoms by the healthcare provider, is challenging.11 Clinical tools have been developed to improve the diagnostic accuracy of SAMS, including the SAMS clinical index (SAMS-CI) tool.12,13 When assessed in the Coenzyme Q10 trial,10 the SAMS-CI had a positive predictive value of 67% and a negative predictive value of 91%, thereby validating this as a clinical aid, particularly in its ability to rule out SAMS.14 Clinical practice guidelines on the management of SAMS have been published by major professional organizations.13,15,16
In this review, we discuss a personalized approach to understanding the molecular and cellular basis for the spectrum of muscle adverse events that are reported in statin-treated patients. The high cardiovascular event rate in patients with indications for statins but who down-titrate or discontinue statins, and the attendant economic burden to society, mandates better understanding of genetic susceptibility. Through this personalized approach, individuals who have a susceptibility to SAMS might be more efficiently transitioned to other LDL cholesterol (LDL-C) lowering treatments. Furthermore, individuals without a genetic susceptibility to SAMS may warrant evaluation for primary muscle disorders.
2. Spectrum of statin-associated muscle symptoms and injury
For many years, adverse muscle symptoms were characterized under the term ‘statin myopathy’.17,18 However, statin myopathy is a neuromuscular term used to describe muscle weakness.13 In 2014, the National Lipid Association Task Force advocated use of specific terminology to describe the spectrum of adverse muscle events (Table 1).13 This terminology was based on symptoms and pathophysiological mechanisms. The term ‘SAMS’, therefore, encompasses a broad spectrum of symptomatic muscle complaints with or without biochemical evidence of muscle damage. Herein, we deliberately use SAMS to indicate the entire spectrum of muscle-related complaints and, where appropriate, define the specific phenotype used in various studies described below. However, it should be noted that there exists considerable heterogeneity and uncertainty in the definitions of SAMS.
Table 1.
Spectrum of statin-associated muscle symptoms (based on 2014 National Lipid Association Task Force13)
| Terms | Descriptions | Histopathological findings |
|---|---|---|
| Myalgia | Muscle discomfort with normal CK | None |
| Myopathy | Muscle weakness | Variable findings:
|
| Myositis | Muscle inflammation | T cells > B cells; macrophages |
| Myonecrosis | CK elevation >three-fold baseline (mild), ≥10-fold baseline (moderate), or ≥50-fold baseline (severe) | Non-specific inflammatory cells with secondary macrophage infiltration |
| Myonecrosis with myoglobinuria (rhabdomyolysis) | As above, plus increase in serum creatinine ≥0.5 mg/dL | Non-specific inflammatory cells with secondary macrophage infiltration |
CK, creatine kinase.
3. Literature review
We searched the PubMed database from to 1966 to March 2018 using multiple search term strategies: statin AND myopathy AND genetics, statin AND muscle AND genetics, statin AND pharmacogen*. Abstracts were reviewed for relevance and, if relevant, full-text articles were retrieved and reviewed.
4. Genetic associations of SAMS
4.1 Candidate gene studies
Early studies focused on candidate genes hypothesized to play a role in SAMS by virtue of their impact on statin metabolism, transport, or action19–22 (Table 2). These studies identified suggestive associations between SAMS and variation in cytochrome P450 genes, including CYP3A4, CYP3A5, CYP2D6, and the vitamin D receptor gene. In general, most of these associations have not been widely replicated.
Table 2.
Genetic variants associated with statin-associated muscle symptom (SAMS) phenotypes
| Gene | Variant | Statin | Phenotype | Odds ratio | Independent replication? | PharmGKB levela | References |
|---|---|---|---|---|---|---|---|
| SLCO1B1 | rs4149056 | Simvastatinb, atorvastatin, rosuvastatin | Myopathy, myalgia, statin discontinuation, ‘intolerance’ | 1.7–4.5 | Yes | 1A | 23–29 |
| COQ2 | rs4693075 | Atorvastatin, rosuvastatin | Myopathy, myalgia | 2.4 | Yes | 2B | 30–32 |
| HTR7 | rs1935349 | Atorvastatin, simvastatin, pravastatin | Myalgia | – | No | 3 | 33 |
| RYR1 | rs118192172 | – | Myopathy | – | No | 3 | 34 |
| GATM | rs9806699 | Simvastatin | Myopathy | 0.6 | No | 3 | 35 , 36 |
| CYP3A4 | rs2740574 | Simvastatin, atorvastatin | Dose reduction or discontinuation | 0.46 | No | 3 | 37 |
| CYP2D6 | *4 | Simvastatin, atorvastatin | Discontinuation, ‘muscle events’ | 1.7–2.5 | Yes | – | 19 , 20 |
| ABCC2 | rs717620 | 1.3 | No | 3 | 38 | ||
| RYR2 | rs2819742 | Cerivastatin | Rhabdomyolysis | 0.24, 1.3 | No | 3 | 39 , 40 |
| CLCN1 | rs55960271 | Myopathy | – | No | – | 41 | |
| VDR | rs731236 | Simvastatin | Myalgia | 4.37 | No | – | 22 |
| ABCG2 | rs2231142 | Atorvastatin | Myalgia | 2.75 | No | – | 42 |
Refers to strength of evidence from the PharmGKB database (https://www.pharmgkb.org).
Strength of association appears to be strongest with simvastatin compared with other statins.
ABC transporters including ABCB1 and ABCG2 are expressed on the canillicular membrane of hepatocytes and are thought to mediate the excretion of statins into bile. Variation in the ABCB1 gene has been reported to be associated with myalgia.43 Variation in ABCG2 impacts the plasma concentrations of statins, in particular, atorvastatin and rosuvastatin.44 Carriers of an ABCG2 variant are reported to have an increased risk of atorvastatin-associated adverse drug reactions (ADRs).42
4.2 Genome-wide association studies
The first genome-wide association study (GWAS) of SAMS was performed in the Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine (SEARCH) study population. SEARCH involved >12 000 individuals with cardiovascular disease who were randomized to treatment with either 80 mg or 20 mg of simvastatin daily.45 Myopathy, defined as creatine kinase (CK) elevation of >10× the upper limit of normal, occurred in 49 participants (0.8%) randomized to 80 mg of simvastatin, and 2 participants (0.03%) randomized to 20 mg of simvastatin. Importantly, the 25-fold excess of myopathy cases in the high- vs. low-dose groups of this randomized control trial confirms the real, though very rare, risk of myopathy associated with statin use, and the influence of dosing on this ADR. An additional 54 cases had ‘incipient myopathy’, defined as CK > 3 × upper limit of normal (ULN) and alanine aminotransferase > 1.7 × the baseline.
A coding variant (p.Val174Ala, rs4149056) in the SLCO1B1 gene was found to be significantly associated with myopathy in this study.23 This association was confirmed in the Heart Protection Study.23 Subsequently, multiple studies have confirmed the association between SLCO1B1 rs4149056 and SAMS,24–27 all with similar odds ratios of 1.7–4.5, making this the most well-validated genetic association for SAMS. The strength of association appears to be greatest in those studies that considered a more severe phenotype as documented by significant elevation in CK (odds ratio 3.2–4.5)23,25,27 compared with studies that included patients with a less severe phenotype based on myalgia or statin discontinuation (odds ratio 1.7–2.05)24,26,28,29
The SLCO1B1 gene product is responsible for hepatic uptake of statins. rs4149056 is a non-synonymous missense variant in exon 5 of the SLCO1B1 gene that changes a conserved valine amino acid to alanine at position 174. The global minor allele frequency of this variant is 12.9%,46 but its frequency varies widely across different ethnic groups, from 2% in individuals of African ancestry to 21% in individuals of Finnish ancestry. At a cellular level, this variant results in reduced transporter activity.47In vivo, individuals carrying this variant have impaired clearance of a number of statins.48,49 In the presence of the loss-of-function rs4149056 variant, plasma levels of statin are increased,48,49 leading to greater systemic exposure, including at the level of muscle, which is thought to be the mechanism for the increased risk of SAMS in these patients (Figure 1).
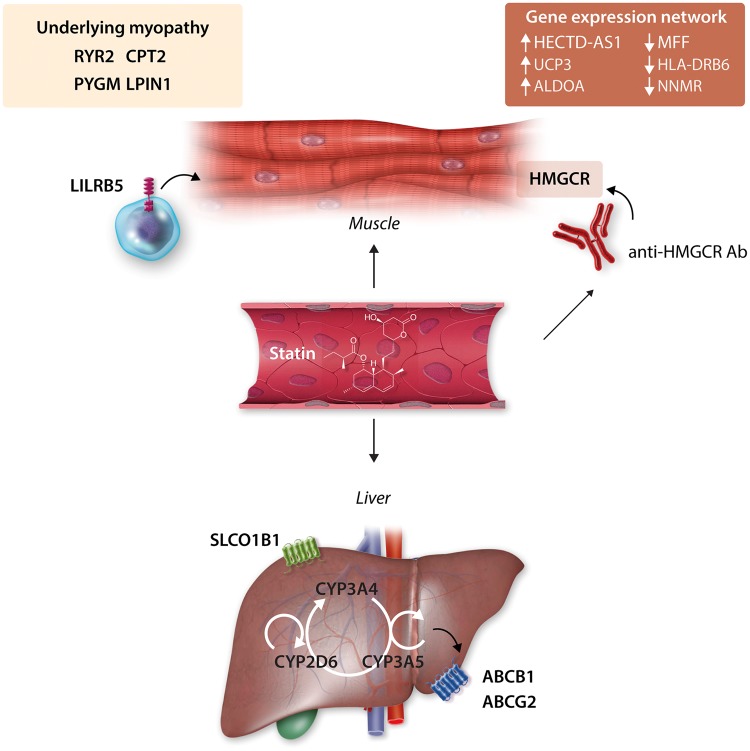
Figure 1.
Biological mechanisms of statin-associated muscle symptoms (SAMS) implicated by genetics. A loss-of-function variant in the SLCO1B1 gene, which encodes a hepatic statin transporter, results in increased plasma statin concentration and increases the risk of SAMS, particularly for simvastatin. Variation in cytochrome P450 metabolic enzymes including CYP3A4, CYP3A5, and CYP2D6 may also predispose to SAMS. Variation in transporters responsible for efflux of statins from hepatocytes including ABCB1 and ABCG2 are also linked to increased plasma concentration and risk of SAMS. At the level of the muscle, statin use may unmask underlying or pre-symptomatic myopathic conditions, for example, those caused by variation in the CPT2, PYGM, RYR2, and LPIN1 genes. The LILRB5 gene, a leucocyte receptor expressed predominantly in immune cells, has been implicated in SAMS, in particular carriers of the p.Asp247 allele. The mechanism is uncertain, but may involve immune-mediated repair of muscle injury. Patients with SAMS also display a specific gene-expression network in muscle that involves pathways related to cellular stress, repair, immune response, and protein catabolism. Finally, statin treatment in immunogenetically susceptible individuals (those with the DRB1*11: 01 allele) can lead to the production of anti-HMG CoA Reductase (anti-HMGCR) antibodies, which may have a direct pathogenic effect on muscle tissue.
The association of rs4149056 with SAMS is statin-specific and appears to be strongest for simvastatin, with a lesser association for atorvastatin or other statins.25,27,50 No association of this variant with clinical myalgia in patients receiving rosuvastatin was reported in the JUPITER trial population.51 This is in keeping with the much greater impact of this variant on plasma levels of simvastatin compared with other statins.48,49 The statin-specificity of this association has implications for management of patients and forms the basis of the guidance from the Clinical Pharmacogenomic Consortium that carriers of rs4149056 should be treated with a statin other than simvastatin.52
A smaller GWAS conducted in cases of cerivastatin-associated rhabdomyolysis identified through legal claims (n = 185) and matched to control patients treated with other statins (n = 645), identified an association with an intronic variant (rs2819742) in the ryanodine receptor 2 (RYR2) gene.39 While it is tempting to hypothesize that RYR2 variation may predispose to statin-induced myopathy in a manner analogous to the role of RYR1 in anaesthetic-induced malignant hyperthermia, such an effect remains speculative, and to date, the association between RYR2 and SAMS has not been independently replicated.
A genome-wide expression quantitative trait locus (eQTL) analysis in lymphoblastoid cell lines (LCLs) identified a cis-eQTL in the GATM gene that interacted with simvastatin treatment.35 A SNP at this locus (rs9806699) was more strongly associated with GATM expression after simvastatin treatment compared with after treatment with control buffer.35 This SNP was associated with a reduced risk of statin-associated myopathy in two patient cohorts.35 However, subsequent studies have failed to replicate this finding.36,53,54
Two GWAS have identified variation in the LILRB5 gene associated with circulating CK levels in the absence of statin use.55,56 In particular, individuals homozygous for the Asp247 allele of the p.Asp247Gly (rs12975366) variant have higher circulating CK levels. Recently, individuals homozygous for the common Asp247 genotype in the GoDARTS cohort were reported to be at increased risk for SAMS, which was defined as CK above the ULN after starting statin therapy (general statin intolerance), or discontinuation of two or more statins at the lowest approved doses irrespective of CK (low-dose intolerance).57 The association of LILRB5 with SAMS was replicated in the PREDICTION-ADR cohort, based on CK >4 × ULN, and in the JUPITER trial cohort, using patient-reported myalgia.57 The overall odds ratio of SAMS with the risk genotype was 1.3. Interestingly, this variant was associated with myalgia in patients assigned to both rosuvastatin and placebo in the JUPITER trial, suggesting that this variant may have statin-independent effects on myalgia.
LILRB5 is an innate immune receptor expressed predominantly in immune cells. How this protein mediates SAMS is unclear. It is hypothesized that LILRB5 may play a role in immune-mediated repair of skeletal muscle damage.58 Direct experimental approaches to study this gene in relevant model systems will be needed to understand its role in SAMS.
GWAS interrogate primarily common variants in the genome, but may not detect rare variants associated with SAMS. One exome sequencing study has been reported in 88 individuals with myopathy, without matched controls. A nonsense variant in the CLCN1 gene (rs55960271, p.Arg894Ter) was identified in four (4.5%) individuals.41 This variant, which has a global minor allele frequency of 0.29%,46 awaits further replication of its association with SAMS.
4.3 HLA basis for statin-associated necrotizing myopathy
Statin-induced muscle complaints usually resolve following statin discontinuation. However, rarely statins can trigger a self-sustaining form of autoimmune myopathy, termed statin-associated autoimmune myopathy. This condition is associated with autoantibodies that recognize HMG-CoA reductase (HMGCR), the pharmacologic target of statins and the expression of which is up-regulated in response to statin therapy.59 These antibodies may have a direct pathogenic effect on muscle tissue expressing HMGCR.60 Affected patients typically present with myalgia, proximal muscle weakness, CK levels over 1000 IU/L, and prominent myofiber necrosis on muscle biopsy. Patients with statin-associated autoimmune myopathy and anti-HMG-CoA reductase autoantibodies often require chronic immunosuppressive therapy to control the disease and prevent flares.
While the SLCO1B1 rs4149056 variant is not a risk factor for developing statin-associated autoimmune myopathy,61 the HLA class II allele DRB1*11:01 appears to be strongly associated with this disease.62,63 Indeed, one study63 showed that 65% of white and 88% of African American anti-HMG-CoA reductase autoantibody positive patients had the DRB1*11:01 allele, while only 7% and 11% of control whites and control African Americans, respectively, had a copy. Thus, the odds ratios for the presence of DRB1*11:01 in anti-HMGCR myopathy patients vs. controls are 24.5 in whites and 56.5 in African Americans. Nonetheless, despite these strong odds ratios, only a tiny fraction of patients with DRB1*11:01 will develop an autoimmune myopathy after statin exposure. As a result, testing for this allele may help confirm the diagnosis in patients suspected of having statin-associated autoimmune myopathy, but would not be clinically useful as a means of screening for patients who are at risk of this rare ADR. It should be noted that the association of statin-associated autoimmune myopathy with DRB1*11:01 is based on observations in a small number of individuals, given the extreme rarity of this condition.
4.4 Markers of underlying neuromuscular disease in SAMS
In a variety of primary neuromuscular disorders, the musculoskeletal effects of statins may unmask muscular symptoms and manifest as SAMS (Table 3). The earliest associations with inflammatory myopathies suggested a causal relationship.64–67,85 However, whether these myositides were in pre-symptomatic or minimally symptomatic states that were made worse by statin exposure, or whether the statins induced the myopathy, is unknown. Statins have been shown to up-regulate atrogin-186,87 and MHC-188 in skeletal myocytes—effects that can, in theory, contribute to weakness and inflammation, respectively.
Table 3.
Neuromuscular diseases and myopathies associated with statin use
| Conditions | Clinical features | References |
|---|---|---|
| Dermatomyositis/polymyositis | Muscle weakness, skin findings | 64–67 , 68–70 |
| Inclusion body myositis | Muscle weakness of proximal and distal muscles | 71 |
| Myasthenia gravis | Fatigable muscle weakness | 72 , 73 |
| Mitochondrial myopathy | Variable; muscle weakness with multi-system involvement | 74 , 75 |
| McArdle disease | Exercise intolerance, fatigue, myalgia, cramps | 76–78 |
| Myotonic dystrophy 1 and 2 | Weakness of facial and hand muscles (DM1) or neck and finger flexors (DM2); myotonia, cataracts | 79 , 80 |
| CPT2 deficiency | Muscle weakness, myoglobinuria | 76 |
| Acid maltase deficiency | Muscle weakness in limb-girdle distribution | 81 |
| Rippling muscle disease | Muscle weakness in limb-girdle distribution | 82 |
| Amyotrophic lateral sclerosis | Upper and lower motor neuron involvement | 83 |
| Muscle phosphorylase b kinase deficiency | Muscle weakness, hepatomegaly, myotonia | 84 |
| Myoadenylate deaminase deficiency | Exertional muscle weakness, cramps and myalgia | 76 |
| Kennedy disease | Weakness of facial, bulbar and limb muscles | 80 |
Patients with underlying neuromuscular disorders are thought to be more susceptible to SAMS but, to our knowledge, this has not been systematically investigated. Early suggestive evidence for such intolerance came from a report identifying an over-representation of heterozygous carrier status for two of the most common inherited myopathies in SAMS cases when compared with the background population frequency (i.e. PYGM, 20-fold; CPT2, 11-fold increase over population frequency).76 Additional genetic myopathies have also been associated with statin intolerance, including acid maltase deficiency,81 phosphorylase b kinase deficiency,84 myotonic dystrophies types 1 and 279,80 mitochondrial myopathies,74 Kennedy disease,80 malignant hyperthermia,34 sporadic rippling muscle disease,82 amyotrophic lateral sclerosis,83 and myasthenia gravis.72,73,89 Individuals heterozygous for mutations in the LPIN1 gene—a cause of childhood rhabdomyolysis—have also been reported to develop myopathy following statin administration.90,91 With many of these case reports, it is unknown whether statin exposure played a causal role in unmasking the neuromuscular phenotype, or whether this was simply the natural progression of the condition.
Rare copy number variations in EYS (28 kb deletion) and LARGE (6.4 kb deletion) have been associated with SAMS.92 Mutations in EYS cause autosomal recessive retinitis pigmentosa and have previously been associated with SAMS.93 However, expression in the spinal cord and possibly the muscle suggest a potential role for the EGF-repeats to exert a Notch-1-like signalling effect on myoblast differentiation and skeletal muscle regeneration after exercise.94 The laminin G domains of EYS may interact with α-dystroglycan which itself is modified by dolichol-dependent N-linked glycosylation, a function possibly impaired by statins.95 Interestingly, mutations in LARGE (acetylglucosaminyltransferase-like protein) cause autosomal recessive congenital muscular dystrophy, due to hypoglycosylation of α-dystroglycan and impaired laminin binding.96
Several questions arise when considering the diverse nature of the reported neuromuscular conditions. First, do these patients harbour the same SAMS susceptibility loci as individuals without underlying neuromuscular disease? Second, if the neuromuscular disorder in fact represents a genetic susceptibility, then what is the magnitude of the risk for SAMS in such patients if exposed to statin? Additional studies in large cohorts of well-phenotyped individuals will be needed to answer these questions.
4.5 Multi-omic networks
Candidate gene and GWAS have identified single genes associated with SAMS, but leave unexplained most of the risk for this condition. This suggests that understanding the molecular mechanisms underlying SAMS will require additional strategies. One approach is the use of multi-omic networks97 to study the contribution of transcription, metabolism, and metabolite levels to SAMS.
A potential role for gene expression networks in SAMS was identified in a recent study that compared transcriptome-wide gene expression patterns from muscle biopsy specimens of patients with reproducible muscle symptoms occurring with statin use, compared with statin-tolerant controls.98 Patients with statin-induced myalgia displayed a distinct programme of gene expression patterns that differentiated them from controls. The most significantly up-regulated genes included HECTD2-AS1, UCP3, and ALDOA, whereas the most significantly down-regulated genes included MFF, HLA-DRB6, and NNMT. The cellular pathways most significantly altered in SAMS cases included cellular stress, immune response, protein catabolism, cholesterol bio-synthesis, protein prenylation, and RAS-GTPase activation. These observations suggest the presence of a unique network of gene expression changes in patients with SAMS and speak to the potential utility of multi-omic-based network analyses to more fully elucidate the molecular mechanisms involved in patients with SAMS.
Transcriptome level data from patient-derived LCLs have been shown to explain a proportion of the variance in LDL-C response to statins and may also have relevance for prediction of SAMS.99 Additional approaches that combine combinations of genomic, transcriptomic, and clinical data may result in improved clinical accuracy in the prediction or diagnosis of SAMS.
While most work to date has focused on transcriptome level data, multi-omic approaches could also include assessment of proteomic, metabolomic, epigenomic, or microbiomic profiles that correlate with SAMS, providing further insight into its molecular causes. For example, statin therapy has been shown to result in profound changes in the gut microbiome in mice,100 and the levels of bacterial-derived bile acids correlate with the magnitude of LDL-C lowering in statin-treated patients.101 Whether statin-induced changes in the microbiome are predictive of SAMS remains to be investigated.
5. Clinical utility of genetic markers for SAMS
To what extent can knowledge of the genetics of SAMS be used to more effectively treat patients and optimize adherence to statins? The SLCO1B1 rs4149056 variant is by far the most robustly associated variant with SAMS, suggesting that testing for this variant may be used to identify or prevent SAMS. However, despite its highly statistical significant association with SAMS, data to support the clinical validity of testing for rs4149056 remain limited. Based on published data, the sensitivity and specificity of one copy of the rs4149056 risk allele is estimated to be 70.4% and 73.7%, respectively.102 The corresponding positive and negative predictive values are 4.1% and 99.4%, respectively. The low positive predictive value relates to the fact that while rs4149056 is common (12.9%), statin myopathy is rare (typically <1 in 10 000 individuals103). These observations suggest that SLCO1B1 genotype, in isolation, lacks sufficient sensitivity and specificity to be used as an accurate diagnostic test for SAMS. The relatively high negative predictive value may be helpful to rule out true statin-induced myopathy, particularly in response to simvastatin.
Notwithstanding this, genetic testing may be of clinical benefit to patients and providers in optimizing medication adherence through provision of reassurance. The potential clinical benefit of genotype-guided statin therapy has been explored in a small, non-randomized trial of 58 patients non-adherent to statins who underwent genotyping of rs4149056 with results provided to the patients and their primary care physicians, as well as 59 control individuals who did not undergo genotyping.104 Patients who underwent genotyping had an increase in their perceived ‘need for statin to prevent sickness’ and a decrease in their perceived ‘concern for statin to disrupt life’. Genotyped patients also had an increased rate of new statin prescriptions, and a greater decrease in LDL-C compared with non-genotyped patients. Interestingly, the benefit among genotyped patients was present in both carriers of the rs4149056 risk allele and non-carriers, suggesting that genetic testing, regardless of the test result, may provide benefit to patients and providers in terms of addressing concerns about perceived risk of ADRs, participating in shared-decision making, and optimizing medication adherence. The observations from this small trial are currently being explored in a larger, randomized trial of rs4149056 genotyping.105,106 Future studies addressing the cost-effectiveness of these approaches would be needed to support broader adoption of this type of pharmacogenetic testing. Already, genotyping for SLCO1B1 is offered by many commercial services and can be accessed by providers in many jurisdictions.
The role of rs4149056 genotyping is also being investigated in a randomized clinical trial in which 400 statin-naive patients will undergo genotyping, and be randomized to receive genotype results immediately, or after 1 year.107 The primary outcome is change in LDL-C, and secondary outcomes will include SAMS, and concordance of statin use with professional society recommendations.
Several healthcare systems and jurisdictions have established pharmacogenetic implementation programmes that include pre-emptive genotyping of SLCO1B1 (i.e. genotyping performed in individuals who do not currently have an indication for the associated medication). These include the PREDICT programme at Vanderbilt,108 the PG4KDS programme at St. Jude Children's Research Hospital,109 the Ubiquitous Pharmacogenomics (U-PGx) programme at several sites in Europe,110 the CLIPMERGE PGx Programme at Mount Sinai,111 the INGENIOUS trial (NCT02297126) at Indiana University School of Medicine,112 and the Right Drug, Right Dose, Right Time—Using Genomic Data to Individualize Treatment (RIGHT) protocol at the Mayo Clinic.113 All of these efforts include strong clinical decision support tools to incorporate genotype into clinical care and rely on clinical practice guideline recommendations for the use of SLCO1B1 genotype in statin prescribing.52
The availability of evidence-based non-statin therapies to reduce LDL-C and lower cardiovascular risk114,115 provides new opportunity to treat patients in whom the presence of SAMS precludes adequate treatment of LDL-C-related risk. For example, in the GAUSS-3 trial of patients with a history of SAMS, 42.6% of patients experienced muscle symptoms while receiving blinded atorvastatin but not placebo. When these same patients were randomized to ezetimibe or evolucumab in Phase B, muscle-related events occurred less frequently (in 20.7% of patients randomized to evolocumab and 28.8% randomized to ezetimibe).9 Evolocumab was associated with a significantly greater reduction in LDL-C compared with ezetimibe. Knowledge of a patient’s genetic risk profile for SAMS may, therefore, aid in the earlier selection of evidence-based, non-statin therapies for patients with high risk for SAMS.
6. Future directions
Given the limitation of SLCO1B1 genotyping in accurately identifying patients at risk of SAMS, it seems likely that new approaches will be required that incorporate other sources of data to improve clinical validity. A score based on the number of risk alleles in three candidate genes, COQ2, ATP2B1, and DMPK, has been shown to provide good discrimination between patients with or without statin-associated myalgia.30 Clinical factors are also known to influence risk for SAMS, including advanced age, female sex, lower body mass index, and renal or hepatic dysfunction.29 A dosing algorithm incorporating SLCO1B1 genotype, ABCG2 genotype, sex, age, body mass index, and time of last dose predicts plasma drug levels of rosuvastatin and atorvastatin.116 Patients predicted to tolerate high-dose atorvastatin using this model had greater adherence to 80 mg of atorvastatin after 1 year compared with patients predicted not to tolerate this dose, although the difference did not reach statistical significance.116 Future studies incorporating clinical variables, risk genotypes, transcriptome data, and metabolite profiling may be able to further refine risk prediction for patients at risk for SAMS.
7. Summary and conclusions
SAMS is a major contributor to under-use of statins and to an increase in preventable morbidity and mortality in patients for whom statin treatment is indicated. The genetic basis of SAMS has been investigated extensively, and points towards multiple pathways that can lead to SAMS (Figure 1), including altered statin pharmacokinetics, immunological response, muscle regeneration, mitochondrial function, and possibly underlying susceptibility to inherited myopathy. Clinical implementation of pharmacogenetic testing for some of these markers is being studied in many healthcare systems, and preliminary findings suggest that this approach may improve patient adherence to statins. Future studies focusing on multi-omic networks will help to further delineate the molecular pathways involved in SAMS and may lead to more personalized approaches to optimize statin use.
Conflict of interest: L.R.B. has served on an advisory board for Sanofi and has received research grants from Sanofi and Amgen. G.B.J.M. has served on advisory boards for Amgen, Sanofi, Boehringer Ingelheim, and Esperion. He has received research grants to institution from Amgen and Merck and has received honoraria from Amgen and Sanofi. R.S.R. has served on advisory boards for Akcea, Amgen, CVS Caremark, Eli Lilly, Regeneron, and Sanofi and has received research grants to his institution from Akcea, Amgen, AstraZeneca, Medicines Company, and Regeneron. He had received honoraria from Akcea, Kowa and Pfizer and has received royalties from UpToDate. S.B. has reported that he has no relationships relevant to the contents of this article to disclose.
Funding
L.R.B. is supported by a CIHR New Investigator Award and is a Michael Smith Foundation for Health Research Scholar.
References
- 1. Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC Jr, Watson K, Wilson PW, Eddleman KM, Jarrett NM, LaBresh K, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC Jr, Tomaselli GF; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129:S1–S45. [DOI] [PubMed] [Google Scholar]
- 2. Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, Hoes AW, Jennings CS, Landmesser U, Pedersen TR, Reiner Z, Riccardi G, Taskinen MR, Tokgozoglu L, Verschuren WM, Vlachopoulos C, Wood DA, Zamorano JL.. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias: the Task Force for the Management of Dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Atherosclerosis 2016;253:281–344. [DOI] [PubMed] [Google Scholar]
- 3. Rosenson RS, Farkouh ME, Mefford M, Bittner V, Brown TM, Taylor B, Monda KL, Zhao H, Dai Y, Muntner P.. Trends in use of high-intensity statin therapy after myocardial infarction, 2011 to 2014. J Am Coll Cardiol 2017;69:2696–2706. [DOI] [PubMed] [Google Scholar]
- 4. Chowdhury R, Khan H, Heydon E, Shroufi A, Fahimi S, Moore C, Stricker B, Mendis S, Hofman A, Mant J, Franco OH.. Adherence to cardiovascular therapy: a meta-analysis of prevalence and clinical consequences. Eur Heart J 2013;34:2940–2948. [DOI] [PubMed] [Google Scholar]
- 5. Serban MC, Colantonio LD, Manthripragada AD, Monda KL, Bittner VA, Banach M, Chen L, Huang L, Dent R, Kent ST, Muntner P, Rosenson RS.. Statin intolerance and risk of coronary heart events and all-cause mortality following myocardial infarction. J Am Coll Cardiol 2017;69:1386–1395. [DOI] [PubMed] [Google Scholar]
- 6. Zhang H, Plutzky J, Shubina M, Turchin A.. Continued statin prescriptions after adverse reactions and patient outcomes. Ann Intern Med 2017;167:221–227. [DOI] [PubMed] [Google Scholar]
- 7. Moriarty PM, Thompson PD, Cannon CP, Guyton JR, Bergeron J, Zieve FJ, Bruckert E, Jacobson TA, Kopecky SL, Baccara-Dinet MT, Du Y, Pordy R, Gipe DA; ODYSSEY ALTERNATIVE Investigators. Efficacy and safety of alirocumab vs ezetimibe in statin-intolerant patients, with a statin rechallenge arm: the ODYSSEY ALTERNATIVE randomized trial. J Clin Lipidol 2015;9:758–769. [DOI] [PubMed] [Google Scholar]
- 8. Parker BA, Capizzi JA, Grimaldi AS, Clarkson PM, Cole SM, Keadle J, Chipkin S, Pescatello LS, Simpson K, White CM, Thompson PD.. Effect of statins on skeletal muscle function. Circulation 2013;127:96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nissen SE, Stroes E, Dent-Acosta RE, Rosenson RS, Lehman SJ, Sattar N, Preiss D, Bruckert E, Ceska R, Lepor N, Ballantyne CM, Gouni-Berthold I, Elliott M, Brennan DM, Wasserman SM, Somaratne R, Scott R, Stein EA; GAUSS-3 Investigators. Efficacy and tolerability of evolocumab vs ezetimibe in patients with muscle-related statin intolerance: the GAUSS-3 randomized clinical trial. JAMA 2016;315:1580–1590. [DOI] [PubMed] [Google Scholar]
- 10. Taylor BA, Lorson L, White CM, Thompson PD.. A randomized trial of coenzyme Q10 in patients with confirmed statin myopathy. Atherosclerosis 2015;238:329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rosenson RS, Baker S, Banach M, Borow KM, Braun LT, Bruckert E, Brunham LR, Catapano AL, Elam MB, Mancini GBJ, Moriarty PM, Morris PB, Muntner P, Ray KK, Stroes ES, Taylor BA, Taylor VH, Watts GF, Thompson PD.. Optimizing cholesterol treatment in patients with muscle complaints. J Am Coll Cardiol 2017;70:1290–1301. [DOI] [PubMed] [Google Scholar]
- 12. Rosenson RS, Miller K, Bayliss M, Sanchez RJ, Baccara-Dinet MT, Chibedi-De-Roche D, Taylor B, Khan I, Manvelian G, White M, Jacobson TA.. The Statin-Associated Muscle Symptom Clinical Index (SAMS-CI): revision for clinical use, content validation, and inter-rater reliability. Cardiovasc Drugs Ther 2017;31:179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rosenson RS, Baker SK, Jacobson TA, Kopecky SL, Parker BA; The National Lipid Association's Muscle Safety Expert Panel. An assessment by the Statin Muscle Safety Task Force: 2014 update. J Clin Lipidol 2014;8:S58–S71. [DOI] [PubMed] [Google Scholar]
- 14. Taylor BA, Sanchez RJ, Jacobson TA, Chibedi-De-Roche D, Manvelian G, Baccara-Dinet MT, Khan I, Rosenson RS.. Application of the statin-associated muscle symptoms-clinical index to a randomized trial on statin myopathy. J Am Coll Cardiol 2017;70:1680–1681. [DOI] [PubMed] [Google Scholar]
- 15. Mancini GB, Baker S, Bergeron J, Fitchett D, Frohlich J, Genest J, Gupta M, Hegele RA, Ng D, Pearson GJ, Pope J, Tashakkor AY.. Diagnosis, prevention, and management of statin adverse effects and intolerance: Canadian Consensus Working Group Update (2016). Can J Cardiol 2016;32:S35–S65. [DOI] [PubMed] [Google Scholar]
- 16. Stroes ES, Thompson PD, Corsini A, Vladutiu GD, Raal FJ, Ray KK, Roden M, Stein E, Tokgözoğlu L, Nordestgaard BG, Bruckert E, De Backer G, Krauss RM, Laufs U, Santos RD, Hegele RA, Hovingh GK, Leiter LA, Mach F, März W, Newman CB, Wiklund O, Jacobson TA, Catapano AL, Chapman MJ, Ginsberg HN, Stroes E, Thompson PD, Corsini A, Vladutiu GD, Raal FJ, Ray KK, Roden M, Stein E, Tokgözoğlu L, Nordestgaard BG, Bruckert E, Krauss RM, Laufs U, Santos RD, März W, Newman CB, John Chapman M, Ginsberg HN, John Chapman M, Ginsberg HN, de Backer G, Catapano AL, Hegele RA, Kees Hovingh G, Jacobson TA, Leiter L, Mach F, Wiklund O; European Atherosclerosis Society Consensus Panel . Statin-associated muscle symptoms: impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur Heart J 2015;36:1012–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Graham DJ, Staffa JA, Shatin D, Andrade SE, Schech SD, La Grenade L, Gurwitz JH, Chan KA, Goodman MJ, Platt R.. Incidence of hospitalized rhabdomyolysis in patients treated with lipid-lowering drugs. JAMA 2004;292:2585–2590. [DOI] [PubMed] [Google Scholar]
- 18. Pasternak RC, Smith SC Jr, Bairey-Merz CN, Grundy SM, Cleeman JI, Lenfant C; American College of Cardiology; American Heart Association; National Heart, Lung and Blood Institute. ACC/AHA/NHLBI clinical advisory on the use and safety of statins. J Am Coll Cardiol 2002;40:567–572. [DOI] [PubMed] [Google Scholar]
- 19. Mulder AB, van Lijf HJ, Bon MA, van den Bergh FA, Touw DJ, Neef C, Vermes I.. Association of polymorphism in the cytochrome CYP2D6 and the efficacy and tolerability of simvastatin. Clin Pharmacol Ther 2001;70:546–551. [DOI] [PubMed] [Google Scholar]
- 20. Frudakis TN, Thomas MJ, Ginjupalli SN, Handelin B, Gabriel R, Gomez HJ.. CYP2D6*4 polymorphism is associated with statin-induced muscle effects. Pharmacogenet Genomics 2007;17:695–707. [DOI] [PubMed] [Google Scholar]
- 21. Wilke RA, Moore JH, Burmester JK.. Relative impact of CYP3A genotype and concomitant medication on the severity of atorvastatin-induced muscle damage. Pharmacogenet Genomics 2005;15:415–421. [DOI] [PubMed] [Google Scholar]
- 22. Ovesjo ML, Skilving I, Bergman P, Rane A, Ekstrom L, Bjorkhem-Bergman L.. Low vitamin D levels and genetic polymorphism in the vitamin D receptor are associated with increased risk of statin-induced myopathy. Basic Clin Pharmacol Toxicol 2016;118:214–218. [DOI] [PubMed] [Google Scholar]
- 23. Link E, Parish S, Armitage J, Bowman L, Heath S, Matsuda F, Gut I, Lathrop M, Collins R.. SLCO1B1 variants and statin-induced myopathy—a genomewide study. N Engl J Med 2008;359:789–799. [DOI] [PubMed] [Google Scholar]
- 24. Voora D, Shah SH, Spasojevic I, Ali S, Reed CR, Salisbury BA, Ginsburg GS.. The SLCO1B1*5 genetic variant is associated with statin-induced side effects. J Am Coll Cardiol 2009;54:1609–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brunham LR, Lansberg PJ, Zhang L, Miao F, Carter C, Hovingh GK, Visscher H, Jukema JW, Stalenhoef AF, Ross CJ, Carleton BC, Kastelein JJ, Hayden MR.. Differential effect of the rs4149056 variant in SLCO1B1 on myopathy associated with simvastatin and atorvastatin. Pharmacogenomics J 2012;12:233–237. [DOI] [PubMed] [Google Scholar]
- 26. Donnelly LA, Doney AS, Tavendale R, Lang CC, Pearson ER, Colhoun HM, McCarthy MI, Hattersley AT, Morris AD, Palmer CN.. Common nonsynonymous substitutions in SLCO1B1 predispose to statin intolerance in routinely treated individuals with type 2 diabetes: a go-DARTS study. Clin Pharmacol Ther 2011;89:210–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Carr DF, O’Meara H, Jorgensen AL, Campbell J, Hobbs M, McCann G, van Staa T, Pirmohamed M.. SLCO1B1 genetic variant associated with statin-induced myopathy: a proof-of-concept study using the clinical practice research datalink. Clin Pharmacol Ther 2013;94:695–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu JE, Liu XY, Chen S, Zhang Y, Cai LY, Yang M, Lai WH, Ren B, Zhong SL.. SLCO1B1 521T > C polymorphism associated with rosuvastatin-induced myotoxicity in Chinese coronary artery disease patients: a nested case-control study. Eur J Clin Pharmacol 2017;73:1409–1416. [DOI] [PubMed] [Google Scholar]
- 29. Bakar NS, Neely D, Avery P, Brown C, Daly AK, Kamali F.. Genetic and Clinical factors are associated with statin-related myotoxicity of moderate severity: a case-control study. Clin Pharmacol Ther 2017; doi:10.1002/cpt.887. [DOI] [PubMed] [Google Scholar]
- 30. Ruano G, Windemuth A, Wu AH, Kane JP, Malloy MJ, Pullinger CR, Kocherla M, Bogaard K, Gordon BR, Holford TR, Gupta A, Seip RL, Thompson PD.. Mechanisms of statin-induced myalgia assessed by physiogenomic associations. Atherosclerosis 2011;218:451–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Oh J, Ban MR, Miskie BA, Pollex RL, Hegele RA.. Genetic determinants of statin intolerance. Lipids Health Dis 2007;6:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Puccetti L, Ciani F, Auteri A.. Genetic involvement in statins induced myopathy. Preliminary data from an observational case-control study. Atherosclerosis 2010;211:28–29. [DOI] [PubMed] [Google Scholar]
- 33. Ruano G, Thompson PD, Windemuth A, Seip RL, Dande A, Sorokin A, Kocherla M, Smith A, Holford TR, Wu AH.. Physiogenomic association of statin-related myalgia to serotonin receptors. Muscle Nerve 2007;36:329–335. [DOI] [PubMed] [Google Scholar]
- 34. Vladutiu GD, Isackson PJ, Kaufman K, Harley JB, Cobb B, Christopher-Stine L, Wortmann RL.. Genetic risk for malignant hyperthermia in non-anesthesia-induced myopathies. Mol Genet Metab 2011;104:167–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mangravite LM, Engelhardt BE, Medina MW, Smith JD, Brown CD, Chasman DI, Mecham BH, Howie B, Shim H, Naidoo D, Feng Q, Rieder MJ, Chen YD, Rotter JI, Ridker PM, Hopewell JC, Parish S, Armitage J, Collins R, Wilke RA, Nickerson DA, Stephens M, Krauss RM.. A statin-dependent QTL for GATM expression is associated with statin-induced myopathy. Nature 2013;502:377–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Luzum JA, Kitzmiller JP, Isackson PJ, Ma C, Medina MW, Dauki AM, Mikulik EB, Ochs-Balcom HM, Vladutiu GD.. GATM polymorphism associated with the risk for statin-induced myopathy does not replicate in case-control analysis of 715 dyslipidemic individuals. Cell Metab 2015;21:622–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Becker ML, Visser LE, van Schaik RH, Hofman A, Uitterlinden AG, Stricker BH.. Influence of genetic variation in CYP3A4 and ABCB1 on dose decrease or switching during simvastatin and atorvastatin therapy. Pharmacoepidemiol Drug Saf 2010;19:75–81. [DOI] [PubMed] [Google Scholar]
- 38. Becker ML, Elens LL, Visser LE, Hofman A, Uitterlinden AG, van Schaik RH, Stricker BH.. Genetic variation in the ABCC2 gene is associated with dose decreases or switches to other cholesterol-lowering drugs during simvastatin and atorvastatin therapy. Pharmacogenomics J 2013;13:251–256. [DOI] [PubMed] [Google Scholar]
- 39. Marciante KD, Durda JP, Heckbert SR, Lumley T, Rice K, McKnight B, Totah RA, Tamraz B, Kroetz DL, Fukushima H, Kaspera R, Bis JC, Glazer NL, Li G, Austin TR, Taylor KD, Rotter JI, Jaquish CE, Kwok PY, Tracy RP, Psaty BM.. Cerivastatin, genetic variants, and the risk of rhabdomyolysis. Pharmacogenet Genomics 2011;21:280–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hubacek JA, Adamkova V, Hruba P, Ceska R, Vrablik M.. Association between polymorphism within the RYR2 receptor and development of statin-associated myalgia/myopathy in the Czech population. Eur J Intern Med 2015;26:367–368. [DOI] [PubMed] [Google Scholar]
- 41. Neřoldová M, Stránecký V, Hodaňová K, Hartmannová H, Piherová L, Přistoupilová A, Mrázová L, Vrablík M, Adámková V, Hubáček JA, Jirsa M, Kmoch S.. Rare variants in known and novel candidate genes predisposing to statin-associated myopathy. Pharmacogenomics 2016;17:1405–1414. [DOI] [PubMed] [Google Scholar]
- 42. Mirošević Skvrce N, Macolić Šarinić V, Šimić I, Ganoci L, Muačević Katanec D, Božina N.. ABCG2 gene polymorphisms as risk factors for atorvastatin adverse reactions: a case-control study. Pharmacogenomics 2015;16:803–815. [DOI] [PubMed] [Google Scholar]
- 43. Fiegenbaum M, da Silveira FR, Van der Sand CR, Van der Sand LC, Ferreira ME, Pires RC, Hutz MH.. The role of common variants of ABCB1, CYP3A4, and CYP3A5 genes in lipid-lowering efficacy and safety of simvastatin treatment. Clin Pharmacol Ther 2005;78:551–558. [DOI] [PubMed] [Google Scholar]
- 44. Keskitalo JE, Zolk O, Fromm MF, Kurkinen KJ, Neuvonen PJ, Niemi M.. ABCG2 polymorphism markedly affects the pharmacokinetics of atorvastatin and rosuvastatin. Clin Pharmacol Ther 2009;86:197–203. [DOI] [PubMed] [Google Scholar]
- 45. Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine (SEARCH) Collaborative Group, Collaborative G, Armitage J, Bowman L, Wallendszus K, Bulbulia R, Rahimi K, Haynes R, Parish S, Peto R, Collins R.. Intensive lowering of LDL cholesterol with 80 mg versus 20 mg simvastatin daily in 12,064 survivors of myocardial infarction: a double-blind randomised trial. Lancet 2010;376:1658–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lek M, Karczewski KJ, Minikel EV, Samocha KE, Banks E, Fennell T, O’Donnell-Luria AH, Ware JS, Hill AJ, Cummings BB, Tukiainen T, Birnbaum DP, Kosmicki JA, Duncan LE, Estrada K, Zhao F, Zou J, Pierce-Hoffman E, Berghout J, Cooper DN, Deflaux N, DePristo M, Do R, Flannick J, Fromer M, Gauthier L, Goldstein J, Gupta N, Howrigan D, Kiezun A, Kurki MI, Moonshine AL, Natarajan P, Orozco L, Peloso GM, Poplin R, Rivas MA, Ruano-Rubio V, Rose SA, Ruderfer DM, Shakir K, Stenson PD, Stevens C, Thomas BP, Tiao G, Tusie-Luna MT, Weisburd B, Won HH, Yu D, Altshuler DM, Ardissino D, Boehnke M, Danesh J, Donnelly S, Elosua R, Florez JC, Gabriel SB, Getz G, Glatt SJ, Hultman CM, Kathiresan S, Laakso M, McCarroll S, McCarthy MI, McGovern D, McPherson R, Neale BM, Palotie A, Purcell SM, Saleheen D, Scharf JM, Sklar P, Sullivan PF, Tuomilehto J, Tsuang MT, Watkins HC, Wilson JG, Daly MJ, MacArthur DG; Exome Aggregation Consortium. Analysis of protein-coding genetic variation in 60,706 humans. Nature 2016;536:285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kameyama Y, Yamashita K, Kobayashi K, Hosokawa M, Chiba K.. Functional characterization of SLCO1B1 (OATP-C) variants, SLCO1B1*5, SLCO1B1*15 and SLCO1B1*15+C1007G, by using transient expression systems of HeLa and HEK293 cells. Pharmacogenet Genomics 2005;15:513–522. [DOI] [PubMed] [Google Scholar]
- 48. Pasanen MK, Neuvonen M, Neuvonen PJ, Niemi M.. SLCO1B1 polymorphism markedly affects the pharmacokinetics of simvastatin acid. Pharmacogenet Genomics 2006;16:873–879. [DOI] [PubMed] [Google Scholar]
- 49. Pasanen MK, Fredrikson H, Neuvonen PJ, Niemi M.. Different effects of SLCO1B1 polymorphism on the pharmacokinetics of atorvastatin and rosuvastatin. Clin Pharmacol Ther 2007;82:726–733. [DOI] [PubMed] [Google Scholar]
- 50. Hou Q, Li S, Li L, Li Y, Sun X, Tian H.. Association between SLCO1B1 gene T521C polymorphism and statin-related myopathy risk: a meta-analysis of case-control studies. Medicine 2015;94:e1268.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Danik JS, Chasman DI, MacFadyen JG, Nyberg F, Barratt BJ, Ridker PM.. Lack of association between SLCO1B1 polymorphisms and clinical myalgia following rosuvastatin therapy. Am Heart J 2013;165:1008–1014. [DOI] [PubMed] [Google Scholar]
- 52. Ramsey LB, Johnson SG, Caudle KE, Haidar CE, Voora D, Wilke RA, Maxwell WD, McLeod HL, Krauss RM, Roden DM, Feng Q, Cooper-DeHoff RM, Gong L, Klein TE, Wadelius M, Niemi M.. The clinical pharmacogenetics implementation consortium guideline for SLCO1B1 and simvastatin-induced myopathy: 2014 update. Clin Pharmacol Ther 2014;96:423–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Carr DF, Alfirevic A, Johnson R, Chinoy H, van Staa T, Pirmohamed M.. GATM gene variants and statin myopathy risk. Nature 2014;513:E1.. [DOI] [PubMed] [Google Scholar]
- 54. Floyd JS, Bis JC, Brody JA, Heckbert SR, Rice K, Psaty BM.. GATM locus does not replicate in rhabdomyolysis study. Nature 2014;513:E1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kristjansson RP, Oddsson A, Helgason H, Sveinbjornsson G, Arnadottir GA, Jensson BO, Jonasdottir A, Jonasdottir A, Bragi Walters G, Sulem G, Oskarsdottir A, Benonisdottir S, Davidsson OB, Masson G, Magnusson OT, Holm H, Sigurdardottir O, Jonsdottir I, Eyjolfsson GI, Olafsson I, Gudbjartsson DF, Thorsteinsdottir U, Sulem P, Stefansson K.. Common and rare variants associating with serum levels of creatine kinase and lactate dehydrogenase. Nat Commun 2016;7:10572.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dube MP, Zetler R, Barhdadi A, Brown AM, Mongrain I, Normand V, Laplante N, Asselin G, Zada YF, Provost S, Bergeron J, Kouz S, Dufour R, Diaz A, de Denus S, Turgeon J, Rheaume E, Phillips MS, Tardif JC.. CKM and LILRB5 are associated with serum levels of creatine kinase. Circ Cardiovasc Genet 2014;7:880–886. [DOI] [PubMed] [Google Scholar]
- 57. Siddiqui MK, Maroteau C, Veluchamy A, Tornio A, Tavendale R, Carr F, Abelega NU, Carr D, Bloch K, Hallberg P, Yue QY, Pearson ER, Colhoun HM, Morris AD, Dow E, George J, Pirmohamed M, Ridker PM, Doney ASF, Alfirevic A, Wadelius M, Maitland-van der Zee AH, Chasman DI, Palmer CNA, Consortium P-A.. A common missense variant of LILRB5 is associated with statin intolerance and myalgia. Eur Heart J 2017;38:3569–3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Marz W, Laufs U.. Leucocyte immunoglobulin-like receptor subfamily-B5 (LILRB5) genetic variation and statin-associated muscle symptoms: another piece in a puzzling puzzle. Eur Heart J 2017;38:3576–3578. [DOI] [PubMed] [Google Scholar]
- 59. Mammen AL. Statin-associated autoimmune myopathy. N Engl J Med 2016;374:664–669. [DOI] [PubMed] [Google Scholar]
- 60. Mohassel P, Mammen AL.. Anti-HMGCR Myopathy. J Neuromuscul Dis 2018;5:11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mammen AL, Chung T, Christopher-Stine L, Rosen P, Rosen A, Doering KR, Casciola-Rosen LA.. Autoantibodies against 3-hydroxy-3-methylglutaryl-coenzyme A reductase in patients with statin-associated autoimmune myopathy. Arthritis Rheum 2011;63:713–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Limaye V, Bundell C, Hollingsworth P, Rojana-Udomsart A, Mastaglia F, Blumbergs P, Lester S.. Clinical and genetic associations of autoantibodies to 3-hydroxy-3-methyl-glutaryl-coenzyme a reductase in patients with immune-mediated myositis and necrotizing myopathy. Muscle Nerve 2015;52:196–203. [DOI] [PubMed] [Google Scholar]
- 63. Mammen AL, Gaudet D, Brisson D, Christopher-Stine L, Lloyd TE, Leffell MS, Zachary AA.. Increased frequency of DRB1*11:01 in anti-hydroxymethylglutaryl-coenzyme A reductase-associated autoimmune myopathy. Arthritis Care Res 2012;64:1233–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Goldman JA, Fishman AB, Lee JE, Johnson RJ.. The role of cholesterol-lowering agents in drug-induced rhabdomyolysis and polymyositis. Arthritis Rheum 1989;32:358–359. [DOI] [PubMed] [Google Scholar]
- 65. Khattak FH, Morris IM, Branford WA.. Simvastatin-associated dermatomyositis. Br J Rheumatol 1994;33:199. [DOI] [PubMed] [Google Scholar]
- 66. Hill C, Zeitz C, Kirkham B.. Dermatomyositis with lung involvement in a patient treated with simvastatin. Aust N Z J Med 1995;25:745–746. [DOI] [PubMed] [Google Scholar]
- 67. Rodriguez-Garcia JL, Serrano Commino M.. Lovastatin-associated dermatomyositis. Postgrad Med J 1996;72:694.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Vasconcelos OM, Campbell WW.. Dermatomyositis-like syndrome and HMG-CoA reductase inhibitor (statin) intake. Muscle Nerve 2004;30:803–807. [DOI] [PubMed] [Google Scholar]
- 69. Noel B, Cerottini JP, Panizzon RG.. Atorvastatin-induced dermatomyositis. Am J Med 2001;110:670–671. [DOI] [PubMed] [Google Scholar]
- 70. Giordano N, Senesi M, Mattii G, Battisti E, Villanova M, Gennari C.. Polymyositis associated with simvastatin. Lancet 1997;349:1600–1601. [DOI] [PubMed] [Google Scholar]
- 71. Supala-Berger A, Fine E, Heffner R, Young-McLain E.. Hyaline inclusion myopathy: unmasked by statin therapy. Muscle Nerve 2009;40:657–661. [DOI] [PubMed] [Google Scholar]
- 72. Cartwright MS, Jeffery DR, Nuss GR, Donofrio PD.. Statin-associated exacerbation of myasthenia gravis. Neurology 2004;63:2188.. [DOI] [PubMed] [Google Scholar]
- 73. Oh SJ, Dhall R, Young A, Morgan MB, Lu L, Claussen GC.. Statins may aggravate myasthenia gravis. Muscle Nerve 2008;38:1101–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Chariot P, Abadia R, Agnus D, Danan C, Charpentier C, Gherasdi RK.. Simvastatin-induced rhabdomyolysis followed by a MELAS syndrome. Am J Med 1993;94:109–110. [DOI] [PubMed] [Google Scholar]
- 75. Phillips PS, Haas RH, Bannykh S, Hathaway S, Gray NL, Kimura BJ, Vladutiu GD, England JD; Scripps Mercy Clinical Research Center. Statin-associated myopathy with normal creatine kinase levels. Ann Intern Med 2002;137:581–585. [DOI] [PubMed] [Google Scholar]
- 76. Vladutiu GD, Simmons Z, Isackson PJ, Tarnopolsky M, Peltier WL, Barboi AC, Sripathi N, Wortmann RL, Phillips PS.. Genetic risk factors associated with lipid-lowering drug-induced myopathies. Muscle Nerve 2006;34:153–162. [DOI] [PubMed] [Google Scholar]
- 77. Livingstone C, Al Riyami S, Wilkins P, Ferns GA.. McArdle's disease diagnosed following statin-induced myositis. Ann Clin Biochem 2004;41:338–340. [DOI] [PubMed] [Google Scholar]
- 78. Lorenzoni PJ, Silvado CE, Scola RH, Luvizotto M, Werneck LC.. McArdle disease with rhabdomyolysis induced by rosuvastatin: case report. Arq Neuropsiquiatr 2007;65:834–837. [DOI] [PubMed] [Google Scholar]
- 79. Screen M, Jonson PH, Raheem O, Palmio J, Laaksonen R, Lehtimaki T, Sirito M, Krahe R, Hackman P, Udd B.. Abnormal splicing of NEDD4 in myotonic dystrophy type 2: possible link to statin adverse reactions. Am J Pathol 2014;184:2322–2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Tsivgoulis G, Spengos K, Karandreas N, Panas M, Kladi A, Manta P.. Presymptomatic neuromuscular disorders disclosed following statin treatment. Arch Intern Med 2006;166:1519–1524. [DOI] [PubMed] [Google Scholar]
- 81. Voermans NC, Lammens M, Wevers RA, Hermus AR, van Engelen BG.. Statin-disclosed acid maltase deficiency. J Intern Med 2005;258:196–197. [DOI] [PubMed] [Google Scholar]
- 82. Baker SK, Tarnopolsky MA.. Sporadic rippling muscle disease unmasked by simvastatin. Muscle Nerve 2006;34:478–481. [DOI] [PubMed] [Google Scholar]
- 83. Zinman L, Sadeghi R, Gawel M, Patton D, Kiss A.. Are statin medications safe in patients with ALS? Amyotroph Lateral Scler 2008;9:223–228. [DOI] [PubMed] [Google Scholar]
- 84. Echaniz-Laguna A, Mohr M, Tranchant C.. Neuromuscular symptoms and elevated creatine kinase after statin withdrawal. N Engl J Med 2010;362:564–565. [DOI] [PubMed] [Google Scholar]
- 85. Schalke BB, Schmidt B, Toyka K, Hartung HP.. Pravastatin-associated inflammatory myopathy. N Engl J Med 1992;327:649–650. [DOI] [PubMed] [Google Scholar]
- 86. Bonifacio A, Mullen PJ, Mityko IS, Navegantes LC, Bouitbir J, Krahenbuhl S.. Simvastatin induces mitochondrial dysfunction and increased atrogin-1 expression in H9c2 cardiomyocytes and mice in vivo. Arch Toxicol 2016;90:203–215. [DOI] [PubMed] [Google Scholar]
- 87. Cao P, Hanai J, Tanksale P, Imamura S, Sukhatme VP, Lecker SH.. Statin-induced muscle damage and atrogin-1 induction is the result of a geranylgeranylation defect. FASEB J 2009;23:2844–2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Singh P, Kohr D, Kaps M, Blaes F.. Skeletal muscle cell MHC I expression: implications for statin-induced myopathy. Muscle Nerve 2010;41:179–184. [DOI] [PubMed] [Google Scholar]
- 89. Parmar B, Francis PJ, Ragge NK.. Statins, fibrates, and ocular myasthenia. Lancet 2002;360:717.. [DOI] [PubMed] [Google Scholar]
- 90. Michot C, Hubert L, Romero NB, Gouda A, Mamoune A, Mathew S, Kirk E, Viollet L, Rahman S, Bekri S, Peters H, McGill J, Glamuzina E, Farrar M, von der Hagen M, Alexander IE, Kirmse B, Barth M, Laforet P, Benlian P, Munnich A, JeanPierre M, Elpeleg O, Pines O, Delahodde A, de Keyzer Y, de Lonlay P.. Study of LPIN1, LPIN2 and LPIN3 in rhabdomyolysis and exercise-induced myalgia. J Inherit Metab Dis 2012;35:1119–1128. [DOI] [PubMed] [Google Scholar]
- 91. Zeharia A, Shaag A, Houtkooper RH, Hindi T, de Lonlay P, Erez G, Hubert L, Saada A, de Keyzer Y, Eshel G, Vaz FM, Pines O, Elpeleg O.. Mutations in LPIN1 cause recurrent acute myoglobinuria in childhood. Am J Hum Genet 2008;83:489–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Stranecky V, Neroldova M, Hodanova K, Hartmannova H, Piherova L, Zemankova P, Pristoupilova A, Vrablik M, Adamkova V, Kmoch S, Jirsa M.. Large copy-number variations in patients with statin-associated myopathy affecting statin myopathy-related loci. Physiol Res 2016;65:1005–1011. [DOI] [PubMed] [Google Scholar]
- 93. Isackson PJ, Ochs-Balcom HM, Ma C, Harley JB, Peltier W, Tarnopolsky M, Sripathi N, Wortmann RL, Simmons Z, Wilson JD, Smith SA, Barboi A, Fine E, Baer A, Baker S, Kaufman K, Cobb B, Kilpatrick JR, Vladutiu GD.. Association of common variants in the human eyes shut ortholog (EYS) with statin-induced myopathy: evidence for additional functions of EYS. Muscle Nerve 2011;44:531–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Conboy IM, Conboy MJ, Smythe GM, Rando TA.. Notch-mediated restoration of regenerative potential to aged muscle. Science 2003;302:1575–1577. [DOI] [PubMed] [Google Scholar]
- 95. Baker SK. Molecular clues into the pathogenesis of statin-mediated muscle toxicity. Muscle Nerve 2005;31:572–580. [DOI] [PubMed] [Google Scholar]
- 96. Longman C, Brockington M, Torelli S, Jimenez-Mallebrera C, Kennedy C, Khalil N, Feng L, Saran RK, Voit T, Merlini L, Sewry CA, Brown SC, Muntoni F.. Mutations in the human LARGE gene cause MDC1D, a novel form of congenital muscular dystrophy with severe mental retardation and abnormal glycosylation of alpha-dystroglycan. Hum Mol Genet 2003;12:2853–2861. [DOI] [PubMed] [Google Scholar]
- 97. Rosenson RS, Brewer HB Jr, Barter PJ, Bjorkegren JLM, Chapman MJ, Gaudet D, Kim DS, Niesor E, Rye KA, Sacks FM, Tardif JC, Hegele RA.. HDL and atherosclerotic cardiovascular disease: genetic insights into complex biology. Nat Rev Cardiol 2018;15:9–19. [DOI] [PubMed] [Google Scholar]
- 98. Elam MB, Majumdar G, Mozhui K, Gerling IC, Vera SR, Fish-Trotter H, Williams RW, Childress RD, Raghow R.. Patients experiencing statin-induced myalgia exhibit a unique program of skeletal muscle gene expression following statin re-challenge. PLoS One 2017;12:e0181308.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Kim K, Bolotin E, Theusch E, Huang H, Medina MW, Krauss RM.. Prediction of LDL cholesterol response to statin using transcriptomic and genetic variation. Genome Biol 2014;15:460.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Caparrós-Martín JA, Lareu RR, Ramsay JP, Peplies J, Reen FJ, Headlam HA, Ward NC, Croft KD, Newsholme P, Hughes JD, O’Gara F.. Statin therapy causes gut dysbiosis in mice through a PXR-dependent mechanism. Microbiome 2017;5:95.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Kaddurah-Daouk R, Baillie RA, Zhu H, Zeng ZB, Wiest MM, Nguyen UT, Wojnoonski K, Watkins SM, Trupp M, Krauss RM.. Enteric microbiome metabolites correlate with response to simvastatin treatment. PLoS One 2011;6:e25482.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Stewart A. SLCO1B1 polymorphisms and statin-induced myopathy. PLoS Curr 2013;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Alfirevic A, Neely D, Armitage J, Chinoy H, Cooper RG, Laaksonen R, Carr DF, Bloch KM, Fahy J, Hanson A, Yue QY, Wadelius M, Maitland-van Der Zee AH, Voora D, Psaty BM, Palmer CN, Pirmohamed M.. Phenotype standardization for statin-induced myotoxicity. Clin Pharmacol Ther 2014;96:470–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Li JH, Joy SV, Haga SB, Orlando LA, Kraus WE, Ginsburg GS, Voora D.. Genetically guided statin therapy on statin perceptions, adherence, and cholesterol lowering: a pilot implementation study in primary care patients. J Pers Med 2014;4:147–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Genetically Guided Statin Therapy. https://ClinicalTrials.gov/show/NCT01894230 (4 June 2018, date last accessed).
- 106. Singh K, Peyser B, Trujillo G, Milazzo N, Savard D, Haga SB, Musty M, Voora D.. Rationale and design of the SLCO1B1 genotype guided statin therapy trial. Pharmacogenomics 2016;17:1873–1880. [DOI] [PubMed] [Google Scholar]
- 107. Integrating Pharmacogenetics in Clinical Care. https://ClinicalTrials.gov/show/NCT02871934 (4 June 2018, date last accessed).
- 108. Pulley JM, Denny JC, Peterson JF, Bernard GR, Vnencak-Jones CL, Ramirez AH, Delaney JT, Bowton E, Brothers K, Johnson K, Crawford DC, Schildcrout J, Masys DR, Dilks HH, Wilke RA, Clayton EW, Shultz E, Laposata M, McPherson J, Jirjis JN, Roden DM.. Operational implementation of prospective genotyping for personalized medicine: the design of the Vanderbilt PREDICT project. Clin Pharmacol Ther 2012;92:87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Hoffman JM, Haidar CE, Wilkinson MR, Crews KR, Baker DK, Kornegay NM, Yang W, Pui CH, Reiss UM, Gaur AH, Howard SC, Evans WE, Broeckel U, Relling MV.. PG4KDS: a model for the clinical implementation of pre-emptive pharmacogenetics. Am J Med Genet C Semin Med Genet 2014;166C:45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Cecchin E, Roncato R, Guchelaar HJ, Toffoli G; Ubiquitous Pharmacogenomics Consortium. Ubiquitous Pharmacogenomics (U-PGx): The time for implementation is now. An Horizon2020 program to drive pharmacogenomics into clinical practice. Curr Pharm Biotechnol 2017;18:204–209. [DOI] [PubMed] [Google Scholar]
- 111. Gottesman O, Scott SA, Ellis SB, Overby CL, Ludtke A, Hulot JS, Hall J, Chatani K, Myers K, Kannry JL, Bottinger EP.. The CLIPMERGE PGx Program: clinical implementation of personalized medicine through electronic health records and genomics-pharmacogenomics. Clin Pharmacol Ther 2013;94:214–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Eadon MT, Desta Z, Levy KD, Decker BS, Pierson RC, Pratt VM, Callaghan JT, Rosenman MB, Carpenter JS, Holmes AM, McDonald CA, Benson EA, Patil AS, Vuppalanchi R, Gufford BT, Dave N, Robarge JD, Hyder MA, Haas DM, Kreutz RP, Dexter PR, Skaar TC, Flockhart DA.. Implementation of a pharmacogenomics consult service to support the INGENIOUS trial. Clin Pharmacol Ther 2016;100:63–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Bielinski SJ, Olson JE, Pathak J, Weinshilboum RM, Wang L, Lyke KJ, Ryu E, Targonski PV, Van Norstrand MD, Hathcock MA, Takahashi PY, McCormick JB, Johnson KJ, Maschke KJ, Rohrer Vitek CR, Ellingson MS, Wieben ED, Farrugia G, Morrisette JA, Kruckeberg KJ, Bruflat JK, Peterson LM, Blommel JH, Skierka JM, Ferber MJ, Black JL, Baudhuin LM, Klee EW, Ross JL, Veldhuizen TL, Schultz CG, Caraballo PJ, Freimuth RR, Chute CG, Kullo IJ.. Preemptive genotyping for personalized medicine: design of the right drug, right dose, right time-using genomic data to individualize treatment protocol. Mayo Clin Proc 2014;89:25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, Sever PS, Pedersen TR; FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017;376:1713–1722. [DOI] [PubMed] [Google Scholar]
- 115. Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, Darius H, Lewis BS, Ophuis TO, Jukema JW, De Ferrari GM, Ruzyllo W, De Lucca P, Im K, Bohula EA, Reist C, Wiviott SD, Tershakovec AM, Musliner TA, Braunwald E, Califf RM; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 2015;372:2387–2397. [DOI] [PubMed] [Google Scholar]
- 116. DeGorter MK, Tirona RG, Schwarz UI, Choi YH, Dresser GK, Suskin N, Myers K, Zou G, Iwuchukwu O, Wei WQ, Wilke RA, Hegele RA, Kim RB.. Clinical and pharmacogenetic predictors of circulating atorvastatin and rosuvastatin concentrations in routine clinical care. Circ Cardiovasc Genet 2013;6:400–408. [DOI] [PMC free article] [PubMed] [Google Scholar]