Abstract
Chemotherapy treats many types of cancer effectively but it often causes side effects. Chemotherapy works on active cells, such as cancer cells, and some healthy cells. Side effects happen when chemotherapy damages these healthy cells. Today, many more drugs are available to treat side effects than in the past. Triptorelin (Decapeptyl) is a gonadotropin-releasing hormone agonist that is reported to have many therapeutic effects besides being an anti-cancer agent. In the current study, intraperitoneal cyclophosphamide (65 mg/kg/day) was administered for 4 weeks to induce marked dystrophic changes in the cerebral cortex and hippocampus of male albino rats. After 4 weeks, we observed significant degeneration of neurocytes with dystrophic changes. Subcutaneous triptorelin (0.05 mg/kg/day) for 4 weeks significantly improved histological signs of degeneration and apoptosis. Anti-Bcl2 staining of sections of the cerebral cortex and hippocampus showed that the apoptotic index was increased. This finding was confirmed by the anti-p53 staining, which showed a significant decrease in the apoptotic index. Ultimately, such improvements were accompanied by significant restoration of normal brain histology, as revealed by hematoxylin and eosin. In conclusion, triptorelin can reverse the apoptotic changes induced by cyclophosphamide therapy, which is more marked in the hippocampus than cerebral cortex.
Keywords: cerebral cortex, cyclophosphamide, hippocampus, p53, triptorelin
1. Introduction
Management of many cancers has been achieved by aggressive chemotherapy and radiotherapy. Generally, chemotherapy is not specific, and places normal and cancer cells at risk by direct and/or indirect mechanisms [1]. Cancer patients can experience various adverse neurological symptoms, including cognitive dysfunction. Several studies reported that this was a real side effect of the disease and its treatment with chemotherapy on the brain function [2,3]. Many studies have focused on the acute effects of chemotherapy on cerebral cortex and hippocampal function and have reported transient acute memory impairment in mice treated with a single dose of cyclophosphamide (CP) [4,5,6]. Recent radiological studies reported that chemotherapy can induce partially reversible pathological changes in the gray and white matter of the cerebral cortex [7]. The hippocampus is one of the rare areas of the brain that exhibits neurogenesis. These new neurons created by the hippocampus are important for memory and learning and require brain-derived neurotrophic factor (BDNF). Many chemotherapeutic agents, such as 5-fluorouracil, significantly reduce the levels of BDNF in the hippocampus of rats [8]. Other chemotherapeutic agents, such as methotrexate, decrease hippocampal cell proliferation in rats following single intravenous injection [9].
CP is a cytotoxic alkylating agent that is most commonly used as an anti-cancer agent [10]. Recent reports reveal that intraperitoneal administration of CP causes oxidative stress in the brain [11] and massive cellular damage [12], consequently triggering apoptosis [13], and death of cancer and healthy cells [14]. CP uptake into healthy cells is higher than in cancer cells, rendering healthy cells more susceptible to damage [15]. The antineoplastic effects of CP are associated with phosphoramide mustard, while acrolein is linked with its toxic side effects [16,17]. Acrolein interferes with the tissue antioxidant defense system [18]. These metabolites reduce cellular resistance to oxidative stress, which can damage the blood–brain barrier [19]. CP can alkylate DNA, which prevents the duplication of the genome in dividing cells, arrests the S-phase of the cell cycle, and induces apoptosis in embryonic neural progenitor cells of the telencephalon 6-12 hours after administration [20,21].
Cancer patients are not the only group who are exposed to the hazards of CP. Pharmacists and nurses are also occupationally exposed to the drug during its production or distribution [22]. Dividing cells are sensitive to the cytotoxic effects of alkylating agents. It has been suggested that inhibition of the pituitary–gonadal axis reduces the rate of spermatogenesis as well as oogenesis [23].
Some immunoreactive studies have reported that gonadotropin releasing hormone (GnRH) receptors were detected in tissues taken from the cerebral cortex and hippocampus, especially the cornu ammonis) [24]. GnRH affects neuronal activity throughout the brain [25,26]. Triptorelin (Decapeptyl) is a GnRH agonist. It is used in the treatment of hormone-responsive cancers, such as prostate or breast cancer [27].
Bcl-2 is an antiapoptotic protein that suppress apoptosis. It is localized mainly in the mitochondrial membrane and plays an important role in protecting tissues from apoptotic cell death [28].
p53 is a tumor suppressor protein that plays a central role in cell cycle arrest and apoptosis [29]. p53 is a proapoptotic short-lived protein (half-life 10–30 minutes) that is constitutively expressed at low levels in most cell types including neurons [30].
2. Materials and Methods
2.1. Animals
Forty male Sprague-Dawley rats (aged 8 weeks, weight 200–250 g) were purchased from the Urology and Nephrology Center, Experimental Animal Center, Mansoura, Egypt. The rats were housed in cages at room temperature (22–25° C) and in a photoperiod of 14 hours light/10 hours dark. The rats were housed in standard animal facility under controlled environmental conditions at room temperature 22±2°C and a 12-hour light-dark cycle and allowed access to food and water ad libitum. All experiments were performed in line with the ethical recommendations of the Faculty of Medicine, Mansoura University, Egypt.
2.2. Experimental protocol
Rats were randomly divided into four groups of 10. Group I: healthy normal control group that received 0.5 mL saline by injection for 4 weeks; Group II: CP control that received intraperitoneal CP (Baxter Oncology, USA, 65 mg/kg/day) for 4 weeks; Group III: triptorelin (Decapeptyl, 1 mg) control that received subcutaneous triptorelin (Ferring, Switzerland, 0.05 mg/kg/day) for 4 weeks; and Group IV: triptorelin-treated group that received intraperitoneal CP (65 mg/kg/day) and subcutaneous triptorelin (0.05 mg/kg/day) for 4 weeks. The rats were anesthetized by inhalation of pentobarbital overdose (200 mg/kg) followed by rapid cervical dislocation and decapitation. This was followed by harvesting of brain tissues, which were placed in 10% formaldehyde.
2.3. Histopathology and immunohistochemical analysis of anti-p53 and anti-Bcl-2 expression
The brains were cut coronally with a microtome (Leica RM 2025; Nassloch, Germany) at 5 μm thickness. Sections were fixed in 10% buffered formalin and embedded in paraffin. Three sets of slides were prepared. At least two different sections were examined per brain sample (cerebral cortex and hippocampus). The first set was stained with hematoxylin and eosin to assess histopathological changes [31]. The second set was stained with immunoperoxidase for evaluation of anti-p53 expression in the nuclei (brown), which indicated positive apoptotic neurocytes. The third set was stained for evaluation of anti-Bcl-2 in the perinuclear membrane (brown), which indicated positive nonapoptotic neurocytes [32]. Scoring of immunohistochemically-stained neurocytes was done under light microscopy and away from lesions. Semi-quantification analysis of the apoptotic index (AI) was determined by counting at least 1000 cells per slide, subdivided into 10 fields chosen randomly, at 400× magnification. AI% = (number of positive cells/total number of calculated cells) × 100, which represented the percentage of positive cells in 1000 cells [33].
2.4. Statistical analysis
All data are presented as mean ± standard error of the mean. All analyses were carried out using SPSS version 17. We used one-way analysis of variance for comparison between the means of the four groups, and the least significant difference test for comparison of each two individual means. A value of p < 0.05 was considered statistically significant. An independent sample t test was used post hoc to compare the four groups that were not paired. A value of p ≤ 0.001 was considered statistically significant.
3. Results
3.1. Cyclophosphamide treated rats (Group II)
Light microscopic examination of the cerebral cortex of CP-treated rats showed degenerated and vacuolated neurocytes with dystrophic changes in the form of shrunken, pyknotic and hyperchromatic nuclei (Figure 1). The CA3 region of the hippocampus showed decreased thickness of the pyramidal layer and severely damaged apoptotic neurocytes in the form of pyknotic, shrunken, vacuolated neurons with hyperchromatic nuclei, and dilated hemorrhagic blood vessels (Figure 4).
Figure 1.
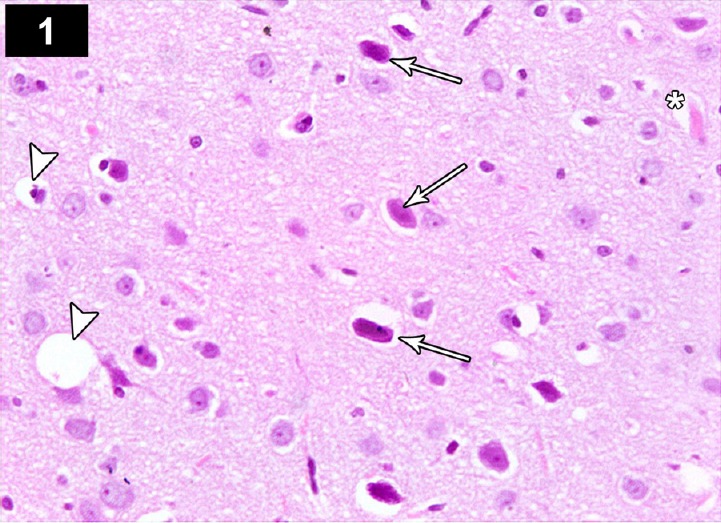
Photomicrograph of the cerebral cortex of a cyclophosphamide-treated rat (Group II) showing neurons with dystrophic changes in the form of shrunken hyperchromatic, irregular with chromatolysis and abnormal Nissl granule distribution (arrows), with dilated blood vessel (*), and degenerate and vacuolated neurocytes (arrow heads). Stain: hematoxylin and eosin; magnification: 400×.
Figure 4.
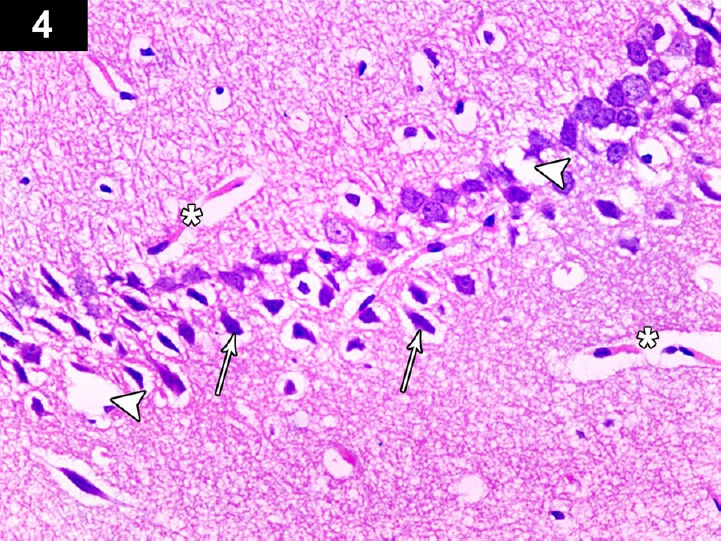
Photomicrograph of hippocampus of a cyclophosphamide-treated rat (Group II) showing decreased thickness of pyramidal cell layer in the CA3 region, with increased apoptotic neurons with dystrophic changes in the form of shrunken hyperchromatic, irregular with chromatolysis and abnormal Nissl granule distribution (arrows) with dilated blood vessel (*), and degenerated and vacuolated neurocytes (arrow head). Stain: hematoxylin and eosin; magnification 400×.
In the cerebral cortex sections stained for anti-Bcl2, some neurocytes showed a weak perinuclear membrane reaction, and a small number of normal neurocytes showed a strong positive reaction (Figure 2). In anti-p53-stained sections, apoptotic neurocytes had a strong positive nuclear reaction, while a small number of normal neurocytes showed a negative reaction (Figure 3). In the CA3 region of the hippocampus stained for anti-Bcl-2, a large number of apoptotic neurocytes showed negative perinuclear membrane immunoreactivity, while a small number of neurocytes showed a positive perinuclear membrane immune reaction (Figure 5). Immunohistochemical staining of the CA3 region with anti-p53 showed positive nuclear immunoreactivity of apoptotic neurocytes (Figures 6, 19 and 20; Table 1).
Figure 2.
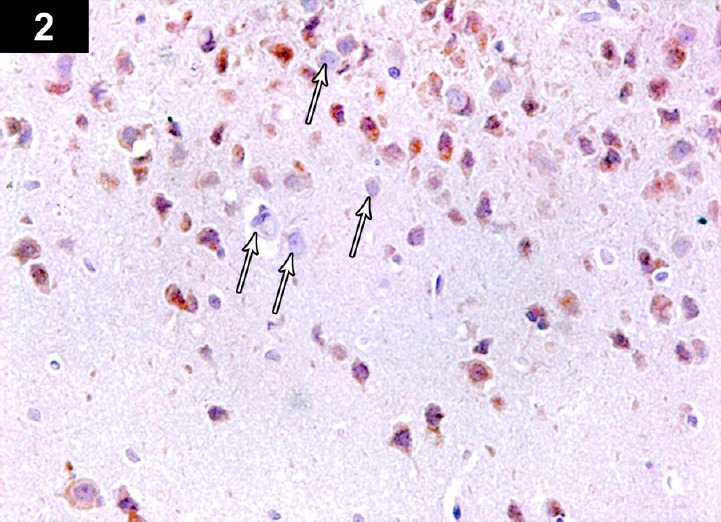
Photomicrograph of an anti-Bcl2-stained section of the cerebral cortex of a cyclophosphamide-treated rat (Group II) showing brown immunoreactive staining of perinuclear membranes of neurocytes. Low immunoreactivity of apoptotic cells in cerebral cortex of a cyclophosphamide-treated rat (arrows). Stain: immunohistochemical anti-Bcl2 staining; magnification: 400×.
Figure 3.
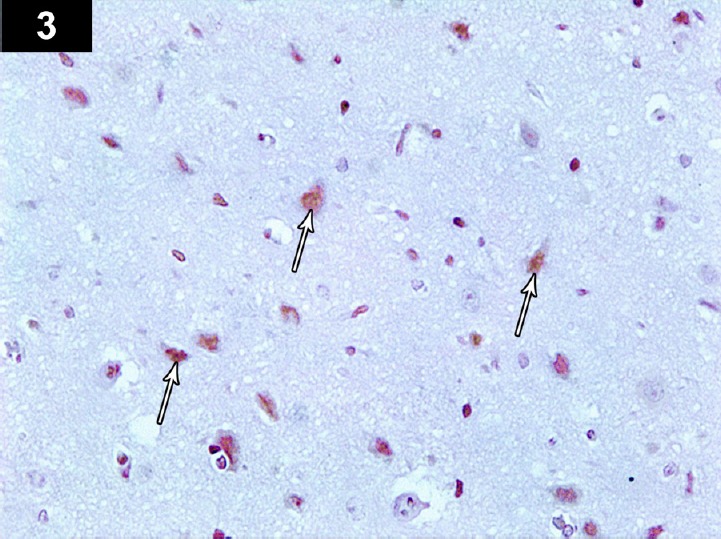
Photomicrograph of anti-p53-stained section of the cerebral cortex of a cyclophosphamide-treated rat (Group II) showing brown positively immunoreactive neurocyte nuclei, with marked expression of positively immunoreactive apoptotic cells (arrows). Stain: immunohistochemical anti-p53 staining; magnification: 400×.
Figure 5.
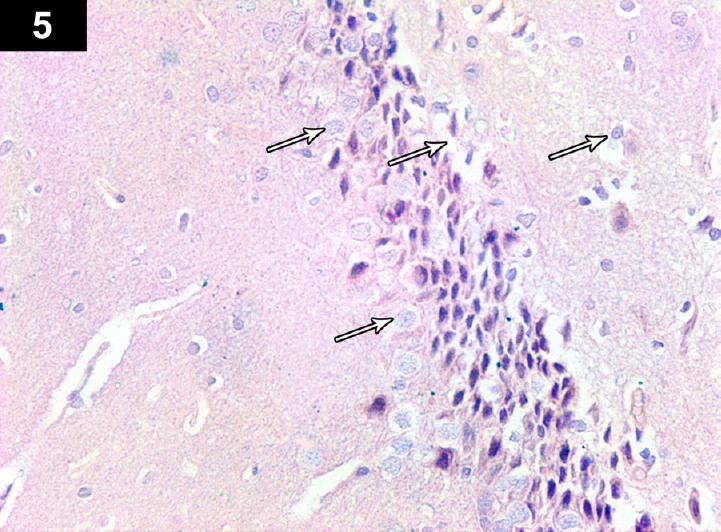
Photomicrograph of an anti-Bcl2-stained section of the hippocampus of a cyclophosphamide-treated rat (Group II) showing brown immunoreactive staining of perinuclear membranes of neurocytes. There was less positive immunoreactivity in some apoptotic cells (arrows). Stain: immunohistochemical anti-Bcl2 staining; magnification: 400×.
Figure 6.
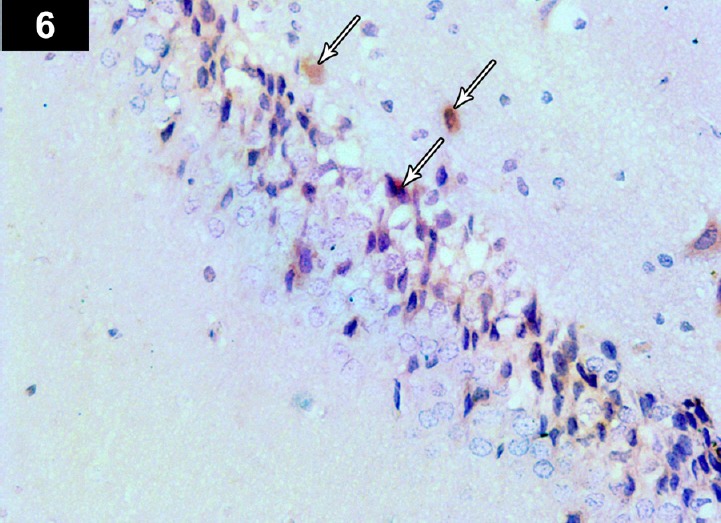
Photomicrograph of an anti-p53-stained section of the hippocampus of a cyclophosphamide-treated rat (Group II) showing brown immunoreactive staining of neurocyte nuclei, with marked expression of apoptotic cells (arrows). Stain: immunohistochemical anti-p53 staining; magnification: 400×.
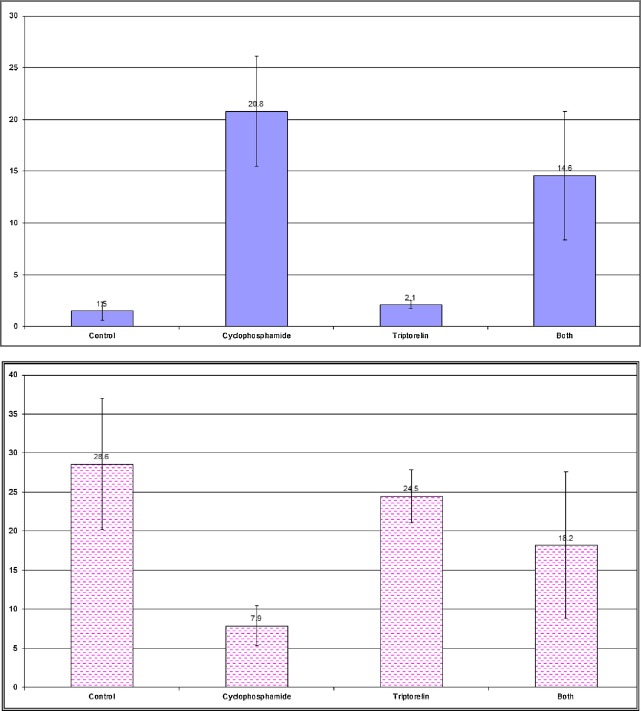
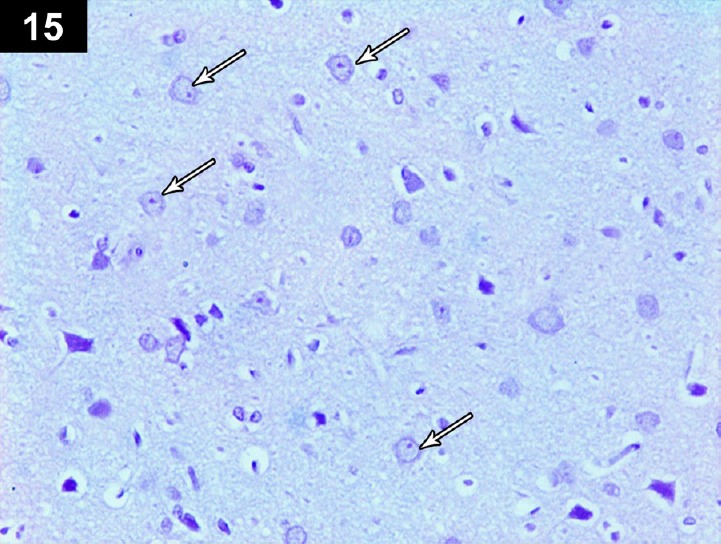
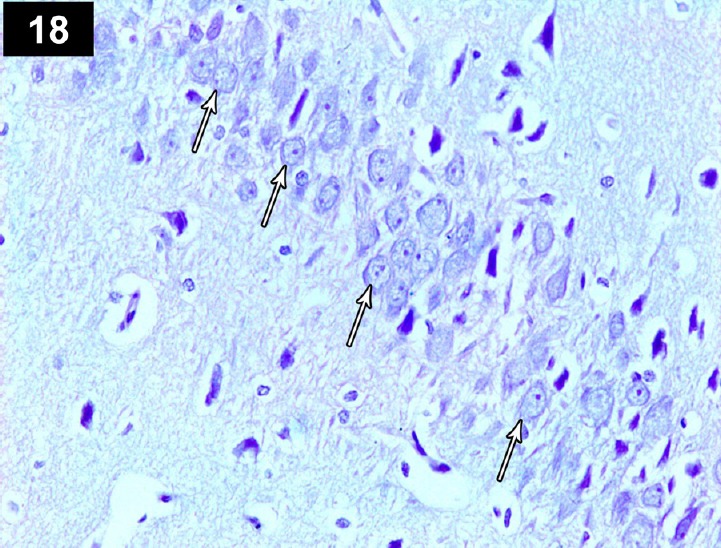
Figure 19.
Changes in the apoptotic index of the neurocytes of the cerebral cortex with (A) anti-p53 (B) anti-Bcl2 staining in adult rats in the control and experimental groups.
Figure 20.
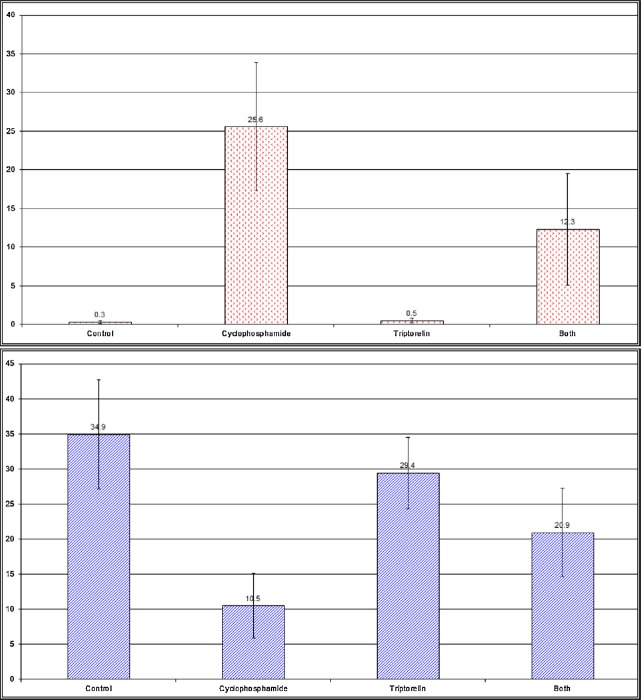
Changes in the apoptotic index of the neurocytes of the hippocampus with (A) anti-p53 (B) anti-Bcl2 staining in adult rats in the control and experimental groups.
Table 1.
Changes in the independent sample t test and probability (p) of the apoptotic index of neurocytes of the cerebral cortex and the hippocampus of adult rats in the control and experimental groups.
| Cerebral cortex | ||||
|---|---|---|---|---|
| IHC | Groups for comparison | t | p | |
| Anti-P53 | Group I (mean ± SD) 1.5 ± 0.9 | Group II | 11.35 | <0.001*** |
| Group III | 1.93 | 0.069 | ||
| Group IV | 6.6 | <0.001*** | ||
| Group II (mean ± SD) 20.8 ± 5.3 | Group III | 11.13 | <0.001*** | |
| Group IV | 2.4 | 0.027* | ||
| Group III (mean ± SD) 2.1 ± 0.4 | Group IV | 6.36 | <0.001*** | |
| Group IV (mean ± SD) 14.6 ± 6.2 | ||||
| Anti-Bcl2 | Group I (mean ± SD) 28.6 ± 8.4 | Group II | 7.44 | <0.001*** |
| Group III | 1.43 | 0.17 | ||
| Group IV | 2.64 | 0.016* | ||
| Group II (mean ± SD) 28.6 ± 8.4 | Group III | 12.26 | <0.001*** | |
| Group IV | 3.41 | 0.003** | ||
| Group III (mean ± SD) 24.5 ± 3.4 | Group IV | 1.99 | 0.061 | |
| Group IV (mean ± SD) 18.2 ± 9.4 | ||||
| Hippocampus | ||||
| IHC | Groups for comparison | t | p | |
| Anti-P53 | Group I (mean ± SD) 0.3 ± 0.2 | Group II | 9.64 | <0.001*** |
| Group III | 16.97 | 0.09 | ||
| Group IV | 5.27 | <0.001*** | ||
| Group II (mean ± SD) 25.6 ± 8.3 | Group III | 9.18 | <0.001*** | |
| Group IV | 3.83 | 0.0012** | ||
| Group III (mean ± SD) 0.5 ± 0.1 | Group IV | 4.74 | <0.001*** | |
| Group IV (mean ± SD) 12.3 ± 7.2 | ||||
| Anti-Bcl2 | Group I (mean ± SD) 34.9 ± 7.8 | Group II | 8.52 | <0.001*** |
| Group III | 1.87 | 0.78 | ||
| Group IV | 4.42 | <0.001*** | ||
| Group II (mean ± SD) 10.5 ± 4.6 | Group III | 8.7 | <0.001*** | |
| Group IV | 4.22 | <0.001*** | ||
| Group III (mean ± SD) 29.4 ± 5.1 | Group IV | 3.32 | 0.003** | |
| Group IV (mean ± SD) 20.9 ± 6.3 | ||||
IHC = immunohistochemistry; SD = standard deviation.
3.2. Triptorelin treated rats (Group III)
Light microscopic examination of the cerebral cortex of triptorelin-treated rats showed healthy neurocytes similar to the control group, with central large vesicular nuclei that contained one or more nucleoli and peripheral distribution of Nissl granules (Figure 7). Light microscopic examination of the hippocampus in the CA3 region showed the thickness of the pyramidal layer and most of the neurocytes appeared normal as the control group (Figure 10). In sections stained for anti-Bcl2, most of the cerebral cortical neurocytes showed positive immunoreactivity in the perinuclear membranes (Figure 8). Most of the nuclei of the cerebral cortical neurocytes showed negative immunoreactivity for anti-p53 (Figure 9). The CA3 region of the hippocampus stained with anti-Bcl2 showed positive immunoreactivity in the perinuclear membrane in most of the neurocytes (Figure 11) but showed negative immunohistochemical reaction for anti-p53 (Figures 12,19 and 20; Table 1).
Figure 7.
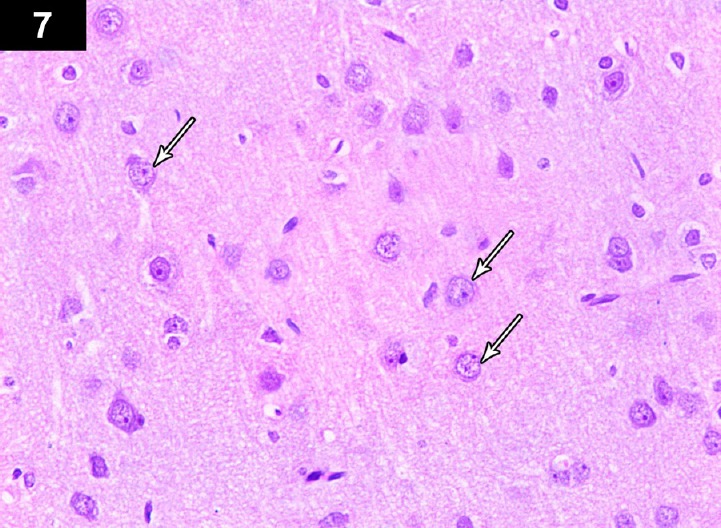
Photomicrograph of cerebral cortex of a triptorelin-treated rat (group III) showing near normal neurons, with central large vesicular nuclei, containing one or more nucleoli, and peripheral distribution of Nissl granules (arrows). Stain: hematoxylin and eosin; magnification: 400 ×.
Figure 10.
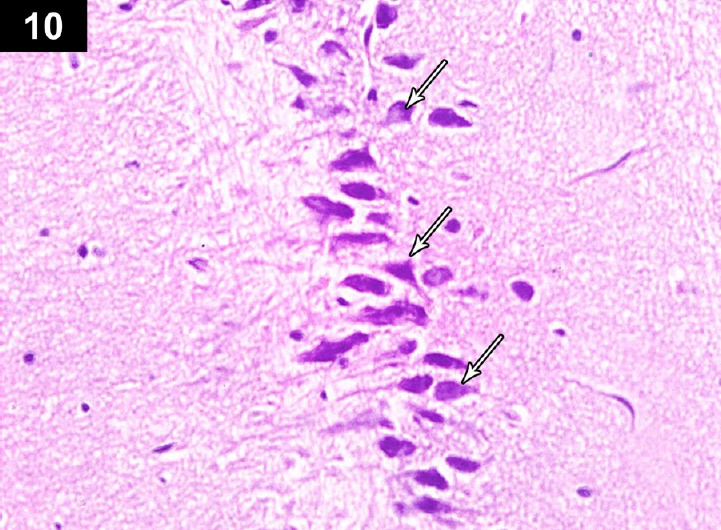
Photomicrograph of hippocampus of a triptorelin-treated rat (Group III) showing near normal thickness of the pyramidal cell layer of the CA3 region, with normal neurons (arrows). Stain: hematoxylin and eosin; magnification: 400×.
Figure 8.
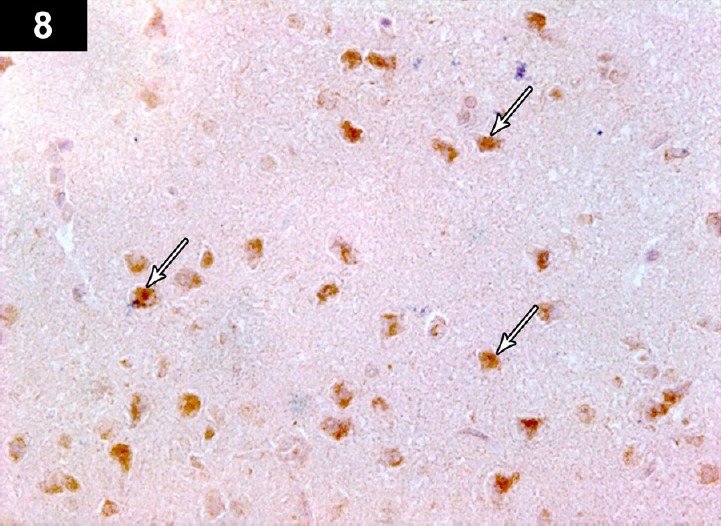
Photomicrograph of an anti-Bcl2-stained section of the cerebral cortex of a triptorelin-treated rat (Group III) showing positive immunoreactivity of neurons in the form of brown staining of neurocyte perinuclear membranes (arrows). Stain: immunohistochemical anti-Bcl2 staining; magnification: 400×.
Figure 9.
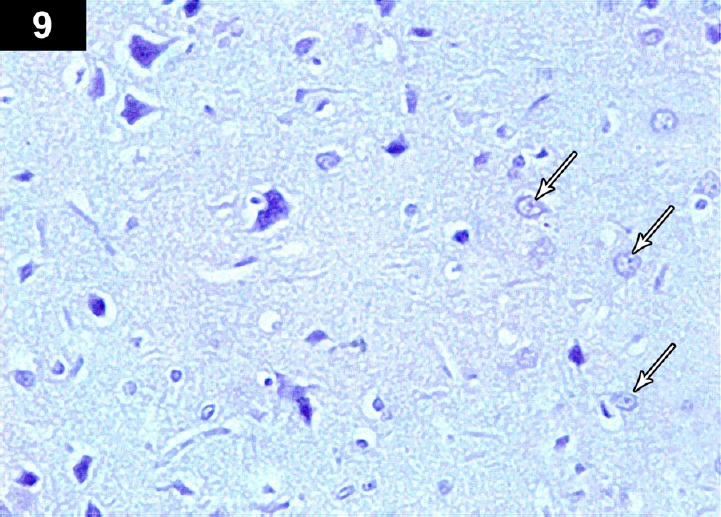
Photomicrograph of an anti-p53-stained section of the cerebral cortex of a triptorelin-treated rat (Group III) showing negative immunoreactivity of neurons in the form of brown staining of cerebral cortex neurocyte nuclei (arrows). Stain: immunohistochemical anti-p53 staining; magnification: 400×.
Figure 11.

Photomicrograph of an anti-Bcl2-stained section of the hippocampus of a triptorelin-treated rat (Group III) showing positive immunoreactivity of neurocyte perinuclear membranes (arrows) Stain: immunohistochemical anti-Bcl2 staining; magnification: 400×.
Figure 12.
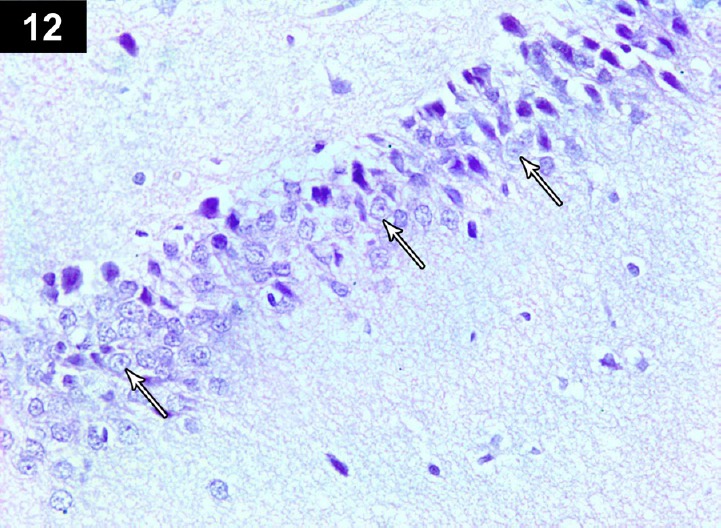
Photomicrograph of an anti-p53-stained section of hippocampus of triptorelin-treated rats (Group III) showing negative immunoreactivityin neurocyte nuclei (arrows) Stain: immunohistochemical anti-p53 staining; magnification: 400×.
3.3. Rats treated with cyclophosphamide and triptorelin (Group IV)
Light microscopic examination of the cerebral cortex of rats treated with CP and triptorelin showed considerable improvement in neurocytes, and most of them appeared normal with large vesicular nuclei containing one or more nucleoli and peripheral distribution of Nissl granules. A small number of neurocytes still showed dystrophic changes in the form of hyperchromatic nuclei with abnormal Nissl granule distribution and vacuolation (Figure 13). Light microscopic examination of the CA3 region of the hippocampus showed a thick pyramidal layer of near normal appearance, and obvious improvement in most of the neurocytes, which showed normal central vesicular nucleoli with normal distribution of Nissl granules. Some neurocytes showed apoptotic figures (Figure 16). Immunohistochemically stained sections of cerebral cortex showed a large number of neurocytes with positive perinuclear membrane immunoreactivity for anti-Bcl2, while a small number of apoptotic neurocytes showed negative immunoreactivity (Figure 14). Most neurocytes showed negative immunoreactivity with anti-p53 staining (Figure 15). Immunohistochemically stained sections of the CA3 region of the hippocampus showed variation in immunoreactivity for Bcl2, Most of the neurocytes showed positive immunoreactivity while a small number of neurons showed apoptotic changes with negative immunoreactivity (Figure 17). In anti-p53 stained sections, nearly all neurocytes showed negative nuclear immunoreactivity (Figures 18,19,20; Table 1).
Figure 13.
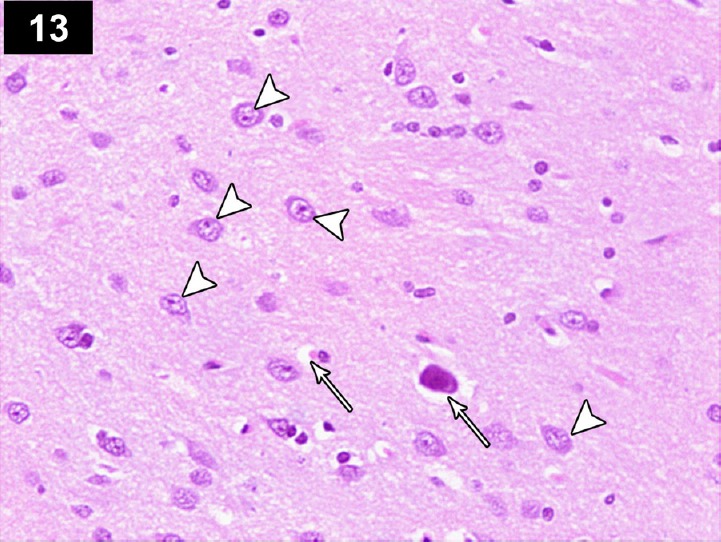
Photomicrograph of cerebral cortex of a cyclophosphamide-treated rat receiving triptorelin (Group IV) showing near normal neurons with central large vesicular nuclei, containing one or more nucleoli, and peripheral distribution of Nissl granules (arrows head), and small number of neurons with dystrophic changes in the form of shrunken hyperchromatic, irregular (arrows), and abnormal Nissl granules distribution. Stain: hematoxylin and eosin; magnification: 400×.
Figure 16.
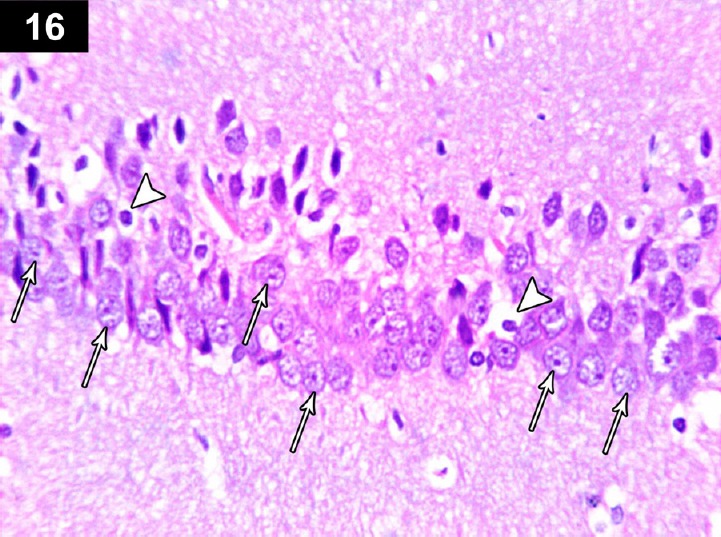
Photomicrograph of the hippocampus of a cyclophosphamide-treated rat receiving triptorelin (Group IV) showing normal neurons n the pyramidal cell layer of the CA3 region (arrows), with a small number of apoptotic neurons (arrow head). Stain: hematoxylin and eosin; magnification: 400×.
Figure 14.
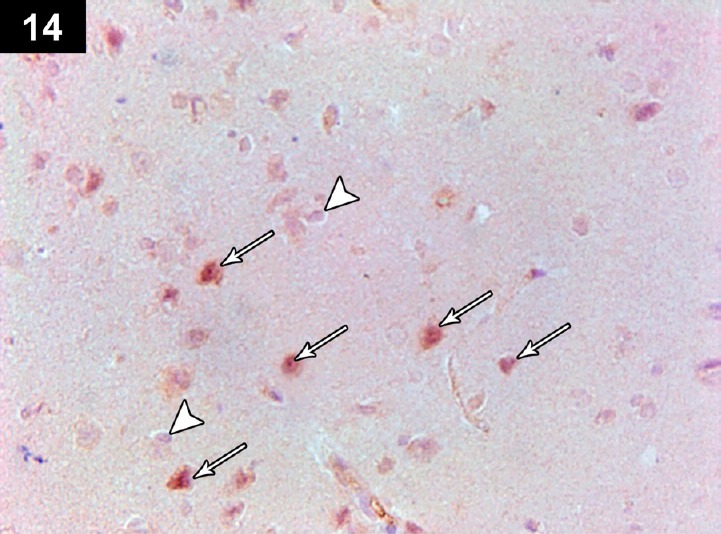
Photomicrograph of an anti-Bcl2-stained section of the cerebral cortex of a cyclophosphamide-treated rat receiving triptorelin (Group IV) showing positive immunoreactivity of most of the neurocyte perinuclear membranes neurons (arrows), with a small number of apoptotic neurons with negative immunoreactivity (arrow head). Stain: immunohistochemical anti-Bcl2 staining; magnification: 400×.
Figure 15.
Photomicrograph of an anti-p53-stained section of the cerebral cortex of a cyclophosphamide-treated rat receiving triptorelin (Group IV) showing negative immunoreactivity in the nuclei of the neurons (arrows). Stain: immunohistochemical anti-p53 staining; magnification: 400 ×.
Figure 17.
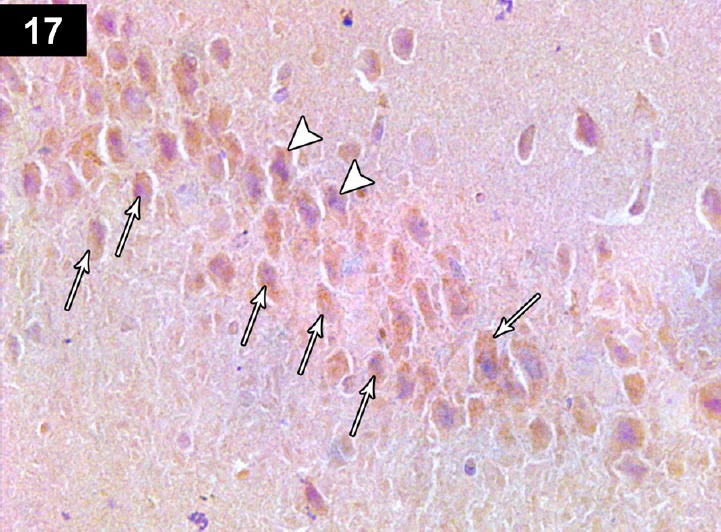
Photomicrograph of an anti-Bcl2-stained section of a cyclophosphamide-treated rat receiving triptorelin (Group IV) showing brown staining of hippocampal neurocyte perinuclear membranes. Positive immunoreactivity of most neurons (arrows), with a small number of apoptotic neurons with negative immunoreactivity (arrow head). Stain: immunohistochemical anti-Bcl2 staining; magnification: 400×.
Figure 18.
Photomicrograph of an anti-p53-stained section of the hippocampus of a cyclophosphamide-treated rat receiving triptorelin (Group IV) showing brown staining of neurocyte nuclei, indicating positive immunoreactivity (arrows). Stain: immunohistochemical anti-p53 staining,; magnification: 400×.
4. Discussion
The number of cancer survivors has increased in the last three decades due to highly efficient chemotherapy regimens. However, 60–75% of cancer survivors face at least one serious side effect of chemotherapy [1]. In this study, we investigated the effect of CP alone and with the protective effect of triptorelin on the cerebral cortex and hippocampus of male albino rats.
Group II showed dilated blood vessels (Figures 1 and 4), this result was confirmed by adjuvant chemotherapy for breast cancer that has been related to transient ischemic attacks and stroke with brain infarctions and cerebral microbleeds. These vascular lesions are indicative of cerebrovascular impairment that could partially explain the association between chemotherapy and cognitive dysfunction [34]. Histopathological study of the same group of rats revealed that the CP induced highly significant dystrophic and apoptotic changes in the neurocytes of the cerebral cortex and hippocampus (Figures 1, 4, 19 and 20; Table 1). In contrast, other studies reported that the hematoxylin and eosin staining did not reveal any unusual hippocampal structure in adult mice 10 days after CP injection. This suggests that acute injection of 40 mg/kg CP does not induce neural apoptosis in the hippocampus of adult mice.
The histopathological results were confirmed by immunohistochemical studies that showed a large number of apoptotic neurons with a positive reaction for p53 and less positive reaction for Bcl2 (Figures 2, 3, 5, 6, 19 and 20; Table 1). This result was in line with an experimental study that had used immunohistochemical markers Ki-67 (a proliferating cell marker) and DCX (an immature neuronal cell marker) for neurogenesis in hippocampus [35]. This work had studied the acute effect of intraperitoneal injection of CP. It showed a significant decrease in the number of Ki-67-and DCX-positive cells at 12 hours after injection of CP, reaching the lowest level at 24 hours after injection. This suggests that CP interrupts hippocampal functions through suppression of neurogenesis. However, during the period from 12 hours to 4 days after injection, CP transiently decreased the number of cells positive for Ki-67 and DCX in the dentate gyrus of adult hippocampi, indicating that CP transiently inhibits adult hippocampal neurogenesis. This suggests that CP only suppresses the generation of new neural cells, possibly by transient arrest of the cell cycle, in the dentate gyrus of the adult hippocampus.
In another study, CP did not induce apoptosis in the adult dentate gyrus, in contrast to induction by irradiation [36].
Computed tomography neuroimaging in patients with breast cancer showed decreased density of gray matter in the cerebral cortex 1 month after completion of chemotherapy, particularly in frontal regions [37]. Chemotherapeutic agents caused increased cell death and decreased cell division in the dentate gyrus of the hippocampus and the corpus callosum in mice [5].
The results of this work revealed the effect of triptorelin on the cerebral cortex and hippocampus (group III). The results showed no significant difference in comparison to that of the control group, in the form of normal neurons in cerebral cortex and hippocampus, with normal thickness of the pyramidal cell layer of CA3 (Figures 7, 10, 19 and 20; Table 1) These results were in association with the anti-Bcl2 stained section of the cerebral cortex and hippocampus, which showed positive immunoreactivity of neurons (Figures 8 and 11), and the anti-p53 stained section, which revealed negative immunoreactivity of neurocytes nuclei (Figures 9 and 12).
The histopathological study of Group IV showed normal neurons but a small number of neurons with dystrophic changes in the neurocytes of cerebral cortex (Figure 13). The CA3 region of the hippocampus of the same group showed a normal pyramidal cell layer and a small number of apoptotic neurons (Figure 16). Anti-Bcl2-stained sections of cerebral cortex and hippocampus showed normal positive immunoreactivity in most of the neuro-cytes, with a small number of negative apoptotic neurons (Figures 14 and 17). However, in the anti-p53-stained sections, the cerebral cortex and hippocampus showed negative immunoreactivity in the nuclei of the neurons (Figures 15 and 18). These results were confirmed by mild to moderately significant changes in the AI of the cerebral cortex in comparison to highly significant changes in the AI of the hippocampus, which indicates the sensitivity of the hippocampal cells (Figures 19 and 20; Table 1).
Immunoreactive GnRH type I receptors in mouse and sheep brains show that staining is restricted to the pyramidal cell layer in the cerebral cortex and hippocampus [24]. Other studies have indicated high densities of GnRH receptors in several regions of the brain including the CA1 and CA3 regions of the hippocampus [38]. Activation of GnRH agonists produces long-lasting enhancement of synaptic transmission mediated by ionotropic glutamate receptors in the CA1 pyramidal neurons of the rat hippocampus [39]. GnRH alters the electrical properties of rat hippocampal pyramidal cells and stimulates increased inositol-1,4,5-trisphosphate production within these cells [39,40]. In the rat and sheep's hippocampal and dentate gyrus GnRHR-expressing neurons co-express estrogen receptor β. Estrogen receptor β is a potent tumor suppressor and plays a crucial role in many cancer types such as prostate cancer [41]. GnRH constitutes an integral component of the neurodegenerative pathology that accompanies Alzheimer's disease [42].
Other histopathological studies on the dentate gyrus and hippocampus in CP-treated show that Decapeptyl has a supporting effect on hippocampal neuronal (hippocampus area neurons) damage in animals that received chemotherapeutic agents [23].
In conclusion, our study shows that triptorelin can reverse the apoptotic changes induced by CP therapy, which is more significant in the hippocampus than in the cerebral cortex.
Conflict of interest
All authors declare no potential conflict of interest including any financial, personal or other relationships with other people or organizations that could have inappropriately influenced, or have been perceived to influence, this study.
Acknowledgments
We thank Prof. Dr. Salwa Gawish (Professor and Head of Histology Department, Faculty of Medicine, Mansoura University, Egypt) for providing us with unlimited advice during the completion our work.
References
- [1].Christina AM, Mini review. How chemotherapy damages the central nervous system. J Biol. 2008;7:11. doi: 10.1186/jbiol73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Wefel JS, Lenzi R, Theriault R, Davis R, Christina AM. The cognitive sequelae of standard dose adjuvant chemotherapy in women with breast cancer: results of a prospective, randomized, longitudinal trial. Cancer. 2004;100:2292–9. doi: 10.1002/cncr.20272. [DOI] [PubMed] [Google Scholar]
- [3].Alvarez JA, Scully RE, Miller TL, Armstrong FD, Constine LS, Friedman DL, et al. Long-term effects of treatments for childhood cancers. Curr Opin Pediatr. 2007;19:23–31. doi: 10.1097/MOP.0b013e328013c89e. [DOI] [PubMed] [Google Scholar]
- [4].Ahles TA, Saykin AJ, Furstenberg CT, Cole B, Mott LA, Skalla K, et al. Neuropsychological impact of standard dose systemic chemotherapy in long-term survivors of breast cancer and lymphoma. J Clin Oncol. 2002;20:485–93. doi: 10.1200/JCO.2002.20.2.485. [DOI] [PubMed] [Google Scholar]
- [5].DietrichJ, Han R, Yang Y, Mayer-Pröschel M, Noble M. CNS progenitor cells and oligodendrocytes are targets of chemotherapeutic agents in vitro and in vivo . J Biol. 2006;5:22. doi: 10.1186/jbiol50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Reiriz AB, Reolon GK, Preissler T, Rosado JO, Henriques JAP, Roesler R, et al. Cancer chemotherapy and cognitive function in rodent models: memory impairment induced by cyclophosphamide in mice. Clin Cancer Res. 2006;12:5000–7. doi: 10.1158/1078-0432.CCR-06-0138. [DOI] [PubMed] [Google Scholar]
- [7].Baudino B, Castellano S, Cauda M. The chemotherapy long-term effect on cognitive functions and brain metabolism in lymphoma patients. QJ Nucl Med Mol Imaging. 2012;56:1–10. [PubMed] [Google Scholar]
- [8].Mustafa S, Walker A, Bennett G, Wigmore PM. 5-flouracil chemotherapy affects spatial working memory and newborn neurons in the adult rat hippocampus. Eur J Neurosci. 2008;2008(28):323–30. doi: 10.1111/j.1460-9568.2008.06325.x. [DOI] [PubMed] [Google Scholar]
- [9].Seigers R, Schagen SB, Beerling W, Boogerd W. Long-lasting suppression of hippocampal cell proliferation and impaired cognitive performance by methotrexate in the rat. Behav Brain Res. 2008;186:168–75. doi: 10.1016/j.bbr.2007.08.004. [DOI] [PubMed] [Google Scholar]
- [10].Tushrendra S, Kasture S, Mohanty BPK, Yusuf J, Manvendra SK, Abhisek A, et al. Cyclophosphamide-induced oxidative stress inbrain: protective effect of Garcinia indicia fruit extract. Int J PharmLife Sci. 2011;2:1035–40. [Google Scholar]
- [11].Bhatia AL, Manda K, Patni Sharma AL. Prophylactic action of linseed (Linum usitatissimum) oil against cyclophosphamide induced oxidative stress on mouse brain. J Med Food. 2006;9:261–4. doi: 10.1089/jmf.2006.9.261. [DOI] [PubMed] [Google Scholar]
- [12].Hanaa AS, Amal O, Hassan AM, Nadia AF. Effect of cyclophosphamide on transcription of SOD1 mRNA and GPX1 mRNA in mice liver and brain tissues. J Appl Biosci. 2010;29:1736–42. [Google Scholar]
- [13].Napolitano J, Singh KK. Mitochondria as targets for detection and treatment of cancer. Expert Rev Mol Med. 2002;9:1–19. doi: 10.1017/S1462399402004453. [DOI] [PubMed] [Google Scholar]
- [14].Stankiewicz A, Skrzydlewska E, Makiela M. Effects of amifostine on liver oxidative stress caused by cyclophosphamide administration to rats. Drug Metabol Drug Interact. 2002;19:67–82. doi: 10.1515/dmdi.2002.19.2.67. [DOI] [PubMed] [Google Scholar]
- [15].Bohnenstengel F, Friedel G, Rilter CA, McCuellan M, Fritz P, Eicheibaum M. Variability of cyclophosphamide up-take into human bronchial carcinoma: consequences for local bio activation. Cancer Chemother Pharmacol. 2000;45:63–8. doi: 10.1007/PL00006745. [DOI] [PubMed] [Google Scholar]
- [16].Ludeman SM. The chemistry of the metabolites of cyclophosphamide. Curr Pharm Des. 1999;5:627–43. [PubMed] [Google Scholar]
- [17].Kern JC, Kehrer JP. A caspase influenced decision between apoptosis and oncosis/necrosis. Chem Biol Interact. 2002;139:79–95. doi: 10.1016/s0009-2797(01)00295-2. [DOI] [PubMed] [Google Scholar]
- [18].Arumugam N, Sivakumar V, Thanislass J, Devaraj H. Effects of acrolein on rat liver antioxidant defense system. Indian J Exp Biol. 1997;35:1373–84. [PubMed] [Google Scholar]
- [19].Subramaniam S, Subramaniam S, Shyamala DC. Erythrocyte antioxidant enzyme activity in CMF treated breast cancer patients. Cancer Biochem Biophys. 1994;14:177–82. [PubMed] [Google Scholar]
- [20].Barton D, Loprinzi C. Novel approaches to preventing chemotherapy induced cognitive dysfunction in breast cancer: the art of the possible. Clin Breast Cancer. 2002;3:121–7. doi: 10.3816/cbc.2002.s.023. [DOI] [PubMed] [Google Scholar]
- [21].Ueno M, Katayama K, Yamauchi H, Nakayama H, Doi K. Cell cycle progression is required for nuclear migration of neural progenitor cells. Brain Res. 2006;1088:57–67. doi: 10.1016/j.brainres.2006.03.042. [DOI] [PubMed] [Google Scholar]
- [22].Baker GL, Kahl LE, Zee BC, Stolzer BL, Agarwal AK, Medsger TA. Malignancy following treatment of rheumatoid arthritis with cyclophosphamide. Am J Med. 1987;83:1–9. doi: 10.1016/0002-9343(87)90490-6. [DOI] [PubMed] [Google Scholar]
- [23].Niakani A, Farah F, Shapour H. Decapeptyl ameliorates cyclophosphamide-induced reproductive toxicity in male Balb/C mice: histomorphometric, stereologic and hormonal evidences. Iran J Reprod Med. 2013;11:791–800. [PMC free article] [PubMed] [Google Scholar]
- [24].Albertson AJ, Navratil A, Mignot M, Dufourny L, Cherrington B, Skinner DC. Immunoreactive GnRH type I receptors in the mouse and sheep brain. J Chem Neuroanat. 2008;35:326–33. doi: 10.1016/j.jchemneu.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Yoshioka K, Suzuki C, Arai S, Iwamura S, Hirose H. Gonadotropin-releasing hormone in third ventricular cerebrospinal fluid of the heifer during the estrous cycle. Biol Reprod. 2001;64:563–70. doi: 10.1095/biolreprod64.2.563. [DOI] [PubMed] [Google Scholar]
- [26].Skinner DC, Caraty A. Measurement and possible function of GnRH in cerebrospinal fluid in ewes. Reprod Suppl. 2002;59:25–39. [PubMed] [Google Scholar]
- [27].Lahlou N, Carel JC, Chaussain JL, Roger M. Pharmacokinetics and pharmacodynamics of GnRH agonists: clinical implications in pediatrics. J Pediatr Endocrinol Metab. 2000;13:723–37. doi: 10.1515/jpem.2000.13.s1.723. [DOI] [PubMed] [Google Scholar]
- [28].Yuan J, Yankner BA. Apoptosis in the nervous system. Nature. 2000;407:802–9. doi: 10.1038/35037739. [DOI] [PubMed] [Google Scholar]
- [29].Polyak K, Xia Y, Zweier JL. A model for p53-induced apoptosis. Nature. 1997;389:300–5. doi: 10.1038/38525. [DOI] [PubMed] [Google Scholar]
- [30].Soussi T. The p53 tumor suppressor gene: from molecular biology to clinical investigation. Ann N Y Acad Sci. 2000;910:121–37. doi: 10.1111/j.1749-6632.2000.tb06705.x. [DOI] [PubMed] [Google Scholar]
- [31].Bancroft JD. Theory and practice of histological techniques. Elsevier Health Sciences. (6th ed) 2008 [Google Scholar]
- [32].Khodeary MF, Sharaf El-Din AI, El Kholy SMS. A histopathological and immunohistochemical study of adult rats’ brain after long-term exposure to amadol (tramadol hydrochloride) Mansoura J Forensic Med Clin Toxicol. 2010;18:1–24. [Google Scholar]
- [33].Xu C, Shu WQ, Qiu ZQ. Protective effects of green tea polyphenols against subacute hepatotoxicity induced by microcystin-LR in mice. Environ Toxicol Pharmacol. 2007;24:140–8. doi: 10.1016/j.etap.2007.04.004. [DOI] [PubMed] [Google Scholar]
- [34].Koppelmans V, De Ruiter MB, Van Der Lijn F, Boogerd W, Seynaeve C, van der Lugt A, et al. Global and focal brain volume in long-term breast cancer survivors exposed to adjuvant chemotherapy. Breast Cancer Res Treat. 2012;132:1099–106. doi: 10.1007/s10549-011-1888-1. [DOI] [PubMed] [Google Scholar]
- [35].Miyoung Y, Kim JS, Myoung-Sub S, Sung-Ho K, Seong Soo K, Chun-Sik B. Cyclophosphamide impairs hippocampus-dependent learning and memory in adult mice: possible involvement of hippocampal neurogenesis in chemotherapy-induced memory deficits. Neurobiol Learning Memory. 2010;93:487–94. doi: 10.1016/j.nlm.2010.01.006. [DOI] [PubMed] [Google Scholar]
- [36].Kim JS, Lee HJ, Kim JC, Kang SS, Bae CS, Shin T, et al. Transient impairment of hippocampus-dependent learning and memory in relatively low-dose of acute radiation syndrome is associated with inhibition of hippocampal neurogenesis. J Rad Res. 2008;49:517–26. doi: 10.1269/jrr.08020. [DOI] [PubMed] [Google Scholar]
- [37].McDonald BC, Conroy SK, Smith DJ, West JD, Saykin AJ. Frontal gray matter reduction after breast cancer chemotherapy and association with executive symptoms: a replication and extension study. Brain Behav Immun. 2013;30:S117–25. doi: 10.1016/j.bbi.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lu F, Yang J, Wu J, Chen Y, Kao Y. Activation of GnRH Receptors produces neuronal excitation in the rat hippocampus. Chinese J Physiol. 1999;42:67–71. [PubMed] [Google Scholar]
- [39].Yang SN, Lu F, Wu JN, Liu DD, Hsieh WY. Activation of GnRH Receptors induces a long-term enhancement of excitatory postsynaptic currents mediated by ionotropic glutamate receptors in the rat hippocampus. Neurosci Lett. 1999;260:33–6. doi: 10.1016/s0304-3940(98)00939-2. [DOI] [PubMed] [Google Scholar]
- [40].Jennes L, Brame B, Centers A, Janovick JA, Conn PM. Regulation of hippocampal gonadotropin releasing hormone (GnRH) receptor mRNA and GnRH-stimulated inositol phosphate production by gonadal steroid hormones. Mol Brain Res. 1995;33:104–10. doi: 10.1016/0169-328x(95)00113-7. [DOI] [PubMed] [Google Scholar]
- [41].Stettner M, Kaulfuss S, Burfeind P, Schweyer S, Strauss A, Ringert RH, et al. The relevance of estrogen receptor-beta expression to the antiproliferative effects observed with histone deacetylase inhibitors and phytoestrogens in prostate cancer treatment. Mol Cancer Ther. 2007;5:2626–33. doi: 10.1158/1535-7163.MCT-07-0197. [DOI] [PubMed] [Google Scholar]
- [42].Atwood CS, Meethal SV, Liu T, Wilson AC, Gallego M, Smith MA, et al. Dysregulation of the hypothalamic-pituitary-gonadal axis with menopause and andropause promotes neurodegenerative senescence. J Neuropathol Exp Neurol. 2005;64:93–103. doi: 10.1093/jnen/64.2.93. [DOI] [PubMed] [Google Scholar]