Abstract
Background
Type 2 diabetes may alter cardiac structure and function. Many patients with type 2 diabetes have diastolic dysfunction with preserved ejection fraction (EF). Recently, this latter measure was criticised. Thus, this research looked at the impact of left ventricular end-diastolic volume and E/e′ ratio variations in patients with type 2 diabetes and preserved EF with the aim to recognise different clinical phenotypes.
Methods
In this cross-sectional study, we evaluated 176 men affected by type 2 diabetes with transthoracic echocardiography. All subjects have preserved EF (>50%). Patients were stratified into four groups based on the median value of both left ventricular end-diastolic volume and E/e′ ratio, and the clinical variables were registered. The independent predictors associated with the groups were analysed by a multinomial logistic regression model.
Results
Diabetes duration, age, estimated glomerular filtration rate and antihypertensive treatments were significantly different among the groups as were EF, left atrial volume index (LAVI), E/A, septum thickness and s′ mean wave. Multinomial regression analysis showed that the groups significantly differed for age, diabetes duration, EF, LAVI, septum thickness and s′ mean wave. The main result of this study was that patients with higher left ventricular volume and higher E/e′ ratio (group 2) showed the worse clinical profile.
Conclusions
Our study might suggest that variations of left ventricular end-diastolic volume along with E/e′ ratio variations, even in the normal range, may allow to recognise phenotypes of patients with type 2 diabetes with worse clinical characteristics. This finding should be tested in prospective studies to assess the predictive roles of these phenotypes.
Keywords: type 2 diabetes, transthoracic echocardiography, left ventricular geometry, preserved ejection fraction, chamber volume.
Significance of this study.
What is already known about this subject?
Type 2 diabetes may alter both heart structure and function.
Heart failure is an increasing complication of type 2 diabetes.
Left ventricular end-diastolic volume modification may be an early marker of alteration of left ventricular geometry.
What are the new findings?
The main result of this study is that phenotyping patients with type 2 diabetes with left ventricular end-diastolic volume and E/e′ ratio may help to categorise patients in groups with different clinical and echocardiographic characteristics.
How might these results change the focus of research or clinical practice?
If confirmed in a prospective setting, our results may help to recognise patients with type 2 diabetes at very high risk of developing heart failure.
Introduction
Diabetes mellitus is a well-known risk factor for the development of heart alterations both in the structure and in the function.1 2 The Framingham Heart Study demonstrated that the frequency of heart failure is five times higher in diabetic women and two times greater in diabetic men compared with age-matched control subjects.3 The most frequent heart alteration in type 2 diabetes is heart failure with preserved ejection fraction (EF).4 However, heart failure with preserved EF was recently questioned5 6 as the EF parameter alone roughly reflects heart performance.7 A MRI study found lower left ventricular (LV) EF, higher values of troponin and other surrogate markers of cardiovascular risk in patients with left ventricular end-diastolic volume (LVEDV) dilation compared with those without LVEDV dilation independently of left ventricular hypertrophy (LVH) pattern, concentric or eccentric.8 Consequently, the authors suggested reclassifying concentric and eccentric LVH into two further groups based on the absence or presence of LVEDV dilation.8
Early diastolic dysfunction is generally referable to an impaired relaxation filling pattern in patients with preserved LVEF.9 As reported in the recommendations for the evaluation of LV diastolic function, it is the combination of elevated LV diastolic pressure and the absence of an increased LVEDV that represents a strong evidence in favour of well-developed diastolic dysfunction.10 It may be misleading to consider isolated diastolic dysfunction without investigating LV dimension, as LV dilation is an important aspect of LV geometric remodelling.7 8
Evidences showed that standard echocardiographic parameters, such as left ventricular end-diastolic volume and E/e′ ratio, are well validated from the pathophysiological and prognostic points of view.11 Therefore, we believe that heart failure with preserved EF may be a quite heterogeneous class and we hypothesise that stratification according to diastolic filling pressure estimated (E/e′ ratio) and LVEDV may help to categorise type 2 diabetes patients.7 11 Consequently, the aim of this study was to test whether stratification of patients affected by type 2 diabetes with preserved LVEF according to E/e′ ratio and LVEDV would be able to separate different phenotypes.
Methods
Subjects
In order to reduce the overall variability of the echocardiographic measures, we studied only white men affected by type 2 diabetes. The sample of the present study was composed of 176 adult male ambulatory patients, out of 215. Ischaemic heart diseases, revascularisation and/or chronic heart failure, valvular heart diseases, atrial fibrillation or atrial flutter, a prior history of cirrhosis, malignancy or overt nephropathy, EF below 50% and absence of some variables were considered exclusion criteria.
Clinical and laboratory variables
Clinical data (duration of diabetes, body mass index and blood pressure) were collected and patients were considered to have hypertension if their blood pressure was ≥140/90 mm Hg or if they were taking some antihypertensive drugs. Venous blood samples were drawn in the morning after an overnight fast. Serum creatinine (measured using a Jaffé rate-blanked and compensated assay) and other biochemical blood measurements were determined using standard laboratory procedures (DAX 96; Bayer Diagnostics, Milan, Italy). Low-density lipoprotein cholesterol was calculated using the Friedewald equation. Haemoglobin A1c (HbA1c) was measured by an automated high-performance liquid chromatography analyser (HA-8140; Menarini Diagnostics, Florence, Italy). The glomerular filtration rate (eGFRCKD-EPI) was estimated by the CKD Epidemiology Collaboration (CKD-EPI) equation.12 Albuminuria was measured by an immuno-nephelometric method on a morning spot urine sample and expressed as the albumin:creatinine ratio. A single ophthalmologist diagnosed diabetic retinopathy using funduscopy after pupillary dilation according to a clinical disease severity scale (no retinopathy, non-proliferative, proliferative or laser-treated retinopathy); the presence of proliferative retinopathy was confirmed by fundus fluorescein angiography. Nephropathy was defined as the presence of eGFR <60 mL/min/1.73 m2 and/or abnormal albuminuria (ie, an albumin:-creatinine ratio ≥30 mg/g creatinine). For all participants, the presence of retinopathy or nephropathy was recorded as microvascular complication, whereas the absence of both complications included those subjects in the category without microvascular complications.
Echocardiography
Transthoracic echocardiographic Doppler evaluation with spectral tissue Doppler analysis (Vivid 7; GE Vingmed, Horten, Norway) were performed in all patients by two experienced cardiologists who were blinded to the participants’ details. Conventional echocardiography was used to measure LV diameters, wall thickness and mass according to standard criteria. LV end-diastolic and end-systolic volumes and LVEF at rest were measured at the apical four-chamber and two-chamber views (by modified Simpson rule).13 Left atrial volume index (LAVI) maximal volume was measured at the end of LV systole from the apical four-chamber and two-chamber views (maximum LA size) using the modified Simpson rule.13 LAVI was calculated as LA volume divided by the body surface area. Pulsed-wave Doppler was used to measure trans-mitral peak early diastolic velocity (E), peak late diastolic velocity (A) and E-wave deceleration time (DTe). Each value was obtained from the average of three measurements. Pulsed-wave tissue Doppler echocardiography of the septal and lateral mitral annulus was used to measure the early peak (e′), and the mean values of septal and lateral annulus measurements were used for analysis (e mean wave).14
In a previous study,15 we have shown that when tissue Doppler imaging signals were remeasured by the same observer, the mean absolute differences (±SD) in tissue velocities within the same observer was 0.19±0.17 cm/s for e′ velocity (p=NS).
Heart failure with preserved ejection fraction (HFpEF) was considered when patients present a LVEF above or equal to 50%.2
Study design
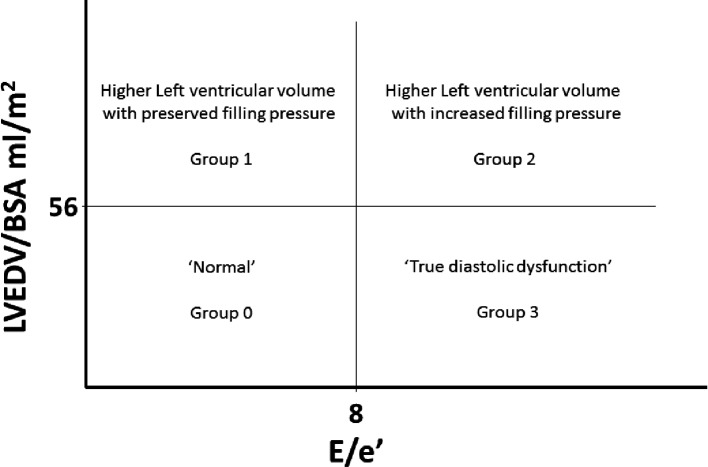
This cross-sectional study sought to assess whether differences in clinical and echocardiographic features existed between patients with type 2 diabetes according to the presence of variations in LVEDV and in E/e′ ratio, estimates of modifications of LV geometry or LV filling pressures, respectively. Four defined groups were thus constituted based on the median value of both LVEDV/body surface area (BSA (56 mL/m2) and averaged E/e′ ratio,8 as reported in figure 1. Subjects with LVEDV/BSA <56 mL/m2 and averaged E/e′ <8 were considered (group 0) ‘normal’ and used as reference category in a multinomial regression model to assess the variables associated with the different groups.
Figure 1.
Categorisation of subjects in four groups according to median values of left ventricular end-diastolic volume (LVEDV) mL/m2 and E/e′. Group 0: LVEDV/body surface area (BSA) <56 mL/m2 and averaged E/e′ ≤8; group 1: LVEDV/BSA ≥56 mL/m2 and averaged E/e′ ≤8; group 2: LVEDV/BSA ≥56 mL/m2 and averaged E/e′ >8; group 3: LVEDV/BSA <56 mL/m2 and averaged E/e′ >8.
Statistical analysis
Data are summarised as means±SD or percentages. Differences in clinical/biochemical characteristics and echocardiographic parameters among groups of patients were compared by the one-way analysis of variance for normally distributed variables and the non-parametric tests for non-normally distributed variables. The χ2 test was used for categorical variables to study differences in proportions or percentages between the groups. To estimate the independent predictors of different phenotypes, a multinomial logistic regression analysis was performed with group 0 as the reference category. Age, duration of diabetes, eGFRCKDEPI, glycosylated haemoglobin, EF E/A, LAVI, septum thickness and s′ mean wave velocity were included as covariates. Covariates for this multinomial logistic regression model were chosen as potential confounding factors based on their significance in univariate analyses. The bivariate correlation between septum thickness and LV mass/BSA was highly significant (r=0.635, p<0.001), and we included only the septum thickness in the multinomial model. A p value<0.05 was assumed to indicate a statistical significance.
Results
We studied 176 men affected by type 2 diabetes with no cardiovascular diseases and preserved ejection fraction (LVEF >50 %) by echocardiography. Considering that the elevated LV diastolic filling pressure with normal LV end-diastolic volume is a strong evidence in favour of well-developed diastolic dysfunction, we categorised subjects according to the median value of LVEDV/BSA (56 mL/m2) and the median value of E/e′ ratio,8 respectively. Therefore, as shown in figure 1, we obtained four phenotypic groups, with group 3 highly indicative of diastolic dysfunction (increased diastolic filling pressure with a normal LVEDV) and group 2 with both increased diastolic filling pressure and higher LVEDV. Subjects with LVEDV/BSA <56 mL/m2 and E/e′ <8 were considered (group 0) ‘normal’ and used as reference category, while groups 1 and 2 showed higher LVEDV. Only very few subjects (3,9%) had a LVEDV above 74 mL/m2, thus satisfying the clinical cut-off for LV dilation.10
Table 1 reports clinical characteristics of the subjects subdivided into the four phenotypic groups. Subjects in group 3 were the oldest, while the longest duration of diabetes was detected in group 2, it being 9–10 years (in average) longer than that of subjects of group 0. Subjects in group 2 showed also the lowest eGFR value, a higher prevalence of subjects treated for hypertension and a tendency to a higher prevalence of subjects with retinopathy. Glycaemic control and lipids levels were comparable among the groups. Oral hypoglycaemic agents were taken by 63.4% of patients, none of them were treated with pioglitazone or SGLT2 inhibitors, while 31.5% were on insulin treatment, and 20% were treated with insulin associated to an oral hypoglycaemic agent. Antihypertensive therapy was reported in 71.8% of patients, while 43% were on statin treatment.
Table 1.
Descriptive statistics for the clinical characteristics of male subjects with type 2 diabetes mellitus stratified by groups
| Group 0(n=53) | Group 1(n=41) | Group 2(n=43) | Group 3(n=39) | P values | |
| Age, years | 64.1±8.8 | 67.3±7.0 | 68.5±8.0 | 70.0±6.4 | 0.003 |
| Diabetes duration, years | 8.4±9.4 | 10.5±8.2 | 17.9±11.2 | 15.2±9.2 | <0.001 |
| BMI, kg/m2 | 28.3±4.0 | 28.1±3.9 | 28.9±4.6 | 27.8±2.9 | 0.559 |
| Pulse pressure, mm Hg | 57.5±12.5 | 64.7±16.5 | 65.3±12.0 | 65.3±14.3 | 0.011 |
| HbA1c, mmol/mol | 52.7±8.7 | 53.6±9.2 | 57.0±17.0 | 57.8±11.5 | 0.118 |
| Total cholesterol, mmol/L | 4.5±1.0 | 4.2±0.9 | 4.3±1.0 | 4.4±1.0 | 0.494 |
| LDL cholesterol, mmol/L | 2.7±0.9 | 2.3±0.8 | 2.4±0.8 | 2.5±0.8 | 0.083 |
| HDL cholesterol, mmol/L | 1.19±0.29 | 1.19±0.30 | 1.18±0.29 | 1.24±0.28 | 0.775 |
| Triglycerides, mmol/L | 1.7±0.7 | 1.5±0.8 | 1.6±0.7 | 1.6±0.7 | 0.838 |
| eGFRCKD-EPI, mL/min/1.73 m2 | 84.6±14.5 | 86.2±11.0 | 75.6±22.1 | 80.7±13.7 | 0.009 |
| Hypertension | 86.8% | 82.9% | 97.7% | 89.7% | 0.157 |
| Retinopathy, of any degree | 15.1% | 4.9% | 25.6% | 15.4% | 0.074 |
| Nephropathy | 24.5% | 31.7% | 34.9% | 28.2% | 0.716 |
| Current smoker | 45.3% | 31.7% | 25.6% | 33.3% | 0.288 |
| Insulin therapy | 39.6% | 24.4% | 34.9% | 46.2% | 0.218 |
| Antihypertensive therapy | 62.5% | 61.0% | 86.0% | 82.1% | 0.011 |
Values are means±SD, percentages or medians (IQR). Hypertension was defined as blood pressure ≥140/90 mm Hg or use of any antihypertensive drugs. Nephropathy was defined as the presence of eGFR <60 mL/min/1.73 m2 and/or abnormal albuminuria. Group 0: LVEDV/BSA <56 mL/m2 and averaged E/e′ ≤8; group 1: LVEDV/BSA ≥56 mL/m2 and averaged E/e′ ≤8; group 2: LVEDV/BSA ≥56 mL/m2 and averaged E/e′ >8; group 3: LVEDV/BSA <56 mL/m2 and averaged E/e′ >8.
BMI, body mass index; BSA, body surface area; eGFRCKD-EPI, estimated glomerular filtration rate; HbA1c, glycosylated haemoglobin; HDL, high-density lipoprotein; LDL, low-density lipoprotein; LVEDV, left ventricular end-diastolic volume.
The echocardiographic characteristics are reported in table 2. The lowest values of EF were observed in subjects in group 2 and in group 1. E/A ratio was significantly different among the four groups and the indexes of systolic performance, annular S′ waves, were lower in subjects in groups 2 and 3. LA volume index was significantly higher in subjects of group 2. Septum thickness and LV mass were higher in groups 2 and 3. No differences were noticed in relative wall thickness.
Table 2.
Echocardiographic characteristics of male subjects with type 2 diabetes mellitus stratified by groups
| Group 0(n=53) | Group 1(n=41) | Group 2(n=43) | Group 3(n=39) | P values | |
| LV ejection fraction, % | 67.2±7.7 | 61.2±4.8 | 59.8±4.8 | 64.7±5.7 | <0.001 |
| S′septum, cm/s | 9.4±2.0 | 9.3±2.2 | 8.0±1.3 | 8.3±1.6 | <0.001 |
| S′lateral, cm/s | 10.5±2.5 | 10.0±2.2 | 9.2±2.2 | 8.7±1.8 | 0.003 |
| Septum thickness, mm | 11.4±1.4 | 11.6±1.3 | 12.3±1.2 | 12.0±1.5 | 0.007 |
| E/A ratio | 0.77±0.22 | 0.74±0.17 | 0.89±0.23 | 0.77±0.14 | 0.003 |
| Deceleration time, ms | 241.4±62.9 | 266.0±74.2 | 244.9±51.6 | 252.7±66.0 | 0.297 |
| E/e′ ratio | 6.3±1.3 | 6.5±1.3 | 10.7±1.9 | 9.9±1.9 | NA |
| LVEDV/BSA, mL/m2 | 47.5 | 64.0 | 64.5 | 48.4 | NA |
| LA volume index, mL/m2 | 24.0±6.8 | 31.2±6.0 | 35.2±8.2 | 30.6±9.4 | <0.001 |
| LV mass/BSA*, g/m2 | 129.5±27.8 | 129.5±24.6 | 144.1±22.7 | 137.3±26.5 | 0.025 |
| Relative wall thickness | 0.43±0.05 | 0.41±0.07 | 0.43±0.06 | 0.43±0.05 | 0.432 |
Values are means±SD or percentages. Group 0: LVEDV/BSA <56 mL/m2 and averaged E/e′ ≤8; group 1: LVEDV/BSA ≥56 mL/m2 and averaged E/e’ ≤8; group 2: LVEDV/BSA ≥56 mL/m2 and averaged E/e′ >8; group 3: LVEDV/BSA <56 mL/m2 and averaged E/e′ >8.
LV mass/BSA was calculated using the following formula: (0.8×(1.04×(LVDd+IVSd+PWd)3−(LVDd)3))+0.6/body surface area (28).
BSA, body surface area; LA, left atrial; LV, left ventricular; LVEDV, left ventricular end-diastolic volume.
In order to identify factors associated with the different phenotypes, we performed a multinomial logistic regression analyses with different phenotypes as dependent variable (group 0 was the reference phenotype), as shown in table 3. Age was a significant predictor associated with all groups, while diabetes duration was significant only in group 2. Decreasing EF and increasing LAVI were significant predictors in groups 1 and 2 compared with group 0; both groups have a higher LVED. E/A ratio was a significant predictor in groups 2 and 3. Septum thickness was a significant predictor in group 2, while a reduction of S′ mean wave was a significant predictor in group 3. The inclusion of either antihypertensive therapy (0=no therapy; 1=therapy) or retinopathy (0=no retinopathy; 1=retinopathy) in the model does not change the results.
Table 3.
Multinomial logistic regression analysis investigating factors associated with groups
| Reference group0 | Group 1OR (95% CI) | P values | Group 2OR (95% CI) | P values | Group 3 | P values |
| Age, years | 1.10 (1.00 to 1.21) | 0.042 | 1.12 (0.97 to 1.25) | 0.058 | 1.16 (1.04 to 1.30) | 0.010 |
| Diabetes duration, years | 0.99 (0.91 to 1.08) | 0.731 | 1.09 (1.00 to 1.18) | 0.041 | 1.06 (0.98 to 1.13) | 0.139 |
| HbA1c | 1.08 (0.53 to 2.20) | 0.831 | 0.66 (0.26 to 1.65) | 0.373 | 1.60 (0.81 to 3.17) | 0.181 |
| eGFRCKD-EPI, mL/min/1.73 m2 | 1.03 (0.97 to 1.09) | 0.294 | 1.00 (0.94 to 1.06) | 0.888 | 1.03 (0.98 to 1.09) | 0.233 |
| Ejection fraction | 0.83 (0.75 to 0.93) | 0.001 | 0.82 (0.72 to 0.93) | 0.002 | 0.96 (0.88 to 1.05) | 0.415 |
| E/A | 1.07 (0.74 to 1.56) | 0.705 | 2.14 (1.39 to 3.31) | 0.001 | 1.54 (1.03 to 2.31) | 0.035 |
| Septum thickness (mm) | 1.04 (0.69 to 1.57) | 0.851 | 1.78 (1.06 to 2.99) | 0.030 | 1.41 (0.90 to 2.23) | 0.106 |
| LA volume index, mL/m2 | 1.16 (1.05 to 1.27) | 0.003 | 1.18 (1.06 to 1.31) | 0.002 | 1.09 (0.99 to 2.19) | 0.131 |
| S′mean, cm/s | 1.05 (0.77 to 1.44) | 0.755 | 0.71 (0.45 to 1.11) | 0.134 | 0.60 (0.39 to 0.92) | 0.019 |
Data are expressed as OR and 95% CI. Group 0: LVEDV/BSA <56 mL/m2 and averaged E/e′ ≤8; group 1: LVEDV/BSA ≥56 mL/m2 and averaged E/e′ ≤8; group 2: LVEDV/BSA ≥56 mL/m2 and averaged E/e′ >8; group 3: LVEDV/BSA <56 mL/m2 and averaged E/e′ >8. Values in bold are statistically significant results.
eGFRCKD-EPI, estimated glomerular filtration rate; HbA1c, glycosylated haemoglobin; LA, left atrial.
Discussion
The results of the present study suggest that in patients affected by type 2 diabetes with preserved EF and without cardiovascular diseases, a higher LVEDV may identify different phenotypes of subjects. Our classification appears to differentiate subjects according to clinical and echocardiographic parameters. Notably, the different phenotypes were predicted by different factors in multinomial logistic regression analysis. In detail, in subjects of group 2 (increase in both LVEDV/BSA and E/e′), the main associated predictors were diabetes duration, LAVI and EF; also, the septum thickness was significantly associated with this group. Regarding the latter association, it is interesting to note that subjects of group 2 were more frequently treated with antihypertensive therapy. Subjects of group 3, those who ideally represent the real diastolic dysfunction with increased diastolic filling pressure and normal LVEDV, showed as main predictors the S′ mean wave and E/A ratio. The factors significantly associated to group 1 were EF and LAVI. It appears that subjects belonging to group 2 have the worse clinical profile with a diabetes of longer duration, a lower eGFRCKD-EPI (16.3% of subjects in this group showed a eGFRCKD-EPI below 60 mL/min/1.73 m2 compared with 7.7% in group 0, 2.4% in group 1% and 10.3% in group 3), a higher pulse pressure, more frequently treated for hypertension and a higher prevalence of retinopathy.
Recent studies conducted in patients with hypertension suggested that LV dilation could identify different phenotypes of LV hypertrophy showing differences in both biomarkers of cardiac performances and prognosis.16 17
Therefore, in the present study, we tested the hypothesis whether LVEDV may identify different phenotypes of patients with type 2o diabetes.
LVEDV is an important parameter of cardiac geometry and even a small increase in volume may have prognostic relevance, as previously shown. A higher LVEDV was shown to independently predict trastuzumab-related cardiotoxicity in patients with HER2-positive early breast cancer.18 In an initially untreated large sample of subjects with hypertension, those with LV dilation, regardless of the hypertrophy pattern, experienced a worse prognosis compared with subjects without LV dilation.19 20 The authors of the Dallas study suggested to subdivide eccentric and concentric LVH into two subgroups based on the presence or absence of LV dilation.16 In this study, subjects with LV dilation were at higher risk of cardiovascular death and heart failure compared with those without LV dilation.16
However, not all studies confirmed the prognostic impact of LV dilation over the LV mass,21 but perhaps this study did not have enough events.
Our study seems to indicate that LVEDV dilation, even though in a normal range, in type 2 diabetes may identify patients with different characteristics. More notable, patients in groups 2 and 3 who differ only for a LVEDV/BSA higher or lower than 56 mL/m2 but with a similar ventricular filling pressure showed similar duration of diabetes and metabolic control level but differed for EF and S′ mean wave, while subjects in group 1 with LVEDV similar to group to 2 but with a normal ventricular filling pressure showed a significant shorter duration of diabetes. These data may suggest that the pathway of cardiac involvement in diabetes might follow different directions: one leading to dilation and the other to diastolic dysfunction. LVEDV may represent the initial bifurcation in this pathway, and it may appear earlier than the diastolic dysfunction. It would be extremely interesting to test this hypothesis in a longitudinal setting with cardiac death and heart failure as outcomes in type 2 diabetes.
Moreover, in a recent study by our group, we also reported an inverse correlation between metabolic control and subclinical systolic dysfunction22 that may also suggest that a better metabolic control could slow the progression of e cardiac disease in type 2 diabetes.
An interesting and recent study by using the cluster analysis on the echocardiographic parameters identified three clusters of cardiac phenotypes: one with low comorbidity, a second with elderly and diastolic dysfunction and a third phenotype with hypertrophic systolic dysfunction. Interestingly, the first cluster showed the lowest value of E/e′ and these patients are similar to those of our group 0, while the third cluster showed the highest left ventricular volume and the lowest strain and these patients are similar to those of our group 2.23
Therefore, considering that the more frequent presentation when the dominant problem is diabetes consists in a setting characterised by abnormal systolic function, as shown by longitudinal strain, despite normal EF,24 the results of our study may have clinical relevance. In fact, a finding of an increasing LVEDV may herald a very initial change in LV geometry that can influence the prognosis of a patient; it would be interesting to test whether a potentiation of therapy at this point may hinder the evolution towards heart failure.
The study is limited by its own cross-sectional design that does allow inferences of the cause–effect relationship, and the use of median value of LVEDV to categorise subjects instead of a clinical cut-off points of normalcies. However, it should be noted that 56 mL/m2 for LVEDV/BSA is close to the mean value for this parameter (54 mL/m2) reported in the recommendations for cardiac chamber quantification.10 Moreover, the absence of a control group does not allow to draw the conclusion of whether this is relevant for patients with type 2 diabetes or also for non-diabetic subjects. Finally, it should be pointed out that due to the low number of subjects in each group, the results should be interpreted with caution since there is a significant risk to find false associations. Nevertheless, our study has some strengths: all subjects had preserved EF and absence of cardiovascular diseases, they were all men eliminating interferences due to sex, and patients were well characterised with respect to diabetes and its complications.
In conclusion, our study might suggest that variations of LVEDV along with E/e′ ratio variations, even in the normal range, may allow to recognise phenotypes of type 2 diabetes patients with worse clinical characteristics. This finding should be tested in further prospective studies to assess the possible predictive roles of these phenotypes.
Footnotes
Contributors: GZ, GT, CB: conception, design and analysis and interpretation of data. GZ: drafting the manuscript. GT, SB, CB, AR, EB: revising critically the manuscript for important intellectual content. MT, LL, AM, LB, LZ, AT: contributed to the analysis and interpretation of data.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: The local ethics committee approved the study protocol.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: All data generated or analysed during this study are included in this published article.
References
- 1. Lehrke M, Marx N. Diabetes mellitus and heart failure. Am J Cardiol 2017;120(1S):S37–S47. 10.1016/j.amjcard.2017.05.014 [DOI] [PubMed] [Google Scholar]
- 2. Ponikowski P, Voors AA, Anker SD, et al. . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016;18:891–975. 10.1002/ejhf.592 [DOI] [PubMed] [Google Scholar]
- 3. Kannel WB, McGee DL. Diabetes and cardiovascular disease. The Framingham study. JAMA 1979;241:2035–8. [DOI] [PubMed] [Google Scholar]
- 4. Boonman-de Winter LJ, Rutten FH, Cramer MJ, et al. . High prevalence of previously unknown heart failure and left ventricular dysfunction in patients with type 2 diabetes. Diabetologia 2012;55:2154–62. 10.1007/s00125-012-2579-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Petrie MC, Caruana L, Berry C, et al. . "Diastolic heart failure" or heart failure caused by subtle left ventricular systolic dysfunction? Heart 2002;87:29–31. 10.1136/heart.87.1.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lindman BR. The diabetic heart failure with preserved ejection fraction phenotype: is it real and is it worth targeting therapeutically? Circulation 2017;135:736–40. 10.1161/CIRCULATIONAHA.116.025957 [DOI] [PubMed] [Google Scholar]
- 7. Konstam MA, Abboud FM. Ejection fraction: misunderstood and overrated (changing the paradigm in categorizing heart failure). Circulation 2017;135:717–9. 10.1161/CIRCULATIONAHA.116.025795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Khouri MG, Peshock RM, Ayers CR, et al. . A 4-tiered classification of left ventricular hypertrophy based on left ventricular geometry: the Dallas heart study. Circulation 2010;3:164–71. 10.1161/CIRCIMAGING.109.883652 [DOI] [PubMed] [Google Scholar]
- 9. Kasner M, Westermann D, Steendijk P, et al. . Utility of Doppler echocardiography and tissue Doppler imaging in the estimation of diastolic function in heart failure with normal ejection fraction: a comparative Doppler-conductance catheterization study. Circulation 2007;116:637–47. 10.1161/CIRCULATIONAHA.106.661983 [DOI] [PubMed] [Google Scholar]
- 10. Lang RM, Badano LP, Mor-Avi V, et al. . Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015;16:233–71. 10.1093/ehjci/jev014 [DOI] [PubMed] [Google Scholar]
- 11. Galderisi M, Esposito R, Trimarco B. Cardiac involvement in diabetes: the dark side of the moon. J Am Coll Cardiol 2017;70:1717–9. 10.1016/j.jacc.2017.08.039 [DOI] [PubMed] [Google Scholar]
- 12. Levey AS, Stevens LA, Schmid CH, et al. . CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nagueh SF, Appleton CP, Gillebert TC, et al. . Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr 2009;22:107–33. 10.1016/j.echo.2008.11.023 [DOI] [PubMed] [Google Scholar]
- 14. Nagueh SF, Smiseth OA, Appleton CP, et al. . Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2016;29:277–314. 10.1016/j.echo.2016.01.011 [DOI] [PubMed] [Google Scholar]
- 15. Bonapace S, Targher G, Molon G, et al. . Relationship between early diastolic dysfunction and abnormal microvolt T-wave alternans in patients with type 2 diabetes. Circ Cardiovasc Imaging 2011;4:408–14. 10.1161/CIRCIMAGING.110.962951 [DOI] [PubMed] [Google Scholar]
- 16. Khouri MG, Peshock RM, Ayers CR, et al. . A 4-tiered classification of left ventricular hypertrophy based on left ventricular geometry: the Dallas heart study. Circ Cardiovasc Imaging 2010;3:164–71. 10.1161/CIRCIMAGING.109.883652 [DOI] [PubMed] [Google Scholar]
- 17. Garg S, de Lemos JA, Ayers C, et al. . Association of a 4-tiered classification of LV hypertrophy with adverse CV outcomes in the general population. JACC Cardiovasc Imaging 2015;8:1034–41. 10.1016/j.jcmg.2015.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bergamini C, Torelli F, Ghiselli L, et al. . Left ventricular end-diastolic volume as early indicator of trastuzumab-related cardiotoxicity in HER2+ breast cancer patients: results from a single-center retrospective study. Minerva Cardioangiol 2017;65:278–87. 10.23736/S0026-4725.16.04278-X [DOI] [PubMed] [Google Scholar]
- 19. Verdecchia P, Angeli F, Mazzotta G, et al. . Impact of chamber dilatation on the prognostic value of left ventricular geometry in hypertension. J Am Heart Assoc 2017;6:pii: e005948 10.1161/JAHA.117.005948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. de Simone G, Izzo R, Aurigemma GP, et al. . Cardiovascular risk in relation to a new classification of hypertensive left ventricular geometric abnormalities. J Hypertens 2015;33:745–54. 10.1097/HJH.0000000000000477 [DOI] [PubMed] [Google Scholar]
- 21. Cuspidi C, Facchetti R, Bombelli M, et al. . Risk of mortality in relation to an updated classification of left ventricular geometric abnormalities in a general population. J Hypertens 2015;33:2133–40. 10.1097/HJH.0000000000000658 [DOI] [PubMed] [Google Scholar]
- 22. Zoppini G, Bergamini C, Bonapace S, et al. . Association between subclinical left ventricular systolic dysfunction and glycemic control in asymptomatic type 2 diabetic patients with preserved left ventricular function. J Diabetes Complications 2017;31:1035–40. 10.1016/j.jdiacomp.2017.01.021 [DOI] [PubMed] [Google Scholar]
- 23. Ernande L, Audureau E, Jellis CL, et al. . Clinical implications of echocardiographic phenotypes of patients with diabetes mellitus. J Am Coll Cardiol 2017;70:1704–16. 10.1016/j.jacc.2017.07.792 [DOI] [PubMed] [Google Scholar]
- 24. Marwick TH, Ritchie R, Shaw JE, et al. . Implications of underlying mechanisms for the recognition and management of diabetic cardiomyopathy. J Am Coll Cardiol 2018;71:339–51. 10.1016/j.jacc.2017.11.019 [DOI] [PubMed] [Google Scholar]