Abstract
Helicobacter pylori (Hp) inhabits the stomach of > 50% of humans and has been established as a major etiological factor in the pathogenesis of chronic gastritis, gastric atrophy, peptic ulcer disease, gastric adenocarcinoma, and gastric mucosa-associated lymphoid tissue lymphoma. The aim of this study was to provide unequivocal information about Hp-associated gastritis grading according to the Sydney grading system and to compare the histopathological features with the endoscopic findings and anti-Hp immunoglobulin (Ig)G serological status. This analytical study was conducted on 157 patients with dyspeptic gastritis. All patients underwent esophagogastroduodenoscopy, and antrum and corpus biopsies were taken. Blood samples were obtained from all participants. Different stains were performed on formalin-fixed, paraffin-embedded tissue blocks that included hematoxylin and eosin and Giemsa stain for histopathological interpretation. The endoscopic findings of gastritis were observed in 120 patients and most of them showed hyperemia (80 patients), whereas seven patients had normal appearing gastric mucosa. Histologically variable numbers of mononuclear inflammatory cellular infiltrates were seen in 150 cases (95.5%). Most of them showed Grade 1 gastritis (80 patients), whereas Grades 2 and 3 were found in 43 and 27 biopsies, respectively. Hp colonization was observed in most of the examined biopsies (93.7%). Hp-IgG seropositivity was found in 80.9% of cases and 19.1% were seronegative. The relationship between endoscopic and histological findings was significant (p < 0.001).
Keywords: Hp, Endoscopic, Histopatholoical & serum IgG, Diagnostic methods
1. Introduction
Infection by Helicobacter pylori (Hp) has been established as a major cause of chronic gastritis. It affects ~50% of the world's population, and it is important in the pathogenesis of other gastrointestinal diseases such as peptic ulcer disease, gastric adenocarcinoma, and gastric lymphoma [1,2,3,4]. Studies reported that its prevalence ranged from < 15% in some populations to virtually 100%, depending on socioeconomic status and country development [5,6].
The National Institute of Health Consensus Development Conference concluded that patients with Hp infection should receive antimicrobial therapy, as the risk of ulcer recurrence and associated complications do not diminish unless Hp infection is cured [7,8]. The inflammatory process has been shown to disappear following eradication of Hp infection, and similar inflammatory changes have been identified in animal models of Hp infection [9]. The histological features of Hp infection are including active inflammation and mononuclear cellular infiltrates which are particularly useful in differentiating bacterial gastritis from non bacterial induced gastritis [10]. Chronic inflammation plays important roles in the development of various cancers, particularly in digestive organs, including Hp-associated gastric cancer (GC) [11]. GC is an important cause of cancer-related death, particularly in Europe, so understanding the pathogenesis of Hp-induced GC may improve risk stratification for prevention and therapy [12,13]. Additionally, circumstantial evidence suggests that infection with Hp may increase the risk of gastric lymphoma, and 60% of cases evolve from chronic gastritis associated with Hp infection [14,15]. In addition to the above, Hp is well recognized as a Class 1 carcinogen because its long-term colonization can provoke chronic inflammation and mucosal atrophy, which can further lead to malignant transformation [16].
Assessing gastritis involves clinical, endoscopic, and histopathological examination [17,18,19]. The Sydney grading system for chronic gastritis and its updated Houston version are the commonly used nomenclature for gastritis but they remain inconsistent [20]. This system categorized gastritis according to intensity of mononuclear inflammatory cellular infiltrates, polymorph activity, atrophy, intestinal metaplasia, and Hp density into mild, moderate and severe categories [20,21]. Nonstandard histology reporting formats are still widely used for gastritis and even specialists are often frustrated by the histological definitions that make it difficult to identify candidates for clinicoendoscopic surveillance [2]. Additionally, there is no simple validated test to quantify the density of Hp infection [22].
Chronic inflammation of the gastric mucosa is also associated with detectable levels of anti-Hp immunoglobulin (Ig)A and IgG classes [23]. Almost all Hp-infected individuals have elevated levels of IgG antibodies, but only in about two-thirds of cases does the IgA titer exceed the cut-off level [24]. Anti-Hp-IgG titer usually remains elevated for long periods after bacterial clearance or eradication [25]. In the same way, its elevated titer is significantly associated with atrophic gastritis and with the presence of GC [26]. This study will supply clinicians, surgeons, and general practitioners with insights into these three diagnostic methods for Hp, including endoscopy, histopathological features of Sydney grading, and serology.
2. Materials and methods
2.1. Patient selection
From August 2012 to July 2014, 157 patients were selected from those who came to the surgery outpatient clinic, Arar Central Hospital, Arar, Saudi Arabia. These patients had symptomatic dyspepsia. The patients were examined clinically, and various routine laboratory investigations were done, including complete blood count, liver and renal function tests, and bleeding and coagulation assessment. Then, the patients underwent esophagogastroduodenoscopy (EGD). Patients were excluded if they had taken Hp eradication treatment such as antibiotics, proton pump inhibitors, or H2 antagonists in the 4 weeks before endoscopy. All the patients gave signed informed consent for biopsies and blood samples.
2.2. Endoscopy
EGD was performed in all our patients in the endoscopy section using Olympus (GFIXQ150, SN:2206196, Olympus corporation, Tokyo) forward viewing EGD under intravenous sedation. A minimum two gastric mucosal tissue biopsies (1 each from the antrum and corpus) were taken. All the patients were examined for findings suggestive of endoscopic gastritis, such as erythema, hyperemia, atrophy, and mucosal nodularity according to the criteria of the Sydney grading system [20]. Additionally, they were examined for the presence of gastric erosion and ulceration. Also, they were evaluated for the anatomical location of gastritis, including antrum, antrum predominant pangastritis, and corpus predominant gastritis. Findings which were suggesting duodenitis, such as hyperemia or ulceration, were considered [27]. Patients were considered endoscopically normal if the gastric mucosa was pink, smooth and lustrous [28].
2.3. Anti-Hp IgG
Blood samples were collected from all the patients, then serological analysis was performed in the Serological Unit of the Department of Virology, Arar Central Hospital for the presence of serum anti-Hp IgG antibody by enzymelinked immunosorbent assay, using Hp-IgG from the UD1-labs (United Diagnostic Industry, KSA; dilution 1:100). The results were recorded as positive or negative according to the manufacturer's directions of OD value of cut-off control which had upper limit of 9.999 and lower limit of 0.480 (UDl-labs; 2013).
2.4. Histopathology
All the obtained biopsies were collected, placed on filter paper, fixed in 10% neutral formalin, and sent for preparation of formalin-fixed, paraffin-embedded tissue blocks. Three-micrometer-thick sections were prepared. One set of tissue sections was stained with hematoxylin and eosin and the other with Giemsa stain for histopathological examination (by 2 experienced pathologists), including detection of Hp in the gastric mucosa. The biopsies were evaluated for the intensity of mononuclear inflammatory cellular infiltrates, inflammatory activity (neutrophilic infiltrations), glandular atrophy, metaplasia, reparative atypia, and dysplasia [21]. Additionally, the cases were graded according to the Houston-updated Sydney system [20], which was graded according to the intensity of mono-nuclear inflammatory cellular infiltrates within the lamina propria: absent inflammation (Grade 0), mild inflammation (Grade 1), moderate inflammation (Grade 2), and severe inflammation (Grade 3; Table 1).
Table 1.
Parameters of gastritis grading according to the updated Sydney system[20].
| Corpus | |||||
|---|---|---|---|---|---|
| No inflammation (G0) | Mild inflammation (G1) | Moderate inflammation (G2) | Severe inflammation (G3) | ||
| ANTRUM | No inflammation (G0) | Grade 0 | Grade I | Grade II | Grade II |
| Mild inflammation (G1) | Grade I | Grade II | Grade II | Grade III | |
| Moderate inflammation (G2) | Grade II | Grade II | Grade III | Grade IV | |
| Severe inflammation (G3) | Grade III | Grade III | Grade IV | Grade IV | |
2.5. Statistical analysis
The statistical analysis was undertaken using SPSS for Windows version 16. A Z test was used for comparison between two proportions. Results were considered to be statistically significant at p < 0.05.
3. Results
3.1. Clinicoendoscopic findings
The patients’ age ranged from 25 years to 80 years with an average of 47 years; 100 patients were male and 57 were female. All patients had heartburn, nausea, and epigastric pain, which did not respond to normal treatment. EGD revealed features of endoscopic gastritis in 120 patients: hyperemia in 80, erosion in 15, ulceration in 10, and nodularity in 15. Regarding the anatomical location of endoscopic gastritis, antral gastritis was found in 90 patients, and antrum predominant pangastritis and corpus predominant gastritis in 15 patients each. All the above findings were significant (p < 0.001). Seven patients (4.5%) had normal appearing gastric mucosa. Endoscopic duodenitis in the form of hyperemia and ulceration was observed in 12 patients, and 10 patients were diagnosed with gastroesophageal reflux disease, and eight with hiatus hernia (Table 2).
Table 2.
Clinical and endoscopic findings.
| Data | No. of cases |
|---|---|
| Age (y) | |
| 25–50 | 100 |
| 51–80 | 57 |
| Sex | |
| Male | 100 |
| Female | 57 |
| Endoscopic findings | |
| 1- Endoscopic gastritis | 120 (76.4) |
| a. Hyperemia | 80 |
| b. Erosions | 15 |
| c. Ulcerations | 10 |
| d. Nodularity | 15 |
| e. Location of endoscopic gastritis | |
| - Antral | 90 |
| - Antrum predominant pangastritis | 15 |
| - Corpus predominant gastritis | 15 |
| 2- Normal gastric mucosa | 7 (4.5) |
| 3- Endoscopic duodenitis | 12 (7.6) |
| 4- Gastroesophageal reflux disease | 10 (6.4) |
| 5- Hiatus hernia | 8 (5.1) |
| Total | 157 |
Data are presented as n or n (%).
3.2. Anti-Hp-IgG
Anti-Hp IgG seropositivity was found in 127 patients (80.9%), with a maximum level of 1189.66%, whereas seronegativity was seen in the remaining 30 patients (19.1%; Table 3).
Table 3.
Frequency of serum levels of Helicobacter pylori immunoglobulin G.
| Frequency | % | Valid % | Cumulative % | |
|---|---|---|---|---|
| Positive | 127 | 80.9 | 80.9 | 80.9 |
| Negative | 30 | 19.1 | 19.1 | 100.0 |
| Total | 157 | 100.0 | 100.0 |
3.3. Histopathological findings
In gastric biopsies, mononuclear inflammatory cellular infiltrates were found in 150 cases (95.5%). The intensity of these inflammatory infiltrates was variable, with Grade 1 gastritis (G1) detected in 80 cases (Figure 1), Grade 2 gastritis (G2) in 43 cases (Figure 2), and Grade 3 gastritis (G3) in 27 cases (Figure 3). In addition, lymphoid follicles with germinal center formation were seen in 40 cases (Figure 4), as well as active inflammation with polymorph infiltration in the lamina propria or inside the glandular lumina identified in 50 cases (Figure 5). Also, 15 cases showed mucosal glandular atrophy (Figure 6); five showedintestinal metaplastic changes (Figure 7); 12 showed reparative atypia of the mucosal glands (Figure 8), whereas, 10 cases exhibited dysplastic changes (Figure 9). Hp colonization was seen in 147 biopsies (Figure 10), among which, three showed gastric adenocarcinoma that developed in addition to chronic Hp-induced gastritis (Figure 11). There was a sign ificant correlation between the various grades of gastritis and Hp colonization (p < 0.001). Hp mononuclear inflammatory cellular infiltrates, lymphoid follicles, and inflammatory activity were observed in most cases (95.5%)with significant correlation (p < 0.001). For comparison between these diagnostic findings, endoscopic positivityfor gastritis was found in 76.4%, and histopathological positivity for Hp infection in 93.6%, whereas IgG seropositivity was detected in 80.9%. So, histopathological identification of Hp was considered more reliable followed by IgG seropositivity and endoscopic findings, yet both endoscopy and histopathology are considered to be invasive techniques (Tables 4 and 5). Regarding the parameters of endoscopy and histopathology, there was insignificant correlation between some variables, including Grade 1 gastritis (G1) and endoscopic hyperemia (p > 0.001), yet the latter revealed a significant relationship with Hp colonization.
Fig. 1.
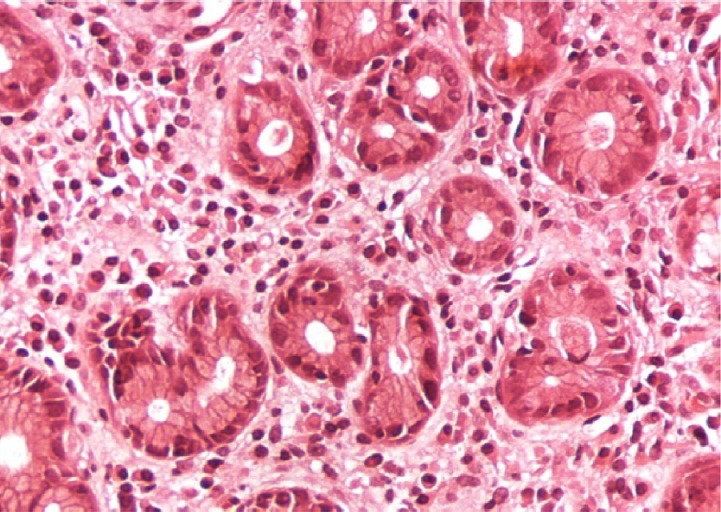
A case of gastric biopsy with Grade 1 gastritis showing mild mononuclear cellular infiltration with excess plasma cells in the lamina propria (hematoxylin and eosin, 200×).
Fig. 2.
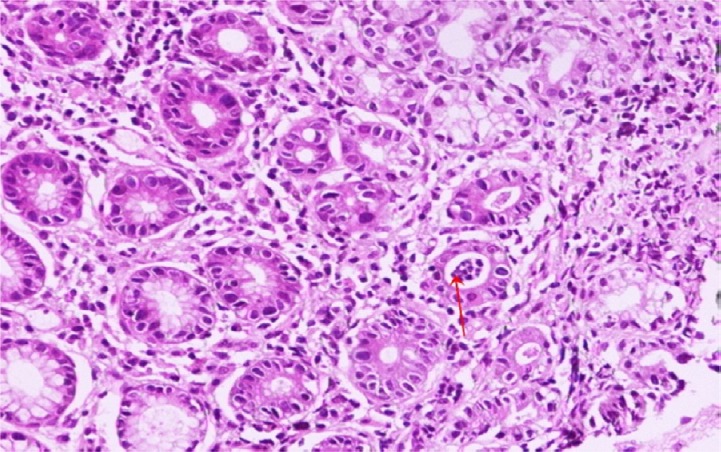
A case of gastric biopsy with Grade 2 gastritis revealing moderate mononuclear cellular infiltration in the lamina propria and cryptitis (arrow) (hematoxylin and eosin, 100×).
Fig. 3.
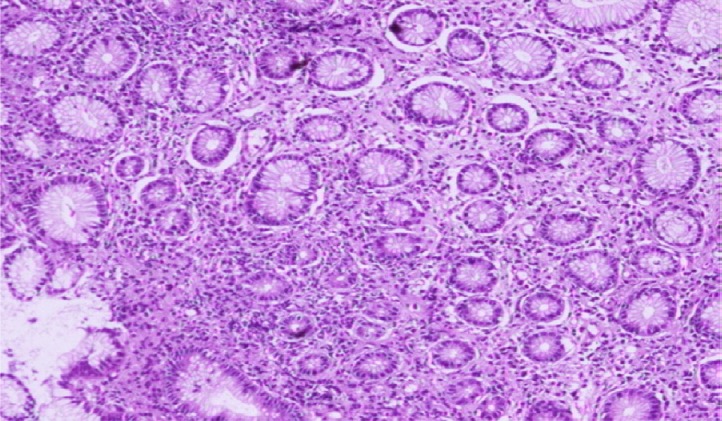
A case of gastric biopsy with Grade 3 gastritis showing severe mononuclear cellular infiltration in the lamina propria (hematoxylin and eosin, 40×).
Fig. 4.
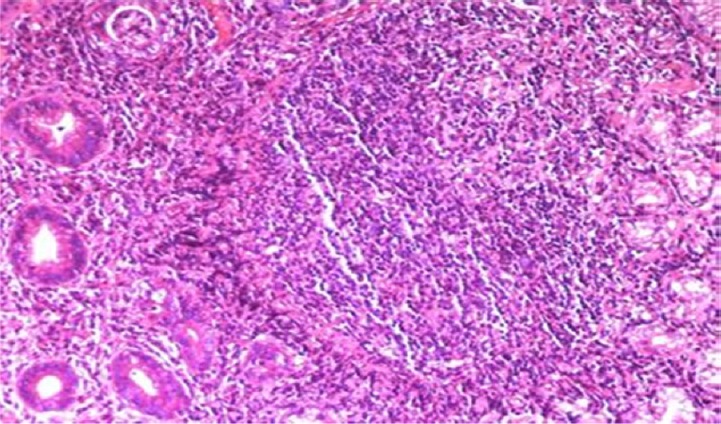
A case of gastric biopsy with Grade 3 gastritis showing severe mononuclear cellular infiltration in the lamina propria with lymphoid follicle formation and glandular atrophy (hematoxylin and eosin, 40×).
Fig. 5.
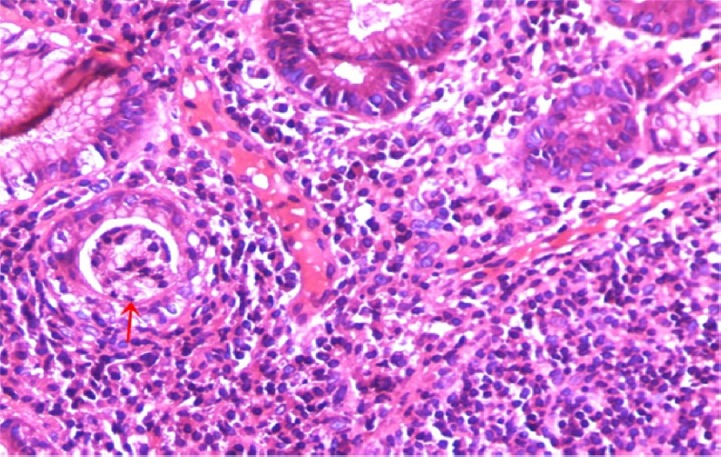
A case of gastric biopsy with Grade 3 gastritis revealing severe mononuclear cellular infiltration in the lamina propria and active inflammation cryptitis formation (arrow) (hematoxylin and eosin, 400×).
Fig. 6.
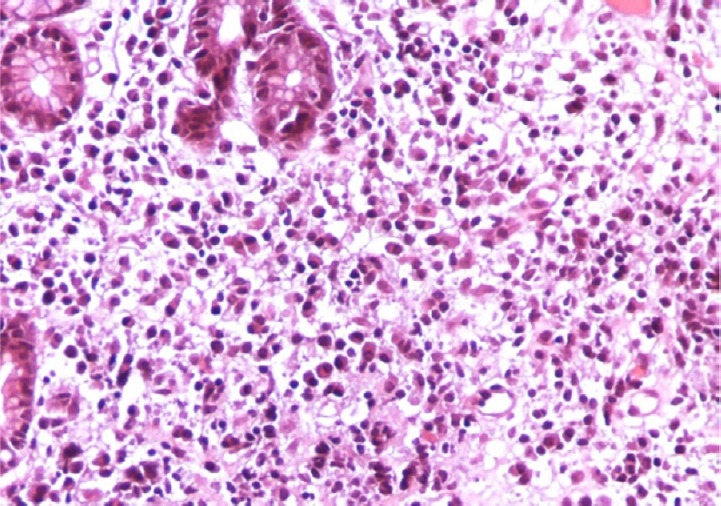
A case of gastric biopsy with Grade 3 gastritis showing severe mononuclear cellular infiltration with glandular atrophy (hematoxylin and eosin, 200×).
Fig. 7.
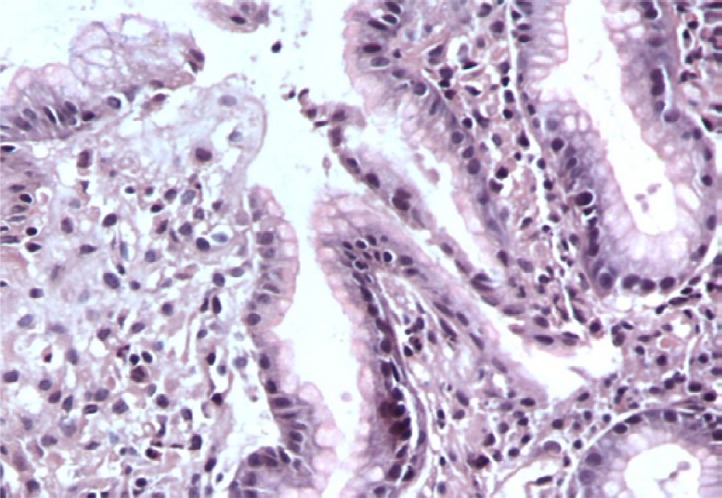
A case of gastric biopsy with Grade 2 gastritis showing moderate mononuclear cellular infiltration with intestinal metaplasia (hematoxylin and eosin, 200×).
Fig. 8.
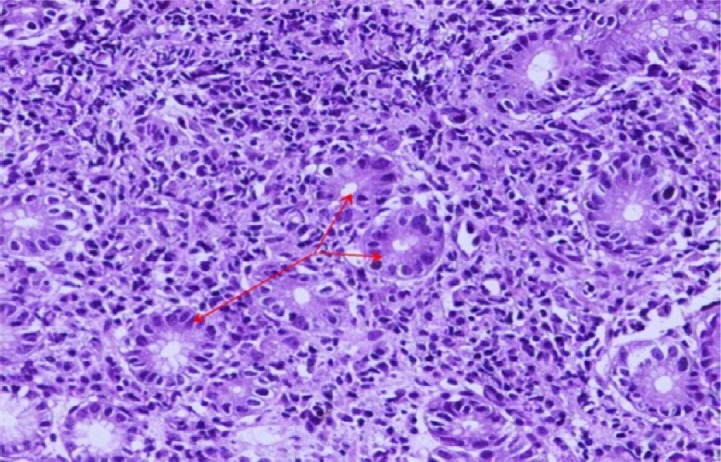
A case of gastric biopsy with Grade 3 gastritis revealing severe mononuclear cellular infiltration and atypical reparative glandular changes (hematoxylin and eosin, 100×).
Fig. 9.
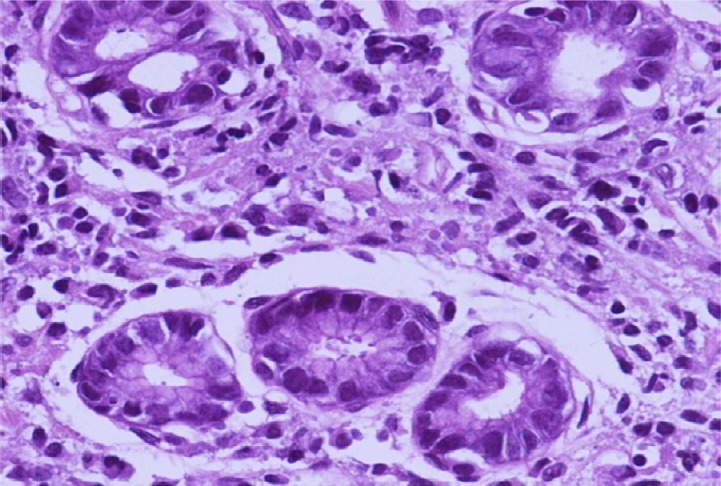
A case of gastric biopsy with Grade 2 gastritis revealing moderate mononuclear cellular infiltration and dysplastic changes (hematoxylin and eosin, 400×).
Fig. 10.
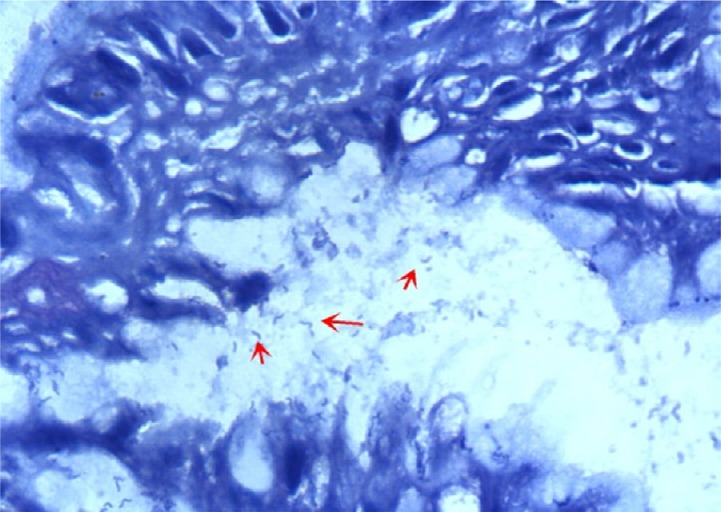
A case of gastric biopsy with Grade 2 gastritis showing severe Helicobacter pylori colonization within the superficial mucus overlying the foveolar epithelial cells (arrows; Giemsa stain, 400×).
Fig. 11.
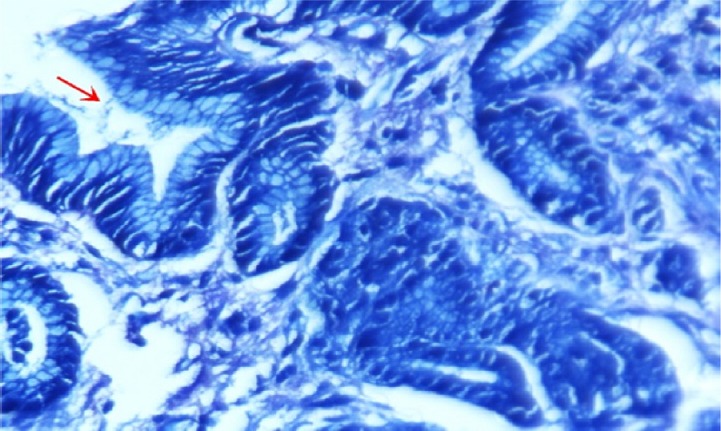
A case of gastric adenocarcinoma revealing malignant glands with Helicobacter pylori colonization within the glandular lumen (arrow; Giemsa stain, 400×).
Table 4.
Distribution of endoscopic and histopathological findings and IgG.
| Endoscopic gastritis | IgG | Normal | |||||
|---|---|---|---|---|---|---|---|
| n = 120 (76.4%) | n = 157 | n = 7 | |||||
| Histopath features n = 157 |
Hyperemia 80 |
Erosions 15 |
Ulcerations 10 |
Nodularity 15 |
+ve | −ve | |
| Inflamm cells G0 = 7 |
– |
– |
– |
– |
– |
7 |
7 |
| G1 = 80 | 58 | 5 | 2 | – | 17 | 10 | – |
| G2 = 43 | 10 | 3 | 1 | 2 | 20 | 7 | – |
| G3 = 27 | 10 | 5 | 2 | 1 | 7 | – | – |
| Activity = 50 | 2 | 2 | 2 | 2 | 39 | 2 | – |
| LFs = 40 | – | – | 2 | 10 | 26 | 2 | – |
| Atrophy = 15 | – | – | 1 | – | 13 | 1 | – |
| Metaplasia = 5 | – | – | – | – | 5 | 1 | – |
| Total | 80 | 15 | 10 | 15 | 127 | 30 | 157 |
Histopath = histopathological; IgG = immunoglobulin G; Inflamm = inflammatory; LF = lymphoid follicle.
Table 5.
Distribution of endoscopic and histopathological features, Helicobacter pylori colonization and IgG of all cases studied.
| Histopathological features | Endoscopic features | Hp | IgG | Normal mucosa | Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inflammation | No | Gastritis | Others | + ve | −ve | + ve | −ve | ||||||
| 150 | 30 | ||||||||||||
| G1 | G2 | G3 | GERD | HH | Do | ||||||||
| 80 | 43 | 27 | 7 | 120 | 10 | 8 | 12 | 147 | 10 | 127 | 30 | 7 | 157 |
Do = duodenitis; G1 = Grade 1 gastritis; G2 = Grade 2 gastritis; G3 = Grade 3 gastritis; GERD = gastroesophageal reflux disease; HH = hiatus hernia.
4. Discussion
Despite its declining incidence, GC (especially the intestinal-type mainly associated with Hp infection) is still a lethal malignancy [12], and primary prevention through Hp eradication is consistently recommended [29,30,31]. Gastric mucosal atrophy is generally considered the “cancerization field” in which GC develops. Based on such a rationale, incorporating the experience gained with the Sydney system and with the international group (Operative Link on Gastritis Assessment) proposed an equivalent grading and staging systems for reporting gastric histology rank gastritis-induced cancer risk according to both the topography and the extent of glandular atrophy [20,32,33,34,35]. The available clinicopathological classifications of gastritis are inconsistently using because most of them are not providing clinicians with immediate prognostic and therapeutic information. In addition, because they lack explicit ranking of severity, the descriptive labels of chronic gastritis carry the risk of being misinterpreted by general practitioners [17].
In this study, the endoscopic findings of gastritis revealed a significant relation between the following variables: hyperemia, erosion, mucosal nodularity, and ulceration (p < 0.005), and the antral type gastritis that is representing the major forms of gastritis (p < 0.001). Our endoscopic findings are in agreement with a study that revealed the presence of erythema in 68% of 300 patients, antral erosion in 7%, duodenal ulcer in 5%, and normal gastric mucosa in 20% [27]. Also, two studies found erythema in 44% and 43% of patients, respectively [36,37]. Yesim et al [28] observed endoscopic gastritis in 54 of 64 (84%) patients, and most (81.3%) showed endoscopic erythematous/exudative gastritis.
Histologically, 150 cases revealed mononuclear inflammatory cellular infiltrates, whereas seven cases were free (G0). Among the former, G1 gastritis was observed in 80 cases, G2 in 43 cases, and G3 in 27 cases. The relation among these grades of gastritis was significant (p < 0.001). Lymphoid follicles were found in 40 cases, inflammatory activity in 50 cases, glandular atrophy in 15 cases, intestinal metaplasia in five cases, and dysplasia in 10 cases, whereas atypical reparative changes were seen in 12 cases, and all these findings revealed a significant relationship (p < 0.001). The relationship between endoscopic findings, inflammatory infiltration, and Hp colonization was significant (p < 0.001), whereas the relation between G0 gastritis and hyperemia was insignificant (p > 0.001). There was a significant correlation between Hp colonization and hyperemia, as well as with gastric erosion (p < 0.001). All grading of gastritis (G1, 2, and 3) showed Hp colonization, whereas G0 was free. Garg et al [27] found mononuclear inflammatory infiltrates in most cases (70%), with 20% of them being endoscopically normal, yet they showed chronic inflammation on histology. Our results are in parallel with those of Khan et al [38], who found 32% of patients with chronic gastritis histologically had normal endoscopic findings, hence emphasizing the role of biopsy even in normal endoscopic cases.
The mucosal nodules detectable by endoscopic examination may not be an essential diagnostic parameter. One study reported that lymphoid follicles were not present at the sites of endoscopically identifiable nodules, and patients endoscopically diagnosed with nodules showed them in areas ranging from the gastric antrum to the gastric angulus, but histological features were observed in areas extending from the antrum to the corpus [39]. Additionally, Matsushia and Aftab [40] found that scores of chronic inflammation, neutrophil activity, glandular atrophy, and intestinal metaplasia were significantly lower with Hp-positive Bangladeshi than in Japanese patients at all gastric sites. They linked this to a hypothesis related to Hp strains and these patients were typically infected with a Western-type Hp [41].
Regarding the anatomical location of endoscopic gastritis, our study revealed that antral type was represented in a higher percentage (57.3%) of patients, whereas, antrum predominant pangastritis and corpus predominant gastritis were each seen in 9.5% of patients. These findings are in agreement with Matsushia et al [42,43], who reported that antral gastritis was present in a higher percentage of cases of endoscopic gastritis, regardless of age. However, our findings are in disagreement with those of Uemura et al [11], who reported that corpus predominant gastritis was present in a higher percentage of Japanese patients. So, the anatomical location of gastritis may be changeable according to the Hp strains and residency.
1n this study the sensitivity of endoscopic abnormalities for gastritis was 76.4%. A previous study performed on 100 patients found sensitivity of 91.7% and another study reported 98.3% [44,45].
Our findings of gastritis grading and Hp colonization revealed an agreement with a study performed by Rugge et al [17], who found G1 gastritis in 51% of patients, G2 in 27.4%, and G3 in 17.2%. That study also found duodenal ulcer associated with gastritis in 86% of patients. Garg et al [27] observed Hp in 37% and 57% of patients with hyperemia and erosion, respectively. They also found mononuclear inflammatory cellular infiltrates in all cases and most of them (70%) exhibited mild inflammation (G1), while 27% showed moderate inflammation (G2). Another study [46] observed mononuclear infiltrates in all biopsies and most revealed moderate inflammation. Garg et al [27] reported that 33% of cases showed activity that is in agreement with our findings. The same authors found glandular atrophy in 12.3% of cases and intestinal metaplasia in 7% of cases, which is close to our findings, as well as being in agreement with other studies [47,48]. In the same theme our findings for microscopical detection of Hp which was seen in 93.7% of all biopsies are near to studies showed positivity in 86.8%, 78% and 65% of cases, respectively [49,50,51]. Additionally, our observations are in agreement with another histopathological study that showed 95% accuracy for detection of Hp [52].
Our study found anti-Hp IgG in 82.8% of cases, whereas the endoscopic evaluation found Hp gastritis in 76.4% of cases and histopathological detection of Hp was found in 93.7% of cases. So, the latter is considered a reliable invasive diagnostic tool in the diagnosis of Hp gastritis. Megraud and Lehours [53] concluded that histopathological detection of Hp is considered the first diagnostic method and is still widely used in suspicious patients with dyspepsia or in regions with high prevalence of Hp. Serologically, there are many tests available for Hp, yet the titers may remain high for months after elimination of Hp by specific therapy. Anti-Hp IgG in some studies revealed a lower specificity for detection [54].
5. Conclusion
Histopathological interpretation of gastric biopsies is a reliable indicator of Hp infection as well as gastritis grading according to the Sydney grading system. Endoscopical findings of Hp-induced gastritis are nearer to the seropositivity of anti-Hp IgG in the environment of study, yet IgG is superior and non-invasive method for Hp diagnosis.
Conflict of interest
Authors declare no conflict of interest.
Acknowledgments
The authors thank all our colleagues, in particular, Dr. Mamdoh Abdul-Aziz, consultant endoscopic surgeon and Dr. Khalid Al-Hamad, endoscopic gastrointestianl physician, as well as staff members of the Department of Pathology, especially the histology technicians, Nasemol, Nisha, and Nijjara, Arar Central Hospital, Saudi Arabia for their help during the preparation of this study.
References
- [1].Rotimi O, Cairns A, Gray S, Mooyyedi P, Dixon MF. Histological identification of Helicobacter pylori: comparison of staining methods. J Clin Pathol. 2000;53:756–9. doi: 10.1136/jcp.53.10.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Rugge M, Pennelli G, Pilozzi E, Fassan M, Ingravallo G, Russo VM, et al. Gastritis: The histology report. Dig Liver Dis. 2011;43(Suppl):S73–384. doi: 10.1016/S1590-8658(11)60593-8. [DOI] [PubMed] [Google Scholar]
- [3].Sipponen P. Helicobacter pylori: a cohort phenomenon. Am J Surg Pathol. 1995;19(Suppl 1):530–6. [PubMed] [Google Scholar]
- [4].Sokwala A, Shah MV, Devoni S, Youga G. Helicobacter pylori: a randomized comparative trial of 7-day versus 14-day triple therapy. S Afr Med J. 2012;102:368–71. doi: 10.7196/samj.5302. [DOI] [PubMed] [Google Scholar]
- [5].Malaty HM. Epidemiology of Helicobacter pylori infection. Best Pract Res. 2007;21:205–14. doi: 10.1016/j.bpg.2006.10.005. [DOI] [PubMed] [Google Scholar]
- [6].Carrasco G, Corvalan AH. Helicobacter pylori-induced chronic gastritis and assessing risks for gastric cancer. Gastroenterol Res Pract. 2013;2013:1–8. doi: 10.1155/2013/393015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].National Health Consensus Development Panel on Helicobacter pyloriin peptic ulcer disease. NH Consensus conference: Helicobacter pyloriin peptic ulcer disease. JAMA. 1994;272:65–9. [PubMed] [Google Scholar]
- [8].Versalovic J. Helicobacter pylori pathology and diagnostic strategies. Am J Clin Pathol. 2003;119:403–12. [PubMed] [Google Scholar]
- [9].El-Zimaity HMT, Graham DY, Al-Assi MT, Malaty H, Karttunen TJ, Graham DP, et al. Inter-observer variation in the histopathological assessment of Helicobacter pylori gastritis. Hum Pathol. 1996;27:35–41. doi: 10.1016/s0046-8177(96)90135-5. [DOI] [PubMed] [Google Scholar]
- [10].Toulaymat M, Marconi BS, Garb MS, Otis C, Nash S. Endoscopic biopsy pathology of Helicobacter pylori gastritis comparison of bacterial detection by immunohistochemistry and genta stain. Arch Pathol Lab Med. 1999;123:778–81. doi: 10.5858/1999-123-0778-EBPOHP. [DOI] [PubMed] [Google Scholar]
- [11].Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784–9. doi: 10.1056/NEJMoa001999. [DOI] [PubMed] [Google Scholar]
- [12].Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- [13].Wen S, Moss SF. Helicobacter pylori virulence factors in gastric carcinogenesis. Cancer Lett. 2009;282:1–8. doi: 10.1016/j.canlet.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Brooks JJ, Enterline HT. Primary gastric lymphoma: a clinicopathologic study of 58 cases with long-term follow up and literature review. Cancer. 1983;51:701–11. doi: 10.1002/1097-0142(19830215)51:4<701::aid-cncr2820510425>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- [15].Parsonnet J, Hansen S, Robringez L, Gelb AB, Warnke RA, Jellum E, et al. Helicobacter pylori infection and gastric lymphoma. N Engl J Med. 1994;330:1267–71. doi: 10.1056/NEJM199405053301803. [DOI] [PubMed] [Google Scholar]
- [16].Lee YC, Chen TH, Chiu HM, Shun CT, Chiang H, Liu TY, et al. The benefit of mass eradication of Helicobacter pylori infection: a community-based study of gastric cancer prevention. Gut. 2013;62:676–82. doi: 10.1136/gutjnl-2012-302240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Rugge M, Meggio A, Pennelli G, Piscioli F, Giacomelli L. Gastritis staging in clinical practice: the OLGA staging system. Gut. 2007;56:631–6. doi: 10.1136/gut.2006.106666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Graham DY, Rugge M. Clinical practice: diagnosis and evaluation of dyspepsia. J Clin Gastroenterol. 2010;44:167–72. doi: 10.1097/MCG.0b013e3181c64c69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lauwers GY, Fujita H, Nagata K, Shimizu M. Pathology of non-Helicobacter pylori gastritis: extending the histopathologic horizons. J Gastroenterol. 2010;45:131–45. doi: 10.1007/s00535-009-0146-3. [DOI] [PubMed] [Google Scholar]
- [20].Dixon MF, Genta RM, Yardley JH, Gorrea P. Classification and grading of gastritis. The updated Sydney system. Am J Surg Pathol. 1996;20:1161–81. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- [21].Dixon MF, Genta RM, Yardley JH, Gorrea P. Histological classification of gastritis and Helicobacter pylori infection: an agreement at last. The International Workshop on the Histopathology of Gastritis? Helicobacter. 1997;2(Suppl 1):S17–24. doi: 10.1111/j.1523-5378.1997.06b09.x. [DOI] [PubMed] [Google Scholar]
- [22].Tummala S, Sheth SG, Goldsmith JD, Goldar-Najafi A, Murphy CK, Osburne MS, et al. Quantifying gastritis Helicobacter pylori infection: a comparison of quantitative culture, urease breath testing and histology. Dig Dis Sci. 2004;52:396–401. doi: 10.1007/s10620-006-9377-9. [DOI] [PubMed] [Google Scholar]
- [23].Rathbone BJ, Wyatt JI, Worsley BW, Shires S SE, Tredojoswiz LK, Heatly R, et al. Systemic and local antibody responses to gastric Campylobacter pyloridis in non-ulcer dyspepsia. Gut. 1986;27:642–7. doi: 10.1136/gut.27.6.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kosunen TU, Seppala K, Sarna S, Sipponen P. Diagnostic value of decreasing IgG, IgA, and IgM antibody titers after eradication of Helicobacter pylori. Lancet. 1992;339:893–5. doi: 10.1016/0140-6736(92)90929-w. [DOI] [PubMed] [Google Scholar]
- [25].Malfertheiner P, Megraud F, Morain CAO, Atherton J, Axon ATR, Bazzoli F, et al. Management of Helicobacter pylori infection – the Maastricht IV/Florence Consensus Report. Gut. 2012;61:646–64. doi: 10.1136/gutjnl-2012-302084. [DOI] [PubMed] [Google Scholar]
- [26].Cirak MY, Akyon Y, Megraud F. Diagnosis of Helicobacter pylori. J Compilation. 2007;12(Suppl 1):4–9. doi: 10.1111/j.1523-5378.2007.00542.x. [DOI] [PubMed] [Google Scholar]
- [27].Garg B, Sandhu V, Sood N, Sood A, Malhotra V. Histopathological analysis of chronic gastritis and correlation of pathological features with each other and with endoscopic findings. Polish J Pathol. 2012;3:172–8. doi: 10.5114/pjp.2012.31501. [DOI] [PubMed] [Google Scholar]
- [28].Yesim Ö, Benal B, Nur A, Rslan E. Upper gastrointestinal endoscopic findings and Helicobacter pylori infection in children with recurrent abdominal pain. Ege Tip Dergisi. 2004;43:165–8. [Google Scholar]
- [29].Correa P, Fontham ET, Bravo JC, Bravo LE, Ruiz B, Zarama G, et al. Chemoprevention of gastric dysplasia: randomized trial of antioxidant supplements and anti-Helicobacter pylori therapy. J Nat Cancer Inst. 2000;92:1881–8. doi: 10.1093/jnci/92.23.1881. [DOI] [PubMed] [Google Scholar]
- [30].Leung WK, Lin SR, Ching JY, To KF, Ng EK, Chan FK, et al. Factors predicting progression of gastric intestinal metaplasia: results of a randomized trial on Helicobacter pylori eradication. Gut. 2004;53:1244–9. doi: 10.1136/gut.2003.034629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Wong BC, Lam SK, Wong WM, Chen JS, Zheng TT, Feng RE, et al. , China Gastric Cancer Study Group.Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA. 2004;291:187–94. doi: 10.1001/jama.291.2.187. [DOI] [PubMed] [Google Scholar]
- [32].Rugge M, Correa P, Dixon MF, Fiocca R, Hattori T, Lechago J, et al. Gastric mucosal atrophy: interobserver consistency using new criteria for classification and grading. Aliment Pharm Ther. 2002;16:1249–59. doi: 10.1046/j.1365-2036.2002.01301.x. [DOI] [PubMed] [Google Scholar]
- [33].Rugge M, Correa P, Di Mario F, El-Omar E, Fiocca R, Geboes K, et al. OLGA staging for gastritis: atutorial. Digest LiverDis. 2008;40:650–8. doi: 10.1016/j.dld.2008.02.030. [DOI] [PubMed] [Google Scholar]
- [34].Rugge M, Kim JG, Mahachai V, Miehlke S, Pennelli G, Russo VM, et al. OLGA gastritis staging in young adults and country-specific gastric cancer risk. Int J Surg Pathol. 2008;16:150–4. doi: 10.1177/1066896907307238. [DOI] [PubMed] [Google Scholar]
- [35].Rugge M, de Boni M, Pennelli G, de Bona M, Giacomelli L, Fas-san M, et al. Gastritis OLGA-staging and gastric cancer risk: a twelve-year clinic-pathological follow-up study. Aliment Pharm Ther. 2010;31:1104–11. doi: 10.1111/j.1365-2036.2010.04277.x. [DOI] [PubMed] [Google Scholar]
- [36].Khakooet SI, Lobo AJ, Shepherd NA, Wilkinson SP. Histological assessment of the Sydney classification of endoscopic gastritis. Gut. 1994;35:1172–5. doi: 10.1136/gut.35.9.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Calabrese C, Di Febo G, Brandi G, Morselli-Labate AM, Areni A, Scialpi C, et al. Correlation between endoscopic features of gastric antrum, histology and Helicobacter pylori infection in adults. Ital J Gastroenterol Hepatol. 1999;31:359–65. [PubMed] [Google Scholar]
- [38].Khan MQ, Alhomsi Z, Al-Momen S, Ahmad M. Endoscopic features of Helicobacter pylori induced gastritis. Saudi J Gastroenterol. 1999;5:9–14. [PubMed] [Google Scholar]
- [39].Nakashima R, Nagata N, Watanabe K, Kobayakawa M, Sakurai T, Akiyama J, et al. Histological features of nodular gastritis and its endoscopic classification. J Digest Dis. 2011;12:436–42. doi: 10.1111/j.1751-2980.2011.00532.x. [DOI] [PubMed] [Google Scholar]
- [40].Matsuhisa T, Aftab H. Observation of gastric mucosa in Bangladesh, the country with the lowest incidence of gastric cancer, and Japan, the country with the highest incidence. Helicobacter. 2012;17:396–401. doi: 10.1111/j.1523-5378.2012.00967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Vilaichone RK, Mahachai V, Tumwasorn S, Wu JY, Graham DY, Yamaoka Y. Molecular epidemiology and outcome of Helicobacter pylori infection in Thailand: a cultural cross roads. Helicobacter. 2004;9:453–9. doi: 10.1111/j.1083-4389.2004.00260.x. [DOI] [PubMed] [Google Scholar]
- [42].Matsuhisa T, Matsukura N, Yamada Y. Topography of chronic active gastritis in Helicobacter pylori positive Asian populations: age-, gender-and endoscopic diagnosis-matched study. J Gastroenterol. 2004;39:324–8. doi: 10.1007/s00535-003-1329-y. [DOI] [PubMed] [Google Scholar]
- [43].Matsuhisa T, Miki M, Yamada N, Sharma SK, Shrestha BM. Helicobacter pylori infection, glandular atrophy, intestinal metaplasia and topography of chronic active gastritis in the Nepalese and Japanese population: the age, gender and endoscopic diagnosis matched study. Kathmandu University Med J. 2007;5:295–301. [PubMed] [Google Scholar]
- [44].Al-Hamdani AA, Fayady AH, Aboul Majeed BA. Helicobacter pylori gastritis: correlation between the endoscopic and histological finding. IJGE. 2001;1:43–8. [Google Scholar]
- [45].Labenz J, Gyenes E. Is H. pylori gastritis a macroscopic diagnosis? DscH Med Wodem Scgr. 1993;118:176–8. doi: 10.1055/s-2008-1059315. [Abstract] [DOI] [PubMed] [Google Scholar]
- [46].Witteman EM, Mravunac M, Becx MJ, Hopman WP, Verschoor JS, Tytgat GN, et al. Improvement of gastric inflammation and resolution of epithelial damage one year after eradication of Helicobacter pylori. J Clin Pathol. 1995;48:250–6. doi: 10.1136/jcp.48.3.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Atisook K Kachinthorn U, Luengrojanakul P. Histology of gastritis and Helicobacter pylori infection in Thailand: a nationwide study of 3776 cases. Helicobacter. 2003;8:132–41. doi: 10.1046/j.1523-5378.2003.00134.x. [DOI] [PubMed] [Google Scholar]
- [48].Hussein NR, Napaki SM, Atherton JC. A study of Helicobacter pylori-associated gastritis patterns in Iraq and their association with strain virulence. Saudi J Gastroenterol. 2009;15:125–7. doi: 10.4103/1319-3767.48971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Kalifehgholi M, Shamsipour F, Ajhdarkosh H, Daryani NE, Pourmand MR, Hosseini M, et al. Comparison of five diagnostic methods for Helicobacter pylori. Iran J Microbiol. 2013;5:396–401. [PMC free article] [PubMed] [Google Scholar]
- [50].Kumar A, Bansal R, Pathak VP, Kishore S, Karya PK. Histopathological changes in gastric mucosa colonized by H. pylori. Indian J Pathol Microbiol. 2006;49:352–6. [PubMed] [Google Scholar]
- [51].Gill HH, Desai HG, Majmudar P, Mehta PR, Prabhu SR. Epidemiology of Helicobacter pylori: the Indian scenario. Indian J Gastroenterol. 1993;12:9–11. [PubMed] [Google Scholar]
- [52].Pourakbari B, Ghazi M, Mahmoudi S, Mamishi S, Azhdarkosh H, Najafi M, et al. Diagnosis of Helicobacter pylori infection by invasive and noninvasive tests. Brazil J Microbiol. 2013;44:795–8. doi: 10.1590/S1517-83822013005000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Megraud F, Lehours P. Helicobacter pylori detection and antimicrobial susceptibility testing. Clin Microbiol Rev. 2007;20:280–322. doi: 10.1128/CMR.00033-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Koletzko S. Noninvasive diagnosis tests for Helicobacter pylori infection in children. Can J Gastroenterol. 2005;19:433–9. doi: 10.1155/2005/213608. [DOI] [PubMed] [Google Scholar]


