Abstract
Colorectal carcinoma is a significant source of major morbidity and mortality. Sonic hedgehog (Shh) is expressed in normal gastrointestinal tract mucosa and in many malignancies. The purpose of the present study is to investigate the relationship between Shh immunoexpression in CRC and clinicopathological characteristics. Paraffin blocks of 155 primary CRCs and 37 nodal metastases were retrieved and tissue microarrays were constructed. Immunohistochemistry was performed using anti-Shh antibody. Immunostaining was scored and results were analysed in relation to the clinicopathological parameters. Shh was overexpressed in primary CRC (p = 0.02) and in nodal metastasis (p = 0.004). There was no difference between Shh immunoexpression in primary CRC and in nodal metastasis (p = 0.941). High Shh immunoexpression was associated with well differentiated tumours (p = 0.004). However, there was no association with other clinicopathological parameters. Shh overexpression was not associated disease free survival (log-rank = 0.079, p = 0.778). Shh is overexpressed in well differentiated CRC. However, Shh is not associated with other clinicopathological and prognostic factors. Loss of Shh may be associated with proliferation and loss of differentiation in CRC. Further molecular studies are required to address the potential importance of Shh signalling in CRC and to test Shh inhibitors and activators as potential therapeutic targets in CRC.
Keywords: CRC, Sonic hedgehog, Immunohistochemistry, Nodal metastasis, Relapse, Distant metastasis
1. Introduction
Colorectal carcinoma (CRC) incidence and mortality rates remain one of the highest among other types of cancer worldwide [1]. Nodal metastasis and tumour stage remain the most important prognostic factors that the treatment plan is decided upon [2]. The pathogenesis of CRC involves a sequential process of genetic and molecular alterations that enhance cellular proliferation and suppress apoptosis [1,3]. It is essential to analyse these steps and correlate them with embryonic basis in order to identify other prognostic factors and modulate new targeted molecular interventions.
Cellular differentiation and proliferation during embryogenesis is regulated through the hedgehog (Hh) signalling pathway [2]. Three members of the mammalian hedgehog were identified, Desert hedgehog (Dhh), Indian hedgehog (Ihh), and Sonic hedgehog (Shh) [4]. Shh expression was reported in normal gastrointestinal tract [2,5]. Continuous Shh signalling activity had been documented in multiple cancers, such as basal cell carcinoma, breast cancer, gastric cancer, pancreatic cancer, and CRC [5,6]. The activity of Shh signalling results in activation and nuclear translocation of the Gli family of transcription factors through multiple intracellular events. These transcription factors control the transcription of hedgehog target genes [7,8].
The aim of the present study is identify the significance of Shh immunoexpression in CRC in relation to the clinicopathological entities and patient outcome.
2. Materials and methods
2.1. Patients
The study included paraffin wax blocks of primary tumour from 155 patients with CRC and corresponding 37 nodal metastases. Blocks were retrieved from the archives of the Department of Pathology at King Abdulaziz University, Jeddah, Saudi Arabia. Clinicopathological characteristics of patients are listed in Table 1. The study was approved by the Research Committee of the Biomedical Ethics Unit, Faculty of Medicine, King Abdulaziz University.
Table 1.
Clinicopathological parameters of cases (n = 155).
| Parameter | Number (%) | |
|---|---|---|
| Sex | Male | 78 (50.3%) |
| Female | 77 (49.7%) | |
| Grade | Well differentiated | 36 (23.2%) |
| Moderately differentiated | 98 (63.2%) | |
| Poorly differentiated | 21 (13.5%) | |
| Age | <60 years | 88 (56.8%) |
| ≥60 years | 67 (43.2%) | |
| Tumour location | Right colon | 41 (26.5%) |
| Left colon | 98 (63.2%) | |
| Rectum | 16 (10.3%) | |
| Tumour size | <5 cm | 68 (43.9%) |
| ≥5 cm | 87 (56.1%) | |
| Primary tumour | T1 | 3 (1.9%) |
| T2 | 22 (14.2%) | |
| T3 | 116 (74.8%) | |
| T4 | 14 (9%) | |
| Nodal metastasis | Negative | 80 (51.6%) |
| Positive | 68 (43.9%) | |
| Cannot be assessed | 7 (4.5%) | |
| Distant metastasis | Negative | 111 (71.6%) |
| Positive | 44 (28.4%) | |
| Lymphovascular invasion | Negative | 132 (85.2%) |
| Positive | 23 (14.8%) | |
| Margin status | Free | 149 (96.1%) |
| Involved | 6 (3.9%) | |
| Disease relapse | Negative | 98 (63.2%) |
| Positive | 57 (36.8%) | |
| Survival | Alive | 94 (60.6%) |
| Dead | 26 (16.8%) | |
| Not available | 35 (22.6%) | |
T1: tumour invades submucosa; T2: tumour invades muscularis propria; T3: tumour invades through the muscularis propria into the subserosa or into non-peritonealised pericolic or perirectal tissues; T4: tumour directly invades other organs or structures, and/or perforates visceral peritoneum.
2.2. Tissue microarray
Tissue microarrays (TMA) were designed and constructed as previously described [9]. Haematoxylin and eosin-stained sections of primary tumours and nodal metastasis were reviewed by an experienced pathologist (WG). Areas of interest were chosen from the original blocks and were marked on the slides. Necrotic areas, autolytic areas and areas containing predominantly the stromal tissue were avoided. Primary CRC and nodal metastasis paraffin-embedded blocks were retrieved and examined for validity to perform TMA. Two tissue cores each 1.5 mm in diameter were punched from each donor block in an automated TMA instrument (TMA Master 1.14 SP3 from 3D Histech Ltd., Budapest, Hungary) and inserted into a recipient paraffin block. Placental tissue was used for orientation. Slides were cut from TMA block and stained with haematoxylin and eosin for initial morphological assessment of accuracy of construction.
2.3. Immunohistochemistry
TMA blocks were cut at 4 μm, and mounted on positive-charged slides (Leica Microsystems Plus Slides). Sections were deparaffinised in xylene and rehydrated in an automated immunostainer (BenchMark XT, Ventana® Medical systems Inc., Tucson, AZ, USA). Pre-treatment was done using CC1 (prediluted cell conditioning solution) for 60 min. Anti-human rabbit anti-Shh polyclonal antibody (Spring™ Bioscience; Cat # E17920) was incubated at 37 °C for 20 min. Ventana® I-view DAB detection kit was used according to kit manufacturer instructions. Subsequently, slides were washed, counterstained with Mayer's haematoxylin and mounted. Negative control (substitution of the primary antibody with Tris–buffered saline) and positive control slides were included.
2.4. Interpretation of Shh immunostaining
Sections were evaluated independently without knowledge of the clinicopathological characteristics of patients. The expression pattern was determined independently by 3 investigators (DG, WG, and AA). Cytoplasmic staining of tumour cells was evaluated. Observer's bias was avoided by repeating the evaluation of protein expression at 2 different time points and without knowledge of patients’ clinical data. Both staining intensity and extent (percentage) were noted. The percentage was calculated by counting the percentage of positive tumour cells within the total number of tumour cells in sections. The percentage was expressed as; (1) when 0–10% of malignant cells were positive, (2) when 11–50% of malignant cells were positive, and (3) when labelling in more than 50% of malignant cells. The staining intensity was reported as; (0) negative; (1) weak; (2) moderate; and (3) high. For statistical purpose, a combination was done between intensity and percentage and was given a numerical 6-scale score. Results were finally dichotomised as low expression when score was 1–3 and high expression when score was 4–6.
2.5. Statistical analysis
Differences between two groups of patients on one variable were tested by using Mann–Whitney test. To test association procedure in three groups of patients on one independent variable the Kruskal–Wallis test was used. Wilcoxon signed rank test was used to test differences between two related groups of paired variables Non-parametric chi-square was used to test variance along one variable. Binary logistic regression analysis was used to predict lymph noel metastasis, distant metastasis, surgical resection margins involvement, lymphovascular invasion, and disease relapse in relation immunoexpression of Shh. Estimated odds ratio {exponential (B)}, 95% confidence interval (CI) for exp(B), and significance denoted for each analysis. The Kaplan–Meier procedure was used to calculate the disease-free survival probabilities and the Log Rank test was used to compare the difference between survivals. Time was calculated from the date of diagnosis to the appearance of disease relapse (or date last seen disease-free). Statistical procedures were performed using SPSS® Release 16.0. Statistical significance was determined at p value of ≤0.05 and was 2-sided.
3. Results
3.1. Shh immunoexpression
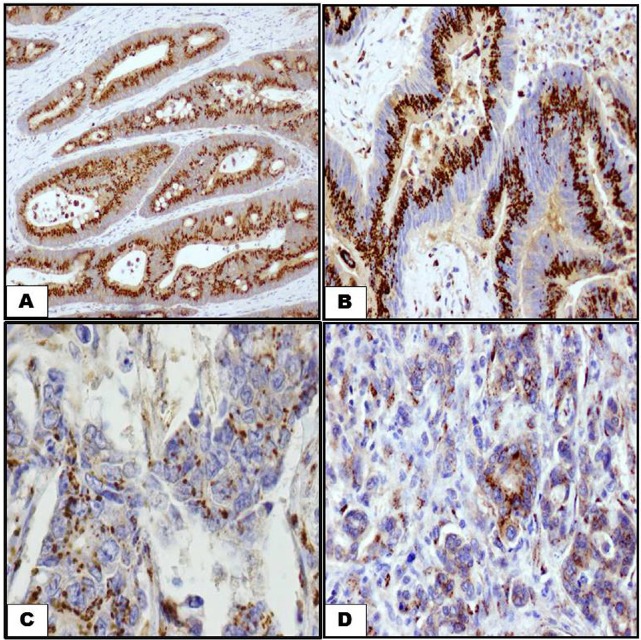
The expression of Shh was defined as cytoplasmic staining with a stippled or granular pattern. In well differentiated carcinomas pattern of Shh expression is seen in within the brush border of tumour cells (Fig. 1A). In moderately differentiated carcinoma, more glandular structures are involved and Shh is observed in the cytoplasm of malignant cells (Fig. 1B). In poorly differentiated carcinoma and nodal metastasis, the infiltrating tumour cells show less number of cytoplasmic Shh immunostaining (Fig. 1C and D). In primary CRC there was a higher incidence of cases with high Shh immunoexpression than low immunoexpression (p = 0.02). In lymph node metastasis, high Shh immunoexpression was higher than low Shh immunoexpression (p = 0.004). However, there was no difference between Shh immunoexpression in primary carcinomas and lymph node metastasis (p = 0.941). Details are shown Table 2.
Fig. 1.
Shh immunostaining labelling in CRC by using anti-Shh antibody. Diaminobenzidine was used as a chromogen and haematoxylin as counterstain. (A) A well differentiated CRC showing extensive brush border immunostaining of Shh (200×). (B) Immunostaining of Shh in a moderately differentiated CRC. Staining is similar to well differentiated CRC (200×). (C, D) Immunostaining of Shh in a poorly differentiated CRC and lymph node metastasis. Staining is less extensive in well and moderately differentiated CRC (200×).
Table 2.
Categories of Shh immunoexpression in primary tumours and nodal metastases.
| Primary tumour (n = 155) | Nodal metastasis (n = 37) | p value | |
|---|---|---|---|
| Low expression | 38 (24.5%) | 4 (10.8%) | 0.941b |
| High expression | 117 (75.5%) | 33 (89.2%) | |
| p value | 0.020a | 0.004a |
aOne sample non-parametric chi-square test.
bMann–Whitney test.
3.2. Relationship between Shh immunoexpression and clinicopathological parameters
There is a statistically significant association between Shh immunoexpression in primary CRC and low tumour grade (p = 0.004). There was no statically significant difference in Shh immunoexpression as regards age, sex, grade, tumour location, depth of invasion (pT), nodal metastasis, distant metastasis, lymphovascular invasion, margin status, disease relapse, or status at end point. Results are shown in Table 3.
Table 3.
Relation of Shh immunoexpression to clinicopathological parameters.
| p value | |
|---|---|
| Age | 0.096b |
| Sex | 0.676b |
| Grade | 0.004a |
| Tumour location | 0.350a |
| Tumour size | 0.618b |
| Depth of invasion (pT) | 0.301a |
| Nodal metastasis | 0.157a |
| Distant metastasis | 0.617b |
| Lymphovascular invasion | 0.850b |
| Margin status | 0.610b |
| Survival | 0.104b |
| Disease relapse | 0.692b |
aKruskal–Wallis test.
bMann–Whitney test.
Binary logistic regression analysis showed that Shh immunoexpression was not an independent predictor of positive margin status, lymphovascular invasion, disease relapse, or nodal and distant metastasis (Table 4). Kaplan–Meier survival analyses showed that Shh immunoexpression in CRC had no significant association with favourable disease-free survival (log-rank = 0.079, p = 0.778) (Fig. 2).
Table 4.
Regression analysis Shh immunoexpression.
| Variable | Exp(β) | 95% CI for exp(β) | p value |
|---|---|---|---|
| Nodal metastasis | 0.545 | 0.270–1.102 | 0.091 |
| Distant metastasis | 1.335 | 0.546–3.262 | 0.526 |
| Surgical resection margins | 0.682 | 0.087–5.373 | 0.716 |
| Lymphovascular invasion | 0.805 | 0.241–2.686 | 0.725 |
| Disease relapse | 0.652 | 0.340–1.250 | 0.198 |
Fig. 2.
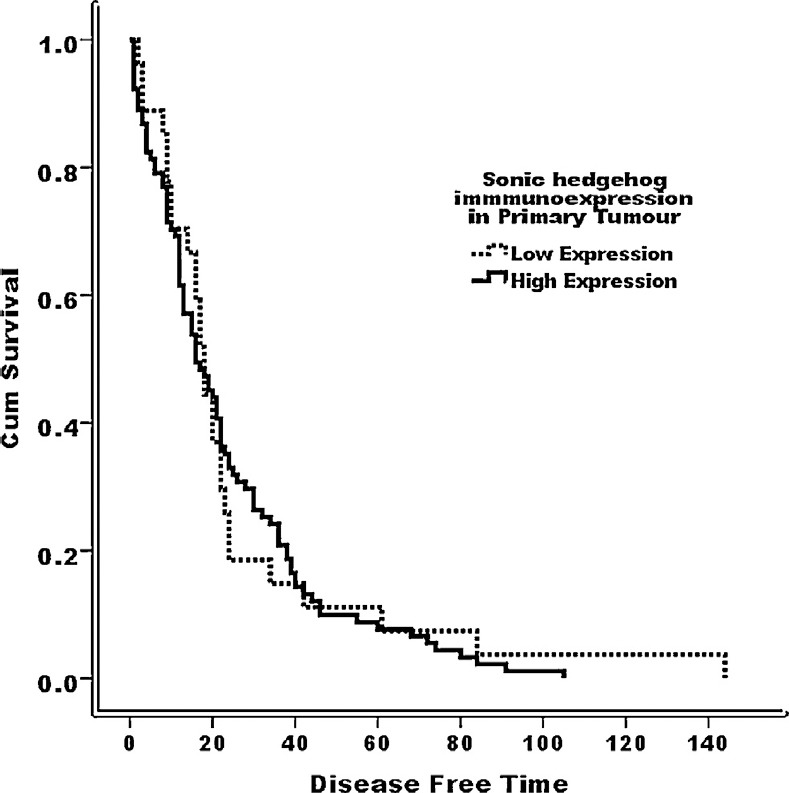
Disease-free survival curve (Kaplan–Meier) according to Shh immunostaining; low Shh immunoexpression; and high Shh immunoexpression (log-rank = 0.079, p = 0.778).
4. Discussion
Concomitant and sequential molecular multistep genetic damages are required for CRC carcinogenesis to occur starting by aberrant crypt proliferation or hyperplasia, adenomas to CRC, and finally metastatic carcinoma [10]. The Hh signalling pathway plays an important role during embryogenesis as well as adult life. It is involved in regulation of proliferation, angiogenesis, matrix remodelling, stem cell renewal and the differentiation in several tissues including the gastrointestinal tract [11,12]. Dysregulation of the Hh pathway is involved in tumour development. Mutations of several components of Hh pathway were found in patients with many types of cancers including CRC [13,14]. Hh pathway involvement in tumorigenesis may be related to the molecular pathways of cancer stem cell [15].
The current study investigated the immunoexpression of Shh in a subset of primary CRC and nodal metastasis. Shh is overexpressed in both primary CRC and nodal metastasis. The results from our study support the previous studies regarding the involvement of the Shh pathway in colorectal carcinogenesis and metastasis [1,6,15,16,17,18]. The Hh is known to have an essential role in cellular proliferation, and cell survival in many tissues [19,20]. Shh has been shown to be localised to areas of increased cellular proliferation in human hyperplastic polyps and that staining was more intense in areas of increased dysplasia in colorectal adenomas and adenocarcinomas [4,21]. Accordingly, the Hh signalling pathway may have a possible role tumour progression [22,23].
In the present study, no correlation was found between Shh immunoexpression and most clinicopathological features. In one study, there were similar findings [24]. In other studies, there were some different results. Shh overexpression correlated to early stage CRC [2,5]. Others found association of Shh with nodal metastasis, disease free survival and overall survival [2], and liver metastasis [6]. The conflicting results may be related to sample size, and methodical issues. Given the paucity of studies featuring the prognostic significance of Shh in CRC, the results from the current study and previous reports need more validation.
In the present study, the high Shh immunoexpression was significantly associated with low grade CRC. In this regard, there were conflicting findings in previous reports. While some reported association of Shh with more undifferentiated CRC [5]. Alinger et al., had shown reduction in Shh expression in CRC than in benign lesions and normal tissues. Also they reported that well differentiated tumours shown more intense Shh expression than high grade carcinomas [1]. Others found that there was no association between Shh and tumour differentiation [2,24]. During embryogenesis, the Hh signalling pathway is essential for organ patterning, cell differentiation and cell proliferation [22]. The Hh signalling pathway is critical to normal mammalian gastrointestinal development and is involved in differentiation in normal colonic tissue [1,6,19,25,26,27]. Shh expression in the gastrointestinal tract is limited to the region of stem cells. Accordingly Shh is related to gastrointestinal epithelial cell differentiation [5]. The findings of the current study as well as previous reports support that Shh overexpression in CRC is related to differentiation more than invasion and aggressiveness. This may result in speculation about the using the Hh signalling activation to be a therapeutic approach in CRC.
The limitation of the present study includes including missing some follow-up data, short survival time in a number of patients. Also, the lack of tissues from normal and dysplastic colonic mucosa is another limitation.
5. Conclusion
Shh is overexpressed in well differentiated CRC. However, Shh is not associated with other clinicopathological and prognostic factors. Loss of Shh may be associated with proliferation and loss of differentiation in CRC. Further molecular studies are required to address the potential importance of Shh signalling in CRC including testing Shh inhibitors and activators as potential therapeutic targets in CRC.
Conflict of interest
The authors confirm that no part of this work has been submitted or published elsewhere and that there are no conflicts of interest.
Acknowledgements
This project was supported by the NSTIP strategic technologies program in the Kingdom of Saudi Arabia – King Abdulaziz City for Science and Technology (KACST) grant 11-BIO1524-03. The authors also, acknowledge with thanks Science and Technology Unit, King Abdulaziz University for technical support.
References
- [1].Alinger B, Kiesslich T, Datz C, Aberger F, Strasser F, Berr F, et al. Hedgehog signaling is involved in differentiation of normal colonic tissue rather than in tumor proliferation. Virchows Arch. 2009;454(4):369–79. doi: 10.1007/s00428-009-0753-7. [DOI] [PubMed] [Google Scholar]
- [2].Xu M, Li X, Liu T, Leng A, Zhang G. Prognostic value of hedgehog signaling pathway in patients with colon cancer. Med Oncol. 2012;29(2):1010–6. doi: 10.1007/s12032-011-9899-7. [DOI] [PubMed] [Google Scholar]
- [3].Sanz-Pamplona R, Berenguer A, Cordero D, Riccadonna S, Sole X, Crous-Bou M, et al. Clinical value of prognosis gene expression signatures in colorectal cancer: a systematic review. PLoS ONE. 2012;7(11):e48877. doi: 10.1371/journal.pone.0048877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Oniscu A, James RM, Morris RG, Bader S, Malcomson RD, Harrison DJ. Expression of Sonic hedgehog pathway genes is altered in colonic neoplasia. J Pathol. 2004;203(4):909–17. doi: 10.1002/path.1591. [DOI] [PubMed] [Google Scholar]
- [5].Monzo M, Moreno I, Artells R, Ibeas R, Navarro A, Moreno J, et al. Sonic hedgehog mRNA expression by real-time quantitative PCR in normal and tumor tissues from colorectal cancer patients. Cancer Lett. 2006;233(1):117–23. doi: 10.1016/j.canlet.2005.03.001. [DOI] [PubMed] [Google Scholar]
- [6].Yoshikawa K, Shimada M, Miyamoto H, Higashijima J, Miyatani T, Nishioka M, et al. Sonic hedgehog relates to colorectal carcinogenesis. J Gastroenterol. 2009;44(11):1113–7. doi: 10.1007/s00535-009-0110-2. [DOI] [PubMed] [Google Scholar]
- [7].Ajani JA, Wang X, Izzo JG, Crane CH, Eng C, Skibber JM, et al. Molecular biomarkers correlate with disease-free survival in patients with anal canal carcinoma treated with chemoradiation. Dig Dis Sci. 2010;55(4):1098–105. doi: 10.1007/s10620-009-0812-6. [DOI] [PubMed] [Google Scholar]
- [8].Dai J, Ai K, Du Y, Chen G. Sonic hedgehog expression correlates with distant metastasis in pancreatic adenocarcinoma. Pancreas. 2011;40(2):233–6. doi: 10.1097/MPA.0b013e3181f7e09f. [DOI] [PubMed] [Google Scholar]
- [9].Gomaa W, Ke Y, Fujii H, Helliwell T. Tissue microarray of head and neck squamous carcinoma: validation of the methodology for the study of cutaneous fatty acid-binding protein, vascular endothelial growth factor, involucrin and Ki-67. Virchows Arch. 2005;447(4):701–9. doi: 10.1007/s00428-005-0002-7. [DOI] [PubMed] [Google Scholar]
- [10].Stryker SJ, Wolff BG, Culp CE, Libbe SD, Ilstrup DM, MacCarty RL. Natural history of untreated colonic polyps. Gastroenterology. 1987;93(5):1009–13. doi: 10.1016/0016-5085(87)90563-4. [DOI] [PubMed] [Google Scholar]
- [11].McMahon AP, Ingham PW, Tabin CJ. Developmental roles and clinical significance of hedgehog signaling. CurrTop Dev Biol. 2003;53:1–114. doi: 10.1016/s0070-2153(03)53002-2. [DOI] [PubMed] [Google Scholar]
- [12].Dessaud E, McMahon AP, Briscoe J. Pattern formation in the vertebrate neural tube: a sonic hedgehog morphogen-regulated transcriptional network. Development. 2008;135(15):2489–503. doi: 10.1242/dev.009324. [DOI] [PubMed] [Google Scholar]
- [13].Evangelista M, Tian H, de Sauvage FJ. The hedgehog signaling pathway in cancer. Clin Cancer Res. 2006;12(20 Pt 1):5924–8. doi: 10.1158/1078-0432.CCR-06-1736. [DOI] [PubMed] [Google Scholar]
- [14].Scales SJ, de Sauvage FJ. Mechanisms of Hedgehog pathway activation in cancer and implications for therapy. Trends Pharmacol Sci. 2009;30(6):303–12. doi: 10.1016/j.tips.2009.03.007. [DOI] [PubMed] [Google Scholar]
- [15].Varnat F, Duquet A, Malerba M, Zbinden M, Mas C, Gervaz P, et al. Human colon cancer epithelial cells harbour active HEDGEHOG-GLI signalling that is essential for tumour growth, recurrence, metastasis and stem cell survival and expansion. EMBO Mol Med. 2009;1(6-7):338–51. doi: 10.1002/emmm.200900039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Shi T, Mazumdar T, Devecchio J, Duan ZH, Agyeman A, Aziz M, et al. cDNA microarray gene expression profiling of hedgehog signaling pathway inhibition in human colon cancer cells. PLoS ONE. 2010;5:10. doi: 10.1371/journal.pone.0013054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mazumdar T, DeVecchio J, Shi T, Jones J, Agyeman A, Houghton JA. Hedgehog signaling drives cellular survival in human colon carcinoma cells. Cancer Res. 2011;71(3):1092–102. doi: 10.1158/0008-5472.CAN-10-2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Douard R, Moutereau S, Pernet P, Chimingqi M, Allory Y, Manivet P, et al. Sonic hedgehog-dependent proliferation in a series of patients with colorectal cancer. Surgery. 2006;139(5):665–70. doi: 10.1016/j.surg.2005.10.012. [DOI] [PubMed] [Google Scholar]
- [19].de Santa Barbara P, van den Brink GR, Roberts DJ. Development and differentiation of the intestinal epithelium. Cell Mol Life Sci. 2003;60(7):1322–32. doi: 10.1007/s00018-003-2289-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kasper M, Schnidar H, Neill GW, Hanneder M, Klingler S, Blaas L, et al. Selective modulation of hedgehog/GLI target gene expression by epidermal growth factor signaling in human keratinocytes. Mol Cell Biol. 2006;26(16):6283–98. doi: 10.1128/MCB.02317-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wang H, Li YY, Wu YY, Nie YQ. Expression and clinical significance of hedgehog signaling pathway related components in colorectal cancer. Asian Pac J Cancer Prev. 2012;13(5):2319–24. doi: 10.7314/apjcp.2012.13.5.2319. [DOI] [PubMed] [Google Scholar]
- [22].Bian YH, Huang SH, Yang L, Ma XL, Xie JW, Zhang HW. Sonic hedgehog-Gli1 pathway in colorectal adenocarcinomas. World J Gastroenterol. 2007;13(11):1659–65. doi: 10.3748/wjg.v13.i11.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Mazumdar T, Devecchio J, Agyeman A, Shi T, Houghton JA. Blocking Hedgehog survival signaling at the level of the GLI genes induces DNA damage and extensive cell death in human colon carcinoma cells. Cancer Res. 2011;71(17):5904–14. doi: 10.1158/0008-5472.CAN-10-4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hu X, Lai D, Chen W, Zi S, Li J, Du P, et al. Differential expression profiles of the Hedgehog signaling pathway between microsatellite-stable and microsatellite-unstable colorectal cancers. Mol Med Rep. 2011;4(5):873–7. doi: 10.3892/mmr.2011.529. [DOI] [PubMed] [Google Scholar]
- [25].Varnat F, Zacchetti G, Ruiz i Altaba A. Hedgehog pathway activity is required for the lethality and intestinal phenotypes of mice with hyperactive Wnt signaling. Mech Dev. 2010;127(1–2):73–81. doi: 10.1016/j.mod.2009.10.005. [DOI] [PubMed] [Google Scholar]
- [26].van den Brink GR, Bleuming SA, Hardwick JC, Schepman BL, Offerhaus GJ, Keller JJ, et al. Indian Hedgehog is an antagonist of Wnt signaling in colonic epithelial cell differentiation. Nat Genet. 2004;36(3):277–82. doi: 10.1038/ng1304. [DOI] [PubMed] [Google Scholar]
- [27].Yoshimoto AN, Bernardazzi C, Carneiro AJ, Elia CC, Martinusso CA, Ventura GM, et al. Hedgehog pathway signaling regulates human colon carcinoma HT-29 epithelial cell line apoptosis and cytokine secretion. PLoS ONE. 2012;7(9):e45332. doi: 10.1371/journal.pone.0045332. [DOI] [PMC free article] [PubMed] [Google Scholar]