Abstract
Diabetes mellitus is one of the oldest disorders that is rapidly emerging as a global health problem. Soy genistein is a legume that has numerous health benefits. This work aimed to study the effect of different doses of genistein on histological, immunohistochemical and morphometrical changes in β-cells of streptozotocin (STZ)-induced diabetic rats and to correlate these effects with plasma glucose and insulin levels. Fifty adult male rats were divided into five equal groups. Group I served as a control. Group II received genistein. Group III comprised STZ-induced diabetic rats. Group IV diabetic animals treated with low dosage genistein. Group V diabetic animals treated with high dosage genistein. Genistein was given for 4 weeks after STZ injection. Rats were sacrificed and pancreatic specimens were taken for light and electron microscopic examination. Blood samples were collected for detection of serum glucose and insulin levels. After diabetic induction, the islets appeared shrunken with cytoplasmic vacuolation of their cells and negative insulin immunoreaction. Ultrastructurally, β-cells showed darkly stained nuclei with marked loss of granules. Morphometrically, significant loss of β-cells was detected. The serum insulin level was decreased with elevation in the serum glucose. High-dose but not low-dose genistein improved the morphology of islets with increased insulin immunoreaction. Genistein also significantly decreased β-cells loss and improved glucose and insulin levels. In conclusion, genistein has a protective effect on pancreatic β-cells damage, possesses the ability to regenerate β-cells and improves serum levels of insulin and glucose in STZ-induced diabetic rats in a dosage-dependent manner.
Keywords: Pancreatic β-cells, Diabetes, Genistein, Oxidative stress
1. Introduction
Diabetes mellitus (DM) is one of the oldest diseases that is rapidly emerging as a global health problem [1]. It is a metabolic state of hyperglycemia resulting from insulin deficiency, insulin resistance or both [2]. World Health Organization (WHO) indicates that DM is one of the major killers of humans in our time and affect 1 –5% of the world population [3,4]. By the year 2025, the number of individuals with diabetes is predicted to be more than 325 million in spite of availability of new drugs, techniques and surgical intervention [5,6].
There are two types of DM. Type 1 (insulin-dependent) that results from damage of pancreatic insulin producing cells and type 2 (non-insulin-dependent) that results from insulin resistance [7]. Among various factors that are being involved in the progression of DM, oxidative stress plays important role in etiology of DM and related complications [8]. Persistent hyperglycemia is responsible for increasing formation of free radicals, autooxidation of glucose and lipid peroxidation as well as disturbance of the antioxidant defense system. The resultant free radicals bring about intracellular oxidative stress [9].
The streptozotocin (STZ) is the most commonly used agent for experimental animals to produce type 1 diabetes by selective damage of pancreatic β-cells. The STZ enters the β-cells via glucose transporter and causes DNA damage [10].
In humans, islets constitute nearly 1–2% of pancreatic tissue and insulin-secreting β-cells represent up to 80% of cells in islets that have the ability to proliferate in both physiological and pathological conditions [11,12]. The balance between transdifferentiation (differentiation of other cell types into β-cells) and neogenesis (differentiation of precursor cells into β-cells) and proliferation of preexisting β-cells are important factors for controlling the mass of β-cells [13].
Based on recent advances, disturbance of regulation of β-cell growth and function are central to the development of two types of diabetes. Hence, many studies are underway to find new effective agents that can increase or preserve islet β-cell mass and function, providing a plane to lower the burden of morbidity from DM and its complications [13,14].
Genistein, a naturally occurring soy isoflavone is a flavonoid in legumes and some herbal medicines [15]. It has numerous health effects [16,17] and its intake is considered safe and nontoxic following pharmacological administration in rats [18] and humans [19]. Previous studies have reported the potential roles of genistein on prevention of hormone-related disorders such as breast cancer, cardiovascular diseases, osteoporosis and chronic renal disease [20,21,22,23].
Recent studies have reported that administration of isoflavone reduced the elevated blood glucose level in diabetic animals [24,25] and postmenopausal women [26]. Genistein has been extensively reported as a compound with many functions through improving the antioxidant defense system and anti-inflammation response [23,27]. However, the studies of the effect of genistein by itself on morphology of pancreatic β-cells in diabetic animals are very limited.
Based on these, we demonstrated in the current study the possible protective effect of different doses of soy isoflavone genistein on histological, immunohistochemical and morphometrical changes in pancreatic β-cells of STZ-induced diabetic rats and correlated these effects with plasma glucose and insulin levels.
2. Materials and methods
2.1. Chemicals
STZ and genistein were obtained from Sigma Chemical Company (St. Louis, Missouri, USA).
STZ was freshly dissolved in 5 mmol/l citrate buffer, pH 4.5 and given intraperitonium (single injection, 60 mg/kg) [28]. Each 100 mg of genistein was dissolved in 10 ml distilled water (10 mg/ml) and given orally by gastric tube. The doses were adjusted on the basis of a previous work [29].
2.2. Animals
Fifty adult male albino rats with body weights of 150-210 g each, at the beginning of experiment were used in the current study. The animals were fed on a standard laboratory food and water ad libitum. They were kept under standard conditions of temperature and humidity. All animal experiments received approval from the ethical committee of Tanta Faculty of Medicine.
2.3. Experiment design
Rats were classified into 5 groups, 10 rats each.
Group I (control group): received 5 mmol/l citrate buffer.
Group II (genistein group): received 20 mg/kg genistein daily.
Group III (diabetic group): this group included STZ-induced diabetic animals.
Group IV (low dosage genistein treated group): diabetic animals received genistein dosage 10 mg/kg/day.
Group V (high dosage genistein treated group): diabetic animals received genistein dosage 20 mg/kg/day.
2.4. Induction of diabetes
Diabetes was induced with single intraperitonium injection of STZ, 60 mg/kg. To overcome the expected hypoglycemia, the animals were allowed to drink 5% glucose solution overnight. After 48 h, the diabetes was confirmed by measuring fasting blood glucose levels from a tail vein using an Ames One Touch Glucometer (LifeScan; Johnson and Johnson, New Brunswick, NJ, USA). Only animals with plasma glucose level above 200 mg/dL [28,30] were chosen for the experiment. Treatment of diabetic rats with genistein (groups IV and V) started 1 week post STZ injection. Low dose probably reflects the daily genistein intake in soy enriched diet in human [31]. All rats were not treated with insulin throughout the study which lasted for 4 weeks after induction of diabetes.
Through the experiment, fluid intake was estimated and finally, the rats were weighed, fasted overnight without water deprivation and then sacrificed under ether inhalation. Histological, immunohistochemical, morphometrical and biochemical studies were conducted.
2.5. Histological and immunohistochemical study
Small pieces were obtained from pancreatic tail, fixed in 10% buffered formalin and processed for examination by light microscopy that were subjected to [32,33].
1- Hematoxylin and eosin (H&E).
2- Immunohistochemical staining for detection of insulin antibody using the avidin–biotin peroxidase system.
The primary antibodies used were mouse monoclonal insulin antibodies (Medico Company, Egypt) at a dilution of 1:100 that incubated with slides for 1 h at room temperature. Then, the sections were counterstained with Meyer's hematoxylin.
For electron microscopy, thin slices of pancreas were fixed in 5% cold glutaraldehyde then washed three times with phosphate buffer (pH 7.2) and post fixed in cold osmium tetroxide for 2 h. Subsequently, the specimens were washed in buffer, dehydrated in a graded series of alcohol and embedded in Epon. Ultrathin sections (50–80 nm) were cut with ultramicrotome, collected on copper grids and stained with uranyl acetate and lead citrate to be examined [34] by (JEM-1010) transmission electron microscopy (TEM; Jeol, Tokyo, Japan) in Tanta University Electron Microscopy Unit.
2.6. Morphometrical study
The image analyzer computer system Leica Qwin 500 in the Central laboratory, Faculty of Medicine, Tanta University was used to evaluate:
1- Number of islets/each pancreatic section.
2- Diameter of the islets.
3- Number of β-cells/islet.
Examination was performed at different magnifications in the non-serial pancreatic sections using H&E. The β- cells were determined by direct counting method at 1000 × magnification. The number of islets was quantified at these sections at 40× magnification. Data were then collected in an Excel sheet and submitted to statistical analysis [35].
2.7. Biochemical study
Venous blood samples from all animals were collected in heparinized tubes and centrifuged at 3000 rpm for 15min to separate plasma used for the determination of [36]:
1- Glucose levels by the glucose oxidase method (kit purchased from Stanbio laboratory, USA).
2- Insulin level by using an enzyme-linked immunosorbent assay, ELISA (kit purchased from DRG International Inc., California, USA).
2.8. Statistics
Statistical analysis were performed using SPSS program, version 17 (IBM Corporation, Somers, New York, USA) in the form of mean (X) ± standard deviation (SD). A one-way analysis of variance (ANOVA) was used for calculation of differences between the groups’ means. P < 0.05 was considered statistically significant.
3. Results
3.1. Body weight and fluid intake
Alterations in body weights and fluid intake in control and experimental groups were shown in Table 1 and Histograms 1 and 2. No significant difference in these parameters in rats treated with genistein alone (group II) comparing with the control one (P > 0.05). After induction of diabetes (group III), significant loss of body weights and increased fluid intake were observed compared to control one (P < 0.001). Treatment with low dosage genistein (group IV) did not significantly prevent these changes. However, high dosage genistein (group V) significantly increased body weights and reduced fluid intake as compared to diabetic group (P < 0.001).
Table 1.
Body weight (g) and fluid intake (ml) in control and experimental groups.
| Groups | Body weight (g) | Fluid intake (ml) |
|---|---|---|
| Group I | 208 ± 3.6 | 31 ± 3 |
| Group II | 204 ± 4.1 | 39 ± 2 |
| Group III | a135 ± 2.3 | a169 ± 7 |
| Group IV | 148 ± 0.3 | 137 ± 4 |
| Group V | b187 ± 2.9 | b76 ± 2 |
Values are expressed as mean ± SD.
a P < 0.001 as compared to control.
b P < 0.001 as compared to diabetic.
Histogram 1.
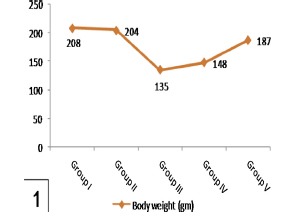
Shows a significant reduction in body weights in group III in comparison with group I and a significant increase in group V compared to group III. Note nonsignificant affection of body weights in group IV comparing with group III.
Histogram 2.
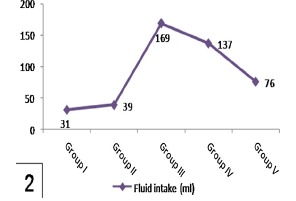
Shows a significant increase of fluid intake in group III compared to group I and a significant reduction in group V compared to group III. Note nonsignificant affection of fluid intake in group IV in comparison to group III.
3.2. Histological and immunohistochemical results
3.2.1. Control & genistein groups (groups I and II)
Pancreatic sections stained with H&E of control and genistein treated rats showed large, regular and well defined islets of Langerhans which represented the endocrine portion of pancreatic lobules. They appeared as pale staining areas surrounded by thin capsule of connective tissue scattered in between the exocrine parenchyma. The islets arranged in anastomosing branching cords with blood capillaries in between and consist of clusters of polygonal cells had pale acidophilic cytoplasm and central rounded vesicular nuclei (Fig. 1).
Fig. 1.
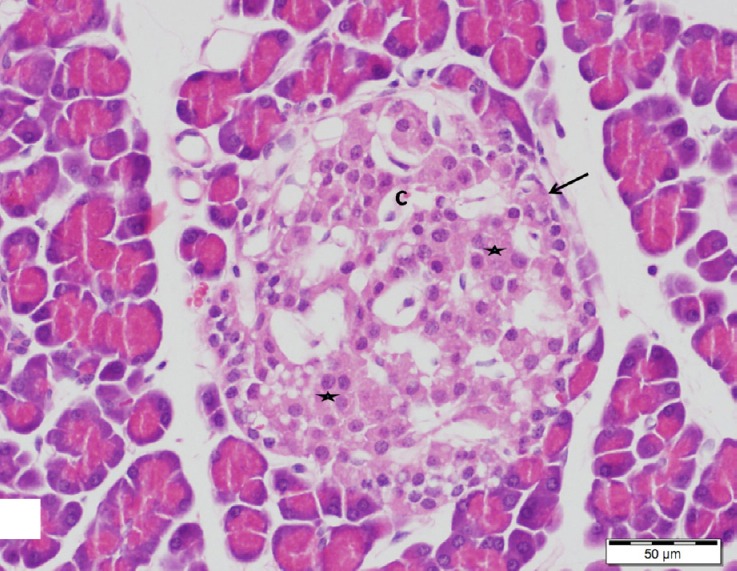
A section of control pancreas showing large well-defined islet of Langerhans (arrow) composed of cords of endocrine cells with rounded vesicular nuclei and pale acidophilic cytoplasm (stars). Note small blood capillaries (C). H&E, scale bar 50 μm.
Regarding the immunohistochemistry, areas of dark brown staining (strong positive) for insulin antibody were seen in islet β-cells cytoplasm which form the major cells population of the islets (Figs. 2 and 3).
Fig. 2.
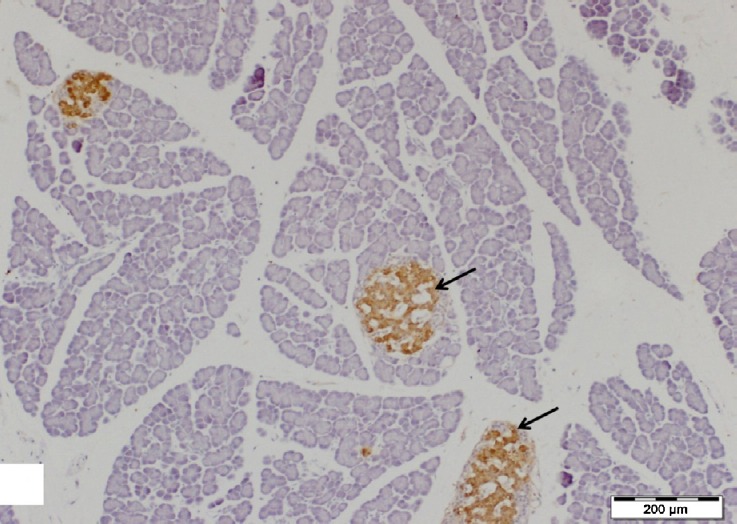
A section of control pancreas showing well-defined islets of Langerhans (arrows) with strong immunoreactions for insulin antibody in their β-cells. Insulin immunostain, scale bar 200 μm.
Fig. 3.
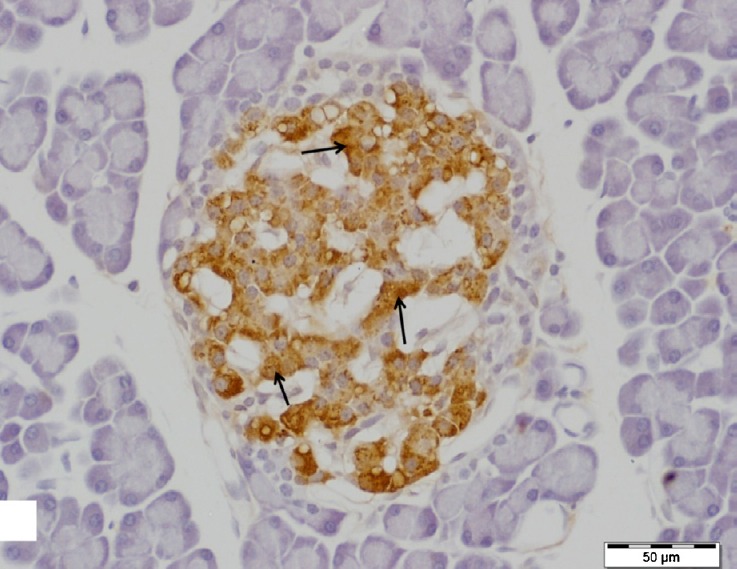
A higher magnification of the previous figure showing highly positive insulin antibody staining (arrows) in the cytoplasm of the majority of cells, β-cells. Insulin immunostain, scale bar 50 μm.
Ultrastructurally, islet β-cells appeared oval or polygonal in shape with indistinct intercellular space had euchromatic rounded nuclei and contained many characteristic β granules in their cytoplasm. An electron lucent halo space surrounded an electron dense core is characteristic feature of the granules. The mitochondria, rough endoplasmic reticulum (rER) and Golgi apparatus were observed among them (Figs. 4 and 5).
Fig. 4.
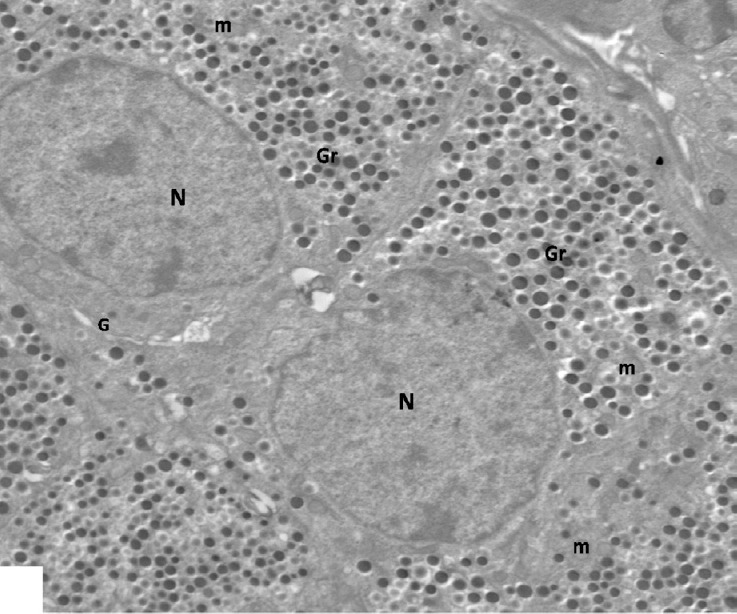
An electron micrograph of control β-cells with indistinct intercellular space showing euchromatic rounded nuclei (N) and characteristic β granules (Gr). Note short profile round mitochondria (m) and Golgi complex (G). ×2000.
Fig. 5.
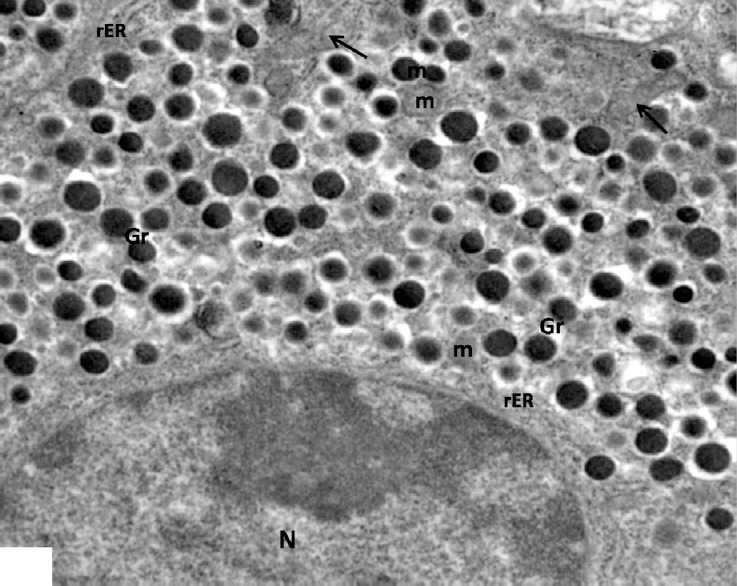
An electron micrograph of a control β-cell showing β-granules with an electron dense core surrounded by an electron lucent halo (Gr), round electron dense mitochondria (m) with arrows indicating elongated mitochondria and rER. Note part of the nucleus (N). ×5000.
3.2.2. Diabetic group (group III)
Pancreatic sections stained with H&E of diabetic group showed few ill-defined islets of Langerhans. Marked histological alterations in the structure of the islets were observed. They appeared shrunken and distorted with marked loss of its cells and normal cellular cord arrangement. Many islets cells revealed marked cytoplasmic vacuolation and pyknotic nuclei and others showed a deeply acidophilic cytoplasm with large darkly stained nuclei (Figs. 6 and 7).
Fig. 6.
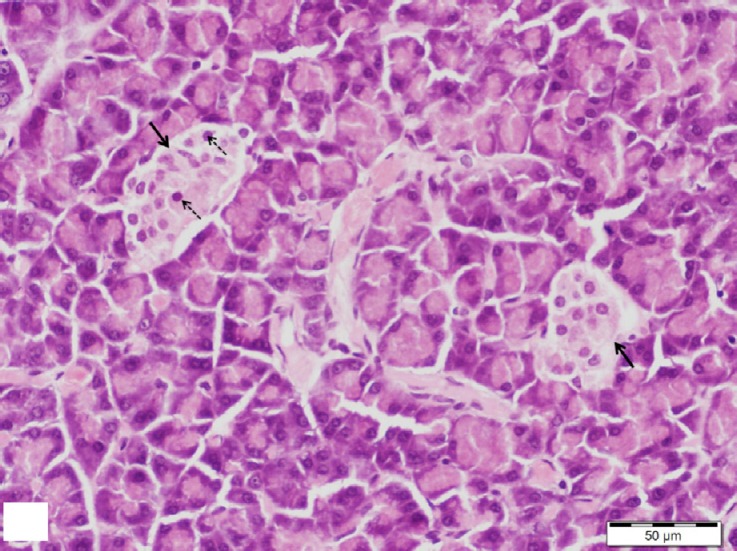
A section of pancreas from the diabetic group showing small islets of Langerhans (arrows) with loss of its normal cell cord arrangement. Many cells show pyknotic nuclei (dotted arrows). H&E, scale bar 50 μm.
Fig. 7.
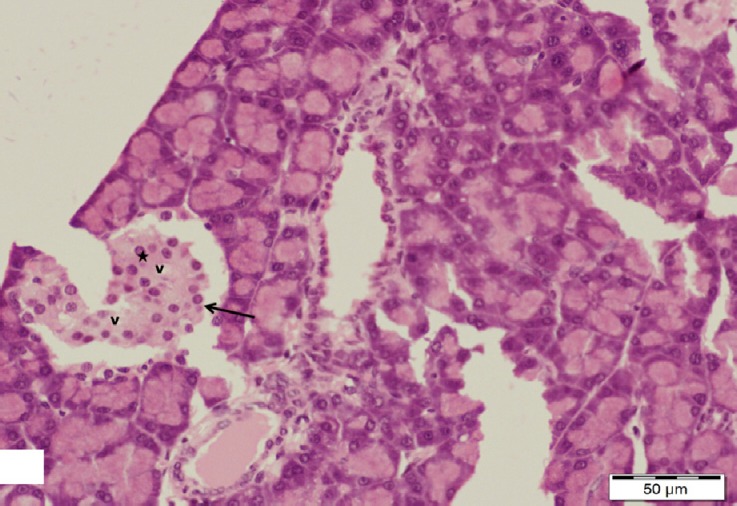
A section of pancreas from the diabetic group showing a shrunken distorted islet of Langerhans with marked loss of its cells (arrow). Note some cells with vacuolated cytoplasm (v) and large darkly stained nuclei (star). H&E, scale bar 50 μm.
Immunohistochemical reactions revealed marked reduction in immunoreactivity for insulin antibody inside islet β-cells and only a few β-cells displayed minimal insulin immunoreaction (Fig. 8).
Fig. 8.
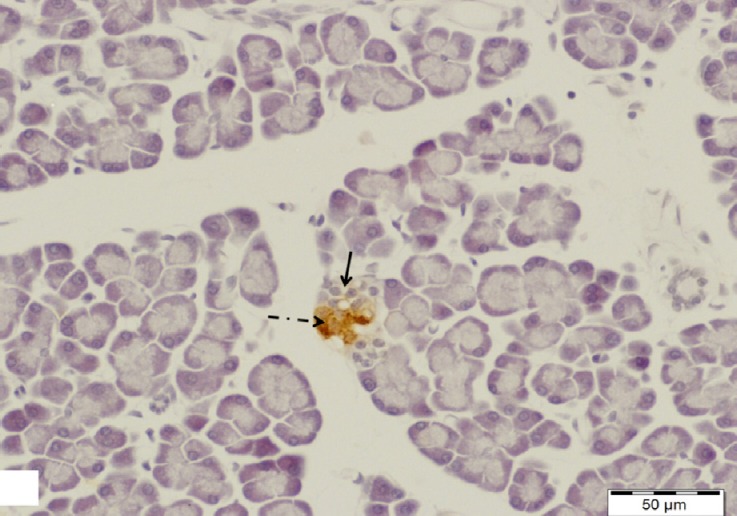
A section of pancreas from the diabetic group showing a small islet of Langerhans with negative insulin immunoreactivity in its β-cells (arrow). Note a few β-cells with minimal insulin immunoreaction (dotted arrow). Insulin immunostain, scale bar 200 μm.
The electron micrographs of pancreas of diabetic rats showed remarkable changes in the islet β-cells, most of the cells exhibited marked loss of their granules in rarified cytoplasm leaving empty spaces or electron lucent areas. Their nuclei showed variable changes, some appeared dark electron dense and others showed marked peripheral aggregation of heterochromatin with irregular nuclear envelope. Golgi apparatus was obviously dilated and the mitochondria were vacuolated with loss of their cristae and matrix. Congested blood capillaries were also seen. β-granules of some cells appeared with increased electron lucent halo around small electron dense cores. Increasing number of empty granules and small mitochondria were also noted (Figs. 9 and 10).
Fig. 9.
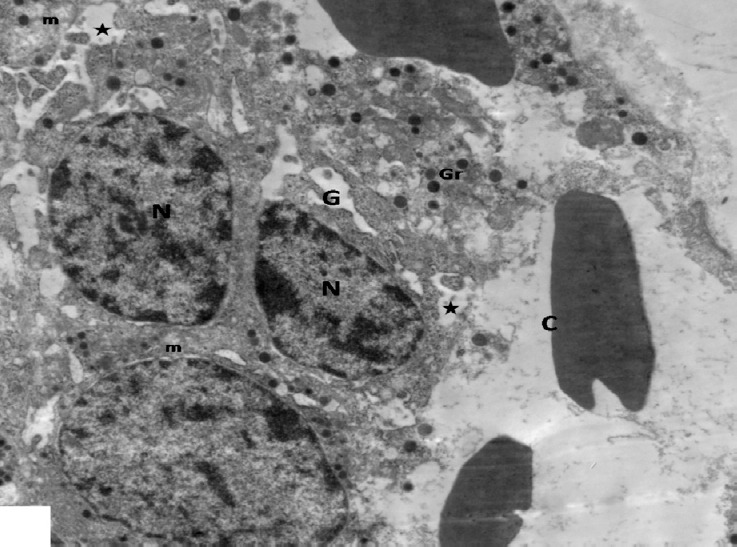
An electron micrograph of pancreas from the diabetic group showing β-cells scattered inbetween congested blood capillaries (C) have rarified cytoplasm with electron lucent areas (stars), few secretory granules (Gr), dilated Golgi complexes (G) and vacuolated mitochondria (m). Note small nuclei (N). × 3000.
Fig. 10.
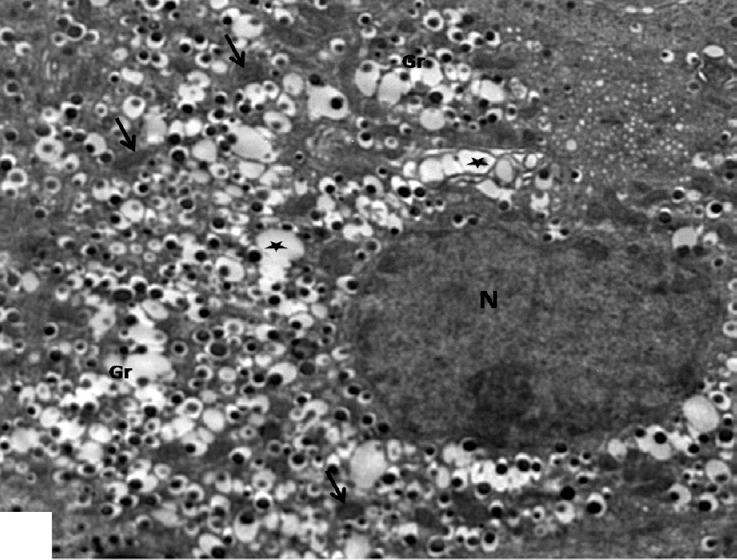
An electron micrograph of a β-cell from the diabetic group showing irregular shaped dark electron dense nucleus (N) and β-granules with increased electron lucent halo (Gr). Note an increased numbers of small mitochondria (arrows) and empty granules (stars). ×2000.
3.2.3. Low dosage genistein treated group (group IV)
By H&E stain, sections of pancreas of diabetic rats treated with low dosage genistein showed the similar changes as those in group III, the islets were still shrunken and ill defined with marked disturbance of cells arrangement. Most cells appeared with marked cytoplasmic vacuolation and pyknotic nuclei (Figs. 11 and 12).
Fig. 11.
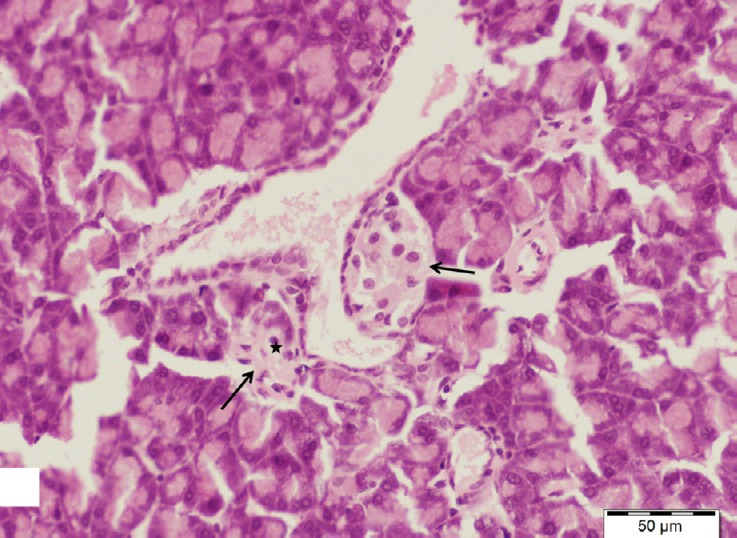
A section of pancreas from low dosage genistein treated group showing shrunken distorted islets of Langerhans (arrows) contained few cells with pyknotic nuclei (star). H&E, scale bar 50 μm.
Fig. 12.
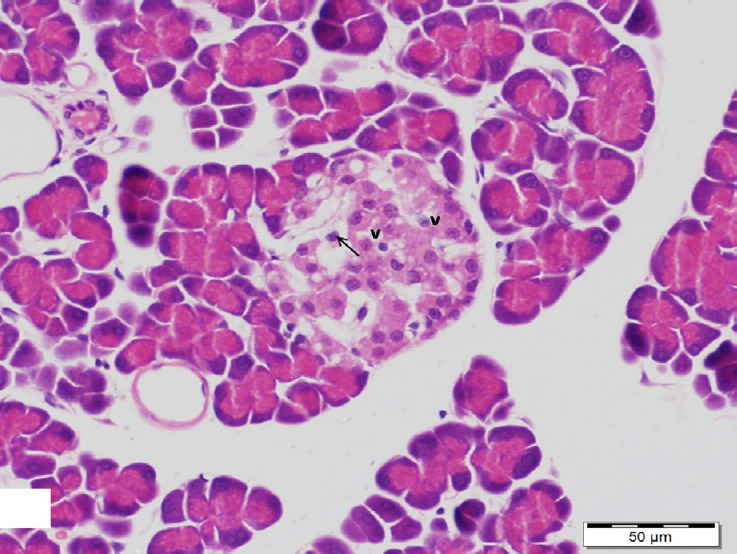
A section of pancreas from low dosage genistein treated group showing a shrunken islet of Langerhans with vacuolated cytoplasm (v) and pyknotic nuclei (arrow) in its cells. H&E, scale bar 50 μm.
Regardless, some islet β-cells displayed mild insulin immunopositivity, most of the cells were still negative for insulin immunoreactivity (Fig. 13).
Fig. 13.
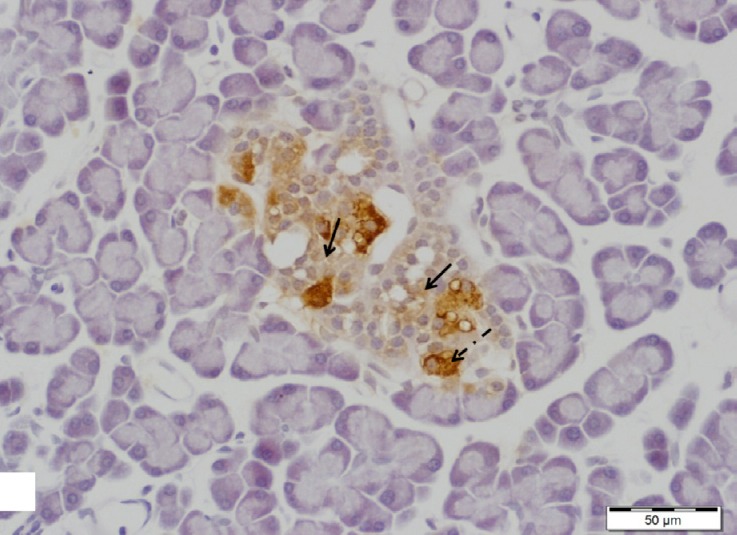
A section of pancreas from low dosage genistein treated group showing an islet of Langerhans with negative insulin immunoreactivity in majority of β-cells (arrows). Note some β-cells with positive insulin immunoreaction (dotted arrow). Insulin immunostain, scale bar 50 μm.
The electron micrographs confirmed the results shown by light microscope as no obvious improvement in the islet β-cells was observed. Most cells were still affected and appeared shrunken with heterochromatic nuclei and disrupted vacuolated cytoplasm that contained decreased amount of β granules and destructed vacuolated mitochondria. Moreover, β secretory granules in the cytoplasm of some cells appeared either empty or with increased halo spaces around less dense cores. Few cells appeared with intact mitochondria and normal characteristic β granules (Figs. 14–16).
Fig. 14.
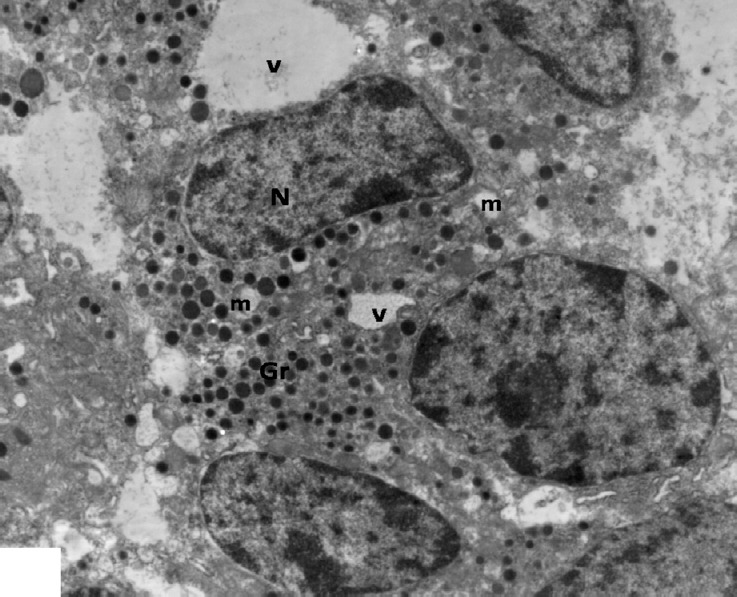
An electron micrograph of an islet β-cells from low dosage genistein treated group showing multiple vacuoles (V) and decreased amount of β-granules (Gr) in the disrupted cytoplasm. Note swollen vacuolated mitochondria (m) and irregular nuclei (N). ×3000.
Fig. 16.
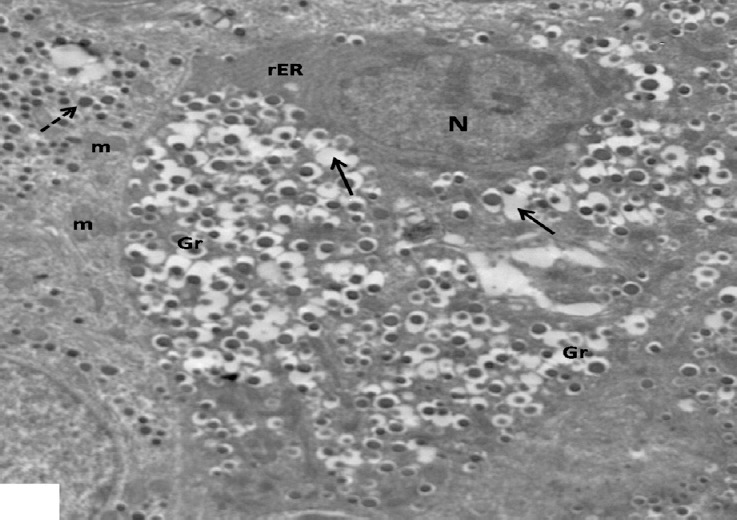
An electron micrograph of β-cells from low dosage genistein treated group showing one cell has dark nucleus with condensed chromatin (N), rER and granules either empty (arrows) or with increased halo spaces (Gr). Note other cells with normal characteristic β granules (dotted arrow) and mitochondria with intact cristae (m). ×2000.
Fig. 15.
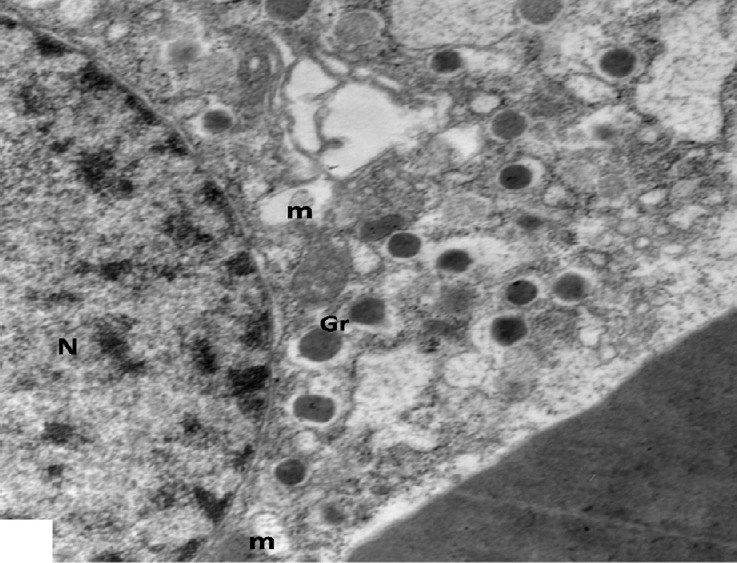
An electron micrograph of a β-cell from low dosage genistein treated group showing decreased β-granules (Gr), vacuolated mitochondria (m) in disrupted cytoplasm and a normal nucleus (N). ×5000.
3.2.4. High dosage genistein treated group (group V)
H&E stained pancreatic sections of the diabetic rats treated with high dosage genistein, showed partial improvement in the histological structure of its islets which appeared more or less normal with an apparent increase in their size. Some of its cells showed minimal cytoplasmic vacuolation and pyknotic nuclei, while others were improved having central vesicular nuclei with pale acidophilic cytoplasm (Fig. 17).
Fig. 17.
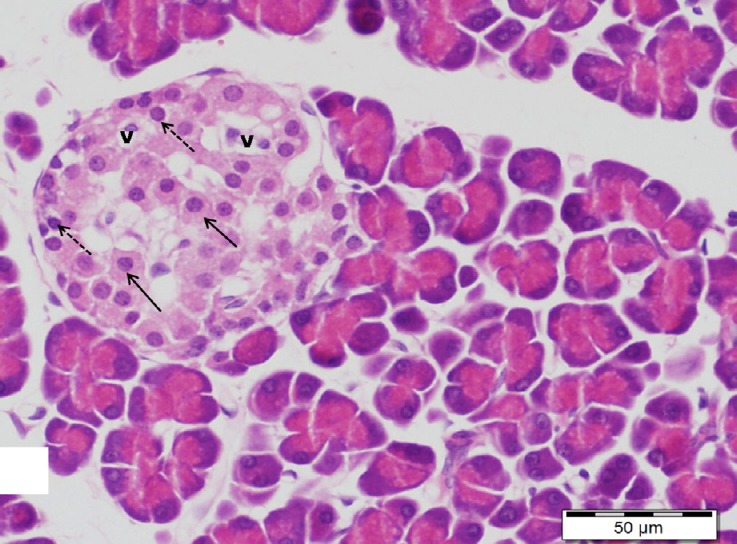
A section of pancreas from high dosage genistein treated group showing an apparent increase in the size of an islet has more or less normal cells (arrows). Note a few cells with vacuolated cytoplasm (v) and pyknotic nuclei (dotted arrows). H&E, scale bar 50 μm.
Regarding the immunohistochemistry, different sizes of islets of pancreas were observed with increasing immunoreaction to insulin antibody in β-cells in comparison to diabetic group (Figs. 18 and 19).
Fig. 18.
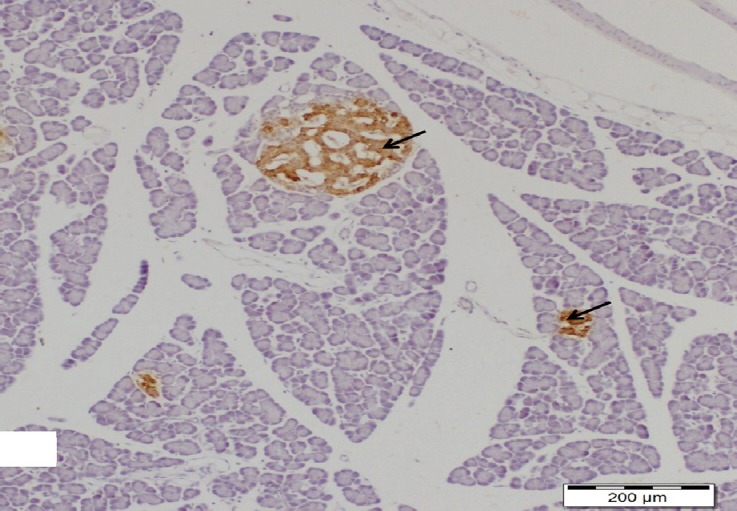
A section of pancreas from high dosage genistein treated group showing appearance of different sizes islets of pancreas with increasing insulin immunoreactivity (arrows). Insulin immunostain, scale bar 200 μm.
Fig. 19.
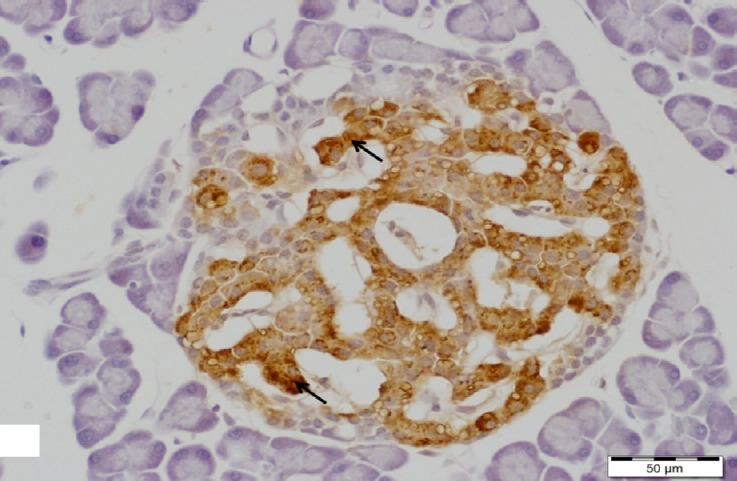
A higher magnification of the previous figure showing an islet of Langerhans with increasing insulin immunoreactivity in cytoplasm of its β-cells (arrows). Insulin immunostain, scale bar 50 μm.
Ultrastructurally, β-islet cells appeared containing euchromatic nuclei with clumps of heterochromatin and an increase in the number of β granules, there were of normal structure as control, having dense core surrounded by lucent halo space of different sizes. Numerous rounded and elongated electron dense mitochondria were observed throughout the cytoplasm as well as proliferating rER (Figs. 20 and 21). However, some cells were still affected and had irregular shaped heterochromatic nuclei and β-granules with increased halo spaces. rER appeared dilated (Fig. 22).
Fig. 20.
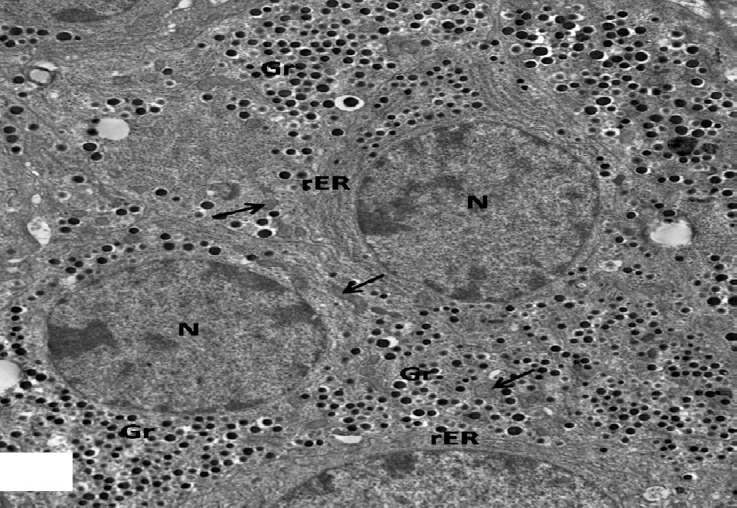
An electron micrograph of an islet of pancreas from high dosage genistein treated group showing euchromatic nuclei (N) of many β-cells with increasing amount of β secretory granules (Gr). Note numerous electron dense mitochondria (arrows) and proliferating rER. × 1500.
Fig. 21.
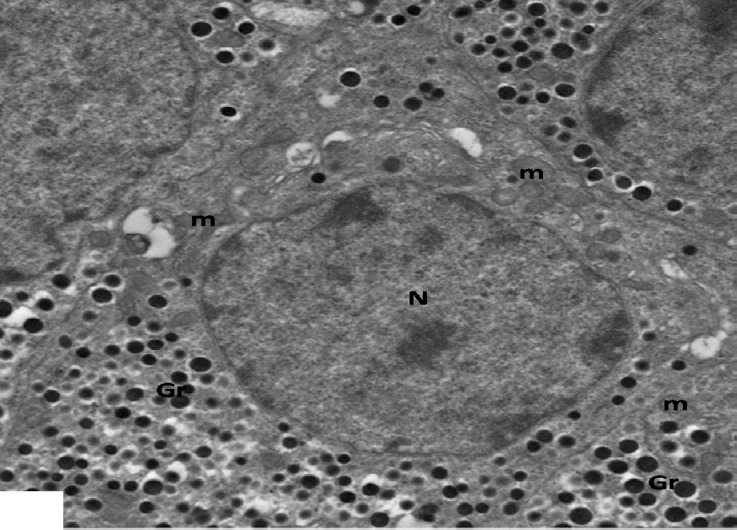
An electron micrograph of an islet of pancreas from high dosage genistein treated group showing β-cells studded with characteristic β secretory granules (Gr), numerous intact mitochondria (m). Note euchromatic nuclei (N). ×2500.
Fig. 22.
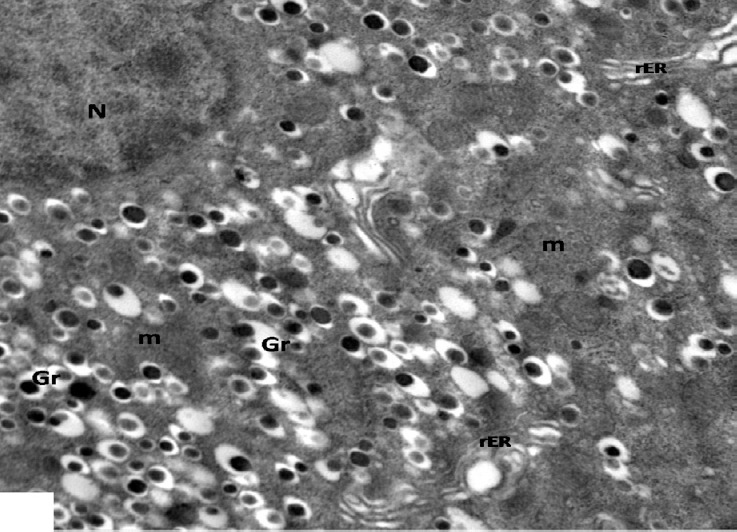
An electron micrograph of a β-cell from high dosage genistein treated group showing β-granules with increased halo spaces (Gr), dilated rER and mitochondria (m). Note part of the nucleus (N). ×4000.
3.3. Morphometric results
Morphometric results (Table 2 and Histograms 3, 4 and 5) showed a significant reduction in the number of islets/pancreas, islet diameter and number of β-cells/islet in diabetic group (group III) compared to that of control one (P <0.001). However, these parameters were significantly increased in high dosage genistein treated group (group V) (P <0.001), but did not significantly change in rats treated with low dose of genistein (group IV) compared to diabetic group. Nonsignificant difference (P >0.05) was observed in these parameters in genistein group (group II) in comparison to the control.
Table 2.
Number of islets/pancreas (N/10 mm2), diameter of islets (μm) and number of β-cells/islet (N/1000 μm2) in control and experimental groups.
| Groups | Number of islets/pancreas (N/10 mm2) | Diameter of islets (μm) | Number of β-cells/islet (N/1000 μm2) |
|---|---|---|---|
| Group I | 18.79 ± 0.719 | 130.74 ± 10.945 | 10.45 ± 0.561 |
| Group II | 18.63 ± 0.731 | 134.39 ± 12.841 | 10.63 ± 0.738 |
| Group III | a4.32 ± 0.395 | a61.87 ± 5.319 | a2.78 ± 0.179 |
| Group IV | 6.98 ± 0.245 | 79.38 ± 3.709 | 4.22 ± 0.636 |
| Group V | b11.42 ± 0.611 | b102.76 ± 7.986 | b7.89 ± 0.473 |
Values are expressed as mean ± SD.
a P < 0.001 as compared to control.
b P < 0.001 as compared to diabetic.
Histogram 3.
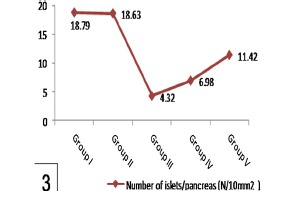
Shows a significant reduction of number of islets/pancreas in group III in comparison to group I and a significant increase in group V when compared with group III. Note nonsignificant affection of number of islets/pancreas in group IV comparing with group III.
Histogram 4.
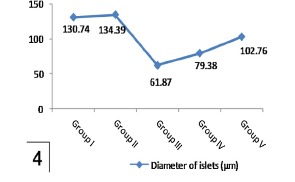
Shows a significant reduction of islet diameter in group III in comparison to group I and a significant increase in group V in comparison with group III. Note nonsignificant affection of islet diameter in group IV comparing with group III.
Histogram 5.
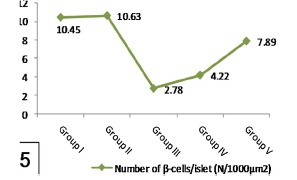
Shows a significant reduction of number of β-cells/islet in group III when compared with group I while it significantly increased in group V in comparison with group III. Note nonsignificant affection of number of β-cells/islet in group IV comparing with group III.
3.4. Biochemical results
Table 3 and Histograms 6 and 7 showed nonsignificant difference (P >0.05) in plasma glucose and insulin levels of the control and genistein groups (groups I and II respectively). In comparison with previous groups, levels of fasting plasma glucose were significantly higher and insulin levels were significantly lower in diabetic group (group III) (P <0.001). High dosage genistein treatment (group V) significantly decreased plasma glucose levels and significantly increased insulin levels (P <0.001) as compared with the diabetic group, but low dosage genistein treatment (group IV) did not significantly reduce plasma glucose levels as well as did not significantly increase insulin levels.
Table 3.
Plasma glucose level (mg/dL) and insulin level (μU/ml) in control and experimental groups.
| Groups | Plasma glucose level (mg/dL) | Insulin level (μU/ml) |
|---|---|---|
| Group I | 94.8 ± 6.21 | 53.6 ± 1.4 |
| Group II | 102.5 ± 2.9 | 49.2 ± 4.3 |
| Group III | a294.2 ± 11.4 | a10.4 ± 1.87 |
| Group IV | 233.7 ± 4.9 | 13.1 ± 4.62 |
| Group V | b178.5 ± 9.3 | b32.9 ± 4.62 |
Values are expressed as mean ± SD.
a P < 0.001 as compared to control.
b P < 0.001 as compared to diabetic.
Histogram 6.
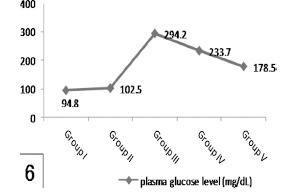
Shows a significant increased glucose level in group III in comparison to group I and a significant reduction in group V in comparison with group III. Note nonsignificant affection of glucose level in group IV comparing with group III.
Histogram 7.
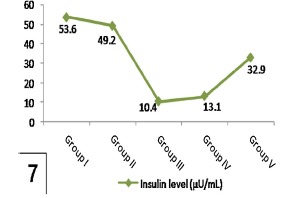
Shows a significant decreased insulin level in group III in comparison to group I and a significant increase in group V when compared to group III. Note nonsignificant affection of insulin level in group IV comparing with group III.
4. Discussion
Diabetes mellitus is one of the most common, dangerous, costly health problems in the twenty- first century. An increase in sedentary life-style, consumption of energy-rich diet and obesity are some of the factors causing the rise in the number of diabetics. Controlling hyperglycemia and oxidative stress inhibition have been suggested as important measures in the management of DM [35,37].
Soy isoflavone genistein may provide new, low cost, greater potential oral hypoglycemic compounds. Therefore, this study was done to investigate the effect of different doses of soy genistein on histological, immunohistochemical and morphometrical changes of pancreatic islets β-cells and correlate these effects with plasma glucose and insulin levels in diabetic rats.
In the current study, all results of genistein group (group II) were similar to the control one indicating safety and non-toxicity of genistein administration. These observations were consistent with results of previous researchers [38] who stated that, there is no any observed toxicity of genistein dosage up to 120 mg/kg/day in rats which is six times more than the highest dosage used in this work.
Experimental diabetes induced by STZ produced severe loss of body weights and an increase of fluid intake which generally occur in diabetes. Dehydration and catabolism of fats as well as proteins with increased catabolic reaction are major causes of these signs [3].
Our study revealed that STZ altered islet morphology which appeared ill defined, damaged and shrunken with marked vacuolated cytoplasm and pyknotic nuclei in most of its endocrine cells in association with marked decrease in insulin antibody positivity in islet β-cells. These results are in agreement with those reported previously [39,40,41].
Moreover, ultrastructural results revealed heterochromatic nuclei, decreased secretory granules, mitochondrial vacuolation and fragmentation as well as dilatation of the rER and Golgi apparatus and multiple vacuoles in the β-cells that in consistence with other investigators [41]. In addition, degranulation of most β-cells and increased halo spaces of granules of other cells were in conforming with the results of other studies [42,43] who found similar ultrastructural changes in rat pancreatic β-cells induced by STZ.
Pancreatic β-cells toxicity induced by STZ has been investigated in several experimental models and is thought to be as a result of production of reactive oxygen species that led to lipid peroxidation and DNA damage [41,42,43,44].
As a result of increasing lipid peroxidation, lipid-containing membranes as Golgi and rER membranes were dilated suggesting the observed cytoplasmic vacuolation of the islet cells seen in the present study. The observed vacuolated mitochondria led to disturbance of mechanism of antioxidation, explaining mitochondrial limited ability to overcome the oxidative stress in β-cells [10].
The histological changes come hand by hand with morphometric results which showed a significant reduction in number of islets and islet diameter as well as number of β-cells/islet in diabetic group compared with the control one. In addition, these observations were consistent with the biochemical finding of a significant increase in serum glucose levels and a significant decrease in insulin secretion. These results were in agreement with previous studies [45,46] that reported significant reduction in the numerical density of islets, volume of islets, and volume of β-cells in the untreated diabetic group of rats when compared with the control one. Previous studies [8,47] reported the same changes in blood glucose and insulin levels in experimental diabetic animals.
The observed degeneration of the islet cells together with significant reduction in the numerical density of islets as well as the decrease in insulin antibody positivity in the diabetic animals, explained decreased insulin level noted in these animals. Consequently, the observed elevation of blood glucose level in this group may be the result of decreasing insulin level.
Diabetes has been shown to be a state of free radicals overproduction resulting from hyperglycemia. Factors that attributed to the formation of free radicals may include not only elevated non-enzymatic and autooxidative glycosylation, but also metabolic stress as a result of alterations in energy metabolism, inflammatory mediators levels and the status of antioxidant defense [41,47]. The resultant decrease in both antioxidant capacity and insulin activity/sensitivity led to commonly observed diabetic complications [47,48].
Comparing with diabetic group, not low dosage treated genistein group (group VI), which corresponding to daily genistein intake after soy enriched diet in humans [31] but high dosage treated genistein group (group V) showed a significant increase in body weights accompanied by a significant decrease in fluid intake. These results were consistent with those of previous study in rats administered soybean seed 400 mg/kg [3] and in mice [49] who reported that soybean by its contents of high percentage of protein and isoflavone, increases metabolic processes in body.
In the present study, genistein causes dose-dependent amelioration of toxic effects of STZ on pancreatic β-cells. Low dosage genistein treated group (group IV) failed to improve the morphological changes in the islets as well as insulin immunoreactivity induced by STZ while, administration of high dosage genistein (group V) resulted in partial improvement of the islets and β-cells associated with increased insulin immunoreactivity.
These results have been supported by electron microscopic findings that revealed appearance of β islets cells with euchromatic nuclei and retention of most β-cells to their normal structures with increasing number of normal β granules, there were of normal structure as control in group V than those observed in group IV.
Studies on whether genistein by itself has an effect on diabetes are very limited and there have been no morphological studies to date examining the pancreatic β-cells ultrastructure in STZ-diabetic rats treated with isoflavone genistein.
Genistein has been reported to be protective agent for diabetes by regulation of oxidative stress and inflammation as it decreases lipid peroxidation, inhibits cyclooxygenase expression and myeloperoxidase activity as well as reacts with free radicals and neutralizes their effects [50,51]. Other researchers stated that the efficiency of genistein as antioxidant is achieved only at high doses (ranging from 25 to 100 μm) and it has not this property at physiological doses as its plasma level through diet intake is not more than 10 μm in both humans and animals [52,53]. The antioxidant effect of soy isoflavones protects cell membranes from damage and increases their stability, which contributes to the increased insulin sensitivity [54].
Moreover, the observed morphometric results confirmed the histological one. In animals treated with low dose genistein (group IV), no significant changes in the number of islets and islet diameter as well as number of β-cells/islet were observed compared with the diabetic one. In contrast, high dosage genistein treatment (group V) was able to reduce STZ islet cells loss and showed significant increase in these parameters.
Accordingly, previous study [14] have shown that genistein is an antidiabetogenic agent through its effect on proliferation of β-cells and insulin secretion by glucose stimulation regardless of its efficiency as antioxidant, an estrogen receptor agonist and tyrosine kinase inhibition. In addition, other data have demonstrated that genistein can act as β-cells growth factor in vivo suggesting a novel mechanism for antidiabetic effect of this agent [53]. This regeneration of the β-cells is probably due to the fact that pancreas contains stable (quiescent) cells which have the capacity of regeneration [35]. Therefore, the surviving cells can proliferate to replace the lost cells.
Other researchers reported that teucrium polium crude extract is able of regeneration of islets of Langerhans and enhancing of insulin secretion [55]. In addition, glabridin has shown the same effects [45].
Our data showed that genistein at high dose (group V) but not in low dose (group IV) can improve both glucose and insulin levels in rats with STZ induced islets damage. This was consistent with results of other researchers who have demonstrated marked improvement in blood glucose and insulin levels after genistein intake for diabetic animals [25,53,56]. Consistent with our observations, a previous study has demonstrated lowering in fasting blood glucose, increasing in plasma insulin and C-peptide and enhancement of insulin staining in the pancreatic islets of STZ-induced diabetic mice supplemented with genistein (200 mg/kg) diets for 9 weeks [57].
The observed increased insulin level and decreased blood glucose concentration may be due to the amelioration of the histological structure of β-cells together with increased insulin immunoreactivity and significant increase in the numerical density of islets that retained the ability to synthesize and secrete insulin.
An increased number of mitochondria that were noted in cytoplasm of β-cells in group V may reflect compensatory adaptation to produce much energy in response of glucose stimulation to maintain glucose homeostasis for synthesis and secretion of insulin [58].
In conclusion, the present study showed that genistein has a protective effect on pancreatic β-cells toxicity induced by STZ in a dose dependent manner. High dosage genistein possesses the ability to regenerate β-cells that results in increasing the lowered serum insulin and consequently decreasing high serum glucose in diabetic rats.
Conflict of interest
The authors declare no conflict of interest.
References
- [1].Hossain P, Kawar B, El Nahas M. Obesity and diabetes in the develop-ing world – a growing challenge. N Engl J Med. 2007;356(3):213–5. doi: 10.1056/NEJMp068177. [DOI] [PubMed] [Google Scholar]
- [2].Limaye PV, Raghuram N, Sivakami S. Oxidative stress andgene expression of antioxidant enzymes in the renal cor-tex of streptozotocin-induced diabetic rats. Mol Cell Biochem. 2003;243(1–2):147–52. doi: 10.1023/a:1021620414979. [DOI] [PubMed] [Google Scholar]
- [3].Aziz OH. Effect of soybean seeds alone or in combination with insulinor glibenclamide on serum lipid profiles in alloxan-induced diabeticrats. Iraqi J Vet Sci. 2009;23(1):17–23. [Google Scholar]
- [4].Kameswararao B, Kesavulu MM, Apparao C. Evaluation of antidia-betic effect of Momordica cymbalaria fruit in alloxan-diabetic rats. Fitoterapia. 2003;74(1–2):7–13. doi: 10.1016/s0367-326x(02)00297-6. [DOI] [PubMed] [Google Scholar]
- [5].Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87(1):4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- [6].Al-Hilfy GHY. Effect of green gea extract on histological structure of kidney, pancreas and adrenal gland in alloxan-induced diabetic malealbino rats. J Al-Nahrain Univ. 2013;16(1):156–65. [Google Scholar]
- [7].Brownlee M. The pathology of diabetic complications: a unifyingmechanism. Diabetes. 2005;54(6):1615–25. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- [8].Prasad SK, Kulshreshtha A, Qureshi TN. Antidiabetic activity of someherbal plants in streptozotocin induced diabetic albino rats. Pak JNutr. 2009;8(5):551–7. [Google Scholar]
- [9].Idris MH, Budin SK, Osman M, Mohamed J. Protective role of Hibiscus sabdariffa calyx extract against streptozotocin induced spermdamage in diabetic rats. EXCLI J. 2012;11:659–69. [PMC free article] [PubMed] [Google Scholar]
- [10].Abdelmeguid NE, Fakhoury R, Kamal SM, AlWafai RJ. Effect of Nigellasativa L. and thymoquinone on streptozotocin induced cellular damage in pancreatic islets of rats. Asian J Cell Biol. 2011;6(1):1–21. [Google Scholar]
- [11].Stoffers DA. The development of beta-cell mass: recent progress andpotential role of GLP-1. Horm Metab Res. 2004;36(11–12):811–21. doi: 10.1055/s-2004-826168. [DOI] [PubMed] [Google Scholar]
- [12].Suarez-Pinzon WL, Yan Y, Power R, Brand SJ, Rabinovitch A. Combi-nation therapy with epidermal growth factor and gastrin increases β-cell mass and reverses hyperglycemia in diabetic NOD mice. Dia-betes. 2005;54(9):2596–601. doi: 10.2337/diabetes.54.9.2596. [DOI] [PubMed] [Google Scholar]
- [13].Tarabra E, Pelengaris S, Khan M. A simple matter of life and death-the trials of postnatal beta-cell mass regulation. Int J Endocrinol. 2012;2012:1–20. doi: 10.1155/2012/516718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gilbert ER, Liu D. Anti-diabetic functions of soy isoflavone geniste:mechanisms underlying effects on pancreatic β-cell function. Food Funct. 2013;4(2):200–12. doi: 10.1039/c2fo30199g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Velasquez MT, Bhathena SJ. Role of dietary soy protein in obesity. Int J Med Sci. 2007;4(2):1449–907. doi: 10.7150/ijms.4.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sacks FM, Lichtenstein A, Van Horn L, Harris W, Kris-Etherton P, Winston M. Soy protein, isoflavones, and cardiovascular health: an American Heart Association Science Advisory for professionals from the Nutrition Committee. Circulation. 2006;113(7):1034–44. doi: 10.1161/CIRCULATIONAHA.106.171052. [DOI] [PubMed] [Google Scholar]
- [17].Si H, Liu D. Genistein, a soy phytoestrogen, upregulates theexpression of human endothelial nitric oxide synthase and low-ers blood pressure in spontaneously hypertensive rats. J Nutr. 2008;138(2):297–304. doi: 10.1093/jn/138.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Roberts D, Veeramachaneni DN, Schlaff WD, Awoniyi CA. Effectsof chronic dietary exposure to genistein, a phytoestrogen, duringvarious stages of development on reproductive hormones and sper-matogenesis in rats. Endocrine. 2000;13(3):281–6. doi: 10.1385/ENDO:13:3:281. [DOI] [PubMed] [Google Scholar]
- [19].Bloedon LT, Jeffcoat AR, Lopaczynski W, Schell MJ, Black TM. Safety and pharmacokinetics of purified soy isoflavones: single-dose administration to postmenopausal women. Am J Clin Nutr. 2002;76(5):1126–37. doi: 10.1093/ajcn/76.5.1126. [DOI] [PubMed] [Google Scholar]
- [20].Pavese JM, Farmer RL, Bergan RC. Inhibition of cancer cellinvasion and metastasis by genistein. Cancer Metastasis Rev. 2010;29(3):465–82. doi: 10.1007/s10555-010-9238-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Gencel VB, Benjamin MM, Bahou SN, Khalil RA. Vascular effects of phytoestrogens and alternative menopausal hormone therapy in car-diovascular disease. Mini-Rev Med Chem. 2012;12(2):149–74. doi: 10.2174/138955712798995020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Stephenson TJ, Setchell KDR, Kendall C, Jenkins WC, Anderson DJA, Fanti JWP. Effect of soy protein-rich diet on renal function inyoung adults with insulin-dependent diabetes mellitus. Clin Nephrol. 2005;64(1):1–11. doi: 10.5414/cnp64001. [DOI] [PubMed] [Google Scholar]
- [23].Kim MJ, Lim Y. Protective effect of short-term genistein supple-mentation on the early stage in diabetes-induced renal damage. Mediators Inflamm. 2013;2013:1–14. doi: 10.1155/2013/510212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ae Park S, Choi MS, Cho SY, Seo JS, Jung UJ. Genistein and daidzeinmodulate hepatic glucose and lipid regulating enzyme activities inC57BL/KsJ-db/db mice. Life Sci. 2006;79(12):1207–13. doi: 10.1016/j.lfs.2006.03.022. [DOI] [PubMed] [Google Scholar]
- [25].Lee JS. Effects of soy protein and genistein on blood glucose, antiox-idant enzyme activities and lipid profile in streptozotocin-induceddiabetic rats. Life Sci. 2006;79(16):1578–84. doi: 10.1016/j.lfs.2006.06.030. [DOI] [PubMed] [Google Scholar]
- [26].Cheng SY, Shaw NS, Tsai KS, Chen CY. The hypoglycemic effects of soy isoflavones on postmenopausal women. J Womens Health (Larchmt) 2004;13(10):1080–6. doi: 10.1089/jwh.2004.13.1080. [DOI] [PubMed] [Google Scholar]
- [27].Behloul N, Wu G. Genistein a promising therapeutic agent for obesity and diabetes treatment. Eur J Pharmacol. 2013;698(1-3):31–8. doi: 10.1016/j.ejphar.2012.11.013. [DOI] [PubMed] [Google Scholar]
- [28].Sen S, Roy M, Chakraborti AS. Ameliorative effects of glycyrrhizin on streptozotocin-induced diabetes in rats. J Pharm Pharmacol. 2011;63(2):287–96. doi: 10.1111/j.2042-7158.2010.01217.x. [DOI] [PubMed] [Google Scholar]
- [29].Yang W, Wang S, Li L, Liang Z, Wang L. Genistein reduces hyperglycemia and islet cell loss in a high-dosage manner in rats with alloxan-induced pancreatic damage. Pancreas. 2011;40(3):396–402. doi: 10.1097/MPA.0b013e318204e74d. [DOI] [PubMed] [Google Scholar]
- [30].Yan JE, Yuan W, Lou X, Zhu T. Streptozotocin-induced diabetic hyperalgesia in rats is associated with upregulation of toll-like receptor 4 expression. Neurosci Lett. 2012;526(1):54–8. doi: 10.1016/j.neulet.2012.08.012. [DOI] [PubMed] [Google Scholar]
- [31].Morton MS, Arisaka O, Miyake N, Morgan LD, Evans BA. Phytoestrogen concentrations in serum from Japanese men and women over forty years of age. J Nutr. 2002;132(10):3168–71. doi: 10.1093/jn/131.10.3168. [DOI] [PubMed] [Google Scholar]
- [32].Bancroft JD, Layton C. The hematoxylin and eosin. In: Suvarna SK, Layton C, Bancroft JD, editors. Bancroft's theory & practice of histo-logical techniques. 7th ed. Ch. 10. Philadelphia: Churchill Livingstoneof Elsevier; 2013. pp. 172–86. [Google Scholar]
- [33].Jackson P, Blythe D. Immunohistochemical techniques. In: Suvarna SK, Layton C, Bancroft JD, editors. Bancroft's theory & practice of histological techniques. 7th ed. Ch. 18. Philadelphia: Churchill Livingstone of Elsevier; 2013. pp. 381–434. [Google Scholar]
- [34].Ayache J, Beaunier L, Boumendil J, Ehret G, Laub D. New York/Dordrecht/Heidelberg/London: Springer; 2010. Sample prepa-ration handbook for transmission electron microscopy techniques. [Google Scholar]
- [35].Adeyemi DO, Komolafe OA, Adewole OS, Obuotor EM, Abiodun A, Adenowo TK. Histomorphological and morphometric studies of the pancreatic islet cells of diabetic rats treated with extracts of Annona muricata. Folia Morphol. 2010;69(2):92–100. [PubMed] [Google Scholar]
- [36].Shim JY, Kim KO, Seo BH, Lee HS. Soybean isoflavone extract improves glucose tolerance and raises the survival rate in streptozotocin- induced diabetic rats. Nutr Res Pract. 2007;1(4):266–72. doi: 10.4162/nrp.2007.1.4.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Haskins K, Bradley B, Powers K, Fadok V, Flores S, Ling X, et al. Oxida-tive stress in type 1 diabetes. In: Sanjeevi CB, Eisenbarth GS, editors. Immunology of diabetes II, pathogenesis from mouse to man, 1005. New York: Annals of the New York Academy of Sciences; 2003. pp. 43–54. [DOI] [PubMed] [Google Scholar]
- [38].Okazaki K, Okazaki S, Nakamura H, Kitamura Y, Hatayama K, Wak-abayashi S, et al. A repeated 28-day oral dose toxicity study of genistein in rats, based on the ‘Enhanced OECD Test Guideline 407’ for screening endocrine-disrupting chemicals. Arch Toxicol. 2002;76(10):553–9. doi: 10.1007/s00204-002-0376-0. [DOI] [PubMed] [Google Scholar]
- [39].Nirmala A, Saroja S, Vasanthi HR, Lalitha G. Hypoglycemic effect of Basella rubra in streptozotocin induced diabetic albino rats. J Pharmacogn Phytother. 2009;1(2):25–30. [Google Scholar]
- [40].Ozdemir O, Akalin PP, Baspinar N, Hatipoglu F. Pathological changes in the acute phase of streptozotocin-induced diabetic rats. Bull Vet Inst Pulawy. 2009;53(4):783–90. [Google Scholar]
- [41].Kanter M, Coskun O, Korkomaz A, Oter S. Effects of Nigella sativa on oxidative stress and beta-cell damage in streptozotocin-induced diabetic rats. Anat Rec A Discov Mol Cell Evol Biol. 2004;279(1):685–91. doi: 10.1002/ar.a.20056. [DOI] [PubMed] [Google Scholar]
- [42].Attia AA. Histological and electron microscopic studies of the effect of beta-carotene on the pancreas of streptozotocin (STZ)-induced diabetic rats. Pak J Biol Sci. 2009;12(4):301–14. doi: 10.3923/pjbs.2009.301.314. [DOI] [PubMed] [Google Scholar]
- [43].Mythili MD, Vyas R, Akila G, Gunasekaran S. Effect of streptozotocin on the ultrastructure of rat pancreatic islets. Microsc Res Tech. 2004;63(5):274–81. doi: 10.1002/jemt.20039. [DOI] [PubMed] [Google Scholar]
- [44].Ahmadi S, Karimian SM, Sotoudeh M, Bahadori M. Histological and immunohistochemical study of pancreatic islet beta cells of diabetic rats treated with oral vanadyl sulphate. Med J Islam Repub Iran. 2002;16(3):173–8. [Google Scholar]
- [45].Abo Gazia MM, Hasan NM. Effect of glabridin on the structure of ileum and pancreas in diabetic rats: a histological, immunohistochemical and ultrastructural study. Nat Sci. 2012;10(3):78–90. [Google Scholar]
- [46].Adewole SO, Caxton Martins EA, Ojewole JAO. Protective effect of quercetin on the morphology of pancreatic β-cells of streptozotocin treated diabetic rats. Afr J Tradit Complement Altern Med. 2007;4(1):64–74. doi: 10.4314/ajtcam.v4i1.31196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Lai MH. Antioxidant effects and insulin resistance improvement of chromium combined with vitamin C and E supplementation for type 2 diabetes mellitus. J Clin Biochem Nutr. 2008;43(3):191–8. doi: 10.3164/jcbn.2008064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Hamdena K, Jaouadib B, Carreauc S, Aouidetd A, Elfekia A. Therapeutic effects of soy isoflavones on a-amylase activity, insulin deficiency, liver-kidney function and metabolic disorders in diabetic rats. Nat Prod Res. 2011;25(3):244–55. doi: 10.1080/14786411003683117. [DOI] [PubMed] [Google Scholar]
- [49].Kim S, Sohn I, Lee YS, Lee YS. Hepatic gene expression profiles are altered by genistein supplementation in mice with diet-induced obesity. J Nutr. 2005;135(1):33–41. doi: 10.1093/jn/135.1.33. [DOI] [PubMed] [Google Scholar]
- [50].Javanbakht MH, Sadria R, Djalali M, Derakhshanian H, Hosseinzadeh P. Soy protein and genistein improves renal antioxidant status in experimental nephrotic syndrome. Nefrologia. 2014;34(4):483–90. doi: 10.3265/Nefrologia.pre2014.Jun.12051. [DOI] [PubMed] [Google Scholar]
- [51].Seibel J, Molzberger AF, Hertrampf T, Laudenbach-Leschowski U, Diel P. Oral treatment with genistein reduces the expression of molecular and biochemical markers of inflammation in a rat model of chronic TNBS-induced colitis. Eur J Nutr. 2009;48(4):213–20. doi: 10.1007/s00394-009-0004-3. [DOI] [PubMed] [Google Scholar]
- [52].Naaz A, Yellayi S, Zakroczymski MA, Bunick D, Doerge DR. The soy isoflavone genistein decreases adipose deposition in mice. Endocrinology. 2003;144(8):3315–20. doi: 10.1210/en.2003-0076. [DOI] [PubMed] [Google Scholar]
- [53].Zhuo F, Zhang W, Zhen W, Lum H, Nadler J. Genistein induces pancreatic β-cell proliferation through activation of multiple signaling pathways and prevents insulin-deficient diabetes in mice. Endocrinology. 2010;151(7):3026–37. doi: 10.1210/en.2009-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Li M, Wei D, Ding W, Baruah B, Crans DC. Anti-diabetic effects of cesium aqua (N,N’ ethylene (salicylideneiminato)-5-sulfonato) oxo-vanadium (IV) dihydrate in streptozotocin-induced diabetic rats. Biol Trace Elem Res. 2008;121(3):226–32. doi: 10.1007/s12011-007-8049-8. [DOI] [PubMed] [Google Scholar]
- [55].Yazdanparast R, Esmaeili MA, Helan JA. Teucrium polium extract effects pancreatic function of streptozotocin diabetic rats: a histopathological examination. Iran Biomed J. 2005;9(2):81–5. [Google Scholar]
- [56].Elmarakby AA, Ibrahim AS, Faulkner J, Mozaffari MS, Liou GI, Abdelsayed R. Tyrosine kinase inhibitor, genistein, reduces renal inflammation and injury in streptozotocin-induced diabetic mice. Vascul Pharmacol. 2011;55(5-6):149–56. doi: 10.1016/j.vph.2011.07.007. [DOI] [PubMed] [Google Scholar]
- [57].Choi MS, Jung UJ, Yeo J, Kim MJ, Lee MK. Genistein and daidzein prevent diabetes onset by elevating insulin level and altering hepatic gluconeogenic and lipogenic enzyme activities in non-obese diabetic (NOD) mice. Diabetes Metab Res Rev. 2008;24(1):74–81. doi: 10.1002/dmrr.780. [DOI] [PubMed] [Google Scholar]
- [58].Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science. 2005;307(5708):384–7. doi: 10.1126/science.1104343. [DOI] [PubMed] [Google Scholar]


