Abstract
Peritoneal dialysis (PD) offers the healthiest way for starting renal replacement therapy (RRT) in End Stage Renal Disease patients, however exposes long-term PD patients to a dangerous complication named encapsulating peritoneal sclerosis (EPS). In this study, we searched for possible risk factors of EPS. Data were collected from two PD centers covering period 1995–2012 and comprised 464 patients. Control group defined as PD patients stayed on PD >42 month (n = 122), and case group was 12 confirmed EPS patients. Associations were analyzed using linear regression analysis. Prevalence and incidence of EPS were 2.59% and 8.9% with an incidence of 0.7% patient-years, respectively. The age at start of PD in EPS patients (32.75 ± 10.8 year) was significantly lower compared with control group (49.61 ± 16.18 year, p = .0001). The mean duration of PD in EPS and control group were 2494.4 ± 940.9 and 1890.2 ± 598.8 days (p = .002). Control group had 145 episodes of peritonitis during total duration of 7686 patient months (peritonitis rate of 1/53). This was 1/26 with a total 38 episodes of peritonitis during the total duration of 997 patient months (p = .01) for EPS group. In regression analysis, PD duration, age at PD start and duration of Ultrafiltration failure (UFF) were associated with EPS. Longer time being on PD, younger age, and higher UFF duration were the risk factors for EPS development.
Keywords: End stage renal disease, peritoneal dialysis, peritoneal fibrosis, encapsulating peritoneal sclerosis, chronic kidney disease
Introduction
Chronic kidney disease (CKD), which is essentially a progressive loss of functional nephrons, is a worldwide public health problem with 8–16% of the adult population suffering from this disease worldwide.1 Only in United States more than 20 million people have some level of CKD.2 A cross sectional study performed in our country reported 8.9% of subjects with CKD stages III–V and 14.5% as CKD stages I–II.3 Although a higher prevalence of 19% has been reported.4 Importantly, CKD may finally progress towards end-stage renal disease (ESRD), in which patients need renal replacement therapy (RRT) in order to survive. Based on latest report, the number of patients being treated for ESRD globally was estimated to be 3,200,000 at the end of 2013 and with a ∼6% growth rate, ESRD continues to increase at a significantly higher rate than the world population.5 By end 2012, in the united state, the prevalent cases of ESRD were 636,905, a figure that demonstrated an increase of 3.7% since 2011.2 Although the exact reasons for the growth of the ESRD program are unknown, but aging population, under-recognition of earlier stages of CKD and risk factors for CKD including hypertension and diabetes may explain this growth.2 Patients with ESRD produce a huge burden on healthcare resources. For example, the total cost of the ESRD program in the united state was approximately $49.3 billion in 2011.2 In addition to huge cost of ESRD management, patients who suffer from ESRD, experience a decreased quality and expectancy of life.
Peritoneal dialysis (PD), as a healthiest way for start of RRT in ESRD patients comprises approximately 11% of dialysis population.6 In our country an increase in number of patients receiving CAPD has been observed over time.7 This modality offers a continuous removal of extracellular fluid and does not need an arteriovenous access. Moreover, PD might work better in preserving the remnant kidney function than hemodialysis. However, one potential risk is development of encapsulating peritoneal sclerosis (EPS) which is a rare but serious clinical complication of long-term PD with high mortality. EPS was first reported by Gandhi and colleagues in 19808 and is characterized by a progressive intra-abdominal inflammatory process that results in sheets of fibrous tissue. These sheets progressively cover and constrict the viscera and impair the intestinal motility and function. Moreover, the peritoneal and systemic inflammation leads to progressive and excessive peritoneal sclerotic thickening. The signs and symptoms of EPS include intermittent, acute, or sub-acute abdominal pain, weight loss, nausea, vomiting, anorexia, constipation, malnutrition, and bloodstained dialysis effluent.9 In most severe cases, the peritoneal membrane turns into a fibrous cocoon wrapping the bowel and causing its partial or complete obstruction.10 If obstruction does not resolve, intestinal dilatation and mural ischemia may develop, resulting in bacterial translocation, systemic inflammatory response syndrome, and sepsis.9 One important finding is loss of dialysis efficacy as peritoneal function usually presents ultrafiltration failure (UFF) which is caused by an increase in peritoneal permeability. The diagnosis of EPS can be confirmed with clinical manifestations as well as radiological or macroscopic findings. EPS may present during PD therapy or after transfer to hemodialysis or renal transplantation.
The exact worldwide incidence of EPS is not known but the incidence augments as time on PD increases. Recent studies reported varied prevalence from 1.1 to 3.3%.11–13 From etiological point, no known single etiologic factor directly related to EPS is known, however time being on PD has been identified by many studies as a risk factor.9–18 Other factors variably introduced as risk factors for EPS includes: bioincompatible dialysate, chlorhexidine disinfectant, povidone-iodine, PD catheters, b-blockers usage, and peritonitis.19–24 There is no single satisfactory intervention to cure the patients with EPS but a multidisciplinary approach, which is based on the EPS staging and includes both medical and surgical intervention, might be helpful.25 It is generally accepted that PD should be discontinued after diagnosis of EPS. Additional interventions include peritoneal lavage, nutritional support, enteral or parenteral nutrition, corticosteroids, tamoxifen, immunosuppression, and enterolysis.25 Despite these interventions the mortality rate is still high and varies between 25.8 and 56.5%.
In our country, we have a computerized PD data system that collects data from all PD centers treating patients throughout the country.7 Using these data, we designed this case-control study in which we used the information from two large PD centers with more comprehensive data, (Shafa center and Shariati hospital, Tehran, Iran) covering period between 1995 and 2012, in order to analyze and compare the basic clinical characteristics and outcomes of patients with and without EPS treated at these two centers and also to search for possible risk factors. To our knowledge this is the first report describing the EPS patients from Iran.
Method
This study reports on all confirmed cases of EPS diagnosed among our PD patients registered in two centers in Tehran (Shafa center and Shariati hospital, affiliated with Tehran University of Medical Sciences, Tehran, Iran) in which we had more comprehensive and precise data, from 1995 to 2012. Ethical approval for the use of registry data was obtained from ethic committee of Tehran University of Medical Sciences.
EPS diagnosis was based on clinical, radiologic, or surgical findings. The clinical criteria for the EPS suspicion included fever, general fatigue, loss of appetite, nausea, vomiting, diarrhea, absent bowel sounds, constipation, abdominal pain, bloody ascites, and a palpable abdominal mass. The ultrasound and CT findings indicating EPS included: peritoneal thickening, loculated ascites, peritoneal calcifications, signs of small bowel obstruction and dilation. The diagnosis of EPS was also made by laparotomy with a fibrotic and thickened membrane covering the bowel, peritoneal thickness, extensive adhesions, encapsulation, and/or the presence of an abdominal cocoon.
The EPS diagnosis was made when there was strong clinical suspicion and that was confirmed by either radiologic or surgical findings. Based on these criteria we confirmed 12 cases of EPS (EPS group).
For control group, we included all patients who started PD at two above mentioned centers from 1995 to 2012, stayed on PD for 42 months or more and did not develop EPS (control group, n = 122). 42 months was chosen, since first EPS patient developed EPS after being on PD for 42 months. All patients were using standard Dianeal dialysis solutions (Baxter Healthcare Corporation, Deerfield, IL).
Demographic and PD-related variables including age at the start of PD, gender, education, body mass index, duration of PD, underlying causes of ESRD, comorbidities, number of cumulative peritonitis episodes, membrane transport status, outcome and cause of death were obtained from Iranian PD registry.
The prevalence of EPS was calculated as the number of EPS patients divided by number of PD patients who were maintained on PD for three or more months. The incidence of EPS was calculated as the number of EPS cases divided by number of patients at risk. Peritonitis rates were calculated as the total number of episodes of peritonitis divided by the total number of months on PD therapy. Time to EPS was defined as the number of months spent on PD before the diagnosis of EPS. UFF was defined as ultrafiltration volume of less than 400 mL in a 4-h 2000 mL dwell with 4.25% glucose dialysate.
Results were summarized as frequencies and percentages for categorical variables, and mean ± sd. for normally distributed continuous variables, Differences between patients with and without EPS were analyzed using chi-square test for categorical data and unpaired t-test for continuous normally distributed data. The predictors of EPS were evaluated by multivariate binary logistic regression analysis.
Analysis of the data was performed using the Stata software application (StataCorp LP, College Station, TX). Differences were considered statistically significant at a level of p < .05.
Results
Clinical and biochemical results
Overall, 464 patients started PD between 1995 and 2012 in these two centers, and of these, 134 patients had been maintained on PD for ≥42 months. During this period, 12 patients were diagnosed with EPS (12/464). The prevalence of EPS was 2.59% and its incidence was 8.9% with an incidence of 0.7% patient-years.
Table 1 describes the anthropometric and clinical characteristics of control and EPS groups. As it is indicated in the table, the age at the start of PD in EPS patients was lower compared with the control patients (32.75 ± 10.8 versus 49.61 ± 16.18 day, p = .0001). The median of age in control group was 50 (16–83 years), and in EPS group was 32 (17–58 years). The gender ratio did not differ between two groups. Fifty percentage of EPS patients (n = 6) were male compared to 48.5% in control (n = 65). The mean duration of PD in EPS patients was 2494.4 ± 940.9 days (median: 2333 days) compared to 1890.2 ± 598.8 in control group (median: 1788 days, p = .002). The mean duration of PD for all PD patients who started their PD during this period (n = 452) but did not develop EPS was 924.15 ± 732.6 days. Only one EPS patient was diabetic. Among the 12 EPS patients, five patients were diagnosed with EPS while on PD; four patients were on hemodialysis and three patients were diagnosed after kidney transplant. The principal reason for conversion to HD and transplant in EPS patients was membrane dysfunction. The major cause of death in our EPS patients (n = 7, 58.3%) was PD complications and only one patient died from heart disease, while among our control group, 45 patients (43/122, 35.24%) died from following causes: heart disease = 24 patients, infection = 2 patients, cancer = 2 patients, cerebrovascular accident = 7 patients, senile = 3 patients, and 5 patients died because of other reasons. No significant difference was present between mortality between EPS and control group (p = .1). Overall mortality among all enrolled PD patients during this period who did not develop EPS was 37.83% (171/452).
Table 1.
Anthropometric characteristics of EPS and control group.
| Characteristic | EPS group (n = 12) | Control Group (n = 122) | p Values |
|---|---|---|---|
| Age (years), Mean (SD) | 32.75 (10.8) | 49.61 (16.2) | .0001 |
| Male/female, n (%) | 6/6 (50/50) | 65/69 (48.5/51.5) | .86 |
| Education, n (%) | .85 | ||
| Illiterate | 0 (0) | 24 (18.3) | |
| ≥ college | 4 (33.3) | 42 (32.1) | |
| University | 8 (66.7) | 65 (49.6) | |
| Weight(kg), Mean (SD) | 63.14 (18.1) | 58.54 (12.1) | .2 |
| BMI (kg/m2), Mean (SD) | 22.2 (5.1) | 23.5 (4.0) | .3 |
| ESRD cause, n (%) | .06 | ||
| Glomerulonephritis | 1 (8.3) | 11, (8.7) | |
| Diabetic nephropathy | 1 (8.3) | 34, (26.8) | |
| Hypertension | 2 (16.7) | 30, (23.6) | |
| Polycystic kidneys | 0, (0) | 10, (7.9) | |
| Collagen vascular | 0, (0) | 3, (2.4) | |
| Others | 7, (58.3) | 14, (11) | |
| Unknown | 1 (8.3) | 25, (19.7) | |
| Comorbidities, n (%) | .97 | ||
| Diabetes mellitus | 0, (0) | 15, (12.3) | |
| Hypertension | 7, (58.3) | 64, (52.4) | |
| Cancer | 0, (0) | 3, (2.4) | |
| CAD | 0 (0) | 20, (16.4) | |
| CVA | 0 (0) | 2, (1.6) | |
| Others | 0 (0) | 12, (9.8) | |
| Without comorbidity | 4 (33.3) | 36, (29.5) | |
| Systolic blood pressure (mmHg), mean (SD) | 127.1 (16.0) | 138.9 (21.1) | .1 |
| Diastolic blood pressure (mmHg), Mean (SD) | 80 (16.7) | 83.7 (10.9) | .4 |
| PD duration (day), Mean (SD) | 2494.4 (940.9) | 1890.2 (598.8) | .002 |
| Mortality rate, n (%) | 7/12 (58.3) | 43/122 (35.24) | .1 |
| Total peritonitis episode | 38 | 145 | .001 |
| Peritonitis rate (patient month) | 1/26 | 1/53 | .01 |
| Outcome, n (%) | .05 | ||
| Recovery | 0 (0) | 1 (1.2) | |
| Stay on PD | 0 (0) | 23 (27.4) | |
| HD | 8 (88.9) | 33 (39.3) | |
| TX | 1 (11.1) | 27 (32.1) |
BMI: Body mass index; ESRD: End stage renal disease; CAD: Coronary artery disease; CVA: Celebrovascular accident; PD: Peritoneal dialysis; HD: Hemodialysis; TX: Kidney transplant.
None of our patients used icodextrin solution as this solution was started at 2014 in our center.
A total of 145 episodes of peritonitis were diagnosed during the total duration of 7686 patient months in our control group, giving an overall peritonitis rate of 1/53. This figure in EPS patients was 1/26 with a total of 38 episodes of peritonitis during the total duration of 997 patient months (p = .01).
Table 2 highlights the laboratory characteristics of these two groups at time of enrollment to PD and at time of EPS diagnosis. The time of EPS diagnosis was defined as the year EPS was diagnosed for EPS patients and the year of death/exit from PD or end of observation on March 31st, 2012 for control group. As it is indicated in the table, the mean age of EPS patients at time enrollment to PD was 32.75 years (17–58), this figure reached to 39.2 years (24–63) at time of EPS diagnosis which was significantly lower than mean age of control group in both time points of enrollment and exit (p = .0001). Other than age, the UFF duration was also significantly higher in EPS group (1440.9 days) compared to control group (985.3 days, p = .03).
Table 2.
Laboratory characteristics of EPS and control groups.
| At time of PD enrollment |
At time of EPS development |
|||||
|---|---|---|---|---|---|---|
| Characteristic | EPS group (n = 12) | Control group (n = 122) | p Values | EPS group (n = 12) | Control group (n = 122) | p Values |
| Age | 32.75 (10.8) | 49.61 (16.2) | .0001 | 39.2 (10.2) | 53.9 (16.3) | .0001 |
| FBS | 97.4 (29.7) | 119.5 (60) | .2 | 100.1 (23.9) | 129.3 (76.5) | .2 |
| Hb (g/dl), mean (SD) | 9.9 (1.9) | 10.5(1.8) | .3 | 10.1 (2.8) | 10.5 (1.8) | .4 |
| Ferritin (ng/ml), mean (SD) | 540.2(376) | 501.8 (396) | .7 | 711.4 (481) | 650 (864) | .8 |
| Albumin (g/dl), mean (SD) | 3.5 (0.5) | 3.89 (0.4) | .02 | 3.3 (0.6) | 3.4 (0.6) | .5 |
| PTH (pg/ml), mean (SD) | 178. 9 (117) | 118.3 (183) | .4 | 186.2 (232) | 78.9 (88) | .1 |
| TG (mg/dl),mean (SD) | 97.6 (43.2) | 170.3(129.8) | .1 | 140.2 (83.4) | 164 (112.7) | .5 |
| Cholesterol (mg/dl), mean (SD) | 171.7 (29.4) | 191.5 (55.7) | .2 | 146.6 (34.5) | 176.9 (50.8) | .04 |
| Na (meq/l), mean (SD) | 140.8 (3.80 | 140.7(3.8) | .9 | 138.3 (4) | 139.3 (4.8) | .5 |
| K (meq/l), mean (SD) | 4.5 (0.7) | 4.6 (0.9) | .8 | 4.1 (0.8) | 4.4(0.9) | .4 |
| Calcium (mg/dl), mean (SD) | 8.8 (1.2) | 9.2 (1.0) | .2 | 9.3 (1.9) | 9.5 (1.1) | .7 |
| Creatinine (mg/dl), mean (SD) | 8.2 (2.6) | 7.9(3.4) | .4 | 10.5 (3.8) | 9.6 93.2) | .3 |
| Phosphorus (mg/dl) | 4.3 (1.2) | 4.8 (1.5) | .2 | 4.5 (2) | 4.7 (1.6) | .6 |
| 24 h urine volume (ml), mean (SD) | 983.3(492) | 866.2 (660) | .6 | 233.3 (375) | 379 (603.7) | .4 |
| Creatinine clearance | ||||||
| Residual | 43.1 (45.4) | 35.3 (33.3) | .5 | 3.5 (5.3) | 11.9 (26.1) | .2 |
| Total | 92.8(46.7) | 81.6 (34.5) | .3 | 59.1 (16.2) | 63.5 (24.1) | .5 |
| Kt/v | ||||||
| Residual Kt/v | 0.4(0.3) | 0.8 (0.7) | .4 | 0.05 (0.1) | 0.2 (0.4) | .2 |
| Total Kt/v | 2.4 (0.4) | 2.24 (0.7) | .6 | 1.8 (0.5) | 1.8 (0.6) | .8 |
| nPCR | 0.85 (0.3) | 0.91 (0.2) | .4 | 0.66 (0.2) | 1.04(3.7) | .7 |
| GFR | 4.3 (3.8) | 4.2 (3.9) | .8 | 0.3 (0.5) | 1.0 (1.6) | .2 |
| 24 h UF, mean (SD) | 990.9 (755) | 946.5 (612) | .8 | 559 (478) | 891 (640) | .08 |
| Transport status, n (%) | – | – | .2 | – | – | .6 |
| Low | 1 (10) | 0 (0) | – | 1 (9.1) | 2 (1.7) | – |
| Low average | 1 (10) | 18 (19.4) | – | 0 (0) | 7 (5.9) | – |
| High average | 6 (60) | 54 (58.1) | – | 2 (18.2) | 42 (35.3) | – |
| High | 2 (20) | 21 (22.5) | – | 8 (72.7) | 68 (57.1) | – |
| UFF duration (day), mean (SD) | – | – | – | 1571 (896) | 974 (643) | .007 |
| UFF, n (%) | – | – | – | 11 (91.6) | 38 (68.8) | .08 |
| Usage of solution n3, n (%) | – | – | – | 39/122 (29.5) | 6/12 (50) | .1 |
| Solution n3 duration (day), mean (SD) | – | – | – | 449.6 (703) | 514.4 (700) | .7 |
FBS: fasting blood glucose; PTH: parathyroid hormon; GFR: glumerular filtration rate; UF: ultrafiltration; UFF: ultrafiltration failure.
EPS patients’ results
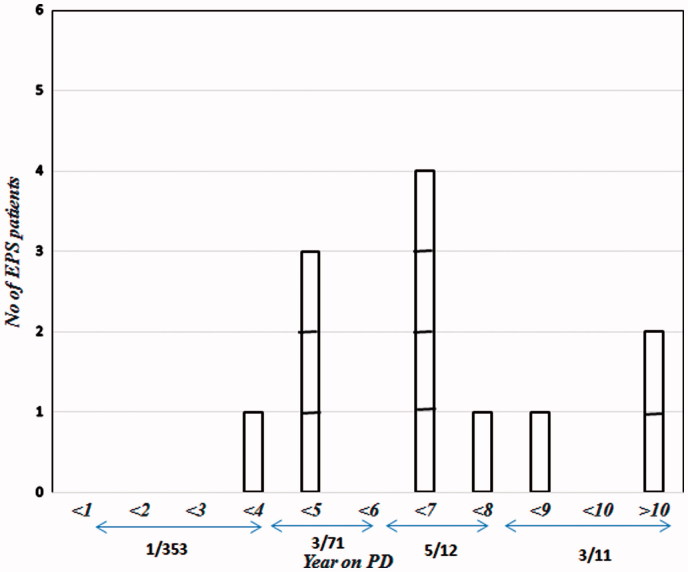
Figure 1 shows the number of EPS patients, and incidence of EPS according to the duration of PD. As it is shown, only one patient developed EPS during the first four years of PD, eight patients developed EPS between 5 and 10 years, and two patients had EPS after they had been on PD for over 10 years (Figure 1). In our observation, 8.6% of patients who stayed for at least four years on PD developed EPS. This figure rose to 10.76%, 23.3%, and 25% for patients who stayed at least 5, 6, and 7 years on PD, respectively. Although a mild decline in percentage of EPS development was observed in patients who used PD for at least eight years (21.4), which might be due to very low number of patients in this category.
Figure 1.
Distribution of the number of patients according to the duration of PD. EPS: encapsulating peritoneal sclerosis; PD: peritoneal dialysis.
Some demographic and clinical information of EPS patients at time of start of PD modality and at time of EPS presentation is shown in Table 3. It should be noted that, because the patient number 10 was paraplegic, he could not attend all the follow ups and therefore most of data for this patient is missing .There was a 43.5% decrease in overtime UF in EPS patients (p = .1). This decrease was 36.4% for CCl (p = .03), 29.4% for Kt/v (p = .008), 79.9% for 24 h urine volume (p = .001) and 93% for GFR (p = .006). The mortality rate was 100% among patients who developed EPS while were on PD.
Table 3.
Clinical characteristics of 12 patients with EPS at beginning of PD and at time of EPS.
| Pt nu. | Age | Sex | ESRD cause | T. peritonitis | PD. D (M) | UFF.D (M) | High transport (M) | Transport status 1 | Transport status 2 | UF1 | UF2 | T.CCl 1 | T.CCl 2 | T.Kt/V1 | T.Kt/V2 | Anuria (M) | RRT at time EPS | Β blocker | Death |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 58 | F | DM | 4 | 53 | 49.7 | 43 | HA | H | 1650 | 300 | 69.7 | 42.9 | 2.3 | 1.13 | 0 | PD | No | Yes |
| 2 | 32 | F | HTN | 1 | 73 | 46.6 | 47 | HA | H | 0 | 1300 | 70.4 | 65.6 | 2.6 | 1.6 | 6 | TX | Yes | No |
| 3 | 28 | M | GN | 3 | 87 | 45.6 | 44 | LA | H | 1000 | 1000 | 102.9 | 55 | 2.8 | 1.4 | 19 | TX | Yes | Yes |
| 4 | 32 | F | ch. Pyelon. | 1 | 96 | 91.3 | 53 | HA | H | 800 | −200 | 74.6 | 72.1 | 2.6 | 2.1 | 18 | HD | No | Yes |
| 5 | 27 | F | Reflux | 5 | 85 | 61.9 | 0 | H | L | 300 | 950 | 224.1 | 32 | 3.0 | 1.2 | 3 | TX | Yes | No |
| 6 | 40 | M | Trauma | 6 | 124 | 99.4 | 84 | – | H | – | −140 | 116 | 43.3 | 1.84 | 1.07 | 92 | PD | No | Yes |
| 7 | 43 | M | Reflux | 2 | 58 | 0 | 0 | HA | HA | 2000 | 600 | 71.79 | 48 | 2.05 | 1.3 | 0 | PD | No | Yes |
| 8 | 33 | F | ch. Pyelon. | 5 | 83 | 75.6 | 40 | L | HA | 1000 | 400 | 75.6 | 64 | 2.6 | 2.26 | 6 | PD | Yes | Yes |
| 9 | 24 | M | Unknown | 1 | 42 | 52.8 | 18 | HA | H | 750 | 100 | 64.9 | 69.6 | 2.1 | 2.2 | 36 | HD | No | No |
| 10 | 23 | M | Reflux | 3 | 151 | – | – | – | – | 0 | 800 | – | – | – | – | – | PD | No | Yes |
| 11 | 17 | M | Unknown | 4 | 42 | 41.1 | 41 | H | H | 2300 | 600 | 60.3 | 86.9 | 1.9 | 2.0 | 60 | HD | Yes | No |
| 12 | 36 | F | HTN | 3 | 46 | 12.1 | 12 | HA | H | 1100 | 1000 | 90.4 | 69.7 | 2.5 | 2.3 | 15 | HD | No | No |
Pt nu: patient number; ESRD: end stage renal disease; T. peritonitis: total peritonitis episodes; PD. D: peritoneal dialysis duration; UFF.D: ultrafiltration failure duration; Transport status1: Transport status at time of PD start; Transport status2: Transport status at time of EPS or exit from PD; H: high transporter; HA: high average transporter; LA: low average transporter; L: low transporter; UF1: 24 h ultrafiltration at time of PD start, UF2: 24 h ultrafiltration at time EPS or exit from PD; T.CCl1: total creatinin clearance at time of PD start; T.CCl2: total creatinin clearance at time EPS or exit from PD; RRT: Renal replacement therapy.
To assess the relation between multiple factors and EPS development, a logistic regression analysis was performed (Table 4). Our data showed that PD duration, age at PD start and duration of UFF were associated with EPS.
Table 4.
Logistic regression analysis.
| Variable | Odds | p Values | 95% CI |
|---|---|---|---|
| Age at peritoneal dialysis start | 0.92 | .02 | 0.9–1.9 |
| Duration of peritoneal dialysis | 1.02 | .02 | 1.00–2.03 |
| Ultrafiltration failure | 4.2 | .2 | 0.5–35 |
| Duration of ultrafiltration failure | 1.00 | .01 | 1.0–2.8 |
| Peritonitis | 1.9 | .9 | 0.2–7.5 |
| Episodes of peritonitis | 1.7 | .8 | 0.1–4.05 |
| High transporter | 1.5 | .5 | 0.4–5 |
| Usage of solution n3 (yes, no) | 2.2 | .2 | 0.6–7.7 |
| Solution n3 duration | 1.00 | .6 | 0.99–2.1 |
| Body mass index | 0.9 | .2 | 0.8–1.4 |
| Systolic blood pressure | 1.06 | .6 | 0.9–1.3 |
| Diastolic blood pressure | 1.02 | .9 | 0.9–1.4 |
| 24 h ultrafiltration | 1.01 | .2 | 0.99–4.3 |
| Total Kt/v | 7.5 | .08 | 0.8–73.9 |
| Total creatinine clearance | 0.9 | .1 | 0.8–1.02 |
Discussion
CKD is a major global public health problem that affects 8–16% of worldwide adult population. Progression of CKD toward ESRD produces a huge burden on health system and forces them to use one of renal replacement modality. PD offers the healthiest way for start of RRT in these patients and global data shows that this modality has a growing rate.7 Although chronic PD patients may face EPS as a serious complication of PD.
This study is a long-term observation of PD patients and patients who developed EPS, in two Iranian centers in which we had more comprehensive data and was done with goal of detailed demographic and biochemical description of EPS patients as well as investigation of the associations of risk factors with EPS development.
In our study, we found 12 patients of EPS among 464 PD patients, which gives us an overall prevalence of 2.6% in 17 years period. This number is in the higher range of what is reported previously in the literature (1.1–3.7%).12,13,26 Although recent data are pointing to an increase in prevalence of EPS compared to previous. For example, a recent study performed in an Italian center27 showed the EPS prevalence at 2.8% in a 34 years period, a number that is close to what was seen in our study. The increase in the prevalence of EPS in recent years might reflect both a longer PD exposure and the higher awareness of EPS.
The incidence of EPS in our study was 8.9% among those who stayed on PD for more than 3.5 years. This high incidence is in accordance with recently reported results from other studies. Gayomali and colleagues showed an incidence of 14.5% among patients stayed on PD >5 years.11 Brown et al. from Scottish Renal Registry reported an EPS rate of at least 8% after 4–5 years of PD exposure.14 However, report from Japanese PD showed a lower incidence (3.8%) for patients stayed on PD for 5–10 years.16 Many factors might be involved in seeing different incidence of EPS across different studies, such as the type of study (survey versus single center using pre-determined criteria), the patient selection criteria for PD, observation time, comorbidities, drugs, etc. Part of higher incidence of EPS in our study might be related to considerably higher period of time spending on PD in our study compared with those in other studies which could potentially greater the exposure risk for EPS development. Another reason might be related to the close monitoring and relatively reliable data that we had from these two centers.
In this study we deliberately did not match the case and control groups for any factors. This kind of study allowed us to evaluate the potential risk factors of EPS during follow-up and in relation to other risk factors. In this context, we compared the anthropometric and biochemical characteristics of two groups at two time points, one at time of PD initiation and one at time of EPS development or exit from PD. We observed that only factors with remarkable difference between two groups were age, PD duration, rate of peritonitis and UFF duration. Accordingly, lower age, PD duration, UFF duration, but not peritonitis nor high transporter showed themselves as independent risk factors when entered into a logistic regression model with EPS as the dependent variable.
Our EPS patients were significantly younger than our control group at time of PD start (by 16 years). Similarly, Yamamoto et al. reported an average age of 35.1 ± 3.3 years for EPS versus 47.3 ± 1.1 years for non EPS patients.28 A difference that reaches 12 years. Johnson et al. reported this difference by 8.9 years.29 Risk of younger age for EPS development in our study did not seem to be related to a longer PD duration. Similar to our study, Korte and colleagues in a multi-center case control study comprised of 63 patients with EPS and 126 control patients found that the younger the patient at the start of the PD, the greater the chance they had of developing EPS.30 They proposed the disrupted peritoneal fibrosis repair process in PD patients with younger age as possible cause of this observation. Mesothelial to mesenchymal transition, as the main mechanism in the induction of peritoneal fibrosis during exposure to PD fluids, represents a complex phenomenon of cellular transdifferentiation that converts the epithelial phenotype into a mesenchymal one, with loss of cell polarization, deconstruction of adherent and tight junctions, and ability to invade the inner layers.31 Whether younger aged patients present with more severe forms of EMT that prone them to an earlier form of peritoneal fibrosis and EPS needs to be investigated.
In this study, the UFF duration was a risk factor for EPS development. To our knowledge this is the first time that UFF duration is highlighted as a risk factor. A progressive reduction in ultrafiltration capacity as a consequence of peritoneal membrane fibrosis and sclerosis commonly occurs in PD patients, but our study showed that as the time of having UFF increases, the risk of EPS development elevates.
Several studies highlighted the association between peritonitis and EPS development,32,33 however in our study although both peritonitis rate and total peritonitis episodes were significantly different between EPS and control groups (Table 1) but neither of these factors were associated with EPS in regression analysis. In Dutch EPS study, Korte and colleagues could not find an association between peritonitis and EPS development. Similarly, Johnson et al. found no relationship between peritonitis frequency and EPS risk.29 Moreover, a single center study from united state indicated that peritonitis is not a prerequisite for the development of EPS.11 It is suggested that other factors related to peritonitis such as organism involved and/or the duration and severity of peritonitis are more effective factors in ESPS development than peritonitis. However, due to inaccuracy of our data for these factors we could not verify them in our study.
As in previous studies, incidence of EPS increased with time spending on PD. We observed that only 0.3% of PD patients developed EPS before four years of PD (1/353), while this figure reached to 4.2% (3/71), 41.6% (5/12) and 27.3% (3/11) for patients who stayed on PD for 4–6, 6–8, and >8 years, respectively. A multicenter survey in Japan showed the elevation of EPS incidence by increasing in the time staying on PD: 0.7%, 2.1%, 5.9%, 15.8%, and 17.2% at 5, 8, 10, 15, and more than 15 years, respectively.16 Similar increase in EPS incidence as a function of being on PD also reported by Rigby et al.15 It should be noted that we had one patient who developed EPS before four years (0.3%) being on PD which might indicate that under certain conditions EPS might develop earlier. Trigka et al.12 and Balasubramanian et al.18 reported an incidence of 14.3% and 11% in patients who had been on PD for less than three years. However, what factors could predispose PD patients to develop early EPS needs further evaluation.
We noticed that mortality rate among all EPS patients who were on PD when their EPS was diagnosed was 100%, while this figure in hemodialysis patients was 25%. This finding raise the hypothesis that early transfer of PD patients who are at increased risk of EPS might be useful in decreasing the mortality among them.
Although there was no significant difference between EPS and control group regarding the cause of ESRD, but only one of 12 EPS patients was diabetic. This observation confirms the previous observation that patients with diabetes rarely develop EPS, despite the fact that similar changes in the peritoneal membrane are present in diabetic patients even before PD treatment is started.34
We found it important to mention that none of our patients were using icodextrin as this PD solution was introduced to our health system on 2014, therefore we cannot comments on risk of using this solution for EPS development.
Conclusion
Our study was designed to compare the chronic PD patients who did and did not develop EPS looking at a large number of demographic and clinical parameters in order to identify independent predictors of EPS. Using regression analysis we found that longer time being on PD, younger age, and higher UFF duration were the risk factors for EPS development. This study is the first comprehensive report of EPS cases from Iran and is strengthen by using, relatively high number of patients in control group, comparability of cases and controls as the cases and the controls without EPS were taken from the same source population, using uniform criteria for diagnosis of EPS, as these two PD centers were supervised by one nephrologist, and for proposing the UFF duration as a risk factor for EPS. However, our study is limited by its retrospective nature and limited data collection such as regular PET for PD patients, severity of comorbidities, dialysate composition, and detailed peritonitis information. Further study for identification of biological markers of EPS for early detection of this disease certainly is needed.
Disclosure statement
The authors report no conflicts of interest.
References
- 1.Jha V, Garcia-Garcia G, Iseki K, et al. Chronic kidney disease: Global dimension and perspectives. Lancet. 2013;382:260–272. [DOI] [PubMed] [Google Scholar]
- 2.Collins AJ, Foley RN, Herzog C, et al. US renal data system 2010 annual data report. Am J Kidney Dis. 2011;57:e1–e526. [DOI] [PubMed] [Google Scholar]
- 3.Najafi I, Shakeri R, Islami F, et al. Prevalence of chronic kidney disease and its associated risk factors: The first report from Iran using both micro albuminuria and urine sediment. Arch Iran Med. 2012;15:70–75. [PubMed] [Google Scholar]
- 4.Hosseinpanah F, Kasraei F, Nassiri AA, Azizi F.. High prevalence of chronic kidney disease in Iran: A large population-based study. BMC Public Health. 2009;9:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anand S, Akter khanam M, Finkelstein F, Global Perspective of kidney disease In: Byham-Gray LD, Burrowes JD, Chertow GM, eds. Nutrition in Kidney Disease. 2nd ed New York: Springer Science + Business Media; 2014:11–23. [Google Scholar]
- 6.Jain AK, Blake P, Cordy P, Garg AX.. Global trends in rates of peritoneal dialysis. J Am Soc Nephrol. 2012;23:533–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Najafi Alatab S, Atabak S, et al. Seventeen years' experience of peritoneal dialysis in Iran: First official report of the Iranian peritoneal dialysis registry. Perit Dial Int. 2014;34:636–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gandhi VC, Humayun HM, Ing TS, et al. Sclerotic thickening of the peritoneal membrane in maintenance peritoneal dialysis patients. Arch Intern Med. 1980;140:1201–1203. [PubMed] [Google Scholar]
- 9.Pollock CA.Diagnosis and management of encapsulating peritoneal sclerosis. Perit Dial Int. 2002;21:S61–S66. [PubMed] [Google Scholar]
- 10.Garosi G, Di Paolo N.. Morphological aspects of peritoneal sclerosis. J Nephrol. 2001;14:S30–S38. [PubMed] [Google Scholar]
- 11.Gayomali C, Hussein U, Cameron SF, Protopapas Z, Finkelstein FO.. Incidence of encapsulating peritoneal sclerosis: A single-center experience with long-term peritoneal dialysis in the United States. Perit Dial Int. 2011;31:279–286. [DOI] [PubMed] [Google Scholar]
- 12.Trigka K, Dousdampanis P, Chu M, et al. Encapsulating peritoneal sclerosis: A single-center experience and review of the literature. Int Urol Nephrol. 2011;43:519–526. [DOI] [PubMed] [Google Scholar]
- 13.Bansal S, Sheth H, Siddiqui N, Bender FH, Johnston JR, Piraino B.. Incidence of encapsulating peritoneal sclerosis at a single U.S. university center. Adv Perit Dial. 2010;26:75–81. [PubMed] [Google Scholar]
- 14.Brown MC, Simpson K, Kerssens JJ, Mactier RA.. Scottish renal registry. Encapsulating peritoneal sclerosis in the new millennium: A national cohort study. Clin J Am Soc Nephrol. 2009;4:1222–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rigby RJ, Hawley CM.. Sclerosing peritonitis: The experience in Australia. Nephrol Dial Transplant. 1998;13:154–159. [DOI] [PubMed] [Google Scholar]
- 16.Kawanishi H, Kawaguchi Y, Fukui H, et al. Encapsulating peritoneal sclerosis in Japan: A prospective, controlled, multicenter study. Am J Kidney Dis. 2004;44:729–737. [PubMed] [Google Scholar]
- 17.Brown EA, Van Biesen W, Finkelstein F.. Length of time on peritoneal dialysis and encapsulating peritoneal sclerosis: Position paper for ISPD. Perit Dial Int. 2009;29:595–600. [PubMed] [Google Scholar]
- 18.Balasubramaniam G, Brown EA, Davenport A, et al. The Pan-Thames EPS study: Treatment and outcomes of encapsulating peritoneal sclerosis. Nephrol Dial Transplant. 2009;24:3204–3215. [DOI] [PubMed] [Google Scholar]
- 19.Slingeneyer A.Preliminary report on a cooperative international study on sclerosing encapsulating peritonitis. Contrib Nephrol. 1987;57:239–247. [DOI] [PubMed] [Google Scholar]
- 20.Dobbie JW.Serositis: Comparative analysis of histological findings and pathogenetic mechanisms in nonbacterial serosal inflammation. Perit Dial Int. 1993;13:256–269. [PubMed] [Google Scholar]
- 21.Moriishi M, Kawanishi H.. Icodextrin and intraperitoneal inflammation. Perit Dial Int. 2008;28:S96–S100. [PubMed] [Google Scholar]
- 22.Moriishi M, Kawanishi H, Tsuchiya S.. Impact on peritoneal membrane of use of icodextrin-based dialysis solution in peritoneal dialysis patients. Adv Perit Dial. 2006;22:24–28. [PubMed] [Google Scholar]
- 23.Lo WK, Chan KT, Leung AC, Pang SW, Tse CY.. Sclerosing peritonitis complicating prolonged use of chlorhexidine in alcohol in the connection procedure for continuous ambulatory peritoneal dialysis. Perit Dial Int. 1991;11:166–172. [PubMed] [Google Scholar]
- 24.Afthentopoulos IE, Passadakis P, Oreopoulos DG, Bargman J.. Sclerosing peritonitis in continuous ambulatory peritoneal dialysis patients: One center’s experience and review of the literature. Adv Ren Replace Ther. 1998;5:157–167. [DOI] [PubMed] [Google Scholar]
- 25.Yamahatsu A, Hamada C, Kaneko K, Io H, Nakata J, Tomino Y.. Long-term outcome of encapsulating peritoneal sclerosis (EPS) patients in a single center. Clin Exp Nephrol. 2015;19:961–967. [DOI] [PubMed] [Google Scholar]
- 26.Lee HY, Kim BS, Choi HY, et al. Sclerosing encapsulating peritonitis as a complication of long-term continuous ambulatory peritoneal dialysis in Korea. Nephrology (Carlton). 2003;8:S33–S39. [DOI] [PubMed] [Google Scholar]
- 27.Vizzardi V, Sandrini M, Zecchini S, Ravera S, Manili L, Cancarini G.. Encapsulating peritoneal sclerosis in an Italian center: Thirty year experience. J Nephrol. 2016;29:259–267. [DOI] [PubMed] [Google Scholar]
- 28.Yamamoto R, Nakayama M, Hasegawa T, et al. High-transport membrane is a risk factor for encapsulating peritoneal sclerosis developing after long-term continuous ambulatory peritoneal dialysis treatment. Adv Perit Dial. 2002;18:131–134. [PubMed] [Google Scholar]
- 29.Johnson DW, Cho Y, Livingston BE, et al. Encapsulating peritoneal sclerosis: Incidence, predictors, and outcomes. Kidney Int. 2010;77:904–912. [DOI] [PubMed] [Google Scholar]
- 30.Korte MR, Sampimon DE, Lingsma HF, et al. Risk factors associated with encapsulating peritoneal sclerosis in Dutch EPS study. Dutch Multicenter EPS Study. Perit Dial Int. 2011;31:269–278. [DOI] [PubMed] [Google Scholar]
- 31.Strippoli R, Moreno-Vicente R, Battistelli C, et al. Molecular mechanisms underlying peritoneal EMT and fibrosis. Stem Cells Int. 2016;2016:3543678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamamoto R, Otsuka Y, Nakayama M, et al. Risk factors for encapsulating peritoneal sclerosis in patients who have experienced peritoneal dialysis treatment. Clin Exp Nephrol. 2005;9:148–152. [DOI] [PubMed] [Google Scholar]
- 33.Lindic J, Rupnik AT, Tomazic J, et al. Encapsulating peritoneal sclerosis in patients on peritoneal dialysis in Slovenia. Ther Apher Dial. 2009;13:282–287. [DOI] [PubMed] [Google Scholar]
- 34.Lindic J, Bren AF, Ferluga D, Drinovec J, Gucek A, Hvala A.. Are there morphological changes of parietal peritoneum in diabetic and nondiabetic uremic patients prior to continuous ambulatory peritoneal dialysis? Artif Organs. 1990;14:285–287. [Google Scholar]