Abstract
Objective: There are limited data on AKI in sub-Saharan Africa. We aim to determine the incidence, characteristics and prognosis of AKI in Cameroon.
Patients and methods: A prospective study including all consenting acute admissions in the internal medicine and the ICU of a tertiary referral hospital in Cameroon from January 2015 to June 2016. Serum creatinine assay was done on admission, days 2 and 7 to diagnose AKI. For patients with AKI, serum creatinine was done on discharge, days 30, 60 and 90. AKI was defined according to the modified KDIGO 2012 criteria as an increase or decrease in serum creatinine of 3 mg/l or greater, or an increase of 50% or more from the reference value obtained at admission or the known baseline value. AKI severity was graded using KDIGO2012 criteria. Outcome measures were renal recovery, mortality and causes of death. Renal recovery was complete if serum creatinine between the first 90 days was less than baseline or reference, partial if less than diagnosis but not baseline or reference, no-recovery if creatinine did not decrease or if the patient remained on dialysis.
Results: Of the 2402 patients included, 536 developed AKI giving a global incidence of 22.3% and annual incidence of 15 per 100 patients-years. Of the 536 patients with AKI, 43.3% were at stage 3, 54.7% were males, median age was 56 years. Pre-renal AKI (61.4%) and acute tubular necrosis (28.9%) were the most frequent forms. Main etiologies were sepsis (50.4%) and volume depletion (31.6%). Renal outcome was unknown in 34% of patients. Of the 354 patients with known renal function at 3 months, 84.2% recovered completely, 14.7% partially and 1.1% progressed to CKD. Global mortality rate was 36.9% mainly due to sepsis.
Conclusions: AKI is frequent in our setting, mainly due to sepsis and hypovolemia. It carries a poor prognosis.
Keywords: Acute kidney injury, incidence, prognosis, Douala, Cameroon
Introduction
Acute kidney injury (AKI) a common worldwide problem is defined as a rapid decline or loss of kidney function. It is encountered in multiple settings but remains a poorly diagnosed disease in the world [1]. The global burden of AKI is estimated at 13.3 million cases per year, with 85% from low-middle-income countries (LMIC) [1,2]. Limited data on the incidence are available worldwide and the data vary widely across studies depending on the setting and the populations investigated. In a recent meta-analysis on world incidence of AKI, including 154 studies with only two from Africa, the reported incidence was 21% in adults and 33.7% in children [3].
In developed countries, hospital acquired AKI is the most frequent form with an incidence of 7–18% and preponderance for elderly patients [4–8]. In contrast, in low income countries (LIC) including Sub-Saharan Africa (SSA), AKI commonly occurs in the community, affecting mostly young adults and children [9–11]. The etiology of AKI in LMIC depend on geographical location and many are preventable. A recent meta-analysis by Wasiu et al. reported that the common causes of AKI in adults in SSA were infections (28%), nephrotoxins (18%), pregnancy related (16%), glomerular disease (8%) and hypovolemia (5%) [11]. The majority of AKI cases are therefore preventable or can be managed by simple measures. Despite advances in medical technique, AKI remains under diagnosed especially in SSA. When diagnosed late, AKI has adverse effects for the individual in general. It is associated with increased length of hospital stay and high cost [12]. The duration and severity of AKI is a risk factor for the development of complications such as a 10-fold increase risk of chronic kidney disease (CKD) and a 3-fold risk of end stage kidney disease (ESKD) [13–15]. Also, AKI is associated with a high risk of death with an annual mortality rate estimated to be greater than prostate cancer, breast cancer, heart failure and diabetes [1].
In SSA, the outcome of patients with AKI is very poor with an overall mortality of 32% in adults. This is extremely high compared to the pooled world mortality of 23.9%. This mortality increases with the severity of AKI which is estimated at 50–60% amongst patients requiring renal replacement therapy (RRT) and to 82% in those in need for dialysis who could not receive it [3,11]. This high mortality in SSA is due to the late presentation of patients with severe disease in the hospital, the non-availability of RRT and the inability to afford treatment as health care costs are covered by out of pocket payment in most SSA countries [10,11,16–20]. Data on the epidemiology of AKI in SSA in general and in Cameroon in particular are limited, but the prevalence of AKI is estimated to be higher than that in developed countries [3,21]. The incidence in this setting is difficult to know due to lack of national registries. Because AKI is associated with high mortality and treatment costly, identifying patients early and intervening to avoid RRT is necessary. Therefore, the objectives of the present study were to determine the incidence, characteristic and outcomes of AKI in Cameroon.
Patients and methods
Study setting
This study was conducted in the intensive care unit (ICU) and internal medicine (IM) ward of the Douala General hospital (DGH). This is the main tertiary reference hospital of the country and a teaching hospital with 320 beds located in the littoral region of Cameroon with approximately three million inhabitants. The hospital has the only public hemodialysis center of the region and therefore the referral hospital for patients with kidney disease. It has a central laboratory for all inpatient and outpatient samples.
Study design
This was an observational prospective study including all patients admitted in the two units from January 2015 to June 2016 (18 months). For each consenting patient, serum creatinine assay was done on admission, days 2 and 7 to diagnose AKI. For patients with AKI, serum creatinine assay was repeated on discharge, days 30, 60 and 90. Creatinine was measured using the Jaffe kinetic method with a spectrophotometer (BIOMERIEUX®, FRANCE) throughout the study period.
Other variables collected were: socio demographic information such as age and gender; clinical data including co morbidities, primary diagnosis, signs and symptoms, of AKI, length of hospital stay and outcomes. Outcomes measures were the need of dialysis, renal recovery and patient mortality.
Definition of operational terms
AKI was defined according to the modified KDIGO 2012 criteria [22] as an increase or decrease in serum creatinine of 0.3 mg/dl or greater, or an increase of 50% or more from the reference value obtained at admission or the known baseline value. AKI severity was graded using KDIGO 2012 criteria [23]. Sepsis was defined as the presence a systemic inflammatory response (fever >38 °C, high white cell count at presentation) an increased C-reactive protein level due to suspected or proven infection (by positive culture or tissue stain) caused by any pathogen or a clinical syndrome associated with a high probability of infection [24]. AKI was community-acquired if patients first presented to the hospital with AKI. Renal recovery was defined as complete if serum creatinine between the first 90 days was equal to or lower than baseline or reference value. Renal recovery was partial if serum creatinine was lower than diagnosis value but not to baseline or reference, and no-recovery if serum creatinine did not decrease or if the patient remained on dialysis.
The diagnosis of acute tubular necrosis was done based on medical history, presence of risk factors, urine indices when available and recovery with a polyuric phase. While for pre-renal AKI medical history, the presence of risk factors, urea/creatinine ratio >20 in the absence of confounders and urine indices when available were used. Obstructive AKI was diagnosed based on history of acute oliguria or anuria and presence of dilatation of urinary tract on ultrasound.
Nephrotoxic AKI was diagnosed based on a history of ingestion of known nephrotoxic drug(s) (NSAID, Angiotensin converting system inhibitors, Cisplatine, Aminoside, Iodine contrast) or an herbal concoction.
Hypertension was considered in any patients on blood pressure lowering medication or blood pressure greater than 140/90 mmHg, while hypotension was blood pressure less than 90/60 mmHg.
Ethical approval was obtained from the ethical committee board of the Douala University and administrative authorization from the DGH.
Statistical analysis
Data were analyzed using Stat view version 5.0 for windows (SAS Institute, Inc., IL, USA). Continuous variables were presented as mean ± standard deviation and/or as median (inter-quartile range). Categorical variable were expressed as percentages. Chi-squared test was used to compare categorical data and t-test or Mann–Whitney test to compare continuous data. A p values <.05 was considered significant.
Results
From January 2015 to June 2016, a total of 2402 patients were admitted with 580 from ICU and 1822 in IM of whom AKI occurred in 536. This corresponded to a global incidence of 22.3% and an annual incidence of 15 per 100 patients-years (Figure 1). Of the 536 patients with AKI, 54.7% were males and the median age was 56 years (14–95). Median length of stay was 8 days (1–54). Main comorbidities were: hypertension (32.2%), diabetes (17.6%), HIV infection (12.6%) and cancer (9.3%). A total of 6.1% (39/536) patients had a known history of CKD (Tables 1 and 2).
Figure 1.
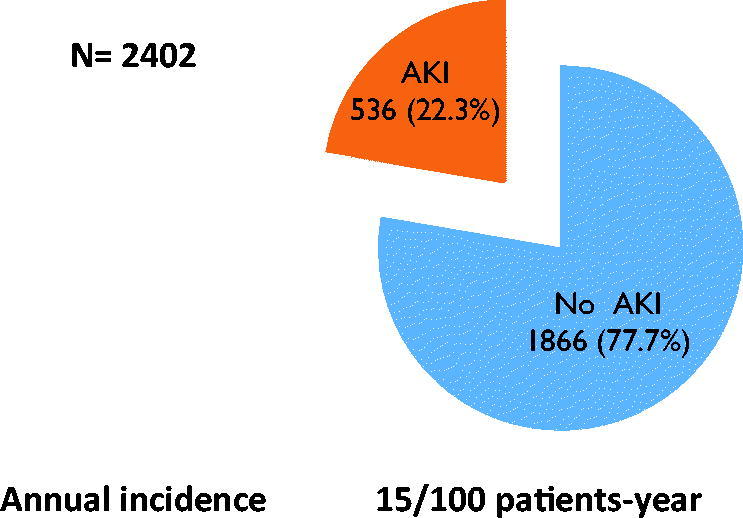
Incidence of AKI.
Table 1.
General characteristics of AKI patients.
| Variables | Total (%) N = 536 | Male (%) N = 293 | Female (%) N = 243 | p |
|---|---|---|---|---|
| Age (years) | ||||
| Mean ± SD | 54.71 ± 18.41 | 55.53 ± 17.39 | 53.72 ± 19.56 | .256 |
| Unit of hospitalization | ||||
| Internal medicine | 407 (75.90) | 230 (42.90) | 177 (33.00) | .170 |
| Intensive care unit | 129 (24.10) | 63 (11.80) | 66 (12.30) | |
| Length of hospital stay (IQ) | ||||
| Median (IQ) | 8 (1–41) | 8 (1–39) | 8 (1–41) | .909 |
| Comorbidity | ||||
| Hypertension | 205 (32.20) | 123 (19.30) | 82 (12.90) | .059 |
| Diabetes mellitus | 112 (17.60) | 64 (10.00) | 48 (7.60) | .584 |
| HIV | 80 (12.60) | 38 (5.90) | 42 (6.70) | .152 |
| Cancer | 59 (9.30) | 24 (3.80) | 35 (5.50) | 1.000 |
| Hepatitis B/C | 51 (8.00) | 38 (5.90) | 13 (2.10) | .003 |
| Heart failure | 47 (7.40) | 26 (4.10) | 21 (3.30) | .946 |
| Known CKD | 39 (6.10) | 26 (4.10) | 13 (2.00) | .096 |
| Liver cirrhosis | 22 (3.40) | 16 (2.50) | 6 (0.80) | .085 |
| Gout | 20 (3.10) | 15 (2.30) | 4 (0.80) | 1.000 |
| Systemic lupus | 2 (0.30) | 0 (0.00) | 2 (0.30) | 1.000 |
HIV: human immunodeficiency virus; CKD: chronic kidney disease.
Bold indicates the significant p values.
Table 2.
Clinical and biological characteristics of the study population.
| Variables | Total (%) | Male (%) | Female (%) | p |
|---|---|---|---|---|
| Urine dipstick on admission (n = 350) | ||||
| Proteinuria | 242 (43.50) | 124 (22.30) | 118 (21.20) | .067 |
| Hematuria/hemoglobinuria | 138 (24.80) | 63 (11.30) | 75 (13.50) | .003 |
| Leucocyturia | 140 (25.20) | 51 (9.20) | 89 (16.0) | <.001 |
| Nitrites | 36 (6.50) | 12 (2.20) | 24 (4.30) | .006 |
| Specific gravity (n = 350) | ||||
| Between 1010 and1020 | 85 (24.30) | 41 (11.70) | 44 (12.60) | |
| >1020 | 167 (47.70) | 108 (30.80) | 59 (16.90) | .023 |
| <1010 | 98 (28.00) | 45 (12.90) | 53 (15.10) | |
| Urine output on admission (n = 437) | ||||
| <100 ml/day | 183 (41.80) | 99 (22.60) | 84 (19.20) | .047 |
| 100–500 ml/day | 187 (42.80) | 88 (20.10) | 99 (22.70) | |
| 500–2500 ml/day | 58 (13.30) | 33 (7.60) | 25 (5.70) | |
| >2500 ml/day | 9 (2.10) | 7 (1.60) | 2 (0.50) | |
| Blood pressure (mmHg) | ||||
| Hypotension | 293 (54.70) | 169 (31.50) | 124 (23.20) | .350 |
| Normal | 184 (34. 30) | 94 (17.50) | 90 (16.80) | |
| Hypertension | 59 (11.00) | 30 (5.60) | 29 (5.40) | |
| Biology (mean ± SD) | ||||
| Urea (n = 536) | 1.29 ± 0.89 | 1.29 ± 0.97 | 1.28 ± 0.78 | .976 |
| Creatinine (n = 536) | 48.44 ± 52.76 | 47.25 ± 53.94 | 49.89 ± 51.38 | .565 |
| Hemoglobin (n = 531) | 9.49 ± 2.98 | 9.77 ± 3.17 | 9.15 ± 2.69 | .016 |
| White blood count (n = 531) | 12.98 ± 21.15 | 12.97 ± 23.48 | 12.99 ± 17.99 | .988 |
| CRP (n = 449) | 147.77 ± 112.68 | 146.03 ± 104.91 | 149.88 ± 121.65 | .727 |
| Sodium (n = 499) | 136.78 ± 9.27 | 136.72 ± 8.23 | 136.86 ± 9.80 | .626 |
| Potassium (n = 499) | 4.53 ± 1.25 | 4.52 ± 1.19 | 4.54 ± 1.31 | .861 |
| Bicarbonate (n = 83) | 18.57 ± 6.72 | 18.93 ± 7.41 | 18.18 ± 7.41 | .614 |
CRP: C-reactive protein.
Bold indicates the significant p values.
AKI was community-acquired in 93.3% (500/536) and 43.3% (232/536) had AKI stage 3 (Table 3). Pre-renal AKI with a frequency of 61.4% (329/536) and acute tubular necrosis 28.9% (155/536) were the most frequent forms. AKI was due to obstruction in 4.10% (22/536) of patients. Main etiologies of AKI were sepsis/bacterial infection, 310/536 (50.4%) and volume depletion 195/536 (31.6%). Nephrotoxins accounted for 62/536 (10.1%), pelvic tumor for 3.0% (18/536) and gynecologic/obstetric causes for 7.1% (38/536). The main sources of infection were urinary 25.1% (88/310), digestive 21.5% (75/310) and pulmonary 18.3% (64/310) (Table 4). Conversely, volume depletion was mainly due to hemorrhage 23.7% (48/186), gastroenteritis 23.1% (47/186), decompensated diabetes 21.7% (44/186) and heart failure 17.7% (36/186) (Table 4). Main causes of obstetric AKI were cervical cancer (34.2%), eclampsia (28.8%) and post-partum hemorrhage (21.1%) while herbal remedies (32.3%) were the main toxins (Table 5).
Table 3.
Nature, mechanism, severity and etiology of AKI amongst participants.
| Variables | Total (%) | Male (%) | Female (%) | p |
|---|---|---|---|---|
| Nature | ||||
| Community acquired | 500 (93.30) | 278 (51.90) | 222 (41.40) | .099 |
| Hospital acquired | 36 (6.70) | 15 (2.80) | 21 (3.90) | |
| Mechanism | ||||
| Pre renal | 329 (61.40) | 195 (36.40) | 134 (25.00) | .004 |
| Acute tubular necrosis | 155 (28.90) | 84 (15.70) | 71 (13.20) | |
| Glomerular | 6 (1.10) | 2 (0.40) | 4 (0.70) | |
| Interstitial | 14 (2.60) | 3 (0.60) | 11 (2.00) | |
| Vascular | 10 (1.90) | 3 (0.60) | 7 (1.30) | |
| Obstructive | 22 (4.10) | 6 (1.10) | 16 (3.00) | |
| Severity AKI | ||||
| 1 | 176 (32.80) | 114 (21.30) | 62 (11.50) | .005 |
| 2 | 128 (23.90) | 65 (12.10) | 63 (11.80) | |
| 3 | 232 (43.30) | 114 (21.30) | 118 (22.00) | |
| Etiologies | ||||
| Sepsis/bacterial infection | 310 (50.40) | 173 (28.10) | 137 (22.30) | |
| Volume depletion | 195 (31.60) | 109 (17.70) | 86 (13.90) | |
| Nephrotoxins | 62 (10.10) | 40 (6.50) | 22 (6.40) | |
| Obstruction | 22 (3.60) | 5 (0.80) | 17 (2.80) | |
| Eclampsia/HELLP | 11 (1.80) | 0 (0.00) | 11 (1.80) | |
| Multiple Myeloma | 5 (0.80) | 3 (0.50) | 2 (0.30) | |
| Malignant hypertension | 5 (0.80) | 3 (0.30) | 2 (0.20) | |
| Systemic lupus | 3 (0.50) | 0 (0.00) | 3 (0.50) | |
| Lymphoma | 1 (0.20) | 1 (0.20) | 0 (0.00) | |
| Unknown | 1 (0.20) | 1 (0.20) | 0 (0.00) | |
Bold indicates the significant p values.
Table 4.
Causes of sepsis/bacterial infection, volume depletion and obstruction amongst the study population.
| Variables | Total (%) | Male (%) | Female (%) | p |
|---|---|---|---|---|
| Sepsis/bacterial infection | 350 | 190 (54.30) | 160 (45.70) | |
| Urinary | 88 (25.10) | 40 (11.40) | 48 (13.70) | |
| Digestive | 75 (21.50) | 47 (13.40) | 28 (8.10) | |
| Pulmonary | 64 (18.30) | 38 (10.90) | 26 (7.40) | .403 |
| Unknown origin | 49 (14.00) | 30 (8.60) | 19 (5.40) | |
| Cutaneous | 37 (10.60) | 19 (5.40) | 18 (5.20) | |
| Meningitis | 18 (5.10) | 5 (1.40) | 13 (3.70) | |
| Septicemia | 13 (3.70) | 9 (2.60) | 4 (1.10) | |
| Gynecology | 4 (1.10) | 0 (0.00) | 4 (1.10) | |
| Endocarditis | 1 (0.30) | 1 (0.30) | 0 (0.00) | |
| Chronic otitis | 1 (0.30) | 1 (0.30) | 0 (0.00) | |
| Volume depletion | 203 | 112 (55.20) | 91 (44.80) | |
| Hemorrhage | 48 (23.70) | 27 (13.30) | 21 (10.40) | |
| Gastroenteritis | 47 (23.10) | 26 (12.70) | 21 (10.40) | |
| Diabetes mellitus | 44 (21.70) | 21 (10.40) | 23 (11.30) | .701 |
| Heart failure | 36 (17.70) | 19 (9.40) | 17 (8.30) | |
| Liver cirrhosis | 14 (6.80) | 9 (4.40) | 5 (2.40) | |
| Diuretics | 6 (2.90) | 5 (2.40) | 1 (0.50) | |
| Burn | 4 (2.00) | 3 (1.50) | 1 (0.50) | |
| Intestinal occlusion | 2 (1.00) | 1 (0.50) | 1 (0.50) | |
| NSAID | 1 (0.50) | 1 (0.50) | 0 (0.00) | |
| Unspecified | 1 (0.50) | 0 (0.50) | 1 (0.00) | |
| Obstructive AKI | 22 | 5 (22.70) | 17 (77.30) | |
| Pelvic tumor | 18 (81.80) | 5 (22.70) | 13 (59.10) | |
| Cervical cancer | 13 (2.10) | 0 (0.00) | 13 (2.10) | |
| Prostate cancer | 4 (0.70) | 4 (0.70) | 0 (0.00) | |
| BPH | 1 (0.20) | 1 (0.20) | 0 (0.00) | .004 |
| Ureters ligation | 2 (9.20) | 0 (0.00) | 2 (9.20) | |
| Retroperitoneal ibrosis | 1 (4.50) | 0 (0.00) | 1 (4.50) | |
| Renal stones | 1 (4.50) | 0 (0.00) | 1 (4.50) |
NSAID: non-steroidal anti-inflammatory drugs; BPH: benign prostatic hyperplasia.
Bold indicates the significant p values.
Table 5.
Nephrotoxins and obstetrical/gynecological causes of AKI amongst study population.
| Variables | Total (%) | Male (%) | Female (%) | p |
|---|---|---|---|---|
| Nephrotoxins | 62 (100%) | 40 (64.50) | 22 (35.50) | .836 |
| Herbal remedies | 20 (32.30) | 13 (21.00) | 7 (11.30) | |
| Unspecified drugs | 11 (17.80) | 7 (11.30) | 4 (6.50) | |
| Rhabdomyolysis | 9 (14.50) | 7 (11.30) | 2 (3.20) | |
| Black water fever | 8 (12.90) | 6 (9.70) | 2 (3.20) | |
| Tumor lysis syndrome | 5 (8.10) | 3 (4.90) | 2 (3.20) | |
| Hemolysis, others | 3 (4.80) | 1 (1.60) | 2 (3.20) | |
| Iodine contrast product | 3 (4.80) | 2 (3.20) | 1 (1.60) | |
| Amphotericine B | 2 (3.20) | 0 (0.00) | 2 (3.20) | |
| ACE inhibitors | 1 (1.60) | 1 (1.60) | 0 (0.00) | |
| Obstetric or gynecology causes | 38 (100) | 0 (0.00) | 38 (100.00) | |
| Cervical cancer | 13 (34.20) | 0 (0.00) | 13 (34.20) | |
| Eclampsia/HELLP | 11 (28.80) | 0 (0.00) | 11 (28.80) | |
| Post-partum hemorrhage | 8 (21.10) | 0 (0.00) | 8 (21.10) | |
| Septic abortion | 2 (5.30) | 0 (0.00) | 2 (5.30) | |
| Endometritis | 2 (5.30) | 0 (0.00) | 2 (5.30) | |
| Bilateral ureters ligation post caesarian section | 2 (5.30) | 0 (0.00) | 2 (5.30) |
ACE: angiotensin converting enzyme.
Amongst the 54 (10.1%) patients with indication for dialysis, 15 (2.8%) could not receive the treatment due to lack of appropriate equipment and denial (Table 6). Renal recovery could not be determined in 34% (182/536) either because the patient died in the acute phase (137/182) or was lost to follow-up (45/182). Of the 354 patients with known renal function at 3 months, 84.2% (298/354) recovered completely, 14.7% (52/354) partially and 1.1% (4/354) progressed to CKD (Table 6). At 3 months a total of 198 patients died giving a global mortality rate of 36.9%. Sepsis was the leading cause of death in 56.9% (119/209) (Table 7).
Table 6.
Management and outcomes of AKI patients.
| Variables | Total (%) | Male (%) | Female (%) | p |
|---|---|---|---|---|
| Use of vasoactive drugs | 71 (13.20) | 39 (7.30) | 32 (5.90) | .988 |
| Blood transfusion | 145 (27.10) | 72 (13.50) | 73 (13.60) | .202 |
| Dialysis indication | 54 (10.10) | 26 (4.90) | 28 (5.20) | |
| Dialysis done | 39 (7.30) | 15 (2.30) | 24 (5.00) | .296 |
| Reasons for non-realization of dialysis (n = 15) | ||||
| Inadequate equipment | 10 (66.80) | 7 (46.80) | 3 (20.00) | 1.000 |
| Denial | 3 (20.00) | 3 (20.00) | 0 (10.00) | 1.000 |
| Early death | 2 (13.20) | 1 (6.60) | 1 (6.60) | 1.000 |
| Renal recovery at 3 months known (n = 354) | ||||
| Complete recovery | 298 (84.20) | 158 (44.60) | 140 (39.60) | .613 |
| Partial recovery | 52 (14.70) | 30 (8.50) | 22 (6.20) | |
| No recovery | 4 (1.10) | 4 (1.10) | 0 (0.00) | |
| Unknown (n = 182) | ||||
| Early death | 137 (75.30) | 78 (42.80) | 59 (32.50) | .613 |
| Loss to follow-up | 45 (24.70) | 23 (12.60) | 22 (12.10) | |
| Patients outcomes | ||||
| Survival | 269 (50.20) | 144 (26.90) | 125 (23.30) | .502 |
| Death | 198 (36.90) | 112 (20.90) | 86 (16.00) | |
| Loss to follow-up | 69 (12.90) | 37 (6.90) | 32 (6.00) | |
Table 7.
Presumed causes of death in the study population.
| Variables | Total n = 209 | Male n = 112 | Female n = 86 | p | |
|---|---|---|---|---|---|
| Presumed causes of death | |||||
| Sepsis | 119 (56.90) | 67 (32.00) | 52 (24.90) | .328 | |
| Multi organ dysfunction | 22 (10.60) | 11 (5.30) | 11 (5.30) | ||
| Hemorrhage | 19 (9.10) | 11 (5.30) | 8 (3.80) | ||
| Cancer | 18 (8.60) | 9 (4.30) | 9 (4.30) | ||
| Liver cirrhosis | 9 (4.30) | 8 (3.80) | 1 (0.50) | ||
| Pulmonary embolism | 8 (3.80) | 4 (1.90) | 4 (1.90) | ||
| Cardiogenic shock | 8 (3.80) | 3 (1.40) | 5 (2.40) | ||
| Absence of dialysis | 5 (2.40) | 3 (1.40) | 2 (1.00) | ||
| Eclampsia | 1 (0.50) | 0 (0.00) | 1 (0.50) | ||
Discussion
To the best of our knowledge, no study on AKI has been performed prospectively in our country. This is the first study to determine the incidence of AKI in Cameroon. The results showed that AKI is frequent with a global incidence of 22.3% and annual incidence of 15/100 patient-year. AKI affects young adults, is mostly community acquired (93.3%) and stage 3 accounts for almost half of case. Pre-renal AKI and acute tubular necrosis from sepsis were the most frequent forms. Dialysis was indicated in 54 patients, but was not done in 15 patients due to lack of adequate materials. Renal outcome was unknown in 1/3 of participants. In those with known renal function, recovery was complete in more than 4/5 and more than 1/3 patients died.
Incidence of AKI
AKI is a public health problem worldwide and in SSA in particular. It is a potentially preventable and reversible disease, often asymptomatic and is a common condition amongst hospitalized patients. In the present study, the global incidence of AKI was 22.3% and an annual incidence of 15/100 patients-years.
Worldwide incidence varies widely across studies depending on the setting and the populations investigated. Data on the incidence of AKI in SSA are very scanty due to lack of registries. Our result was higher compared to the recent reported pooled world incidence of 21% in adults [3] and also much higher as the results of recent hospital based studies in the developed world that reported AKI in 3.2–9.6% of admissions [25,26]. In our study, 93.3% of AKI cases were community acquired. This is in accordance with reported findings in LIC where contrary to developed countries, AKI is mostly community acquired and affects more young adults and children [9,11,27]. One explanation of this figure may be the underlying causes of AKI in LIC such as sepsis, hypovolemia due to diarrhea and nephrotoxic drugs. All these factors are prevalent in the community [11,17,28]. Almost half of our patients were at AKI stage 3 with various symptoms; this is mainly due to the severity of the underlying disease and late presentation of patients in our hospital that is a reference hospital for severe medical cases in the region. Our result is in accordance with reports in SSA [11].
Etiology of AKI
The etiology of AKI in LMIC is dependent on geographical location. A recent meta-analysis from Wasiu et al. reported that the two leading causes in SSA were infections and nephrotoxin [11]. In the present study, pre-renal AKI (61.4%) and acute tubular necrosis (28.9%) were the most frequent forms. Sepsis from bacterial infection was the main precipitating factor followed by hypovolemia mainly from dehydration and hemorrhage. Similar findings were reported in developed and developing countries [18,29–33]. The main source of infection in our study population was the urinary tract (88/310), followed by gastroenteritis (75/310) and pneumonia (64/310). These three sources have been reported in the literature but with differences in proportion [34–36]. Pneumonia was the leading cause in the study of Madav et al. in Nepal followed by gastroenteritis and urinary tract infection [35]. In contrast, gastroenteritis was the first etiology in the studies of Praskash et al. (60%) in India and Arogundade et al. (36.9%) in Nigeria [17,28]. Toxic AKI is common in SSA with a global prevalence of 18% [11]. We found that 62 (10.1%) cases of AKI were due to nephrotoxins mainly herbal remedies (20 patients). This is similar to reported findings in LIC [27,37,38]. We found 22 cases (4.10%) of obstructive AKI, similar to the reported prevalence in SSA (5%), of Singhal et al. (5%) and Nagamani et al. (4%) [11,32,39]. In SSA, the major causes of obstruction are renal stone, prostate hypertrophy and less malignancy [11]. In our study, malignancy mainly solid pelvic cancer (18 cases) was the leading cause of obstruction. This high proportion of cancer can be explained by the fact that our hospital has a referral center for oncology included in the internal medicine ward and patients are usually referred at late stage of the disease in that setting with complications [40]. The estimated prevalence of obstetric AKI in SSA is 16% mainly due to septic abortion, eclampsia and post-partum hemorrhage [11]. With the improved maternal care, the incidence has decreased. We found an incidence of obstetric AKI of 7.1% (38 patients) with eclampsia (11 patients) post-partum hemorrhage (eight cases) and sepsis (four patients) being the major causes. This is similar to other studies in SSA [30,41].
Dialysis and outcome
The pooled rate of dialysis requirement for AKI in the world was estimated at 2.3% [3]. In total, 10.1% (54/536) of our participants needed dialysis and this is much lower compared to most studies in SSA that reported 70% of adults in need of dialysis [11]. One major explanation of the low rate of dialysis need in the present study is that contrary to studies in SSA, this was a prospective study based on incidence. Patients were screened on admission and therefore AKI was diagnosed and managed earlier reducing the need of dialysis. Dialysis could not be realized in 15 patients (2.8%), patients mostly due to lack of adequate materials. Scarcity of resources is already known as a frequent reason for not providing dialysis in LIC [10,17,22,30,42]. For patients with known renal function at 3 months, full renal recovery was achieved in 298 (84.2%), partial in 52 (14.7%) and four (1.1%) progressed to CKD. Renal outcome is not routinely reported in studies from SSA and the true rate is unknown. Tariq et al. reported higher rate of full recovery (92.5%), partial (7%) and no recovery in 0.6% of patients [29]. Renal recovery was estimated at 55% in adult in the study of Wasiu et al. with 13% of patients with residual CKD [11]. In contrast our renal recovery rate was extremely high compared to one study in the same setting that reported full recovery only in 55.4% of patient [30]. This difference can be explained by the retrospective nature of that study and the small sample size (108 patients). Renal recovery could not be determined in 182 (34%) patients either because the patient died in the acute phase or was loss of follow-up similar to finding of Tariq et al. [29].
Despite advances in medical techniques, AKI is relatively common and still linked to adverse outcomes such as high in-hospital and long-term mortality rate [25,26,43] with a high risk for CKD development in those who survived [14]. The mortality rate in this study was estimated at 36.9% and this is extremely high compared to the pooled world AKI-associated all-cause mortality of 23.9% [3]. But our rate is comparable to the findings in SSA where the pooled rate in adults was estimated at 32% [11]. The high mortality rate could be explained mostly by the severity of the underlying disease and also the late referral and presentation of patients to the hospital with severe disease as HGD is the main tertiary referral hospital in Cameroon. The reasons for late presentation could be the silent evolution of AKI and especially the lack of funds as medical costs are covered by out of pocket payment for the majority in our setting and in most countries in SSA [16,17,20,42]. The high mortality rate associated with AKI is alarming. The International Society of Nephrology has set a goal ‘0 by 25’ that zero people should die of untreated AKI in the poorest parts of the world by 2025 (1, 2) and implementation of this program in low-resource settings will surely reduce the burden of AKI. In this study, sepsis from bacterial infection was responsible of more than half of death. This is in accordance with other findings in the literature where sepsis is reported as the major cause of death among patients with AKI especially in ICU [35,44]. Studies have reported significantly worse outcomes of patients with sepsis and AKI compared to non-septic AKI or sepsis alone [36,34,45–47].
Strength and limitations
There are limited data addressing the epidemiology and causes of AKI in LIC. To our knowledge this is the first study that provides a convenience sample of the incidence of AKI in Cameroon. One limitation is that the study was carried out in a single referral tertiary hospital. Furthermore, urine indices were not recorded. But given the prospective nature of data collection and the population studied, we assume that these results are representative. It will bridge the gap of knowledge on AKI incidence and outcome in SSA, raise awareness of AKI and provide caregivers with knowledge to identify and adequately manage patients at risk. Consequently, preventable deaths associated with AKI could be reduced in our setting.
Conclusions
Data on incidence of AKI in SSA are scanty and this study showed that AKI is a frequent condition in Cameroon; it is mostly community-acquired from bacterial infections and hypovolemia. Most patients recovered their renal function, but mortality rate was extremely high mainly due to sepsis. Prevention remains the key to reduce incidence and mortality due to AKI. This result may help health care providers for service planning and provide information to them for early action to prevent deterioration of renal function in hospitalized patients. The poor prognosis reported raises the need of implementing effective programs for AKI prevention, early detection and treatment in SSA and also determine factors contributing to this poor outcome as most etiologies of AKI in this setting are preventable.
Acknowledgements
We thank all the patients who participated in this study and the staff of the ICU and Internal medicine ward.
Disclosure statement
The authors declare no conflict of interest.
References
- 1.Lewington AJ, Cerda J, Mehta RL.. Raising awareness of acute kidney injury: a global perspective of a silent killer. Kidney Int. 2013;84:457–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lameire NH, Bagga A, Cruz D, et al. Acute kidney injury: an increasing global concern. Lancet. 2013;382:170–179. [DOI] [PubMed] [Google Scholar]
- 3.Susantitaphong P, Cruz DN, Cerda J, et al. World incidence of AKI: a meta-analysis. Clin J Am Soc Nephrol. 2013;8:1482–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nash K, Hafeez A, Hou S.. Hospital-acquired renal insufficiency. Am J Kidney Dis. 2002;39:930–936. [DOI] [PubMed] [Google Scholar]
- 5.Thomas M, Sitch A, Dowswell G.. The initial development and assessment of an automatic alert warning of acute kidney injury. Nephrol Dial Transplant. 2010;26:2161–2168. [DOI] [PubMed] [Google Scholar]
- 6.Uchino S, Bellomo R, Goldsmith D, et al. An assessment of the RIFLE criteria for acute renal failure in hospitalized patients. Crit Care Med. 2006;34:1913–1917. [DOI] [PubMed] [Google Scholar]
- 7.Wonnacott A, Meran S, Amphlett B, et al. Epidemiology and outcomes in community-acquired versus hospital-acquired AKI. Clin J Am Soc Nephrol. 2014;9:1007–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yilmaz R, Erdem Y.. Acute kidney injury in the elderly population. Int Urol Nephrol. 2010;42:259–271. [DOI] [PubMed] [Google Scholar]
- 9.Ibrahim A, Ahmed MM, Kedir S, et al. Clinical profile and outcome of patients with acute kidney injury requiring dialysis – an experience from a haemodialysis unit in a developing country. BMC Nephrol. 2016;17:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jha V, Parameswaran S.. Community-acquired acute kidney injury in tropical countries. Nat Rev Nephrol. 2013;9:278–290. [DOI] [PubMed] [Google Scholar]
- 11.Olowu WA, Niang A, Osafo C, et al. Outcomes of acute kidney injury in children and adults in sub-Saharan Africa: a systematic review. Lancet Glob Health. 2016;4:e242–e250. [DOI] [PubMed] [Google Scholar]
- 12.Chertow GM, Burdick E, Honour M, et al. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365–3370. [DOI] [PubMed] [Google Scholar]
- 13.Chawla LS, Amdur RL, Amodeo S, et al. The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney Int. 2011;79:1361–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coca SG, Singanamala S, Parikh CR.. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2012;81:442–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishani A, Nelson D, Clothier B, et al. The magnitude of acute serum creatinine increase after cardiac surgery and the risk of chronic kidney disease, progression of kidney disease, and death. Arch Intern Med. 2011;171:226–233. [DOI] [PubMed] [Google Scholar]
- 16.Anochie IC, Eke FU.. Acute renal failure in Nigerian children: Port Harcourt experience. Pediatr Nephrol. 2005;20:1610–1614. [DOI] [PubMed] [Google Scholar]
- 17.Arogundade F, Sanusi A, Okunola O, et al. Acute renal failure (ARF) in developing countries: which factors actually influence survival. Cent Afr J Med. 2006;53:34–39. [DOI] [PubMed] [Google Scholar]
- 18.Chijioke A, Makusidi A.. Severe acute kidney injury in adult Nigerians from university of Ilorin teaching hospital, Ilorin, Kwara state. BOMJ. 2011;8:1. [Google Scholar]
- 19.Obiagwu PN, Abdu A.. Peritoneal dialysis vs. haemodialysis in the management of paediatric acute kidney injury in Kano, Nigeria: a cost analysis. Trop Med Int Health. 2015;20:2–7. [DOI] [PubMed] [Google Scholar]
- 20.Olowu WA.Renal failure in Nigerian children: factors limiting access to dialysis. Pediatric Nephrol. 2003;18:1249–1254. [DOI] [PubMed] [Google Scholar]
- 21.Mehta RL, Cerda J, Burdmann EA, et al. International Society of Nephrology's 0by25 initiative for acute kidney injury (zero preventable deaths by 2025): a human rights case for nephrology. Lancet. 2015;385:2616–2643. [DOI] [PubMed] [Google Scholar]
- 22.Mehta RL, Burdmann EA, Cerdá J, et al. Recognition and management of acute kidney injury in the International Society of Nephrology 0by25 Global Snapshot: a multinational cross-sectional study. Lancet. 2016;387:2017–2025. [DOI] [PubMed] [Google Scholar]
- 23.Khwaja A.KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120:c179–c184. [DOI] [PubMed] [Google Scholar]
- 24.Goldstein B, Giroir B, Randolph A.. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatric Critical Care Med. 2005;6:2–8. [DOI] [PubMed] [Google Scholar]
- 25.Fang Y, Ding X, Zhong Y, et al. Acute kidney injury in a Chinese hospitalized population. Blood Purif. 2010;30:120–126. [DOI] [PubMed] [Google Scholar]
- 26.Lafrance JP, Miller DR.. Acute kidney injury associates with increased long-term mortality. J Am Soc Nephrol. 2010;21:345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jha V, Rathi M.. Natural medicines causing acute kidney injury. Semin Nephrol. 2008;28:416–428. [DOI] [PubMed] [Google Scholar]
- 28.Prakash J, Tripathi K, Malhotra V, et al. Acute renal failure in eastern India. Nephrol Dial Transplant. 1995;10:2009–2012. [PubMed] [Google Scholar]
- 29.Ali T, Khan I, Simpson W, et al. Incidence and outcomes in acute kidney injury: a comprehensive population-based study. J Am Soc Nephrol. 2007;18:1292–1298. [DOI] [PubMed] [Google Scholar]
- 30.Fouda H, Ashuntantang G, Halle M, et al. The epidemiology of acute kidney injury in a Tertiary Hospital in Cameroon: a 13 months review. J Nephrol Ther. 2016;6:2161. [Google Scholar]
- 31.Metcalfe W, Simpson M, Khan I, et al. Acute renal failure requiring renal replacement therapy: incidence and outcome. QJM. 2002;95:579–583. [DOI] [PubMed] [Google Scholar]
- 32.Nagamani R, Sudarsi K, Amaravati K, et al. A study on clinical profile of acute kidney injury. Ratio. 2015;8:3. [Google Scholar]
- 33.Prakash J, Singh TB, Ghosh B, et al. Changing epidemiology of community-acquired acute kidney injury in developing countries: analysis of 2405 cases in 26 years from Eastern India. Clin Kidney J. 2013;6:150–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bagshaw SM, Uchino S, Bellomo R, et al. Septic acute kidney injury in critically ill patients: clinical characteristics and outcomes. Clin J Am Soc Nephrol. 2007;2:431–439. [DOI] [PubMed] [Google Scholar]
- 35.Ghimire M, Pahari B, Sharma SK, et al. Outcome of sepsis-associated acute kidney injury in an intensive care unit: an experience from a tertiary care center of central Nepal. Saudi J Kidney Dis Transplant. 2014;25:912. [DOI] [PubMed] [Google Scholar]
- 36.Hoste EA, Lameire NH, Vanholder RC, et al. Acute renal failure in patients with sepsis in a surgical ICU: predictive factors, incidence, comorbidity, and outcome. J Am Soc Nephrol. 2003;14:1022–1030. [DOI] [PubMed] [Google Scholar]
- 37.Adelekun TA, Ekwere TR, Akinsola A.. The pattern of acute toxic nephropathy in Ife, Nigeria. West Afr J Med. 1999;18:60–63. [PubMed] [Google Scholar]
- 38.Luyckx VA, Steenkamp V, Stewart MJ.. Acute renal failure associated with the use of traditional folk remedies in South Africa. Ren Fail. 2005;27:35–43. [PubMed] [Google Scholar]
- 39.Singal, et al. Clinical profile of acute renal failure. JAPI. 2002;50:71–73. [Google Scholar]
- 40.Halle MP, Toukep LN, Nzuobontane SE, et al. The profile of patients with obstructive uropathy in Cameroon: case of the Douala General Hospital. Pan Afr Med J. 2016;23:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hachim K, Badahi K, Benghanem M, et al. Obstetrical acute renal failure. Experience of the nephrology department, Central University Hospital ibn Rochd, Casablanca. Nephrologie. 2001;22:29–31. [PubMed] [Google Scholar]
- 42.Chijioke A, Makusidi A, Rafiu M.. Factors influencing hemodialysis and outcome in severe acute renal failure from Ilorin, Nigeria. Saudi J Kidney Dis Transplant. 2012;23:391. [PubMed] [Google Scholar]
- 43.Murugan R, Kellum JA.. Acute kidney injury: what’s the prognosis? Nat Rev Nephrol. 2011;7:209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neveu H, Kleinknecht D, Brivet F, et al. Prognostic factors in acute renal failure due to sepsis. Results of a prospective multicentre study. The French Study Group on Acute Renal Failure. Nephrol Dial Transplant. 1996;11:293–299. [DOI] [PubMed] [Google Scholar]
- 45.Bagshaw SM, George C, Bellomo R.. Early acute kidney injury and sepsis: a multicentre evaluation. Crit Care. 2008;12:R47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bagshaw SM, Lapinsky S, Dial S, et al. Acute kidney injury in septic shock: clinical outcomes and impact of duration of hypotension prior to initiation of antimicrobial therapy. Intensive Care Med. 2009;35:871–881. [DOI] [PubMed] [Google Scholar]
- 47.Oppert M, Engel C, Brunkhorst F-M, et al. Acute renal failure in patients with severe sepsis and septic shock: a significant independent risk factor for mortality: results from the German Prevalence Study. Nephrol Dial Transplant. 2007;23:904–909. [DOI] [PubMed] [Google Scholar]


