Abstract
Background
Interpretation of serum vitamin D biomarkers across pregnancy is complex due to limited understanding of pregnancy adaptations in vitamin D metabolism. During pregnancy, both gestational age and serum 25-hydroxyvitamin D [25(OH)D] concentrations may influence the concentrations of 1,25-dihydroxyvitamin D [1,25(OH)2D], 24,25-dihydroxyvitamin D [24,25(OH)2D], and parathyroid hormone (PTH).
Objective
We aimed to identify predictors of change in serum 25(OH)D across gestation in pregnant adolescents and to assess the contribution made by cholecalciferol (vitamin D3) supplementation. We sought to determine whether gestational age and 25(OH)D concentration interacted to affect serum 1,25(OH)2D, 24,25(OH)2D, or PTH.
Methods
Pregnant adolescents (n = 78, 59% African American, mean ± SD age: 17 ± 1 y) living in Rochester, NY (latitude 43°N) were supplemented with 200 IU or 2000 IU vitamin D3/d and allowed to continue their daily prenatal supplement that contained 400 IU vitamin D3. Serum was collected at study entry (18 ± 5 wk of gestation), halfway through study participation, and at delivery (40 ± 2 wk). Serum concentrations of the biochemical markers were modeled with linear mixed-effects regression models.
Results
Vitamin D3 supplement intake and season of delivery determined change in 25(OH)D across pregnancy. Fall-winter delivery was associated with a decline in 25(OH)D unless vitamin D3 supplement intake was >872 IU/d. The interaction of gestational age and 25(OH)D affected 24,25(OH)2D concentrations. For a given 25(OH)D concentration, model-predicted serum 24,25(OH)2D increased across gestation except when 25(OH)D was <13 ng/mL. Below this threshold, 24,25(OH)2D was predicted to decline over time. Mean serum 1,25(OH)2D was elevated (>100 pg/mL) throughout the study.
Conclusion
Our results suggest that when maternal serum 25(OH)D was low, its catabolism into 24,25(OH)2D decreased or remained stable as pregnancy progressed in order to maintain persistently elevated serum 1,25(OH)2D. Furthermore, in adolescents living at latitude 43°N, standard prenatal supplementation did not prevent a seasonal decline in 25(OH)D during pregnancy. This study was registered at clinicaltrials.gov as NCT01815047.
Keywords: vitamin D; cholecalciferol; 25-hydroxyvitamin D; 24,25-dihydroxyvitamin D; pregnancy; adolescence; maternal nutrition; perinatal nutrition
Introduction
Pregnancy involves adaptations in maternal vitamin D metabolism and calcium homeostasis. Yet, it is unclear if these changes are sufficient to satisfy physiologic demands when the availability of vitamin D or both vitamin D and calcium is limited (1). Stress to the maternal vitamin D economy may be more evident in pregnant adolescents. Vitamin D is important for skeletal health, and skeletal mass increases throughout adolescence (2). Moreover, many US adolescent girls have low serum 25-hydroxyvitamin D [25(OH)D] (3), and most have inadequate intakes of vitamin D and calcium (4).
Pregnancy adaptations in vitamin D metabolism are incompletely understood. Most studies report that serum 25(OH)D remains stable across pregnancy while plasma volume expands and serum vitamin D binding protein concentration increases (5). In early pregnancy, concentrations of the hormone 1,25-dihydroxyvitamin D [1,25(OH)2D] rise 100–150% (5) and efficiency of intestinal calcium absorption doubles (6). These increases are sustained or augmented late in pregnancy when fetal calcium accrual peaks (7). Current thought is that 1,25(OH)2D may contribute to, but is not required for, gestational increases in calcium absorption (8–10). Furthermore, in the nonpregnant state, renal activation of 25(OH)D into 1,25(OH)2D is stimulated primarily by parathyroid hormone (PTH). However, PTH is often found to be low in pregnancy, and another factor may drive the persistent elevation of 1,25(OH)2D during pregnancy (8). Although PTH is frequently used as a physiologic indicator of vitamin D status in nonpregnant individuals, PTH as an indicator of the vitamin D economy during pregnancy has not been confirmed (11).
Serum 25(OH)D is used to assess vitamin D status, but optimal concentrations for health in the general population remain controversial. During pregnancy, interpretation of serum 25(OH)D is further complicated by profound changes to the calcium and vitamin D economies (5, 12). Improved assays have renewed interest in assessment of serum 24,25-dihydroxyvitamin D [24,25(OH)2D], the primary product of 25(OH)D catabolism. Recent data from nonpregnant populations suggest that serum 24,25(OH)2D and the ratio of 24,25(OH)2D to 25(OH)D are useful indicators of vitamin D deficiency (13), but the utility of these indicators during pregnancy has not been addressed. The few recent data on serum 24,25(OH)2D during human pregnancy are limited to late gestation and suggest that it may be lower in the pregnant state (14, 15). This is plausible since hormones and other signals, including PTH, reciprocally regulate production of 1,25(OH)2D and 24,25(OH)2D (i.e., vitamin D activation and inactivation) in support of calcium/phosphorus homeostasis (16). Prior studies have assessed 25(OH)D and 1,25(OH)2D across pregnancy, but there is little information on longitudinal change in 24,25(OH)2D during pregnancy.
To our knowledge, no study to date has estimated the independent effects of gestational age on calcitropic hormones and 24,25(OH)2D and assessed whether maternal serum 25(OH)D status modifies these possible effects. To advance interpretation of serum vitamin D biomarkers during pregnancy, we longitudinally assessed serum 24,25(OH)2D, 25(OH)D, 1,25(OH)2D, and PTH in pregnant adolescents. We sought to identify predictors of longitudinal change in serum 25(OH)D and to determine if gestational age and maternal 25(OH)D interact to affect 1,25(OH)2D, 24,25(OH)2D, or PTH concentration across adolescent pregnancy.
Methods
Study participants
Participants were recruited from the population receiving care at the Rochester Adolescent Maternity Program, a program that provides specialized prenatal care for pregnant adolescents in Rochester, NY (latitude 43°N). Adolescents were eligible to enroll if 13–18 y of age, carrying a singleton pregnancy, and between 12 and 29 wk of gestation at enrollment. Exclusion criteria included use of tobacco, steroids, or medications that influence vitamin D or calcium metabolism as well as HIV infection, malabsorption disease, diabetes, history of an eating disorder, history of drug abuse, or medical history of elevated blood lead. The institutional review boards at the University of Rochester and Cornell University approved the study (NCT01815047), which complied with the Declaration of Helsinki—Ethical Principles for Medical Research Involving Human Subjects. A study coordinator obtained written informed consent, and assent with parental consent was obtained from the 1 participant who was <15 y of age at enrollment.
Study outcomes
The original data analysis plan was to assess change in the serum markers as a function of assignment to cholecalciferol (vitamin D3) supplement group. However, because of low adherence to the intervention, we had to treat the study as an observational cohort study. We identified predictors of the change in 25(OH)D across pregnancy and tested whether gestational age and maternal 25(OH)D status interacted to affect serum 1,25(OH)2D, 24,25(OH)2D, or PTH concentration.
Study procedures
A study coordinator allocated participants by alternate assignment to 2 parallel groups that received either 200 IU or 2000 IU of vitamin D3 daily in addition to their prescribed prenatal supplement containing 400 IU of vitamin D3. We expected that 5 mo of supplementation with 2400 IU vitamin D3/d would increase baseline serum 25(OH)D by ∼17 ng/mL (17). In our previous study of adolescents recruited from the same prenatal clinic, mean serum 25(OH)D was 22 ng/mL throughout pregnancy (18). The 2000 IU/d treatment was chosen to provide a dose well below the tolerable upper intake level (9) that could substantially shift the 25(OH)D distribution in 1 study arm. The 200 IU supplement was used to provide some potential benefit to participants in the other arm and to bring their total supplemental vitamin D3 intake to 600 IU/d (the recommended dietary allowance).
At enrollment, participants received a 6-wk supply of vitamin D3 supplements (Tishcon Corporation, Westbury, NY). Participants, providers, and study personnel were blinded to supplement group identity. At each monthly prenatal visit, we supplied a new supplement bottle and collected the previous bottle along with any remaining pills, which were counted. To encourage participation, study personnel sent weekly text messages and provided educational handouts at prenatal visits. Participants were remunerated for each bottle that was returned regardless of adherence. We calculated the total number of pills consumed by each participant based on the number of bottles and pills returned. If a bottle was not returned, then we determined that the participant consumed 0 pills from that bottle. We calculated the cumulative intake of vitamin D3 from the intervention by multiplying the number of pills consumed by either 200 or 2000 IU. To aid interpretation, cumulative intake was rescaled to an estimate of daily intake by dividing it by the overall mean number of days in the study. Intervention adherence was defined as the number of pills consumed divided by the number of pills dispensed. The vitamin D3 content of a randomly selected supplement from each study arm was assessed annually by HPLC at an external lab (Heartland Laboratories, Ames, IA).
Structured questionnaires were used to record demographic information, health history, and use of prenatal and other dietary supplements. Dietary intake was assessed by a 24-h recall at entry and at the second study visit. Study assistants entered 24-h recalls into the Nutrition Data System for Research 2014 (University of Minnesota, Minneapolis, MN) in duplicate. Usual nutrient intake was defined as the average from the two 24-h recalls, and the prevalence of inadequate intake was the proportion of participants whose usual nutrient intake was less than the estimated average requirement (19).
A non-fasted blood sample was collected at participant entry (any time between 12 and 29 wk of gestation), approximately halfway through study participation, and upon entering the hospital for delivery. This flexible visit schedule was used to maximize recruitment and retention and resulted in what are known as longitudinal data with irregularly spaced measurement occasions. Season of blood collection was defined as fall-winter (November–March) or spring-summer (April–October) based on monthly UV index in Rochester and potential for endogenous vitamin D3 production in the northeastern United States (20). Serum was isolated and stored at −80°C until analyses.
Biochemical analyses
Vitamin D metabolites (25-hydroxy-ergocalciferol [25(OH)D2], 25-hydroxycholecalciferol [25(OH)D3], 1,25-dihydroxyergocalciferol, 1,25-dihydroxycholecalciferol, and 24,25-dihydroxycholecalciferol [24,25(OH)2D3]) in pristine serum were analyzed simultaneously by LC with tandem MS at the Department of Laboratory Medicine at the University of Washington (Seattle, WA) following published methods (21). Most samples (n = 202) were analyzed in a single batch, and 4 were analyzed in a second batch. Interassay CVs were between 3.5% and 10.4%. Serum 25(OH)D and 24,25(OH)2D3 were calibrated to National Institute of Standards and Technology standard reference material 972a. No standard reference material exists for 1,25(OH)2D. Serum PTH (intact PTH 1–84) was measured by ELISA (ALPCO, Salem, NH), and kit controls and an in-house control were run in duplicate with each ELISA. The intra- and interassay CVs were 5.7% and 8.2%, respectively. Serum total calcium and phosphorus at entry and at the second study visit were measured at a Clinical Laboratory Improvement Amendments-certified laboratory at the University of Rochester Medical Center and evaluated in relation to reference ranges (8.1–10.4 mg/dL for calcium and 2.7–5.5 mg/dL for phosphorus) (22). Maternal anthropometry and pregnancy and birth outcomes were abstracted from electronic health records.
Statistical analyses
Analyses were conducted with SAS software version 9.4 and JMP Pro 12 (SAS Institute Inc., Cary, NC). The study blind was maintained until completion of analyses. A 2-sided alpha level of 0.05 denoted statistical significance. Participant characteristics at entry and delivery were compared by supplement group, and characteristics at entry were compared by study completion status. Continuous variables were compared with 2-sample t test when normally distributed or with Wilcoxon’s rank-sum test when skewed. Categorical variables were compared with chi-square test or, when any category had expected counts <5, with Fisher's exact test. The effect of supplement group assignment on Δ-25(OH)D [serum 25(OH)D at delivery minus at entry] was tested with 1-factor ANOVA.
The serum 25(OH)D concentration across pregnancy was modeled with a linear mixed-effects regression model that included a continuous fixed effect of gestational age in weeks, a random subject intercept, a random slope of gestational age, and a random covariance parameter between intercept and slope. Potential determinants of longitudinal change in 25(OH)D, including intake of vitamin D3 from the intervention, adherence to the prenatal supplement, intakes of calcium and vitamin D from foods and beverages, baseline serum 25(OH)D, season of delivery, race, pre-pregnancy BMI, pre-pregnancy obesity, and gestational weight gain, were tested independently by including each as a fixed effect that interacted with the fixed effect of gestational age. When the result had P < 0.2, these variables were tested simultaneously and eliminated by backward selection until significant predictors remained. If not yet in the model, known determinants of 25(OH)D concentration, including intakes of calcium and vitamin D from foods and beverages, season of delivery, race, pre-pregnancy BMI, and pre-pregnancy obesity, were then tested as main effects, eliminated by backward selection, and retained if P < 0.05. Finally, triple-interactions were tested.
We wanted to estimate the effects of gestational age on 1,25(OH)2D, 24,25(OH)2D, and PTH and to assess whether these potential effects depend on the supply of 25(OH)D. To do this, we constructed a linear mixed-effects regression model for each of these biochemical outcomes that included the aforementioned random effects and continuous fixed effects for gestational age, 25(OH)D, and their interaction term. The fixed effect of gestational age is the independent association of gestational age with the outcome (e.g., PTH). The fixed effect of 25(OH)D is the independent association of 25(OH)D with the outcome. The interaction term tests whether the association of gestational age with the outcome depended on 25(OH)D concentration. When necessary, the outcome was loge transformed to meet the assumptions of the model.
To assess relations between all biochemical markers we used Pearson correlation coefficients, which estimated the linear associations between markers at each stage of pregnancy. Because the first and second study visits were irregularly spaced, we binned the biochemical data from the first and second study visits according to trimester of pregnancy. If any participant contributed to the same bin more than once, only 1 measurement occasion (earliest gestational age) was used. The stages of pregnancy were defined as trimester 1, trimester 2, trimester 3 (not delivery), and delivery, since delivery can be considered a physiologically distinct stage of pregnancy, and all delivery visits occurred within a very narrow window of gestation.
Sensitivity analyses
Results for PTH, 1,25(OH)2D, and 24,25(OH)2D at delivery were excluded from statistical analyses if blood was collected after emergency delivery (n = 4), as previous studies showed that serum PTH, 1,25(OH)2D, and 24,25(OH)2D can change substantially in the early postpartum period (23–25). We checked whether excluding these observations influenced results. In this study, serum 25(OH)D refers to total 25(OH)D [both 25(OH)D3 and 25(OH)D2] and serum 1,25(OH)2D refers to total 1,25(OH)2D. In contrast, serum 24,25(OH)2D refers to only 24,25(OH)2D3 because 24,25(OH)2D2 cannot be measured by the assay and is not in the National Institute of Standards and Technology standard reference material. For both 25(OH)D and 1,25(OH)2D, the vitamin D3 form predominated in all samples. At entry, 25(OH)D2 comprised (median) 0.8% (IQR: 0.5%, 1.4%) of total 25(OH)D. At delivery, this proportion was 1.1% (IQR: 0.6%, 1.5%). However, because 24,25(OH)2D2 concentrations were unavailable, we repeated the regression model of 24,25(OH)2D using 25(OH)D3 in place of total 25(OH)D. Finally, each regression model was repeated using only participants with complete data.
Results
Participant flow and characteristics
Participants were recruited between December 2012 and August 2015. Supplemental Figure 1 documents the flow of the 150 adolescents assessed for eligibility. Of these, 78 were allocated to a supplement group and received the intervention. Mean ± SD gestational age at entry was 18 ± 5 wk, and 75% of teens entered the study at ≤20 wk of gestation. Mean pre-pregnancy BMI (kg/m2) was 25. The prevalence of pre-pregnancy overweight was 40% and of pre-pregnancy obesity was 18%. Most of this cohort (59%) identified as African American or multiracial including African American. The remaining participants were classified as white/other race because they identified as white (n = 16), other (n = 14), American Indian (n = 1), or multiracial (n = 1). Twenty-two participants were of Hispanic ethnicity.
Serum 25(OH)D concentration at study entry ranged from 8.6 to 45.1 ng/mL. No statistically significant differences existed between the groups at entry (Table 1). There were notable (although statistically nonsignificant) differences in racial composition and, perhaps as a result, the distribution of serum 25(OH)D. Specifically, in the 200 IU/d group, a larger proportion of participants was African American (P = 0.17) and mean serum 25(OH)D was lower (P = 0.06). African American teens had a significantly greater prevalence (50%) of serum 25(OH)D < 20 ng/mL at entry compared with white/other teens (13%). Measures of dietary intake did not differ significantly by supplement group. Mean vitamin D intake from foods and beverages was 216 IU/d, and the prevalence of inadequate intake (<400 IU/d) was 93%. For calcium, the breakdown was 938 mg/d and 76% (<1100 mg/d).
TABLE 1.
Characteristics of pregnant adolescents at study entry and delivery1
| 200 IU/d | 2000 IU/d | |
|---|---|---|
| Maternal age, y | 17.5 ± 1.1 [39] | 17.3 ± 1.2 [39] |
| Gestational age at entry, wk | 17.0 ± 4.3 [39] | 18.4 ± 4.6 [39] |
| Pre-pregnancy BMI, kg/m2 | 24.1 ± 4.9 [39] | 25.8 ± 6.8 [37] |
| African American race, n (%) | 26 (66.7) | 20 (51.3) |
| Hispanic ethnicity, n (%) | 11 (28.2) | 11 (29.0) |
| Serum 25(OH)D at entry, ng/mL | 23.1 ± 9.1 [39] | 26.9 ± 9.1 [39] |
| <12 ng/mL, n (%) | 4 (10.3) | 1 (2.6) |
| 12 to <20 ng/mL, n (%) | 13 (33.3) | 9 (23.1) |
| 20 to <30 ng/mL, n (%) | 14 (35.9) | 14 (35.9) |
| ≥30 ng/mL, n (%) | 8 (20.5) | 15 (38.5) |
| Serum 25(OH)D at delivery, ng/mL | 23.5 ± 10.2 [30] | 30.5 ± 14.1 [32]* |
| <12 ng/mL, n (%) | 2 (6.7) | 5 (15.6) |
| 12 to <20 ng/mL, n (%) | 11 (36.7) | 3 (9.4)* |
| 20 to <30 ng/mL, n (%) | 10 (33.3) | 7 (21.9) |
| 30 to ≤50 ng/mL, n (%) | 6 (20.0) | 15 (46.9)* |
| >50 ng/mL, n (%) | 1 (3.3) | 2 (6.3) |
| Number of vitamin D supplements consumed2 | 65 (27, 107) [30] | 75 (0, 117) [32] |
| Fall–winter delivery, n (%) | 16 (53.3) | 14 (43.8) |
| Gestational weight gain, kg | 13.6 ± 4.5 [30] | 17.2 ± 6.4 [32]* |
| Baby birth weight, g | 3233 ± 368 [29] | 3351 ± 486 [31] |
| Low birth weight (<2500 g), n (%) | — | 2 (6.5) |
| Preeclampsia/pregnancy-induced hypertension, n (%) | 4 (13.3) | 2 (6.3) |
1Values are means ± SDs [n] unless otherwise indicated. *Different from 200 IU/d, P < 0.05. IU, International Units; 25(OH)D, 25-hydroxyvitamin D.
2Median (25th,75th percentiles) [n].
Serum was collected from all 78 participants at study entry, 65 at a second study visit, and 62 at delivery. The study flow diagram (Supplemental Figure 1) includes the reasons why blood was not collected at delivery. The proportion of teens that completed the delivery visit did not differ between supplement groups. Those who did not complete the delivery visit were more likely to be African American (P = 0.04), and their mean 25(OH)D at entry was lower (P = 0.02) than that of participants who completed the study. Mean gestational age at delivery was 39.6 ± 1.6 wk and this did not differ between groups. The supplement groups were not different at delivery with respect to relevant characteristics shown in Table 1, except the 2000 IU/d group gained statistically significantly more weight over pregnancy.
Intervention adherence and treatment effect
The higher-dose and lower-dose supplements contained (mean ± SD) 2345 ± 146 and 225 ± 13 IU of vitamin D3, respectively. In the 62 participants followed to delivery, mean number of days in the study was 153 ± 35 d, with no statistically significant difference by supplement group. Intervention adherence was low in both groups. Median adherence was 44% (IQR: 0%, 63%) in the 2000 IU/d group and 37% (IQR: 15%, 58%) in the 200 IU/d group. Most teens (66%) reported daily adherence to the prescribed prenatal supplement, and prenatal supplement adherence was positively associated with intervention adherence.
Between entry and delivery, 25(OH)D increased on average (± SD) 2.3 ± 11.4 ng/mL in the 2000 IU/d group and decreased 0.7 ± 8.0 ng/mL in the 200 IU/d group, a treatment effect of 3.0 (95% CI: −2.1, 8.0; P = 0.2; n = 62). However, when we considered intervention adherence or the number of pills consumed, we did detect a difference in the Δ-25(OH)D between groups. In the 2000 IU/d group, the number of pills consumed was statistically significantly associated with Δ-25(OH)D (+0.08 ng/mL per pill consumed; P = 0.02). There was no such association in the 200 IU/d group (P = 0.44). All serum calcium and phosphorus values were within normative ranges.
Linear mixed-effects models of vitamin D metabolites and PTH across pregnancy
Because of low intervention adherence, the remaining results are from observational data analyses in which intake of vitamin D3 from the intervention refers to intake based on pill counts. The observed concentrations of 25(OH)D, 1,25(OH)2D, 24,25(OH)2D, and PTH across gestation are shown in Supplemental Figure 2. Results of the mixed model of serum 25(OH)D are reported in Table 2 and illustrated in Figure 1. The predictors of change in 25(OH)D across pregnancy are the variables that statistically significantly interacted with gestational age. These included intake of vitamin D3 from the intervention and season of delivery. For teens who delivered in fall–winter, model-predicted 25(OH)D declined across pregnancy unless vitamin D3 supplement intake was ≥873 IU/d. Table 2 shows the model coefficients and equation used to calculate this value. In contrast, serum 25(OH)D was predicted to increase across pregnancy in spring–summer deliveries, and the magnitude of the increase depended on vitamin D3 supplement intake. African American race was associated with having a 6.2-ng/mL lower 25(OH)D throughout pregnancy, but change in 25(OH)D over time did not differ by race.
TABLE 2.
Predictors of serum 25-hydroxyvitamin D in pregnant adolescents1
| Fixed effects | β ± SE | P value |
|---|---|---|
| Intercept | 23.1 ± 1.7 | <0.0001 |
| Gestational age,2 wk | −0.169±0.081 | 0.0423 |
| Season of delivery (spring-summer)3 | −0.396 ± 2.1 | 0.85 |
| Gestational age × season of delivery | 0.332 ± 0.11 | 0.0027 |
| Intake of intervention vitamin D,4 1000 IU/d | 5.69 ± 1.6 | 0.0007 |
| Gestational age × intake of intervention vitamin D5 | 0.222 ± 0.074 | 0.0043 |
| Maternal race (white/other)6 | 6.19 ± 2.0 | 0.0038 |
1Values are fixed-effects estimates from a random coefficients model of serum 25-hydroxyvitamin D concentration conducted using SAS PROC MIXED with an unstructured covariance matrix, n = 67 subjects, 194 observations. IU, International Units.
2Gestational age is centered at mean 28.7 wk.
3Effect of spring-summer (April–October) delivery compared with the reference category, fall-winter (November–March) delivery.
4Daily intake of vitamin D3 from the supplementation intervention is centered at the median 0.111 (i.e., 111 IU).
5The gestational age coefficient −0.1692 is the slope of gestational age when season is fall-winter and intake of intervention vitamin D is 0.111 (1000 IU/d). The slope of gestational age in fall-winter becomes nonnegative (i.e., is zero) when intake of intervention vitamin D is 0.873 (1000 IU/d) or 873 IU/d: 0 = 0.111 – (−0.1692 ÷ 0.2220) – X. Solve for X = 0.873 or 873 IU/d.
6Effect of white/other race compared with reference category, African American race.
FIGURE 1.
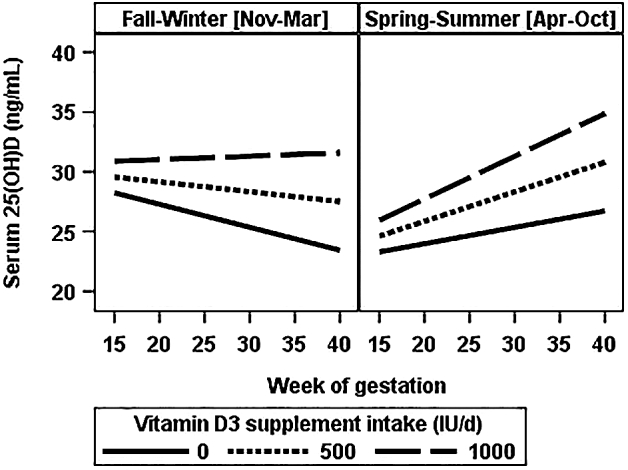
Model-predicted serum 25(OH)D concentration across gestation by season of delivery and daily intake of intervention vitamin D3 in pregnant adolescents. Values are marginal predictions for 25(OH)D concentration across gestation when vitamin D3 supplement intake was set at 0, 500, or 1000 IU/d. Data were analyzed with a regression coefficients model with an unstructured covariance matrix (Table 2). IU, International Units; 25(OH)D, 25-hydroxyvitamin D.
Loge serum PTH was inversely associated with 25(OH)D (P = 0.01) and positively associated with gestational age (P < 0.01). Holding 25(OH)D constant, PTH increased an estimated 4.4 pg/mL between 18 and 40 wk of gestation. This increase did not depend on 25(OH)D concentration, i.e., there was no interaction of gestational age and 25(OH)D (P-interaction = 0.46). Loge serum 1,25(OH)2D was weakly positively associated with 25(OH)D (P < 0.001), but the gestational age and interaction terms were not significant, meaning neither 1,25(OH)2D nor its association with 25(OH)D changed linearly over gestation. After removing the nonsignificant interaction term from each of these models, we tested if maternal race helped to predict PTH or 1,25(OH)2D by including it as a main effect alone or in interaction terms (retained if P < 0.05). Race improved the model of PTH but not 1,25(OH)2D. The increase in PTH across pregnancy depended on race and was greater in African Americans (P = 0.048) (Supplemental Table 1).
Gestational age and serum 25(OH)D interacted to affect 24,25(OH)2D (P-interaction < 0.0001). For a given 25(OH)D concentration, the model-predicted slope of 24,25(OH)2D across pregnancy was negative when 25(OH)D was <12.6 ng/mL, zero when it was 12.6 ng/mL, and greater with each unit increase in 25(OH)D above this threshold. Figure 2 illustrates the interaction by showing the model-predicted concentrations of serum 24,25(OH)2D as a function of gestational age and serum 25(OH)D. The 12.6 ng/mL threshold was calculated from the model coefficients and equation provided in Table 3. Race did not confound or modify these effects. The results of sensitivity analyses supported all inferences reported.
FIGURE 2.

Model-predicted serum 24,25(OH)2D concentration as a function of gestational age and serum 25(OH)D concentration in pregnant adolescents. Values are marginal predictions for serum 24,25(OH)2D across gestation when 25(OH)D concentration was set at 10, 20, 30, 40, or 50 ng/mL. Data were analyzed with a regression coefficients model with an unstructured covariance matrix (Table 3). 24,25(OH)2D, 24,25 dihydroxyvitamin D; 25(OH)D, 25-hydroxyvitamin D.
TABLE 3.
Effects of gestational age and serum 25(OH)D concentration on serum 24,25-dihydroxyvitamin D in pregnant adolescents1
| Fixed effects | β ± SE | P value |
|---|---|---|
| Intercept | 1.51 ± 0.043 | <0.0001 |
| Gestational age,2 wk | 0.0130 ± 0.0022 | <0.0001 |
| Serum 25(OH)D,3 ng/mL | 0.0667 ± 0.0027 | <0.0001 |
| Gestational age × serum 25(OH)D4 | 0.000853 ± 0.00020 | <0.0001 |
1Values are fixed-effects estimates from a random coefficients model of serum 24,25-dihydroxyvitamin D concentration conducted using SAS PROC MIXED with an unstructured covariance matrix, n = 78 subjects, 201 observations. 25(OH)D, 25-hydroxyvitamin D.
2Gestational age is centered at mean 28.7 wk.
3Serum 25(OH)D is centered at mean 27.8 ng/mL.
4The gestational age coefficient 0.0130 is the slope of gestational age when serum 25(OH)D is centered at mean 27.8 ng/mL. The slope of gestational age is zero when 25(OH)D is 12.6 ng/mL: 0 = 27.8 – (0.0130 ÷ 0.000853) – X. Solve for X = 12.6 ng/mL. Below this 25(OH)D concentration, the slope of gestational age is negative.
Correlations between biochemical markers by stage of pregnancy
Table 4 includes the correlations between all biochemical markers by stage of pregnancy. At delivery only, PTH was inversely correlated with 25(OH)D, 24,25(OH)2D, and the ratio of 24,25(OH)2D to 25(OH)D. Serum calcium correlated positively with serum phosphorus in each trimester and with 1,25(OH)2D in the third trimester only. Neither PTH nor 25(OH)D was significantly correlated with 1,25(OH)2D at any stage of pregnancy. In contrast, 25(OH)D was highly positively correlated with 24,25(OH)2D and the ratio of 24,25(OH)2D to 25(OH)D. Both of these correlations became stronger as pregnancy progressed.
TABLE 4.
Correlations between biochemical markers by stage of pregnancy in adolescents1
| 25(OH)D | PTH | 1,25(OH)2D | 24,25(OH)2D | 24,25:25 | Ca | Pi | |
|---|---|---|---|---|---|---|---|
| 25(OH)D | |||||||
| T1 | −0.08 | 0.25 | 0.92**** | 0.38 | −0.35 | −0.08 | |
| T2 | 0.10 | 0.17 | 0.86**** | 0.51**** | −0.01 | −0.16 | |
| T3 | −0.20 | 0.20 | 0.92**** | 0.71**** | 0.25 | 0.06 | |
| D | −0.27* | 0.21 | 0.92**** | 0.74**** | NA | NA | |
| PTH | |||||||
| T1 | 0.17 | −0.21 | −0.11 | −0.23 | 0.30 | ||
| T2 | 0.11 | 0.03 | −0.08 | −0.25 | −0.16 | ||
| T3 | −0.08 | −0.23 | −0.14 | −0.18 | −0.23 | ||
| D | −0.12 | −0.30* | −0.29* | NA | NA | ||
| 1,25(OH)2D | |||||||
| T1 | 0.22 | 0.10 | 0.05 | 0.22 | |||
| T2 | 0.06 | −0.10 | −0.14 | −0.13 | |||
| T3 | 0.15 | 0.01 | 0.48*** | 0.15 | |||
| D | 0.03 | −0.12 | NA | NA | |||
| 24,25(OH)2D | |||||||
| T1 | 0.70*** | −0.22 | −0.11 | ||||
| T2 | 0.84**** | −0.04 | −0.22 | ||||
| T3 | 0.89**** | 0.10 | −0.03 | ||||
| D | 0.90**** | NA | NA | ||||
| 24,25:25 | |||||||
| T1 | 0.03 | −0.02 | |||||
| T2 | −0.03 | −0.16 | |||||
| T3 | −0.07 | −0.13 | |||||
| Ca | |||||||
| T1 | 0.51* | ||||||
| T2 | 0.35** | ||||||
| T3 | 0.36** | ||||||
1Each test used 1 data point per participant (earliest available) at each stage of pregnancy, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Ca, total serum calcium; D, delivery (n = 58, except for 25(OH)D n = 62 and PTH n = 56); NA, not available; Pi, total serum phosphorus; T1, trimester 1 (n = 19, except for PTH n = 17); T2, trimester 2 (n = 57, except for PTH n = 52); T3, trimester 3 (not delivery visit) (n = 53, except for PTH, calcium, and phosphorus n = 52); 1,25(OH)2D, 1,25-dihydroxyvitamin D; 24,25(OH)2D, 24,25-dihydroxyvitamin D; 24,25:25, the molar ratio of 24,25(OH)2D to 25(OH)D; 25(OH)D, 25-hydroxyvitamin D.
Discussion
In this cohort of pregnant adolescents, daily supplementation with 200 IU of vitamin D3 plus a standard prenatal supplement (400 IU) failed to prevent seasonal decline in 25(OH)D across pregnancy. Maternal PTH increased over the study period and was inversely correlated with 25(OH)D at delivery only. Similar to data obtained from pregnant adults, serum 1,25(OH)2D was 130% higher than the nonpregnant reference median (45 pg/mL) at both entry and delivery. Regarding serum 24,25(OH)2D, our results indicate that from ∼12 wk of gestation onward, this catabolic product increases as a function of gestational age unless maternal serum 25(OH)D status remains <13 ng/mL.
As expected at latitude 43°N, season influenced maternal serum 25(OH)D concentration. Fall–winter delivery was associated with decline in 25(OH)D over the study period, and ≥873 IU of vitamin D3/d was required to prevent this seasonal decline. This dose is more than twice the vitamin D3 content of a typical prenatal supplement. A recent large vitamin D supplementation trial in England, The Maternal Vitamin D Osteoporosis Study, reported a similar finding (26). Women on placebo (plus prenatal supplementation) who delivered in winter or spring experienced a decline in 25(OH)D, but the women in the 1000 IU vitamin D3/d group avoided this seasonal decline.
Prevention of seasonal decline in 25(OH)D is probably most important in gravidae whose serum 25(OH)D at conception is low relative to target concentrations. For optimal bone health in the general population, the US National Academy of Medicine associates serum 25(OH)D <12 ng/mL with risk of vitamin D deficiency and <20 ng/mL with risk of inadequacy (9). The Endocrine Society recommends concentrations ≥30 ng/mL for vitamin D sufficiency (27). Whether optimal 25(OH)D concentrations differ in pregnant females has not been adequately researched (12). Interpretation of serum 25(OH)D is complicated by pregnancy, which involves physiologic adaptations to maintain maternal calcium homeostasis while supporting fetal skeletal consolidation. The literature indicates there are redundant hormonal mechanisms that increase calcium absorption and serum 1,25(OH)2D during pregnancy (28, 29), which casts doubt on the essentiality of the vitamin D-PTH axis during pregnancy. On the other hand, the serum 1,25(OH)2D concentration is elevated 1- to 2-fold during normal human pregnancy, and evidence suggests that the vitamin D-PTH axis does help to maximize maternal mineral absorption and fetal growth (29–32). Our data on the PTH-25(OH)D relation further document vitamin D-PTH activity during pregnancy. In addition, our results suggest that the 25(OH)D concentrations associated with inadequacy in the general population also cannot support gestational increase in 24,25(OH)2D, presumably because of the need to conserve 25(OH)D and meet the elevated 1,25(OH)2D production demands of pregnancy.
Maternal PTH concentrations in this adolescent cohort were in the low-normal part of the nonpregnant reference range at study entry and increased slightly but statistically significantly as pregnancy progressed. Many previous studies (8, 18, 33, 34) but not all (35, 36) found that PTH gradually increased across pregnancy. One advantage of our mixed model was the capacity to estimate the average change in PTH across pregnancy while controlling for fluctuation in serum 25(OH)D over time. The observed increase may indicate heightened PTH activity in late pregnancy when turnover of the maternal calcium pool and renal calcium conservation have been shown to increase (6, 34). Longitudinal increase in PTH was greater in African American teens, as their PTH was lower in early pregnancy but similar at delivery relative to white/other teens. Racial differences in PTH are frequently observed in pregnant and nonpregnant populations but are not well understood (11). Given sample size limitations, it was difficult for us to isolate if this effect was entirely due to race or if it was partly related to lower 25(OH)D in African Americans.
Serum 25(OH)D and PTH were significantly inversely correlated only at delivery, when fetal calcium demands are maximal. The PTH-25(OH)D correlations in this study and our previous adolescent pregnancy cohort (18) are on the same order as those measured in pregnant adults in the third trimester (r = −0.33) (37) and in nonpregnant adults (r = −0.20 to −0.30) (38). Our results support the concept that, despite adjustments to the calcium economy, PTH provides information about physiologic vitamin D status at least during late pregnancy. Kramer et al. (37) used the PTH-25(OH)D relation at 30 wk of gestation to identify a threshold for vitamin D adequacy, an approach common to studies of nonpregnant populations. They determined that PTH began to rise when 25(OH)D was <33 ng/mL, and this threshold was not significantly different in the same women postpartum. However, the PTH-25(OH)D relation was less curvilinear in pregnancy. Our plot of the PTH-25(OH)D relation at delivery revealed an inverse association but no obvious threshold behavior.
In these adolescents, a weak positive association between 25(OH)D and 1,25(OH)2D was noted, but this relation was not statistically significant at any individual stage of pregnancy. Hollis et al. (39) reported that in pregnant women, 1,25(OH)2D was positively associated with 25(OH)D and reached a plateau when 25(OH)D was >40 ng/mL. We saw no plateau effect in our data, but relatively few adolescents achieved 25(OH)D > 40 ng/mL despite receiving ≤2000 IU of additional vitamin D3/d. Serum 1,25(OH)2D was elevated above the nonpregnant reference limit at study entry, tended to peak around 29 wk, and then declined slightly to entry values at delivery. We modeled 1,25(OH)2D linearly over time because participants were measured a maximum of 3 times. However, a model that included time as a quadratic term suggested that 1,25(OH)2D may actually follow a curvilinear trajectory across adolescent pregnancy.
To the best of our knowledge, ours is the largest longitudinal study that has assessed serum 24,25(OH)2D concentrations across pregnancy. Gestational age and serum 25(OH)D interacted to affect 24,25(OH)2D. Model-predicted serum 24,25(OH)2D increased across pregnancy except when maternal serum 25(OH)D was low. Specifically, our results predict that when serum 25(OH)D is less than ∼13 ng/mL, its catabolism will decline as pregnancy progresses, presumably to support elevated 1,25(OH)2D production. Recent studies in nonpregnant populations have found that 24,25(OH)2D production shuts down markedly in adult men and women with vitamin D deficiency (13). Thus, we believe that metabolic conservation of 25(OH)D plausibly explains the effect we observed. In our data this effect was detected at maternal 25(OH)D concentrations in the range of 10–20 ng/mL (the exact threshold depended on the model or subset of participants used).
Our data cannot explain why serum 24,25(OH)2D concentration increased 20% on average over time, independently of change in 25(OH)D. This effect of gestational age on 24,25(OH)2D was not explained by supplement intake or season of delivery and may be due to physiologic changes across pregnancy. The 24,25(OH)2D molecule has no established biologic activity, but studies indicate that it may inhibit PTH secretion and have direct effects on bone (40). We found 3 prior studies that measured serum 24,25(OH)2D throughout most of pregnancy (41), 2 of which were longitudinal in a small number of women (24, 42). In these studies, the pattern of change in 24,25(OH)2D over gestation mirrored that of 25(OH)D, likely because these metabolites are highly correlated. None of the studies isolated the independent effect of gestational age on 24,25(OH)2D or tested whether maternal 25(OH)D status modifies this effect. Methods for longitudinal data analysis and assessment of 24,25(OH)2D have advanced considerably since the publication of these reports decades ago.
Few studies, if any, have assessed 25(OH)D, PTH, 1,25(OH)2D, and 24,25(OH)2D from early pregnancy to term in a population with a high prevalence of low serum 25(OH)D. Our results may help to improve evaluation of serum vitamin D biomarkers during pregnancy. Yet, the following should be considered when interpreting study findings. The results are subject to limitations such as lack of causal inference that challenge most observational studies. African American race was associated with lower 25(OH)D and greater increase in PTH over time. Otherwise, race did not confound or modify the effects reported. However, our study population included only adolescents, mostly of minority race, so our findings may not extend to all pregnant adolescents or to any pregnant adults. Although calcium turnover may be greater during adolescent compared with adult pregnancy, research has shown that pregnancy-related increases in serum 1,25(OH)2D, calcium absorption, and calcium excretion are similar in adolescents (43). Regardless, a large multiracial cohort should test whether our findings extend to pregnant adults. Finally, assessment of other hormones known to affect mineral metabolism, including PTH-related peptide, prolactin, placental lactogen, estrogen, calcitonin, and fibroblast growth factor 23, may have improved the interpretation of study findings.
Our results indicate that vitamin D supplementation with doses greater than that in a standard prenatal supplement may be required to prevent seasonal decline in 25(OH)D across pregnancy in adolescents at higher latitudes. We found that gestational age and maternal serum 25(OH)D concentration interacted to affect 24,25(OH)2D concentration. Future research should investigate whether the mean increase in 24,25(OH)2D that we observed (that was independent of change in 25(OH)D) has functional significance. Moreover, 20% of this group of mostly minority adolescents had a serum 25(OH)D <13 ng/mL at some point during pregnancy, and these low concentrations of 25(OH)D could not support the gestational increase in 24,25(OH)2D. This may indicate that pregnant adolescents with such low serum 25(OH)D experience a strained vitamin D economy. Future research should address this hypothesis and examine whether stress to the vitamin D economy during pregnancy has implications for maternal and child health.
Supplementary Material
Acknowledgments
We thank study coordinators Allison McIntyre, Lauren Cowen, Melissa Miller, and Jessica Gulliver and the Rochester Adolescent Maternity Program staff. Lynn Johnson contributed statistical advice. The lab of Andrew Hoofnagle measured the vitamin D metabolites. The authors’ responsibilities were as follows—KOO, EKP, RAQ, and EC: designed and oversaw the research; CMB: conducted the research; CMB and FV: performed statistical analyses; CMB and KOO: wrote the article and were responsible for the final content; and all authors: read and approved the final manuscript.
Notes
Supported by USDA award 2011–03424 and NIH award T32-DK007158.
Author disclosures: CMB, EKP, RAQ, EC, FV, and KOO, no conflicts of interest.
The funding sources were not involved in this research or its publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the NIH.
Supplemental Figures 1 and 2 and Supplemental Table 1 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used: PTH, parathyroid hormone; Δ-25(OH)D, serum 25(OH)D at delivery minus serum 25(OH)D at study entry; 1,25(OH)2D, 1,25-dihydroxyvitamin D; 24,25(OH)2D, 24,25-dihydroxyvitamin D; 24,25(OH)2D3, 24,25-dihydroxycholecalciferol; 25(OH)D, 25-hydroxyvitamin D; 25(OH)D2, 25-hydroxyergocalciferol; 25(OH)D3, 25-hydroxycholecalciferol.
References
- 1. Olausson H, Goldberg GR, Laskey MA, Schoenmakers I, Jarjou LM, Prentice A. Calcium economy in human pregnancy and lactation. Nutrition Res Rev 2012;25:40–67. [DOI] [PubMed] [Google Scholar]
- 2. Baxter-Jones AD, Faulkner RA, Forwood MR, Mirwald RL, Bailey DA. Bone mineral accrual from 8 to 30 years of age: an estimation of peak bone mass. J Bone Miner Res 2011;26:1729–39. [DOI] [PubMed] [Google Scholar]
- 3. Ginde AA, Sullivan AF, Mansbach JM, Camargo CA Jr.. Vitamin D insufficiency in pregnant and nonpregnant women of childbearing age in the United States. Am J Obstet Gynecol 2010;202:436.e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Berner LA, Keast DR, Bailey RL, Dwyer JT. Fortified foods are major contributors to nutrient intakes in diets of US children and adolescents. J Acad Nutr Diet 2014;114:1009–22.e8. [DOI] [PubMed] [Google Scholar]
- 5. Brannon PM, Picciano MF. Vitamin D in pregnancy and lactation in humans. Annu Rev Nutr 2011;31:89–115. [DOI] [PubMed] [Google Scholar]
- 6. Heaney RP, Skillman TG. Calcium metabolism in normal human pregnancy. J Clin Endocrinol Metab 1971;33:661–70. [DOI] [PubMed] [Google Scholar]
- 7. Givens MH, Macy IG. The chemical composition of the human fetus. J Biol Chem 1933;102:7–17. [Google Scholar]
- 8. Kovacs CS. Calcium metabolism during pregnancy and lactation. In: De Groot LJ, Chrousos G, Dungan K, Feingold KR, Grossman A, Hershman JM, Koch C, Korbonits M, McLachlan R, New Met al., editors. Endotext. South Dartmouth, MA: MDText.com, Inc., 2000. [Google Scholar]
- 9. Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes for calcium and vitamin D. Washington (DC): The National Academies Press, 2011. [PubMed] [Google Scholar]
- 10. Salles JP. Bone metabolism during pregnancy. Ann Endocrinol (Paris) 2016;77:163–8. [DOI] [PubMed] [Google Scholar]
- 11. Wagner CL, Hollis BW. Beyond PTH: assessing vitamin D status during early pregnancy. Clin Endocrinol (Oxf) 2011;75:285–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kovacs CS. Vitamin D in pregnancy and lactation: maternal, fetal, and neonatal outcomes from human and animal studies. Am J Clin Nutr 2008;88:520s–8s. [DOI] [PubMed] [Google Scholar]
- 13. Cashman KD, Hayes A, Galvin K, Merkel J, Jones G, Kaufmann M, Hoofnagle AN, Carter GD, Durazo-Arvizu RA, Sempos CT. Significance of serum 24,25-dihydroxyvitamin D in the assessment of vitamin D status: a double-edged sword? Clin Chem 2015;61:636–45. [DOI] [PubMed] [Google Scholar]
- 14. Jones KS, Assar S, Prentice A, Schoenmakers I. Vitamin D expenditure is not altered in pregnancy and lactation despite changes in vitamin D metabolite concentrations. Sci Rep 2016;6:26795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Park H, Brannon PM, West AA, Yan J, Jiang X, Perry CA, Malysheva OV, Mehta S, Caudill MA. Vitamin D metabolism varies among women in different reproductive states consuming the same intakes of vitamin D and related nutrients. J Nutr 2016;146:1537–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jones G, Prosser DE, Kaufmann M. Cytochrome P450-mediated metabolism of vitamin D. J Lipid Res 2014;55:13–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Heaney RP, Davies KM, Chen TC, Holick MF, Barger-Lux MJ. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr 2003;77:204–10. [DOI] [PubMed] [Google Scholar]
- 18. Young BE, McNanley TJ, Cooper EM, McIntyre AW, Witter F, Harris ZL, O'Brien KO. Vitamin D insufficiency is prevalent and vitamin D is inversely associated with parathyroid hormone and calcitriol in pregnant adolescents. J Bone Miner Res 2012;27:177–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Murphy SP, Barr SI. Practice paper of the American Dietetic Association: using the Dietary Reference Intakes. J Am Diet Assoc 2011;111:762–70. [DOI] [PubMed] [Google Scholar]
- 20. Holick MF. Environmental factors that influence the cutaneous production of vitamin D. Am J Clin Nutr 1995;61:638s–45s. [DOI] [PubMed] [Google Scholar]
- 21. Laha TJ, Strathmann FG, Wang Z, de Boer IH, Thummel KE, Hoofnagle AN. Characterizing antibody cross-reactivity for immunoaffinity purification of analytes prior to multiplexed liquid chromatography-tandem mass spectrometry. Clin Chem 2012;58:1711–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Abbassi-Ghanavati M, Greer LG, Cunningham FG. Pregnancy and laboratory studies: a reference table for clinicians. Obstet Gynecol 2009;114:1326–31. [DOI] [PubMed] [Google Scholar]
- 23. Seki K, Makimura N, Mitsui C, Hirata J, Nagata I. Calcium-regulating hormones and osteocalcin levels during pregnancy: a longitudinal study. Am J Obstet Gynecol 1991;164:1248–52. [DOI] [PubMed] [Google Scholar]
- 24. Reddy GS, Norman AW, Willis DM, Goltzman D, Guyda H, Solomon S, Philips DR, Bishop JE, Mayer E. Regulation of vitamin D metabolism in normal human pregnancy. J Clin Endocrinol Metab 1983;56:363–70. [DOI] [PubMed] [Google Scholar]
- 25. Markestad T, Ulstein M, Bassoe HH, Aksnes L, Aarskog D. Vitamin D metabolism in normal and hypoparathyroid pregnancy and lactation. Case report. Br J Obstet Gynaecol 1983;90:971–6. [DOI] [PubMed] [Google Scholar]
- 26. Cooper C, Harvey NC, Bishop NJ, Kennedy S, Papageorghiou AT, Schoenmakers I, Fraser R, Gandhi SV, Carr A, D'Angelo Set al. Maternal gestational vitamin D supplementation and offspring bone health (MAVIDOS): a multicentre, double-blind, randomised placebo-controlled trial. Lancet Diabetes Endocrinol 2016;4:393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011;96:1911–30. [DOI] [PubMed] [Google Scholar]
- 28. Fudge NJ, Kovacs CS. Pregnancy up-regulates intestinal calcium absorption and skeletal mineralization independently of the vitamin D receptor. Endocrinology 2010;151:886–95. [DOI] [PubMed] [Google Scholar]
- 29. Kirby BJ, Ma Y, Martin HM, Buckle Favaro KL, Karaplis AC, Kovacs CS. Upregulation of calcitriol during pregnancy and skeletal recovery after lactation do not require parathyroid hormone. J Bone Miner Res 2013;28:1987–2000. [DOI] [PubMed] [Google Scholar]
- 30. Kovacs CS, Woodland ML, Fudge NJ, Friel JK. The vitamin D receptor is not required for fetal mineral homeostasis or for the regulation of placental calcium transfer in mice. Am J Physiol Endocrinol Metab 2005;289:E133–44. [DOI] [PubMed] [Google Scholar]
- 31. Rummens K, van Cromphaut SJ, Carmeliet G, van Herck E, van Bree R, Stockmans I, Bouillon R, Verhaeghe J. Pregnancy in mice lacking the vitamin D receptor: normal maternal skeletal response, but fetal hypomineralization rescued by maternal calcium supplementation. Pediatr Res 2003;54:466–73. [DOI] [PubMed] [Google Scholar]
- 32. Cardot-Bauters C. Hypoparathyroidism and pregnancy. Ann Endocrinol (Paris) 2016;77:172–5. [DOI] [PubMed] [Google Scholar]
- 33. Singh HJ, Mohammad NH, Nila A. Serum calcium and parathormone during normal pregnancy in Malay women. J Matern Fetal Med 1999;8:95–100. [DOI] [PubMed] [Google Scholar]
- 34. Vargas Zapata CL, Donangelo CM, Woodhouse LR, Abrams SA, Spencer EM, King JC. Calcium homeostasis during pregnancy and lactation in Brazilian women with low calcium intakes: a longitudinal study. Am J Clin Nutr 2004;80:417–22. [DOI] [PubMed] [Google Scholar]
- 35. Ardawi MS, Nasrat HA, BA'Aqueel HS. Calcium-regulating hormones and parathyroid hormone-related peptide in normal human pregnancy and postpartum: a longitudinal study. Eur J Endocrinol 1997;137:402–9. [DOI] [PubMed] [Google Scholar]
- 36. Ritchie LD, Fung EB, Halloran BP, Turnlund JR, Van Loan MD, Cann CE, King JC. A longitudinal study of calcium homeostasis during human pregnancy and lactation and after resumption of menses. Am J Clin Nutr 1998;67:693–701. [DOI] [PubMed] [Google Scholar]
- 37. Kramer CK, Ye C, Hanley AJ, Connelly PW, Sermer M, Zinman B, Retnakaran R. The relationship between parathyroid hormone and 25-hydroxyvitamin D during and after pregnancy. J Clin Endocrinol Metab 2016;101:1729–36. [DOI] [PubMed] [Google Scholar]
- 38. Lips P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr Rev 2001;22:477–501. [DOI] [PubMed] [Google Scholar]
- 39. Hollis BW, Johnson D, Hulsey TC, Ebeling M, Wagner CL. Vitamin D supplementation during pregnancy: double-blind, randomized clinical trial of safety and effectiveness. J Bone Miner Res 2011;26:2341–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Carpenter TO. CYP24A1 loss of function: clinical phenotype of monoallelic and biallelic mutations. J Steroid Biochem Mol Biol 2017;173:337–40. [DOI] [PubMed] [Google Scholar]
- 41. Reiter EO, Braunstein GD, Vargas A, Root AW. Changes in 25-hydroxyvitamin D and 24,25-dihydroxyvitamin D during pregnancy. Am J Obstet Gynecol 1979;135:227–9. [DOI] [PubMed] [Google Scholar]
- 42. Markestad T, Ulstein M, Aksnes L, Aarskog D. Serum concentrations of vitamin D metabolites in vitamin D supplemented pregnant women. A longitudinal study. Acta Obstet Gynecol Scand 1986;65:63–7. [DOI] [PubMed] [Google Scholar]
- 43. O'Brien KO, Nathanson MS, Mancini J, Witter FR. Calcium absorption is significantly higher in adolescents during pregnancy than in the early postpartum period. Am J Clin Nutr 2003;78:1188–93. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


