Abstract
Acrylamide (ACR) is one of the most important contaminants occurring in foods heated at high temperatures. The aim of this study is to investigate the protective efficacy of extra virgin olive oil (EVOO), a main component of the Mediterranean diet, against nephrotoxicity induced by ACR. Rats have received by gavage during 21 days either ACR (40 mg/kg body weight) or ACR-associated with EVOO (300 μl) or only EVOO (300 μl). Acrylamide induced nephrotoxicity as evidenced by an increase in malondialdehyde (MDA), hydrogen peroxide (H2O2), protein carbonyls (PCOs) and a decrease in glutathione, non-protein thiols (NPSHs), and vitamin C levels. Activities of catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GPx) were also decreased. Lactate dehydrogenase (LDH) activity, creatinine, urea, and uric acid, urinary volume and creatinine clearance levels were modified. EVOO supplementation improved all the parameters indicated above. Kidney histoarchitecture confirmed the biochemical parameters and the beneficial role of EVOO. EVOO, when added to the diet, may have a beneficial role against kidney injury by scavenging free radicals and by its potent antioxidant power.
Keywords: Acrylamide, rats, nephrotoxicity, antioxidant status, extra virgin olive oil
Introduction
Acrylamide (ACR) is widely used in polyacrylamide synthesis and in other industrial products. In addition to industrial and laboratory uses, ACR is formed spontaneously during Maillard browning reaction between reducing sugars (glucose and fructose) and free amino acids mainly asparagine. 1 High levels of ACR have been found in foods heated at high temperatures, especially in potato products, such as French fries and potato crisps; cereal products, and roasted coffee. 2 , 3 Following exposure via oral ingestion or inhalation, ACR accumulates at higher levels in the blood than in any other tissues. 4 It can be rapidly distributed to all tissues and transformed into glycidamide (GA), a more toxic form than ACR, by CYP2E1. 5 The latter is one of several CYPs known to cause bio-activation metabolizing a range of exogenous substances, including ACR. GA can be transformed by hydrolysis to glyceramide or glutathione conjugation leading to the formation of two mercapturic acid products excreted in the urine. 5 , 6 Moreover, ACR attacks the biological molecules through the interaction between its vinyl group, SH and NH2 of proteins mainly hemoglobin. 7 , 8 It has been known to be neurotoxic, genotoxic, and carcinogenic. 9 It has been reported that ACR causes disturbances in redox status and enzyme activities in rats. 10 , 11 An imbalance between the production of reactive oxygen species (ROS) and antioxidant capacity increases oxidative stress which plays a critical role in the toxicity induced by ACR. 11–13
To overcome oxidative stress, antioxidants and plant phenolics are considered as the chemoprotective agents against oxidative stress-related diseases. Extra virgin olive oil (EVOO) has been recognized for its antioxidant properties and its positive effects against oxidative stress. 14 Previous studies have demonstrated the ability of EVOO to modify cellular membrane structure and to reduce oxidative injury. 15 , 16 The beneficial health effects of olive oil have been mainly attributed to its high oleic acid content and to its powerful antioxidant capacity due to the presence of phenolic compounds. 17 EVOO appears to be a functional food with various components such as monounsaturated fatty acids (MUFA) with nutritional benefits. It contains 80% of omega-9 MUFA, oleic acid, and other minor components such as aliphatic alcohols, sterols, and polyphenols (α-tocopherols, tyrosol, oleuropein and hydroxytyrosol). 18 In vivo and in vitro studies suggested that phenolic hydroxytyrosol (HTy) and oleuropein compounds of EVOO are effective antioxidants through the inhibition of lipid peroxidation and by scavenging of free radicals. 19 In addition to their direct antioxidant capacity, their effects on cardiovascular system are beneficial to health due to the anti-inflammatory, anti-thrombotic, and anti-hypertensive actions. 20 To our knowledge, this is the first study that evaluates the protective effects of EVOO against kidney damage and histological changes in ACR treated rats.
Materials and methods
Chemicals
ACR, glutathione (oxidized and reduced), nicotinamide adenine dinucleotide phosphate reduced form (NADPH), 5, 5′-dithiobis-2-nitrobenzoic acid (DTNB) and thiobarbituric acid (TBA) were purchased from Sigma (St. Louis; MO). All other chemicals, of analytical grades, were purchased from standard commercial suppliers.
Oil samples analysis
Biologic EVOO samples were obtained from a Chetoui variety cultivar grown in the North of Tunisia. To verify the quality criteria of olive oil, free acidity, conventionally expressed in oleic acid (g/100 g), peroxide value (PV) (meqO2/kg), and UV absorption characteristics (K232 and K270) were determined according to the International Olive Oil Council. 21
Different constituents of EVOO were analyzed:
Fatty acids were converted into fatty acid methyl esters (FAMEs) prepared by dissolving 0.1 g of EVOO in methanol and incubated for 1 h. Individual FAMES were separated and quantified by gas chromatography using model 5890 series II instrument (Hewlett-Packard Ca Palo Alto, Calif.) equipped with a flame ionization detector and a fused silica capillary column HP – INNOWAX (30 m length ×0.25 mm i.d. and 0.25 μm of film thickness). The temperature was programmed to increase from 170 to 270 °C at a rate of 5 °C/min. Nitrogen ultra was used as carrier gas. The results were expressed as a relative area percent of the total FAMEs. 22
Carotenoids and chlorophylls (mg/kg oil) were determined at 470 and 670 nm, respectively, in cyclohexane using the specific extinction values according to the method of Minguez Mosquera’s et al. 23
The phenolic compounds were extracted, estimated colorimetrically at 765 nm using the Folin–Ciocalteau reagent, and expressed as hydroxytyrosol equivalents as reported by Montedoro et al. 24
α-Tocopherol was evaluated according to the method of Gimeno et al. 25 Each oil sample was diluted with n-hexane (1:10), the mixture was vortexed and 200 μl were transferred to a test tube containing 600 μl of methanol and 200 μl of internal standard (300 μg/ml). HPLC separation was carried out on a Hewlett-Packard system (Waldbronn, Germany) equipped with a HP-1100 pump, a Rheodyne model 7725 injector (Cotati, CA, loop volume 20 μl), a HP-1200 M multi-array detector and a Supelcosil ODS-2 column (150 × 4.5 mm id., film thickness 5 μm).
Animals and treatment
Female Wistar rats, weighing 160 ± 10 g, were obtained from the Central Pharmacy (SIPHAT, Tunisia). They were housed at ambient temperature (22 ± 2 °C) in a 12-h light/dark cycle and a minimum relative humidity of 40%. Food (SNA, Sfax, Tunisia) and water were available ad libitum. One week after acclimatization to laboratory conditions, the rats were randomly divided into four groups of six each.
Group 1: serving as negative controls where rats received daily distilled water.
Group 2: rats received daily by gavage ACR at a dose of 40 mg/kg BW.
Group 3: rats received daily by gavage both ACR and 300 μL of EVOO.
Group 4: serving as positive controls where rats received daily by gavage 300 μl of EVOO.
Water and food intake by rats and their body weights were recorded daily. Twenty-four hours before the sacrifice day, the rats were placed individually in metabolic cages for urine collection. After recording 24 h urine volumes, samples were centrifuged at 5000 × g for 5 min to eliminate the probable presence of excrements. The supernatants were collected and then stored at –20 °C for further analysis. At the end of the experimental period (21 days), all rats were euthanized by cervical decapitation to avoid stress. The trunk blood was collected into heparinized tubes and centrifuged at 2200 × g for 10 min. Plasma samples were drawn and stored at –80 °C until analysis. Kidneys were dissected out, cleaned and weighed. Some portions were rinsed, homogenized in Tris–HCl buffer (pH = 7.4) and centrifuged. The resulting supernatants were kept at –80 °C for biochemical assays. Other kidney samples were immediately removed, cleaned, fixed in 10% buffered formalin solution and embedded in paraffin for histological studies.
The experimental procedures were carried out according to the General Guidelines on the Use of living Animals in Scientific Investigations 26 and approved by the Ethical Committee of Sciences Faculty, Sfax University.
Biochemical assays
Protein quantification
Kidney protein contents were measured according to the method of Lowry et al. 27 using bovine serum albumin as standard.
Assay of oxidative stress and antioxidant defenses in kidney tissue
The concentrations of malondialdehyde (MDA), an index of lipid peroxidation, in kidney tissue were determined according to the method of Draper and Hadley. 28 Hydrogen peroxide (H2O2) generation was assessed as described by Ou and Wolff method. 29 PCO content was measured using the dinitrophenyl hydrazine (DNPH) method of Reznick and Packer. 30
The reduced glutathione (GSH) content in kidney tissue was assayed according to the method of Ellman 31 modified by Jollow et al. 32 Kidney non-protein thiol (NPSH) levels were determined by the method of Ellman 31 and results were expressed as nmol/mg protein. Ascorbic acid level in kidney was performed as described by Jacques-Silva et al. 33 Catalase (CAT), superoxide dismutase (SOD) and glutathione peroxidase (GPx) activities were measured according to the methods of Aebi, 34 Beauchamp and Fridovich 35 and Flohe and Gunzler 36 , respectively.
Estimation of urea, uric acid, creatinine and creatinine clearance in plasma and urine
The levels of urea, uric acid, and creatinine in plasma and urine were assayed spectrophotometrically using commercially available diagnostic kits (Biomaghreb, Tunisia, Ref. 20143, 20095, 20151, respectively). Creatinine clearance was calculated using UV/P equation, 37 where U is the urinary creatinine level, V the urinary volume collected within 24 h, and P the plasma creatinine level.
Plasma and kidney lactate dehydrogenase activities
Lactate dehydrogenase (LDH) activities in plasma and kidney were measured using a commercially available diagnostic kit (Biomaghreb, Tunisia, Ref. 200125).
Histological studies
Some kidney samples, intended for histological examination by light microscopy, were immediately fixed in formalin solution (10%) and processed in a series of graded ethanol solutions. Then, they were embedded in paraffin. Blocks were made and sectioned at a thickness of 5 μm and stained with hematoxylin and eosin, then examined under light microscopy (ZEISS, Axiolab) and were fitted with Canon Power Shot camera (A640) to capture images for histological studies.
Statistical analysis
The data were analyzed using the statistical package program Stat view 5 Software for Windows (SAS Institute, Berkley, CA). Statistical analysis was performed using one-way analysis of variance (ANOVA) followed by Fisher’s protected least significant difference (PLSD) test as a post hoc test for comparison between groups. Student unpaired t-test was also used when comparison between two groups was required. All values were expressed as means ± SE. The .05 level was selected as the point of minimal statistical significance.
Results
Quality criteria of olive oil
The quality criteria of olive oil (acidity, peroxide values and UV specific extinction) were determined. The values of these parameters (Table 1) were included in the ranges established for EVOO category (International Olive Oil Council 21 ).
Table 1.
Analytical parameters of EVOO.
| Analytical parameters | Mean values |
|---|---|
| Free acidity (g/100 g) | 0.41 |
| K232 | 2 |
| K270 | 0.16 |
| PV (meqO2/kg) | 7.5 |
| Fatty acid (%) | |
| Palmitic acid (C16:0) | 12.68 ± 0.12 |
| Palmitoleic acid (C16:1w7) | 0.54 ± 0.04 |
| Stearic acid (C18:0) | 2.80 ± 0.06 |
| Oleic acid (C18:1w9) | 66.35 ± 0.39 |
| Linoleic acid (C18:2w6) | 16.03 ± 0.41 |
| Linolenic acid (C18:3w3) | 0.70 ± 0.03 |
| Arachidonic acid (C20:0) | 0.44 ± 0.02 |
| Gadoleic acid (C20:1w-9) | 0.34 ± 0.10 |
| SFA | 15.98 ± 0.13 |
| MUFA | 67.23 ± 0.37 |
| PUFA | 16.73 ± 0.43 |
| MUFA/PUFA | 4.02 ± 0.13 |
| Antioxidant content (mg/kg) | |
| Chlorophylls | 7.40 ± 0.06 |
| β-Carotene | 9.17 ± 0.51 |
| Total polyphenols | 486.01 ± 62.26 |
| α-tocopherol | 1242.66 ± 75.26 |
| β-tocopherol | 46.26 ± 10.01 |
MUFA: monounsaturated fatty acid; PUFA: polyunsaturated fatty acid; SFA: saturated fatty acids.
Analytical parameters of EVOO and its compounds
EVOO analytical parameters (fatty acid and antioxidant composition) are presented in Table 1. EVOO contained 15.98% of saturate (palmitic and stearic acids), 67.23% of monounsaturate (mainly oleic acid), and 17% of polyunsaturate fatty acids. Chlorophyl pigments, total phenolic compounds, and tocopherols were calculated in EVOO and reported in Table 1.
Evaluation of body and absolute kidney weights
Death was not observed during the experimental period. As shown in Table 2, our results recorded a significant decrease in the body weight gain and in the absolute kidney weight of treated rats with ACR when compared with those of controls (p < .001). When EVOO was supplemented to ACR treated rats, a recovery occurred in body and in absolute kidney weights (Table 2).
Table 2.
Initial and final body weights, absolute kidney weight, daily food and water consumption in control and treated rats with ACR, EVOO and their combination (ACR + EVOO) during 21 days.
| Parameters and treatment | Controls | ACR | ACR + EVOO | EVOO |
|---|---|---|---|---|
| Initial body weights (g) | 161 ± 2.88 | 162.31 ± 1.49 | 162.69 ± 1. 19 | 161.5 ± 3.81 |
| Final body weight (g) | 180.33 ± 6.81 | 167.72 ± 1.96 *** | 182. 5 ± 2.97+++ | 185.51 ± 5.32 |
| Absolute kidney weights (g) | 1.22 ± 0.11 | 0.97 ± 0.07 *** | 1.18 ± 0.03++ | 1.27 ± 0.03 |
| Food consumption (g/day/rat) | 12.39 ± 2.23 | 9.86 ± 1.44 *** | 12.51 ± 1.11+++ | 11.75 ± 0.89 |
| Drinking water intake (ml/day/rat) | 27.92 ± 4.32 | 22.81 ± 2.13 *** | 25.96 ± 1.64 * +++ | 26.17 ± 2.03 |
Values are means ± SE for six rats in each group.
ACR; (ACR + EVOO) and EVOO treated groups vs. control group: *p < .05; ***p < .001.
(ACR + EVOO) group vs. ACR group: ++p < .01; +++p < .001.
Food and water intake
Compared with controls, food and water consumption by ACR treated rats were reduced by 20 and 18%, respectively and no changes were observed in rats treated only by EVOO.
Co-administration of EVOO to ACR treated rats increased significantly food and water intake (Table 2).
Estimation of MDA, H2O2, PCO and non-enzymatic antioxidant levels in kidney
Our results showed a significant increase in lipid peroxidation, H2O2, and protein oxidation by 188, 63, and 108% in ACR treated rats when compared with those of controls. When EVOO was administered with ACR, a significant reduction of these biomarkers in the kidney was observed when compared with ACR treated group (Table 3).
Table 3.
MDA, H2O2, PCO, GSH, NPSH and vitamin C levels in the kidney of control and treated rats with ACR, EVOO and their combination during 21 days of treatment.
| Parameters & treatment |
Controls |
ACR |
ACR + EVOO |
EVOO |
| MDA a | 2.03 ± 0.43 | 5.85 ± 0.75*** | 2.96 ± 0.34+++* | 2.61 ± 0.36 |
| H2O2 b | 29.03 ± 2.45 | 47.34 ± 2.97*** | 26.61 ± 2.51+++ | 30.92 ± 1.94 |
| PCO b | 1.56 ± 0.22 | 3.25 ± 0.34*** | 1.76 ± 0.19+++ | 1.45 ± 0.08 |
| GSH c | 9.71 ± 0.52 | 6.25 ± 0.75*** | 9.35 ± 0.92+++ | 9.46 ± 0.76 |
| NPSH b | 31.65 ± 3.03 | 22.99 ± 3.15*** | 29.42 ± 2.47++ | 29.96 ± 2.24 |
| Vitamin C d | 1.51 ± 0.13 | 0.83 ± 0.17*** | 1.41 ± 0.13+++ | 1.46 ± 0.19 |
nmoles of MDA/mg protein.
nmoles/mg protein.
μg/mg protein.
μmol/mg protein.
Values are means ± SE for six rats in each group.
ACR; (ACR + EVOO) and EVOO treated groups vs. control group: *p < .05; ***p < .001.
(ACR + EVOO) group vs. (ACR) group: ++ p < .01; +++p < .001.
A significant decrease in GSH (36%), NPSH (27%), and vitamin C (45%) levels was shown in rats exposed to ACR when compared with controls. These modifications were significantly alleviated following co-administration of EVOO (Table 3).
Enzymatic antioxidant status in the kidney
ACR treatment resulted in a significant decrease of CAT (−41%), SOD (−17%), and GPx activities (−35%) when compared with those of controls. Co-treatment of ACR treated rats with EVOO improved enzymatic antioxidant activities (Figure 1).
Figure 1.
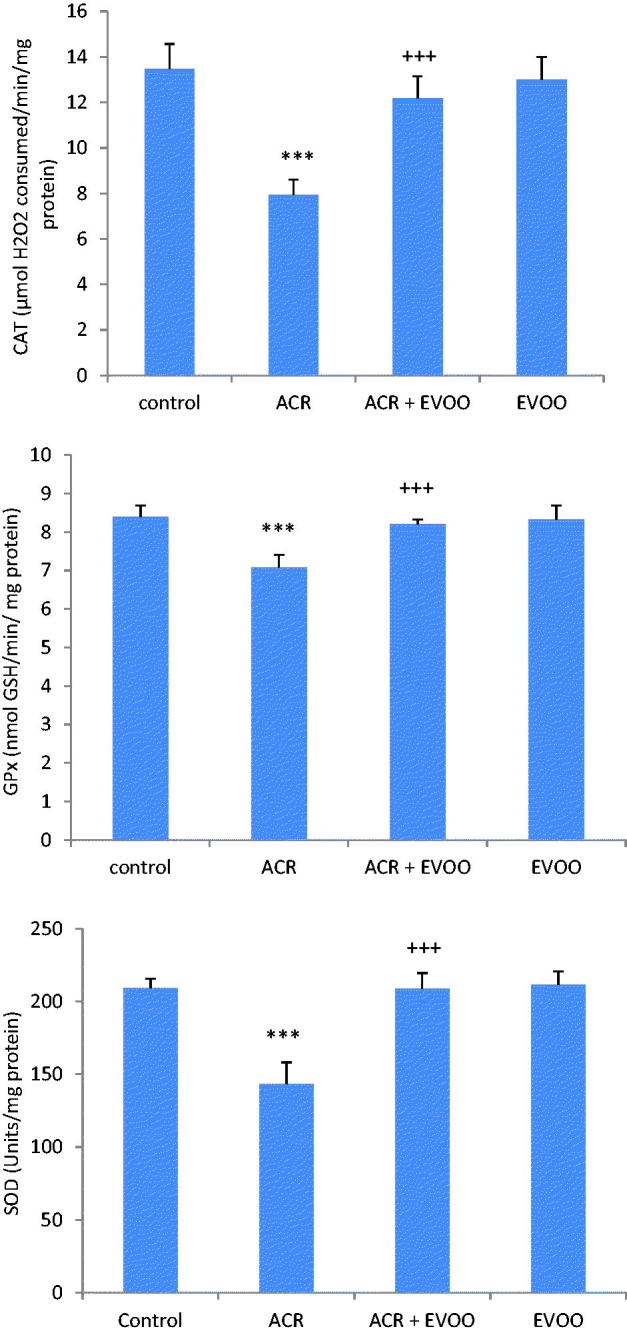
Antioxidant enzyme activities (CAT, GPx and SOD) in the kidney of control and treated rats with ACR, EVOO and their combination (ACR + EVOO) during 21 days. Values are means ± SE for six rats in each group. ACR, (ACR + EVOO) and EVOO treated groups vs. control group: ***p < .001. (ACR + EVOO) group vs. ACR group: +++p < .001.
Biomarkers of kidney damage
In ACR treated rats, LDH activity, a biomarker of membrane kidney damage, was increased in plasma by 25% and decreased in kidney by 40% when compared with those of controls. Co-treatment with EVOO improved LDH activities when compared with those of ACR treated rats (Figure 2).
Figure 2.
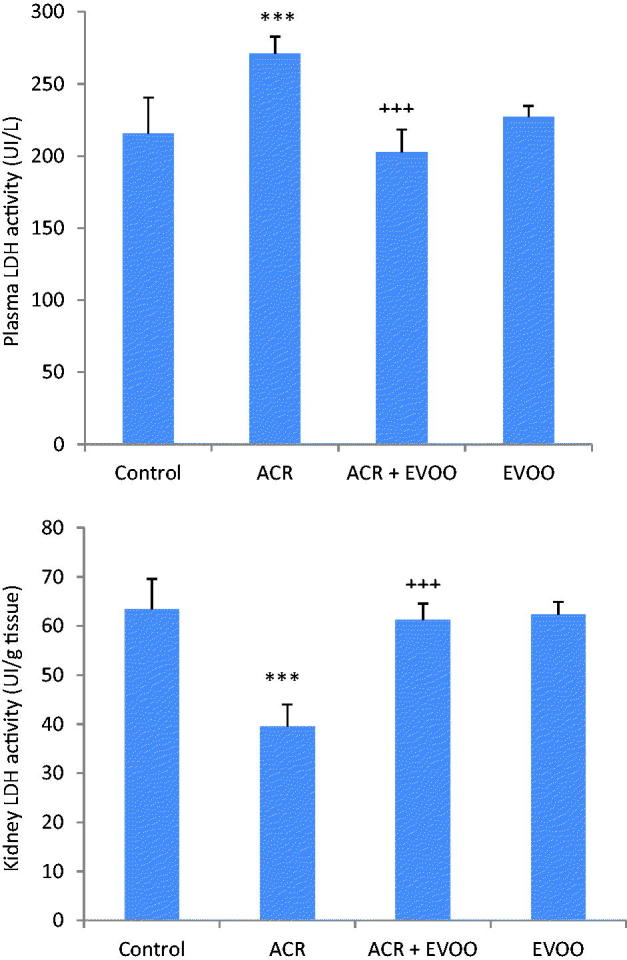
LDH activities in plasma and in kidney of control and treated rats with ACR, EVOO and their combination (ACR + EVOO) during 21 days. Values are means ± SE for six rats in each group. ACR, (ACR + EVOO) and EVOO treated groups vs. control group: ***p < .001. (ACR + EVOO) group vs. ACR group: +++p < .001.
Other kidney specific biomarkers were analyzed. Our results showed that creatinine and urea levels of ACR treated rats were higher in plasma (+31 and +52%, respectively) and lower in urine (−44 and −66%) than those of the controls (Table 4). While, the level of uric acid, a nitrogenous waste product, was decreased in plasma (−10%) and its urinary excretion was increased (+38%) when compared with controls. ACR-treated rats showed also a constellation of disorders in renal function witnessed by an increased urinary volume (+18%) and a reduction of creatinine clearance, considered as an indicator of glomerular dysfunction (−51%). Co-treatment with EVOO extract improved renal biomarkers when compared with those of ACR treated rats (Table 4).
Table 4.
Plasma and urinary levels of creatinine, urea and uric acid and creatinine clearance of control and treated rats with ACR, (EVOO) and their combination (ACR + EVOO) during 21 days.
| Parameters and treatments | Control | ACR | ACR + EVOO | EVOO |
|---|---|---|---|---|
| Creatinine (μmol/l) | ||||
| Plasma | 129.58 ± 4.77 | 170.15 ± 6.02*** | 130.33 ± 6.53+++ | 132.69 ± 3.37 |
| Urine | 4612 ± 423.58 | 2586 ± 261.41*** | 4530 ± 367.85+++ | 4565.5 ± 211.42 |
| Urea (mmol/l) | ||||
| Plasma | 4.73 ± 0.39 | 7.2 ± 0.46*** | 5.53 ± 0.32+++* | 4.81 ± 0.23 |
| Urine | 439.36 ± 44.99 | 146.8 ± 13.72*** | 423.2 ± 30.63+++ | 427.4 ± 20.57 |
| Uric acid (μmol/l) | ||||
| Plasma | 255.67 ± 3.95 | 229.72 ± 4.47*** | 248.11 ± 5.39* +++ | 252.71 ± 4.11 |
| Urine | 1420 ± 277.49 | 1969 ± 165.62** | 1474.8 ± 87.21+++ | 1488 ± 80.75 |
| Urinary volume | 9.68 ± 0.81 | 11.4 ± 0.71** | 10.02 ± 0.53++ | 10.16 ± 0.59 |
| Creatinine clearance | 357.51 ± 26.52 | 174.09 ± 17.15*** | 374.01 ± 17.09+++ | 366.54 ± 25.26 |
Values were expressed as means ± SD. The number of determinations was n = 6.
ACR, (ACR + EVOO) and EVOO groups vs. control group: *p < .05; **p < .01; ***p < .001.
(ACR + EVOO) vs. ACR: ++p < .01; +++p < .001.
Histopathological studies
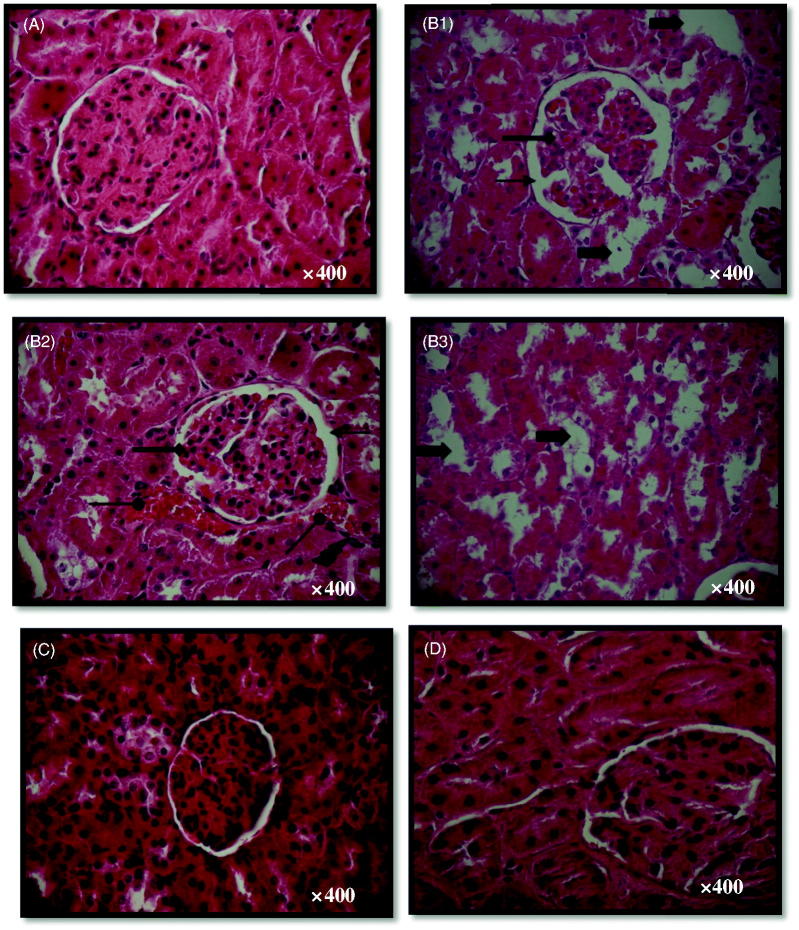
The light microscopy evaluation of kidney in control group showed normal renal parenchyma and glomeruli (Figure 3(A)). While renal histoarchitecture of ACR treated rats showed a glomeruli fragmentation, an enlargement of glomeruli Bowman’s space, a marked hemorrhage, an infiltration of leucocytes and a tubular dilatation (Figure 3(B1–B3)). This pathological aspect was alleviated by the administration of EVOO to ACR treated group (Figure 3(C)). There were no histological alterations in the kidney of positive controls treated with EVOO alone (Figure 3(D)).
Figure 3.
Histological kidney sections of control (A) and treated rats with ACR (B1, B2 and B3), EVOO and ACR (C) and EVOO (D) during 21 days. Arrows indicate:  glomeruli showing enlarged Bowman’s space,
glomeruli showing enlarged Bowman’s space, tubular dilatation,
tubular dilatation,  infiltration of leucocyte cells,
infiltration of leucocyte cells,  hemorrhage
hemorrhage  glomeruli fragmentation
glomeruli fragmentation
Discussion
ACR is one of the most important contaminants occurring in heated food products. Exposure of humans and animals to ACR via their diet is currently one of the most serious global health concerns. Natural dietary antioxidants have been given attention as possible protective agents and as a health food supplement against toxicity induced by dietary contaminants. Our study was planned to evaluate the protective effect of EVOO against kidney damage and histological changes induced by ACR in rats.
The exposure of rats to ACR was hazardous to physical variables based on body and organ weights. In the present investigation, ACR treatment led to a significant decrease in body and absolute kidney weights. The reduction of daily food and water consumption in ACR-treated rats was in line with a decline of body and kidney weights. Similar results have been found by Motamedshariaty et al. 38 in ACR treated rats at a dose of 50 mg/kg body weight for 11 days. The decline of body weight could be explained by a metabolic disorder causing energy metabolism pathways which interfere with ACR. 39 In our experimental study, administration of EVOO to ACR-treated group improved body and kidney weights, which could be attributed to an increase in daily food and water intakes as reported in our previous studies performed on liver and heart toxicity induced by both aluminum and ACR. 40 , 41
These morphological changes of body and kidney weights in ACR treated rats were accompanied with an alteration of renal function. The increase of blood urea and serum creatinine is considered as the marker of kidney impairment according to Karahan et al. 42 Moreover, creatinine, a by-product of the muscle energy metabolism, is filtered through glomeruli and excreted into the urine. 43 In the present study, an increase of plasma creatinine, urea and a decrease of creatinine clearance reflected the diagnosis of renal failure. Similar results have been reported by Raju et al. 44 in ACR treated rats with 5, 10, or 50 mg/kg of diet for 10 weeks. Furthermore, kidney dysfunction, marked mainly by a glomerular filtration decline, was accompanied by an increase in a 24-h urinary volume output in ACR treated rats. Another biochemical marker, used in the current study to evaluate kidney function, was uric acid levels in plasma and urine. This compound is the end product of purine catabolism which can reduce oxidative stress by scavenging various reactive oxygen species. 45 In our experimental study, treatment of rats by ACR resulted in a decrease of plasma uric acid levels and an increase in its urinary excretion. Co-administration of EVOO to ACR treated rats attenuated the kidney impairment as suggested by a significant improvement of the renal biomarkers indicated above. This protection may be attributed according to De la Torre 46 to the ω-9 MUFA oleic acid and anti-oxidative polyphenols in EVOO.
Reactive oxygen species (ROS) are considered as the important modulators of renal blood flow and glomerular filtration rate. They are produced during normal cellular function to maintain an appropriate balance between ROS and antioxidants in order to ensure the structural integrity of cellular constituents. Disturbances in the normal redox state of cells can cause toxic effects on biological structures such as lipids, proteins and DNA. Our results indicated that the pro-oxidant effect of ACR altered membrane integrity and fluidity in the cells of adult rats through the generation of ROS. Their enhanced production led to membrane disruption and oxidation of poly unsaturated fatty acids after ACR treatment as revealed by a marked elevation in kidney MDA, index of lipid peroxidation. Oxidative injury may cause molecular disorganization of lipids resulting in increased membrane permeability and leakage of cellular enzymes into blood. 47 In addition, LDH, an intracellular enzyme, decreased in kidney tissue and increased in the plasma of ACR treated group, confirming the increase in membrane permeability. Furthermore, ACR generated kidney oxygen radical production like H2O2 suggesting the dysfunction of the mitochondrial respiration chain. Free radicals which are responsible for lipid peroxidation can also cause deleterious effects on proteins. The oxidative damage of proteins was reflected in our study by an increase of PCO levels in ACR treated group. In the current investigation, the increase of lipid peroxidation, protein carbonylation and H2O2 formation support the hypothesis that nephrotoxicity induced by ACR is related to involvement of reactive oxygen species. In fact, mitochondria are the main producers of ROS and also the primary targets of oxidative damage. ACR induced mitochondrial damage including inhibition of electron transport chain, ATPase and SOD activities and the depolarization of mitochondrial membrane potential as reported by Zhu et al. 13 Previous studies have found a significant increase in kidney MDA of ACR treated rats. 11 , 12 The potential role of oxidative stress following ACR exposure suggests that antioxidant supplementation may mitigate ACR-induced toxicity. In our experimental study, EVOO may protect kidney from oxidative damage by scavenging reactive species and/or preventing their formation. Previous studies have shown that EVOO increases the resistance to lipid and protein oxidation and the antioxidant defense system due to its high content of phenolic. 40 , 41 , 48 The phenolic compounds of olive oil are located at the surface of the phospholipid bilayer in membrane cells in order to delay the peroxidation of phospholipids initiated by free radicals from the aqueous phase, to chelate peroxyl radicals and to regenerate of α-tocopherol. 49
To alleviate the effects of oxidative stress, the cells have several ways either by repairing or by reducing the occurrence of oxidative damage by means of enzymatic and non-enzymatic antioxidants. In our study, we analyzed kidney redox status, since it plays a pivotal role in the human health due to its role in the release of xenobiotics such as ACR. The decreased activities of antioxidant enzymes (SOD, CAT, and GPx) in renal tissue indicated the failure of antioxidant defense system to overcome the influx of ROS induced by ACR. Similar results have been reported by Abdel-Daim et al. 11 in treated rats with ACR at a dose of 20 mg/kg body weight. Administration of EVOO to ACR-treated rats caused a modulation in the activities of antioxidant enzymes due to the phenolic compounds found in the Chetoui variety, such as secoiridoids (aglycon forms of oleuropein and ligstroside) and lignans which are able to quench reactive oxygen species and radicals before reaching their cellular targets. 50 Besides, the non-enzymatic antioxidant system like GSH, NPSH, and vitamin C complements the activity of the enzymatic antioxidant system when oxidative stress is in excess. Glutathione plays an important role in maintaining cellular redox status and its level is considered as a significant marker of oxidative stress. ACR, an unsaturated amide, can react with cellular nucleophiles like GSH. Previous studies have reported the detoxification of ACR by conjugation with GSH. 51 , 52 In addition, another product NPSH, considered as one of the important primary defenses that counteract oxidative stress, decreased significantly in kidney of ACR treated rats compared with the controls. However, oral administration of EVOO to ACR-treated rats caused a modulation of non-enzymatic antioxidant levels. As reported in our previous study 41 , the beneficial effects of EVOO in the Mediterranean diet could be attributed not only to the close relationship between unsaturated and saturated fatty acids (SFA), but also to the antioxidant properties of its phenolic compounds.
To substantiate the biochemical findings, kidney histological examination was undertaken. Modifications in its histoarchitecture, seen in ACR treated rats, were characterized by a glomeruli fragmentation, an enlargement of glomeruli Bowman’s space, a marked hemorrhage, an infiltration of leucocytes, and a tubular dilatation. The histopathological aspect could be due to the accumulation of free radicals as the consequence of the increased H2O2 products and MDA levels in the kidney tissue of ACR treated rats. The mechanism underlying the kidney protection of EVOO might be related to its radical scavenging properties and its effect as a regulator of antioxidative systems.
Conclusion
The underlying mechanism for nephrotoxicity, caused by ACR, may be related to the generation of free radicals, the imbalance in the redox state and the marked oxidation of membrane lipids and proteins leading to the loss of membrane kidney integrity. Concomitant administration of EVOO mitigated all biochemical and histological changes due to its powerful antioxidant potential and by its richness in MUFA, carotenoids, tocopherols, and polyphenols.
Acknowledgements
The authors are indebted to Mr Menaa Assili and Mr Chedli Tmar for their skillful technical assistance.
Disclosure statement
The authors declare that they have no competing interests to disclose.
References
- 1. Tareke E, Rydberg P, Karlsson P, Eriksson S, Törnqvist M.. Analysis of acrylamide, acarcinogen formed in heated foodstuffs. J Agric Food Chem. 2002;50:4998–5006. [DOI] [PubMed] [Google Scholar]
- 2. Bortolomeazzi R, Munari M, Anese M, Verardo G. Rapid mixed mode solid phase extraction method for the determination of acrylamide in roasted coffee by HPLC–MS/MS. Food Chem. 2012;135:2687–2693. [DOI] [PubMed] [Google Scholar]
- 3. Brunton NP, Gormley R, Butler F, et al. A survey of acrylamide precursors in Irish ware potatoes and acrylamide levels in French fries. Food Sci Technol. 2007;40:1601–1609. [Google Scholar]
- 4. Shipp A, Lawrence G, Gentry R, et al. Acrylamide: Review of toxicity data and dose-response analyses for cancer and noncancer effects. Crit Rev Toxicol. 2006;36:481–608. [DOI] [PubMed] [Google Scholar]
- 5. Fennell TR, Sumner SC, Snyder RW, et al. Metabolism and hemoglobin adduct formation of acrylamide in humans. Toxicol Sci. 2005;85:447–459. [DOI] [PubMed] [Google Scholar]
- 6. Dybing E, Farmer PB, Andersen M, et al. Human exposure and internal dose assessments of acrylamide in food. Food Chem Toxicol. 2005;43:365–410. [DOI] [PubMed] [Google Scholar]
- 7. Adamsa A, Hamdania S, Van Lanckera F, MeALjrib S, De Kimpe N.. Stability of acrylamide in model systems and its reactivity with selected nucleophiles. Food Res Int. 2010;43:1517–1522. [Google Scholar]
- 8. Xie J, Terry KL, Poole EM, et al. Acrylamide hemoglobin adduct levels and ovarian cancer risk: A nested case-control study. Cancer Epidemiol Biomarkers Prev. 2013;22:653–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. IARC IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Lyon; 1994:1. [PMC free article] [PubMed] [Google Scholar]
- 10. Yousef MI, El-Demerdash FM.. Acrylamide-induced oxidative stress and biochemical perturbations in rats. Toxicology. 2006;219:133–141. [DOI] [PubMed] [Google Scholar]
- 11. Abdel-Daim MM, Abd Eldaim MA, Hassan AG.. Trigonella foenum-graecum ameliorates acrylamide-induced toxicity in rats: Roles of oxidative stress, proinflammatory cytokines, and DNA damage. Biochem Cell Biol. 2015;93:192–198. [DOI] [PubMed] [Google Scholar]
- 12. Zhang L, Wang E, Chen F, Yan H, Yuan Y.. Potential protective effects of oral administration of allicin on acrylamide-induced toxicity in male mice. Food Funct. 2013;4:1229–1236. [DOI] [PubMed] [Google Scholar]
- 13. Zhao M, Wang P, Zhu Y, Liu X, Hu X, Chen F.. The chemoprotection of a blueberry anthocyanin extract against the acrylamide-induced oxidative stress in mitochondria: Unequivocal evidence in mice liver. Food Funct. 2015;6:3006–3012. [DOI] [PubMed] [Google Scholar]
- 14. Fito M, de la Torre R, Covas MI.. Olive oil and oxidative stress. Mol Nutr Food Res. 2007;51:1215–1224. [DOI] [PubMed] [Google Scholar]
- 15. Vissers MN, Zock PL, Katan MB.. Bioavailability and antioxidant effects of olive oil phenols in humans: A review. Eur J Clin Nutr. 2004;58:955–965. [DOI] [PubMed] [Google Scholar]
- 16. Lopez S, Bermudez B, Montserrat-de la Paz S, et al. Membrane composition and dynamics: A target of bioactive virgin olive oil constituents. Biochim Biophys Acta. 2014;1838:1638–1656. [DOI] [PubMed] [Google Scholar]
- 17. Visioli F, Galli C.. Biological properties of olive oil phytochemicals. Crit Rev Food Sci Nutr. 2002;42:209–221. [DOI] [PubMed] [Google Scholar]
- 18. Huang CL, Sumpio BE.. Olive oil, the mediterranean diet, and cardiovascular health. J Am Coll Surg. 2008;207:407–416. [DOI] [PubMed] [Google Scholar]
- 19. Leger CL, Kadri-Hassani N, Descomps B.. Decreased superoxide anion production in cultured human promonocyte cells (THP-1) due to polyphenol mixtures from olive oil processing wastewaters. J Agric Food Chem. 2000;48:5061–5067. [DOI] [PubMed] [Google Scholar]
- 20. Covas MI. Olive oil and the cardiovascular system. Pharmacol Res. 2007;55:175–186. [DOI] [PubMed] [Google Scholar]
- 21. IOOC (International Olive Oil Council) Trade Standard Applying to Olive Oils and Olive-pomace Oils; 2008. [Google Scholar]
- 22. Dabbou S, Issaoui M, Servili M, et al. Characterisation of virgin olive oils from European olive cultivars introduced in Tunisia. Eur J Lipid Sci Technol. 2009;111:392–401. [Google Scholar]
- 23. Minguez-Mosquera MI, Rejano-Navarro L, Gandul-Rojas B, Sanchez-Gomez AH, Garrido-Fernandez J.. Color pigment correlation in virgin olive oil. J Am Oil Chem Soc. 1991;68:332–336. [Google Scholar]
- 24. Montedoro GF, Servili M, Baldioli M, Miniati E.. Simple and hydrolyzable phenolic compounds in virgin olive oil.1. Their extraction, separation and quantitative and semi-quantitative evaluation by HPLC. J Agric Food Chem. 1992;40:1571–1576. [Google Scholar]
- 25. Gimeno E, Castellote AI, Lamuela-Raventos RM, de la Torre MC, Lopez-Sabater MC.. Rapid determination of vitamin E in vegetable oils by reversed-phase high-performance liquid chromatography. J Chromatogr A. 2000;881:251–254. [DOI] [PubMed] [Google Scholar]
- 26. Council of European Communities. Council instructions about the protection of living animals used in scientific investigations. Off J Eur Commun JO 86/609/CEE. 1986;358:1–18. [Google Scholar]
- 27. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ.. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:269–275. [PubMed] [Google Scholar]
- 28. Draper HH, Hadley M.. Malondialdehyde determination as index of lipid peroxidation . Methods Enzymol. 1990;186:421–431. [DOI] [PubMed] [Google Scholar]
- 29. Ou P, Wolff SP.. A discontinuous method for catalase determination at near physiological concentrations of H2O2 and its application to the study of H2O2 fluxes within cells. J Biochem Biophys Methods. 1996;31:59–67. [DOI] [PubMed] [Google Scholar]
- 30. Reznick Z, Packer L. Oxidative damage to proteins: Spectrophotometric method for carbonyl assay . Method Enzymol. 1994;233:357–363. [DOI] [PubMed] [Google Scholar]
- 31. Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–77. [DOI] [PubMed] [Google Scholar]
- 32. Jollow DJ, Mitchell JR, Zampaglione N, Gillette JR. Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology. 1974;11:151–169. [DOI] [PubMed] [Google Scholar]
- 33. Jacques-Silva MC, Nogueira CW, Broch LC.. Diphenyl diselenide and ascorbic acid changes deposition of selenium and ascorbic acid in liver and brain of mice . Pharmacol. Toxicol 2001;88:119–125. [DOI] [PubMed] [Google Scholar]
- 34. Aebi H. Catalase in vitro . Methods Enzymol. 1984;105:121–126. [DOI] [PubMed] [Google Scholar]
- 35. Beauchamp C, Fridovich I.. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971;44:276–287. [DOI] [PubMed] [Google Scholar]
- 36. Flohe L, Gunzler WA.. Assays of glutathione peroxidase. Methods. Enzymol. 1984;108:114–121. [DOI] [PubMed] [Google Scholar]
- 37. Charrel M. Urée et creatinine In: Semiologie biochimique (Urea and creatinin). Paris: Ellipses Publishers; 1991:124. [Google Scholar]
- 38. Motamedshariaty VS, Amel Farzad S, Nassiri-Asl M, Hosseinzadeh H.. Effects of rutin on acrylamide-induced neurotoxicity. Daru. 2014;22:27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tanii H, Hashimoto K.. Effect of acrylamide and related compounds on glycolytic enzymes of rat brain. Toxicol Lett. 1985;26:79–84. [DOI] [PubMed] [Google Scholar]
- 40. Ghorbel I, Elwej A, Jamoussi K, Boudawara T, Kamoun NG, Zeghal N.. Potential protective effects of extra virgin olive oil on the hepatotoxicity induced by co-exposure of adult rats to acrylamide and aluminum. Food Funct. 2015a;6:1126–1135. [DOI] [PubMed] [Google Scholar]
- 41. Ghorbel I, Khemakhem M, Boudawara O, et al. Effects of dietary extra virgin olive oil and its fractions on antioxidant status and DNA damage in the heart of rats co-exposed to aluminum and acrylamide. Food Funct. 2015b;6:3098–3108. [DOI] [PubMed] [Google Scholar]
- 42. Karahan I, Atessahin A, Yilmaz S, Ceribasi AO, Sakin F.. Protective effect of lycopene on gentamicin-induced oxidative stress and nephrotoxicity in rats. Toxicology. 2005;215:198–204. [DOI] [PubMed] [Google Scholar]
- 43. Pandey PC, Mishra AP.. Novel potentiometric sensing of creatinine. Sens. Actuators B Chem. 2004;99:230–235. [Google Scholar]
- 44. Raju J, Roberts J, Taylor M, et al. Toxicological effects of short-term dietary acrylamide exposure in male F344 rats. Environ Toxicol Pharmacol. 2015;39:85–92. [DOI] [PubMed] [Google Scholar]
- 45. Regoli F, Winste GW.. Quantification of total oxidant scavenging capacity of antioxidants for peroxynitrite peroxyl radicals and hydroxyl radicals. Toxicol Appl Pharmacol. 1999;156:96–105. [DOI] [PubMed] [Google Scholar]
- 46. De la Torre R. Bioavailability of olive oil phenolic compounds in humans. Inflammopharmacology. 2008;16:245–247. [DOI] [PubMed] [Google Scholar]
- 47. Celik I, Suzek H.. The hematological effects of methyl parathion in rats. J Hazard Mater. 2008;153:1117–1121. [DOI] [PubMed] [Google Scholar]
- 48. Bonilla-Polo A, Murillo-Ramos JJ, Gonzalez-Bonilla J, Sanz-Perez B.. Variations in fatty acids, tocopherol and other quality parameters of virgin olive oil subjected to refining process. Nutr Hosp. 1997;12:309–311. [PubMed] [Google Scholar]
- 49. Paiva-Martins F, Gordon MH, Gameiro P.. Activity and location of olive oil phenolic antioxidants in liposomes. Chem Phys Lipids. 2003;24:23–36. [DOI] [PubMed] [Google Scholar]
- 50. Ben Hassine K, Taamalli A, Ben Slama M, et al. Characterization and preference mapping of autochthonous and introduced olive oil cultivars in Tunisia. Eur J Lipid Sci Technol. 2015;117:112–121. [Google Scholar]
- 51. Awad ME, Abdel‐Rahman MS, Hassan SA.. Acrylamide toxicity in isolated rat hepatocytes. Toxicol in Vitro. 1998;12:699–704. [DOI] [PubMed] [Google Scholar]
- 52. Tong GC, Cornwell WK, Means GE.. Reactions of acrylamide with glutathione and serum albumin. Toxicol Lett. 2004;147:127–131. [DOI] [PubMed] [Google Scholar]