Abstract
Background: The reported prevalence rates and etiologies of acute kidney injury (AKI) are quite variable in different regions of the world. The current study was planned to determine the etiology, clinical profile, and short-term outcome of pediatric AKI at our hospital.
Methods: A prospective, observational study was carried out from April 2014 to March 2015. All pediatric patients (1 month to ≤15 years) diagnosed as AKI using modified pRIFLE criteria were studied and followed for 3 months to document short-term outcome.
Results: AKI was diagnosed in 116 children. The mean age was 7.5 ± 4.4 years and males were predominant (60.3%). At presentation, 83.6% had oliguria/anuria, 37.1% hypertension and 17.2% severe anemia. Etiology included primary renal (74/116; 63.8%), postrenal (28/116; 24.1%) and prerenal (11/116; 9.5%) causes. Postinfectious glomerulonephritis (PIGN) and crescentic glomerulonephritis in primary renal, obstructive urolithiasis in postrenal and sepsis in prerenal, were the most common etiologies. At presentation, 89/116 (76.7%) patients were in pRIFLE Failure category. Regarding outcome, 68 (58.6%) patients recovered, six (5.2%) died, 18 (15.5%) developed chronic kidney disease (CKD) and 22 (19%) end-stage renal disease (ESRD). Comparison of recovered and unrecovered AKI showed that characteristics such as hypertension, severe anemia, edema, volume overload, requirement of mechanical ventilation, initiation of dialysis and need of >5 sessions of dialysis had statistically significant (p <0.05) association with nonrecovery.
Conclusion: Glomerulonephritides (PIGN and crescentic) and obstructive urolithiasis are major causes of pediatric AKI at our center. A fairly high percentage of cases recovered and these mainly comprised of PIGN and obstructive urolithiasis.
Keywords: Acute kidney injury, children, etiology, clinical profile, outcome, Pakistan
Introduction
Acute kidney injury (AKI) is a major contributor to childhood morbidity and mortality. Survivors of AKI are at risk of limited kidney reserves and residual abnormalities like proteinuria, hypertension and chronic kidney disease (CKD).1–4 The burden of AKI has increased in both developed and developing countries, with the reported incidence found to be quite variable in different regions of the world. A multicenter study from China reported an incidence of 0.32%, whereas a single-center study from India revealed an incidence of 25.1%. Yearly incidence of 0.8/100,000 population was reported from United Kingdom and figures from multicenter study at USA were found as 0.39%.5–10 Etiology of AKI categorized as prerenal, intrinsic renal and postrenal causes, is also variably reported from developed and developing world. A prospective multicenter investigation from China reported urolithiasis as the most common cause of AKI followed by acute glomerulonephritis (GN) and severe dehydration.5 A large-scale study of a national pediatric cohort from across the United States revealed shock followed by septicemia as the most common risk factors associated with AKI.6 These profound variations point to the need of highlighting epidemiology and prevalent causes at regional level, in order to improve the recognition and timely management of this silent killer.
Sindh Institute of Urology and Transplantation (SIUT) is a publicly funded tertiary care facility, where all treatment is provided free of cost to all patients. This attracts a large volume of patients, from both rural and urban areas, who are referred mostly when they require specialized care or dialysis. At national level in Pakistan, pediatric AKI data based on standardized AKI definition is lacking. Therefore, the current study was designed prospectively to determine the etiology, clinical profile and short-term outcome of pediatric patients presenting with AKI at our hospital.
Methods
Study design and location
It was a descriptive, prospective, observational study, carried out at pediatric nephrology department of SIUT for a period of 12 months, from April 2014 to March 2015. Ethical clearance was obtained from institutional ethical review committee of SIUT (SIUT-ERC.ERC-A12–2015). Informed consent was obtained from parents/guardians of all participants.
Patient recruitment and methods
All children aged 1 month to 15 years, diagnosed with AKI, were enrolled for the study. Children presenting with CKD, known renal comorbids, such as nephrotic syndrome or tubular disorders, were excluded. The diagnosis of AKI was based on pRIFLE criteria11 and estimated creatinine clearance (eCCl) was determined by calculating estimated glomerular filtration rate (eGFR) using Schwartz equation.12
All enrolled patients were subjected to further standard workup of AKI. Serum chemical and serological markers were tested as per indication. All patients received standard etiology-specific treatment, which was not influenced by financial status of the patients, as hospital offers free of cost medical, surgical management and dialysis facility. Indication for dialysis included one or more of following features: symptoms of volume overload, severe metabolic acidosis, advanced uremia and severe electrolyte imbalance not responding to medical regimen. Hemodialysis was the preferred modality in all children who were more than 10 kg in weight, as hemodialysis is available 24 h at our hospital. Peritoneal dialysis was opted for in children weighing less than 10 kg and with unstable hemodynamics. For patients requiring admission, urine output and serum creatinine were monitored daily till discharge. After discharge, frequency of follow-up was individualized depending on the severity of the illness.
Data collection and definitions
For each patient, a predesigned proforma was filled recording relevant details. Severity of AKI at presentation was graded using pRIFLE criteria as follows: R for risk, I for injury, and F for failure. Etiology of AKI was grouped as prerenal, renal diseases, and postrenal. For outcome, each patient was followed till recovery or three months postdiagnosis date and categorized as follows: recovered (eGFR, >90 mL/min); CKD stage 2–4 (eGFR, 15–89 mL/min), ESRD (eGFR, <15 mL/min); death; and lost to follow-up.
Statistical analysis
Data were analyzed using Statistical Package for the Social Sciences (SPSS) version 20.0 (IBM corporation, Armonk, NY). Continuous variables were expressed as mean ± standard deviation (SD) and categorical variables were expressed as percentage or number of cases and compared using Chi-square test. Statistical significance was considered at p values <.05.
Results
During the 12-month study period, 116 children were diagnosed to have AKI, out of which, 70 (60.3%) were males and 46 (39.7%) females. Male-to-female ratio was 1.5:1. Mean age at presentation was 7.5 ± 4.4 years and age range was 1.5 months to 15 years. Majority of patients, 77 (66.4%), were older than five years.
Etiology of AKI
Primary renal disease-related AKI was most frequent, found in 74 (63.8%) children, followed by post-renal in 28 (24.1%) and prerenal in 11 (9.5%) cases. In three (2.6%) children, etiology was unidentified. Overall, looking at individual etiological diseases, three most common causes were: post-infectious glomerulonephritis (PIGN) in 26/116 (22.4%), obstructive urolithiasis in 24/116 (20.7%) and crescentic GN in 20/116 (17.2%) cases.
Primary renal disease principally comprised of PIGN (26/74; 35.1%) and crescentic GN (20/74; 27%). Hemolytic uremic syndrome (HUS) was identified in 8/74 (10.8%) cases, of which one patient lacked peripheral hematological manifestation, but biopsy was consistent with thrombotic microangiopathy (TMA). Envenomation accounted for 8/74 (10.8%) cases, of which five were secondary to scorpion bite and three to snake bite. Malarial nephropathy was found in 6/74 (8.1%) patients, all secondary to Plasmodium (P) vivax except 1 case with both P. viax and P. falciparum infection. In all, 3/74 (4%) patients showed acute tubular necrosis (ATN) as the cause of AKI. Two of these were post-surgical in etiology and one showed this complication in the background podocytopathic disorder. Drug/toxin-induced AKI was identified in 2/74 (2.7%) children. In one case, paraphenylenediamine (PPD) poisoning was implicated in a 15-year-old girl, whereas the other case was of ifosfamide toxicity in a child who received chemotherapy for sarcoma. Only one (1.3%) patient showed mesangiocapillary GN as the underlying cause of AKI.
Postrenal etiological group mainly comprised of obstructive urolithiasis (21/28; 75%), whereas pelviureteric junction obstruction (PUJO) (2/28; 7.1%) and posterior uretheral valves (PUV) (2/28; 7.1%) accounted for minority of cases. Prerenal AKI group principally included sepsis (8/11; 72.7%) and one case (9.1%) each of gastroenteritis, shock, and cholemic nephrosis (Table 1).
Table 1.
Etiology of acute kidney injury (AKI) in 116 children.
| Etiologies of pediatric AKI | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre-renal |
Renal |
Postrenal |
|||||||
|
n = 11 (9.5%) |
n = 74 (63.8%) |
n = 28 (24.1%) |
Unknown etiology | ||||||
| Etiology | n | % | Etiology | n | % | Etiology | n | % | n = 3 (2.6%) |
| Sepsis | 8 | 72.7% | PIGN | 26 | 35.1% | Obstructive urolithiasis | 24 | 85.7% | |
| Gastroenteritis | 1 | 9.1% | Crescentic GN | 20 | 27% | Bilateral PUJO | 2 | 7.1% | |
| Liver-related disease | 1 | 9.1% | HUS | 8 | 10.8% | Posterior urethral valves | 2 | 7.1% | |
| Shock | 1 | 9.1% | Envenomation | 8 | 10.8% | ||||
| Malarial nephropathy | 6 | 8.1% | |||||||
| Drugs/Toxins | 2 | 2.7% | |||||||
| ATN | 3 | 4.0% | |||||||
| Mesangiocapillary GN | 1 | 1.3% | |||||||
ATN: acute tubular necrosis; GN: glomerulonephritis; HUS: hemolytic uremic syndrome; PIGN: post infectious GN; PUJO: pelvi–ureteric junctional obstruction.
Clinical characteristics
The main clinical features of AKI children are shown in Table 2. A decrease in urine output, either oliguria or anuria, was the most frequent (97; 83.6%) presenting symptom. Other presenting features are shown in Table 2. On examination, 60 (51.7%) children had edema and 43 (37.1%) were hypertensive. Initiation of dialysis was needed in 62 (53.4%) subjects (hemodialysis in 60 and peritoneal dialysis in 2) and majority required more than 5 sessions of hemodialysis.
Table 2.
Clinical characteristics and supportive care required for acute kidney injury (AKI) cases.
| Characteristics | Frequency |
|---|---|
| Presenting Features | |
| Oliguria/anuria | 97 (83.6%) |
| Fever | 76 (65.5%) |
| Edema | 60 (51.7%) |
| Vomiting | 42 (36.2%) |
| Clinical features at presentation | |
| Severe anemia (Hb <6 g/dl) | 20 (17.2%) |
| Hypertension (BP >95th centile) | 43 (37.1%) |
| Volume overload | 19 (16.4%) |
| Supportive care | |
| Ventilatory support | 9 (7.8%) |
| Dialysis | 62 (53.4%) |
Severity of AKI at presentation
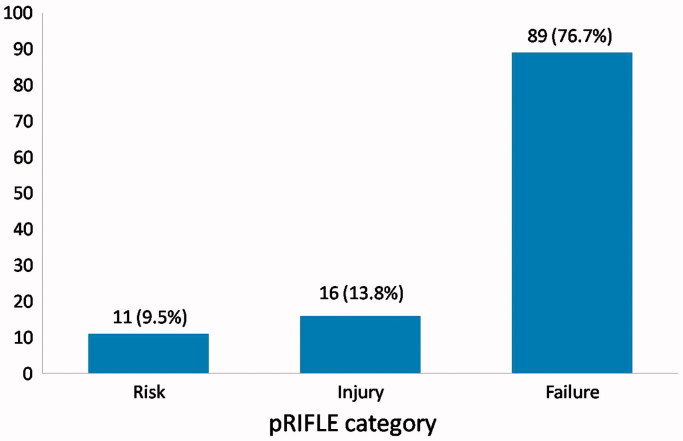
At presentation, most of the patients were in pRIFLE category Failure, 89 (76.7%), while 16 (13.8%) were in injury and 11 (9.5%) patients in risk category (Figure 1). Mean serum creatinine at presentation was 6.5 ± 5.1 mg/dl (range: 0.79 to 21.4 mg/dl).
Figure 1.
pRIFLE Category at Presentation of AKI cases.
Outcome
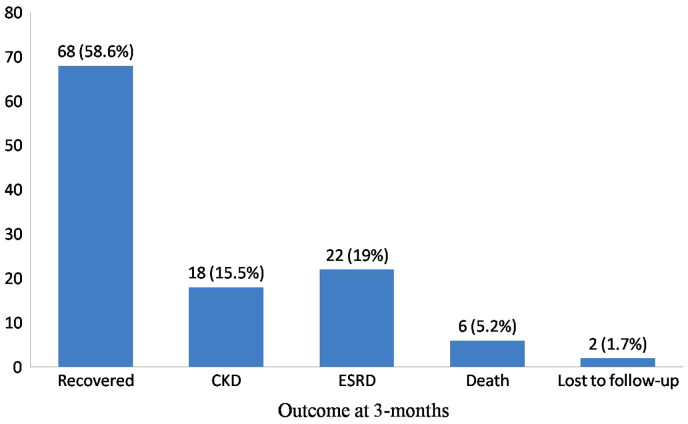
In majority of AKI cases (68:58.6%), renal functions returned to normal (Figure 2). The mean duration of recovery was 17.8 ± 26.8 days (range: 3 to 90 days). Mortality occurred in six (5.2%) cases; the causes being sepsis in four cases, frank pulmonary hemorrhage with antineutrophil cytoplasmic antibody (ANCA)-positive crescentic GN in one case and underlying cause of AKI was unidentified in one case. Three months post-AKI diagnosis, 22 (19%) patients were found to be dialysis dependent (ESRD) and 18 (15.5%) were CKD (seven patients, CKD stage 2; nine patients, CKD stage 3; and two patients, CKD stage 4). Final outcome was not established in two (1.7%) patients as they were lost to follow-up.
Figure 2.
The outcome of children with AKI at 3 months of follow-up.
Table 3 shows outcome as regards to pRIFLE category at presentation. It is evident from this table that the vast majority of patients belonging to risk and injury categories recovered. Table 4 highlights outcome according to etiological groups, showing that children with PIGN and obstructive urolithiasis constituted the majority of recovered group.
Table 3.
Outcome according to pRIFLE category at presentation.
| Outcome (n = 116) |
|||||
|---|---|---|---|---|---|
| pRIFLE category n = 116 | Recovered n = 68 | CKD n = 18 | ESRD n = 22 | Death n = 6 | Lost to follow-up n = 2 |
| Risk, n = 11 | 11 (100%) | – | – | – | – |
| Injury, n = 16 | 12 (75%) | 4 (25%) | – | – | – |
| Failure, n = 89 | 45 (50.5%) | 14 (15.7%) | 22 (24.7%) | 6 (6.7%) | 2 (2.2%) |
CKD: chronic kidney disease; ESRD: end-stage renal disease.
Table 4.
Outcome according to etiological category of renal disease.
| Outcome n = 116 |
|||||
|---|---|---|---|---|---|
| Etiology of AKI n = 116 | Recovered n = 68 | CKD n = 18 | ESRD n = 22 | Death n = 6 | Lost to follow-up n = 2 |
| PIGN, n = 26 | 22 (84.6%) | 4 (15.4%) | – | – | – |
| Obstructive urolithiasis, n = 24 | 20 (83.3%) | 2 (8.3%) | – | 2 (8.3%) | – |
| Crescentic GN, n = 20 | 1 (5%) | 4 (20%) | 14 (70%) | 1 (5%) | – |
| Sepsis, n = 8 | 1 (12.5%) | 2 (25%) | 2 (25%) | 2 (25%) | 1 (12.5%) |
| HUS, n = 8 | 3 (37.5%) | 2 (25%) | 2 (25%) | – | 1 (12.5%) |
| Envenomation, n = 8 | 6 (75%) | – | 2 (25%) | – | – |
| Malarial nephropathy, n = 6 | 2 (33.3%) | 3 (50%) | 1 (16.6%) | – | – |
| PUJO & PUV, n = 4 | 4 (100%) | – | – | – | – |
| ATN, n = 3 | 2 (66.6%) | – | 1 (33.3%) | – | – |
| Drugs & toxins, n = 2 | 1 (50%) | 1 (50%) | – | – | – |
| Acute gastroenteritis, n = 1 | 1 (100%) | – | – | – | – |
| Shock, n = 1 | 1 (100%) | – | – | – | – |
| Mesangiocapillary GN, n = 1 | 1 (100%) | – | – | – | – |
| Cholemic nephrosis, n = 1 | 1 (100%) | – | – | – | – |
| Unknown etiology, n = 3 | 2 (66.6%) | – | – | 1 (33.3%) | – |
ATN: acute tubular necrosis; GN: glomerulonephritis; HUS: hemolytic uremic syndrome; PIGN: post infectious GN; PUJO: pelvi–ureteric junctional obstruction; PUV: posterior uretheral valves.
Comparison of variables in recovered versus unrecovered AKI subjects
Severe anemia (hemoglobin (Hb) < 6 g/dl), hypertension, edema, volume overload, pRIFLE category Failure at presentation, requirement of mechanical ventilation, need of initiation of dialysis and requirement of >5 sessions of dialysis were found to have statistically significant (p < .05) association with unrecovered AKI cases as compared to those who recovered, while younger age (<5 years) and change in urine output (oliguria/anuria) had statistically insignificant (p = .14) association (Table 5).
Table 5.
Independent variables in recovered versus unrecovered acute kidney injury (AKI) cases.
| Variables | Recovered n = 69 (59.5%) | Unrecovered n = 47 (40.5%) | p value |
|---|---|---|---|
| Age less than 5 years | 27 (39.7%) | 12 (25.0%) | .09 |
| Oliguria/anuria | 54 (79.4%) | 43 (89.6%) | .14 |
| Edema | 29 (42.6%) | 31 (64.6)%) | .02 |
| Hypertension (BP >95th centile) | 17 (25.0%) | 26 (54.2%) | .001 |
| Severe anemia (Hb <6 g/dl) | 7 (10.3%) | 13 (27.1%) | .01 |
| Volume overload | 5 (7.4%) | 14 (29.2%) | .002 |
| pRIFLE category failure | 45 (66.2%) | 44 (91.7%) | .001 |
| Required dialysis | 21 (30.9%) | 41 (85.4%) | <.001 |
| Required >5 dialysis sessions | 3 (4.4%) | 32 (66.7%) | <.001 |
| Mechanical ventilator support | 1 (1.5%) | 8 (16.7%) | .003 |
Discussion
This study describes pediatric AKI from a tertiary care pediatric nephrology center of Pakistan. Most of the children were older than 5 years which differs from that reported by other developing countries where pediatric AKI is seen mainly in younger age groups;5,7,9,13 while studies from most of developed countries observed pediatric AKI in >5 years age group.6,14–16 Possible explanation of this contrasting finding could be that our center lacks facility to manage neonates and young infants; therefore, younger children are under-represented in our study population. Most dominant pRIFLE score at presentation was failure category. We graded AKI considering baseline eCCl as 100 mL/min/1.73 m2; because baseline serum creatinine was not available in nearly all cases.
Etiological spectrum of AKI as reviewed in medical literature shows wide variation between developed and developing countries. In developed world, AKI is usually a hospital-acquired disease, whereas in developing countries, community-acquired AKI is frequently reported.17–20 A cross-sectional analysis of 2009 Kids Inpatient Database from United States reported shock, septicemia, mechanical ventilation and extracorporeal support to be associated with AKI.6 Likewise, Bresolin et al. found shock and sepsis as predominant etiology in pediatric AKI cohort.21 On the contrary, a different spectrum of etiologies is seen in developing countries. A prospective multicenter study from China revealed urolithiasis as the most dominant AKI etiology followed by acute GN and severe dehydration.5 A review of Nigerian publications regarding pediatric AKI from 1990 to 2012 postulated nephrotoxins and infections as predominant AKI etiology.22 Krishnamurthy et al. reported infections, PIGN, snake envenomation, and hemolytic uremic syndrome (HUS) as common causes of AKI.7 Our study from a developing country, encountered mainly community acquired AKI. Three most common etiologies identified were PIGN followed by obstructive urolithiasis and crescentic GN, revealing high burden of glomerulonephritides and stone disease causing AKI in our setup. Nearly, similar etiological spectrum of AKI is reported from a large pediatric cohort of neighboring country, China.5 However, an earlier study from our country describing renal and postrenal causes of pediatric AKI reported HUS as predominant cause of AKI in <2 years old children and urolithiasis as major etiology in children >2 years of age.23 Gastroenteritis and sepsis-related AKI are less commonly seen in present study, which is most probably an underestimation as the current study was conducted at pediatric nephrology center and such cases are mainly admitted at general pediatric hospitals.
We documented a significantly low mortality rate (5.2%) in contrast to that reported from India (46.3%)7 and Nigeria (50.4%),22 whereas high recovery and low mortality figures observed in our study are quite similar to that reported from multicenter study in China.5 Low mortality figure can be an underestimation as we have recorded 3-month outcome and many of ESRD patients may have died over next few months. Overall high recovery rate observed may be because of the fact that the majority of our AKI cohort comprised of PIGN and obstructive urolithiasis cases, who recovered normal renal function with supportive care and urgent surgical decompression, respectively.
This study has some limitations. Firstly, data in this study are from a single pediatric nephrology center; therefore, it does not truly reflect etiological spectrum of pediatric AKI prevalent throughout the country. Secondly, our study did not cover neonatal AKI, as our hospital lacks facility of neonatal ICU. Post-surgical cases are also not included as study population did not include cases from pediatric surgery and cardiac surgery units. Nevertheless, our study describes very relevant information on pediatric AKI spectrum encountered at a large tertiary care referral center in a developing country.
Conclusions
Our study revealed that GN followed by obstructive stone disease constitutes the major bulk of pediatric AKI burden at a single tertiary care center. We found a high recovery and low mortality at short-term follow-up. Further multicenter studies are needed to elaborate this subject and plan preventive strategies to reduce childhood morbidity and mortality from this disease.
Acknowledgements
We greatly acknowledge the guidance and help of Dr Masood Hussain Rao, Principal Research Officer, Dow University of Health and Sciences, for his help in the statistical analysis of the study.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee at which the studies were conducted (IRB approval number: SIUT-ERC.ERC-A12–2015) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from parents/guardians of all individual participants included in the study.
References
- 1.Askenazi DJ, Feig DI, Graham NM, Hui Stickle S, Goldstein SL. 3–5 year longitudinal follow-up of pediatric patients after acute renal failure. Kidney Int. 2006;69:184–189. [DOI] [PubMed] [Google Scholar]
- 2.Sinha R, Nandi M, Tallus K, Marks SD, Taraphder A.. Ten year follow-up of children after acute renal failure from a developing country. Nephrol Dial Transplant. 2009;24:829–833. [DOI] [PubMed] [Google Scholar]
- 3.Schneider J, Khemani R, Grushkin C, Bart R.. Serum creatinine as stratified in the RIFLE score for acute kidney injury is associated with mortality and length of stay for children in the pediatric intensive care unit. Crit Care Med. 2010;38:933–939. [DOI] [PubMed] [Google Scholar]
- 4.Mammen C, Al Abbas A, Skippen P, et al Long-term risk of CKD in children surviving episodes of acute kidney injury in the intensive care unit: a prospective cohort study. Am J Kidney Dis. 2012;59:523–530. [DOI] [PubMed] [Google Scholar]
- 5.Cao Y, Yi ZW, Zhang H, Dang XQ, Wu XC, Huang AW. Etiology and outcomes of acute kidney injury in Chinese children: A prospective multicentre investigation. BMC Urol. 2013; 13:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sutherland SM, Ji J, Sheikhi FH, et al AKI in hospitalized children: Epidemiology and clinical associations in a National cohort. Clin J Am Soc Nephrol. 2013; 8:1661–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krishnamurthy S, Narayanan P, Prabha S, et al Clinical profile of acute kidney injury in a pediatric intensive care unit from Southern India: A prospective observational study. Indian J Crit Care Med. 2013;17:207–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jenssen GR, Bangstad HJ, Nygard K, Vold L, Bjerre A.. Incidence and etiology of acute kidney injury in children in Norway between 1998 and 2008. Acta Pediatrica. 2014;103:1192–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Esezobor CI, Ladapo TA, Osinaike B, Lesi FEA.. Pediatric acute kidney injury in a tertiary hospital in Nigeria: Prevalance, cause and mortality rate. PLoS One. 2012;7:e51229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moghal NE, Brocklebank JT, Meadow SR.. A review of acute renal failure in children: incidence, etiology and outcome. Clin Nephrol. 1998;49:91–95. [PubMed] [Google Scholar]
- 11.Akcan-Arikan A, Zappitelli M, Loftis LL, Washburn KK, Jefferson LS, Goldstein SL.. Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int. 2007;71:1028–1035. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz GJ, Brion LP, Spitzer A.. The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children and adolescents. Pediatr Clin North Am. 1987; 34:571–590. [DOI] [PubMed] [Google Scholar]
- 13.Gheissari A, Mehrasa P, Merrikhi A, Madihi Y.. Acute kidney injury: A pediatric experience over 10 years at a tertiary care center. J Nephropathol. 2012;1:101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Albar H.Acute kidney injury in critically ill children at pediatric intensive care unit. CKD-211. 2013;40:891–893. [Google Scholar]
- 15.Cerda J, Bagga A, Kher V, Chakravarthi RM.. The contrasting characteristics of acute kidney injury in developed and developing countries. Nat Clin Pract Nephrol. 2008;4:138–153. [DOI] [PubMed] [Google Scholar]
- 16.Bailey D, Phan V, Litalien C, et al Risk factor of acute renal failure in critically ill children: A prospective descriptive epidemiological study. Pediatr Crit Care Med. 2007;8:29–35. [DOI] [PubMed] [Google Scholar]
- 17.Lewington Andrew JP, Cerda J, Mehta RL.. Raising awareness of acute kidney injury: A global perspective of a silent killer. Kidney Int. 2013;84:457–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lameire N, Biesen WV, Vanholder R.. The changing epidemiology of acute renal failure. Nat Clin Pract Nephrol. 2006;2:364–377. [DOI] [PubMed] [Google Scholar]
- 19.Abraham G, Gupta RK, Senthiselvan A, van der Meulen J, Johny KV.. Cause and prognosis of acute renal failure in Kuwait: A 2-year prospective study. J Trop Med Hyg. 1989; 92:325–329. [PubMed] [Google Scholar]
- 20.Anochie IC, Eke FU. Acute renal failure in Nigerian children: Port Harcourt experience. Pediatr Nephrol. 2005;20:1610–1614. [DOI] [PubMed] [Google Scholar]
- 21.Bresolin N, Silva C, Halllal A, et al Prognosis for children with acute kidney injury in the intensive care unit. Pediatr Nephrol. 2009;24:537–544. [DOI] [PubMed] [Google Scholar]
- 22.Olowu WA.Acute kidney injury in children in Nigeria. Clin Nephrol. 2015;83:70–74. [DOI] [PubMed] [Google Scholar]
- 23.Jamal A, Ramzan A. Renal and post-renal causes of acute renal failure in children. J Coll Physicians Surg Pak. 2004;14:411–415. [PubMed] [Google Scholar]