Abstract
Introduction: Granulomatosis with polyangiitis (GPA) is a rare necrotizing vasculitis, which usually involves the upper and lower respiratory systems and kidneys and often have a relapsing course. Neutrophil/lymphocyte ratio (NLR) has been shown to be a useful marker predicting not only progressive disease, but also mortality in various inflammatory diseases. We aimed to investigate the roles of NLR in predicting the extend of clinical involvement and prognosis of patients with GPA.
Materials and methods: Consecutive newly diagnosed GPA patients who had follow-up for at least 6 months between 2010 and 2016 at Gazi University Internal Medicine-Rheumatology clinic were retrospectively analyzed.
Results: Fifty-three newly diagnosed GPA patients were studied. NLR was significantly higher in the GPA group compared with the control group (4.50 [min–max: 0.07–34.81] vs 1.77 [min–max: 1.04–2.90], respectively, p < .001). NLR significantly correlated with ESR and CRP levels (r = .40 and r = .48, respectively, p < .001 for both).
Discussion: GPA is a vasculitis with a significant morbidity and mortality (REF). Renal involvement usually presents with crescentric glomerulonephritis, resulting in significant and permanent loss of renal functions and end-stage kidney disease. Higher NLR at baseline is associated with worse renal outcome. Our findings suggest that baseline NLR could have a predictive value for renal prognosis. We have also demonstrated a significant correlation between NLR and BVAS activity scores. Our data suggest that GPA patients with a significantly high NLR at baseline might need closer follow-up for persistent disease activity.
Keywords: NLR, granulomatosis with polyangiitis, renal failure
Introduction
Granulomatosis with polyangiitis (GPA) is a rare necrotizing vasculitis of small-to-medium-sized vessels, which usually involves the upper and lower respiratory systems and kidneys and often have a relapsing course.1 Its clinical presentation varies from a limited non-vital organ involvement to a life threatening systemic vasculitis.2 Erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) are the acute phase reactants used in the clinical evaluation of disease activity, however, their roles in predicting the extend of disease involvement or prognosis are limited.3 Neutrophil/lymphocyte ratio (NLR) has been shown to be a useful marker predicting not only progressive disease, but also mortality in various inflammatory diseases.4 To the best of our knowledge, its role in GPA has not been previously reported. We aimed to investigate the roles of NLR in predicting the extend of clinical involvement and prognosis of patients with GPA.
Materials and methods
Study patients
Consecutive newly diagnosed GPA patients who had follow-up for at least 6 months between 2010 and 2016 at Gazi University Internal Medicine-Rheumatology clinic were retrospectively analyzed. Those who fulfilled the 2012 Chapel Hill consensus conference criteria were included as the GPA group. Control group consisted of age and sex matched patients who presented to the same clinic during the same period with non-inflammatory conditions. Those with active infections or malignant diseases were excluded. Birmingham Vasculitis Activity Score (BVAS) system was used for the evaluation of the disease activity status in the GPA group. GPA group was sub-grouped into renal and non-renal GPA groups. Baseline and 6-month follow-up levels of hemoglobin, serum creatinine and CRP, neutrophil, lymphocyte and platelet counts, ESRs, as well as creatinine clearance rates (calculated using The Modification of Diet in Renal Disease method) were retrieved, and NLRs were calculated.
Statistics
Levene’s and Shapiro–Wilk’s tests were applied to test the normality and variance homogeneity of data. Independent-samples T and Paired sample T-tests were used to analyze the differences of two numerical and normally distributed parameters between two independent and dependent groups, respectively. One-way analysis of variance parametric test was used to test the differences of means between 3 or more groups. The comparisons of continuous variables were also analyzed by Wilcoxon, Kruskal–Wallis and Mann–Whitney U nonparametric tests. Statistically significance was tested by using Chi-square analysis categorical variables. Normally distributed numerical parameters were shown as mean ± standard deviation, skew distributed ones were presented as median and (min–max.) percentiles. Categorical variables were expressed as frequencies and percentages. To determine the optimal cutoff values of WBC, CRP, ESR and N/L variables, the receiver operating characteristic (ROC) curve analysis was performed. Statistical analysis was conducted by using Statistical Package for the Social Sciences 15.0 (SPSS Inc., Chicago, IL) and MedCalc software, Version 9.2.0.1. p Values <.05 were considered as statistically significant.
Results
Fifty-three newly diagnosed GPA patients were studied. NLR was significantly higher in the GPA group compared with the control group (4.50 [min–max: 0.07–34.81] vs 1.77 [min–max: 1.04–2.90], respectively, p < .001). Baseline characteristics of the patients and control groups are shown in Table 1. NLR significantly correlated with ESR and CRP levels (r = .40 and r = .48, respectively, p < .001 for both).
Table 1.
General characteristics and results of inflammatory markers of the patients in the GPA and the control groups.
| GPA group (n = 53) | Control group (n = 39) | p Values | |
|---|---|---|---|
| Age, mean ± SD | 48.5 ± 14.3 | 49.5 ± 13.7 | .728 |
| Gender, % males | 58.5 | 51.3 | .53 |
| NLR, (N/L count) | 4.61 (0.62–46) | 1.77 (1.04–2.9) | <.001 |
| ESR, mm/h | 46.5 (3–131) | 8 (1–34) | <.001 |
| CRP, mg/l | 28.0 (1.27–300) | 3.2 (1.16–7.4) | <.001 |
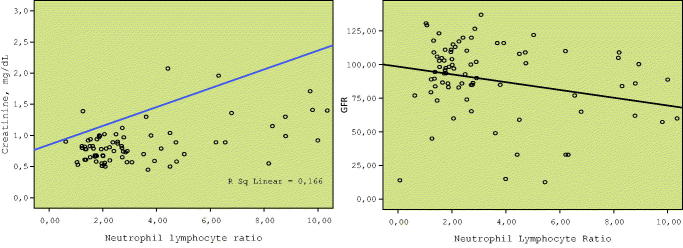
Sub-group analyses were performed with respect to the renal involvement in the GPA group. There was no significant difference between the renal and non-renal groups with respect to the baseline ESR or CRP levels. However, NLR was significantly higher in the renal GPA sub-group compared with the non-renal sub-group (Table 2). In the renal GPA group, ROC of NLR is demonstrated in Figure 1. Baseline NLR correlated with the serum creatinine level at baseline (Spearman’s rho. = 0.487, p < .001). Moreover, there was also a significant inverse correlation between baseline NLR and GFR at 6-month follow-up (Spearman’s rho = −0.296, p = .005) (Figure 2). At 6-month follow-up, those at remission according to the BVA system had significantly lower NLR, lower creatinine level at baseline compared with GPA patients who had active or persistent disease (Table 3).
Table 2.
Comparison of patients in the renal and non-renal subgroups of the GPA patients at baseline.
| Renal GPA (n = 29) | Non-renal GPA (n = 24) | p Values | |
|---|---|---|---|
| Age | 47.0 ± 15.6 | 50.2 ± 12.8 | .434 |
| NLR | 8.79 (1.26–46) | 2.48 (0.62–8.17) | <.001 |
| ESR, mm/h | 49 (3–108) | 38.5 (7.28–131) | .919 |
| CRP, mg/dl | 38.8 (1.27–300) | 13.35 (5.51–250) | .371 |
| Serum creatinine, mg/dl | 2.07 (0.79–12.9) | 0.75 (0.45–1.02) | <.001 |
| GFR ml/min | 33 (2.7–110) | 98.65 (60–122) | <.001 |
| BVAS | 13 (0–24) | 2.5 (0–21) | .009 |
The variables showing normal distribution are presented as mean ± SD while the skew distrusted variables are as median (min–max.).
Figure 1.
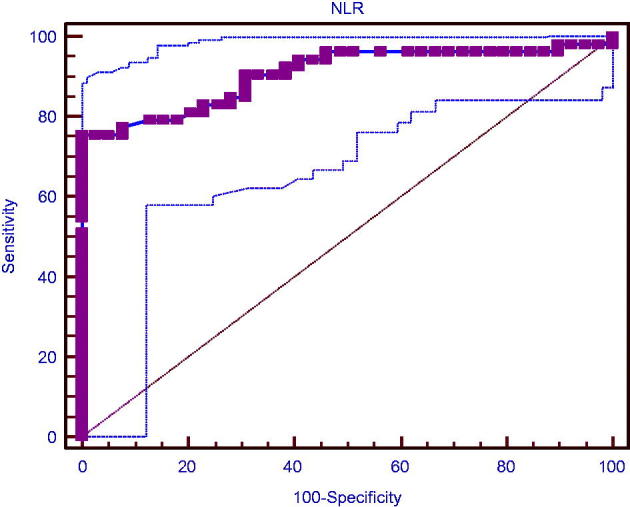
ROC analysis for NLR (AUC was 0.906 for >2.9 of NLR with 75% sensitivity, 100% specificity).
Figure 2.
Baseline NLR correlated with serum creatinine level at baseline (Spearman’s rho. = 0.487, p < .001). Moreover, there was also a significant inverse correlation between baseline NLR and GFR at 6-month follow-up (Spearman’s rho = −0.296, p = .005).
Table 3.
Comparison of GPA patients who have active or persistent disease with those in remission at 6-month follow-up according to the BVAS system.
| Active or persistent disease (n = 16) | Remission (n = 13) | p Values | |
|---|---|---|---|
| NLR | 6.55 (1.26–46) | 3.64 (0.62–14.01) | .004 |
| Serum creatinine, mg/dl | 1.71 (0.5–13.91) | 0.89 (0.45–7.30) | .001 |
| GFR ml/min | 36 (2.7–120) | 86 (6.9–137) | .009 |
The variables showing skew distribution are presented as median (min–max.).
Discussion
GPA is a vasculitis with a significant morbidity and mortality. Renal involvement usually presents with crescentric glomerulonephritis, resulting in significant and permanent loss of renal functions and end-stage kidney disease. End-stage kidney disease has been shown to be associated with increased cardiovascular risk and shortened life expectancy.5 Moreover, impairment in renal functions and advanced age are reported to adversely affect the mortality rates in GPA patients. Therefore, it is crucial to manage renal disease taking risk factors into consideration in GPA patients with poor prognosis in order to reduce their mortality. Available tests, including ANCA titers, are not adequate and new clinical and/or laboratory parameters are needed to predict renal prognosis.
Widely used acute phase reactants, such as, ESR and CRP levels are significantly elevated in patients with active GPA, however, impaired kidney functions might affect their results. Their role in predicting long-term outcome is unclear. There is a need for new markers that could be used to predict renal outcome and prognosis. NLR is a readily available and reliable test, which is also cost-effective. It has been shown to be associated with inflammation. Higher NLR in patients with active renal disease could be due to the prolonged lifespan of neutrophils secondary to the impaired apoptosis and increased neutrophil count associated with elevated G-CSF (granulocyte-colony stimulating factor) levels. Lowered lymphocyte counts could also lead to higher NLR. It has been previously reported that systemic infections and stress might cause lymphopenia resulting in a rise in NLR.6,7 Lymphocyte apoptosis in sepsis might lead to lymphopenia.8 NLR also rises in acute bacterial infections, therefore, we excluded cases with acute infections at baseline. NLR is expected to rise with glucocorticoid use, therefore, we also excluded patients who are already on steroids at presentation to our center. One of the factors affecting NLR is renal failure. Some of the evidence in the literature has demonstrated that in patients with acute or chronic renal failure may cause elevated NLR by the reason of that inflammation plays a major role in the development of renal failure.9,10 In our study, the NLRs were different and creatinine levels were similar between non-renal GPA patients and control groups (p < .001, p = .826, respectively) and non-renal GPA patients had higher NLR than control.
NLR has been demonstrated to be an independent risk factor for cardiovascular diseases.11,12 NLR has also been shown to rise in a variety of conditions including cirrhosis, Alzheimer’s disease, thyroid papillary carcinoma, as well as colorectal cancers.13–16 Celikbilek et al.,17 have reported that NLR could be helpful in predicting severe disease in patients with ulcerative colitis. Similarly, high levels have been previously reported in inflammatory diseases such as, rheumatoid arthritis and familial Mediterranean disease.18,19 Moreover, Makay et al.,20 have demonstrated a correlation between NLR and disease activity and risk of gastrointestinal bleeding in patients with Henoch–Schonlein purpura. In conclusion, we have demonstrated that there is a correlation between baseline NLR and 6-month serum creatinine levels. Higher NLR at baseline is associated with worse renal outcome. Our findings suggest that baseline NLR could have a predictive value for renal prognosis. We have also demonstrated a significant correlation between NLR and BVAS activity scores. Our data suggest that GPA patients with a significantly high NLR at baseline might need closer follow-up for persistent disease activity. Prospective studies with larger number of patients are needed to demonstrate its value in clinical practice to guide therapy.
Disclosure statement
Authors declare no conflict of interest.
References
- 1.Arslan S, Isik AU, Mungan S, Ural A, Sari RA. Wegener’s granulomatosis with an atypical presentation as acute tonsillitis. ORL J Otorhinolaryngol Relat Spec. 2014;76:57–61. [DOI] [PubMed] [Google Scholar]
- 2.de Luna G, Chauveau D, Aniort J, et al. Plasma exchanges for the treatment of severe systemic necrotizing vasculitides in clinical daily practice: Data from the French Vasculitis Study Group. J Autoimmun. 2015;65:49–55. [DOI] [PubMed] [Google Scholar]
- 3.Hobbs KF, Cohen MD.. Rheumatoid arthritis disease measurement: A new old idea. Rheumatology (Oxford). 2012;51:vi21–vi27. [DOI] [PubMed] [Google Scholar]
- 4.Ruggiero C, Metter EJ, Cherubini A, et al. White blood cell count and mortality in the Baltimore longitudinal study of aging. J Am Coll Cardiol. 2007;49:1841–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sipahioglu MH, Kucuk H, Unal A, et al. Impact of arterial stiffness on adverse cardiovascular outcomes and mortality in peritoneal dialysis patients. Perit Dial Int. 2012;32:73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wyllie DH, Bowler IC, Peto TE.. Relation between lymphopenia and bacteraemia in UK adults with medical emergencies. J Clin Pathol. 2004;57:950–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Menges T, Engel J, Welters I, et al. Changes in blood lymphocyte populations after multiple trauma: Association with posttraumatic complications. Crit Care Med. 1999;27:733–740. [DOI] [PubMed] [Google Scholar]
- 8.Hotchkiss RS, Swanson PE, Freeman BD, et al. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit Care Med. 1999;27:1230–1251. [DOI] [PubMed] [Google Scholar]
- 9.Abe T, Kato S, Tsuruta Y, et al. Neutrophil/lymphocyte ratio as a predictor of cardiovascular events in incident dialysis patients: A Japanese prospective cohort study. Clin Exp Nephrol. 2015;19:718–724. [DOI] [PubMed] [Google Scholar]
- 10.Erdem E.Neutrophil lymphocyte ratio in aute renal failure. Indian J Nephrol. 2015;25:126–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nunez J, Nunez E, Bodi V, et al. Usefulness of the neutrophil to lymphocyte ratio in predicting long-term mortality in ST segment elevation myocardial infarction. Am J Cardiol. 2008;101:747–752. [DOI] [PubMed] [Google Scholar]
- 12.Tamhane UU, Aneja S, Montgomery D, Rogers EK, Eagle KA, Gurm HS. Association between admission neutrophil to lymphocyte ratio and outcomes in patients with acute coronary syndrome. Am J Cardiol. 2008;102:653–657. [DOI] [PubMed] [Google Scholar]
- 13.Kuyumcu ME, Yesil Y, Ozturk ZA, et al. The evaluation of neutrophil-lymphocyte ratio in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2012;34:69–74. [DOI] [PubMed] [Google Scholar]
- 14.Kim JY, Park T, Jeong SH, et al. Prognostic importance of baseline neutrophil to lymphocyte ratio in patients with advanced papillary thyroid carcinomas. Endocrine. 2014;46:526–531. [DOI] [PubMed] [Google Scholar]
- 15.Walsh SR, Cook EJ, Goulder F, Justin TA, Keeling NJ. Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol. 2005;91:181–184. [DOI] [PubMed] [Google Scholar]
- 16.Biyik M, Ucar R, Solak Y, et al. Blood neutrophil-to-lymphocyte ratio independently predicts survival in patients with liver cirrhosis. Eur J Gastroenterol Hepatol. 2013;25:435–441. [DOI] [PubMed] [Google Scholar]
- 17.Celikbilek M, Dogan S, Ozbakir O, et al. Neutrophil-lymphocyte ratio as a predictor of disease severity in ulcerative colitis. J Clin Lab Anal. 2013;27:72–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mercan R, Bitik B, Tufan A, et al. The association between neutrophil/lymphocyte ratio and disease activity in rheumatoid arthritis and ankylosing spondylitis. J Clin Lab Anal. 2016;30:597–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uslu AU, Deveci K, Korkmaz S, et al. Is neutrophil/lymphocyte ratio associated with subclinical inflammation and amyloidosis in patients with familial Mediterranean fever? Biomed Res Int. 2013;2013:185317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Makay B, Gucenmez OA, Duman M, Unsal E. The relationship of neutrophil-to-lymphocyte ratio with gastrointestinal bleeding in Henoch-Schonlein purpura. Rheumatol Int. 2014;34:1323–1327. [DOI] [PubMed] [Google Scholar]