Abstract
Background: Morphine is an opioid analgesic drug often used for pain relief in cancer patients. However, there is growing evidence that morphine may modulate tumor growth, progression and metastasis. Unfortunately, the results obtained by these studies are still contradictory.
Methods: In this study, we investigated the effect of morphine in human clear cell renal cell carcinoma 786-O, RLC-310 cells and whether morphine affects on tumor growth in human clear cell renal cell carcinoma 786-O, RLC-310 cells. The cell proliferation was determined by MTT assay, cell proliferation, migration and invasion assays. Immunofluorescence staining and Q-PCR was used to determine the Survivin expression.
Results: It was shown that morphine enhances proliferation of 786-O, RLC-310 cells, whereas morphine promoted the growth and aggressive phenotype of 786-O and RLC-310 cells in vitro though Survivin-dependent signaling.
Conclusions: Our data showed that morphine promotes RCC growth and increases RCC progression via over-expression of Survivin.
Keywords: Clear cell renal cell carcinoma, morphine, renal cell carcinoma, Survivin
Introduction
Clear cell renal cell carcinoma (ccRCC) is the most common primary tumor arising from the kidney in adults.1 Approximately 10–28% of ccRCC will develop a local recurrence or distant metastasis RCC (mRCC) after curative nephrectomy.2,3 mRCC often causes pain and discomfort, especially in advanced stages of the disease. Therefore, the experience of pain in cancer patients is widely accepted as a major threat to quality of life, and the relief of pain has emerged as a priority in mRCC care. The principles of pain management should be the same as those used for other cancer-related pain, which includes the vigilant assessment of the pain and active pain therapy commensurate with cancer pain treatment guidelines. Opioids, such as morphine, are the most powerful analgesics, which have been the most frequently used to relieve pain in cancer pain with cancer metastasis, including mRCC. However, emerging evidence showed that morphine had extra analgesic effects that appeared to alter tumor progression.4–10
Morphine produces strong analgesic effects by stimulating opioid receptor signaling in neurons, which is largely used to relieve pains of patients with cancer in terminal phases, in order to improve their quality of life.11 However, emerging evidence showed that morphine had extra analgesic effects that appeared to alter tumor progression by activating non-classical opioid receptor signaling. Therefore, understanding the contribution of morphine to cancer growth is an important question because existing reports conflict.9,12 Morphine inhibits cisplatin-induced apoptosis and suppression of tumor growth in nasopharyngeal carcinoma xenografts.6 Morphine also activates MAPK/ERK by phosphorylation via PTX-sensitive GPCRs and NO, which leads to the promotion of tumor growth in breast cancer.8 On the other hand, morphine can inhibit migration of tumor-infiltrating leukocytes and suppresses angiogenesis associated with tumor growth in mice.9 In addition to these well-recognized effects, various studies have suggested that morphine elicits a variety of biological effects that appear to be independent of its analgesic properties and may affect cell survival or proliferation. Unfortunately, at present the role of morphine in the regulation of tumor cell growth is not yet correctly established. Morphine has been demonstrated to inhibit the growth of various animal models10,13,14 or human cancer cell lines.6,15 On the contrary, morphine can protect astrocytes from apoptosis triggered by apoptosis-promoting agents16 and promote the growth of tumor cells.5,12 Until now, no studies have examined the effects of morphine in RCC. In this study, we aimed to investigate the role of morphine in RCC.
Materials and methods
Cell lines and cell culture
The human RCC cancer cell lines 786-O, RLC-310 and Chinese hamster ovary (CHO) cells were obtained from the American Type Culture Collection (ATCC). All cells were grown at 37 °C in a humidified atmosphere containing 5% CO2 in RPMI 1640 medium supplemented with 10% FBS, penicillin (100 IU/mL), and streptomycin (100 mg/ml). Unless otherwise specified, cells were seeded at a density of 2 × 104/well in 24-well culture plates, or 2 × 105/well in 6-well culture plates, and after an overnight incubation for adherence, were treated with 1 nM to 10 mM of morphine. After 48 h of incubation, cells were harvested for assay or continued for further experiments.
Cell proliferation assay
We seeded 786-O, RLC-310 and CHO overnight at 5000/well in a 24-well plate or 50,000/well in a six-well plate. For serum-replete conditions, cells were incubated with inducers/inhibitors for 48 h in complete culture medium without the growth factor. For serum-depleted conditions, cells were serum and growth factor starved overnight and then incubated for an additional 48 h without serum and growth factor but with morphine. Cells were enumerated using a Coulter counter and with WST-8 assay kit (Dojindo Molecular Technologies, Gaithersburg, MD), which forms a colored formazan by the activity of cellular dehydrogenases of viable cells. Optical density obtained was extrapolated for the number of cells using calibration curves for known number of cells.
MTT assay
MTT (Sigma, San Francisco, CA) assay was used to assess the growth of RCC cells. Cells (2.5–5 × 103) were plated in 96-well flat bottom plates in a final volume of 200 μl. When attached to the flat, cells were exposed to drugs for 24–48 h. Cell survival was assessed as the manufacturer’s instructions.
Transwell migration and invasion assays
For migration assay, cells (5 × 104) pretreated with morphine (0, 1, 10 μM) for 4 days were resuspended in culture medium with the same concentration of morphine and placed into uncoated membrane in the upper chamber (24-well insert, 8 μm, Corning Costar, Corning, NY). DMEM supplemented with 10% FBS was used as an attractant in the lower chamber. After being incubated for 24 h, cells migrated through the membrane were fixed with 4% paraformaldehyle (Santa Cruz Biotechnology, Santa Cruz, CA) and stained with 1% crystal violet (Shanghai Sangon Company, Shanghai, China). The stained cell images were captured by microscope (Olympus, Osaka, Japan), and five random fields at 10 × magnification were counted. Results represented the average of triplicate samples from three independent experiments. For invasion assays, cells (8 × 104) were placed into 50 μl matrigel-(BD Biosciences, Franklin Lakes, NJ) coated membrane in upper chamber and being incubated for 36 h. Following steps were similar with migration assays.
Quantitative reverse transcriptase polymerase chain reaction
Total RNA was extracted by using TRIzol reagent (Invitrogen, Carlsbad, CA), which was used to generate cDNA by using SuperScript III RT (Invitrogen) with an oligo-dT primer. Q-PCR was performed using Platinum SYBR Green qPCR SuperMix (Invitrogen) as recommended by the manufacturer. The primers used were as follows: Survivin, 5′-CGACCCCATAGAGGAACATAAA-3′ and 5′-GGAATAAACCCTGGAAGTGGTG-3′. And β-actin (forward, 5′- CATCCTGCGTCTGGACCTGG -3′; reverse, 5′- TAATGTCACGCACGATTTCC -3′) as control.
Immunofluorescence staining
Immunofluorescence staining of cells was performed as previously described. Briefly, cells were fixed in 4% para-formaldehyde-PBS at room temperature for 20 minutes and permeabilized in 0.5% Triton X-100 in PBS for 10 minutes at 4 °C. Cells were then blocked with 3% BSA and incubated with primary antibody against polyclonal survivin (Abcam, Abcam Biotechnology, Abcam, Cambridge, England) and β-catenin (Millipore, Billerica, MA) followed by a FITC conjugated second antibody (Invitrogen), counterstained with DAPI (1 μg/ml) and visualized using a confocal microscope (Leica, Lahn, Germany).
Statistical analysis
Each experiment was performed in triplicate and repeated at least three times. All of the data are expressed as mean ± SD. A p values less than .05 was considered statistically significant (*P < .05, #P < .01). Student’s t-test was used to compare the expressions of relative mRNA levels, proliferation cells, migrated cells and invaded cells.
Results
Morphine stimulate RCC cells proliferation
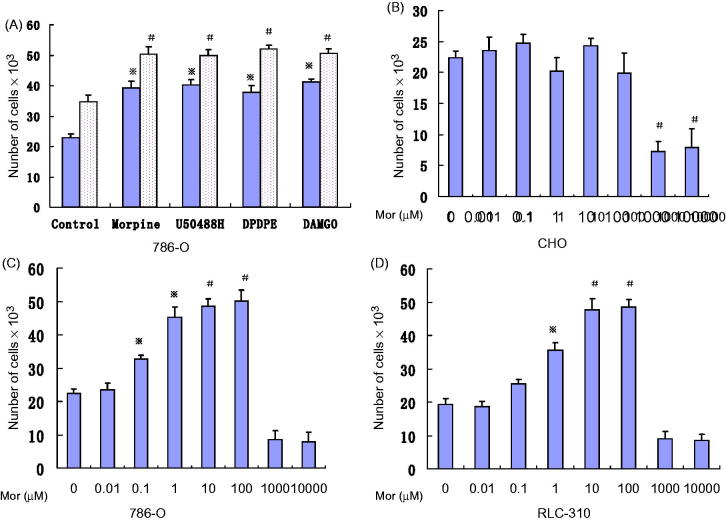
We studied the effect of morphine and specific opioid receptor agonists on human on RCC cells. We first confirmed the effect of morphine, D50488H, DAMGO, and DPDPE on 786-O cells. Our results show morphine as well as MOR, DOR, and KOR agonists (at 50 μM) induced significant 786-O proliferation under both serum-free and serum-replete conditions (Figure 1(A)). The degree of stimulation by most individual agonists was similar in both serum-replete and serum-depleted conditions. None of these opioids had any effect on wild-type Chinese hamster ovary cells. Therefore, morphine, and MOR, DOR, and KOR agonists induce RCC cells proliferation directly, and MOR agonist also potentiates the serum-induced proliferation.
Figure 1.
Morphine and opioid receptor agonists stimulate 786-O proliferation. (A) after 48 h of incubation, 50μM each of morphine, MOR, DOR, and KOR agonists (DAMGO,DPDPE {[D-Pen (2, 5)]-Enkephalin} and U-50488H {trans-()-3,4-Dichloro-N-methyl-N-(2-[1-Pyrrolidinyl] Cyclohexyl) -Benzeneacetamide}, respectively) stimulated 786-O proliferation, under serum-free (▪) as well asserum-replete (□)conditions. ※, P < .05; #, P < .01. (B) Morphine cannot stimulate CHO cell proliferation. (C,D) Morphine concentration-dependent stimulation of 786-O,RLC-310 proliferation after 48 h of incubation. ※, P < .05; #, P < .01 compared to without morphine. Each experiment was repeated three times in triplicates, and each value indicates mean; bars ± SD.
We next examined the effect of morphine concentration (1 nM to 10 mM) on RCC cells proliferation. Morphine is used clinically in doses of 10–2450 mg/day, resulting in serum concentrations that are only 2 nM to 3.5 μM. Wild-type Chinese hamster ovary cells, which do not express any opioid receptors; our data show there were little proliferative effect (Figure 1(B)). On the other hand, we found that a significant proliferative effect occurred in the range of 10 nM to 100 μM morphine (P < .01 versus control; Figure 1(C and D)). We could find that the ideal morphine concentration was located between 10 nM and 100 μM (Figure 1(C and D)). Therefore, we used 50 μM of morphine in later experiments.
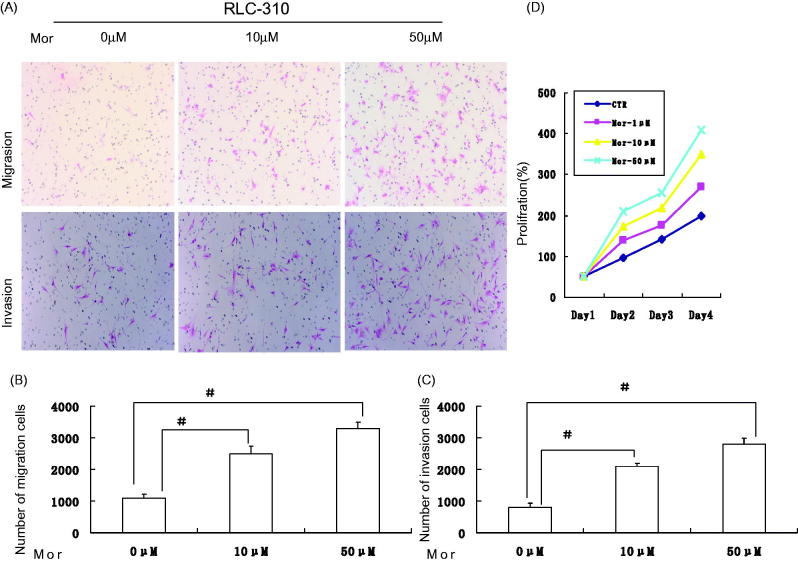
Morphine promotes the migration/invation ability of RCC cells in vitro
We next examined whether ectopic additional morphine was sufficient to promote the migration/invation capability of RCC cells. After morphine added, the migration/invation capability was significantly increased to approximately 3.5- and 4.0-fold in vitro (P < .01; Figure 2(A–C)), respectively. However, no significant difference was observed between the absence of morphine group and the control group. These results were also confirmed by MTT assay (Figure 2(D)). Taken together, our data showed that morphine promotes metastasis in RCC cells. The same results were also obtained for 786-O cells (data not shown).
Figure 2.
Morphine augments the migration/invation ability of RCC cells in vitro. (A) Representative images of the migrate RLC-310 cells in Transwell migration and invasion assays were taken at ×200 magnification. (B, C) Numbers of the invaded/migrated RLC-30 cells per chamber.D:MTT assay results show an enhancement of proliferation in RCC cells treated with morphine with respect to control cells. Data are presented as the means ± SD from three independent experiments. ※, P <.05; #, P <.01.
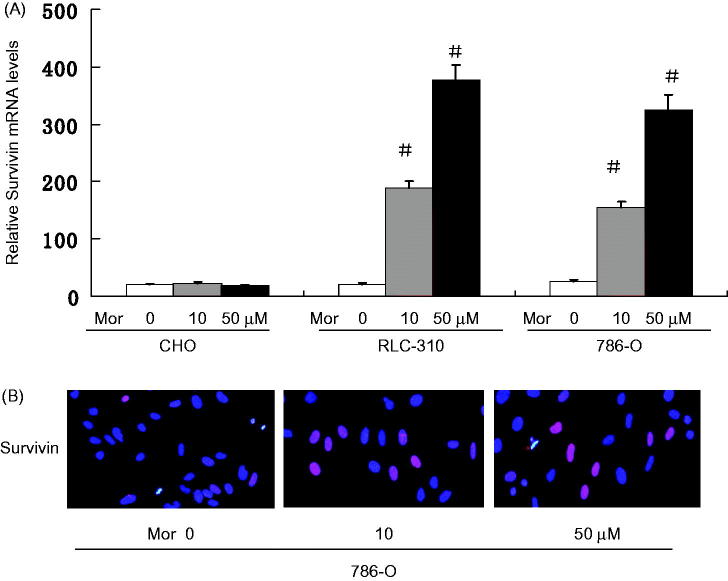
Morphine increases the expression of survivin
Survivin is a member of the inhibitor of apoptosis (IAP) family. Survivin protein functions to inhibit caspase activation, thereby leading to a negative regulation of apoptosis. Therefore, it has been characterized to have a strong anti-apoptotic activity. Recently, increased expression of Survivin has been found to be associated with invasion and metastasis of various types of cancers, including RCC.17 Other contributing effects of morphine include activation of the survival signal PKB/Akt, inhibition of apoptosis, and promotion of cell cycle progression by increasing cyclin D1.8 Survivin is a bifunctional inhibitor of apoptosis protein that has been implicated in protection from apoptosis and regulation of mitosis.18,19 Consistent with these effects, to explore the underlying mechanism by which morphine promotes the properties of RCC cells, we examined the expression of Survivin following morphine treatment. We examined the mRNA levels of Survivin in RLC-310 and 786-O cells treated with morphine by Q-PCR. Morphine significantly increased the mRNA levels of Survivin in both RLC-310 and 786-O cells. In comparison to untreated controls, the mRNA levels of Survivin were increased 19.18 ± 0.85 folds in RLC-310 cells (Figure 3(A)), while 14.92 ± 1.47 folds in 786-O cells (Figure 3(A)). Consistently, Immunofluorescence staining showed that morphine dose-dependent increased the protein levels of Survivin in RLC-310 and 786-O cells; Our results show that dense tumor cytoplasmic and membrane were staining for survivin (Figure 3(B)). These data suggest that morphine may promote RCC cell properties by up-regulating Survivin.
Figure 3.
Morphine increases the expression of Survivin. (A) The mRNA levels of Survivin in CHO,786-O and RLC-310 cells were measured by Q-PCR after treating with morphine (0, 10, 50 μM) for 4 days. Error bars represent mean ± SD of triplicates. (B) Immunofluorescence was performed using FITC-labeled phalloidin, Survivin. Nuclei were stained with DAPI (Scale bar, 20 μm). ※, P < .05; #, P < .01.
Discussion
Cancer pain is one of the most common symptoms in cancer patients experienced at some point during the course of their illness. However, morphine contributes to the proliferation, invasion and metastasis of cancer cells, so it is important to control cancer pain for the aim at enhancement of life quality originally, leaving out hastening or delaying death. But with the successful achievement of analgesics in cancer pain, the effects on non-neural cells, such as endothelial cells, tumor cells, and mast cells become worthwhile.8,20,21 However, the results obtained in the studies assessing cancer cell growth in vitro or in vivo are still controversial. Many reports showed that morphine was able to inhibit the growth of various human cancer cell lines, including breast cancer, gastric cancer, lung cancer and prostate cancer.7,22–24 On the contrary, other studies have shown that morphine increases tumor cell growth in vivo5,8 and in vitro.12 In this study, it has been demonstrated that morphine significantly contributes to the proliferation, invasion and metastasis of RCC cell through a Survivin-dependent mechanism.
These contrasting results are probably associated with different morphine doses used, route of administration, and/or plasma doses achieved at steady state. In fact, in vitro and in vivo studies demonstrated that tumor-enhancing effects with morphine occur after administration of low daily doses or single dose of morphine,25 while tumor suppression occurs after chronic high doses of morphine.13,14
Survivin is a newly identified member of the inhibitor of apoptosis (IAP) gene family that has been implicated in suppression of apoptotic cell death and regulation of cell division.26 Over-expression of Survivin protein could inhibit tumor cell apoptosis, promote metastatic ability of tumor cells, and increase genomic instability, thereby boosting malignant phenotypes, such as local invasion and distant metastasis17,27,28 Recent studies demonstrated that Survivin expression was associated with advanced clinico-pathological stages and grades of ccRCC, while ccRCC patients with low Survivin levels had a better survival rate compared to patients with high Survivin-expressed tumor.17,29 In our research, the Q-PCR showed that the morphine increase the expression of Survivin in RLC-310,786-O RCC cells, while the immunofluorescence staining showed the similar results.
Currently, both morphine and anti-cancer drugs have been simultaneously given to patients, especially those patients with cancer metastasis. Morphine activates MAPK/ERK by phosphorylation via PTX-sensitive GPCRs and NO, which leads to the promotion of tumor growth in breast cancer.8 Morphine also induces phosphorylation of epidermal growth factor receptor (EGFR) via opioid receptors, promotes cell proliferation and increases cell invasion.30 In addition, morphine promotes breast cancer cell migration and invasion by increasing the expression of NET1.10 Until now, little attention has been paid to the RCC during application of morphine. Our study showed that morphine promoted the RCC cells phenotype and induced Survivin over-expression, which could contribute to the cancer development.
It has been proposed that morphine plays also a role in tumor apoptosis. Apoptosis is a form of cell death in which a programmed sequence of events leads to the elimination of cells without releasing harmful substances into the surrounding area. On the other side, Survivin is a member of the inhibitor of apoptosis (IAP) family. Survivin negatively regulates apoptosis by interfering with caspase-9 processing.27 Survivin may be closely linked to escape from apoptosis of RCC cells and the development of RCC. Our results show morphine augments the growth and aggressive phenotype of renal cancer cells in vitro. We also found that Survivin was the target gene of morphine in RCC cell lines. The results suggest that morpine could play an important role in tumorigenesis and progression of RCC.
Disclosure statement
The authors have declared that no competing interests exist.
References
- 1.Jonasch E, Gao J, Rathmell WK.. Renal cell carcinoma. Bmj. 2014;349:g4797. doi: 10.1136/bmj.g4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rini BI, Campbell SC, Escudier B.. Renal cell carcinoma. Lancet 2009; 373:1119–1132. [DOI] [PubMed] [Google Scholar]
- 3.Liu S, Qi L, Han W, et al. . Overexpression of wip1 is associated with biologic behavior in human clear cell renal cell carcinoma. PLoS One 2014;9:e110218. doi: 10.1371/journal.pone.0110218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Afsharimani B, Cabot P, Parat MO.. Morphine and tumor growth and metastasis. Cancer Metastasis Rev. 2011;30:225–238. [DOI] [PubMed] [Google Scholar]
- 5.Bimonte S, Barbieri A, Rea D, et al. . Morphine promotes tumor angiogenesis and increases breast cancer progression. Biomed Res Int. 2015;2015:161508. doi: 10.1155/2015/161508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao LH, Li HT, Lin WQ, et al. . Morphine, a potential antagonist of cisplatin cytotoxicity, inhibits cisplatin-induced apoptosis and suppression of tumor growth in nasopharyngeal carcinoma xenografts. Sci Rep. 2016;6:18706. doi: 10.1038/srep18706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ge ZH, Wang ZX, Yu TL, et al. . Morphine improved the antitumor effects on MCF-7 cells in combination with 5-Fluorouracil. Biomed Pharmacother. 2014;68:299–305. [DOI] [PubMed] [Google Scholar]
- 8.Gupta K, Kshirsagar S, Chang L, et al. . Morphine stimulates angiogenesis by activating proangiogenic and survival-promoting signaling and promotes breast tumor growth. Cancer Res. 2002;62:4491–4498. [PubMed] [Google Scholar]
- 9.Koodie L, Yuan H, Pumper JA, et al. . Morphine inhibits migration of tumor-infiltrating leukocytes and suppresses angiogenesis associated with tumor growth in mice. Am J Pathol. 2014;184:1073–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nguyen J, Luk K, Vang D, et al. . Morphine stimulates cancer progression and mast cell activation and impairs survival in transgenic mice with breast cancer. Br J Anaesth. 2014;113S1:i4–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paice JA, Ferrell B.. The management of cancer pain. CA Cancer J Clin. 2011;61:157–182. [DOI] [PubMed] [Google Scholar]
- 12.Niu DG, Peng F, Zhang W, et al. . Morphine promotes cancer stem cell properties, contributing to chemoresistance in breast cancer. Oncotarget 2015;6:3963–3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harimaya Y, Koizumi K, Andoh T, et al. . Potential ability of morphine to inhibit the adhesion, invasion and metastasis of metastatic colon 26-L5 carcinoma cells. Cancer Lett. 2002;187:121–127. [DOI] [PubMed] [Google Scholar]
- 14.Sasamura T, Nakamura S, Iida Y, et al. . Morphine analgesia suppresses tumor growth and metastasis in a mouse model of cancer pain produced by orthotopic tumor inoculation. Eur J Pharmacol. 2002;441:185–191. [DOI] [PubMed] [Google Scholar]
- 15.Tegeder I, Grosch S, Schmidtko A, et al. . G protein-independent G1 cell cycle block and apoptosis with morphine in adenocarcinoma cells: Involvement of p53 phosphorylation. Cancer Res. 2003;63:1846–1852. [PubMed] [Google Scholar]
- 16.Kim MS, Cheong YP, So HS, et al. . Protective effects of morphine in peroxynitrite-induced apoptosis of primary rat neonatal astrocytes: Potential involvement of G protein and phosphatidylinositol 3-kinase (PI3 kinase). Biochem Pharmacol. 2001;61:779–786. [DOI] [PubMed] [Google Scholar]
- 17.Liu S, Qi L, Yu Q, et al. . Survivin and HLA-I expression predicts survival of patients with clear cell renal cell carcinoma. Tumour Biol. 2014;35:8281–8288. [DOI] [PubMed] [Google Scholar]
- 18.Duffy MJ, O’Donovan N, Brennan DJ, et al. . Survivin: A promising tumor biomarker. Cancer Lett. 2007;249:49–60. [DOI] [PubMed] [Google Scholar]
- 19.Mita AC, Mita MM, Nawrocki ST, Giles FJ.. Survivin: Key regulator of mitosis and apoptosis and novel target for cancer therapeutics. Clin Cancer Res. 2008;14:5000–5005. [DOI] [PubMed] [Google Scholar]
- 20.Vincent L, Vang D, Nguyen J, et al. . Mast cell activation contributes to sickle cell pathobiology and pain in mice. Blood. 2013;122:1853–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farooqui M, Geng ZH, Stephenson EJ, et al. . Naloxone acts as an antagonist of estrogen receptor activity in MCF-7 cells. Mol Cancer Ther. 2006;5:611–620. [DOI] [PubMed] [Google Scholar]
- 22.Kampa M, Bakogeorgou E, Hatzoglou A, et al. . Opioid alkaloids and casomorphin peptides decrease the proliferation of prostatic cancer cell lines (LNCaP, PC3 and DU145) through a partial interaction with opioid receptors. Eur J Pharmacol. 1997;335:255–265. [DOI] [PubMed] [Google Scholar]
- 23.Maneckjee R, Minna JD.. Opioid and nicotine receptors affect growth regulation of human lung cancer cell lines . Proc Natl Acad Sci USA. 1990;87:3294–3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qin Y, Chen J, Li L, et al. . Exogenous morphine inhibits human gastric cancer MGC- 803 cell growth by cell cycle arrest and apoptosis induction. Asian Pac J Cancer Prev. 2012;13:1377–1382. [DOI] [PubMed] [Google Scholar]
- 25.Zong J, Pollack GM.. Morphine antinociception is enhanced in mdr1a gene-deficient mice. Pharm Res. 2000;17:749–753. [DOI] [PubMed] [Google Scholar]
- 26.Ambrosini G, Adida C, Altieri DC.. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997;3:917–921. [DOI] [PubMed] [Google Scholar]
- 27.Garg H, Suri P, Gupta JC, et al. . Survivin: A unique target for tumor therapy. Cancer Cell Int. 2016;16:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen X, Chen XG, Hu X, et al. . MiR-34a and miR-203 inhibit survivin expression to control cell proliferation and survival in human osteosarcoma cells. J Cancer. 2016;7:1057–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zamparese R, Pannone G, Santoro A, et al. . Survivin expression in renal cell carcinoma. Cancer Invest. 2008;26:929–935. [DOI] [PubMed] [Google Scholar]
- 30.Fujioka N, Nguyen J, Chen C, et al. . Morphine-induced epidermal growth factor pathway activation in non-small cell lung cancer. Anesth Analg. 2011;113:1353–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]