Abstract
Large conductance calcium and voltage‐activated potassium channels (BKC a) are transmembrane proteins, ubiquitously expressed in the majority of organs, and play an active role in regulating cellular physiology. In the heart, BKC a channels are known to play a role in regulating the heart rate and protect it from ischemia–reperfusion injury. In vascular smooth muscle cells, the opening of BKC a channels results in membrane hyperpolarization which eventually results in vasodilation mediated by a reduction in Ca2+ influx due to the closure of voltage‐dependent Ca2+ channels. Ex vivo studies have shown that BKC a channels play an active role in the regulation of the function of the majority of blood vessels. However, in vivo role of BKC a channels in cardiovascular function is not completely deciphered. Here, we have evaluated the rapid in vivo role of BKC a channels in regulating the cardiovascular function by using two well‐established, rapid‐acting, potent blockers, paxilline and iberiotoxin. Our results show that BKC a channels are actively involved in regulating the heart rate, the function of the left and right heart as well as major vessels. We also found that the effect on BKC a channels by blockers is completely reversible, and hence, BKC a channels can be exploited as potential targets for clinical applications for modulating heart rate and cardiac contractility.
Keywords: BKCa channels, cardiac function, echocardiography, paxilline
Introduction
BKCa channels are voltage and calcium‐activated potassium channels found in a multitude of cells throughout the body, including vascular smooth muscle cells (VSMCs) and neurons in which they function to regulate cell tone and excitability (Singh et al. 2012; Toro et al. 2014; Balderas et al. 2015). On intracellular injections of Ca2+, Meech in 1970s reported an increase in K+ conductance in nerve cells which set the foundation for the existence of BKCa channels (Brown et al. 1970). The opening of BKCa channels results in fast repolarization of the cellular membrane and hence, closure of voltage‐dependent Ca2+ channels, resulting in reduced Ca2+ entry into the cell and increased Ca2+ extrusion by the Na+–Ca2+ exchanger. Therefore, the primary function of BKCa is to exert a negative feedback on the membrane potential and on intracellular Ca2+ (Toro et al. 2014).
BKCa channels are heterogeneously expressed in the cardiovascular system (Singh et al. 2012; Balderas et al. 2015). The pore‐forming α‐subunit encoded by a gene, Kcnma1, is present in the membranes of many cell types, except adult cardiomyocytes where they are exclusively present in mitochondria (Xu et al. 2002; Singh et al. 2013; Soltysinska et al. 2014; Toro et al. 2014). The regulatory β subunit (encoded by four genes, Kcnmb1‐4) is expressed in the heart and vascular smooth muscle cells. A recently identified γ subunit (LRRC26) is also present in cerebral artery smooth muscle cells (Evanson et al. 2014). BKCa channels are considered key players in the vascular system, where they play a major role in the regulation of vascular tone. During depolarization of VSMCs, BKCa channels open to guard against excessive vasoconstriction. Ex vivo experiments revealed that blockage of BKCa channels with pharmacologic agents results in aortic and carotid artery constriction. In cardioprotection from ischemia–reperfusion studies, activation of BKCa channels results in a reduction in myocardial infarction, whereas blocking by Paxilline (PAX) ablated cardioprotection from ischemic preconditioning (Singh et al. 2013; Toro et al. 2014; Balderas et al. 2015).
Over the last decade, significant progress has been made to understand the role of BKCa channels in cardiac function. The α‐subunit of the channel has been shown to be involved in regulation of heart rate through the sinoatrial (SA) nodal cell regulation (Imlach et al. 2010; Lai et al. 2014). However, the role of BKCa channels in the regulation of cardiac function is not deciphered. In order to understand the role of BKCa channels in cardiac and vascular function of major vessels, we have used a combination of pharmacology and echocardiography. Using a rat model, we have injected either PAX (cell permeable) or iberiotoxin (IBTX, cell impermeable) into the left femoral vein. In this study, we have used comprehensive 2D echocardiography to evaluate the effects of the highly specific inhibitors PAX and IBTX on several physiologic parameters of left and right ventricular function (Gao et al. 2000, 2011; Kohut et al. 2016), as well as the hemodynamic status of the cardiovascular system. Echocardiography images were acquired under anesthesia, and various parameters of cardiovascular function were evaluated.
We have found that blocking BKCa channels reversibly alters the cardiovascular function by reducing the heart rate and increases contraction of major vessels. We also found that the inhibitory effect of BKCa is completely reversible. Our study is the first in vivo comprehensive study to establish the role of BKCa channels in cardiovascular function.
Methods
Animals
In this study, 2‐month‐old Sprague–Dawley male rats (Charles River Laboratories, Wilmington, MA) weighing 300–330 g were used for experiments. The experimental procedures were designed in accordance with National Institutes of Health and American Association for the Accreditation of Laboratory Animal Care (AAALAC) guidelines and approved by the Drexel University College of Medicine Institutional Animal Care and Use Committee (IACUC), Philadelphia, PA.
Cardiovascular function analysis
A high‐frequency, high‐resolution digital imaging platform with linear array technology and Color Doppler Mode for in vivo high‐resolution micro‐imaging was used for echocardiography as per previously published guidelines (Kohut et al. 2016) (Vevo® 2100 Imaging System, FUJIFILM VisualSonics Inc., Toronto, Canada). For assessing the cardiovascular function of rats, a high‐frequency transducer probe (VisualSonics MS250 with a frequency range of 18–38 MHz) was utilized as it provides appropriate resolution and depth of penetration needed.
Anesthesia was achieved using 2.5% (v/v) isoflurane mixed with oxygen. Rats were evaluated with a toe pinch to ensure a complete anesthesia. After achieving an adequate anesthesia, anthropomorphic measurements were taken. Total body weight and nose to anus length were recorded, and rats were secured on a prewarmed (37°C) imaging platform. Hairs from the ventral chest were removed using commercially available hair removal cream (Nair™, © Church & Dwight Co). After securing each rat to the imaging platform, anesthesia concentration was titrated from 1% to 3% to maintain a minimum heart rate of 300 beats/min.
Baseline echocardiograms were obtained for each rat. After baseline echocardiogram, anesthesia was maintained, and the left femoral vein of each rat was dissected and exposed. Using a 23‐gage needle and 1‐mL syringe, rats were injected with 1 mL/kg of a solution of PAX (final concentrations, 5.7 and 115 nmol/L) or IBTX (final concentrations, 1 and 10 μmol/L), and DMSO (control). An echocardiogram was repeated at 1 and 15 min after injection. Mean arterial pressure was measured by Miller transducer inserted into the carotid. A total of 4–6 rats were assigned to each group. The total anesthesia time for each animal did not exceed 25 min, with each echocardiogram taking 5–10 min to complete.
Image analysis
All echocardiograms were reviewed using the VisualSonic Vevo Lab software in conjunction with a clinical echocardiographer to ensure optimal image quality and analysis.
Specific views were captured using various echo imaging modalities to obtain the physiologic parameters of interest (Gao et al. 2000, 2011; Kohut et al. 2016). Figure 1 shows B mode views of the rat heart obtained during 2D echocardiography. Figure 1A shows the parasternal long‐axis view of the heart acquired using B mode. From this view, left ventricle (LV) functional parameters were measured including LV ejection fraction (LVEF). In addition, the postimaging analysis included LV strain analysis of the parasternal long‐axis (PSLAX) views to calculate Global Longitudinal Strain (GLS). Figure 1B shows the parasternal short‐axis (PSSAX) view at the level of the aortic valve toward the base of the heart. The pulmonic valve and tricuspid valve are also seen here, and this view is ideal for assessing right ventricular outflow tract and pulmonary artery diameter. Doppler echocardiography is also obtained from this view with sample volume in the pulmonary artery at the valve leaflet tips. Figure 1C shows the apical four‐chamber view. Other parameters of LV systolic function, such as Mitral Annular Plane Systolic Excursion (MAPSE) and Tissue Doppler imaging, can be obtained by placing the sample volume at the lateral mitral annulus and angulating the M‐Mode or Doppler plane along the red line.
Figure 1.
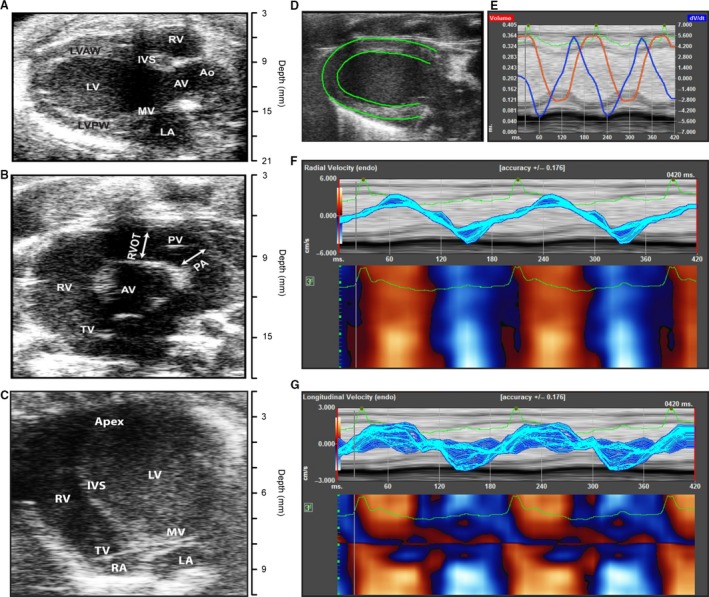
Echocardiographic analysis of the heart. (A) Parasternal long‐axis view of the heart. (B) Short‐axis view of the heart. (C) Apical four‐chamber view of the heart. (D) Tracing the left ventricular myocardial borders for strain analysis. (E) Strain analysis indicating left ventricular volume (red) and derivative of tissue velocity (blue) wall motion in two cardiac cycles. The green line indicates the corresponding EKG. (F) Radial strain for two long‐axis LV contractions. (G) Longitudinal strain for two long‐axis LV contractions. Ao, aorta; AV, aortic valve; IVS, interventricular septum; LA, left atrium; LV, left ventricle; LVAW, left ventricular anterior wall; LVPW, left ventricular posterior wall; MV, mitral valve; PA, pulmonary artery; PV, pulmonic valve; RA, right atrium; RV, right ventricle; RVOT, right ventricular outflow tract; TV, tricuspid valve.
Statistical analysis
For physiologic parameters of interest, four measurements for each animal were taken and averaged. Notably, at high doses of PAX, several animals experienced cardiac arrest prior to the 15‐min time interval. Hemodynamic data at the 15‐min interval was assigned a value corresponding to 1% of the baseline to avoid calculations involving null values.
All values were entered into Microsoft Excel, and Student's t‐test was used to compare means for statistical significance. All values are reported as mean with standard error.
Results
Inhibition of BKCa causes bradycardia
Inhibition of BKCa channels with PAX and lolitrem B is known to reduce heart rate in wild‐type but not in Kcnma1 −/− mice (Imlach et al. 2010). Ex vivo studies in rat hearts also showed a reduction in heart rate when perfused with PAX and lolitrem B. We tested the role of BKCa in the regulation of heart rate in in vivo model. Heart rate was measured at both 1‐ and 15‐min intervals after injecting PAX and IBTX. As mentioned previously, prior studies have noted the negative chronotropic effect of BKCa channel inhibition on heart rate and have also elucidated the mechanism of this relative bradycardia via an effect on SA nodal cells (Lai et al. 2014). Both high‐ (50 ng/mL) and low‐dose (2.5 ng/mL) PAX groups experienced significant reductions in heart rate in rats (Fig. 2).
Figure 2.
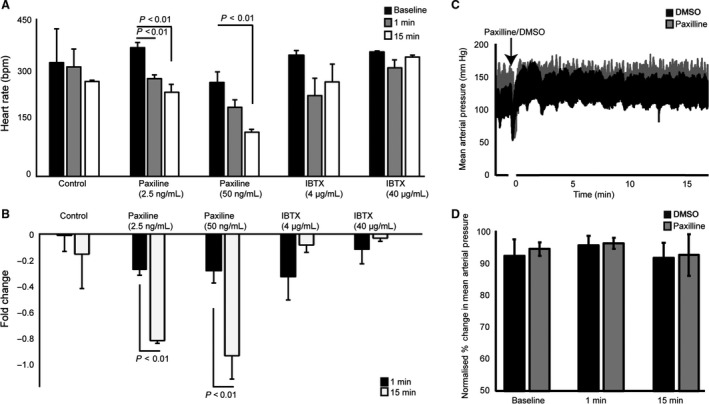
Change in heart rate (beats per minute) after administration of Paxilline or Iberiotoxin. (A) Absolute reduction in heart rate after administration of DMSO control compared with low‐dose and high‐dose Paxilline or Iberiotoxin at baseline (n = 5–7), 1 min, and 15 min after injection. (B) Fold change in heart rate from baseline for the three groups at 1 min and 15 min after injection. Treatment with Paxilline at low dose resulted in decreased heart rate at 1 and 15 min. At high doses, a decrease in heart rate was also seen. Treatment with the higher dose of Paxilline showed a greater fold reduction in heart rate at 15 min compared with the low‐dose Paxilline. The use of Iberiotoxin did not significantly affect the heart rate. (C) Representative mean arterial pressure traces with or without (50 ng/mL Paxilline, n = 4). (D) Percentage change of MAP normalized to MAP before Paxilline injection. There was no difference observed in the MAP with Paxilline as compared with the DMSO control.
Immediately after injection of PAX (after 1 min) both 2.5‐ and 50‐ng/mL groups showed a ~1–25% reduction in heart rate (Fig. 2), but animals injected with 50 ng/mL showed ~40–50% reduction and was fatal in over 50% animals injected with PAX. Surprisingly, IBTX showed no change in heart rate after 1 or 15 min at lower (4 μg/mL) or higher dose (40 μg/mL) (Fig. 2). To test whether the reduction in heart rate is mediated by a change in blood pressure, we measured mean arterial pressure after Paxilline (5 ng/mL). As shown by other groups, we did not detect any changes in the MAP within 1 or 15 min of Paxilline injection (Imlach et al. 2010). Our results agree with ex vivo and knock out studies showing the role of BKCa in regulating the heart rate and also provided us valuable pharmacological tools to evaluate cardiovascular function in vivo.
Inhibition of BKCa causes left ventricular dysfunction
We performed a comprehensive analysis of the LV of rats after injecting DMSO (control), PAX, or IBTX. Changes in LVEF in response to DMSO, PAX, and IBTX were measured using the PSLAX view. DMSO had no impact on the cardiac ejection fraction. As shown in Figure 3A and B, there were no significant changes in the LVEF with control, PAX, or IBTX. PAX at 15 min showed a small but not statistically significant reduction in LVEF. Qualitatively, injection of PAX did induce abnormalities in wall motions.
Figure 3.
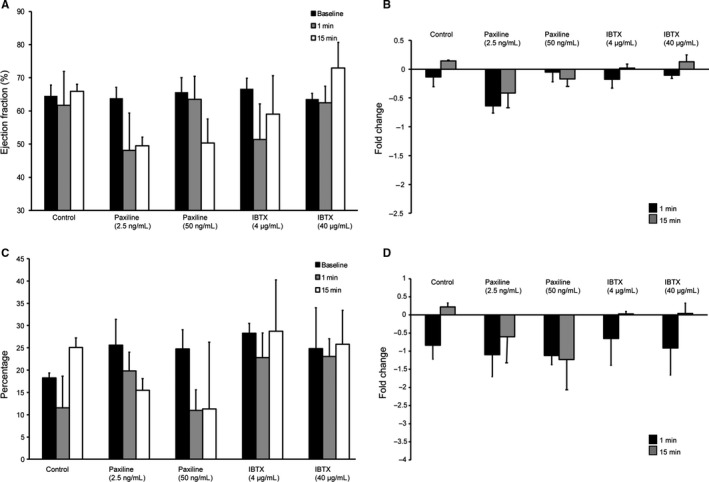
Evaluation of left ventricular function after administration of Paxilline (n = 7) or Iberiotoxin (n = 4). (A) Bar graphs showing an absolute reduction in left ventricular ejection fraction. (B) Fold change in left ventricular ejection fraction obtained from A. (C) Bar graph showing changes in absolute global longitudinal strain values. (D) Fold change in global longitudinal strain calculated from C. No statistically significant reduction in left ventricular ejection fraction was found. Although not statistically significant, strain analysis revealed a trend toward reduction in left ventricular function seen with Paxilline at low and high doses at both 1 and 15 min after drug administration.
To analyze the LV function, we also performed global left ventricle strain analysis (Figs. 1D–G and 3C, and D). Myocardial strain analysis allows for detection of more subtle changes in LV function than an assessment of LVEF alone (Figs. 3C and D). In agreement with LVEF, strain analysis did not yield any significant differences between control or experimental (PAX or IBTX) animals. As observed for LVEF, LV strain also showed reduction with PAX, but it was not statistically significant. In a null mutant mouse, a small reduction in LVEF (from ~60% to ~55%) was reported (Frankenreiter et al. 2017). However, a slight reduction in LV function with PAX and wall movement abnormalities indicated a possible dysfunction of coronary arteries which we tested further in detail.
Effect of BKCa inhibition on coronary artery flow
BKCa channels are abundantly expressed in the plasma membrane of coronary artery SMC where they are predicted to play a role in regulating coronary flow. They are known to play an active role in protecting the heart from IR injury (Ahn et al. 1994). However, most of the studies are carried out in isolated cells, vessels, or heart. For the first time, we performed in vivo analysis of the coronary artery flow by using PAX and IBTX (Fig. 4). Color Doppler movies were acquired and flow rates were measured. We found that inhibition of BKCa by PAX significantly (P < 0.01) reduced mean coronary velocity and coronary mean gradient at 50 ng of PAX within 1 min but was completely reversible after 15 min (Fig. 4). Similarly, IBTX showed a significant (P < 0.05) reduction in the mean velocity and mean gradient (Fig. 4) at 4 as well as 40 μg/mL which completely reversed within 15 min. These results implicate BKCa channels in regulating the coronary perfusion.
Figure 4.
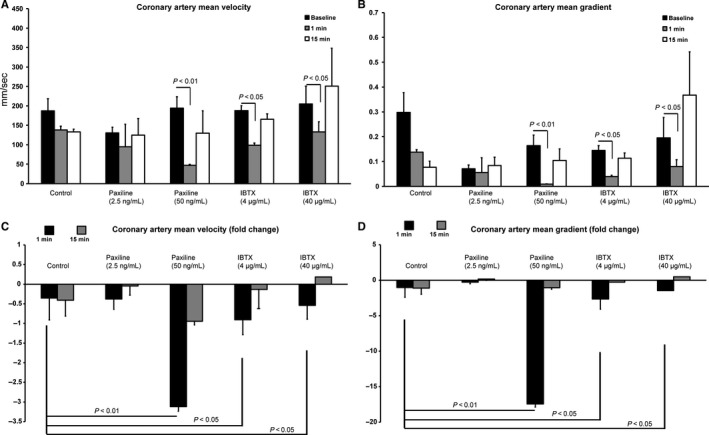
Evaluation of coronary flow after administration of Paxilline (n = 5) or Iberiotoxin (n = 4). (A) Absolute reduction in mean coronary velocity. (B) Absolute reduction in mean coronary gradient. (C) Fold change in mean coronary velocity. (D) Fold change in mean coronary gradient. Velocity through the left main coronary artery was found to be decreased 1 min after administration of high‐dose Paxilline. This reduction in flow normalized at 15 min. Mean coronary gradient in this particular group was also found to be decreased.
Effect of BKCa inhibition on pulmonary function
Previous studies in in vitro using isolated vessels have indicated that activation of BKCa channel with NS1619 results in pulmonary artery (PA) dilation found in patients with pulmonary hypertension (Vang et al. 2010). To identify the role of BKCa in pulmonary arteries, we measured right ventricular outflow tract (RVOT) and PA diameter at baseline and then 1 and 15 min post injection. We interestingly did not find any significant changes in either RVOT or PA diameter (Fig. 5) as would have been expected from ex vivo work (Vang et al. 2010).
Figure 5.
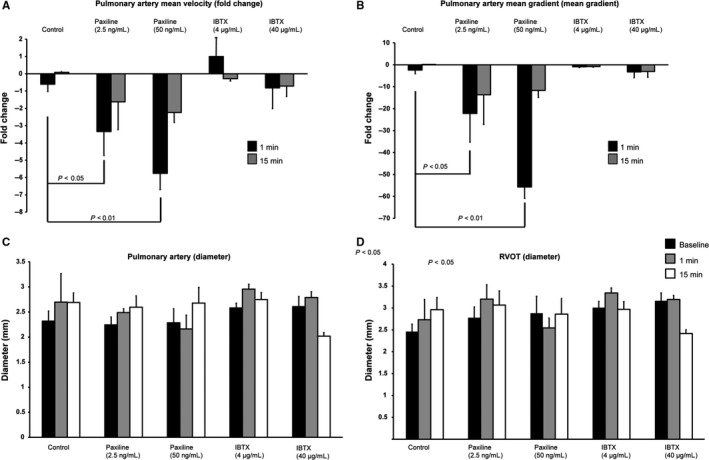
Evaluation of pulmonic artery and right heart function after administration of Paxilline (n = 5) or Iberiotoxin (n = 4). (A) Bar graph showing fold change in mean pulmonary velocity. (B) Bar graph representing fold change in mean pulmonary gradient. (C) Absolute change in pulmonary artery diameter. (D) Absolute change in right ventricular outflow tract diameter. Velocity across the pulmonic valve was decreased 1 min after administration of both low‐dose and high‐dose Paxilline. In both dosages, velocity began to show normalization by 15 min. Mean pulmonary gradient showed a similar trend. In both cases, Iberiotoxin did not show an effect. No differences were seen in pulmonary artery diameter and right ventricular outflow tract diameter.
To study the functional consequence of inhibition of BKCa channels, we measured mean pulmonary velocity and mean gradient using color Doppler. PAX at both lower (2.5 ng/mL) and higher (50 ng/mL) concentrations showed a significant reduction in the mean pulmonary velocities and mean gradient (Fig. 5). These reductions in pulmonary velocities showed near complete reversibility after 15 min. IBTX, however, showed no change in mean pulmonary velocity at lower or higher concentrations even after 15 min (Fig. 5).
Discussion
BKCa channels are ubiquitously expressed in the plasma membrane of vascular smooth muscle cells (Jia et al. 2013), cardiac fibroblasts (Li et al. 2009), and endothelial cells (Rusko et al. 1992; Ungvari et al. 2002). Surprisingly in adult cardiomyocytes, a unique BKCa channel splice variant is exclusively present in the mitochondria (Singh et al. 2012, 2013). The pore‐forming α‐subunit interacts with regulatory β‐subunits, and β‐subunits are also widely expressed in the heart and VSMCs (Brenner et al. 2000). Several ex vivo studies have shown that in the vascular system, BKCa channels are the key players in the regulation and maintenance of vascular tone (Ghatta et al. 2006). Interaction of α‐subunit with regulatory β‐subunits enhances the Ca2+ sensitivity of the BKCa channel which allows basal activity under physiological conditions of negative membrane potential of VSMCs (Tanaka et al. 1997) and possibly in mitochondrial membranes. BKCa channels are known to provide an endogenous compensatory mechanism to buffer vasoconstriction by opening in response to the depolarization of VSMCs and the rise in cytosolic Ca2+ concentrations (Nelson et al. 1995). However, the role of BKCa channels in cardiovascular function is marred with controversies ranging from the absence of BKCa channels at the plasma membrane on adult cardiomyocytes to whether vasodilator effect of BKCa channels in VSMCs is reduced, is persistent, or increases under pathophysiological conditions (Rusch 2009). The key reason for these discrepancies are due to focus of most of the studies on ex vivo experimental approaches such as isolated VSMCs or vessels, and complex signaling mechanisms (Singh et al. 2016) involving BKCa channels.
In a swine model, NS1619, an agonist for BKCa channels induced significantly smaller coronary arteriolar dilation in metabolic syndrome as compared with controls indicating a reduction in functional BKCa channels (Borbouse et al. 2009). In addition, changes in expression and properties of BKCa have been reported in different pathophysiological conditions, such as hypoxia, hypertension, and diabetes (England et al. 1993; Sobey 2001), indicating that BKCa plays a direct role in maintaining vascular physiology. In the heart, BKCa has been shown to play a direct role in cardioprotection from ischemia–reperfusion injury (Singh et al. 2013; Soltysinska et al. 2014; Frankenreiter et al. 2017). In sinoatrial node cells (SANCs), expression of functional BKCa channels was characterized and pharmacological inhibition by PAX in vivo was shown to be involved in cardiac pacing (Imlach et al. 2010; Lai et al. 2014). Using genetic and pharmacological tools, BKCa channels were implicated in SANCs firing rate (Lai et al. 2014). In a recent study, cardiomyocyte‐specific BKCa null mutant mice exhibited mild hypotension, a slight decrease in heart rate, as well as left ventricular ejection fraction and fractional shortening (Frankenreiter et al. 2017). This was a surprising finding as mechanisms involving baroreceptors, renal, and neuroendocrine systems in blood pressure expressed normal levels of BKCa, indicating that BKCa present in cardiomyocytes causes cardiac dysfunction and blood pressure dampening.
In this study, our focus was to decipher the role of BKCa channels in the cardiovascular system in vivo. While in vitro and ex vivo investigation into the role of BKCa channels has previously been performed, in our study we were able to gain insight into in vivo function of this channel. BKCa channels inhibition had a statistically significant negative chronotropic effect on heart rate in agreement with earlier studies (Imlach et al. 2010; Lai et al. 2014). While we also believe it to possibly have a negative inotropic effect evidenced by a small reduction in LVEF and GLS (Frankenreiter et al. 2017), our results did not show statistical significance. Inhibition of BKCa channels showed a significant effect on coronary flow. Furthermore, the effects of BKCa channels inhibition extend to the right heart as well with reduced pulmonary artery velocity, VTI, and right heart stroke volume.
Bradycardia mediated by inhibition of BKCa channels is the direct result of SA nodal cell inhibition and reduction of SA nodal action potential generation (Imlach et al. 2010; Lai et al. 2014). In our study, we found that PAX treatment, but not IBTX treatment, resulted in a lower heart rate as shown earlier (Imlach et al. 2010; Lai et al. 2014). In myocardial tissue, BKCa channels have been shown to be found only intracellularly, localized to the mitochondria (Singh et al. 2013). PAX is able to cross the plasma membrane (Manzanares et al. 2011) and bind to intracellular BKCa channels in addition to plasma membrane components. IBTX does not cross the external plasma membrane (Bingham et al. 2006) and typically only binds to BKCa channels at the external plasma membrane. The fact that no persistent differences in heart rate or mean arterial pressure was seen in the DMSO group or the IBTX group confirms that the BKCa channels mediated reduction in heart rate could also be due to intracellular BKCa channels and not a vagal mediated effect or reflexive tachycardia in response to the peripheral vascular tone. The mechanisms by which BKCa channels inhibition affects myocytes is complex. Within the SA node, the inhibition of BKCa channels yields prolonged diastolic depolarization and decreased SA nodal cell excitability, resulting in bradycardia (Lai et al. 2014). Initial studies establishing that BKCa inhibition results in bradycardia showed that in addition to PAX, high‐dose IBTX slowed the heart rate in isolated rat hearts, suggestive that the target BKCa receptor is in the plasma membrane of cardiac cells (Imlach et al. 2010; Lai et al. 2014). However, the subsequent finding that BKCa is only present in the mitochondria of adult cardiac myocytes challenges this assumption (Singh et al. 2013).
Mitochondrial BKCa channels (Singh et al. 2012) like other mitochondrial ion channels regulate many intracellular activities, including ROS production, calcium accumulation, and modulation of ATP production (Ponnalagu and Singh 2017; Gururaja Rao et al. 2018). Activation of mitochondrial BKCa channels in cardiomyocytes results in reduced ROS production, increased calcium retention capacity, and delay in mitochondrial permeability transition pore opening, and inhibition of BKCa channels in isolated mitochondria has been shown to have the opposite effect (Singh et al. 2012; Toro et al. 2014; Balderas et al. 2015). Presumably, it is the effect of calcium accumulation which leads to delayed depolarization. Furthermore, differential expression of the β subunit may also be a factor in the effects exerted by various drugs in different tissues. Out of four known β subunits, cardiac tissue contains predominantly the β3 and β4 subunits (Li and Yan 2016). Presence of the β4 subunit confers resistance to IBTX (Meera et al. 2000), and its presence in cardiac myocytes may explain why PAX but not IBTX showed a significant effect in our study. A third mechanism that may contribute to the bradycardic effect of PAX but not IBTX is the central nervous system (CNS) effect of PAX – neurons within the CNS preferentially express the β4 subunit and are very resistant to the effects of IBTX (Wang et al. 2014). BKCa inhibition of the sympathetic nerve cells results in prolonged depolarization and decreased action potential firing, resulting in decreased sympathetic innervation to the heart and decreased heart rate.
In addition, it is possible that extracardiac BKCa channels within cardiac neurons can play a role in heart rate modulation and this may explain why some studies have seen an effect with IBTX. Cardiac neurons have been shown to play a role in mediating vasomotor tone in response to electromechanical forces within the ventricle and function to preserve heart rate and ventricular contractility (Arora et al. 2001). They are both PAX and IBTX sensitive and have already been demonstrated to have effects in cardiac ischemia–reperfusion injury (Scornik et al. 2001; Perez et al. 2013; Wojtovich et al. 2013). Although our study was supportive of the effects of BKCa inhibition being mediated wholly by intracardiac mitochondrial BKCa, further investigation is needed to discern the role of both populations of BKCa in cells.
Expression of BKCa channels in coronary arteries diminishes with age in rats and human beings without affecting the biophysical or pharmacological properties (Marijic et al. 2001). In contrast, exercise ameliorates expression of BKCa channels in coronary arteries in aged rats (Albarwani et al. 2010). In agreement with a reduction in expression of BKCa channels, a significant reduction was reported in contraction capacity in coronary arteries of aged rats in ex vivo experiments (Marijic et al. 2001). Our results for the first time show that inhibition of BKCa channels plays a significant role in maintaining coronary function. Coronary blood flow exhibits a typical dose–response curve to increasing heart rate. Blood flow per single cardiac cycle, however, is reduced at increased heart rate, reflecting the decrease in diastolic duration. Thus, the bradycardia incurred from PAX is not a contributing factor to our decrease in coronary flow, but rather an impairment of BKCa channels mediated vasodilation.
Even though the role of BKCa channels in cardiac mitochondria in cardioprotection from ischemia–reperfusion injury has been demonstrated by independent groups (Singh et al. 2012, 2013; Soltysinska et al. 2014; Frankenreiter et al. 2017), at least one group has indicated the role of noncardiomyocyte BKCa channels in cardioprotection (Wojtovich et al. 2013). A recent study has also shown that BKCa channels present in adult cardiomyocytes play a direct role in cardioprotection by using cardiomyocyte‐specific knockout mice (Frankenreiter et al. 2017). However, the role of BKCa channels present in other cells and organs could also be partially contributing to the cardioprotection and cardiac function. Regulation of BKCa channels in maintaining the coronary artery flow may also support the finding of reduced infarct size in animal models that undergo BKCa channels—activation prior to or immediately after an ischemic injury (Singh et al. 2013). A reduction in blood flow is well‐characterized to slow oxygen delivery which may limit production of free radicals in the cardiac tissue. Increase in free radicals which also results in Ca2+ overload mediated by blocking BKCa channels are well‐characterized and established factors for the cardiac ischemia–reperfusion injury (Stowe et al. 2006; Heinen et al. 2007; Aldakkak et al. 2008; Singh et al. 2012, 2013).
Evaluation of the cardiac function was performed by echocardiography, and global longitudinal strain analysis was carried out. Although this study represents a significant step forward in elucidating the physiologic effects of pharmacologic regulation of BKCa, there are some limitations to the usage of echocardiography. Echocardiography is inherently subject to some variability in interpretation. To minimize variability in interpretation, quantitative measurements were used, computed by delineation of the endocardial border. Our quantitative measures of LV function did not show any statistically significant data, although qualitative changes in wall motion were seen. However, we chose not to report specific details of qualitative measures like wall motion abnormality (WMA) due to the high degree of interobserver variability when judging their severity and significance. Moreover, WMAs are generally consequences of decreased coronary perfusion and flow, parameters which were measured quantitatively and found to be reduced after exposure to PAX and IBTX.
Our LVEF and strain data sets did not bear out statistical significance, and strain analysis showed cardiac dysfunction with PAX which was reversible after 15 min of injection. Though LVEF is the most commonly used marker to assess for LV dysfunction, there are some limitations to its use in cardiac studies. Strain analysis allows for more sensitive evaluation of myocardial contractility and can detect differences in LV function that may be missed when using LVEF alone. Another advantage is that strain analysis can be performed retroactively during postimaging analysis as long as high‐quality images have been captured using a standard imaging protocol. We also evaluated the role of BKCa channels on the right heart as the right side of the heart in the context of BKCa channels is not extensively studied. In terms of right heart, we noted a statistically significant decrease in pulmonary flow velocity and mean pulmonary gradient. These changes appear to occur independently of vascular tone as we did not witness a significant change in RVOT or PA diameter. This would suggest that the decreases in flow were an extension of a decrease in cardiac output driven primarily by PAX‐induced bradycardia.
Given the widespread cardiac effects of BKCa channels inhibition observed, the translation ability of these findings is promising. However, the most significant and consistently observed reversible effect of PAX is sinus bradycardia. It seems that many of the cardiac effects we observed were likely related to the BKCa channels in SNACs and coronary arteries. This opens the door for BKCa channels blockade as a means of antiarrhythmic control in patients who do not tolerate beta blockers or other classes of heart rate‐controlling medications (Zimetbaum 2012; Maan et al. 2013). An optimal dose of BKCa channels blocker/inhibitor such as PAX could therapeutically lower heart rate without significantly affecting other cardiac variables could prove useful. The most significant finding is that this impact on BKCa channels is completely reversible. In summary, using pharmacological tools, we have performed in vivo comprehensive evaluation and showed the role of BKCa channels on cardiovascular function.
Conflict of Interest
Authors have no conflicts of interest to disclose.
Patel N. H., Johannesen J., Shah K., Goswami S. K., Patel N. J., Ponnalagu D., Kohut A. R., Singh H.. Inhibition of BKCa negatively alters cardiovascular function. Physiol Rep, 6 (12), 2018, e13748, https://doi.org/10.14814/phy2.13748
Funding Information
This work was supported by the Commonwealth Universal Research Enhancement (CURE) Program Grants to HS, a grant from the W. W. Smith Charitable Trust, American Heart Association National Scientist Development Grant (11SDG230059), American Heart Association Grant‐in‐Aid (16GRNT29430000), National Institute of Health (R01‐HL133050), and Drexel University College of Medicine startup funds to HS.
References
- Ahn, D. S. , Kim Y. B., Lee Y. H., Kang B. S., and Kang D. H.. 1994. Fatty acids directly increase the activity of Ca(2 + )‐activated K+ channels in rabbit coronary smooth muscle cells. Yonsei Med. J. 35:10–24. [DOI] [PubMed] [Google Scholar]
- Albarwani, S. , Al‐Siyabi S., Baomar H., and Hassan M. O.. 2010. Exercise training attenuates ageing‐induced BKCa channel downregulation in rat coronary arteries. Exp. Physiol. 95:746–755. [DOI] [PubMed] [Google Scholar]
- Aldakkak, M. , Stowe D. F., Chen Q., Lesnefsky E. J., and Camara A. K.. 2008. Inhibited mitochondrial respiration by amobarbital during cardiac ischaemia improves redox state and reduces matrix Ca2 + overload and ROS release. Cardiovasc. Res. 77:406–415. [DOI] [PubMed] [Google Scholar]
- Arora, R. C. , Hirsch G. M., Johnson Hirsch K., Hancock Friesen C., and Armour J. A.. 2001. Function of human intrinsic cardiac neurons in situ. Am. J. Physiol. Regul. Integr. Comp. Physiol. 280:R1736–R1740. [DOI] [PubMed] [Google Scholar]
- Balderas, E. , Zhang J., Stefani E., and Toro L.. 2015. Mitochondrial BKCa channel. Front. Physiol. 6:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingham, J. P. , Bian S., Tan Z. Y., Takacs Z., and Moczydlowski E.. 2006. Synthesis of a biotin derivative of iberiotoxin: binding interactions with streptavidin and the BK Ca2 + ‐activated K+ channel expressed in a human cell line. Bioconjug. Chem. 17:689–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borbouse, L. , Dick G. M., Asano S., Bender S. B., Dincer U. D., Payne G. A., et al. 2009. Impaired function of coronary BK(Ca) channels in metabolic syndrome. Am. J. Physiol. Heart Circ. Physiol. 297:H1629–H1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner, R. , Perez G. J., Bonev A. D., Eckman D. M., Kosek J. C., Wiler S. W., et al. 2000. Vasoregulation by the beta1 subunit of the calcium‐activated potassium channel. Nature 407:870–876. [DOI] [PubMed] [Google Scholar]
- Brown, H. M. , Hagiwara S., Koike H., and Meech R. M.. 1970. Membrane properties of a barnacle photoreceptor examined by the voltage clamp technique. J. Physiol. 208:385–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England, S. K. , Wooldridge T. A., Stekiel W. J., and Rusch N. J.. 1993. Enhanced single‐channel K+ current in arterial membranes from genetically hypertensive rats. Am. J. Physiol. 264:H1337–H1345. [DOI] [PubMed] [Google Scholar]
- Evanson, K. W. , Bannister J. P., Leo M. D., and Jaggar J. H.. 2014. LRRC26 is a functional BK channel auxiliary gamma subunit in arterial smooth muscle cells. Circ. Res. 115:423–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankenreiter, S. , Bednarczyk P., Kniess A., Bork N. I., Straubinger J., Koprowski P., et al. 2017. cGMP‐elevating compounds and ischemic conditioning provide cardioprotection against ischemia and reperfusion injury via cardiomyocyte‐specific BK channels. Circulation 136:2337–2355. [DOI] [PubMed] [Google Scholar]
- Gao, X. M. , Dart A. M., Dewar E., Jennings G., and Du X. J.. 2000. Serial echocardiographic assessment of left ventricular dimensions and function after myocardial infarction in mice. Cardiovasc. Res. 45:330–338. [DOI] [PubMed] [Google Scholar]
- Gao, S. , Ho D., Vatner D. E., and Vatner S. F.. 2011. Echocardiography in mice. Curr. Protoc. Mouse Biol. 1:71–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghatta, S. , Nimmagadda D., Xu X., and O'Rourke S. T.. 2006. Large‐conductance, calcium‐activated potassium channels: structural and functional implications. Pharmacol. Ther. 110:103–116. [DOI] [PubMed] [Google Scholar]
- Gururaja Rao, S. , Ponnalagu D., Patel N. J., and Singh H.. 2018. Three decades of chloride intracellular channel proteins: from organelle to organ physiology. Curr. Protoc. Pharmacol. 80:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinen, A. , Aldakkak M., Stowe D. F., Rhodes S. S., Riess M. L., Varadarajan S. G., et al. 2007. Reverse electron flow‐induced ROS production is attenuated by activation of mitochondrial Ca2 + ‐sensitive K+ channels. Am. J. Physiol. Heart Circ. Physiol. 293:H1400–H1407. [DOI] [PubMed] [Google Scholar]
- Imlach, W. L. , Finch S. C., Miller J. H., Meredith A. L., and Dalziel J. E.. 2010. A role for BK channels in heart rate regulation in rodents. PLoS ONE 5:e8698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia, X. , Yang J., Song W., Li P., Wang X., Guan C., et al. 2013. Involvement of large conductance Ca(2 + )‐activated K (+) channel in laminar shear stress‐induced inhibition of vascular smooth muscle cell proliferation. Pflugers Archiv. 465:221–232. [DOI] [PubMed] [Google Scholar]
- Kohut, A. , Patel N., and Singh H.. 2016. Comprehensive echocardiographic assessment of the right ventricle in murine models. J. Cardiovasc. Ultrasound 24:229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai, M. H. , Wu Y., Gao Z., Anderson M. E., Dalziel J. E., and Meredith A. L.. 2014. BK channels regulate sinoatrial node firing rate and cardiac pacing in vivo. Am. J. Physiol. Heart Circ. Physiol. 307:H1327–H1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Q. , and Yan J.. 2016. Modulation of BK channel function by auxiliary beta and gamma subunits. Int. Rev. Neurobiol. 128:51–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, G. R. , Sun H. Y., Chen J. B., Zhou Y., Tse H. F., and Lau C. P.. 2009. Characterization of multiple ion channels in cultured human cardiac fibroblasts. PLoS ONE 4:e7307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maan, A. , Mansour M., Ruskin J. N., and Heist E. K.. 2013. Current evidence and recommendations for rate control in atrial fibrillation. Arrhythmia Electrophysiol. Rev. 2:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzanares, D. , Gonzalez C., Ivonnet P., Chen R. S., Valencia‐Gattas M., Conner G. E., et al. 2011. Functional apical large conductance, Ca2 + ‐activated, and voltage‐dependent K+ channels are required for maintenance of airway surface liquid volume. J. Biol. Chem. 286:19830–19839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marijic, J. , Li Q., Song M., Nishimaru K., Stefani E., and Toro L.. 2001. Decreased expression of voltage‐ and Ca(2 + )‐activated K(+) channels in coronary smooth muscle during aging. Circ. Res. 88:210–216. [DOI] [PubMed] [Google Scholar]
- Meera, P. , Wallner M., and Toro L.. 2000. A neuronal beta subunit (KCNMB4) makes the large conductance, voltage‐ and Ca2 + ‐activated K+ channel resistant to charybdotoxin and iberiotoxin. Proc. Natl Acad. Sci. USA 97:5562–5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson, M. T. , Cheng H., Rubart M., Santana L. F., Bonev A. D., Knot H. J., et al. 1995. Relaxation of arterial smooth muscle by calcium sparks. Science 270:633–637. [DOI] [PubMed] [Google Scholar]
- Perez, G. J. , Desai M., Anderson S., and Scornik F. S.. 2013. Large‐conductance calcium‐activated potassium current modulates excitability in isolated canine intracardiac neurons. Am. J. Physiol. Cell Physiol. 304:C280–C286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponnalagu, D. , and Singh H.. 2017. Anion channels of mitochondria. Handb. Exp. Pharmacol. 240:71–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusch, N. J. 2009. BK channels in cardiovascular disease: a complex story of channel dysregulation. Am. J. Physiol. Heart Circ. Physiol. 297:H1580–H1582. [DOI] [PubMed] [Google Scholar]
- Rusko, J. , Tanzi F., van Breemen C., and Adams D. J.. 1992. Calcium‐activated potassium channels in native endothelial cells from rabbit aorta: conductance, Ca2 + sensitivity and block. J. Physiol. 455:601–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scornik, F. S. , Merriam L. A., and Parsons R. L.. 2001. Number of K(Ca) channels underlying spontaneous miniature outward currents (SMOCs) in mudpuppy cardiac neurons. J. Neurophysiol. 85:54–60. [DOI] [PubMed] [Google Scholar]
- Singh, H. , Stefani E., and Toro L.. 2012. Intracellular BK(Ca) (iBK(Ca)) channels. J. Physiol. 590:5937–5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, H. , Lu R., Bopassa J. C., Meredith A. L., Stefani E., and Toro L.. 2013. mitoBKCa is encoded by the Kcnma1 gene, and a splicing sequence defines its mitochondrial location. Proc. Natl Acad. Sci. USA 110:10836–10841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, H. , Li M., Hall L., Chen S., Sukur S., Lu R., et al. 2016. MaxiK channel interactome reveals its interaction with GABA transporter 3 and heat shock protein 60 in the mammalian brain. Neuroscience 317:76–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobey, C. G. 2001. Potassium channel function in vascular disease. Arterioscler. Thromb. Vasc. Biol. 21:28–38. [DOI] [PubMed] [Google Scholar]
- Soltysinska, E. , Bentzen B. H., Barthmes M., Hattel H., Thrush A. B., Harper M. E., et al. 2014. KCNMA1 encoded cardiac BK channels afford protection against ischemia‐reperfusion injury. PLoS ONE 9:e103402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowe, D. F. , Aldakkak M., Camara A. K., Riess M. L., Heinen A., Varadarajan S. G., et al. 2006. Cardiac mitochondrial preconditioning by Big Ca2 + ‐sensitive K+ channel opening requires superoxide radical generation. Am. J. Physiol. Heart Circ. Physiol. 290:H434–H440. [DOI] [PubMed] [Google Scholar]
- Tanaka, Y. , Meera P., Song M., Knaus H. G., and Toro L.. 1997. Molecular constituents of maxi KCa channels in human coronary smooth muscle: predominant alpha + beta subunit complexes. J. Physiol. 502(Pt 3):545–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toro, L. , Li M., Zhang Z., Singh H., Wu Y., and Stefani E.. 2014. MaxiK channel and cell signalling. Pflugers Archiv. 466:875–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari, Z. , Csiszar A., and Koller A.. 2002. Increases in endothelial Ca(2+) activate K(Ca) channels and elicit EDHF‐type arteriolar dilation via gap junctions. Am. J. Physiol. Heart Circ. Physiol. 282:H1760–H1767. [DOI] [PubMed] [Google Scholar]
- Vang, A. , Mazer J., Casserly B., and Choudhary G.. 2010. Activation of endothelial BKCa channels causes pulmonary vasodilation. Vascul. Pharmacol. 53:122–129. [DOI] [PubMed] [Google Scholar]
- Wang, B. , Jaffe D. B., and Brenner R.. 2014. Current understanding of iberiotoxin‐resistant BK channels in the nervous system. Front. Physiol. 5:382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtovich, A. P. , Nadtochiy S. M., Urciuoli W. R., Smith C. O., Grunnet M., Nehrke K., et al. 2013. A non‐cardiomyocyte autonomous mechanism of cardioprotection involving the SLO1 BK channel. PeerJ 1:e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, W. , Liu Y., Wang S., McDonald T., Van Eyk J. E., Sidor A., et al. 2002. Cytoprotective role of Ca2 + ‐ activated K+ channels in the cardiac inner mitochondrial membrane. Science 298:1029–1033. [DOI] [PubMed] [Google Scholar]
- Zimetbaum, P. 2012. Antiarrhythmic drug therapy for atrial fibrillation. Circulation 125:381–389. [DOI] [PubMed] [Google Scholar]


