Abstract
This study was conducted to investigate how far dietary zinc (Zn) modifies the histomorphological alterations induced by diabetes in rat kidneys. The animals were divided into negative control group (10 rats). Diabetes was induced in thirty animals by streptozotocin. After confirming diabetes, the animals were divided into three groups (n = 10). Group II served as the positive control group (fed on standard diet), group III was fed on Zn deficient diet, and group IV was fed on Zn supplemented diet. Caspase-3 immune staining was used to estimate the caspase activity. Stereological procedures were used to measure the quantity of the immune stain and the surface area of the Bowman’s space. The renal cortices of group II rats revealed apparent widening of Bowman’s spaces with few apoptotic figures. The filtration barrier showed thickening of the basement membrane. The proximal convoluted tubules showed patchy loss of the apical microvilli with swollen mitochondria. The distal convoluted tubules revealed area of irregular basal enfolding. The picture was aggravated by Zn deficiency in group III besides areas of cortical interstitial fibrosis. The histopathological alterations were minimal in the cortices of group IV. A significant increase of the Bowman’s space surface area in group II and IV while decrease in group III compared with group I. The expression of Caspase-3 density was significantly increased in group II and III compared with group I while in group IV was non significant. In conclusion, dietary Zn modulated renal cortical changes caused by diabetes in rats.
Keywords: Zinc, diabetes, diet, kidney, pathology
Introduction
Zinc (Zn) is a well-known essential trace element, however, its specific roles in the cells are not well understood.1 It is an essential component of enzyme molecules, proteins, and biomembranes.2 Even though, its quantities are very small in body tissue, the structure and hence the stability of proteins such as insulin and insulin receptors depend on the presence of this element.2,3 Cellular activities such as DNA synthesis, gene expression, enzymatic catalysis, as well as hormonal release, and storage are regulated by intracellular Zn.4,5 Also acts as a coordinate regulator of cell mitosis and cell suicide process of apoptosis.4,6
Because our bodies cannot store Zn, continuous Zn supplementation is essential to maintain normal body functions.7 Zn deficiency is one of the commonest trace elements deficiencies particularly in developing countries.8 Many pathophysiological conditions are accompanied with Zn deficiency as in increased loss (hyperzincuria and sweating),9 increased demands (severe burns, pregnancy and lactation),10,11 heavy metal poisoning, aging, and diseases as Down’s syndrome.4 Diet supplementation with iron and calcium lead to decrease in Zn concentration in the circulation.12 Diabetics always suffer low tissue concentration of Zn, due to defective absorption and increased loss concomitant with Zn metabolism imbalance.13–16
Diabetes mellitus (DM) is a metabolic disorder associated with severe oxidative stress caused by the high glucose levels in the plasma which induces production of oxygen free radicals that damage different body cells.17–21 This oxidative stress has been implicated in the pathogenesis of diseases of most vital organs, particularly the kidney. Almost all renal structures; glomerular basement membranes,22 tubular basement membrane,23 renal arterioles, and podocytes,24,25 even the interstitium26 are affected. These changes finally lead to irreversible renal damage.27
Antioxidants play a beneficial role in preventing diabetic complications by decreasing production and/or increasing scavenging of the free radicals.28 Trace elements such as Zn act as a cellular antioxidative defense together with an action in insulin signal transduction and glucose metabolism.29 Deficiency of antioxidants exacerbates oxidative stress associated with diabetes.30–33
The accompanied Zn depletion with diabetics is expected to aggravate renal pathological changes. The present work was conducted to investigate the renal histopathological changes that accompany dietary Zn deficiency versus its supplementation in diabetic rats.
Material and methods
Experimental animals
A total of number 40 male Wistar rats weighing 80–100 g were obtained from the Mansoura University Animal House. They were kept during the study under standard laboratory conditions with maintained supply of water ad libitum and diets prepared by dietary specialist at the animal house. All the performed procedures follow the guideline of the national institute of health (NIH) for the care and use of laboratory animals (NIH Publication 85-23 Rev. 1985).
Diets
Standard diet: It was prepared according to Swenerton and Hurley.34 The diet contained a ration of soybean protein, sucrose, corn oil, mineral, and vitamin mixture in drinking tap water.
Zinc deficient diet: The Zn content of the standard diet was reduced by treatment of the soybean protein by the tetrasodium salt of ethylenediaminetetraacetic acid (Na4EDTA) according to35 by suspension of soybean protein with EDTA.
Zinc supplemented Diet: Zn was supplied as ZnSO4 (HEALTHAID LTD, Healthaid House, Marborough Hill, Harrow, Middlesex, HA1 1UD England) at a dose of 5 mg/kg in drinking tap water.36
Procedure
Ten animals, served as the negative control group (Group I) were maintained on the standard diet for 10 weeks. Diabetes was induced in 30 animals by 5 doses (20 mg/kg) of STZ (Sigma, St. Louide, Mo) in 5 consecutive days.37 Diabetes was confirmed (blood glucose over 200 mg/dL) in whole blood obtained from the tail vein one week after, using the glucose reagent strips supplied by Sigma Chemical Co. Diabetic rats were randomly divided into 3 groups (10 rats each). Group II was used as the positive control group; and were fed the standard diet. Group III was fed with Zn deficient diet, group IV was fed with Zn supplemented diet. Ten weeks after confirmation of diabetes, all rats were sacrificed under pentobarbital anesthesia (5 mg/100 g). Kidneys were collected, and longitudinally dissected into two equal halves, one half was processed for LM and the other for EM.
Tissue preparation
For LM, kidney specimens were fixed in 4% paraformaldehyde, then immersed in paraffin. Serial sections of 5 μm stained by hematoxylin and eosin according to the standard methods.
For Caspase-3 immune staining, paraffin-embedded biopsies were deparafinized and hydrated by xylene and alcohol. To block endogenous peroxidase, the sections were treated with 3% hydrogen peroxide followed by incubation over night at 4 °C with primary antibody raised against Caspase-3 (Clone Caspase-3; Sigma, Saint Lous, MO), then counterstained with hematoxylin and finally dehydrated, cleared and mounted.38
For transmission EM, specimens from the kidneys were immersed in 2.5% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.3) for 4 h and then post fixed in 1% osmium tetroxide in 0.1 M cacodylate buffer (pH 7.3) for 2 h. The specimens were dehydrated in ascending grades of alcohol, and passed in two changes of propylene oxide to be embedded in Epon. Semi-thin sections (1 μm thick) were stained with toluidine blue to select the proper sites for ultra-thin sections (60–80 nm thick) which were cut, double stained with 2% uranyl acetate and 2% lead citrate and examined with the transmission electron microscope.39 Preparing specimens were done in the Electron Microscopy Unit, Mansoura University.
Measurements
By using digital camera (CH-9435 DFC 290, Germany), quantification data of five randomly selected slides, at 400× magnification, were collected using a package of image analysis (Leica Q Win standard, digital camera CH-9435 DFC 290, Germany). Images were saved as TIFF and analyzed on Intel® Core I3® based computer using Video Test Morphology® software (Russia) with a particular built-in routine for calibrated area measurement and immune staining quantification. The scoring of the percentage of the positively brown stained cells to the negatively stained cells was determined by image analysis program.
The surface areas of Bowman's space were measured to assess any micro-anatomical alterations by using Image J software version 1.42 (National Institutes of Health, Bethesda, MD) analysis system at ×400 magnification in 15 fields of hematoxylin-stained renal sections.
Statistics
By using Statistical Package for Social Science (SPSS) version 15.0, the data were statistically analyzed. Unpaired t-test was used for comparison between two groups. For intergroup comparison, ANOVA test was applied with the least significance (LSD) post hoc analysis. Significance was considered when p values <.001.
Results
LM examination of group I (control group) demonstrated most of the components of the renal cortex including; renal corpuscles, proximal convoluted tubular cell lining with rounded nuclei and acidophilic cytoplasm and apical brush border, distal convoluted tubules, macula densa and collecting tubules (Figure 1(A)).
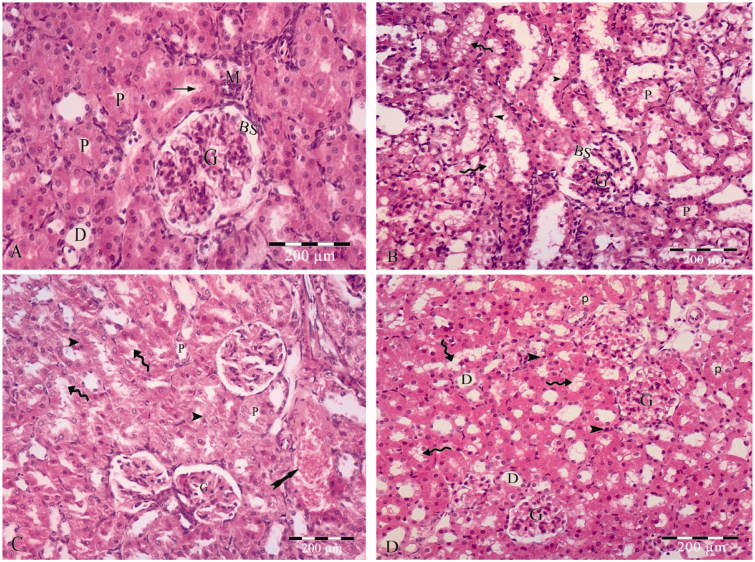
Figure 1.
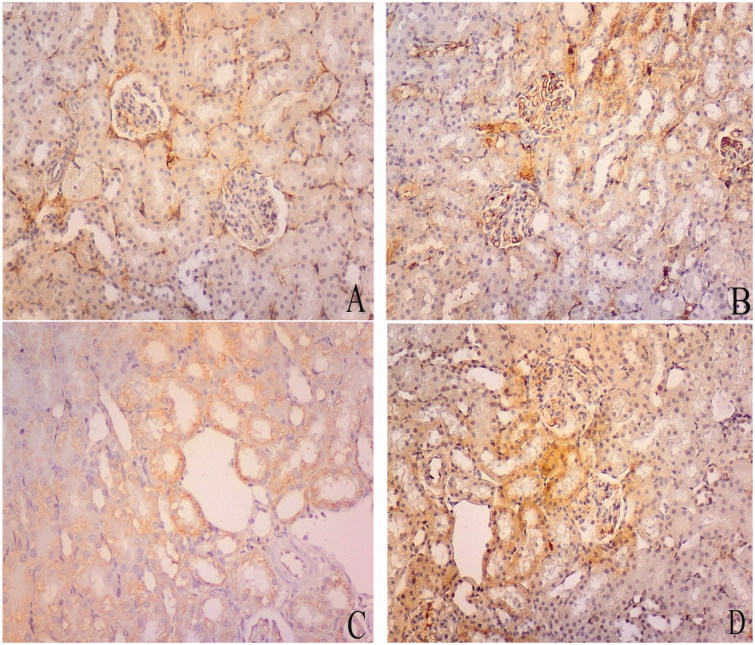
(A) photomicrograph of hematoxylin and eosin stained paraffin sections; (A) Control rat renal cortex showing glomerular capillary (G), Bowmans space (BS), proximal convoluted tubules (P) lined with cuboidal cells with acidophilic cytoplasm, rounded open face nuclei and apical brush border (arrow), distal convoluted tubules (D) and macula densa (M). (B) The sections of group II show apparent widded Bowmans space (BS) of the glomerulous (G). The cell linning of the proximal convoluted tubules (P) with lost apical brush border, apoptotic linning (arrow heads) cells having small darkly satined nuceil and slighly eosophillic vaculated cytoplasm (curved arrows). (C) The sections of group III shows of the glomerulous (G). The cell linning of the proximal convoluted tubules (P) is distorted with lost apical brush border, apoptotic linning (arrow heads) cells having small darkly satined nuceil and slighly eosophillic vaculated cytoplasm (curved arrows). Areas of haemrrage are also detected (tailed arrow). (D) Section in zinc treated renal cortex demonstrates apparently normal glomerular capillaries (G) and minimal vaculation in proximal tubular (P) lining cells (curved arrows) with small darkly stained nuclei (arrow heads). The distal convoluted tubules (D) appears normal.
Renal cortices of group II showed marked changes as widened Bowman’s spaces, scattered patchy loss of the apical brush border with some apoptotic figures in the form of darkly stained nuclei, vacuolated eosinophilic cytoplasm (Figure 1(B)).
The renal cortices of group III rats showed more dramatic histopathological changes in comparison to those of groups I and II. The cellular lining of the proximal convoluted tubules showed numerous apoptotic figures with wide spread areas of apical border loss. Areas of mild blood extravasations were also evident (Figure 1(C)).
On contrary, group IV renal cortices showed mild changes in the form of less modifiable apoptotic figures in the cellular lining of the proximal convoluted tubules and apparently normal Bowman’s corpuscles and distal convoluted tubules (Figure 1(D)).
Transmission electron microscopic examination of the renal cortices of group I (Figure 2(A)) showed all the components of the normal renal corpuscles including podocytes with large irregular nuclei. Apparently normal filtration barrier was formed of regular minor processes of the podocytes, regular capillary endothelium and uniform basement membrane forming filtration barrier. The proximal convoluted tubular lining cells showed regular basal infoldings with numerous mitochondria, large euchromatic nuclei and areas of large apical microvilli (Figure 3(A)). The distal convoluted tubular cells appeared normal with well-defined cell border (Figure 4(A)).
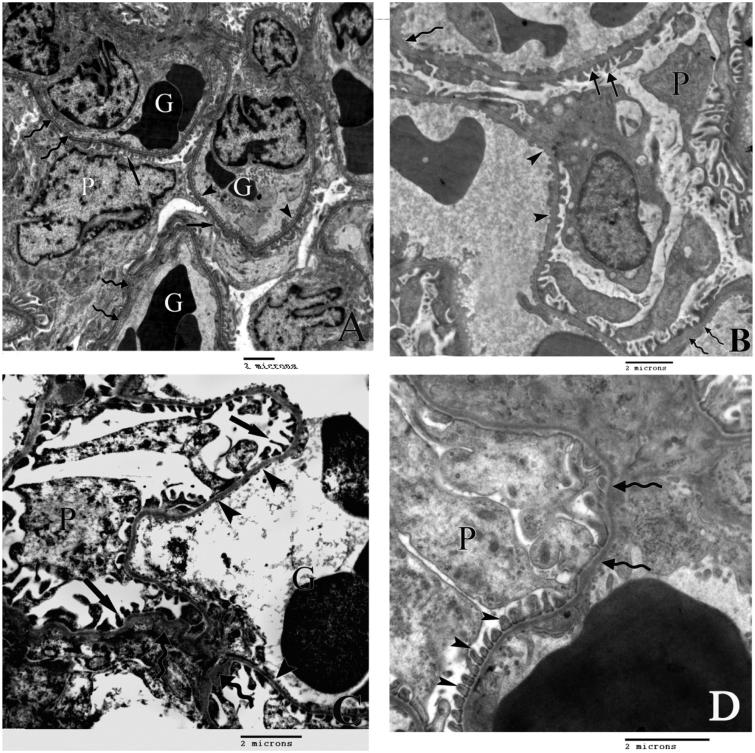
Figure 2.
A) An electron micrograph demonstrating the renal glomerular corpuscle of the control rat with a part of the cell body of podocytes (P) and glomerular capillaries (G). The filtration barrier is formed of minor processes of the podocytes (arrows), fenestrated endothelium of the glomerular capillaries (arrow heads) separated by uniformly thick basement membrane (curved arrows). B) An electron micrograph of the renal corpuscle of group II rats showing irregular minor podocytic processes (arrows) and areas of markedly thickened basement membrane (curved arrows). Arrow heads point to the irregular fenestrated endothelium. C) An electron micrograph of the renal corpuscle of group III rats showing a part of the body of podocte (P) with irregular minor podocytic processes (arrows) and areas of markedly thickened basement membrane (curved arrows) and irregular fenestrated endothelium (arrow heads). D) An electron micrograph of the renal corpuscle of group IV rats demenestrating slight thickneneing of the basement memebrane (curved arrows) and regular minor processes (arrow heads) of podocytes (P).
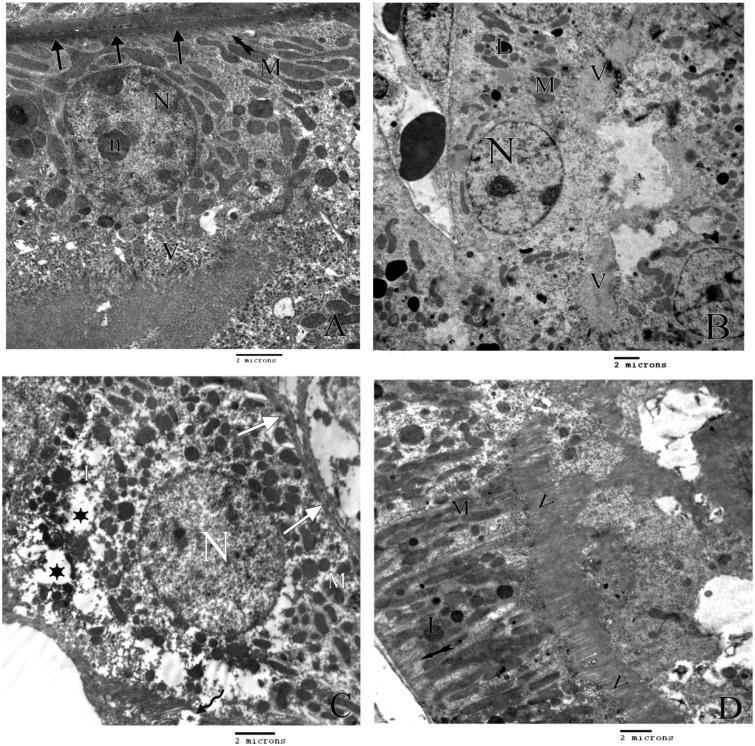
Figure 3.
A) An electron micrograph of the lining cells of proximal tubules of control rat with thick basement membrane (arrows), rounded euchromatic nucleus (N) with prominent nucleolus (n), numerous mitochondria (M) with regular basal infolding (tailed arrow) and apical microvilli (V). B) An electron micrograph of proximal tubules of group II rats showing, area of microvilli (V) with some areas of detachment, swollen bizarre shape mitochondria (M), secondary lysosomes (L) and normal nucleus (N). C) An electron micrograph of proximal tubules of group III rats showing, area of detached microvilli (curved arrow), swollen bizarre shape mitochondria (M), wide cytoplasmic spaces (stars) multiple secondary lysosomes (L) and apparently normal nucleus (N). D) An electron micrograph of the lining cells of proximal tubulus of group IV rats showing regular elongated mitochonderia (M), secondary lysosomes (L) with normal basal infolding (tailed arrow) and normal apical microvilli (V).
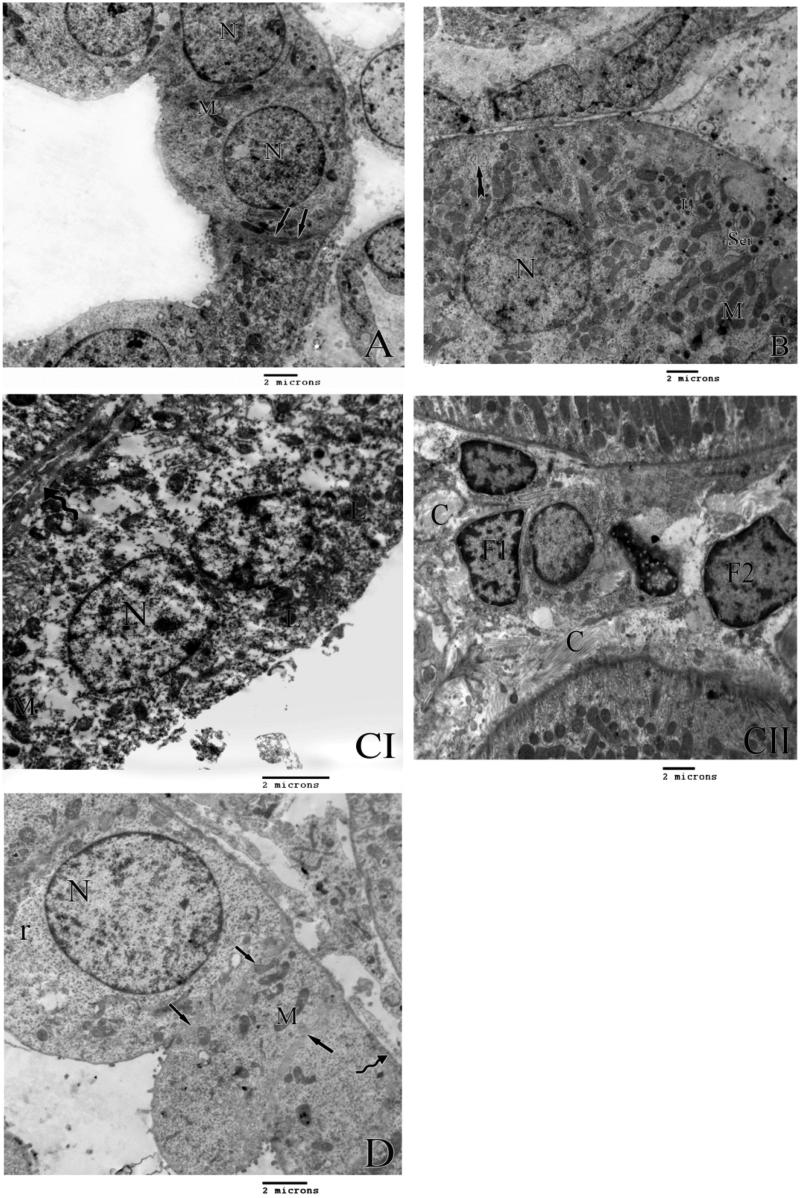
Figure 4.
A) An electron micrograph of the control rat distal tubule lining cells showing definite cell border (arrows) and elongated mitochondria (M) and rounded nuclei (N). B) An electron micrograph of the lining cells of the distal tubules of group II rats showing distorted basal enfolding (tailed arrow) bizarre shape mitochondria (M) swollen smooth endoplasmic reticulum (Ser) secondary lysosomes (L) and rounded nucleus (N). C) An electron micrograph of the lining cells of the distal tubules of group III rats showing irregular thickening of the basement membrane (curved arrow), bizarre shape mitochondria (M), secondary lysosomes (L) and rounded nucleus (N). II) shows areas of fibrosis in the interstitial tissue (C) with primary and secondary fibrocytes (F1 and F2 respectively). D) An electron micrograph of lining cells of distal tubules of group C rats showing rounded nucleus (N) numerous free ribosomes (r) with distict cell border (arrows), normal mitochonderia (M) with normal thickened basement membrane (curved arrow).
Group II renal cortices revealed an obvious mild changes in the filtration barrier in the form of irregular minor podocytic processes, irregular fenestrated endothelium together with areas of basement membrane thickening (Figure 2(B)). The cells of proximal and distal convoluted tubules showed swollen mitochondria and few lysosomes. Areas of detached apical microvilli in the proximal convoluted tubular lining cells were noticed (Figure 3(B)). Areas of decreased basal infoldings in the distal convoluted tubules were also seen (Figure 4(B)).
The renal cortices of group III (Figure 2(C)) showed extensive changes demonstrated by areas of excessive thickening of the basement membrane with irregular fenestrated endothelium. Numerous swollen bizarre shaped mitochondria and lysosomes were present in cellular lining cells of both proximal and distal convoluted tubules besides areas of interacytoplasmic spaces (Figures 3(C) and 4, CI). Areas of excessive deposition of collagen bundles were observed in the cortical interstitium (Figure 4, CII).
Ultra structural examinations of the renal cortices of group IV were almost normal with minor changes in the form of areas of slight thickening of the basement membrane of the filtration barrier (Plate 2, D). The cytoplasm of the cellular lining of both proximal and distal convoluted tubules appeared more or less normal apart from multiple secondary lysosomes (Figures 3(D) and 4(D)).
On analysis of semi-quantitative measurements of Caspase-3 density of different groups, its expression showed a highly significant change on comparing group II and III to the control group (group I). Group IV showed non significant change when compared with the control one (group I). The different experimental groups showed inter group significant changes when compared with each other (Table 1, graph 1, Figure 5).
Table 1.
Caspase-3 density of the 4 groups.
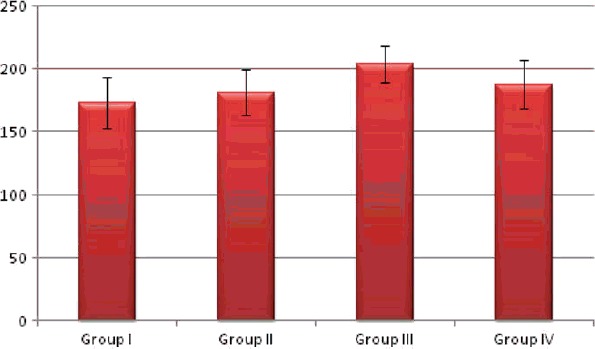
| Group | Mean | ±SD | Significance |
|---|---|---|---|
| Group I | 173.0 | 20.645 | p < .001 |
| Group II | 181.5 | 18.006 | |
| Group III | 203.5a,b | 14.554 | |
| Group IV | 187.7b,c | 19.029 |
p: Probability; SD: standard deviation; Test used: ANOVA followed by post-hoc tukey.
Significance versus group I.
Significance versus group II.
Significance versus group III.
Figure 5.
Representative photomicrographs of caspase-3 expression determined by immunohistochemistry. Mean scoring of caspase-3 in the renal cortical areas of the control rats was 173.0 ± 21. It increased in group II (181.5 ± 18). Group III showed significant increase (203.5 ± 14). The score returns near normal in group IV (187.7 ± 19). Caspase-3 density (Table 1, graph 1). Initial increase in group II followed by significant increase in group III and group IV, being more evident in group III. Bowman's space (Table 2 graph 2). Significant increase in group II, III and IV, while significantly decreased in group III.
The Bowman’s space area showed a significant increase in group II and IV and significant decrease in group III compared with the control group (group I). On comparing the different groups with each other, highly significant changes were found in-between all groups (Table 2, graph 2 , Figure 5).
Table 2.
Bowman’s space of the 4 groups.
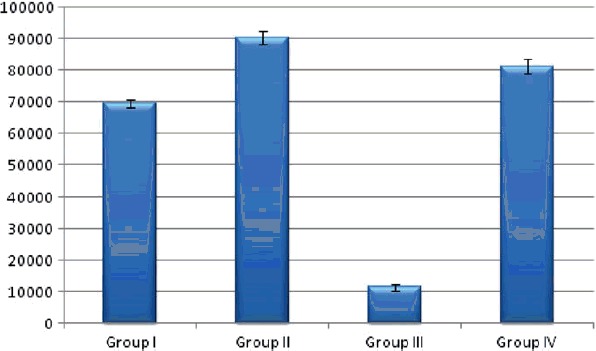
| Group | Mean | ±SD | Significance |
|---|---|---|---|
| Group I | 69466.5 | 1136.330 | p < .001 |
| Group II | 90415.5a | 2017.575 | |
| Group III | 11328.2ab | 1140.380 | |
| Group IV | 81241.6abc | 2226.433 |
p: Probability; SD: standard deviation; Test used: ANOVA followed by post-hoc tukey.
Significance versus group I.
Significance versus group II.
Significance versus group III.
Discussion
Zn is an essential trace element which is important for maintaining the integrity of the normal cellular functions. Because our bodies can't store the minimal required quantity of this important element, there is a continuous call for its adequate dietary supplement.
Diabetic nephropathy is the leading cause of death among diabetics mainly due to end stage renal failure.40,41 It was proofed that all diabetics suffer Zn deficiency particularly at the late stage.42,43 This fact was explained by increased Zn renal loss or decreased absorption.16
The current study was conducted to establish narrative data about the histopathological changes in various components of renal cortex and how far dietary Zn supplementation can modify these changes.
Although Zn levels in the kidney tissues were not determined, it is settled that diabetes in non Zn supplemented rats caused highest kidney zinc values with significant decrease in its plasma levels.44,45 This finding was explained by the enhanced urinary zinc excretion in experimentally-induced diabetes.14
Compared with negative control group (Group I), marked pathological findings were observed in the positive one (Group II). The renal cortices showed widened Bowman's space. The filtration barrier had an irregular of podocytic minor processes with marked thickening of the basement membrane and irregular fenestrated endothelium. The cell lining of the proximal convoluted tubules showed areas of lost apical brush border with signs of apoptosis in the form of small darkly stained nuclei and vacuolated cytoplasm. The cytoplasm contained bizarre shape mitochondria and secondary lysosomes. The distal tubules had distorted basal enfolding, bizarre shape mitochondria with swollen smooth endoplasmic reticulum and numerous secondary lysosomes. These findings were supported by the statistical analysis that demonstrated significant increase in Caspase-3 expression together with a significant increase in the surface area of Bowman's space.
Similar findings were reported in previous studies.41,46–49 The associated cellular injuries are explained by that fact that diabetes is associated with activation of increased free radical production together with impaired production of antioxidants with subsequent increase in tissue oxidative injuries.50–52
Histological and morphometric analysis of kidneys of the diabetic rats fed on Zn deficient diet (group III) revealed more extensive degenerative changes in the form of numerous apoptotic figures, areas of blood extravasations together with areas of extensive thickening of the basement membrane. The proximal convoluted tubules showed more extensive loss of the apical microvilli. The cytoplasm cellular lining of both proximal and distal convoluted tubules showed swollen mitochondria, secondary lysosomes and intracytoplasmic spaces. Collagen bundle deposition was observed in the cortical interstitium. These changes were confirmed by morphometric analysis of Caspase-3 density which showed a highly significant expression compared with the other groups, while Bowman's space surface area showed significant decrease compared with the other groups. These exacerbated histopathological changes induced by Zn deficiency in diabetics was attributed to increased chronic cellular oxidative stress.53–57 The librated reactive oxygen species attack lipids and lipoproteins of the membranes, starting a series of chain reactions of lipid per oxidation inducing the cellular changes ending in cell death23,58
Areas of interstitial fibrosis in-between distal convoluted tubules were also detected by the ultra structural examination. The increased fibrosis was also described by Li et. al.59 who specifies this fibrosis by the increased collagen IV accumulation. Other studies reported endothelial cell damage and hyaline mass formation in diabetes.52
Group IV (Zn supplemented diets) showed minimal changes being more or less normal. Secondary lysosomes were the main finding. Apoptotic figures were not evident which was supported by the non significant change in Caspase-3 expression when compared with the group (Group I).
The protective effect of Zn is explained by the fact that Zn is a reactive oxygen species antagonist. It acts by protecting oxidation of the sulfhydryl groups and also by scavenging for oxygen species antagonist16,60 or by preventing the increase of free radical formation.61 Other studies reported similar protective effects on diabetic kidney59 and on other organs as the lungs.62
Semi-quantitative assessment of the Bowman’s space surface area showed significant increase in group II and IV, while a significant decrease in group III when compared to group I. This significant increase in group II can be explained by a decrease in Bowman’s space pressure and damage to the parietal epithelial cells which caused by diabetes.63 In spite of the postulated protective effect of Zn in group IV, the paradoxical increase in Bowman’s space surface area may be due to the fact that the mechanism by which diabetes produces the increased surface area is not compensated by Zn. On the other hand, the significant decrease in Group III Bowman's space surface area is explained by the increased levels of serum glucocorticoids accompanying Zn deficiency with subsequent stress response in the affected cells.64 The high level of glucocorticoids induces mesangial hypercellularity, thickening of the Bowman's capsule together with adhesions in-between the glomerular tufts and the Bowman’s capsule.65
Caspases are mediators in the process of cell apoptosis.66 They are proenzymes that control a cascade of proteolytic events ending in cell death.67 Caspase-3 is particularly involved in the process of apoptosis by controlling Ca/Mg-dependent endonuclease which is involved in segmenting DNA ending in chromatin condensation and DNA fragmentation.68–70 It is normally expressed in the cells indicating normal apoptotic changes. These processes together with mitosis keep the tissues and organ sizes and shapes.71
Our study showed significant increased caspase-3 expression in groups II and III compared with group I indicating increased apoptosis in the renal tubular cells. This is explained by the cytotoxicity caused by increased reactive oxygen species leading to activation of the mitochondrial protein kinases ending in apoptosis. Deficiency of Zinc had been associated with increased apoptosis in other organs as intestine, retina, thymus, testis, pancreas, bone, and neural epithelium.4,72–76 Other studies reported increased apoptosis in cells cultured in Zn deficient media.71,77,78
On the other hand, group IV Caspase-3 expression showed non significant change indicating the protective effect of Zn supplementation.
Conclusion
The current study proofed the potential protective role of Zn in diabetic-induced renal damage in rats as evidenced by histomorphological observations
Recommendation
Antioxidants are important to control diabetic nephropathy and zinc supplementation is important to control progression of diabetic nephropathy.
Disclosure statement
There is no conflict of interest.
References
- 1.Bermudez L, Garcia-Vicent Lopez CJ, Torro MI, Lurbe E.. Assessment of ten trace elements in umbilical cord blood and maternal blood: Association with birth weight. J Transl Med. 2015;13:291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berg JM, Shi Y.. The galvanization of biology: A growing appreciation for the roles of zinc. Science. 1996;271:1081–1085. [DOI] [PubMed] [Google Scholar]
- 3.McCall KA, Huang C, Fierke CA.. Function and mechanism of zinc metalloenzymes . J Nutr. 2000;130:1437S–1446S. [DOI] [PubMed] [Google Scholar]
- 4.Truong-Tran AQ, Ho LH, Chai F, Zalewski PD.. Cellular zinc fluxes and the regulation of apoptosis/gene-directed cell death . J Nutr. 2000;130:1459S–1466S. [DOI] [PubMed] [Google Scholar]
- 5.Vallee BL, Falchuk KH.. The biochemical basis of zinc physiology. Physiol Rev. 1993;73:79–118. [DOI] [PubMed] [Google Scholar]
- 6.Chai F, Truong-Tran AQ, Ho LH, Zalewski PD.. Regulation of caspase activation and apoptosis by cellular zinc fluxes and zinc deprivation: A review . Immunol Cell Biol. 1999;77:272–278. [DOI] [PubMed] [Google Scholar]
- 7.Rink L, Gabriel P.. Zinc and the immune system. Proc Nutr Soc. 2000;59:541–552. [DOI] [PubMed] [Google Scholar]
- 8.Hambidge M.Human zinc deficiency . J Nutr. 2000;130:1344S–1349S. [DOI] [PubMed] [Google Scholar]
- 9.Cunningham JJ, Fu A, Mearkle PL, Brown RG.. Hyperzincuria in individuals with insulin-dependent diabetes mellitus: Concurrent zinc status and the effect of high-dose zinc supplementation. Metabolism. 1994;43:1558–1562. [DOI] [PubMed] [Google Scholar]
- 10.Chaffee BW, King JC.. Effect of zinc supplementation on pregnancy and infant outcomes: A systematic review. Paediatr Perinat Epidemiol. 2012;26:118–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dempsey C, McCormick NH, Croxford TP, Seo YA, Grider A, Kelleher SL.. Marginal maternal zinc deficiency in lactating mice reduces secretory capacity and alters milk composition. J Nutr. 2012;142:655–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jayalakshmi S, Platel K.. Compromised zinc status of experimental rats as a consequence of prolonged iron & calcium supplementation. Indian J Med Res. 2016;143:238–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Honnorat J, Accominotti M, Broussolle C, Fleuret AC, Vallon JJ, Orgiazzi J.. Effects of diabetes type and treatment on zinc status in diabetes mellitus. Biol Trace Elem Res. 1992;32:311–316. [DOI] [PubMed] [Google Scholar]
- 14.Levine AS, McClain CJ, Handwerger BS, Brown DM, Morley JE.. Tissue zinc status of genetically diabetic and streptozotocin-induced diabetic mice . Am J Clin Nutr. 1983;37:382–386. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura T, Higashi A, Nishiyama S, Fujimoto S, Matsuda I.. Kinetics of zinc status in children with IDDM. Diabetes Care. 1991;14:553–557. [DOI] [PubMed] [Google Scholar]
- 16.Salmonowicz B, Krzystek-Korpacka M, Noczynska A.. Trace elements, magnesium, and the efficacy of antioxidant systems in children with type 1 diabetes mellitus and in their siblings. Adv Clin Exp Med. 2014;23:259–268. [DOI] [PubMed] [Google Scholar]
- 17.Baynes JW.Role of oxidative stress in development of complications in diabetes. Diabetes. 1991;40:405–412. [DOI] [PubMed] [Google Scholar]
- 18.Giugliano D, Ceriello A, Paolisso G.. Oxidative stress and diabetic vascular complications. Diabetes Care. 1996;19:257–267. [DOI] [PubMed] [Google Scholar]
- 19.Ha H, Lee HB.. Oxidative stress in diabetic nephropathy: Basic and clinical information . Curr Diab Rep. 2001;1:282–287. [DOI] [PubMed] [Google Scholar]
- 20.Wolff SP.Diabetes mellitus and free radicals. Free radicals, transition metals and oxidative stress in the aetiology of diabetes mellitus and complications . Br Med Bull. 1993;49:642–652. [DOI] [PubMed] [Google Scholar]
- 21.Yu BP.Cellular defenses against damage from reactive oxygen species. Physiol Rev. 1994;74:139–162. [DOI] [PubMed] [Google Scholar]
- 22.Reddi AS, Bollineni JS.. Selenium-deficient diet induces renal oxidative stress and injury via TGF-beta1 in normal and diabetic rats. Kidney Int. 2001;59:1342–1353. [DOI] [PubMed] [Google Scholar]
- 23.Farid N, Inbal D, Nakhoul N, Evgeny F, Miller-Lotan R, Levy AP, Rabea A.. Vitamin E and diabetic nephropathy in mice model and humans. World J Nephrol. 2013;2:111–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calcutt NA, Cooper ME, Kern TS, Schmidt AM.. Therapies for hyperglycaemia-induced diabetic complications: From animal models to clinical trials. Nat Rev Drug Discov. 2009;8:417–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maiti R, Agrawal NK.. Atherosclerosis in diabetes mellitus: Role of inflammation. Indian J Med Sci. 2007;61:292–306. [PubMed] [Google Scholar]
- 26.Fioretto P, Mauer M.. Diabetic nephropathy: Diabetic nephropathy-challenges in pathologic classification. Nat Rev Nephrol. 2010;6:508–510. [DOI] [PubMed] [Google Scholar]
- 27.Molitch ME, DeFronzo RA, Franz MJ, Keane WF, Mogensen CE, Parving HH, Steffes MW.. Nephropathy in diabetes. Diabetes Care. 2004;27:S79–S83. [DOI] [PubMed] [Google Scholar]
- 28.Makuc J, Petrovic D.. A review of oxidative stress related genes and new antioxidant therapy in diabetic nephropathy. Cardiovasc Hematol Agents Med Chem. 2011;9:253–261. [DOI] [PubMed] [Google Scholar]
- 29.Lin CC, Huang YL.. Chromium, zinc and magnesium status in type 1 diabetes. Curr Opin Clin Nutr Metab Care. 2015;18:588–592. [DOI] [PubMed] [Google Scholar]
- 30.Johnson RJ, Couser WG, Chi EY, Adler S, Klebanoff SJ.. New mechanism for glomerular injury. Myeloperoxidase-hydrogen peroxide-halide system. J Clin Investig. 1987;79:1379–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neale TJ, Ojha PP, Exner M, Poczewski H, Ruger B, Witztum JL, Davis P, Kerjaschki D.. Proteinuria in passive Heymann nephritis is associated with lipid peroxidation and formation of adducts on type IV collagen. J Clin Investig. 1994;94:1577–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shah SV.The role of reactive oxygen metabolites in glomerular disease . Annu Rev Physiol. 1995;57:245–262. [DOI] [PubMed] [Google Scholar]
- 33.Yoshioka T, Ichikawa I, Fogo A.. Reactive oxygen metabolites cause massive, reversible proteinuria and glomerular sieving defect without apparent ultrastructural abnormality. J Am Soc Nephrol. 1991;2:902–912. [DOI] [PubMed] [Google Scholar]
- 34.Swenerton H, Hurley LS.. Severe zinc deficiency in male and female rats . J Nutr. 1968;95:8–18. [DOI] [PubMed] [Google Scholar]
- 35.Davis PN, Norris LC, Kratzer FH.. Iron deficiency studies in chicks using treated isolated soybean protein diets. J Nutr. 1962;78:445–453. [DOI] [PubMed] [Google Scholar]
- 36.Tang Y, Yang Q, Lu J, et al. Zinc supplementation partially prevents renal pathological changes in diabetic rats. J Nutr Biochem. 2010;21:237–246., [DOI] [PubMed] [Google Scholar]
- 37.Parvizi MR, Parviz M, Tavangar SM, et al. Protective effect of magnesium on renal function in STZ-induced diabetic rats. J Diabetes Metab Disord. 2014;13:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carter MR, Hornick JL, Lester S, Fletcher CD.. Spindle cell (sarcomatoid) carcinoma of the breast: A clinicopathologic and immunohistochemical analysis of 29 cases. Am J Surg Pathol. 2006;30:300–309. [DOI] [PubMed] [Google Scholar]
- 39.Hayat MA, Principles and techniques of electron microscopy Biological Applications. 3rd ed., 24–74. Hampshire and London: CRC Press; 1989. [Google Scholar]
- 40.Schultz CJ, Konopelska-Bahu T, Dalton RN, et al. Microalbuminuria prevalence varies with age, sex, and puberty in children with type 1 diabetes followed from diagnosis in a longitudinal study. Oxford Regional Prospective Study Group. Diabetes Care. 1999;22:495–502. [DOI] [PubMed] [Google Scholar]
- 41.Teoh SL, Abd LA, Das S.. Histological changes in the kidneys of experimental diabetic rats fed with Momordica charantia (bitter gourd) extract. Rom J Morphol Embryol. 2010;51:91–95. [PubMed] [Google Scholar]
- 42.Basaki M, Saeb M, Nazifi S, Shamsaei HA.. Zinc, copper, iron, and chromium concentrations in young patients with type 2 diabetes mellitus. Biol Trace Elem Res. 2012;148:161–164. [DOI] [PubMed] [Google Scholar]
- 43.Jansen J, Rosenkranz Overbeck E, Warmuth S, et al. Disturbed zinc homeostasis in diabetic patients by in vitro and in vivo analysis of insulinomimetic activity of zinc. J Nutr Biochem. 2012;23:1458–1466. [DOI] [PubMed] [Google Scholar]
- 44.Lau AL, Failla ML.. Urinary excretion of zinc, copper and iron in the streptozotocin-diabetic rat . J Nutr. 1984;114:224–233. [DOI] [PubMed] [Google Scholar]
- 45.Sivrikaya A, Bicer M, Akil M, Baltaci AK, Mogulkoc R.. Effects of zinc supplementation on the element distribution in kidney tissue of diabetic rats subjected to acute swimming. Biol Trace Elem Res. 2012;147:195–199. [DOI] [PubMed] [Google Scholar]
- 46.Grover JK, Vats V, Rathi SS, Dawar R.. Traditional Indian anti-diabetic plants attenuate progression of renal damage in streptozotocin induced diabetic mice. J Ethnopharmacol. 2001;76:233–238. no. [DOI] [PubMed] [Google Scholar]
- 47.Grover JK, Yadav SP, Vats V.. Effect of feeding Murraya koeingii and Brassica juncea diet on [correction] kidney functions and glucose levels in streptozotocin diabetic mice. J Ethnopharmacol. 2003;85:1–5. [DOI] [PubMed] [Google Scholar]
- 48.Kim DH, Choi BH, Ku SK, Park JH, Oh E, Kwak MK.. Beneficial effects of sarpogrelate and rosuvastatin in high fat diet/streptozotocin-induced nephropathy in mice. PLoS One. 2016;11:e0153965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yadav UC, Moorthy K, Baquer NZ.. Combined treatment of sodium orthovanadate and Momordica charantia fruit extract prevents alterations in lipid profile and lipogenic enzymes in alloxan diabetic rats. Mol Cell Biochem. 2005;268:111–120. [DOI] [PubMed] [Google Scholar]
- 50.Harding AH, Wareham NJ, Bingham SA, et al. Plasma vitamin C level, fruit and vegetable consumption, and the risk of new-onset type 2 diabetes mellitus: The European prospective investigation of cancer–Norfolk prospective study. Arch Intern Med. 2008;168:1493–1499. [DOI] [PubMed] [Google Scholar]
- 51.Prakasam A, Sethupathy S, Pugalendi KV.. Antiperoxidative and antioxidant effects of Casearia esculenta root extract in streptozotocin-induced diabetic rats. Yale J Biol Med. 2005;78:15–23. [PMC free article] [PubMed] [Google Scholar]
- 52.Rashid S, Qamar K, Tassaduq I.. Role of vitamin E in preventing arteriohyalinization in kidneys of streptozotocin induced diabetic mice. J Pak Med Assoc. 2015;65:1085–1088. [PubMed] [Google Scholar]
- 53.Fushimi H, Inoue T, Yamada Y, et al. Zinc deficiency exaggerates diabetic osteoporosis. Diabetes Res Clin Pract. 1993;20:191–196. [DOI] [PubMed] [Google Scholar]
- 54.Kim HJ, Vaziri ND.. Contribution of impaired Nrf2-Keap1 pathway to oxidative stress and inflammation in chronic renal failure. Am J Physiol Renal Physiol. 2010;298:F662–F671. [DOI] [PubMed] [Google Scholar]
- 55.Suzuki M, Betsuyaku T, Ito Y, et al. Down-regulated NF-E2-related factor 2 in pulmonary macrophages of aged smokers and patients with chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2008;39:673–682. [DOI] [PubMed] [Google Scholar]
- 56.Zhang C, Lu X, Tan Y, et al. Diabetes-induced hepatic pathogenic damage, inflammation, oxidative stress, and insulin resistance was exacerbated in zinc deficient mouse model. PLoS One. 2012;7:e49257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhao Y, Tan Y, Dai J, et al. Exacerbation of diabetes-induced testicular apoptosis by zinc deficiency is most likely associated with oxidative stress, p38 MAPK activation, and p53 activation in mice. Toxicol Lett. 2011;200:100–106. [DOI] [PubMed] [Google Scholar]
- 58.Levy AP, Hochberg I, Jablonski K, et al. Haptoglobin phenotype is an independent risk factor for cardiovascular disease in individuals with diabetes. The Strong Heart Study. J Am Coll Cardiol. 2002;40:1984–1990. [DOI] [PubMed] [Google Scholar]
- 59.Li B, Cui W, Tan Y, et al. Zinc is essential for the transcription function of Nrf2 in human renal tubule cells in vitro and mouse kidney in vivo under the diabetic condition. J Cell Mol Med. 2014;18:895–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tapiero H, Tew KD.. Trace elements in human physiology and pathology: Zinc and metallothioneins . Biomed Pharmacother. 2003;57:399–411. [DOI] [PubMed] [Google Scholar]
- 61.Bicer M, Akil M, Sivrikaya A, Kara E, Baltaci AK, Mogulkoc R.. Effect of zinc supplementation on the distribution of various elements in the serum of diabetic rats subjected to an acute swimming exercise. J Physiol Biochem. 2011;67:511–517. [DOI] [PubMed] [Google Scholar]
- 62.Mehta AJ, Joshi P, Fan CX, et al. Zinc supplementation restores PU.1 and Nrf2 nuclear binding in alveolar macrophages and improves redox balance and bacterial clearance in the lungs of alcohol-fed rats. Alcohol Clin Exp Res. 2011;35:1519–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tobar A, Ori Y, Benchetrit S, et al. Proximal tubular hypertrophy and enlarged glomerular and proximal tubular urinary space in obese subjects with proteinuria. PLoS One. 2013;8:e75547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fraker PJ, Telford WG.. A reappraisal of the role of zinc in life and death decisions of cells . Proc Soc Exp Biol Med. 1997;215:229–236. [DOI] [PubMed] [Google Scholar]
- 65.Waters CB, Adams LG, Scott-Moncrieff JC, et al. Effects of glucocorticoid therapy on urine protein-to-creatinine ratios and renal morphology in dogs. J Vet Intern Med. 1997;11:172–177. [DOI] [PubMed] [Google Scholar]
- 66.Hassan HA, Edrees GM, El-Gamel EM, El-Sayed EA.. Proanthocyanidin and fish oil potent activity against cisplatin-induced renal cell cycle arrest and apoptosis in rats. Ren Fail. 2015;37:1356–1362. [DOI] [PubMed] [Google Scholar]
- 67.Thornberry NA, Lazebnik Y.. Caspases: Enemies within. Science. 1998;281:1312–1316. [DOI] [PubMed] [Google Scholar]
- 68.Blanc C, Deveraux Q, Krajewski LS, et al. Caspase-3 is essential for procaspase-9 processing and cisplatin-induced apoptosis of MCF-7 breast cancer cells. Cancer Res. 2000;60:4386–4390. [PubMed] [Google Scholar]
- 69.Cummings BS, Schnellmann RG.. Cisplatin-induced renal cell apoptosis: Caspase 3-dependent and -independent pathways . J Pharmacol Exp Ther. 2002;302:8–17. [DOI] [PubMed] [Google Scholar]
- 70.Janicke RU, Sprengart ML, Wati MR, Porter AG.. Caspase-3 is required for DNA fragmentation and morphological changes associated with apoptosis . J Biol Chem. 1998;273:9357–9360. [DOI] [PubMed] [Google Scholar]
- 71.Wyllie AH.Apoptosis: An overview . Br Med Bull. 1997;53:451–465. [DOI] [PubMed] [Google Scholar]
- 72.Duvall E, Wyllie AH.. Death and the cell. Immunol Today. 1986;7:115–119. [DOI] [PubMed] [Google Scholar]
- 73.Record IR, Tulsi RS, Dreosti IE, Fraser FJ.. Cellular necrosis in zinc-deficient rat embryos. Teratology. 1985;32:397–405. [DOI] [PubMed] [Google Scholar]
- 74.Rogers JM, Taubeneck MW, Daston GP, et al. Zinc deficiency causes apoptosis but not cell cycle alterations in organogenesis-stage rat embryos: Effect of varying duration of deficiency. Teratology. 1995;52:149–159. [DOI] [PubMed] [Google Scholar]
- 75.Sunderman FW, Jr., The influence of zinc on apoptosis . Ann Clin Lab Sci. 1995;25:134–142. [PubMed] [Google Scholar]
- 76.Zalewski PD, Millard SH, Forbes IJ, et al. Video image analysis of labile zinc in viable pancreatic islet cells using a specific fluorescent probe for zinc. J Histochem Cytochem. 1994;42:877–884. [DOI] [PubMed] [Google Scholar]
- 77.Aiuchi T, Mihara S, Nakaya M, Masuda Y, Nakajo S, Nakaya K.. Zinc ions prevent processing of caspase-3 during apoptosis induced by geranylgeraniol in HL-60 cells. J Biochem. 1998;124:300–303. [DOI] [PubMed] [Google Scholar]
- 78.Perry DK, Smyth MJ, Stennicke HR, et al. Zinc is a potent inhibitor of the apoptotic protease, caspase-3. A novel target for zinc in the inhibition of apoptosis . J Biol Chem. 1997;272:18530–18533. [DOI] [PubMed] [Google Scholar]