Abstract
Background: The aim of this study was to compare the incidence of post-procedural acute kidney injury (AKI) and other renal outcomes in patients undergoing transapical (TA) and transfemoral (TF) approaches for transcatheter aortic valve replacement (TAVR).
Methods: All consecutive adult patients undergoing TAVR for aortic stenosis from 1 January 2008 to 30 June 2014 at a tertiary referral hospital were included. AKI was defined based on Kidney Disease Improving Global Outcomes (KDIGO) criteria. Logistic regression adjustment, propensity score stratification, and propensity matching were performed to assess the independent association between procedural approach and AKI.
Results: Of 366 included patients, 171 (47%) underwent TAVR via a TA approach. AKI occurrence in this group was significantly higher compared to the TF group (38% vs. 18%, p < .01). The TA approach remained significantly associated with increased risk of AKI after logistic regression (OR 3.20; CI 1.68–4.36) and propensity score adjustment: OR 2.83 (CI 1.66–4.80) for stratification and 3.82 (CI 2.04–7.44) for matching. Nonetheless, there was no statistically significant difference among the TA and TF groups with respect to major adverse kidney events (MAKE) or estimated glomerular filtration rate (eGFR) at six months post-procedure.
Conclusion: In a cohort of patients undergoing TAVR for aortic stenosis, a TA approach significantly increases the AKI risk compared with a TF approach. However, the TAVR approach did not affect severe renal outcomes or long-term renal function.
Keywords: Acute kidney injury, transfemoral, transapical, approach, transcatheter aortic valve replacement, AKI, TAVR
Introduction
Acute kidney injury (AKI) is relatively prevalent in patients undergoing transcatheter aortic valve replacement (TAVR).1,2 It is also independently associated with 30-day and 1-year mortality.3,4 AKI after TAVR is associated with reported mortality rate as high as 55%.3–9 Potential risk factors for AKI after TAVR have already been identified, such as pre-existing chronic kidney disease (CKD), diabetes, contrast media exposure, hypotension, periprocedural bleeding, and blood transfusion.2,3,5,10 Despite efforts to prevent AKI after TAVR, the incidence in patients with severe aortic stenosis following TAVR from 15 to 57%.3,5–7
Transfemoral (TF) and transapical (TA) access approaches are the most commonly used approaches for TAVR. Patients undergoing TA-TAVR have been shown to have more complications, compared to patients undergoing TF-TAVR, including AKI and worse prognosis.11–18 However, patients selected for TA-TAVR typically have higher comorbidities, especially peripheral vascular atherosclerotic diseases. Therefore, these results may be confounded by selection bias.10,17
The aim objective of this propensity-matched study was to compare the incidence of AKI and other renal outcomes following TAVR in patients undergoing TA and TF approaches.
Materials and methods
Study population
This was a single-center retrospective cohort study conducted at a tertiary referral hospital. All consecutive adult patients (age 18 years or older) who underwent TAVR via TA or TF approach for aortic stenosis from 1 January 2008 to 30 June 2014 at Mayo Clinic in Rochester, MN, USA were included. For patients with multiple aortic valve replacement procedures, only the first intervention during the study period was included in the analysis. Patients with end-stage renal disease, received any dialysis modalities within 14 days prior to intervention, or did not provide research authorization were excluded. Almost all TAVR procedures (99%) in our institution during study period were performed using balloon-expandable valve under general anesthesia. This study was approved by the Mayo Clinic Institutional Review Board (IRB). Informed consent was waived for patients who provided research authorization.
Data collection
Clinical characteristics, laboratory data, echocardiographic data, procedural data, and post-procedural data were collected using manual and automated retrieval from the electronic medical record. The risk of operative mortality was calculated based on patient demographics, pre-operative clinical characteristics, and procedures utilizing the Society of Thoracic Surgeons (STS) risk score.19–21 The estimated glomerular filtration rate (eGFR) was derived using the Chronic Kidney Disease Epidemiology Collaboration equation.22
Clinical outcomes
The primary outcome was post-operative AKI. AKI was identified and staged based on the serum creatinine (SCr) criterion of the Kidney Disease Improving Global Outcomes (KDIGO) definition.23 AKI was defined as an increase in SCr of ≥0.3 mg/dL (≥26.5 μmol/L) within 48 h of intervention or a relative increase of ≥50% from baseline within seven days after intervention. The baseline values for SCr were obtained as follows: if outpatient SCr measurement between 180 and seven days before intervention was available, the most recent value was used. Otherwise, the lowest value within five days prior to intervention was used. Secondary outcomes included in-hospital mortality, need for renal replacement therapy (RRT) at any time during hospitalization, the composite of major adverse kidney events (MAKE), and six-month mortality. MAKE were defined as the composite of in-hospital death, use of RRT, or persistence of renal dysfunction (defined by SCr ≥200% of reference) at hospital discharge.
Patient vital status was reviewed using registration data and the electronic medical record. For patients with unknown vital status at six months after ICU admission, the Social Security Death Index was used.24 Among surviving patients at six months after intervention, renal function was assessed by eGFR calculation at six months and evaluation of the need for chronic dialysis. eGFR values of 5 mL/min/1.73 m2 at six months were imputed for patients who needed chronic dialysis.
Statistical analysis
All continuous variables were reported as mean ± standard deviation (SD). All categorical variables were reported as counts with percentages. The differences in clinical characteristics and outcomes of patients in the TA and TF groups were tested using the Student’s t-test for continuous variables and the Chi-squared test, or Fisher’s exact test, for categorical variables, as appropriate. A two-sided p values of <.05 was considered statistically significant. Unless specified, analyses were performed using JMP statistical software (SAS, Cary, NC).
When estimating the effect of the TA approach on post-procedural AKI compared to the TF approach, three separate statistical analyses to account for pre-operative differences between these two groups: (1) Multivariate logistic regression adjusting for variables with statistically significant differences in the univariate analysis: age, body mass index (BMI), dyslipidemia, stroke, peripheral vascular disease, chronic lung disease, previous percutaneous coronary intervention (PCI), previous cardiac surgery, previous coronary bypass graft surgery (CABG), and pre-operative statin use. (2) Propensity score stratification into quintiles. The pooled risk of AKI after stratification by propensity score was assessed using the Cochran–Mantel–Haenzel method. (3) Matching on a 1:1 basis on the logit of propensity score with a caliper width equal to 0.25 of the SD of the logit of the propensity score. Propensity scores were estimated using logistic regression with the TA approach as the dependent variable on the following covariates: STS risk score, eGFR, and variables with statistically significant differences in univariate analysis between the two groups, as described above. Propensity score generation, stratification, and matching for patients in the TA and TF groups were performed using R statistical software version 3.1.1 (Vienna, Austria).25
Results
A total of 366 patients were included in the initial cohort prior to matching: 171 in the TA group and 195 in the TF group. In the unmatched cohort, the TA group was significantly older, had higher BMI, and more prevalence of dyslipidemia, as well as stroke, peripheral vascular disease, history of previous PCI, cardiac surgery, CABG, and pre-operative statin use (Table 1). Intraoperatively, there was no significant difference in procedure duration or the need for intra-aortic balloon pump. However, the TA group more frequently required RBC transfusion than the TF group (Table 2).
Table 1.
Baseline characteristics between patients with transfemoral versus transapical approach.
| Before matching |
After matching |
|||||
|---|---|---|---|---|---|---|
| Characteristics | Transfemoral (n = 195) | Transapical (n = 171) | p Values | Transfemoral (n = 108) | Transapical ( n = 108) | p Values |
| STS risk score | 7.9 ± 7.0 | 8.9 ± 5.0 | .12 | 8.3 ± 8.3 | 8.3 ± 5.0 | .98 |
| Age (year) | 80 ± 9 | 82 ± 7 | .01 | 81 ± 8 | 82 ± 7 | .34 |
| Male sex | 110 (56) | 97 (57) | .95 | 68 (63) | 55 (51) | .07 |
| White | 190 (97) | 164 (96) | .41 | 106 (98) | 106 (98) | 1.00 |
| Body mass index (kg/m2) | 32.1 ± 8.2 | 28.4 ± 6.0 | <.001 | 30.1 ± 5.7 | 29.4 ± 6.5 | .37 |
| eGFR (mL/min/1.73m2) | 56 ± 20 | 54 ± 21 | .19 | 54 ± 18 | 54 ± 21 | .98 |
| NYHA class III–IV | 171 (88) | 145 (85) | .42 | 91 (84) | 94 (87) | .56 |
| Comorbidity | ||||||
| Diabetes mellitus | 77 (39) | 70 (41) | .78 | 39 (36) | 44 (41) | .48 |
| Hypertension | 174 (89) | 157 (92) | .40 | 101 (94) | 101 (94) | 1.00 |
| Dyslipidemia | 164 (84) | 162 (95) | .001 | 102 (94) | 100 (93) | .58 |
| Myocardial infarction | 63 (32) | 69 (40) | .11 | 46 (43) | 34 (31) | .09 |
| Congestive heart failure | 108 (55) | 100 (58) | .55 | 59 (55) | 58 (54) | .89 |
| Stroke | 44 (23) | 60 (35) | .01 | 34 (31) | 31 (29) | .66 |
| Peripheral vascular disease | 86 (44) | 125 (73) | <.001 | 63 (58) | 66 (61) | .68 |
| Anemia | 7 (4) | 2 (1) | .14 | 3 (3) | 2 (2) | .65 |
| Chronic lung disease | 128 (66) | 95 (56) | .048 | 64 (59) | 65 (60) | .89 |
| Smoking within 1 year | 4 (2) | 4 (2) | .85 | 1 (1) | 2 (2) | .56 |
| Prior cardiac intervention | ||||||
| PCI | 87 (45) | 104 (61) | .002 | 63 (58) | 61 (56) | .78 |
| Cardiac surgery | 78 (40) | 96 (56) | .002 | 57 (53) | 49 (45) | .28 |
| CABG | 70 (36) | 90 (53) | .001 | 54 (50) | 45 (42) | .22 |
| Valve surgery | 46 (24) | 31 (18) | .20 | 24 (22) | 18 (17) | .30 |
| Aortic valve surgery | 7 (4) | 3 (2) | .28 | 4 (4) | 2 (2) | .41 |
| Echocardiographic finding | ||||||
| Ejection fraction | 56 ± 13 | 55 ± 13 | .62 | 54 ± 14 | 56 ± 13 | .49 |
| Aortic valve gradient | 48 ± 15 | 48 ± 13 | .67 | 47 ± 15 | 49 ± 13 | .34 |
| Aortic valve insufficiency | 111 (57) | 90 (53) | .41 | 66 (61) | 61 (57) | .49 |
| Mitral valve dysfunction | 152 (78) | 135 (79) | .82 | 90 (83) | 77 (71) | .06 |
| Preoperative medication | ||||||
| ACEI/ARB | 78 (40) | 72 (42) | .68 | 45 (42) | 48 (44) | .68 |
| Beta-blocker | 137 (70) | 116 (68) | .62 | 84 (78) | 70 (65) | .06 |
| Statin | 125 (64) | 137 (80) | .001 | 86 (80) | 84 (78) | .74 |
| Aspirin | 141 (72) | 127 (74) | .67 | 87 (81) | 80 (74) | .26 |
| Normal sinus rhythm | 142 (73) | 123 (72) | .85 | 81 (75) | 78 (72) | .64 |
| Elective surgery | 189 (97) | 160 (94) | .13 | 105 (97) | 99 (92) | .07 |
Continuous variables are reported as mean ± SD; Categorical variables are reported as count (percentage).
Abbreviations: ACEI: angiotensin-converting enzyme inhibitor; AKI: acute kidney injury; ARB: angiotensin II receptor blocker; CABG: coronary artery bypass graft surgery; eGFR: estimated glomerular filtration rate; kg: kilogram; m: meter; min: minutes; mL: milliliter; NYHA: New York Heart Association; PCI: percutaneous coronary intervention; STS: Society of Thoracic Surgeons.
Table 2.
Intraoperative characteristics between patients with transfemoral versus transapical approach.
| Before matching |
After matching |
|||||
|---|---|---|---|---|---|---|
| Characteristics | Transfemoral (n = 195) | Transapical (n = 171) | p Values | Transfemoral (n = 108) | Transapical (n = 108) | p Values |
| Procedure duration (min) | 131 ± 56 | 122 ± 47 | .09 | 132 ± 66 | 118 ± 42 | .07 |
| RBC transfusion needed | 51 (26) | 69 (40) | .004 | 32 (30) | 42 (39) | .15 |
| Intra-aortic balloon pump | 5 (3) | 2 (1) | .33 | 5 (5) | 2 (2) | .25 |
Continuous variables are reported as mean ± SD; Categorical variables are reported as count (percentage).
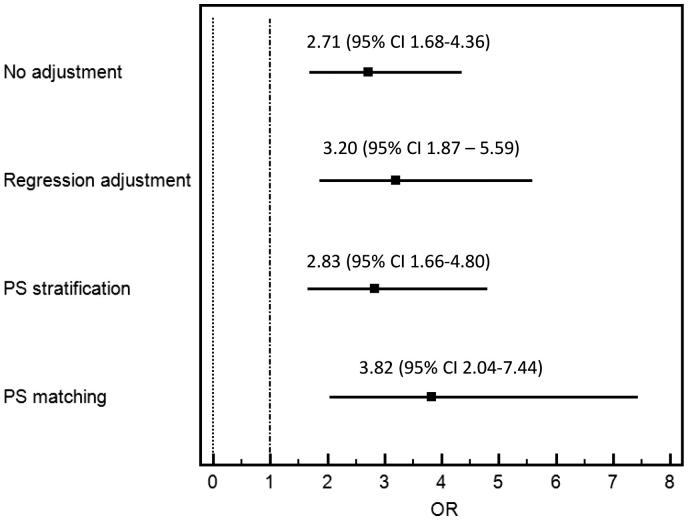
In the unmatched cohort, post-operative AKI occurred in 38% (N = 65) of the TA group: 32% stage 1, 2% stage 2, and 4% stage 3. In contrast, post-operative AKI occurred in 18% (N = 36) of the TF group: 14% stage 1, 2% stage 2, and 3% stage 3 (Table 3). In unadjusted analysis, AKI occurrence in the TA group was significantly higher than in the TF group (OR = 2.71; 95% CI 1.68–4.36; p < .001). There was no significant difference in the need for RRT, in-hospital mortality, MAKE, stroke/TIA, vascular complication, six-month mortality, as well as eGFR at six months. In adjusted analysis, TA approach remained significantly associated with increased risk of AKI: OR 3.20 (CI 1.87–5.59) (Figure 1).
Table 3.
Outcomes between patients with transfemoral versus transapical approach.
| Before matching |
After matching |
|||||
|---|---|---|---|---|---|---|
| Characteristics | Transfemoral (n = 195) | Transapical (n = 171) | p Values | Transfemoral (n = 108) | Transapical (n = 108) | p Values |
| Acute kidney injury | 36 (18) | 65 (38) | <.001 | 17 (16) | 45 (42) | <.001 |
| AKI stages | <.001 | <.001 | ||||
| No AKI | 159 (82) | 106 (62) | 91 (84) | 63 (58) | ||
| Stage 1 | 27 (14) | 55 (32) | 14 (13) | 37 (34) | ||
| Stage 2 | 3 (2) | 3 (2) | 1 (1) | 2 (2) | ||
| Stage 3 | 6 (3) | 7 (4) | 2 (2) | 6 (6) | ||
| Dialysis in hospital | 4 (2) | 5 (3) | .59 | 1 (1) | 3 (3) | .62 |
| In-hospital mortality | 6 (3) | 4 (2) | .66 | 2 (2) | 3 (3) | .66 |
| Major adverse kidney events | 9 (5) | 7 (4) | .81 | 3 (3) | 4 (4) | .70 |
| eGFR at 6 monthsa | 56 ± 20 | 53 ± 20 | .16 | 53 ± 19 | 52 ± 20 | .58 |
| Stroke/TIA | 3 (1.5) | 4 (2.3) | .58 | 2 (1.9) | 1 (0.9) | .56 |
| Vascular complication | 3 (1.5) | 0 (0) | .10 | 1 (0.9) | 0 (0) | .32 |
| 6-month mortality | 21 (11) | 14 (8) | .40 | 10 (9) | 8 (7) | .62 |
Continuous variables are reported as mean ± SD; Categorical variables are reported as count (percentage).
Analysis was limited in survivor six months after procedure.
Figure 1.
Odds ratio for AKI of the transapical versus transfemoral approach according to various analyses.
Propensity score matching created a matched cohort of 108 patients in each group. The matching rigorously reduced the difference in covariates between two groups (Figures S1 and S2). In this matched cohort, baseline characteristics and pre-operative variables were comparable between the TA and TF groups (Table 1). There was no significant difference in procedure duration, need for RBC transfusion, or need for intra-aortic balloon during procedure between the two groups (Table 2).
In the matched cohort, AKI occurred in 42% (N = 45) of the TA group: 34% stage 1, 2% stage 2, and 6% stage 3. In contrast, AKI occurred in 16% (N = 17) of the TF group: 13% stage 1, 1% stage 2, and 2% stage 3 (Table 3). The risk of AKI was significantly higher in the TA group (OR = 3.82; CI 2.04–7.44; p < .001) (Figure 1). A similar association was observed after propensity score stratification with OR of 2.83 (CI 1.66–4.80). There was no significant difference in the need for the RRT, in-hospital mortality, MAKE, six-month mortality, as well as eGFR at six months.
Discussion
Comprehensive propensity score analysis in a cohort of severe AS patients undergoing TAVR was used to demonstrate a TA approach significantly increased the risk of AKI compared to a TF approach with an overall 3.82-fold increased the risk of AKI. However, there were no significant differences between these two approaches in the setting of severe AKI with respect to RRT, in-hospital mortality, or six-month mortality. Before matching, patients in the TA group were significantly older, had a higher BMI, and more prevalence of dyslipidemia, as well as stroke, peripheral vascular disease, history of previous PCI, cardiac surgery, CABG, and pre-operative statin use.
Despite comparable patient populations in propensity score match analysis, TA-TAVR still carried a significantly higher AKI risk compared to TF-TAVR. Therefore, the mechanisms of this observation of higher AKI risk are likely beyond the fact that patients are undergoing TA-TAVR usually have more severe peripheral vascular disease. It is possible that the instrumentation of the aorta during the TA-TAVR may lead to cholesterol crystal emboli and the dislodgement of calcium plaques, resulting in AKI.10 TF-TAVR can also be performed under moderate sedation and local anesthetics, while TA-TAVR is usually performed under general anesthesia, which can contribute to renal hypoperfusion.26
Although patients undergoing TA-TAVR developed AKI more frequently compared to TF-TAVR, the primary outcome difference was in stage 1 AKI and did not translate into more major adverse events or six-month mortality. Comparable mortality risk among patients undergoing TA-TAVR and TF-TAVR was recently demonstrated by Schymik et al.27 Using propensity score matching, no difference was reported in mortality risk between patients undergoing TA and TF approaches in a high volume treatment center. An experienced heart team was postulated to be an important explanation for this observation. However, in PARTNER 2 trial,28 which studied intermediate-risk patients, TAVR resulted in a lower rate of death or disabling stroke than surgery in the TF-access cohort, whereas outcomes were similar in the two groups in the transthoracic-access cohort.
There are several limitations to our study. (1) As we utilized data from a prospectively collected database and electronic health records, our study has an observational design. Our results establish an association, not causality. Additionally, this study was conducted in a tertiary referral center serving a predominantly Caucasian population, potentially introducing selection bias and limiting the generalizability of the results. (2) We did not include urine output criterion for AKI diagnosis. The inclusion of urine output criterion would increase the sensitivity of AKI definition. However, an indwelling urinary catheter was not used to obtain accurate hourly urine output data for a significant percentage of patients in our cohort. Also, diuretics were commonly utilized in TAVR patients enrolled in our study. Therefore, it could reduce the accuracy of urine output criterion for AKI diagnosis.
In conclusion, TA-TAVR is associated with higher incidence of AKI compared to TF-TAVR. However, this increased frequency of mild AKI associated with TA-TAVR does not translate into higher mortality, need for RRT, or long-term renal function.
Supplementary Material
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- 1.Thongprayoon C, Cheungpasitporn W, Srivali N, et al. AKI after transcatheter or surgical aortic valve replacement . J Am Soc Nephrol. 2016;27:1854–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scherner M, Wahlers T.. Acute kidney injury after transcatheter aortic valve implantation. J Thorac Dis. 2015;7:1527–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elhmidi Y, Bleiziffer S, Deutsch MA, et al. Acute kidney injury after transcatheter aortic valve implantation: Incidence, predictors and impact on mortality. Arch Cardiovasc Dis. 2014;107:133–139. [DOI] [PubMed] [Google Scholar]
- 4.Thongprayoon C, Cheungpasitporn W, Gillaspie EA, Greason KL, Kashani KB.. The risk of acute kidney injury following transapical versus transfemoral transcatheter aortic valve replacement: A systematic review and meta-analysis. Clin Kidney J. 2016;9:560–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thongprayoon C, Cheungpasitporn W, Srivali N, et al. Acute kidney injury after transcatheter aortic valve replacement: A systematic review and meta-analysis. Am J Nephrol. 2015;41:372–382. [DOI] [PubMed] [Google Scholar]
- 6.Adams DH, Popma JJ, Reardon MJ, et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med. 2014;370:1790–1798. [DOI] [PubMed] [Google Scholar]
- 7.Thongprayoon C, Cheungpasitporn W, Srivali N, Kittanamongkolchai W, Greason KL, Kashani KB.. Incidence and risk factors of acute kidney injury following transcatheter aortic valve replacement. Nephrology (Carlton, Vic.). 2015. [Epub ahead of print]. doi: 10.1111/nep.12704. [DOI] [PubMed] [Google Scholar]
- 8.Thongprayoon C, Cheungpasitporn W, Podboy AJ, Gillaspie EA, Greason KL, Kashani KB.. The effects of contrast media volume on acute kidney injury after transcatheter aortic valve replacement: A systematic review and meta-analysis. J Evid Based Med. 2016. [Epub ahead of print]. doi: 10.1111/jebm.12208. [DOI] [PubMed] [Google Scholar]
- 9.Cheungpasitporn W, Thongprayoon C, Kashani K.. Transcatheter aortic valve replacement: A kidney's perspective. J Renal Inj Prev. 2016;5:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Najjar M, Salna M, George I.. Acute kidney injury after aortic valve replacement: Incidence, risk factors and outcomes. Expert Rev Cardiovasc Ther. 2015;13:301–316. [DOI] [PubMed] [Google Scholar]
- 11.Di Mario C, Eltchaninoff H, Moat N, et al. The 2011–12 pilot European sentinel registry of transcatheter aortic valve implantation: In-hospital results in 4,571 patients. EuroIntervention. 2013;8:1362–1371. [DOI] [PubMed] [Google Scholar]
- 12.Thomas M, Schymik G, Walther T, et al. Thirty-day results of the SAPIEN aortic Bioprosthesis European Outcome (SOURCE) Registry: A European registry of transcatheter aortic valve implantation using the Edwards SAPIEN valve. Circulation. 2010;122:62–69. [DOI] [PubMed] [Google Scholar]
- 13.Eltchaninoff H, Prat A, Gilard M, et al. Transcatheter aortic valve implantation: Early results of the FRANCE (FRench Aortic National CoreValve and Edwards) registry. Eur Heart J. 2011;32:191–197. [DOI] [PubMed] [Google Scholar]
- 14.Zahn R, Gerckens U, Grube E, et al. Transcatheter aortic valve implantation: First results from a multi-centre real-world registry. Eur Heart J. 2011;32:198–204. [DOI] [PubMed] [Google Scholar]
- 15.Tamburino C, Capodanno D, Ramondo A, et al. Incidence and predictors of early and late mortality after transcatheter aortic valve implantation in 663 patients with severe aortic stenosis. Circulation. 2011;123:299–308. [DOI] [PubMed] [Google Scholar]
- 16.Rodes-Cabau J, Webb JG, Cheung A, et al. Long-term outcomes after transcatheter aortic valve implantation: Insights on prognostic factors and valve durability from the Canadian multicenter experience. J Am Coll Cardiol. 2012;60:1864–1875. [DOI] [PubMed] [Google Scholar]
- 17.Aregger F, Wenaweser P, Hellige GJ, et al. Risk of acute kidney injury in patients with severe aortic valve stenosis undergoing transcatheter valve replacement. Nephrol Dial Transplant. 2009;24:2175–2179. [DOI] [PubMed] [Google Scholar]
- 18.Saia F, Ciuca C, Taglieri N, et al. Acute kidney injury following transcatheter aortic valve implantation: Incidence, predictors and clinical outcome. Int J Cardiol. 2013;168:1034–1040. [DOI] [PubMed] [Google Scholar]
- 19.Shahian DM, Edwards FH.. The society of thoracic surgeons 2008 cardiac surgery risk models: Introduction. Ann Thorac Surg. 2009;88:S1. [DOI] [PubMed] [Google Scholar]
- 20.O'Brien SM, Shahian DM, Filardo G, et al. The society of thoracic surgeons 2008 cardiac surgery risk models: Part 2–isolated valve surgery. Ann Thorac Surg. 2009;88(1Suppl):S23–S42. [DOI] [PubMed] [Google Scholar]
- 21.Shahian DM, O'Brien SM, Filardo G, et al. The society of thoracic surgeons 2008 cardiac surgery risk models: Part 3-valve plus coronary artery bypass grafting surgery. Ann Thorac Surg. 2009;88:S43–S62. [DOI] [PubMed] [Google Scholar]
- 22.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.KDIGO AKI Work Group KDIGO clinical practice guidelines for acute kidney injury. Kidney Int. 2012;2:1–138. [Google Scholar]
- 24.Wentworth DN, Neaton JD, Rasmussen WL.. An evaluation of the social security administration master beneficiary record file and the National Death Index in the ascertainment of vital status. Am J Public Health. 1983;73:1270–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sekhon JS.Multivariate and propensity score matching software with automated balance optimization: The matching package for R. J Stat Softw. 2011;42:1–52. [Google Scholar]
- 26.Ruparelia N, Prendergast BD.. Transcatheter aortic valve implantation – what the general physician needs to know. Clin Med (Lond). 2015;15:420–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schymik G, Wurth A, Bramlage P, et al. Long-term results of transapical versus transfemoral TAVI in a real world population of 1000 patients with severe symptomatic aortic stenosis. Circ Cardiovasc Interv. 2015;8:e000761. [DOI] [PubMed] [Google Scholar]
- 28.Leon MB, Smith CR, Mack MJ, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2016;374:1609–1620. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.