Abstract
OBJECTIVE
This study evaluated the effectiveness of a community health worker (CHW) diabetes self-management education (DSME) program, followed by two different approaches to maintain improvements in HbA1c and other clinical and patient-centered outcomes over 18 months.
RESEARCH DESIGN AND METHODS
The study randomized 222 Latino adults with type 2 diabetes and poor glycemic control from a federally qualified health center to 1) a CHW-led, 6-month DSME program or 2) enhanced usual care (EUC). After the 6-month program, participants randomized to the CHW-led DSME were further randomized to 1) 12 months of CHW-delivered monthly telephone outreach (CHW-only) or 2) 12 months of weekly group sessions delivered by peer leaders (PLs) with telephone outreach to those unable to attend (CHW+PL). The primary outcome was HbA1c. Secondary outcomes were blood pressure, lipid levels, diabetes distress, depressive symptoms, understanding of diabetes self-management, and diabetes social support. Assessments were conducted at baseline and at 6, 12, and 18 months.
RESULTS
Participants in the CHW intervention at the 6-month follow-up had greater decreases in HbA1c (−0.45% [95% CI −0.87, −0.03]; P < 0.05) and in diabetes distress (−0.3 points [95% CI −0.6, −0.03]; P < 0.05) compared with EUC. CHW+PL participants maintained HbA1c improvements at 12 and 18 months, and CHW-only participants maintained improvements in diabetes distress at 12 and 18 months. CHW+PL participants also had significantly fewer depressive symptoms at 18 months compared with EUC (−2.2 points [95% CI −4.1, −0.3]; P < 0.05). Participants in CHW-led DSME had significant improvements in diabetes social support and in understanding of diabetes self-management at 6 months relative to EUC, but these intervention effects were not sustained at 18 months.
CONCLUSIONS
This study demonstrates the effectiveness of a 6-month CHW intervention on key diabetes outcomes and of a volunteer PL program in sustaining key achieved gains. These are scalable models for health care centers in low-resource settings for achieving and maintaining improvements in key diabetes outcomes.
Introduction
Type 2 diabetes (T2D) disproportionately affects low-income racial and ethnic minority groups and is a growing public health problem. Diabetes self-management education (DSME) is necessary but often insufficient to sustain the substantial self-management effort needed during a lifetime with diabetes (1). Many patients need on-going diabetes self-management support (DSMS) (2). DSMS is defined as “activities that assist the individual with diabetes to implement and sustain the on-going behaviors needed to manage their illness (3).”
As trusted members of their communities, trained community health workers (CHWs) are particularly effective in reaching and providing both DSME and DSMS to members of communities of color who face numerous barriers to diabetes self-management (4–7). CHW interventions in diabetes have led to improved self-monitoring, self-care, lifestyle change, and blood glucose control outcomes compared with control groups (3–5,7,8). Most studies, however, report postintervention outcomes only at 6 and 12 months. A key challenge is to sustain immediate gains after a DSME intervention over longer periods of follow-up (4,8).
One particularly promising approach to sustain gains from more intensive CHW interventions is to offer less intensive support services by peer leaders (PLs) (4,8). PLs share important characteristics with participants, including having diabetes, and are volunteers or receive small stipends to reimburse any expenses (9–12). Some PL-led interventions have resulted in improvements in HbA1c compared with control groups (13). Other PL interventions did not result in greater improvements in HbA1c than control groups but found improved self-empowerment scores, self-care indicators (9), patient activation (12), and diabetes-related distress (10). One study found a PL program sustained gains in glycemic control and several key patient-centered outcomes achieved through a CHW-led DSMS program, but there was no comparison with a usual care group (14).
Given the effectiveness and potential low cost of PL-led DSMS, further research is needed to test whether PLs may be effective in helping to maintain gains achieved through more intensive DSME programs led by CHWs compared with usual care. Therefore, the current study examined the effectiveness of a CHW intervention in improving clinical outcomes (HbA1c, blood pressure, and lipid levels), psychosocial outcomes (depressive symptoms and diabetes-related distress), diabetes self-management behaviors, and understanding of diabetes self-management compared with enhanced usual care (EUC) immediately after the intervention at 6 months. We then examined two alternative lower-intensity approaches from 6 to 18 months of follow-up. Specifically, we examined whether an additional PL intervention, implemented after the conclusion of the 6-month CHW-led intervention, enhanced maintenance of improved outcomes compared with monthly CHW-only follow-up or EUC alone.
Research Design and Methods
Study Population
Founded in 1999, the Racial and Ethnic Approaches to Community Health (REACH) Detroit Partnership is a community-based participatory research (CBPR) coalition of community organizations, academic institutions, and health care systems that has used CBPR approaches to design, implement, and evaluate interventions aimed at improving diabetes care and outcomes in east and southwest Detroit (15). All work has been conducted with the Community Health and Social Services Center (CHASS), a federally qualified community health center serving the predominantly Latino community in southwest Detroit, and guided by a steering committee of partnership members.
We developed a culturally tailored DSME curriculum, “Journey to Health/El Camino a la Salud,” grounded in the empowerment approach that emphasizes a collaborative approach to facilitate self-directed behavior change of patients (15,16). Empowerment-based approaches are effective in improving chronic disease self-management among racial and ethnic minority patients (17).
We evaluated the 6-month CHW intervention in two prior cohorts of adult African American and Latino patients with poor glycemic control. In the first, we compared the effectiveness of the CHW intervention with a concurrent observational control group and found significant improvements in HbA1c and other clinical outcomes (18). Our second cohort study, using a randomized, 6-month delayed control group design, also found significant improvements in HbA1c and other patient-centered outcomes compared with the delayed control group (8). Both studies were limited to outcomes measured immediately after the conclusion of the 6-month CHW-led program. The current study was conducted with a third cohort of participants with poorly controlled diabetes.
Study Procedures
The University of Michigan Institutional Review Board approved this study. From October 2009 to February 2013, we reviewed computer-generated lists of all potentially eligible CHASS patients with physician-diagnosed diabetes who were at least 21 years old and self-identified as Latina/o. Individuals with physical limitations preventing participation, terminal health conditions, serious psychiatric illness, and self-reported excessive alcohol or illicit drug use were excluded.
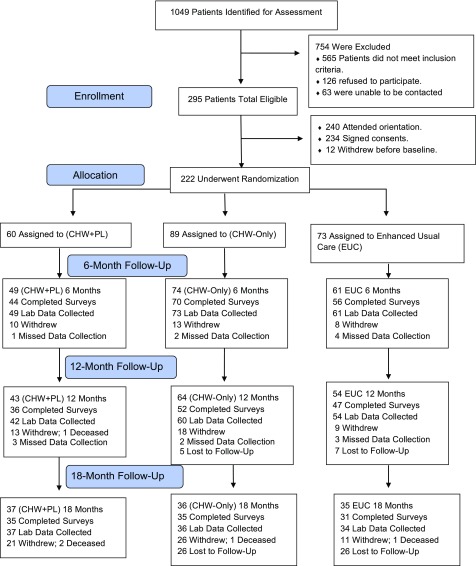
From the initial list of 1,049 patients, 295 were eligible based on intake screening, 565 did not meet inclusion criteria, 126 refused to participate, and 63 were unable to be contacted (see Fig. 1). There were no differences in sex and ethnicity between eligible participants and nonparticipants, but nonparticipants were an average of 3.7 years older than participants (P = 0.012). Of the 295 eligible, 234 initially signed consents and 12 withdrew before baseline, leaving 222 to be randomized. After participants provided informed consent, laboratory and anthropometric measurements were collected, and participants completed baseline questionnaires. The 222 participants were first randomized into the CHW intervention arm (n = 149) or the EUC (n = 73) arm using a computer-generated process with concealed allocation. At 6 months (immediately after the CHW intervention), CHW intervention participants were further randomized into the CHW-only intervention (n = 89) or the CHW+PL intervention (n = 60) groups.
Figure 1.
Consolidated Standards of Reporting Trials flow diagram.
EUC
The EUC group received a 2-h class conducted by a research assistant covering how to interpret their clinical and anthropometric results. EUC participants were contacted once each month to update contact information.
CHW-Led DSME
Three trained CHWs conducted activities during the initial 6 months of the intervention period. The CHWs were all Spanish-fluent Latinas who had completed high school or had a GED and were recruited from the southwest Detroit community. The CHWs underwent >160 h of CHW training, >80 h of diabetes education, including home visit experiences, and training in human subjects protocols, behavior modification strategies, cultural competency, and CPBR principles. CHWs were trained in empowerment-based approaches to inform their approach to each component of the intervention (19), including motivational interviewing (20), which is used to elicit participants’ goals and help participants formulate their own action plans.
During the 6-month intervention, CHWs conducted 1) DSME classes, 2) two 60-min home visits each month, and 3) one clinic visit with the participant and his or her primary care provider. The diabetes self-management classes, Journey to Health/El Camino a la Salud, were culturally tailored group classes in both English and Spanish (8,16). Eleven 2-h group sessions of 8–10 participants were held every 2 weeks at community locations. The development, implementation, and evaluation of these curricula are described in depth elsewhere (16). CHWs used the empowerment approach to diabetes education by eliciting participants’ experiences and requests for information (17). CHWs also helped participants improve communication skills with their providers and facilitated necessary referrals to other service systems. During home visits, and phone calls every 2 weeks, CHWs helped participants set goals using the five-step goal-setting model, which included 1) exploring a participant-identified problem, 2) discussing the emotional impact of the problem, 3) selecting a self-management goal, 4) developing an action plan, and 5) executing and evaluating the action plan (21).
CHW+PL
The PLs were recruited by the CHWs among patients with diabetes who had previously successfully completed the Journey to Health/Camino a la Salud program. They completed 46 h of training led by the CHWs over 12 weeks (11). Besides the initial training, the CHWs led monthly booster support sessions with the PLs and provided them with ongoing supervision.
All participants in the PL intervention completed the CHW intervention from baseline to 6 months and then were randomized to participate in the PL intervention. Adapted from the Lifelong Management program of Tang et al. (22), the PL intervention was designed to provide patients with ongoing emotional and behavioral support through weekly drop-in group-based sessions and follow-up telephone contacts from 6 to 18 months. Participants were invited to attend weekly group diabetes self-management sessions as often as needed. Based on patient-empowerment principles, discussion topics were driven by patients’ self-identified priorities, questions, and concerns (19). The PLs sought to complete the following five tasks at each session: discuss recent self-management challenges, share feelings about these challenges and other aspects of living with diabetes, engage in group-based problem solving, address questions about diabetes and its care, and set self-management goals. The PLs helped participants set goals using the same five-step goal-setting model described above. PLs also provided support to participants by discussing psychosocial concerns, identifying facilitators and barriers to behavior change, taking inventory of support sources, and developing strategies to navigate the health care system.
To ensure regular contact with each participant, PLs made a telephone support call to any participant who had not attended a session over 3 consecutive weeks. During the telephone support calls, PLs facilitated a conversation that closely mirrored support activities conducted in the group setting.
CHW-Only
After the 6-month intervention, participants randomized to this group received monthly telephone calls from a CHW who had led their DSME group to check in and assess continued progress in setting and meeting diabetes care goals.
All Participants
Information about community activities that were free and publicly available was provided to all study participants, who all were receiving ongoing health care at CHASS.
Study Measures
The primary clinical outcome was HbA1c, measured with a Bayer DCA 2000+ Analyzer (23). This assay has a test coefficient of variation <5% as required by the National Diabetes Data Group. Secondary clinical outcomes included a lipid panel (total cholesterol, LDL cholesterol [LDLc], and HDL cholesterol [HDLc]), using the Cholestech LDX (Cholestech, Hayward, CA) point-of-care machine that meets National Cholesterol Education Program guidelines for measuring lipid levels (24). Systolic and diastolic blood pressures were taken with two readings on a Welch Allyn Speidel & Keller sphygmomanometer; the averaged readings were used in the analysis. All participants were weighed on an EverWeigh lithium digital scale. Height and waist circumference were measured by the same technician at each time point. BMI was calculated as weight in kilograms divided by the square of height in meters. Waist circumference was measured at the umbilical waist using the Tech-Med model (cat. no. 4414) measuring tape.
We assessed diabetes-related distress using the Diabetes Distress Scale, a 17-item instrument that assesses emotional distress and functioning specific to living with diabetes, with higher scores indicating higher levels of distress (25). We assessed diabetes-specific social support with an adapted version of the Diabetes Support Scale, a six-item instrument that assesses perceived social support as it relates to meeting emotional needs, seeking advice, and obtaining information, with higher scores indicating more support (26). Depressive symptoms were assessed with the Patient Health Questionnaire-9 (27). Understanding of diabetes self-management was computed from 16 questions from the Diabetes Care Profile (28,29).
Statistical Methods
All baseline characteristics were compared between the intervention groups and EUC group with the Fisher F test for one-way ANOVA (30). The log-rank test was used to compare “diabetes duration (years)” between the two groups (31). Categorical variables were compared between groups with the Pearson χ2 test or with the Fisher exact test for rare outcomes.
Participants were analyzed as part of their original group assignments. The outcomes were evaluated for intervention effects by using linear mixed models (LMMs) to account for repeated measures. Because education differed significantly between the treatment groups, all outcome analyses were adjusted with a binary indicator for high school graduation.
The outcomes in the LMMs were measurements at baseline and at 6, 12, and 18 months, with covariates of indicator variables for time (baseline, 6, 12, 18 months), group, group × time interaction, and education. Medication intensification was included for HbA1c and cholesterol but omitted for blood pressure because of minimal change in medications during the study period (32,33). To ensure that medication treatment intensification did not confound the intervention effect, HbA1c and lipids outcomes were analyzed with and without medication intensification (i.e., change in number or dose of medicine). Changes in medications were calculated from baseline to 6 months, 6 to 12 months, and 12 to 18 months.
Changes in physical and psychosocial outcomes (and 95% CI) from baseline to each follow-up time point were estimated for the intervention and control groups by post hoc contrasts. From baseline to 6 months, there were two treatment groups, EUC and CHW. After 6 months, the CHW group split into the CHW-only and CHW+PL groups. Thus, estimates after 6 months compared the EUC, CHW-only, and CHW+PL groups. To check for multiple comparisons, all P values for the contrasts were double-checked by using Monte Carlo simulation (34). For each outcome analysis, we included all available data, consistent with the intent-to-treat principle. No demographic or baseline outcome measures differed significantly by whether HbA1c was missing at the 18-month follow-up. All significance tests were two-tailed using α = 0.05. SAS 9.4 software was used for all analyses.
To ensure that missing data were not biasing the results, all outcomes were analyzed with all available data and by using multiple imputations with chained equations (35). Twenty imputations were included to obtain >99% relative efficiency, with no changes in results (36).
Results
Table 1 presents the baseline characteristics of the study participants. Educational status differed by treatment group and was therefore included as a covariate in outcome analyses, coded as a binary indicator for high school graduation. No physical or psychosocial outcomes significantly differed between groups at baseline.
Table 1.
Baseline characteristics of REACH Detroit, cohort 3
| Group |
Total | P value for between groups | |||
|---|---|---|---|---|---|
| EUC | CHW-only | CHW+PL | |||
| Characteristic | (n = 73) | (n = 89) | (n = 60) | (N = 222) | |
| Age (years) | 48.5 (10.0) | 48.2 (10.7) | 50.2 (11.1) | 48.9 (10.6) | 0.488a |
| Female, n (%) | 49 (67.1) | 54 (60.7) | 32 (53.3) | 135 (60.8) | 0.269b |
| High school graduate, n (%) | 32 (43.8) | 19 (21.3) | 17 (28.3) | 68 (30.6) | 0.008b |
| Employed full or part time, n (%) | 32 (43.8) | 37 (41.6) | 26 (43.3) | 95 (42.8) | 0.954b |
| Antihyperglycemic medication, n (%) | |||||
| No medication | 3 (4.1) | 4 (4.5) | 3 (5.0) | 10 (4.5) | 0.839c |
| Only oral diabetes medication | 49 (67.1) | 64 (71.9) | 45 (75.0) | 158 (71.2) | |
| Insulin, with or without medication | 21 (28.8) | 21 (23.6) | 12 (20.0) | 54 (24.3) | |
| Physiological measures | |||||
| HbA1c (NGSP %) | 7.7 (1.8) | 7.7 (1.7) | 8.2 (2.2) | 7.8 (1.9) | 0.136a |
| LDLc (mg/dL) | 95.4 (28.9) | 92.2 (29.8) | 102.1 (35.3) | 95.8 (31.2) | 0.219a |
| HDLc (mg/dL) | 37.6 (11.6) | 40.7 (13.6) | 40.5 (16.8) | 39.6 (13.9) | 0.347a |
| BP (mmHg) | |||||
| Systolic | 133.3 (15.8) | 131.7 (17.5) | 134.8 (17.8) | 133.1 (17.0) | 0.550a |
| Diastolic | 80.1 (10.9) | 78.9 (10.0) | 81.2 (10.1) | 79.9 (10.3) | 0.402a |
| BMI (kg/m2) | 32.3 (5.4) | 33.9 (7.3) | 33.1 (7.6) | 33.2 (6.8) | 0.299a |
| Waist circumference (inches) | 39.5 (5.4) | 40.9 (5.6) | 41.8 (6.3) | 40.7 (5.8) | 0.071a |
| Psychological measures | |||||
| DDSd | 2.0 (1.0) | 2.2 (1.1) | 2.0 (1.0) | 2.1 (1.0) | 0.657a |
| DSSe | 4.0 (1.1) | 4.3 (1.0) | 4.0 (1.2) | 4.1 (1.1) | 0.098a |
| PHQf | 4.8 (4.3) | 5.8 (5.2) | 6.5 (6.1) | 5.7 (5.2) | 0.187a |
| DCPg | 2.8 (0.9) | 2.8 (0.8) | 2.9 (0.8) | 2.8 (0.8) | 0.673a |
Data are mean (SD) unless otherwise indicated. BP, blood pressure; DCP, Diabetes Care Profile; DDS, Diabetes Distress Scale; DSS, Diabetes Support Scale; PHQ, Patient Health Questionnaire.
Bold values are statistically significant (P < 0.05).
aF test for equal means.
bPearson χ2 test.
cFisher exact test.
dDDS: <2, little or no distress; 2 to <3, moderate distress; ≥3, high distress.
eDSS: 1 = strongly disagree to 6 = strongly agree.
fInterpretation of depression level from the PHQ: 1–4, minimal; 5–9, mild; 10–14, moderate; 15–19, moderately severe; 20–27, severe.
gDCP, which measures understanding of diabetes self-management: 1 = poor to 5 = excellent.
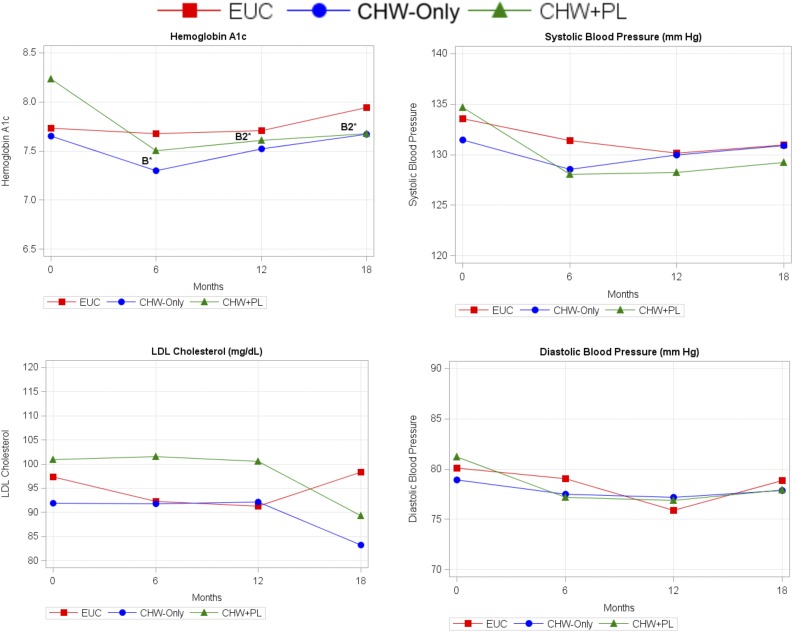
Figure 2 shows change in HbA1c levels over time. Among participants receiving the CHW intervention, mean HbA1c decreased by −0.51% (95% CI −0.75, −0.26; P < 0.001) from baseline to 6 months. A significant intervention effect was demonstrated compared with EUC (0.45% [95% CI −0.87, −0.03]; P < 0.05). From 6 to 12 months, improvements in HbA1c were sustained for participants randomized to the CHW+PL group (−0.63% [95% CI −1.06, −0.19]; P < 0.01) but not the CHW-only or the EUC groups. The intervention effect for the CHW+PL group at 12 months continued to be significant (−0.60% [95% CI −1.18, −0.01]; P < 0.05). From 12 to 18 months, the CHW+PL group maintained reductions in HbA1c (−0.56% [95% CI −1.06, −0.05]; P < 0.05), and the intervention effect at 18 months was also significant (−0.76% [95% CI −1.48, −0.05]; P < 0.05). HbA1c results were similar after adjusting for medication intensification.
Figure 2.
Trajectory of physical outcomes over time from LMM. Mean estimates from LMM. Covariates include time point, treatment group, interaction between time and treatment group, and high school education. B, significant intervention effect relative to EUC (control) group and CHW-combined (CHW-only and CHW+PL groups) at 6 months; B1, significant intervention effect for CHW-only group at 12 or 18 months; B2, significant intervention effect for CHW+PL group at 12 or 18 months. *P < 0.05.
A different pattern was apparent for LDLc and blood pressure levels. There were no differences in LDLc levels among groups from baseline to 6 and to 12 months. At 18 months, significant decreases in LDLc from baseline in the CHW+PL group (−12.3 mg/dL [95% CI −23.1, −1.6]; P < 0.05) were observed. However, the intervention effect for LDLc was not significant. Intervention effects were not found for HDLc, total cholesterol, or BMI. Similarly, no intervention effects were found for blood pressure; only within-group differences were observed over time. No intervention effects were observed for waist circumference or the waist-to-hip ratio.
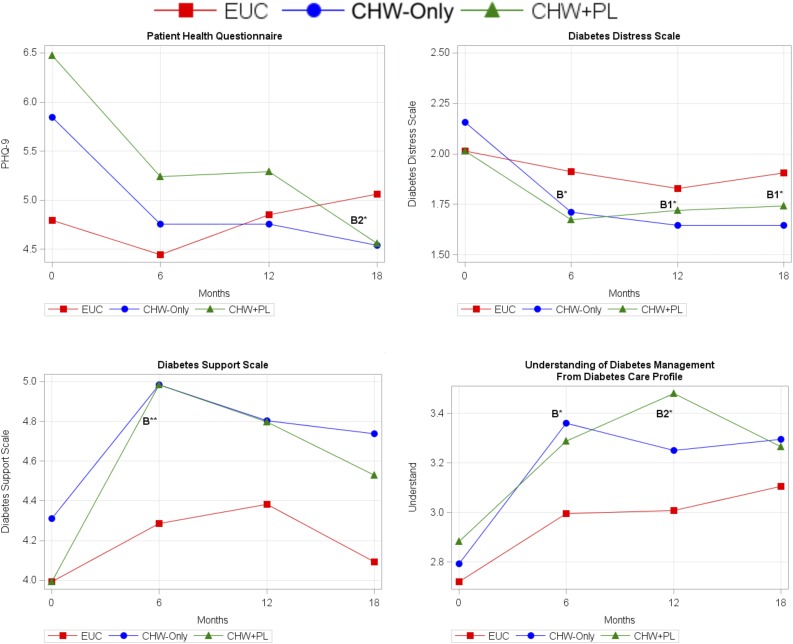
Figure 3 shows the mean estimates over time from LMMs for the psychosocial outcomes. Depressive symptoms significantly decreased for participants receiving the CHW intervention from baseline to 6 months (−1.1 points [95% CI −1.9, −0.4]; P < 0.01), but no intervention effect was observed. At 18 months, the CHW-only and the CHW+PL groups both maintained the reductions in depression symptoms compared with baseline. At 18 months, only the CHW+PL group had a significant intervention effect compared with EUC (−2.2 [95% CI −4.1, −0.3]; P < 0.05).
Figure 3.
Trajectory of psychological outcomes over time from LMM. Mean estimates from LMM. Covariates include time point, treatment group, interaction between time and treatment group, and high school education. PHQ-9, Patient Health Questionnaire-9. B, significant intervention effect relative to EUC (control) group and CHW-combined (CHW-only and CHW+PL groups) at 6 months. B1, significant intervention effect for CHW-only group at 12 or 18 months; B2, significant intervention effect for CHW+PL group at 12 or 18 months. *P < 0.05; **P < 0.01.
Diabetes-related distress significantly decreased from baseline to 6 months for participants receiving the CHW intervention (−0.4 points [95% CI −0.6, −0.2]; P < 0.001), with an intervention effect of −0.3 (95% CI −0.6, −0.03; P < 0.05). Improved distress scores were maintained at 12 and 18 months for the CHW-only and the CHW+PL groups. In addition, intervention effects were found at 12 months (−0.3 [95% CI −0.6, −0.01]; P < 0.05) and 18 months (−0.4 [95% CI −0.7, −0.1]; P < 0.05) for the CHW-only group but not for the CHW+PL group.
From baseline to 6 months, diabetes-related support increased significantly for both the EUC (0.3 points [95% CI 0.01, 0.6]; P < 0.05) and the CHW group (0.8 [95% CI 0.6, 1.0]; P < 0.001). However, the CHW group had a significantly higher increase than the EUC group, with an intervention effect of 0.5 (95% CI 0.2, 0.8; P < 0.01). There were no significant differences across groups for the later time periods.
The EUC and CHW groups significantly improved in their understanding of diabetes management from baseline to 6 months, with a significant intervention effect for the CHW group relative to EUC (0.2 points [95% CI 0.01, 0.4]; P < 0.05). The EUC, CHW-only, and the CHW+PL groups each maintained improvement in the understanding of diabetes management at 12 and 18 months. An intervention effect was found in the CHW+PL group (0.3 [95% CI 0.1, 0.6]; P < 0.05) compared with EUC at 12 months but not in the CHW-only group compared with EUC (0.2 [95% CI −0.1, 0.4]). However, there was no significant intervention effect at 18 months. None of the significant P values lost significance after being recomputed with Monte Carlo simulation.
Conclusions
This study found a significant intervention effect for HbA1c among low-income, urban Latino adults with T2D, with decreases at 6 months among participants receiving the CHW DSME intervention. Participants in the CHW+PL group maintained these improvements at 12 and 18 months. Although the CHW+PL group sustained significant improvements in LDLc and in systolic and diastolic blood pressure at 18 months, differences between groups in these clinical measures were not significant. The CHW+PL group had a significant intervention effect on reducing depressive symptoms at 18 months. Diabetes-related distress decreased significantly for CHW intervention participants at 6 months, with intervention effects at both 12 and 18 months for the CHW-only group. Diabetes-related support had a significant intervention effect at 6 months. Understanding of diabetes management showed a significant intervention effect at 6 months, and the intervention effect was sustained to 12 months in the CHW+PL group.
These findings build on prior research in several key ways. Most significantly, the study confirms previous research by the REACH Detroit Partnership on the effectiveness of the culturally tailored, CHW-led DSME program immediately after the intervention at 6 months (8–18,37). A strength of this study is that it extends those findings by demonstrating that clinical and psychosocial outcomes can be sustained to 18 months through the ongoing support of PLs trained and supervised by CHWs compared with usual care. Although CHW diabetes interventions have demonstrated varying degrees of short-term success (5), very few studies have examined outcomes for an 18-month period. There is overwhelming evidence that for many adults with diabetes, gains achieved through short-term diabetes self-management programs are not sustained without ongoing support (38,39). The combination of CHW and PL services after an empowerment-based DSME provides an efficient and low-cost means for continued support for diabetes self-management.
This study reinforces the importance of examining outcomes beyond the conclusion of short-term diabetes self-management education programs. Although HbA1c, diabetes-related distress, diabetes-related support, and self-management knowledge improved immediately after the 6-month CHW intervention, LDLc and depressive symptom outcomes did not show a significant intervention effect until 18 months. It is important to note that our intervention did not target LDLc or depression as primary outcomes, although information on both was provided through the curriculum. The intervention also did not target weight loss, which may account for the nonsignificant results for BMI and waist circumference. Increasing education and support for these outcomes as part of our intervention could improve these outcomes sooner and more effectively. Improvements at 18 months are encouraging for LDLc and depressive symptoms.
The current study also demonstrates that a linked CHW+PL intervention, with CHWs providing initial and monthly booster training and supervision for PLs, can be a successful model. We did not include an arm that received only PL services. Although this could be considered a limitation, the comparison of these two nonprofessional interventions for DSME was not the intent of this study. Rather, we found a cooperative model using CHWs and PLs was effective in sustaining gains achieved through a more intensive, short-term DSME program led by CHWs. This is potentially a scalable and sustainable model for health care centers in low-resource settings and provides volunteer opportunities for patients who successfully complete CHW-led DSME programs and would like to support other patients grappling with diabetes.
Several limitations are notable. First, Latino participants were recruited from one federally qualified health center in southwest Detroit. Thus, the generalizability of this study is limited to this population. Second, psychological and behavioral measures were self-reported. Third, we experienced attrition in our sample through 18 months. Although expected, the reduced sample size may have affected our ability to detect some statistically significant results. Future studies should consider multisite or national randomized controlled trials with larger sample sizes with representation from the diverse populations of Latinos in the U.S. Fourth, as with other interventions, those willing to participate likely differ significantly from nonparticipants, with nonparticipants often having worse clinical measures. We also excluded patients less likely to be able to participate in the intervention, further limiting generalizability.
Notwithstanding these limitations, our findings provide further evidence for culturally appropriate, theory-based CHW and PL interventions aimed at improving diabetes self-management among Latinos with T2D. The study offers encouragement for underresourced health centers seeking to provide effective services to low-income Latino communities. Our findings also demonstrate the feasibility of conducting rigorous research involving low-income communities of color using CBPR principles and methods. Finally, our study contributes to the literature on the need for expanded and sustained CHW programs and the hybrid CHW+PL model that we tested. The spread of these models has been slowed by inadequate and unstable funding. Policy changes supporting sustainable funding and reimbursement models for CHW and PL programs, as well as ongoing research on the cost effectiveness of these programs, could lead to a greater reach in communities that seek to provide culturally appropriate care and achieve health equity.
Article Information
Acknowledgments. The authors thank the REACH Detroit Partnership and the dedicated staff at CHASS, including the outstanding CHWs and PLs. The authors thank the patients who participated in this intervention.
Funding. This research was supported by a Peers for Progress grant from the American Association of Family Physicians Foundation, by the National Institute of Diabetes and Digestive and Kidney Diseases (grant P30-DK092926 to the Michigan Center for Diabetes Translational Research and grant R18-DK-0785501A1), and by the Centers for Disease Control and Prevention (cooperative agreement no. U50/CCU417409). The funding sources had no role in the study design; data collection; administration of the interventions; analysis, interpretation, or reporting of data; or decision to submit the findings for publication.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. M.S.S., E.C.K., and M.H. contributed to study conception and design, data analysis and interpretation, and manuscript preparation and were the principal investigators. B.S. contributed to study conception and design, data collection, analysis, and interpretation, and manuscript preparation. G.Pi., J.H., A.L., and N.E. contributed to study conception and design, data analysis and interpretation, and manuscript preparation. G.Pa. contributed to study conception and design, data collection, study implementation, and manuscript preparation. T.T. and M.F. contributed to study conception and design of the PL intervention. M.S.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the American Public Health Association Annual Meeting and Expo, Denver, CO, 29 October–2 November 2016.
Footnotes
Clinical trial reg. no. NCT00800410, clinicaltrials.gov.
References
- 1.Piette JD, Glasgow R. Strategies for improving behavioral health outcomes among patients with diabetes: self-management, education. In Evidence-Based Diabetes Care. Gerstein HC, Haynes RB, Eds. Hamilton, Ontario, Canada, BC Decker, 2001, pp. 207–251 [Google Scholar]
- 2.Haas L, Maryniuk M, Beck J, et al.; 2012 Standards Revision Task Force . National standards for diabetes self-management education and support. Diabetes Care 2012;35:2393–2401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Norris SL, Chowdhury FM, Van Le K, et al. Effectiveness of community health workers in the care of persons with diabetes. Diabet Med 2006;23:544–556 [DOI] [PubMed] [Google Scholar]
- 4.Palmas W, Findley SE, Mejia M, et al. Results of the northern Manhattan diabetes community outreach project: a randomized trial studying a community health worker intervention to improve diabetes care in Hispanic adults. Diabetes Care 2014;37:963–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim K, Choi JS, Choi E, et al. Effects of community-based health worker interventions to improve chronic disease management and care among vulnerable populations: a systematic review. Am J Public Health 2016;106:e3–e28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah M, Kaselitz E, Heisler M. The role of community health workers in diabetes: update on current literature. Curr Diab Rep 2013;13:163–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American Public Health Association. Community health workers [article online]. Available from https://www.apha.org/apha-communities/member-sections/community-health-workers. Accessed 22 March 2017
- 8.Spencer MS, Rosland AM, Kieffer EC, et al. Effectiveness of a community health worker intervention among African American and Latino adults with type 2 diabetes: a randomized controlled trial. Am J Public Health 2011;101:2253–2260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baig AA, Benitez A, Locklin CA, et al.; Little Village Community Advisory Board . Picture good health: a church-based self-management intervention among Latino adults with diabetes. J Gen Intern Med 2015;30:1481–1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Vries L, van der Heijden AA, van ’t Riet E, et al. Peer support to decrease diabetes-related distress in patients with type 2 diabetes mellitus: design of a randomised controlled trial. BMC Endocr Disord 2014;14:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang TS, Nwankwo R, Whiten Y, Oney C. Outcomes of a church-based diabetes prevention program delivered by peers: a feasibility study. Diabetes Educ 2014;40:223–230 [DOI] [PubMed] [Google Scholar]
- 12.Xue L. Effectiveness of a Peer Leader Supported Diabetes Self-Management Support Program on Patient Assessment of Care for Chronic Conditions (PACIC) Pittsburgh, University of Pittsburgh, 2014 [Google Scholar]
- 13.Thom DH, Ghorob A, Hessler D, De Vore D, Chen E, Bodenheimer TA. Impact of peer health coaching on glycemic control in low-income patients with diabetes: a randomized controlled trial. Ann Fam Med 2013;11:137–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang TS, Funnell M, Sinco B, et al. Comparative effectiveness of peer leaders and community health workers in diabetes self-management support: results of a randomized controlled trial. Diabetes Care 2014;37:1525–1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kieffer EC, Willis SK, Odoms-Young AM, et al. Reducing disparities in diabetes among African-American and Latino residents of Detroit: the essential role of community planning focus groups. Ethn Dis 2004;14(Suppl. 1):S27–S37 [PubMed] [Google Scholar]
- 16.Feathers JT, Kieffer EC, Palmisano G, et al. The development, implementation, and process evaluation of the REACH Detroit Partnership’s diabetes lifestyle intervention. Diabetes Educ 2007;33:509–520 [DOI] [PubMed] [Google Scholar]
- 17.Funnell MM, Anderson RM. Empowerment and self-management of diabetes. Clin Diabetes 2004;22:123–127 [Google Scholar]
- 18.Two Feathers J, Kieffer EC, Palmisano G, et al. Racial and Ethnic Approaches to Community Health (REACH) Detroit partnership: improving diabetes-related outcomes among African American and Latino adults. Am J Public Health 2005;95:1552–1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson RM, Funnell MM, Arnold MS. Using the empowerment approach to help patients change behavior. In Practical Psychology for Diabetes Clinicians. 2nd ed. Anderson B, Rubin RL, and Rubin RR, Eds. Alexandria, VA, American Diabetes Association, 2003, pp. 3–12 [Google Scholar]
- 20.Emmons KM, Rollnick S. Motivational interviewing in health care settings. Opportunities and limitations. Am J Prev Med 2001;20:68–74 [DOI] [PubMed] [Google Scholar]
- 21.Funnel MM, Anderson RM. Behavior change strategies. In Medical Management of Type 2 Diabetes. 5th ed. Burant CF, Ed. Alexandria, VA, American Diabetes Association, 2004, pp. 124–129 [Google Scholar]
- 22.Tang TS, Funnell MM, Noorulla S, Oh M, Brown MB. Sustaining short-term improvements over the long-term: results from a 2-year diabetes self-management support (DSMS) intervention. Diabetes Res Clin Pract 2012;95:85–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arsie MP, Marchioro L, Lapolla A, et al. Evaluation of diagnostic reliability of DCA 2000 for rapid and simple monitoring of HbA1c. Acta Diabetol 2000;37:1–7 [DOI] [PubMed] [Google Scholar]
- 24.Rogers EJ, Misner L, Ockene IS, Nicolosi RJ. Evaluation of seven Cholestech L.D.X analyzers for total cholesterol determinations. Clin Chem 1993;39:860–864 [PubMed] [Google Scholar]
- 25.Polonsky WH, Fisher L, Earles J, et al. Assessing psychosocial distress in diabetes: development of the diabetes distress scale. Diabetes Care 2005;28:626–631 [DOI] [PubMed] [Google Scholar]
- 26.Barrera M Jr, Glasgow RE, McKay HG, Boles SM, Feil EG. Do internet-based support interventions change perceptions of social support?: an experimental trial of approaches for supporting diabetes self-management. Am J Community Psychol 2002;30:637–654 [DOI] [PubMed] [Google Scholar]
- 27.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fitzgerald JT, Anderson RM, Gruppen LD, et al. The reliability of the Diabetes Care Profile for African Americans. Eval Health Prof 1998;21:52–65 [DOI] [PubMed] [Google Scholar]
- 29.Fitzgerald JT, Davis WK, Connell CM, Hess GE, Funnell MM, Hiss RG. Development and validation of the Diabetes Care Profile. Eval Health Prof 1996;19:208–230 [DOI] [PubMed] [Google Scholar]
- 30.Fisher RA. The Design of Experiments. Edinburgh, U.K., Oliver and Boyd, 1942 [Google Scholar]
- 31.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. 2nd ed. [Internet], 2011. Hoboken, NJ, John Wiley & Sons; Available from https://onlinelibrary.wiley.com/doi/book/10.1002/9781118032985. Accessed 24 March 2017 [Google Scholar]
- 32.Piatt GA, Anderson RM, Brooks MM, et al. 3-year follow-up of clinical and behavioral improvements following a multifaceted diabetes care intervention: results of a randomized controlled trial. Diabetes Educ 2010;36:301–309 [DOI] [PubMed] [Google Scholar]
- 33.Piatt GA, Orchard TJ, Emerson S, et al. Translating the chronic care model into the community: results from a randomized controlled trial of a multifaceted diabetes care intervention. Diabetes Care 2006;29:811–817 [DOI] [PubMed] [Google Scholar]
- 34.Edwards D, Berry JJ. The efficiency of simulation-based multiple comparisons. Biometrics 1987;43:913–928 [PubMed] [Google Scholar]
- 35.van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res 2007;16:219–242 [DOI] [PubMed] [Google Scholar]
- 36.Rubin DB. Multiple Imputation for Nonresponse in Surveys. Hoboken, NJ, John Wiley & Sons, 2004 [Google Scholar]
- 37.Wagner EH. The role of patient care teams in chronic disease management. BMJ 2000;320:569–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Norris SL, Lau J, Smith SJ, Schmid CH, Engelgau MM. Self-management education for adults with type 2 diabetes: a meta-analysis of the effect on glycemic control. Diabetes Care 2002;25:1159–1171 [DOI] [PubMed] [Google Scholar]
- 39.Norris SL, Engelgau MM, Narayan KM. Effectiveness of self-management training in type 2 diabetes: a systematic review of randomized controlled trials. Diabetes Care 2001;24:561–587 [DOI] [PubMed] [Google Scholar]