Abstract
OBJECTIVE
Lesbian and bisexual (LB) women are more likely than heterosexual women to exhibit risk factors for type 2 diabetes, but studies estimating the burden of type 2 diabetes among LB women are uncommon and limited to cross-sectional designs. This study investigated incidence of type 2 diabetes in LB women and heterosexual women in a large, longitudinal U.S. cohort.
RESEARCH DESIGN AND METHODS
Women participating in the Nurses’ Health Study II (NHS II) ages 24–44 years in 1989 were prospectively followed through 2013. Self-reported clinician diagnosis of type 2 diabetes was assessed every other year to identify incidence. Of the participants, 1,267 identified as lesbian or bisexual and 92,983 identified as heterosexual. Cox proportional hazards regression was used to model incidence of type 2 diabetes.
RESULTS
LB women had a 27% higher risk of developing type 2 diabetes than heterosexual women (adjusted incidence rate ratio [IRR] 1.27, 95% CI 1.05, 1.54). Differences between LB women and heterosexual women in risk of type 2 diabetes were greater during younger ages (sexual orientation–by-age interaction, P < 0.001). BMI mediated the relationship between sexual orientation and type 2 diabetes; the IRR was completely attenuated when BMI was added to the model (IRR 0.85, 95% CI 0.70, 1.03).
CONCLUSIONS
Findings indicate that LB women develop type 2 diabetes at younger ages than heterosexual women. Higher BMI in LB women is an important contributor to this disparity. Public health and clinical efforts to prevent, detect, and manage obesity and type 2 diabetes among LB women are warranted.
Introduction
Although the majority of lesbian and bisexual (LB) women living in the U.S. are no less healthy than their heterosexual female counterparts, the population of LB women has health disparities and unique health needs (1). For example, extensive research demonstrates that LB women are more likely than heterosexual women to experience mental health and substance use problems (2–4). Less extensive is research examining potential differences between LB women and heterosexual women in their risk of developing chronic physical health problems; results from such studies have been inconclusive thus far (5,6). For example, findings from cross-sectional studies investigating differences between LB women and heterosexual women in risk for type 2 diabetes have been mixed, with some studies finding differences by sexual orientation (7–9) and other studies finding no difference (10–15). Most studies detecting sexual orientation differences found higher risk for type 2 diabetes in LB women compared with heterosexual women (7,8).
Despite inconclusive findings, there is reason to suspect that LB women may have disparities in chronic physical health conditions, including type 2 diabetes, because they are more likely than heterosexual women to have risk factors such as obesity (16), tobacco smoking (3,16), heavy alcohol drinking (3,16), and stress-related exposures (e.g., discrimination, violence victimization, and psychological distress) (3,17–19). Consistent with findings from population-based studies, analyses with data from the Nurses’ Health Study II (NHS II), the longitudinal cohort examined in the current study, have found that LB women are more likely than heterosexual women to report obesity, cigarette smoking, heavy alcohol use, depression, and childhood victimization (20–22).
Minority stress is theorized to be a central reason why LB women are at elevated risk for physical health problems including type 2 diabetes (23). Minority stress, or the external (e.g., discrimination) and internal (e.g., expectations of rejection) stressors resulting from a socially stigmatized sexual orientation (18), can negatively affect coping, emotion regulation, and social, interpersonal, and cognitive processes (24). Both physiological and behavioral pathways link minority stress to type 2 diabetes risk. Regarding physiological pathways, high allostatic load, or the cumulative wear and tear on the body resulting from chronic exposure to stress, can increase risk for disease (25). Chronic stress is associated with type 2 diabetes through dysregulation of at least three biological pathways: the hypothalamic-pituitary-adrenal axis, the autonomic nervous system, and the inflammatory system (26). Dysregulation of the hypothalamic-pituitary-adrenal axis results in elevated cortisol, which leads to intra-abdominal adiposity and insulin resistance, both factors contributing to increased risk of type 2 diabetes. Also, overactivation of both the autonomic nervous system and the inflammatory response can lead to insulin resistance. Regarding behavioral pathways, exposure to minority stress may be associated with a greater likelihood of engaging in maladaptive coping behaviors such as cigarette smoking or stress-related eating, which may increase risk for type 2 diabetes (27–29).
According to an Institute of Medicine report published in 2011 (1), research is needed to understand how minority sexual orientation intersects with other sociodemographic factors to influence health. Age and rural residence are two such factors that could modify sexual orientation–related differences in risk for type 2 diabetes. Regarding age, exposure to minority stress and resulting risk factors for type 2 diabetes may be heightened during adolescence and young adulthood when LB women typically become aware of their minority sexual orientation (30). Thus, differences between LB women and heterosexual women in risk of developing type 2 diabetes may be accentuated during younger ages. In support of this hypothesis, research suggests that when age modifies relationships between minority sexual orientation and behavioral, mental, or physical health outcomes, disparities are larger during younger versus older ages (31,32). However, there is some inconsistency, as an analysis of the representative California Health Interview Survey (CHIS) found that compared with heterosexual women of similar age, lesbian women aged 60 years or older had elevated risk for type 2 diabetes, but lesbian women younger than aged 60 years did not (31). Regarding rural residence, LB women living in rural areas may have unique challenges related to minority stress that have the potential to exacerbate risk for type 2 diabetes. Although research is inconclusive, rural-residing LB women may have lower social support, less access to affirming health care providers, and less comfort disclosing their minority sexual orientation to others compared with LB women living in urban areas (1,33). A previous study found that rural-residing lesbian women had significantly higher BMIs than urban-residing lesbian women (34).
A limitation of previous studies investigating differences between LB and heterosexual women in risk for type 2 diabetes is a lack of longitudinal designs. Another limitation is that studies frequently had low power to detect statistically significant differences between LB women and heterosexual women. As a consequence, effect sizes in some studies have suggested potential elevated risk for type 2 diabetes in lesbian or bisexual women, but estimates were not statistically significant and crossed the null value (12,14). To address these limitations and build on the current literature, we analyzed longitudinal data from the NHS II to compare age-specific incidence of type 2 diabetes during ages 24–68 years among LB and heterosexual women. We also examined intersections of sexual orientation with age and rural living status to assess potential differences in type 2 diabetes risk by these factors. Lastly, because of the robust evidence that LB women have higher BMI than heterosexual women and BMI’s strong positive relationship with risk of type 2 diabetes, we examined its mediating influence in explaining the relationship between sexual orientation and type 2 diabetes.
Research Design and Methods
Study Population
The NHS II is a prospective cohort of female registered nurses established in 1989. Participants aged 24–44 years were recruited from 15 of the most populous states in the U.S. using state nursing boards. The baseline questionnaire was mailed to 517,000 women, of which 123,000 (24%) responded. Those who had incomplete surveys or were ineligible (including those who reported breast cancer) were excluded, yielding a total of 116,671 in the cohort. Biennial mailed questionnaires were used for follow-up. The follow-up rate has exceeded 90% for every 2-year period. Data collected from 1989 to 2013 comprised the analyses.
Analytic Sample
Participants were excluded from analyses if they had a diagnosis of diabetes before 1989, self-reported diabetes in 1989, reported a history of gestational diabetes in 1989, had type 1 diabetes, or reported type 2 diabetes without providing a date of diagnosis. Participants were also excluded if they did not report a heterosexual, lesbian, or bisexual sexual orientation. After exclusions, a total of 94,250 women remained in the analytic sample; 1,267 lesbian or bisexual women contributed 29,984 person-years and 92,983 heterosexual women contributed 2,230,488 person-years during ages 24–68 years to the analytic models.
Measures
Type 2 Diabetes
Participants were asked if and when they had been diagnosed with type 2 diabetes by a clinician on each biennial questionnaire. Participants reporting diabetes were mailed a supplementary questionnaire assessing symptoms, diagnostic tests, and hypoglycemic therapy to confirm self-reported diagnoses. As recommended (35), confirmation of diabetes required at least one of the following: 1) at least one classic symptom (e.g., excessive thirst, polyuria) plus fasting plasma glucose (PG) ≥126 mg/dL or random PG ≥200 mg/dL; or 2) at least two elevated PG levels on different occasions (fasting PG ≥126 mg/dL and/or random PG ≥200 and/or PG ≥200 at 2 h on oral glucose tolerance testing) in the absence of symptoms; or 3) treatment with hypoglycemic medication (insulin or oral hypoglycemic agent). This is a validated method of identifying type 2 diabetes in the NHS II, as assessed via a substudy that confirmed by medical record review more than 98% of self-reported diabetes cases (36). Because participants are nurses who virtually all have access to health care, prevalence of undiagnosed diabetes in the NHS II is rare. Another validation substudy found that only 1 out of 200 randomlyselected participants not previously reporting a diabetes diagnosis had an elevated fasting PG or plasma fructosamine level in the diabetic range (37).
Sexual Orientation
Participants identified their sexual orientation in 1995 and 2009 using the following question: “Whether or not you are currently sexually active, what is your sexual orientation or identity? (Please choose one answer).” Response options included “Heterosexual”; “Lesbian, gay, or homosexual”; “Bisexual”; “None of these”; and “Prefer not to answer.” Consistent with our previous work (38,39), reported sexual orientation in 2009 was used, except where there was missing information (i.e., not identifying as heterosexual, lesbian, or bisexual), in which case sexual orientation reported in 1995 was used. A sensitivity analysis using an alternative assignment of sexual orientation, where sexual orientation reported in 1995 was used for waves 1991 to 2007 and sexual orientation reported in 2009 was used for the 2011 and 2013 waves, was performed (data not shown). Results of two different sexual orientation categorizations were consistent, thus analyses based on most recent report of sexual orientation are presented. For this study, we also combined women reporting their sexual orientation as lesbian or bisexual into one category to increase the statistical power to detect differences by sexual orientation. Prior to collapsing lesbian and bisexual women into one category, preliminary analyses keeping these groups separate were performed. Because effect estimates were consistent for these groups, they were combined in analyses.
BMI
NHS II participants reported their height and weight at baseline and in each follow-up questionnaire, and those reports were used to calculate their BMI (kg/m2). For analysis, BMI was updated at each assessment and modeled as a continuous variable. Because BMI is a causal factor for type 2 diabetes, it was conceptualized and modeled as a mediator in the relationship between minority sexual orientation and type 2 diabetes.
Covariates
Age (continuous), family history of diabetes (assessed at baseline), race/ethnicity (non-Hispanic white vs. all other race/ethnicities), region of residence (Northeast, Midwest, South, and West), rural status (rural, defined as population density <500 people per square mile, vs. nonrural), menopausal status (premenopausal, postmenopausal and never used postmenopausal hormones, postmenopausal and previously used postmenopausal hormones, and postmenopausal and currently using postmenopausal hormones), and whether a participant visited a health care provider during the past 2 years were all included as potential confounders in analyses. Time-varying confounders (e.g., region of residence, menopausal status, visited health care provider during the past 2 years) were updated with each new data collection. Behaviors such as physical activity, diet, smoking, and alcohol use were not included as covariates because these factors are likely on the causal pathway between sexual orientation and incident type 2 diabetes. Inclusion of these factors as covariates would have yielded overadjustment bias (40).
Data Analysis
Distributions of the independent variables were computed for LB women and heterosexual women. Age-specific Cox proportional hazards regression models were then fit to examine the association between sexual orientation and incident type 2 diabetes. Study time for these models began in 1989 (baseline) and continued until age of type 2 diabetes diagnosis, death, loss to follow-up, or end of follow-up. The Wald test and likelihood ratio test were used to compute P values. We tested for departures from the proportional hazards assumption (i.e., effect modification by age) using likelihood ratio tests comparing models with and without age interaction terms. In addition to examining effect modification by age, we examined effect modification by rural living status using a sexual orientation–by-rural term in the model. To test whether BMI mediated the relationship between sexual orientation and type 2 diabetes, we compared estimates from models that included and excluded BMI updated at each assessment to assess for changes in model parameter estimates. Incidence rate ratios (IRR) and 95% CIs comparing LB women to heterosexual women are reported.
Analyses were conducted using SAS software, version 9.3, with a significance level of 0.05. Participants provided informed consent for this study, which was approved by the Institutional Review Board at Brigham and Women’s Hospital and the Human Subjects Committee at the Harvard T. H. Chan School of Public Health. Analyses for this study were performed between 2016 and 2018.
Results
Sociodemographic characteristics of the study sample are presented in Table 1. The average age of women during follow-up was about 46 years. The majority of women were non-Hispanic white and were premenopausal during most of the follow-up period. Compared with heterosexual women, LB women were less likely to have lived in the Midwest and in rural areas and they had a higher mean BMI.
Table 1.
Sociodemographic characteristics of heterosexual and LB women in the NHS II, 1989–2013
| Heterosexual | LB | |
|---|---|---|
| Sample size, n | 92,983 | 1,267 |
| Person-years, n | 2,230,488 | 29,984 |
| Age in years during follow-up | 45.7 (8.8) | 46.3 (8.8) |
| Non-Hispanic white | 95 | 95 |
| Region of residence | ||
| Northeast | 33 | 33 |
| Midwest | 33 | 22 |
| South | 19 | 18 |
| West | 15 | 27 |
| Rural status (population density <500 people/square mile) | 32 | 24 |
| Menopausal status | ||
| Premenopausal | 69 | 66 |
| Postmenopausal, never used postmenopausal hormones | 9 | 11 |
| Postmenopausal, previously used postmenopausal hormones | 12 | 13 |
| Postmenopausal, currently using postmenopausal hormones | 10 | 10 |
| Visited health care provider during past 2 years | 94 | 93 |
| Family history of diabetes | 15 | 16 |
| BMI, kg/m2 | 26.0 (5.8) | 27.5 (6.8) |
Data are mean (SD) or percent unless otherwise indicated.
During the follow-up period (1989–2013), 6,399 out of 94,250 women developed type 2 diabetes. Table 2 presents the IRR for type 2 diabetes comparing LB women to heterosexual women. Before adjusting for potential confounders, the crude IRR suggested that LB women had a 22% greater risk of developing type 2 diabetes than heterosexual women. After adjusting for family history of diabetes, race/ethnicity, region of residence, rural status, and menopausal status, LB women continued to have a significantly higher incidence of type 2 diabetes than heterosexual women (IRR 1.27, 95% CI 1.05, 1.54; P = 0.014).
Table 2.
Results of Cox proportional hazards regression models estimating the IRR for type 2 diabetes comparing LB women to heterosexual women in the NHS II, 1989–2013
| IRR (95% CI) |
P value | ||
|---|---|---|---|
| Heterosexual | LB | ||
| Crude | Referent group | 1.22 (1.01, 1.48) | 0.043 |
| Adjusted for confoundersa | Referent group | 1.27 (1.05, 1.54) | 0.014 |
| Adjusted for BMIb | Referent group | 0.85 (0.70, 1.03) | 0.091 |
aAdjusted for age, family history of type 2 diabetes, race/ethnicity, region of residence, rural status, menopausal status, and visit to health care provider in the past 2 years.
bAdjusted for confounders and updated BMI as a mediator.
Results of the mediation analysis shown in Table 2 indicated that the relationship between sexual orientation and type 2 diabetes was completely attenuated after BMI was added to the model. This finding suggests that higher BMI among LB women completely explained their greater incidence of type 2 diabetes.
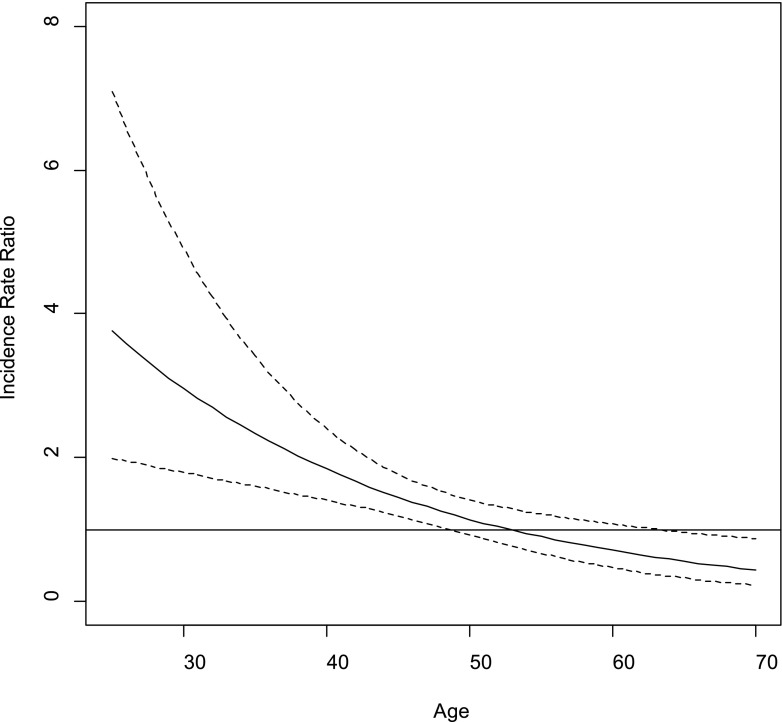
Figure 1 illustrates the sexual orientation–by-age interaction on incident type 2 diabetes. According to the figure, the incidence of type 2 diabetes for LB women was significantly higher than for heterosexual women until approximately age 50, after which risk among LB and heterosexual women became more similar. A statistical test of the sexual orientation–by-age interaction indicated it was significant (P = 0.0007). Results of age-stratified models are shown in Table 3. When women were younger than age 40, incidence of type 2 diabetes was more than two times higher among LB women compared with heterosexual women, whereas incidence was approximately 30% higher among LB women during ages 40–49 years. After age 50, incidence of type 2 diabetes was similar for both sexual orientation groups. Adjustment for BMI attenuated associations between sexual orientation and incidence of type 2 diabetes during ages 24–39 years and ages 40–49 years. A test of a sexual orientation–by-rural statistical interaction indicated that differences between LB women and heterosexual women in risk of type 2 diabetes were similar irrespective of rural status (sexual orientation–by-rural interaction, P = 0.21).
Figure 1.
Type 2 diabetes IRR plot for sexual orientation–by-age interaction. The solid line represents the IRR for type 2 diabetes comparing LB women to heterosexual women across age (in years). The dotted lines represent the 95% CI for the IRR.
Table 3.
Results of age-stratified Cox proportional hazards regression models estimating the IRR for type 2 diabetes comparing LB women to heterosexual women in the NHS II, 1989–2013
| IRR (95% CI) |
|||
|---|---|---|---|
| Ages 24–39 years | Ages 40–49 years | Ages 50–68 years | |
| Crude | 2.52 (1.60, 3.98) | 1.32 (0.99, 1.76) | 0.91 (0.67, 1.24) |
| Adjusted for confoundersa | 2.44 (1.54, 3.86) | 1.36 (1.02, 1.81) | 0.96 (0.70, 1.31) |
| Adjusted for BMIb | 1.46 (0.92, 2.33) | 0.90 (0.68, 1.20) | 0.67 (0.49, 0.92) |
Referent group is heterosexual women.
aAdjusted for age, family history of type 2 diabetes, race/ethnicity, region of residence, rural status, menopausal status, and visit to health care provider in the past 2 years.
bAdjusted for confounders and updated BMI as a mediator.
Conclusions
Findings from the NHS II cohort suggest that LB women have a higher incidence of type 2 diabetes than heterosexual women. Our study also found that differences between LB women and heterosexual women in risk of type 2 diabetes were greater during younger ages than older ages. When participants were ages 24–39 years, LB women had more than twice the risk of developing type 2 diabetes than heterosexual women, whereas risk for developing type 2 diabetes was similar for both groups of women by the time they reached age 50 years. In contrast, we did not find evidence that rural LB women were especially vulnerable to developing type 2 diabetes. Findings indicated that differences between LB women and heterosexual women in risk for developing type 2 diabetes were similar in rural and nonrural settings. Finally, our study found that greater BMI among LB women prospectively explained their elevated risk for type 2 diabetes. This finding helps to establish the critical role of obesity in contributing to LB women’s greater risk of developing type 2 diabetes.
Our study has important advantages compared with previous studies examining type 2 diabetes among LB women. First, prior studies have been limited to cross-sectional designs (7–15) that were only able to examine prevalence of disease. In contrast, our study followed women over a significant portion of adulthood to identify sexual orientation differences in the development of new cases of type 2 diabetes. Second, our study had greater statistical power than prior studies, which strengthened the ability to detect differences. Third, in contrast to prior studies, which had a significant portion of participants who were relatively young, our study followed a substantial proportion of women into their 60s. Because type 2 diabetes is a disease that develops with age, it may be difficult to detect sexual orientation–related differences in existing cross-sectional samples.
The results of this study have important clinical and public health implications. Clinically, type 2 diabetes is a complex condition requiring careful management, which can include lifestyle therapy, medication adherence, and personal monitoring (41). Most complications related to type 2 diabetes (e.g., retinopathy, neuropathy, and cardiovascular disease) are dependent on the duration of time people live with the disease and poor disease management (42). Given the significantly higher risk of developing type 2 diabetes before age 50 years among LB women, and their potentially longer duration of living with type 2 diabetes, LB women may also be more likely to experience complications compared with heterosexual women. However, research on potential sexual orientation–related differences in disease management is lacking and there exists no information on the degree that typically prescribed therapies and interventions are effective for LB women. Additional research on the health of LB women after diagnosis is vital to understanding the implications of disease on their subsequent health risks and prognosis. Research is also needed to aid in developing effective clinical practices for treatment of type 2 diabetes in this population.
A related issue that may affect LB women’s detection and management of type 2 diabetes includes concerns around access to care. Research has demonstrated unique challenges related to sexual orientation stigma that hinder LB women from accessing health care (43), which can adversely impact diabetes diagnosis and management. Compared with general population–based surveys, which had mixed findings with regard to sexual orientation–related differences in type 2 diabetes prevalence, our study found significantly higher incidence among LB women. This discrepancy could be due to high levels of health care access in our study population, which may have resulted in better detection of type 2 diabetes among women in the NHS II compared with women in the general population. Improving health care access may help improve data quality by reducing nondetection bias. Greater access may also improve disease management and prevention of type 2 diabetes complications for LB women.
Findings from this study underscore the importance of developing public health prevention efforts for LB women. Given the large societal and personal costs of type 2 diabetes, and the clinical burden of managing this disease, there is a need to develop and test intervention strategies for LB women to prevent and address risk factors for type 2 diabetes including obesity, stress, and behavioral factors. Similar to studies of women in general, a cross-sectional study found that sexual minority women who exhibited sufficient physical activity and healthy eating habits had lower risk for obesity, chronic conditions, and poor quality of life than those exhibiting less favorable behaviors (44). Although it is important to address behavioral factors such as physical activity, sedentary behavior, and dietary intake, focusing on these factors alone may not be sufficient to eliminate LB women’s disparities in chronic disease. In the NHS II cohort, LB women reported healthier diet quality (38) and greater physical activity (39) than heterosexual women. Although LB women in NHS II also reported greater sedentary behavior (e.g., sitting time) (39), it does not appear that behavioral factors alone explain LB women’s greater BMI and greater incidence of type 2 diabetes. Given the detrimental health impacts of minority stress, it may also be necessary to address societal stigma and psychosocial stress.
Our study identified obesity as a potentially modifiable factor contributing to LB women’s elevated incidence of type 2 diabetes. Because LB women’s greater risk of obesity begins early in the life span (45), efforts to prevent high BMI beginning in adolescence should be prioritized. In addition, although studies suggest weight loss is difficult to achieve and maintain (46), intensive behavioral interventions containing evidence-based strategies to improve dietary intake and promote physical activity have been found to reduce obesity and improve fitness (47). Nonetheless, the extent to which behavioral interventions developed for the general population are effective for LB women is unknown, and few interventions to promote healthy weight and fitness have been developed specifically for LB women. A notable exception is a multisite collaborative of researchers and community organizations funded by the Office of Women’s Health that developed and pilot tested five 12- to 16-week-long interventions tailored for LB women over age 40 years (48). Although the specific components varied by site, each intervention addressed four Institute of Medicine recommendations: increasing physical activity, promoting healthy dietary intake, promoting limited alcohol intake, and reducing consumption of sugar-sweetened beverages. Evaluation of these programs found that 58% of participants achieved at least 3 out of 9 health objectives, and improvements in physical activity and waist-to-height ratio were observed in some program participants relative to those in a comparison group (49). The authors concluded that tailored programs can improve weight-related outcomes among LB women. Results of the current study as well as other studies (31,32) suggest efforts should also focus on LB women younger than age 40 years given their disproportionate burden of experiencing health disparities in multiple domains.
Our study is not without its shortcomings. Because the NHS II comprises professional nurses who are primarily non-Hispanic white, findings may not generalize to ethnic or racial minority women or women in low or high socioeconomic positions. Additional research using longitudinal data from diverse, population-based samples are needed to better understand disease risk among sexual minority women. Research is also needed to identify the influence of other modifiable risk and protective factors, including dietary intake, sedentary behaviors, physical activity, and minority stress factors, in contributing to LB women’s differential risk of developing type 2 diabetes. Although the NHS II has collected information on some of these modifiable factors, the inability to examine their influences in the current study represents a limitation. Another limitation of our study is that measures were based on self-report; therefore, measurement error and misclassification are possible. It is also possible that not all confounders were measured, which could lead to bias in estimates related to uncontrolled confounding. Also, further confirmation of the observation of effect modification by age with other data sources would lend evidence to this study’s finding. However, our study has important strengths. One advantage is that women were not recruited based on their sexual orientation, which presumably results in less selection bias than studies sampling participants from lesbian, gay, bisexual, and transgender community settings. In addition, cohort retention is high, as more than 90% of women continue to participate, which likely results in low bias from attrition.
In summary, the elevated incidence of type 2 diabetes among LB women, particularly at younger ages, is alarming and highlights the critical need to expand prevention efforts, screening and detection, and clinical management to this population impacted by health disparities. Additional work is needed to identify appropriate mechanisms for intervention and to continue refining and testing tailored approaches. A better understanding of disease management challenges LB women experience after diagnosis is also needed to help reduce the clinical burden of type 2 diabetes complications. Lastly, expanding efforts by the public health and medical professions to address the detrimental health impacts of social stigma and minority stress may lead to improved health and wellness among LB women.
Article Information
Acknowledgments. The authors thank the women participating in the NHS II who provided the information about their lives enabling this study to be carried out. The authors also thank Donna Spiegelman, Harvard T.H. Chan School of Public Health, for providing guidance on statistical analysis.
Funding. This study was supported by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases through award numbers R01DK099360 and R01DK1129401 and the National Cancer Institute through UM1CA176726.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. H.L.C. conceived the study and obtained funding. H.L.C. and N.A.V. wrote the manuscript. H.-J.J. and B.H. conducted data analysis. M.W. supervised data analysis. H.L.C., N.A.V., S.B.A., and F.B.H. edited the manuscript. All authors reviewed and approved the manuscript. H.L.C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article is featured in a podcast available at http://www.diabetesjournals.org/content/diabetes-core-update-podcasts.
References
- 1.Institute of Medicine. The Health of Lesbian, Gay, Bisexual, and Transgender People: Building a Foundation for Better Understanding. Washington, DC, The National Academies Press, 2011. [PubMed] [Google Scholar]
- 2.Bostwick WB, Boyd CJ, Hughes TL, McCabe SE. Dimensions of sexual orientation and the prevalence of mood and anxiety disorders in the United States. Am J Public Health 2010;100:468–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gonzales G, Przedworski J, Henning-Smith C. Comparison of health and health risk factors between lesbian, gay, and bisexual adults and heterosexual adults in the United States: results from the National Health Interview Survey. JAMA Intern Med 2016;176:1344–1351 [DOI] [PubMed] [Google Scholar]
- 4.McCabe SE, Hughes TL, Bostwick WB, West BT, Boyd CJ. Sexual orientation, substance use behaviors and substance dependence in the United States. Addiction 2009;104:1333–1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eliason MJ. Chronic physical health problems in sexual minority women: review of the literature. LGBT Health 2014;1:259–268 [DOI] [PubMed] [Google Scholar]
- 6.Simoni JM, Smith L, Oost KM, Lehavot K, Fredriksen-Goldsen K. Disparities in physical health conditions among lesbian and bisexual women: a systematic review of population-based studies. J Homosex 2017;64:32–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diamant AL, Wold C, Spritzer K, Gelberg L. Health behaviors, health status, and access to and use of health care: a population-based study of lesbian, bisexual, and heterosexual women. Arch Fam Med 2000;9:1043–1051 [DOI] [PubMed] [Google Scholar]
- 8.Dilley JA, Simmons KW, Boysun MJ, Pizacani BA, Stark MJ. Demonstrating the importance and feasibility of including sexual orientation in public health surveys: health disparities in the Pacific Northwest. Am J Public Health 2010;100:460–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ward B, Dahlhamer J, Galinsky A, Joestl S. Sexual Orientation and Health Among U.S. Adults: National Health Interview Survey, 2013. Hyattsville, MD, National Center for Health Statistics, 2014 [PubMed] [Google Scholar]
- 10.Blosnich JR, Farmer GW, Lee JGL, Silenzio VMB, Bowen DJ. Health inequalities among sexual minority adults: evidence from ten U.S. states, 2010. Am J Prev Med 2014;46:337–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cochran SD, Mays VM. Physical health complaints among lesbians, gay men, and bisexual and homosexually experienced heterosexual individuals: results from the California Quality of Life Survey. Am J Public Health 2007;97:2048–2055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conron KJ, Mimiaga MJ, Landers SJ. A population-based study of sexual orientation identity and gender differences in adult health. Am J Public Health 2010;100:1953–1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farmer GW, Jabson JM, Bucholz KK, Bowen DJ. A population-based study of cardiovascular disease risk in sexual-minority women. Am J Public Health 2013;103:1845–1850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fredriksen-Goldsen KI, Kim HJ, Barkan SE, Muraco A, Hoy-Ellis CP. Health disparities among lesbian, gay, and bisexual older adults: results from a population-based study. Am J Public Health 2013;103:1802–1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garland-Forshee RY, Fiala SC, Ngo DL, Moseley K. Sexual orientation and sex differences in adult chronic conditions, health risk factors, and protective health practices, Oregon, 2005–2008. Prev Chronic Dis 2014;11:E136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzales G, Henning-Smith C. Health disparities by sexual orientation: results and implications from the Behavioral Risk Factor Surveillance System. J Community Health 2017;42:1163–1172 [DOI] [PubMed] [Google Scholar]
- 17.Mays VM, Cochran SD. Mental health correlates of perceived discrimination among lesbian, gay, and bisexual adults in the United States. Am J Public Health 2001;91:1869–1876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meyer IH. Prejudice, social stress, and mental health in lesbian, gay, and bisexual populations: conceptual issues and research evidence. Psychol Bull 2003;129:674–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schneeberger AR, Dietl MF, Muenzenmaier KH, Huber CG, Lang UE. Stressful childhood experiences and health outcomes in sexual minority populations: a systematic review. Soc Psychiatry Psychiatr Epidemiol 2014;49:1427–1445 [DOI] [PubMed] [Google Scholar]
- 20.Austin SB, Jun HJ, Jackson B, et al. Disparities in child abuse victimization in lesbian, bisexual, and heterosexual women in the Nurses' Health Study II. J Womens Health (Larchmt) 2008;17:597–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Case P, Austin SB, Hunter DJ, et al. Sexual orientation, health risk factors, and physical functioning in the Nurses’ Health Study II. J Womens Health (Larchmt) 2004;13:1033–1047 [DOI] [PubMed] [Google Scholar]
- 22.Jun HJ, Corliss HL, Nichols LP, Pazaris MJ, Spiegelman D, Austin SB. Adult body mass index trajectories and sexual orientation: the Nurses’ Health Study II. Am J Prev Med 2012;42:348–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lick DJ, Durso LE, Johnson KL. Minority stress and physical health among sexual minorities. Perspect Psychol Sci 2013;8:521–548 [DOI] [PubMed] [Google Scholar]
- 24.Hatzenbuehler ML, Nolen-Hoeksema S, Dovidio J. How does stigma “get under the skin”? The mediating role of emotion regulation. Psychol Sci 2009;20:1282–1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McEwen BS. Stress, adaptation, and disease: allostasis and allostatic load. Ann N Y Acad Sci 1998;840:33–44 [DOI] [PubMed] [Google Scholar]
- 26.Hackett RA, Steptoe A. Type 2 diabetes mellitus and psychological stress—a modifiable risk factor. Nat Rev Endocrinol 2017;13:547–560 [DOI] [PubMed] [Google Scholar]
- 27.Gruskin EP, Byrne KM, Altschuler A, Dibble SL. Smoking it all away: influences of stress, negative emotions, and stigma on lesbian tobacco use. J LGBT Health Res 2008;4:167–179 [DOI] [PubMed] [Google Scholar]
- 28.Lehavot K, Simoni JM. The impact of minority stress on mental health and substance use among sexual minority women. J Consult Clin Psychol 2011;79:159–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mason TB, Lewis RJ. Minority stress, body shame, and binge eating among lesbian women. Psychol Women Q 2016;40:428–440 [Google Scholar]
- 30.Calzo JP, Antonucci TC, Mays VM, Cochran SD. Retrospective recall of sexual orientation identity development among gay, lesbian, and bisexual adults. Dev Psychol 2011;47:1658–1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boehmer U, Miao X, Linkletter C, Clark MA. Health conditions in younger, middle, and older ages: are there differences by sexual orientation? LGBT Health 2014;1:168–176 [DOI] [PubMed] [Google Scholar]
- 32.Bränström R, Hatzenbuehler ML, Pachankis JE. Sexual orientation disparities in physical health: age and gender effects in a population-based study. Soc Psychiatry Psychiatr Epidemiol 2016;51:289–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fisher CM, Irwin JA, Coleman JD. LGBT health in the midlands: a rural/urban comparison of basic health indicators. J Homosex 2014;61:1062–1090 [DOI] [PubMed] [Google Scholar]
- 34.Barefoot KN, Warren JC, Smalley KB. An examination of past and current influences of rurality on lesbians’ overweight/obesity risks. LGBT Health 2015;2:154–161 [DOI] [PubMed] [Google Scholar]
- 35.The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus . Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997;20:1183–1197 [DOI] [PubMed] [Google Scholar]
- 36.Manson JE, Rimm EB, Stampfer MJ, et al. Physical activity and incidence of non-insulin-dependent diabetes mellitus in women. Lancet 1991;338:774–778 [DOI] [PubMed] [Google Scholar]
- 37.Field AE, Coakley EH, Must A, et al. Impact of overweight on the risk of developing common chronic diseases during a 10-year period. Arch Intern Med 2001;161:1581–1586 [DOI] [PubMed] [Google Scholar]
- 38.VanKim NA, Austin SB, Jun HJ, Hu FB, Corliss HL. Dietary patterns during adulthood among lesbian, bisexual, and heterosexual women in the Nurses’ Health Study II. J Acad Nutr Diet 2017;117:386–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.VanKim NA, Austin SB, Jun HJ, Corliss HL. Physical activity and sedentary behaviors among lesbian, bisexual, and heterosexual women: findings from the Nurses' Health Study II. J Womens Health (Larchmt) 2017;26:1077–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology 2009;20:488–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2012;55:1577–1596 [DOI] [PubMed] [Google Scholar]
- 42.Nathan DM. Long-term complications of diabetes mellitus. N Engl J Med 1993;328:1676–1685 [DOI] [PubMed] [Google Scholar]
- 43.Alencar Albuquerque G, de Lima Garcia C, da Silva Quirino G, et al. Access to health services by lesbian, gay, bisexual, and transgender persons: systematic literature review. BMC Int Health Hum Rights 2016;16:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.VanKim NA, Erickson DJ, Eisenberg ME, Lust K, Rosser BR, Laska MN. Relationship between weight-related behavioral profiles and health outcomes by sexual orientation and gender. Obesity (Silver Spring) 2016;24:1572–1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Austin SB, Nelson LA, Birkett MA, Calzo JP, Everett B. Eating disorder symptoms and obesity at the intersections of gender, ethnicity, and sexual orientation in US high school students. Am J Public Health 2013;103:e16–e22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hall KD, Kahan S. Maintenance of lost weight and long-term management of obesity. Med Clin North Am 2018;102:183–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Williamson DA. Fifty years of behavioral/lifestyle interventions for overweight and obesity: where have we been and where are we going? Obesity (Silver Spring) 2017;25:1867–1875 [DOI] [PubMed] [Google Scholar]
- 48.Fogel SC, McElroy JA, Garbers S, et al. Program design for healthy weight in lesbian and bisexual women: a ten-city prevention initiative. Womens Health Issues 2016;26(Suppl. 1):S7–S17 [DOI] [PubMed] [Google Scholar]
- 49.McElroy JA, Haynes SG, Eliason MJ, et al. Healthy weight in lesbian and bisexual women aged 40 and older: an effective intervention in 10 cities using tailored approaches. Womens Health Issues 2016;26(Suppl. 1):S18–S35 [DOI] [PubMed] [Google Scholar]