Abstract
OBJECTIVE
This study evaluated the association between hemoglobin A1c (A1C) and wound outcomes in patients with diabetic foot ulcers (DFUs).
RESEARCH DESIGN AND METHODS
We conducted a retrospective analysis of an ongoing prospective, clinic-based study of patients with DFUs treated at an academic institution during a 4.7-year period. Data from 270 participants and 584 wounds were included in the analysis. Cox proportional hazards regression was used to assess the incidence of wound healing at any follow-up time in relation to categories of baseline A1C and the incidence of long-term (≥90 days) wound healing in relation to tertiles of nadir A1C change and mean A1C change from baseline, adjusted for potential confounders.
RESULTS
Baseline A1C was not associated with wound healing in univariate or fully adjusted models. Compared with a nadir A1C change from baseline of −0.29 to 0.0 (tertile 2), a nadir A1C change of 0.09 to 2.4 (tertile 3) was positively associated with long-term wound healing in the subset of participants with baseline A1C <7.5% (hazard ratio [HR] 2.07; 95% CI 1.08–4.00), but no association with wound healing was seen with the mean A1C change from baseline in this group. Neither nadir A1C change nor mean A1C change were associated with long-term wound healing in participants with baseline A1C ≥7.5%.
CONCLUSIONS
There does not appear to be a clinically meaningful association between baseline or prospective A1C and wound healing in patients with DFUs. The paradoxical finding of accelerated wound healing and increase in A1C in participants with better baseline glycemic control requires confirmation in further studies.
Introduction
Given their high attendant risk of amputation, lower-extremity ulcers are a dreaded complication of diabetes. The prevalence of foot ulcers in patients with diabetes is 4–10%, and the lifetime incidence is as high as 25% (1). Among amputations in patients with diabetes, 85% are preceded by a foot ulcer (2). Major risk factors for diabetic foot ulcers (DFUs) include loss of protective sensation (LOPS) from advanced peripheral neuropathy, peripheral vascular disease (PVD), changes in foot structure, poor glycemic control, cigarette smoking, and history of DFU or amputation (1,3,4). In addition to impairment in quality of life, DFUs are associated with reduced life expectancy, with 5-year mortality rates as high as 55% for ischemic ulcers and 77% for those with a previous lower-limb amputation (5,6).
Glycemic control is an established method of primary prevention of microvascular complications (7) and has been shown to reduce amputation rates when combined with other cardiovascular disease prevention strategies (8). The role of glycemic control for secondary prevention of DFUs (i.e., preventing ulceration in patients with established neuropathy) or tertiary prevention (i.e., prevention of amputation in patients with DFUs) is less clear. A systematic review of nine randomized controlled trials (RCTs) found that intensive glycemic control was associated with a 35% reduced risk of amputations in patients with “diabetic foot syndrome” (9). Studies have shown mixed results regarding the effect of glycemic control on wound healing, time to wound healing, and amputation rate. For instance, some observational studies have shown a direct association between baseline hemoglobin A1c (A1C) and rate of wound healing (10,11), baseline A1C and amputation rate (12), and mean A1C and amputation rate (13,14). Most of these studies, however, found no association between glycemic control and wound outcome (15–18), and a meta-analysis of five RCTs found that baseline A1C was not associated with wound healing in patients with neuropathic DFUs (19).
A limitation of previous studies in this area was that measures of glycemic control were generally collected before wound treatment, making it difficult to draw inferences regarding the effect of glycemic control during wound treatment on wound outcomes. The objective of this study was therefore to evaluate not only the association between baseline A1C and wound healing but also the association of wound healing with change in A1C from baseline by using prospectively collected A1C measures. Furthermore, unlike previous studies, our intervention included diabetes specialists as integral members of the multidisciplinary diabetic foot and wound team, which facilitated timely collection of A1C measurements and individualization of glycemic targets. We hypothesized that tighter glycemic control would be associated with shorter time to wound healing.
Research Design and Methods
Study Design
This was a clinic-based observational study of patients with DFUs seen at the Johns Hopkins Multidisciplinary Diabetic Foot and Wound Clinic between 3 July 2012 and 7 March 2017. Briefly, the integrated clinic includes specialists from vascular surgery (surgeon, physician assistant), podiatry (surgical podiatrist), endocrinology (physicians, nurse practitioner), and wound care (nurse) (20). All interventions and study-related procedures reflected standard of care for management of DFUs. For participants with glycemic control at target, visits with the diabetes specialist occurred approximately every 3 months but as frequently as weekly with other team members if needed for wound care needs. For patients with uncontrolled diabetes, the frequency of visits was determined predominantly by wound care needs rather than by diabetes management needs. The Johns Hopkins Institutional Review Board approved the study. Written informed consent was obtained from each participant.
Study Population
Adult patients with a diagnosis of diabetes and lower-extremity wound(s) were eligible for participation. Exclusion criteria included 1) patient inability or unwillingness to adhere to treatment recommendations, 2) presence of lymphedema/venous stasis, 3) wound requiring minor podiatric or conservative treatment considered more appropriate for a general podiatry clinic, 4) patients seeking only a one-time second opinion who wished to continue outside care with established vascular surgeon or endocrinologist, and 5) stage 5 wounds according to the Society for Vascular Surgery WIfI (Wound, Ischemia and foot Infection) wound staging classification system (20,21) deemed to require immediate major amputation.
A1C Measures
Glycemic control was assessed using serum or point-of-care A1C measurements, which were obtained at a goal of 90-day intervals in accordance with the standard of care. Point-of-care A1C was measured using the Alere Afinion AS100 Analyzer, which meets performance standards established by the National Glycohemoglobin Standardization Program. Quality control of A1C testing by clinical staff was overseen by the Johns Hopkins Department of Pathology Point-of-Care Testing office.
Baseline A1C was defined as the most recent A1C result within the interval of −365 to +30 days from date of the initial wound assessment. Any A1C measurement obtained after the date of the baseline A1C was considered a prospective A1C. Two prospective measures of A1C were used to calculate the change in A1C from baseline: “nadir A1C change” was defined as the difference between the baseline A1C and the single lowest prospective A1C measurement, and “mean A1C change” was defined as the difference between the baseline A1C and the average of all prospectively collected A1C measurements. For wounds with only one prospective A1C measurement, nadir and mean A1C (and corresponding changes from baseline) were equivalent.
Wound Assessment and Interventions
All wounds were staged at initial presentation by the vascular surgeon using the validated WIfI staging system, which takes into account wound size, PVD, and underlying infection and accurately predicts the need for major amputation (20–23). Standardized Infectious Diseases Society of America guidelines were followed for the treatment of infected wounds (24). Interventions were classified as wound care only and surgery, defined as debridement, minor amputation (distal to ankle), split-thickness skin grafting, endovascular intervention, open bypass, and endarterectomy. The need for major amputation (above the level of the ankle) was based on the severity of wound and determined by the surgeons.
Covariates
Potential confounders in the association between glycemic control and wound outcomes were evaluated. Diabetes type and duration and presence of relevant comorbidities were confirmed by the diabetes team based on the participant’s reported history and medical record review. Every participant had a detailed neurological assessment by the podiatrist, including 10-g monofilament proprioception, Achilles and patellar deep tendon reflexes, vibratory sensation, sharp/dull discrimination, and motor coordination of heel to patella to ankle bilaterally. Abnormality in at least two of these tests was used to define LOPS (25,26). Wound severity was evaluated using the categorical WIfI stage. We also adjusted for A1C targets (<7% or 7.0–7.5%) because the participant’s A1C levels were individualized at the discretion of the diabetes specialist based on participant comorbidities, diabetes duration, life expectancy, established vascular complications, risk of hypoglycemia, and participant motivation and support (27,28).
Study Outcome
The primary outcome was time to wound healing, which was defined as complete epithelialization of the wound with restoration of sustained functional and anatomic continuity (29,30). For healed wounds that reopened within the subsequent 6 weeks, the wound outcome was changed to unhealed, and the wound observation period was extended to the last visit date. Wounds requiring major amputation were considered treatment failure. A participant who did not have an outcome during the wound observation time, died, or withdrew from the study before experiencing a wound outcome was censored on the date of the last clinic visit. Because A1C levels are usually obtained at 90-day intervals, participants with wounds that healed before 90 days from the baseline assessment may not have had an opportunity to have a repeat A1C collected. Therefore, although baseline A1C was evaluated as a predictor of wound healing at any time during follow-up, the association of A1C change measures and wound healing was limited to long-term (≥90 days) outcomes.
Statistical Analysis
Summary statistics were calculated by using means, SD, medians, and interquartile ranges for continuous variables. Statistical significance was evaluated with the Student t test or Wilcoxon rank sum test for continuous variables and the Fisher exact test or χ2 test for proportions. Normality was assessed using the Shapiro-Wilk test. Some participants had multiple wounds, and we used a pseudorandom number generator function to randomly select one wound per study participant to ensure independence of wound outcomes and other covariates when reporting baseline characteristics (Table 1), because related samples cannot be examined in univariate analyses.
Table 1.
Baseline characteristics of wounds by wound outcome
| One wound per participant (random) |
||||
|---|---|---|---|---|
| Not healed | Healed | All | ||
| Characteristics | (n = 68) | (n = 202) | (N = 270) | P value* |
| Time to wound outcome, days | 98 (82) | 84 (130) | 91 (121) | 0.89 |
| Sex, % | 0.22 | |||
| Female | 47.1 | 38.6 | 40.7 | |
| Male | 52.9 | 61.4 | 59.3 | |
| Age, years | 60.7 ± 12.4 | 57.4 ± 11.0 | 58.3 ± 11.4 | 0.04 |
| Race, %† | 0.14 | |||
| White/Caucasian | 43.3 | 30.2 | 33.4 | |
| Black | 53.7 | 66.8 | 63.6 | |
| Other | 3.0 | 3.0 | 3.0 | |
| BMI, kg/m2‡ | 29.6 (12.8) | 31.7 (10.5) | 31.1 (10.9) | 0.58 |
| Diabetes type, % | 0.89 | |||
| Type 1 | 5.9 | 5.4 | 5.6 | |
| Type 2 | 94.4 | 94.6 | 94.4 | |
| Diabetes duration, years | 15.2 (11.5) | 16.1 (14.2) | 15.7 (12.9) | 0.81 |
| Baseline A1C, % | 7.8 (3.6) | 8.3 (3.5) | 8.1 (3.5) | 0.79 |
| Nadir A1C, % | 7.0 (2.2) | 7.1 (2.2) | 7.1 (2.2) | 0.33 |
| Mean A1C, % | 7.5 (2.8) | 7.8 (2.8) | 7.7 (2.9) | 0.35 |
| Nadir A1C change from baseline | −0.5 (2.2) | −0.5 (1.5) | −0.5 (1.7) | 0.41 |
| Mean A1C change from baseline | −0.2 (1.2) | −0.2 (1.2) | −0.2 (1.2) | 0.26 |
| Target A1C, % | 0.48 | |||
| <7.0% | 32.3 | 37.1 | 35.9 | |
| 7.0–7.5% | 67.7 | 62.9 | 64.1 | |
| Observed-to-expected A1C per 90 days | 1.0 (0.2) | 1.0 (0.2) | 1.0 (0.2) | 0.55 |
| N (%) with ≥1 prospective A1C | 60 (88.2) | 174 (86.1) | 234 (86.7) | 0.66 |
| Antihyperglycemic medications, % | ||||
| Metformin | 25.0 | 39.6 | 35.9 | 0.03 |
| DPP-4 inhibitors | 2.9 | 5.9 | 5.2 | 0.34 |
| GLP-1 agonists | 2.9 | 1.0 | 1.5 | 0.26 |
| Thiazolidinediones | 1.5 | 0.5 | 0.7 | 0.44 |
| SGLT-2 inhibitors | 0 | 0 | 0 | — |
| Sulfonylureas, % | 14.7 | 16.3 | 15.9 | 0.75 |
| Sulfonylurea types, % | 0.38 | |||
| Glyburide | 0 | 9.4 | 7.3 | |
| Glimepiride | 44.4 | 21.9 | 26.8 | |
| Glipizide | 55.6 | 68.8 | 65.9 | |
| Insulin, % | 75.0 | 63.9 | 66.7 | 0.09 |
| Total insulin dose (units/kg/day), % | 0.01 | |||
| 0.00 | 23.5 | 35.3 | 32.3 | |
| 0.07–0.23 | 5.9 | 8.4 | 7.8 | |
| 0.23–0.46 | 14.7 | 21.9 | 20.1 | |
| 0.46–0.82 | 22.1 | 19.4 | 20.1 | |
| 0.82–3.28 | 33.8 | 14.9 | 19.7 | |
| Comorbidities, % | ||||
| Coronary artery disease | 27.9 | 23.3 | 24.4 | 0.44 |
| Prior myocardial infarction | 11.8 | 12.4 | 12.2 | 0.89 |
| PVD | 42.7 | 37.6 | 38.9 | 0.46 |
| Prior amputation | 27.9 | 32.2 | 31.1 | 0.51 |
| Hypertension | 80.9 | 83.2 | 82.6 | 0.67 |
| LOPS | 94.1 | 92.6 | 93.0 | 0.67 |
| Retinopathy | 30.9 | 23.3 | 25.2 | 0.21 |
| Dialysis | 16.2 | 9.1 | 11.1 | 0.12 |
| Prior kidney transplant | 13.2 | 7.9 | 9.3 | 0.19 |
| eGFR categories, %|| | 0.66 | |||
| G1–2 | 31.3 | 31.8 | 31.7 | |
| G3 | 28.1 | 34.1 | 32.5 | |
| G4 | 18.8 | 18.4 | 18.5 | |
| G5 | 21.9 | 15.6 | 17.3 | |
| Current smoker, % | 64.7 | 55.5 | 57.8 | 0.18 |
| WIfI stage, %¶ | 0.04 | |||
| 1 | 19.1 | 33.7 | 30.0 | |
| 2 | 17.7 | 15.4 | 15.9 | |
| 3 | 27.9 | 30.2 | 29.6 | |
| 4 | 35.3 | 20.8 | 24.4 | |
| Uninfected wounds at baseline (n = 128) | 0.95 | |||
| No antibiotics, n/N (%) | 22/28 (78.6) | 78/100 (78.0) | 100/128 (78.1) | |
| Antibiotic use, n/N (%) | 6/28 (21.4) | 22/100 (22.0) | 28/128 (21.9) | |
| Infected wounds at baseline (n = 142) | 0.37 | |||
| No antibiotics, n/N (%) | 0/40 (0) | 2/102 (1.9) | 2/142 (1.4) | |
| Antibiotic use, n/N (%) | 40/40 (100) | 100/102 (98.1) | 140/142 (98.6) | |
| Antibiotic use, % | 67.7 | 60.4 | 62.2 | 0.29 |
| Wound intervention, % | 0.66 | |||
| Wound care | 44.1 | 41.1 | 41.9 | |
| Surgery | 55.9 | 58.9 | 58.2 | |
Continuous data are shown as mean ± SD or median (interquartile range) and categorical data as indicated.
DPP-4, dipeptidyl peptidase 4; GLP-1, glucagon-like peptide 1; n, number of wounds; SGLT-2, sodium–glucose cotransporter 2.
*P values were calculated using the Student t test for continuous variables with normal distribution and the Wilcoxon rank sum test for nonnormally distributed variables. Fisher exact test or χ2 tests were used for categorical variables. P values were not reported for “All wounds” because of lack of independence of characteristics for multiple wounds per participant. Bold values indicate P < 0.05.
†Race missing one observation in “Not healed” category.
‡BMI missing one observation in “Healed” category.
||eGFR categories were based on Kidney Disease Improving Global Outcomes guidelines. Laboratory results were missing for 4 observations in the “Not healed” category and for 23 observations in the “Healed” category.
¶WIfI classification of the Society for Vascular Surgery.
The association between baseline A1C, evaluated as a categorical variable (<6.5%, 6.5–8.0%, and 8.0%), and wound healing was evaluated in univariate and multivariable Cox regression models (Table 2). These baseline A1C categories were selected because they were felt to reflect clinical treatment targets for this patient population, whereby the reference group of 6.5–8.0% would be considered acceptable glycemic control for most of the participants, <6.5% indicative of tight control, and >8% indicative of inadequate control. Baseline A1C was also evaluated as a continuous measure but was not significantly associated with wound healing (data not shown). Covariates known to be clinically significant predictors of wound healing and those that showed a univariate association with wound healing at a significance level of ≤0.10 were entered as covariates into multivariate proportional hazards regression models for wound healing. Model 1 was adjusted for age, sex, and race. Model 2 was additionally adjusted for smoking status, prior amputation, LOPS, quartiles of insulin doses, metformin use, sulfonylurea use, wound intervention (surgery vs. wound care), estimated glomerular filtration rate (eGFR) category (based on Kidney Disease Improving Global Outcomes guidelines), kidney transplant status, target A1C, and antibiotic use. We checked the Cox proportional hazards assumption with graphs of Schoenfeld residuals and tested for nonproportionality. Because nonproportionality was observed in the fully adjusted model (model 2), we stratified this model by WIfI and PVD.
Table 2.
Association of baseline A1C and wound healing in multivariable Cox models
| Unadjusted (n = 584) | Model 1* (n = 583) | Model 2† (n = 528) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline A1C | N | HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value |
| 6.5–8.0% | 162 | 1.00 | Ref | — | 1.00 | Ref | — | 1.00 | Ref | — |
| <6.5% | 149 | 1.00 | 0.69–1.47 | 0.96 | 0.99 | 0.69–1.43 | 0.96 | 0.97 | 0.65–1.44 | 0.89 |
| >8.0% | 298 | 1.18 | 0.89–1.58 | 0.26 | 1.13 | 0.83–1.54 | 0.44 | 0.97 | 0.70–1.36 | 0.87 |
N, number of wounds.
P values <0.05 are statistically significant.
*Model 1 adjusted for age, sex, and race; one observation was dropped due to missing race.
†Model 2 adjusted for age, sex, race, smoking status, neuropathy (LOPS), prior amputation, quartiles of insulin dose (units/kg/day), metformin use, sulfonylurea use, wound intervention (surgery vs. wound care only), eGFR category, kidney transplant, A1C target (<7.5% vs. ≥7.5%), and antibiotic use; 56 observations were dropped from model 2: 54 missing eGFR, 1 missing race, and 1 missing insulin dose.
The association between A1C change measures and long-term (≥90 days) wound healing was evaluated in univariate and multivariable Cox regression models, stratified by baseline A1C status of <7.5% (Table 3) and ≥7.5% (Table 4). We stratified by baseline A1C for two reasons. First, inclusion of both A1C change and baseline A1C as covariates in a regression model would result in nonindependence of these variables because calculation of A1C change includes baseline A1C. Second, because the degree of A1C change is expected to be different based on the variance of baseline A1C from target A1C, analysis of wounds by baseline A1C status allows clinically meaningful inferences to be made about the effect of A1C change in relation to the median target A1C of this population (7.5%). Nadir A1C change and mean A1C change were both evaluated as categorical variables (tertiles), with the reference group being the middle tertile (i.e., least amount of change from baseline) to make results more clinically meaningful. Similarly, insulin doses were categorized as tertiles, because larger numbers of quantiles resulted in insufficient numbers of wounds in each quantile in the models.
Table 3.
Association of A1C change from baseline and long-term (≥90 day) wound healing among wounds with baseline A1C <7.5%
| Unadjusted (n = 129) |
Model 1 (n = 129) |
Model 2 (n = 118) |
Model 3 (n = 117) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | |
| ΔNadir A1C | |||||||||||||
| Tertile 1 (−1.7 to −0.3) | 47 | 0.67 | 0.41–1.09 | 0.11 | 0.64 | 0.36–1.14 | 0.13 | 0.85 | 0.48–1.50 | 0.58 | 0.85 | 0.48–1.51 | 0.29 |
| Tertile 2 (−0.29 to 0.0) | 54 | 1.00 | Ref | — | 1.00 | Ref | — | 1.00 | Ref | — | 1.00 | Ref | — |
| Tertile 3 (0.09–2.4) | 28 | 1.29 | 0.77–2.17 | 0.34 | 1.30 | 0.76–2.22 | 0.34 | 1.90 | 1.03–3.53 | 0.04 | 2.07 | 1.08–4.00 | 0.03 |
| ΔMean A1C | |||||||||||||
| Tertile 1 (−1.4 to 0.0) | 69 | 1.02 | 0.54–1.95 | 0.07 | 0.96 | 0.39–2.38 | 0.93 | 0.78 | 0.30–2.02 | 0.61 | 0.85 | 0.27–2.72 | 0.79 |
| Tertile 2 (0.02–0.3) | 17 | 1.00 | Ref | — | 1.00 | Ref | — | 1.00 | Ref | — | 1.00 | Ref | — |
| Tertile 3 (0.35–4.25) | 43 | 1.20 | 0.62–2.35 | 0.55 | 1.19 | 0.45–3.10 | 0.73 | 0.97 | 0.33–2.84 | 0.96 | 1.06 | 0.32–3.56 | 0.93 |
N, number of wounds; Δ, change (from baseline).
Bold values indicate statistical significance (P < 0.05).
Model 1: adjusted for WIfI classification system (stage 1–4) and wound intervention (wound care vs. surgery). Model 2: adjusted for WIfI, wound intervention, age, smoking status, antibiotic use, eGFR stages, history of kidney transplant, and history of amputation; 11 observations were dropped due to missing eGFR data. Model 3: adjusted for WIfI, wound intervention, age, smoking status, antibiotic use, eGFR stages, history of kidney transplant, history of amputation, and total insulin dose (units/kg) assessed in tertiles (tertile 1: 0.00; tertile 2: 0.11–0.26; tertile 3: 0.27–3.28); 12 observations were dropped (11 missing eGFR data and 1 missing insulin dose).
Table 4.
Association of A1C change from baseline and long-term (≥90 day) wound healing among wounds with baseline A1C ≥7.5%
| Unadjusted (n = 143) |
Model 1 (n = 143) |
Model 2 (n = 141) |
Model 3 (n = 141) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | |
| ΔNadir A1C | |||||||||||||
| Tertile 1 (−9 to −2.5) | 48 | 1.22 | 0.76–1.94 | 0.41 | 1.23 | 0.75–2.02 | 0.42 | 1.58 | 0.91–2.75 | 0.10 | 1.85 | 0.91–3.79 | 0.09 |
| Tertile 2 (−2.5 to −1.0) | 50 | 1.00 | Ref | — | 1.00 | Ref | — | 1.00 | Ref | — | 1.00 | Ref | — |
| Tertile 3 (−0.9 to 2.6) | 45 | 1.24 | 0.77–2.00 | 0.38 | 1.19 | 0.66–2.12 | 0.56 | 1.51 | 0.84–2.73 | 0.17 | 1.53 | 0.82–2.87 | 0.18 |
| ΔMean A1C | |||||||||||||
| Tertile 1 (−6 to −1.5) | 48 | 1.23 | 0.77–1.97 | 0.38 | 1.35 | 0.81–2.26 | 0.25 | 1.60 | 0.92–2.79 | 0.10 | 1.86 | 0.91–3.85 | 0.09 |
| Tertile 2 (−1.5 to −0.3) | 48 | 1.00 | Ref | — | 1.00 | Ref | — | 1.00 | Ref | — | 1.00 | Ref | — |
| Tertile 3 (−0.2 to 2.7) | 47 | 1.09 | 0.67–1.75 | 0.74 | 1.22 | 0.68–2.17 | 0.50 | 1.22 | 0.74–2.40 | 0.34 | 1.33 | 0.73–2.43 | 0.35 |
N, number of wounds; Δ, change (from baseline).
P values <0.05 are statistically significant.
Model 1: adjusted for WIfI classification system (stages 1–4) and wound intervention (wound care vs. surgery). Model 2: adjusted for WIfI, wound intervention, age, smoking status, antibiotic use, eGFR stages, history of kidney transplant, and history of amputation. Model 3: adjusted for WIfI, wound intervention, age, smoking status, antibiotic use, eGFR stages, history of kidney transplant, history of amputation, and total insulin dose (units/kg) assessed in tertiles (tertile 1: 0.00–0.23; tertile 2: 0.23–0.56; tertile 3: 0.57–3.28). Two observations were dropped from models 2 and 3 due to missing eGFR data.
When selecting covariates, we sought to achieve an event-to-predictor ratio of <10 to minimize the risk of overfitting (31), favored covariates known to be strong clinical predictors, and omitted covariates that were felt to be captured in other variables (e.g., PVD was not included because it is already a component of the WIfI stage variable). Model 1 was adjusted for WIfI stage and wound intervention. Model 2 was additionally adjusted for age, smoking status, antibiotic use, eGFR stages, history of kidney transplant, and prior amputation. Model 3 was additionally adjusted for total insulin dose in tertiles of units per kilogram per day. The Cox proportional hazards assumption was met, so stratification was not required.
Because wounds in the same participant are not independent events, robust estimation of SEs for clustered data were performed. Collinearity for each of the covariates in the Cox models was evaluated using the variance inflation factors, and there was no evidence of collinearity. A flowchart of the wounds included in the Cox analyses with information about missing prospective A1C measurements is shown in Supplementary Fig. 1. Statistical analyses were performed using Stata 14 (StataCorp, College Station, TX) and SAS 9.3 (SAS Institute, Cary, NC) statistical software.
Results
Description of Study Participants and Wounds
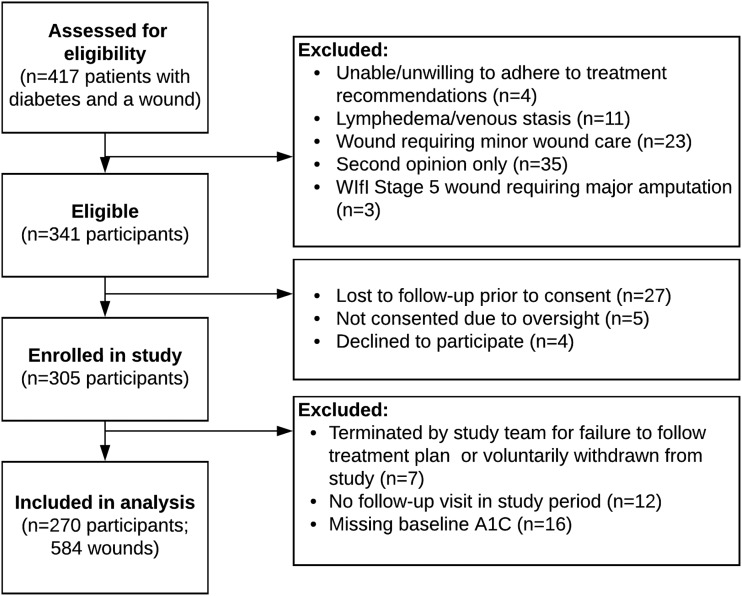
All eligible patients were approached for enrollment in the study. Among the 417 patients with a DFU seen in the clinic during the study period, 341 (82%) were deemed eligible for participation (Fig. 1). Four candidates declined to participate, 27 were lost to follow-up before consent could be obtained, and 5 were not consented due to oversight, resulting in 305 enrolled participants and a recruitment yield of 90%. After excluding 7 participants for failure to adhere to treatment plan or voluntary withdrawal, 12 participants for lack of follow-up, and 16 participants with missing baseline A1C results, data from 270 participants and 584 wounds were included in the analysis.
Figure 1.
Flowchart of study participants.
Table 1 summarizes the baseline characteristics and wound outcomes using one randomly selected wound per participant. Baseline characteristics for all wounds are reported in Supplementary Table 1. There were no significant differences when all wounds were compared with a random selection of wounds. The study population consisted of a high-risk group of obese, middle-aged participants with predominantly type 2 diabetes and advanced diabetes-related complications. There was a slight preponderance of men and African Americans in this cohort, and consistent with their long diabetes duration, most participants required insulin. Older age and higher total insulin doses and WIfI stage were negatively associated with wound healing. Metformin use was positively associated with wound healing, but otherwise, there were no differences in use of antihyperglycemic medications by wound outcome. No differences were found in the prevalence of comorbid conditions, smoking status, wound intervention type (surgery vs. wound care only), or antibiotic use by wound outcome. Interestingly, although PVD was not significantly associated with wound healing, the WIfI stage, which incorporates the presence of ischemia together with wound size and infection, was a significant predictor.
Glycemic control at study entry was poor, with median baseline A1C of 8.1%. An A1C was obtained per 90-day interval for nearly all participants (observed-to-expected A1C ratio of 1.0), and the frequency of A1C testing did not differ by wound outcome. Most participants were targeted to an A1C of 7.0–7.5%, with no differences in A1C target by wound outcome. The median nadir A1C during wound treatment was 7.1%, representing a median change from baseline of −0.5%. The median of the mean A1C was 7.7%, representing a median change from baseline of −0.2%.
Wound Healing
Among the 584 wounds, 450 (77.1%) had evidence of wound healing on the basis of complete epithelialization. Among these 450 wounds, 411 (85.6%) were confirmed to have sustained wound closure at a follow-up visit ≥6 weeks. The remaining 39 wounds, which were considered healed, were of participants who had no follow-up (n = 29) or who had a follow-up visit at an interval <6 weeks (n = 10) after confirmation of initial wound closure.
Baseline A1C and Wound Healing
Table 2 reports the association of baseline A1C and wound healing. Compared with the reference group of baseline A1C 6.5–8.0%, there were no differences in the unadjusted and adjusted hazard ratio (HR) for wound healing in wounds with a baseline A1C of <6.5% or >8.0%. Although data are not reported, we did not see any association between baseline A1C and incidence of wound healing in relation to short-term (<90 day) or long-term (≥90 day) outcomes.
Change in A1C and Long-term Wound Healing
Table 3 reports the association of A1C change measures and long-term (≥90 day) wound healing in participants with baseline A1C of <7.5%. Of the 129 wounds, 34 (26.4%) had a single prospective A1C measurement; thus, nadir and mean A1C were equivalent. Univariate analyses showed no association between nadir A1C change or mean A1C change from baseline and wound healing. There was also no association seen after adjusting for WIfI stage and wound intervention (model 1). On one hand, model 2, which was adjusted for a greater number of confounders, showed a paradoxical association with nadir A1C change: the highest tertile of change (i.e., A1C increase from baseline) was associated with a HR of 1.90 (95% CI 1.03–3.53; P = 0.04) for wound healing, which persisted in the fully adjusted model accounting for insulin doses (HR 2.07; 95% CI 1.08–4.00). On the other hand, no association was seen between the mean A1C change from baseline in any of the models.
Table 4 reports the association of A1C change measures and long-term (≥90 day) wound healing in participants with baseline A1C ≥7.5%. Of the 143 wounds, 17 (11.9%) had one prospective A1C measurement, with equivalent nadir and mean A1C values. In this group, no association between nadir A1C change or mean A1C change was seen in any of the models. Supplementary Table 2 (one wound per participant) and Supplementary Table 3 (all wounds) report the baseline characteristics of the wounds used in the analyses in Tables 3 and 4.
Conclusions
In this long-term, prospective, clinic-based study of DFUs, we did not observe an association with baseline A1C and wound healing, which is consistent with previous studies. Similarly, change in A1C measures during wound treatment were generally not associated with accelerated wound healing. We did, however, observe an unexpected positive association of long-term wound healing and increased A1C from baseline in the subset of wounds from participants with better glycemic control at baseline (A1C <7.5%). This paradoxical finding was limited to change in the nadir A1C and was not seen with change in the mean A1C.
Why an increase in A1C was associated with accelerated wound healing only in participants with better glycemic control at baseline and why the same pattern was not seen with increase in mean A1C is not readily apparent. Although it is possible that the baseline level of glycemic control at the initial visit in a multidisciplinary diabetic wound clinic could modify the timing and intensity of treatment modalities offered (i.e., poorer glycemic control delaying surgery out of concern for postsurgical infection), the inverse association between nadir A1C and wound healing persisted even after adjustment for comorbid conditions, interventions, and insulin doses. As expected, the magnitude of change in a single A1C value from baseline (nadir A1C change) was more pronounced than the average of multiple values from baseline (mean A1C change). Thus, the phenomenon of regression to the mean could partly explain why the association was not seen with mean A1C change.
Chronic hyperglycemia is known to disrupt wound healing in patients with diabetes (32–34); thus, a positive association between an increase in A1C from baseline and wound healing is counterintuitive. Although we did not formally monitor hypoglycemic episodes in this study, one possible explanation for this unexpected association may be that unrecognized hypoglycemia could contribute to a stress response that impairs wound healing via immune dysregulation (35–38). We treated medications as time-fixed (i.e., baseline) variables and were thus unable to account for the effect of medication dose adjustments during wound treatment. Insulin is an anabolic agent that could theoretically accelerate wound healing through its effects on protein synthesis, inflammation, and other processes; accordingly, intensification of insulin doses during wound treatment could possibly modify A1C measures (exposure) and wound healing (outcome). However, intensification of insulin doses would be expected to be less pronounced in participants with better glycemic control at baseline, and, if insulin were mediating the inverse association observed, one would expect decreases in insulin doses to result in increases in A1C, rather than the contrary. We did not find an interaction between change in A1C measures and baseline insulin doses but suspect that such an interaction would have been observed had insulin doses been collected prospectively and treated as a time-varying covariate.
Rapid improvement in glycemic control has been linked to treatment-induced neuropathy of diabetes (“insulin neuritis”) that results in severe pain and autonomic dysfunction (39,40). Most of our participants already had LOPS, but it is plausible that rapid A1C lowering could contribute to worse wound outcomes via effects on endoneurial edema, ischemia, and neuronal injury (41). Again, although this mechanism could be invoked to explain a paradoxical association of A1C increase and favorable wound outcome, it is less likely to be encountered among participants with better glycemic control at baseline and seems an inadequate explanation for our findings.
The totality of these data does not support a clear association of better glycemic control and favorable wound outcomes in DFUs. Most studies have shown a neutral or favorable association of A1C and wound healing time (10–12,15–18), and there are no completed RCTs of intensive compared with conventional glycemic control in the management of DFUs. To definitively evaluate the effect of glycemic control on patients with established DFUs would require an RCT; however, selecting A1C targets higher than that recommended for the general population would be ethically problematic, because intentionally exposing participants to higher levels of glycemia could accelerate risk of other microvascular complications even if potentially beneficial in the short-term for wound healing. Alternatively, assigning hyperglycemic patients to different rates of A1C lowering could shed light on this association, but such a study would be difficult to achieve from a practical standpoint without strict adherence to medication titration algorithms relative to blood glucose targets. In any case, collecting information about antihyperglycemic medications prospectively is important, because variations in A1C during wound follow-up are likely mediated by medication dose changes.
Our study has several strengths. This was a large cohort study performed over a long period of time (>4.5 years). Loss to follow-up bias was minimized by the very low dropout rate, and findings are generalized to a multidisciplinary limb salvage clinic. Unlike most studies in this area, which have generally used baseline A1C as the measure of glycemic exposure, our study included both baseline and prospective A1C measures, with nearly complete ascertainment.
Some limitations should be considered in the interpretation of our results. We attempted to adjust for key covariates but were unable to account for baseline hemoglobin (which may influence A1C measurements), level of physical activity, or nutritional status, and there may be as yet unmeasured confounders. Exclusion of patients with very small or minor wounds may have biased our results. We did not have information about the specialists seen at each visit, and it is possible that the frequency of clinic visits was influenced by the need for more intensive glycemic management rather than wound care needs. We believe this is unlikely to be a significant confounder based on the set up of our clinic model. It is possible that a small number of wound outcomes were misclassified due to inability to verify sustained wound closure for 6 weeks for some participants because of lack of follow-up; however, because our clinic has real-time access to emergency department visits and hospitalizations for all study participants, the possibility that a participant would have a wound recurrence in our health system without our knowledge is minimized. Finally, although we attempted to collect A1C measurements at 90-day intervals, participants whose wounds healed <90 days were less likely to return to the clinic for a follow-up A1C; thus, determining how change in A1C measures affect short-term (<90 day) outcomes was not possible in this study. To overcome this limitation, we partitioned our data set at the 90-day interval, which resulted in a decrease in the sample size and possible loss of power. A study with a defined protocol for A1C measurements, irrespective of timing of wound outcomes, would be better designed to address the potential role of on-going glycemic control and wound outcomes in this population.
To our knowledge, our study is one of the larger prospective studies looking specifically at the role of glycemic control on wound outcomes during DFU treatment. Although prospective A1C measures were generally not associated with wound healing after adjusting for multiple confounders, we observed a paradoxical association of accelerated wound healing in participants with better baseline glycemic control who had an increase in their A1C. Further studies are needed to confirm this finding. In the interim, in the absence of any clear benefit of more intensive glycemic control, our findings support a conservative A1C target in this high-risk patient population.
Supplementary Material
Article Information
Acknowledgments. The authors acknowledge assistance for clinical data coordination and retrieval from the Center for Clinical Data Analytics, supported in part by the Johns Hopkins Institute for Clinical and Translational Research (UL1-TR-001079) from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the Johns Hopkins Institute for Clinical and Translational Research, NCATS, or NIH.
Funding. This study was supported by NIH grants T32-DK-062707 to B.K.F., P30-DK-079637 (from National Institute of Diabetes and Digestive and Kidney Diseases Diabetes Research Centers) to S.L., and 5KL2-TR-001077-02 (from NCATS) to N.M.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. B.K.F. collected and analyzed data and wrote the manuscript. C.J.A. contributed to the study design, collected data, and edited the manuscript. K.F.H., R.S., P.F., S.L., and J.C. collected data and contributed to discussion. K.C.L. collected data and reviewed the manuscript. S.M.H. and G.J. researched data and reviewed the manuscript. C.W.H. researched data and contributed to discussion. S.Y. edited the manuscript and researched data. N.M. designed the study, collected and analyzed the data, and wrote and edited the manuscript. N.M. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc17-1683/-/DC1.
References
- 1.Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA 2005;293:217–228 [DOI] [PubMed] [Google Scholar]
- 2.Apelqvist J, Larsson J. What is the most effective way to reduce incidence of amputation in the diabetic foot? Diabetes Metab Res Rev 2000;16(Suppl. 1):S75–S83 [DOI] [PubMed] [Google Scholar]
- 3.Gregg EW, Gu Q, Williams D, et al. Prevalence of lower extremity diseases associated with normal glucose levels, impaired fasting glucose, and diabetes among U.S. adults aged 40 or older. Diabetes Res Clin Pract 2007;77:485–488 [DOI] [PubMed] [Google Scholar]
- 4.Boyko EJ, Ahroni JH, Stensel V, Forsberg RC, Davignon DR, Smith DG. A prospective study of risk factors for diabetic foot ulcer. The Seattle Diabetic Foot Study. Diabetes Care 1999;22:1036–1042 [DOI] [PubMed] [Google Scholar]
- 5.Fortington LV, Geertzen JH, van Netten JJ, Postema K, Rommers GM, Dijkstra PU. Short and long term mortality rates after a lower limb amputation. Eur J Vasc Endovasc Surg 2013;46:124–131 [DOI] [PubMed] [Google Scholar]
- 6.Moulik PK, Mtonga R, Gill GV. Amputation and mortality in new-onset diabetic foot ulcers stratified by etiology. Diabetes Care 2003;26:491–494 [DOI] [PubMed] [Google Scholar]
- 7.Hemmingsen B, Lund SS, Gluud C, et al. Targeting intensive glycaemic control versus targeting conventional glycaemic control for type 2 diabetes mellitus. Cochrane Database Syst Rev 2013:CD008143. [DOI] [PubMed] [Google Scholar]
- 8.Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 2008;358:580–591 [DOI] [PubMed] [Google Scholar]
- 9.Hasan R, Firwana B, Elraiyah T, et al. A systematic review and meta-analysis of glycemic control for the prevention of diabetic foot syndrome. J Vasc Surg 2016;63(2 Suppl.):22S–28S.e1–2 [DOI] [PubMed] [Google Scholar]
- 10.Markuson M, Hanson D, Anderson J, et al. The relationship between hemoglobin A(1c) values and healing time for lower extremity ulcers in individuals with diabetes. Adv Skin Wound Care 2009;22:365–372 [DOI] [PubMed] [Google Scholar]
- 11.Christman AL, Selvin E, Margolis DJ, Lazarus GS, Garza LA. Hemoglobin A1c predicts healing rate in diabetic wounds. J Invest Dermatol 2011;131:2121–2127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pscherer S, Dippel FW, Lauterbach S, Kostev K. Amputation rate and risk factors in type 2 patients with diabetic foot syndrome under real-life conditions in Germany. Prim Care Diabetes 2012;6:241–246 [DOI] [PubMed] [Google Scholar]
- 13.Miyajima S, Shirai A, Yamamoto S, Okada N, Matsushita T. Risk factors for major limb amputations in diabetic foot gangrene patients. Diabetes Res Clin Pract 2006;71:272–279 [DOI] [PubMed] [Google Scholar]
- 14.Lepore G, Maglio ML, Cuni C, et al. Poor glucose control in the year before admission as a powerful predictor of amputation in hospitalized patients with diabetic foot ulceration. Diabetes Care 2006;29:1985. [DOI] [PubMed] [Google Scholar]
- 15.Moffat AD, Worth ER, Weaver LK. Glycosylated hemoglobin and hyperbaric oxygen coverage denials. Undersea Hyperb Med 2015;42:197–204 [PubMed] [Google Scholar]
- 16.Yesil S, Akinci B, Yener S, et al. Predictors of amputation in diabetics with foot ulcer: single center experience in a large Turkish cohort. Hormones (Athens) 2009;8:286–295 [DOI] [PubMed] [Google Scholar]
- 17.Tabur S, Eren MA, Çelik Y, et al. The major predictors of amputation and length of stay in diabetic patients with acute foot ulceration. Wien Klin Wochenschr 2015;127:45–50 [DOI] [PubMed] [Google Scholar]
- 18.Pickwell K, Siersma V, Kars M, et al. Predictors of lower-extremity amputation in patients with an infected diabetic foot ulcer. Diabetes Care 2015;38:852–857 [DOI] [PubMed] [Google Scholar]
- 19.Margolis DJ, Kantor J, Santanna J, Strom BL, Berlin JA. Risk factors for delayed healing of neuropathic diabetic foot ulcers: a pooled analysis. Arch Dermatol 2000;136:1531–1535 [DOI] [PubMed] [Google Scholar]
- 20.Mathioudakis N, Hicks CW, Canner JK, et al. The Society for Vascular Surgery Wound, Ischemia, and foot Infection (WIfI) classification system predicts wound healing but not major amputation in patients with diabetic foot ulcers treated in a multidisciplinary setting. J Vasc Surg 2017;65:1698–1705.e1 [DOI] [PubMed] [Google Scholar]
- 21.Mills JL Sr., Conte MS, Armstrong DG, et al. The Society for Vascular Surgery lower extremity threatened limb classification system: risk stratification based on Wound, Ischemia, and foot Infection (WIfI). J Vasc Surg 2014;59:220–234.e1–2. [DOI] [PubMed] [Google Scholar]
- 22.Causey MW, Ahmed A, Wu B, et al. Society for Vascular Surgery limb stage and patient risk correlate with outcomes in an amputation prevention program. J Vasc Surg 2016;63:1563–1573.e2 [DOI] [PubMed] [Google Scholar]
- 23.Zhan LX, Branco BC, Armstrong DG, Mills JL Sr. The Society for Vascular Surgery lower extremity threatened limb classification system based on Wound, Ischemia, and foot Infection (WIfI) correlates with risk of major amputation and time to wound healing. J Vasc Surg 2015;61:939–944 [DOI] [PubMed] [Google Scholar]
- 24.Lipsky BA, Berendt AR, Cornia PB, et al.; Infectious Diseases Society of America . 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis 2012;54:e132–e173 [DOI] [PubMed] [Google Scholar]
- 25.Handelsman Y, Bloomgarden ZT, Grunberger G, et al. American Association of Clinical Endocrinologists and American College of Endocrinology--clinical practice guidelines for developing a diabetes mellitus comprehensive care plan--2015. Endocr Pract 2015;21(Suppl. 1):1–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boulton AJ, Armstrong DG, Albert SF, et al. Comprehensive foot examination and risk assessment. A report of the Task Force of the Foot Care Interest Group of the American Diabetes Association, with endorsement by the American Association of Clinical Endocrinologists. Phys Ther 2008;88:1436–1443 [DOI] [PubMed] [Google Scholar]
- 27.American Diabetes Association Glycemic targets. Sec 6. In Standards of Medical Care in Diabetes—2017. Diabetes Care 2017;40(Suppl. 1):S48–S56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cahn A, Raz I, Kleinman Y, et al. Clinical assessment of individualized glycemic goals in patients with type 2 diabetes: formulation of an algorithm based on a survey among leading worldwide diabetologists. Diabetes Care 2015;38:2293–2300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lazarus GS, Cooper DM, Knighton DR, Percoraro RE, Rodeheaver G, Robson MC. Definitions and guidelines for assessment of wounds and evaluation of healing. Wound Repair Regen 1994;2:165–170 [DOI] [PubMed] [Google Scholar]
- 30.Margolis DJ, Berlin JA, Strom BL. Interobserver agreement, sensitivity, and specificity of a “healed” chronic wound. Wound Repair Regen 1996;4:335–338 [DOI] [PubMed] [Google Scholar]
- 31.Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 1996;15:361–387 [DOI] [PubMed] [Google Scholar]
- 32.Falanga V. Wound healing and its impairment in the diabetic foot. Lancet 2005;366:1736–1743 [DOI] [PubMed] [Google Scholar]
- 33.Leung PC. Diabetic foot ulcers--a comprehensive review. Surgeon 2007;5:219–231 [DOI] [PubMed] [Google Scholar]
- 34.Dinh T, Tecilazich F, Kafanas A, et al. Mechanisms involved in the development and healing of diabetic foot ulceration. Diabetes 2012;61:2937–2947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kiecolt-Glaser JK, Marucha PT, Malarkey WB, Mercado AM, Glaser R. Slowing of wound healing by psychological stress. Lancet 1995;346:1194–1196 [DOI] [PubMed] [Google Scholar]
- 36.Marucha PT, Kiecolt-Glaser JK, Favagehi M. Mucosal wound healing is impaired by examination stress. Psychosom Med 1998;60:362–365 [DOI] [PubMed] [Google Scholar]
- 37.Norman D. The effects of stress on wound healing and leg ulceration. Br J Nurs 2003;12:1256–1263 [DOI] [PubMed] [Google Scholar]
- 38.Godbout JP, Glaser R. Stress-induced immune dysregulation: implications for wound healing, infectious disease and cancer. J Neuroimmune Pharmacol 2006;1:421–427 [DOI] [PubMed] [Google Scholar]
- 39.Gibbons CH, Freeman R. Treatment-induced neuropathy of diabetes: an acute, iatrogenic complication of diabetes. Brain 2015;138:43–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gibbons CH, Freeman R. Treatment-induced diabetic neuropathy: a reversible painful autonomic neuropathy. Ann Neurol 2010;67:534–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ohshima J, Nukada H. Hypoglycaemic neuropathy: microvascular changes due to recurrent hypoglycaemic episodes in rat sciatic nerve. Brain Res 2002;947:84–89 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.