Abstract
OBJECTIVE
In cystic fibrosis (CF), hemoglobin A1c (HbA1c) is thought to underestimate glycemia. However, few studies have directly assessed the relationship between HbA1c and average glucose in CF. We determined the relationships among glycemic markers—HbA1c, fructosamine (FA), glycated albumin (%GA), and 1,5-anhydroglucitol (1,5-AG)—and continuous glucose monitoring (CGM) in CF, hypothesizing that alternate markers would better predict average sensor glucose (ASG) than HbA1c.
RESEARCH DESIGN AND METHODS
CF participants and a group of healthy control subjects (HCs), ages 6–25 years, wore CGM for up to 7 days. Pearson correlations assessed the relationships between CGM variables and HbA1c, FA, %GA, and 1,5-AG. The regression line between HbA1c and ASG was compared in CF versus HC. Linear regressions determined whether alternate markers predicted ASG after adjustment for HbA1c.
RESULTS
CF (n = 93) and HC (n = 29) groups wore CGM for 5.2 ± 1 days. CF participants were 14 ± 3 years of age and 47% were male, with a BMI z score −0.1 ± 0.8 and no different from HCs in age, sex, or BMI. Mean HbA1c in CF was 5.7 ± 0.8% (39 ± 9 mmol/mol) vs. HC 5.1 ± 0.2% (32 ± 2 mmol/mol) (P < 0.0001). All glycemic markers correlated with ASG (P ≤ 0.01): HbA1c (r = 0.86), FA (r = 0.69), %GA (r = 0.83), and 1,5-AG (r = −0.26). The regression line between ASG and HbA1c did not differ in CF versus HC (P = 0.44). After adjustment for HbA1c, %GA continued to predict ASG (P = 0.0009) in CF.
CONCLUSIONS
HbA1c does not underestimate ASG in CF as previously assumed. No alternate glycemic marker correlated more strongly with ASG than HbA1c. %GA shows strong correlation with ASG and added to the prediction of ASG beyond HbA1c. However, we are not advocating use of HbA1c for diabetes screening in CF based on these results. Further study will determine whether glycemic measures other than ASG differ among different types of diabetes for a given HbA1c.
Introduction
A strong linear relationship between average glucose and hemoglobin A1c (HbA1c) in adults with type 1 and type 2 diabetes (1,2) has been well described. The Diabetes Control and Complications Trial (DCCT) and Epidemiology of Diabetes Interventions and Complications (EDIC) follow-up study have also demonstrated clear correlations between HbA1c and the development of microvascular and macrovascular complications in these populations (3,4). However, studies of the association between HbA1c and average glucose in individuals with cystic fibrosis (CF) have been limited, and there is controversy surrounding the utility of HbA1c in individuals with CF (5–7). HbA1c has been described as insensitive for diabetes screening compared with the oral glucose tolerance test (OGTT) in CF (5,8). There has also been speculation that HbA1c underestimates average glucose in CF owing to an increased rate of red blood cell turnover (9,10) and high vitamin E intake, resulting in decreased glycosylation (11). However, the evidence for these explanations is limited and the exact reasons behind why HbA1c might perform differently in individuals with CF are not well understood.
There is growing interest in the use of alternate markers of glycemia—fructosamine (FA), glycated albumin (GA), and 1,5-anhydroglucitol (1,5-AG)—for diabetes screening and glucose management (12–14), particularly in settings where HbA1c may be unreliable (15,16). With the exception of a small study including FA in adults with CF (7), the association between these alternate markers and average glucose in individuals with CF has not been studied.
Our group previously found that alternate glycemic markers correlate with multiple continuous glucose monitoring (CGM) variables (17) and that these markers are sensitive for detecting type 2 diabetes in obese youth with prediabetes (14). Therefore, we enrolled participants with CF and a group of healthy control subjects (HCs) into a study—the Glycemic Monitoring in Cystic Fibrosis Study (GeM-CF)—aimed at characterizing free-living glucose patterns across the glycemic spectrum and have collected alternate markers of glycemia in this cohort. Our objectives here were to examine the relationships among CGM measures (including measures of average glucose, hyperglycemia, and glucose variability) and HbA1c, FA, GA, and 1,5-AG in CF compared with HC groups. We also specifically examined the relationship between average sensor glucose (ASG) and HbA1c and compared our findings with those previously described in the DCCT and A1c-Derived Average Glucose (ADAG) cohorts (1,2). Given the speculation surrounding the relationship between HbA1c and average glucose in individuals with CF, we hypothesized that alternate measures of glycemia would better predict average glucose than HbA1c in this population. Furthermore, because studies in adults with type 1 and type 2 diabetes have suggested that these alternate markers, particularly 1,5-AG and GA, may better predict glucose variability than HbA1c (18,19), we also assessed whether alternate markers would outperform HbA1c in predicting glycemic variability—specifically, SD, coefficient of variation (CV), and mean amplitude of glycemic excursions (MAGE)—measured by CGM in our CF participants. Despite existing concerns regarding the HbA1c–average glucose relationship in CF, HbA1c is still commonly used for monitoring glycemic control in individuals with CF-related diabetes (CFRD). Given the importance of glycemic control in reducing pulmonary function decline and mortality in CF (20,21), the findings from this study will better define the optimal tool for monitoring of glycemic control in this population.
Research Design and Methods
Study Population
CF and HC participants ages 6–25 years were recruited. CF participants were recruited from our pulmonary and diabetes clinics. HC participants were identified from general endocrine clinics and with recruitment flyers and emails sent to faculty, staff, and students at the University of Colorado Anschutz Medical Campus. Inclusion criteria for CF participants included a diagnosis of CF by newborn screen, sweat chloride testing, or genetic testing. CF patients with glucose abnormalities along the entire glycemic spectrum (i.e., those with normal glycemia, abnormal glycemia, and CFRD) were included. Exclusion criteria for CF included a BMI >85th percentile, known type 1 or type 2 diabetes, use of medications affecting glucose other than insulin (e.g., systemic steroids) in the prior 3 months, changes in insulin dosing in the past 3 months, hospitalization in the prior 6 weeks, or pregnancy. Exclusion criteria for the HC group included known diagnosis of diabetes or prediabetes, BMI ≥85th percentile, chronic disease, acute illness, or pregnancy. This study was approved by the Colorado Multiple Institutional Review Board (Aurora, CO), and appropriate consent and assent were obtained.
Study Visit
Study visits took place in the Clinical and Translational Research Center at Children’s Hospital Colorado. Height, weight, BMI, and hip and waist circumference were obtained and physical exam and Tanner staging were completed by a pediatric endocrinologist. CF genotype, presence of pancreatic insufficiency, gastrostomy tube feedings, and use of a CFTR modulator, as well as baseline lung function data from the most recent pulmonary clinic visit, were collected via chart review.
All participants wore a blinded iPro2 CGM (Medtronic, Inc., Northridge, CA) for a minimum of 3 and up to 7 days. They were provided a glucometer (OneTouch; LifeScan) and trained to collect capillary blood glucoses four times daily—prior to meals and at bedtime—and to keep a food log during the week of CGM wear. HC participants underwent an OGTT and collection of baseline laboratories, including HbA1c and alternate glycemic markers—FA, GA, and 1,5-AG—on the day of CGM placement. CF participants returned 1 week after CGM placement to undergo the OGTT and collection of HbA1c and alternate glycemic markers. The rational for two study visits for CF patients was to collect CGM data preceding the venipuncture for collection of alternate markers. For example, 1,5-AG reflects glucose patterns in the preceding 2–4 days. However, as HC subjects are not expected to have significant CGM variability, and to minimize the burden associated with coming in for multiple study visits, we combined the CGM placement, blood draw, and OGTT into a single visit, with the CGM device returned by mail.
Laboratory Procedures
Participants arrived to the outpatient research center between 8:00 a.m. and 10:00 a.m. after a minimum of 8 h of fasting. CF participants with known diabetes on insulin were asked to withhold long-acting insulin for 24 h prior to the visit and short-acting insulin within 4 h of the study visit. Fasting blood glucose was obtained. Glucola was administered at a dose of 1.75 g/kg (maximum dose of 75 g) followed by a 1-h glucose measurement in CF participants and a 2-h glucose measurement in HC and CF participants. HbA1c and OGTT results were used to exclude prediabetes and diabetes in HC participants, and OGTT results were used to classify participants with CF into categories based on glycemic status: normal glycemia (fasting plasma glucose <100 mg/dL, 1-h glucose value <200 mg/dL, and 2-h glucose value <140 mg/dL), abnormal glycemia (fasting plasma glucose 100–125 mg/dL, 1-h glucose >200 mg/dL, and/or 2-h glucose 140–199 mg/dL), and CFRD (fasting plasma glucose ≥126 mg/dL and/or 2-h glucose ≥200 mg/dL).
HbA1c was measured on a DCA Vantage Analyzer (Siemens, Deerfield, IL), a DCCT-aligned instrument, with an interday CV of 2.8%. FA was measured on the Roche Modular P Chemistry Analyzer (Roche Diagnostics Corporation) using a colorimetric assay, with interassay CV of 3%. GA was measured with the Lucica GA-L assay (Asahi Kasei Pharma, Tokyo, Japan), an enzymatic method adapted to the Roche Analyzer and calculated as the percentage of GA relative to total albumin (%GA), with an interassay CV of 2.1% (mean 22.7%). Both FA and GA tests were run in Dr. Michael Steffes’ laboratory at the University of Minnesota. Because FA is also dependent on total protein but not routinely corrected for this, we used albumin as an estimate for total protein and also assessed FA-adjusted albumin by including albumin as a covariate in the model. 1,5-AG was measured with GlycoMark (Tomen America, New York, NY), a commercially available colorimetric assay, with an interassay CV of 4.1% at 4.67 μg/mL.
CGM Measures
CGM summary variables were calculated with R, version 3.1.1, software (R Foundation for Statistical Computing, Vienna, Austria [https://www.r-project.org]) after manual review of raw glucose values downloaded from CGM software. Analysis of CGM data has previously been described (22). Briefly, CGM measures were calculated in each participant in contiguous 24-h intervals to include an equal percentage of daytime versus nighttime sensor glucoses (288 sensor glucose values per day). Sensor data dependent on total duration of CGM wear, including time spent above/under a glucose cut point, area under the curve (AUC), and number of excursions, were averaged over the total days of CGM wear. MAGE was calculated using EasyGV, version 9.0.R2 (University of Oxford).
Statistical Analysis
Descriptive statistics were calculated by cohort (HC vs. CF). Group comparisons were performed using Satterthwaite two-sample t tests and Kolmogorov-Smirnov tests for continuous variables and χ2 and Fisher exact tests for categorical variables. Pearson correlation coefficients were calculated for HbA1c, 1,5-AG, FA, and %GA versus CGM measures for all CF participants. Multiple linear regression models were used to determine whether the alternative markers predicted a given CGM measure (e.g., mean glucose and measures of glycemic variability such as SD and MAGE) after adjustment for HbA1c. To test whether the relationship between ASG and HbA1c was similar in the CF cohort compared with HC, we used a linear regression model with an interaction term for HbA1c and HC. To evaluate whether the relationship between ASG and HbA1c was similar in the CF cohort compared with the DCCT and ADAG cohorts, we used the test described by Clogg et al. (23).
Results
A total of 135 youth and young adults (n = 101 CF and n = 34 HC) were enrolled. Of those with CF, four had incomplete CGM data, one had a diagnosis of CFTR-related metabolic syndrome, one did not return the glucometer for CGM calibration, and two were diagnosed with pulmonary exacerbations and started on steroids shortly after CGM placement. Among the HCs, four had incomplete CGM data and one had a prediabetes result on the OGTT and was excluded from analysis. Data from the remaining 93 CF and 29 HC participants were included in the final analysis. Descriptive statistics are presented in Table 1. There were no significant differences in age, BMI z score, Tanner stage, race, or sex between HC and CF participants. Of the participants with CF, 24 had normal glycemia, 41 had abnormal glycemia, and 28 had CFRD. CF participants had higher fasting and 1- and 2-h OGTT glucose values, higher HbA1c, %GA, and lower 1,5-AG than HC. FA values were higher in the overall group of CF participants compared with HC, but this did not reach statistical significance (P = 0.06).
Table 1.
Demographics and CGM measures
| CF | HC | P | |
|---|---|---|---|
| n | 93 | 29 | |
| Age (years) | 14 ± 3 | 14 ± 4 | 0.99 |
| Male | 44 (47) | 12 (41) | 0.58 |
| BMI z score | −0.09 ± 0.8 | −0.29 ± 0.7 | 0.21 |
| Weight (kg) | 49.4 ± 13.3 | 46.2 ± 14.7 | 0.31 |
| Height (cm) | 157.8 ± 13.3 | 155.0 ± 16.1 | 0.39 |
| Race | 0.20 | ||
| White | 83 (89) | 25 (86) | |
| Hispanic | 9 (10) | 2 (7) | |
| Other | 1 (1) | 2 (7) | |
| Genotype | |||
| Classes I–III | 84 (90) | ||
| Classes IV and V | 4 (4) | ||
| Unidentified | 5 (6) | ||
| Pancreatic insufficient | 90 (97) | ||
| Gastronomy tube feedings | 18 (19) | ||
| CFTR modulator use | 28 (30) | ||
| Insulin use | 23 (25) | ||
| FVC (%) | 98 ± 14 | ||
| FEV1 (%) | 90 ± 13 | ||
| Fasting plasma glucose (mg/dL) | 97 ± 22 | 88 ± 8 | <0.0007 |
| 1-h plasma glucose (mg/dL) | 201 ± 61 | 112 ± 26 (n = 12) | <0.0001 |
| 2-h plasma glucose (mg/dL) | 150 ± 50 | 107 ± 22 | <0.0001 |
| CF and normal glycemia | 24 (26) | ||
| CF and abnormal glycemia | 41 (44) | ||
| CF-related diabetes | 28 (30) | ||
| HbA1c [% (mmol/mol)] | 5.7 ± 0.8 (39 ± 9) | 5.1 ± 0.2 (32 ± 2) | <0.0001 |
| FA (mmol/L) | 243 ± 34 | 234 ± 16 | 0.06 |
| Total albumin (g/dL) | 4.2 ± 0.4 | 4.4 ± 0.3 | 0.03 |
| GA (%) | 13.0 ± 2.7 | 12.1 ± 0.9 | 0.01 |
| 1,5-AG (μg/mL) | 18.9 ± 7.4 | 23.7 ± 8.2 | 0.008 |
| CGM measures | |||
| Average glucose (mg/dL) | 116 ± 29 | 101 ± 10 | <0.0001 |
| Average day glucose (mg/dL) | 118 ± 31 | 101 ± 10 | <0.0001 |
| Average night glucose (mg/dL) | 112 ± 27 | 99 ± 9 | 0.0001 |
| Maximum day glucose (mg/dL) | 217 ± 59 | 148 ± 23 | <0.0001 |
| Maximum night glucose (mg/dL) | 170 ± 52 | 135 ± 23 | <0.0001 |
| Minimum glucose (mg/dL) | 63 ± 13 | 66 ± 10 | 0.21 |
| Average AUC/day (mg/min/dL) | 1.7 × 105 ± 4.2 × 104 | 1.4 × 105 ± 1.4 × 104 | <0.0001 |
| Excursions >140/day | 2.8 ± 1.5 | 0.8 ± 1 | <0.0001 |
| Excursions >200/day | 0.5 ± 0.7 | 0 ± 0 | <0.0001 |
| % time >140 mg/dL | 14 ± 17 | 1.9 ± 4.6 | <0.0001 |
| % time >200 mg/dL | 3 ± 13 | 0 ± 0 | 0.03 |
| % time <70 mg/dL | 2 ± 4 | 1.6 ± 2.4 | 0.18 |
| % time <60 mg/dL | 1 ± 3 | 0.2 ± 0.5 | 0.01 |
| SD (mg/dL) | 25 ± 13 | 13 ± 3 | <0.0001 |
| CV | 0.2 ± 0.06 | 0.1 ± 0.03 | <0.0001 |
| MAGE (mg/dL) | 58 ± 28 | 27 ± 8 | <0.0001 |
Data are mean ± SD or n (%) unless otherwise indicated. FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity.
CGM results are presented in Table 1. CF and HC wore CGM for a mean ± SD of 5.2 ± 1 days and obtained 4.0 ± 1 glucometer readings/day for calibration. CGM measures of average glucose, hyperglycemia (maximum glucose, % time spent >140 and >200 mg/dL, and excursions >140 and >200 mg/dL), and glycemic variability (SD, CV, and MAGE) were greater in the CF cohort compared with HC. Minimum sensor glucose and % time spent with glucose level <70 mg/dL were no different between the two groups. CGM results for the CF participants grouped by glycemic category are included in Supplementary Table 1.
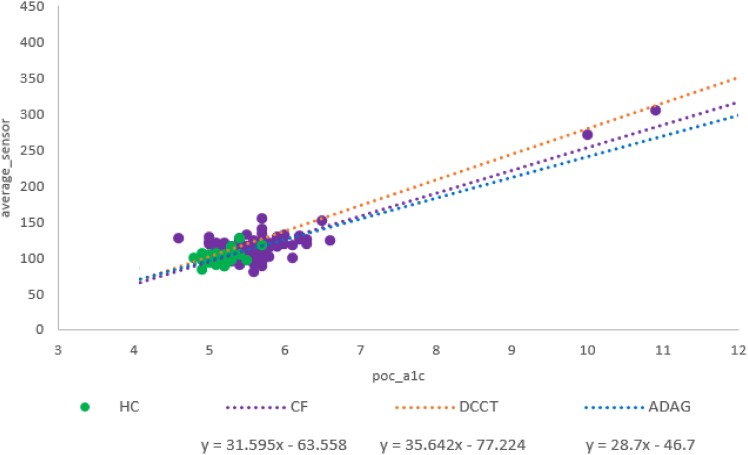
Figure 1 compares the regressions of HbA1c against average glucose in our CF cohort and in two historical studies of glucose profiles in adults with type 1 diabetes from the DCCT, as well as the ADAG study, which included adults with type 1 and type 2 diabetes as well as control subjects without diabetes. Data from HCs are also plotted. There was no difference in the relationship between HbA1c and average glucose between our CF and HC participants (P = 0.44). We next compared the relationship between HbA1c and average glucose in our CF group with that published in the ADAG study (1), and there were no significant differences in the slope (P = 0.15) or intercept (P = 0.16) between the two cohorts. We then compared the relationship between HbA1c and average glucose in our CF group with that published in the DCCT (2), and although there was a difference in the slope of the regression lines (P = 0.04), there was no significant difference in the intercept of the regression lines (P = 0.22), between the two cohorts.
Figure 1.
Scatterplots of HbA1c vs. ASG. ADAG = adults with type 1 diabetes and type 2 diabetes and control subjects in the study by Rohlfing et al. (2). DCCT = adults with type 1 diabetes in the study by Nathan et al. (1). CF and HC = our cohort.
Table 2 presents Pearson correlation coefficients between CGM variables and HbA1c and alternate glycemic markers in CF participants. All four glycemic measures correlated well with multiple CGM variables, including measures of average glucose, hyperglycemia, and glycemic variability. The magnitude of the correlation coefficients between these glycemic estimates and CGM variables was no greater with any of the alternative markers than with HbA1c. All four glycemic measures also correlated with fasting plasma glucose and the 2-h glucose on OGTT, and none of the alternate markers correlated with OGTT measures more strongly than HbA1c.
Table 2.
Pearson correlation coefficients between HbA1c and alternative markers versus CGM variables in CF participants
| CGM variable | HbA1c | 1,5-AG | FA | %GA |
|---|---|---|---|---|
| Average glucose | 0.86§ | −0.26* | 0.69§ | 0.83§ |
| Average day glucose | 0.87§ | −0.28† | 0.66§ | 0.83§ |
| Average night glucose | 0.77§ | −0.17 | 0.70§ | 0.78§ |
| Maximum day glucose | 0.51§ | −0.23* | 0.34† | 0.49§ |
| Maximum night glucose | 0.59§ | −0.18 | 0.48§ | 0.62§ |
| Minimum sensor glucose | −0.26* | 0.26* | −0.26* | −0.32† |
| Average AUC/day | 0.86§ | −0.26* | 0.69§ | 0.83§ |
| Excursions >140/day | −0.08 | 0.15 | −0.17 | −0.09 |
| Excursions >200/day | 0.31† | −0.18 | 0.16 | 0.27* |
| % time >140 mg/dL | 0.77§ | −0.23* | 0.61§ | 0.73§ |
| % time >200 mg/dL | 0.91§ | −0.36‡ | 0.78§ | 0.90§ |
| % time <70 mg/dL | −0.02 | −0.09 | −0.03 | −0.02 |
| % time <60 mg/dL | −0.01 | −0.03 | −0.05 | −0.02 |
| SD | 0.77§ | −0.34‡ | 0.55§ | 0.74§ |
| CV | 0.40§ | −0.30† | 0.21* | 0.34‡ |
| MAGE | 0.61§ | −0.32† | 0.41§ | 0.60§ |
| OGTT measures | ||||
| Fasting plasma glucose | 0.62§ | −0.27† | 0.61§ | 0.62§ |
| 1-h glucose | 0.29† | −0.23* | 0.18 | 0.26* |
| 2-h glucose | 0.57§ | −0.30† | 0.34‡ | 0.46§ |
*P < 0.05;
†P < 0.01;
‡P < 0.001;
§P < 0.0001.
Table 3 presents results of regression of the alternate glycemic markers on ASG, SD, CV, and MAGE, with adjustment for HbA1c in the CF cohort. Only %GA predicted variability in average glucose and SD beyond that explained by HbA1c alone. Assays for %GA take into account total albumin levels, an important consideration in individuals with CF, where malnutrition is a concern and albumin levels are often decreased. Our cohort of CF participants had significantly lower albumin levels than HC (P = 0.03, Table 1). However, FA measurements, which are also dependent on total protein, are not routinely adjusted for total albumin. Given the lower levels of albumin in our CF cohort, we performed further analysis of FA with adjustment for albumin. However, despite this correction, FA still failed to predict ASG, SD, CV, and MAGE beyond that explained by HbA1c.
Table 3.
Regression of alternate markers on CGM measures of glycemia in CF
| Model | Covariate | Outcome | Regression coefficient ± SE* | P |
|---|---|---|---|---|
| 1 | FA | Mean glucose | 0.09 ± 0.07 | 0.21 |
| 2 | FA | SD | −0.02 ± 0.04 | 0.63 |
| 3 | FA | MAGE | −0.07 ± 0.11 | 0.48 |
| 4 | FA | CV | −3 × 10–4 ± 3 × 10–4 | 0.29 |
| 5 | GA | Mean glucose | 3.79 ± 1.07 | 0.0009 |
| 6 | GA | SD | 1.51 ± 0.61 | 0.015 |
| 7 | GA | MAGE | 3.08 ± 1.71 | 0.08 |
| 8 | GA | CV | 0.003 ± 0.005 | 0.40 |
| 9 | 1,5-AG | Mean glucose | 0.18 ± 0.23 | 0.43 |
| 10 | 1,5-AG | SD | −0.15 ± 0.12 | 0.24 |
| 11 | 1,5-AG | MAGE | −0.48 ± 0.34 | 0.17 |
| 12 | 1,5-AG | CV | −0.001 ± 0.001 | 0.11 |
| 13 | FA-albumin adjusted | Mean glucose | 0.11 ± 0.08 | 0.16 |
| 14 | FA-albumin adjusted | SD | 0.02 ± 0.04 | 0.64 |
| 15 | FA-albumin adjusted | MAGE | 0.04 ± 0.12 | 0.76 |
| 16 | FA-albumin adjusted | CV | 2 × 10–5 ± 3 × 10–4 | 0.94 |
*Adjusted for HbA1c.
Conclusions
Our report is the largest to date to describe the relationship between average glucose and HbA1c in CF, and it is the first study to examine the relationships among three nontraditional markers of glycemia and multiple CGM measures in CF. Importantly, in contrast to previous reports in the literature, we found that HbA1c correlated well with multiple glycemic measures on CGM in CF and that HbA1c did not behave differently in CF than in HCs or in adults with type 1 or type 2 diabetes. Therefore, these results support a strong correlation between HbA1c and average glucose similar to that seen in individuals without diabetes, as well as those with type 1 and type 2 diabetes (1), and verify findings from a smaller previous report (6) in adults with CF. All nontraditional glycemic markers correlated with average glucose, but of the three, %GA showed the strongest relationship with average glucose, with a correlation similar to that seen between HbA1c and average glucose in this population.
We also found correlations between these glycemic markers and multiple components of the CGM profile. Notably, however, none of the alternate markers correlated more strongly with any CGM measure than HbA1c. Only one other article has examined the relationship between an alternate glycemic marker, FA, and average glucose in individuals with CFRD (7), but the sample size was small (n = 13) and HbA1c distribution narrow (mean ± SD 6.4 ± 0.6% and range 5.5–7.3%). These authors concluded that there was no relationship between FA and average glucose in CF. However, they also did not find a significant relationship between FA and average glucose in their equally small group of participants with type 1 diabetes (n = 15), a correlation that has been well documented in larger studies, suggesting that their findings were constrained by low power. No studies have previously examined GA or 1,5-AG as a tool for monitoring glycemic control in any CF population. Although both correlated with multiple CGM variables, a novel finding in our study is that GA performed comparably with HbA1c and, additionally, added to the prediction of mean glucose and SD even after adjustment for HbA1c. 1,5-AG has been touted as a useful marker of glycemic excursions (24); however, in this cohort of CF youth and young adults, it did not outperform HbA1c in predicting SD, CV, or MAGE. Notably, CF participants on insulin were instructed to hold long-acting insulin for 24 h and short-acting insulin for 4 h before the study visit, which conceivably could have impacted 1,5-AG results. However, we would have expected this to bias results in favor of this short-term alternate marker, which was not seen. Therefore, in scenarios where HbA1c may be unreliable, such as anemia or hemoglobinopathies in individuals with CF, GA, reported as %GA with correction for total albumin, appears to be a suitable alternative. However, its utility may be limited until the test becomes more widely available.
The relationship between HbA1c and average glucose has been called into question based on reports describing poor sensitivity of HbA1c for diagnosing CFRD detected by OGTT (5,25,26). Lanng et al. (26) reported a normal HbA1c in 70% of 46 patients diagnosed with CFRD by OGTT. Holl et al. (25) found that only 1 of 13 patients with CF diagnosed with diabetes by American Diabetes Association and World Health Organization criteria had an HbA1c >6.5%, while 3 had an HbA1c between 5.7 and 6.5% and 9 had a normal HbA1c (<5.7%); the 2-h glucose for patients with HbA1c <5.7% was no different than for those with HbA1c ≥5.7%. Burgess et al. (8) found that a lower HbA1c threshold of 5.8% appeared to improve sensitivity of this test to 93% for detecting diabetes by OGTT; in contrast, Boudreau et al. (5) retested this threshold, and in their cohort, HbA1c only had a sensitivity of 68% for identifying CFRD by OGTT. However, the conclusion that HbA1c therefore underestimates glycemia in CF based on comparisons with the OGTT is inaccurate, as HbA1c and OGTT are in fact measuring two different components of glycemia and are not interchangeable. HbA1c reflects a weighted mean of glucose levels over the past 3 months, while the OGTT 2-h glucose is a single measurement of an individual’s response to an oral glucose load. Therefore, a low concordance for diagnosing diabetes between the two tests in CF should not lead to the conclusion that HbA1c underestimates average glucose. Moreover, poor sensitivity of HbA1c in diagnosing diabetes by OGTT is not confined to CF and has been documented in other populations (27,28).
Only two small studies, to our knowledge, have attempted to directly examine the relationship between HbA1c and average glucose in CF, with contradictory results. Godbout et al. (7) did not find a relationship between HbA1c and mean plasma glucose (measured by capillary blood glucose testing pre- and postmeal, bedtime, and overnight), collected 3 days/month over 3 months in 13 adults with CF. However, the study may have been underpowered and without a wide enough distribution of HbA1c values (5.5–7.3% [37–56 mmol/mol]). In contrast, in a pilot study by Brennan et al. (6) on the relationship between HbA1c and mean glucose measured by CGM over a 48-h period in 20 adults with CF, 10 with CFRD, and a wider range of HbA1c values (4 to >9% [20 to >75 mmol/mol]), the authors found a relationship between HbA1c and mean glucose similar to that described in the DCCT. Our study confirms the findings of the latter article, shows an HbA1c and average glucose relationship similar to that in other populations with diabetes, and includes a much larger sample size of individuals with CF and wider range of HbA1c values (4.6–10.9%, 27–96 mmol/mol).
Increased red blood cell turnover in CF has been proposed as an explanation for lower HbA1c values in individuals with CF; however, evidence to support this claim is limited. Only two small studies on red blood cell half-life in adults with CF have been reported, one of which was a conference abstract (9,10). The study by Wagener et al. (10) found “minimally decreased” red blood cell turnover in 6 of 10 patients with CF. The conference abstract found no differences in red blood cell half-life in nine adults with CF compared with normal values, although two patients studied during acute exacerbation did have more rapid red blood cell turnover. In contrast, iron deficiency anemia has been reported as common in CF (29), and iron deficiency is typically associated with higher HbA1c owing to decreases in red blood cell turnover (11), further confounding this relationship. Furthermore, it is important to remember that significant interindividual variability in glycation rates exists and this phenomenon has been well described in individuals with type 1 diabetes (1,15). A recent study modeling hemoglobin glycation and red blood cell kinetics suggests that all glucose-independent variation in HbA1c may in fact be explained by interindividual differences in red blood cell turnover (30). Although iron indices and red blood cell kinetics were not assessed in our cohort, our findings suggest that the relationship between HbA1c and average glucose in individuals with CF is not significantly different from that seen within the general population.
Whether average glucose is the best measure of glycemic control is also a subject of debate. Other glycemic measures such as glucose variability—as measured by the amplitude and duration of glycemic excursions—are not reflected by HbA1c and have been proposed as potentially important determinants of micro- and macrovascular disease (31–33) in type 1 and type 2 diabetes. We previously reported associations between glycemic variability captured by CGM and retrospective lung function decline in CF (22). Nontraditional markers of glycemia have been proposed as potentially better indicators of acute glucose fluctuations (34) and glucose excursion (18) than HbA1c in other populations with diabetes. Therefore, we also assessed the correlations between HbA1c and our alternate markers, with SD, CV, and MAGE, and found good correlations, although only GA continued to explain some of this variability after adjustment for HbA1c.
Limitations
Our estimates for average glucose were generated from 1 week of CGM wear, while HbA1c represents a weighted measure of average glucose over the preceding 3 to 4 months. Glucose levels in the preceding 30 days have been found to contribute to ∼50% of the final HbA1c result, while glucose levels from 90 to 120 days prior only contribute ∼10% (2). Although the DCCT (via seven-point capillary blood glucose profiles submitted at quarterly visits) and ADAG studies collected measures of average glycemia intermittently over the preceding months, published reports have validated the Nathan equation and HbA1c–to–average blood glucose relationship from only 24 h of CGM data (35). In addition to red blood cell turnover, renal failure also impacts HbA1c owing to a complex interplay of contributing factors including uremia and alterations in red blood cell life span (11). We did not obtain measures of renal function, another limitation of this study, although renal failure is not a typical complication seen in adolescents with CF and would not be expected in an asymptomatic, healthy child. Most individuals in this study had HbA1c values <7%, which makes extrapolation of the HbA1c and average glucose relationship more difficult. We attempted to enroll as many participants as possible with higher HbA1c values, but given the early stage of disease in most youth with CF, we were limited by the range of CFRD severity in our clinic population. Although we would not expect physiology underlying the HbA1c–mean glucose relationship to change at different HbA1c values, whether adults with CF with lower pulmonary function or individuals with frequent pulmonary exacerbations and extended periods of chronic inflammation might have greater red blood cell turnover and a different HbA1c-ASG relationship requires further study. Furthermore, whether individuals with CF, compared with those with prediabetes or type 2 diabetes, for example, have greater hyperglycemia or glucose variability by measures other than average glucose for a given HbA1c requires further study.
Notably, whether HbA1c is an appropriate screening test for CFRD was not specifically addressed in this analysis, and we are not advocating use of HbA1c for routine CFRD screening based on this study’s findings. When OGTT cut points by American Diabetes Association criteria are applied to define CFRD, prior studies have found that HbA1c underperforms. Although the OGTT identifies individuals with CF with diabetes and clinical decline that improves after initiation of insulin therapy (36,37), current cut points were derived from populations with type 2 diabetes with the goal of identifying those at risk for retinopathy, and the best tests for screening and diagnosing CFRD are yet to be determined. Whether CFRD might be redefined by CF-specific cut points including abnormalities of intermediate OGTT time points (38) or CGM (39), in order to better identify those at risk for pulmonopathy or nutritional decline from abnormalities in glucose metabolism, requires further study.
Conclusion
Both HbA1c and nontraditional markers of glycemia correlate well with multiple glycemic patterns on CGM in CF. None of the alternate markers outperformed HbA1c at predicting average glucose, but we have extended the literature showing that GA is also correlated with glycemic control in CF. Prospective studies examining the relationship between glycemic outcomes other than average glucose or HbA1c and CF-relevant clinical outcomes are needed.
Supplementary Material
Article Information
Acknowledgments. The authors thank the patients and families as well as the Cystic Fibrosis Center at Children’s Hospital Colorado and the Colorado Clinical and Translational Sciences Institute (CCTSI) personnel.
Funding. This research was supported by National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (NIH), grant DK-094712-04; NIH grants TR-000154 (to CCTSI) and UL1-TR-001082 (to REDCap [Research Electronic Data Capture]); and Cystic Fibrosis Foundation Therapeutics grants CHAN16A0 and CHAN16GE0. Materials for the GA assay were provided by Asahi Kasei Pharma.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Industry contributors had no role in design, conduct, or reporting of this study.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. C.L.C. designed the study, researched data, and wrote the manuscript. E.H., J.T., T.V., and L.P. researched data and reviewed and edited the manuscript. P.S.Z. and K.J.N. designed the study, contributed to the discussion, and reviewed and edited the manuscript. C.L.C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the Western Medical Research Conference, Carmel, CA, 25–27 January 2018.
Footnotes
Clinical trial reg. no. NCT02211235, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc17-2419/-/DC1.
References
- 1.Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D, Heine RJ; A1c-Derived Average Glucose Study Group . Translating the A1C assay into estimated average glucose values. Diabetes Care 2008;31:1473–1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rohlfing CL, Wiedmeyer HM, Little RR, England JD, Tennill A, Goldstein DE. Defining the relationship between plasma glucose and HbA(1c): analysis of glucose profiles and HbA(1c) in the Diabetes Control and Complications Trial. Diabetes Care 2002;25:275–278 [DOI] [PubMed] [Google Scholar]
- 3.Nathan DM, Genuth S, Lachin J, et al.; Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 4.Nathan DM, Cleary PA, Backlund JY, et al.; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group . Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boudreau V, Coriati A, Desjardins K, Rabasa-Lhoret R. Glycated hemoglobin cannot yet be proposed as a screening tool for cystic fibrosis related diabetes. J Cyst Fibros 2016;15:258–260 [DOI] [PubMed] [Google Scholar]
- 6.Brennan AL, Gyi KM, Wood DM, Hodson ME, Geddes DM, Baker EH. Relationship between glycosylated haemoglobin and mean plasma glucose concentration in cystic fibrosis. J Cyst Fibros 2006;5:27–31 [DOI] [PubMed] [Google Scholar]
- 7.Godbout A, Hammana I, Potvin S, et al. . No relationship between mean plasma glucose and glycated haemoglobin in patients with cystic fibrosis-related diabetes. Diabetes Metab 2008;34:568–573 [DOI] [PubMed] [Google Scholar]
- 8.Burgess JC, Bridges N, Banya W, et al. . HbA1c as a screening tool for cystic fibrosis related diabetes. J Cyst Fibros 2016;15:251–257 [DOI] [PubMed] [Google Scholar]
- 9.Hardin DS, Grilley K, Baron B, Hale KA. Accelerated red blood cell turnover can invalidate the use of hemoglobin A1c as a diagnostic test for cystic fibrosis related diabetes (Abstract). Pediatr Res 1999;45:90A [Google Scholar]
- 10.Wagener JS, McNeill GC, Taussig LM, Corrigan JJ, Lemen R. Ferrokinetic and hematologic studies in cystic fibrosis patients. Am J Pediatr Hematol Oncol 1983;5:153–160 [PubMed] [Google Scholar]
- 11.Radin MS. Pitfalls in hemoglobin A1c measurement: when results may be misleading. J Gen Intern Med 2014;29:388–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nathan DM, McGee P, Steffes MW, Lachin JM; DCCT/EDIC Research Group . Relationship of glycated albumin to blood glucose and HbA1c values and to retinopathy, nephropathy, and cardiovascular outcomes in the DCCT/EDIC study. Diabetes 2014;63:282–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Selvin E, Francis LM, Ballantyne CM, et al. . Nontraditional markers of glycemia: associations with microvascular conditions. Diabetes Care 2011;34:960–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan CL, Pyle L, Kelsey M, Newnes L, Zeitler PS, Nadeau KJ. Screening for type 2 diabetes and prediabetes in obese youth: evaluating alternate markers of glycemia - 1,5-anhydroglucitol, fructosamine, and glycated albumin. Pediatr Diabetes 2016;17:206–211 [DOI] [PubMed] [Google Scholar]
- 15.Cohen RM, Holmes YR, Chenier TC, Joiner CH. Discordance between HbA1c and fructosamine: evidence for a glycosylation gap and its relation to diabetic nephropathy. Diabetes Care 2003;26:163–167 [DOI] [PubMed] [Google Scholar]
- 16.Desouza CV, Rosenstock J, Zhou R, Holcomb RG, Fonseca VA. Glycated albumin at 4 weeks correlates with A1c levels at 12 weeks and reflects short-term glucose fluctuations. Endocr Pract 2015;21:1195–1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan CL, Pyle L, Kelsey MM, et al. . Alternate glycemic markers reflect glycemic variability in continuous glucose monitoring in youth with prediabetes and type 2 diabetes. Pediatr Diabetes 2017;18:629–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshiuchi K, Matsuhisa M, Katakami N, et al. . Glycated albumin is a better indicator for glucose excursion than glycated hemoglobin in type 1 and type 2 diabetes. Endocr J 2008;55:503–507 [DOI] [PubMed] [Google Scholar]
- 19.Sun J, Dou JT, Wang XL, et al. . Correlation between 1,5-anhydroglucitol and glycemic excursions in type 2 diabetic patients. Chin Med J (Engl) 2011;124:3641–3645 [PubMed] [Google Scholar]
- 20.Adler AI, Shine B, Haworth C, Leelarathna L, Bilton D. Hyperglycemia and death in cystic fibrosis-related diabetes. Diabetes Care 2011;34:1577–1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bismuth E, Laborde K, Taupin P, Velho G, Ribault V, Jennane F, et al. . Glucose tolerance and insulin secretion, morbidity, and death in patients with cystic fibrosis. J Pediatr 2008;152:540–545, 545.e1 [DOI] [PubMed] [Google Scholar]
- 22.Chan CL, Vigers T, Pyle L, Zeitler PS, Sagel SD, Nadeau KJ. Continuous glucose monitoring abnormalities in cystic fibrosis youth correlate with pulmonary function decline. J Cyst Fibros. 23 March 2018 [Epub ahead of print]. DOI: 10.1016/j.jcf.2018.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clogg CC, Petkova E, Haritou A. Statistical methods for comparing regression coefficients between models. Am J Sociol 1995;100:1261–1293 [Google Scholar]
- 24.Dungan KM, Buse JB, Largay J, et al. . 1,5-anhydroglucitol and postprandial hyperglycemia as measured by continuous glucose monitoring system in moderately controlled patients with diabetes. Diabetes Care 2006;29:1214–1219 [DOI] [PubMed] [Google Scholar]
- 25.Holl RW, Buck C, Babka C, Wolf A, Thon A. HbA1c is not recommended as a screening test for diabetes in cystic fibrosis. Diabetes Care 2000;23:126. [DOI] [PubMed] [Google Scholar]
- 26.Lanng S, Hansen A, Thorsteinsson B, Nerup J, Koch C. Glucose tolerance in patients with cystic fibrosis: five year prospective study. BMJ 1995;311:655–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Picón MJ, Murri M, Muñoz A, Fernández-García JC, Gomez-Huelgas R, Tinahones FJ. Hemoglobin A1c versus oral glucose tolerance test in postpartum diabetes screening. Diabetes Care 2012;35:1648–1653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee JM, Wu EL, Tarini B, Herman WH, Yoon E. Diagnosis of diabetes using hemoglobin A1c: should recommendations in adults be extrapolated to adolescents? J Pediatr 2011;158:947–952.e1–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ater JL, Herbst JJ, Landaw SA, O’Brien RT. Relative anemia and iron deficiency in cystic fibrosis. Pediatrics 1983;71:810–814 [PubMed] [Google Scholar]
- 30.Malka R, Nathan DM, Higgins JM. Mechanistic modeling of hemoglobin glycation and red blood cell kinetics enables personalized diabetes monitoring. Sci Transl Med 2016;8:359ra130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hirsch IB. Glycemic variability and diabetes complications: does it matter? Of course it does! Diabetes Care 2015;38:1610–1614 [DOI] [PubMed] [Google Scholar]
- 32.Dasari PS, Gandomani BS, Teague AM, Pitale A, Otto M, Short KR. Glycemic variability is associated with markers of vascular stress in adolescents. J Pediatr 2016;172:47–55.e2 [DOI] [PubMed] [Google Scholar]
- 33.Monnier L, Mas E, Ginet C, et al. . Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA 2006;295:1681–1687 [DOI] [PubMed] [Google Scholar]
- 34.Buse JB, Freeman JL, Edelman SV, Jovanovic L, McGill JB. Serum 1,5-anhydroglucitol (GlycoMark ): a short-term glycemic marker. Diabetes Technol Ther 2003;5:355–363 [DOI] [PubMed] [Google Scholar]
- 35.O’Riordan SM, Danne T, Hanas R, Peters CJ, Hindmarsh P. Paediatric estimated average glucose in children with type 1 diabetes. Diabet Med 2014;31:36–39 [DOI] [PubMed] [Google Scholar]
- 36.Lanng S, Thorsteinsson B, Nerup J, Koch C. Diabetes mellitus in cystic fibrosis: effect of insulin therapy on lung function and infections. Acta Paediatr 1994;83:849–853 [DOI] [PubMed] [Google Scholar]
- 37.Moran A, Pekow P, Grover P, et al.; Cystic Fibrosis Related Diabetes Therapy Study Group . Insulin therapy to improve BMI in cystic fibrosis-related diabetes without fasting hyperglycemia: results of the cystic fibrosis related diabetes therapy trial. Diabetes Care 2009;32:1783–1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brodsky J, Dougherty S, Makani R, Rubenstein RC, Kelly A. Elevation of 1-hour plasma glucose during oral glucose tolerance testing is associated with worse pulmonary function in cystic fibrosis. Diabetes Care 2011;34:292–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hameed S, Morton JR, Jaffé A, et al. . Early glucose abnormalities in cystic fibrosis are preceded by poor weight gain. Diabetes Care 2010;33:221–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.