Abstract
OBJECTIVE
Hispanics/Latinos have the highest risks for metabolic syndrome (MetS) in the U.S. and are also at increased risk for Alzheimer disease. In this study, we examined associations among neurocognitive function, MetS, and inflammation among diverse middle-aged and older Hispanics/Latinos.
RESEARCH DESIGN AND METHODS
Cross-sectional data (2008–2011) from the Hispanic Community Health Study/Study of Latinos (HCHS/SOL) were analyzed to examine associations between neurocognition and MetS among diverse Hispanics/Latinos (N = 9,136; aged 45–74 years).
RESULTS
MetS status was associated with lower global neurocognition, mental status, verbal learning and memory, verbal fluency, and executive function. Age significantly modified the associations between MetS and learning and memory measures. Significant associations between MetS and neurocognition were observed among middle-aged Hispanics/Latinos, and all associations remained robust to additional covariates adjustment.
CONCLUSIONS
We found that MetS was associated with lower neurocognitive function, particularly in midlife. Our findings support and extend current hypotheses that midlife may be a particularly vulnerable developmental period for unhealthy neurocognitive aging.
Introduction
The metabolic syndrome (MetS) is a cluster of five overlapping risk factors for diabetes, heart disease, and neurocognitive impairment and disorders (1,2). MetS emerges in midlife and is related to the development of diabetes, heart disease, and stroke (3). MetS affects about one-third of U.S. adults and occurs earlier and more commonly among Hispanics/Latinos compared with other racial/ethnic groups (4). Like MetS, neurocognitive decline, mild cognitive impairment, and Alzheimer disease and related dementias (ADRD) are currently thought to emerge in midlife and progress with age (5–7). Although age and apoE4 genotypes are leading risks of ADRD, they are not modifiable. Thus, there is heightened interest in preventing neurocognitive decline and ADRD by identifying and modifying cardiovascular disease (CVD) risks earlier in the life course. As such, MetS prevention and control may also serve as effective means for reducing not only CVD, but also potentially neurocognitive decline and dementias (8), particularly among Hispanics/Latinos who are at high risk for MetS, ADRD, and low health care access (9).
Previous MetS studies reported associations with neurocognitive impairment and ADRD in older adults (10,11), but principally among those with high levels of inflammatory biomarkers (12–14). Less is known about middle age, when MetS emerges and neurocognitive function is thought to become vulnerable (8,15). In terms of primary prevention, middle age is likely when effective behavioral and pharmacological interventions are indicated to arrest MetS from further developing into diabetes, CVD events, and brain pathologies; that is, provided adequate health care is available, especially to the most vulnerable populations.
In this study, we examined associations among neurocognitive function, MetS, and inflammation among diverse middle-aged and older Hispanics/Latinos. Given that current opinions suggest that neurocognitive decline and ADRD pathology begins in midlife, we sought to compare midlife and later life associations among neurocognitive function, MetS, and inflammation in this important and vulnerable population of Hispanics/Latinos.
Research Design and Methods
Study Sample
We used data from the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). The HCHS/SOL is a large, epidemiological study of Hispanic/Latino men and women from diverse backgrounds. Recruitment and data collection occurred between 2008 and 2011 and included 16,415 participants from four major U.S. communities (Bronx, NY; Chicago, IL; Miami-Dade, FL; and San Diego, CA) with known large Hispanic/Latino concentrations. Study participants were sampled to ensure representation of Hispanic/Latino adults, aged 18–74 years at recruitment into the study, in the target populations. Sample size was set to allow for appropriate inferences to six major Hispanic/Latino groups, including Dominicans, Central Americans, Cubans, Mexicans, Puerto Ricans, South Americans, and other. Briefly, the HCHS/SOL was designed to collect detailed demographic, sociocultural, and health data through survey questionnaires and rich biological specimens (e.g., blood and urine) from consenting participants. The design of the study included a two-stage probability sampling approach that oversampled adults 45–74 years of age to allow appropriate inferences to this age-group given the age distributions of Hispanics/Latinos in the four communities of interest. A detailed discussion of the study objectives, design, and implementation has been published elsewhere (16,17).
Neurocognitive Tests
Neurocognitive tests were administered in quiet testing rooms in each of the four HCHS/SOL clinics. Participants were tested in their preferred language (English or Spanish) during face-to-face interviews by trained, bilingual research assistants. Four neurocognitive tests were used in this study: 1) Six-Item Screener (SIS) (18), 2) Brief Spanish English Verbal Learning Test (B-SEVLT) (19,20), 3) Word Fluency (WF) test of the Multilingual Aphasia Examination (21,22), and 4) Digit Symbol Subtest (DSS) (23). Three neurocognitive tests were only available in English (SIS, WF, and DSS). Therefore, they were translated by a certified translator and reviewed by the HCHS/SOL Translation Committee to ensure the Spanish translations were appropriate for diverse, Pan-American Latinos. In addition, the Neurocognitive Reading Center principal investigator (H.M.G.) reviewed the original and translated tests to ensure translation accuracy and test fidelity. The neurocognitive testing and scoring procedures used in HCHS/SOL have been previously described (24). Briefly, the SIS (range 0–6) is a mental status test that was scored dichotomously with a reported value of ≤4 representing cognitive impairment. The cut point reflects previous validation work in non-Latino white patients with dementia (18). However, because the SIS was not validated for dementia in Hispanics/Latinos, we will refer to a low SIS score plainly as low mental status. The B-SEVLT is an episodic learning and memory test with two scores: 1) the summed total correctly learned items across three learning trials (B-SEVLT-sum; range 0–45) and 2) total correctly recalled items (B-SEVLT-recall; range 0–15) following an interference trial. WF is a language and verbal fluency test (continuous) of summed correctly generated words (beginning with letters F and A) within 1 min (i.e., for each letter). DSS is a mental processing speed and executive function test that was a continuous total of correct responses (range 0–90).
We used generalized structural equation modeling for survey data to fit a confirmatory factor using the four continuous neurocognitive measures and the dichotomous mental status measure and subsequently derive a factor score (mean 50; SD 10) for a latent global neurocognition measure. In addition to modeling global neurocognition as a primary outcome, we also independently examined each of the four neurocognitive tests as well as low mental status (SIS ≤4). All continuous neurocognitive outcomes were standardized (Z scored) to facilitate cross-test result comparisons and interpretation of effect sizes in SD terms.
MetS
The MetS was measured following International Diabetes Federation (IDF) specifications (25). A participant was classified as meeting MetS if they satisfied criteria for abdominal obesity (≥94 cm for men and ≥80 cm for women) plus any two of four factors including 1) elevated triglyceride (TG) level (≥150 mg/dL) or treatment for TG abnormalities; 2) low HDL cholesterol (<40 mg/dL for men and <50 mg/dL for women) or treatment for HDL abnormalities; 3) elevated blood pressure (BP) (systolic BP ≥130 mmHg or diastolic BP ≥85 mmHg), based on the average of three 1-min separated BP readings, or use of antihypertensive medications; and 4) impaired fasting glucose (≥100 mg/dL), previous diabetes diagnosis, or use of antidiabetic medications.
Covariates
We accounted for four sociodemographic variables: age, sex, level of education (less than high school, high school or equivalent, and more than high school), and the six Hispanic/Latino backgrounds (i.e., Dominican, Central American, Cuban, Mexican, Puerto Rican, and South American, and other). We also controlled for depressive symptoms using the 10-item Center for Epidemiological Studies Depression Scale (CES-D-10) and for field center site to account for potential variations due to locales or study protocol implementation.
To examine differential effects due to inflammation, we used a joint modeling approach (equivalent to split modeling for stratified analyses) interacting inflammation groups (high vs. normal) hsCRP levels (hsCRP <3 vs. ≥3 mg/L) with all model covariates. This hsCRP threshold is associated with high CVD risk (26). In additional sensitivity analysis, we also controlled for nonsteroidal anti-inflammatory drug use because they are associated with inflammation reduction.
Analytic Procedures
All analyses were done using procedures for complex surveys in the Stata software package (14.1; StataCorp, College Station, TX). HCHS/SOL sampling weights were adjusted for nonresponse and calibrated to the 2010 U.S. Census. All estimates and inferences were generated using methods appropriate for subpopulation analyses and accounted for the stratification, clustering, and probability weighting in HCHS/SOL to allow correct generalizations to the target population. Our analytic sample included 9,136 Hispanic/Latino participants; Supplementary Fig. 1 details the study’s inclusion criteria. The study protocol was reviewed and approved by the institutional review boards at University of California, San Diego, and all other participating sites.
Our data analyses were conducted in three steps. First, we generated descriptive statistics to characterize the target subpopulation overall and by MetS status (Table 1). We tested for significant differences by MetS status using survey-adjusted χ2 tests for the categorical covariates (e.g., education) and two-tailed t tests for the continuous measures (e.g., CES-D-10). Second, we used survey generalized linear models (assuming a Gaussian distribution for continuous outcomes and logistic for the dichotomous low mental status indicator) to examine the associations between the MetS and neurocognition (Table 2). For each outcome, we fit two incremental models to 1) test age- and sex-adjusted associations and 2) examine attenuations due to control for education, Hispanic/Latino background, depressive symptoms, and study site. Given the importance of age to both neurocognition and metabolic health, we used interactions to account and test for differential nonadditive effects of continuous age on the associations between MetS status and neurocognition. To facilitate the interpretation of results, we calculated the marginal means of the continuous neurocognitive outcomes and marginal probabilities for the dichotomous SIS by MetS status and plotted them across the age continuum (Fig. 1). In sensitivity analyses, we independently adjusted for 1) antidiabetic and antihypertensive medications and 2) nonsteroidal anti-inflammatory drug use. Adjustment for these medications did not have any measurable effect on the reported results (H.M.G., W.T., unpublished observations).
Table 1.
Characteristics of the HCHS/SOL target population by MetS status
| No MetS (n = 4,270) | MetS (n = 4,866) | Total (n = 9,136) | P value | |
|---|---|---|---|---|
| Sex (%) | ||||
| Female | 52.9 | 56.3 | 54.7 | 0.0221 |
| Education (%) | 0.000 | |||
| Less than HS | 35.0 | 43.4 | 39.5 | |
| HS or equivalent | 21.7 | 20.7 | 21.2 | |
| More than HS | 43.3 | 35.9 | 39.3 | |
| Background (%) | 0.0039 | |||
| Dominican | 9.8 | 8.4 | 9.0 | |
| Central American | 6.4 | 6.7 | 6.6 | |
| Cuban | 25.2 | 29.9 | 27.7 | |
| Mexican | 32.8 | 29.4 | 30.9 | |
| Puerto Rican | 17.5 | 18.7 | 18.1 | |
| South American | 6.2 | 4.7 | 5.4 | |
| Other | 2.1 | 2.3 | 2.2 | |
| Language preference | ||||
| Spanish | 83.3 | 88.3 | 86.0 | 0.000 |
| Antihypertensives (%) | ||||
| Yes | 12.5 | 39.9 | 27.3 | 0.000 |
| Antidiabetics (%) | ||||
| Yes | 4.9 | 26.7 | 16.7 | 0.000 |
| Age, years (mean) | 54.8 (9.4) | 57.8 (9.9) | 56.5 (9.9) | 0.000 |
| CES-D-10 (mean) | 7.2 (7.7) | 7.8 (7.9) | 7.5 (7.8) | 0.001 |
| MetS components (%) | 0.000 | |||
| Abdominal obesity | 67.5 | 100.0 | 85.0 | |
| Impaired TG | 13.9 | 59.2 | 38.3 | |
| Impaired HDL | 15.1 | 60.0 | 39.2 | |
| Impaired BP | 35.7 | 76.3 | 57.6 | |
| Impaired fasting glucose | 23.9 | 73.3 | 50.5 | |
| MetS components count (%) | 0.000 | |||
| None | 8.6 | 0.0 | 4.0 | |
| 1 | 33.4 | 0.0 | 15.4 | |
| 2 | 52.9 | 0.0 | 24.4 | |
| 3 | 3.7 | 48.6 | 27.8 | |
| 4 | 1.4 | 34.4 | 19.2 | |
| 5 | 0.0 | 17.1 | 9.2 | |
| MetS components sum (mean) | 1.6 (0.9) | 3.7 (0.9) | 2.7 (1.6) | 0.000 |
| Cognitive outcomes | ||||
| B-SEVLT-sum (mean) | 22.9 (6.9) | 22 (6.8) | 22.4 (6.9) | 0.000 |
| B-SEVLT-recall (mean) | 8.2 (3.6) | 7.9 (3.5) | 8.1 (3.5) | 0.000 |
| WF (mean) | 19.2 (9) | 17.6 (8.6) | 18.3 (8.8) | 0.000 |
| DSS (mean) | 36.1 (16.7) | 32 (15.8) | 33.9 (16.4) | 0.000 |
| Global cognition (mean) | 51.1 (11.3) | 49.3 (11.2) | 50.2 (11.3) | 0.000 |
| SIS ≤4 (%) | 14.1 | 17.5 | 16.0 | 0.003 |
MetS and its components are defined using the IDF specifications. HS, high school.
Table 2.
Association of MetS with neurocognitive function among Hispanics/Latinos of diverse backgrounds
| β (SE) |
|||
|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |
| B-SEVLT-sum | |||
| MetS | −0.07 (0.025)** | 0.42 (0.162)* | 0.53 (0.153)*** |
| Age | −0.03 (0.002)*** | −0.02 (0.002)*** | −0.02 (0.002)*** |
| MetS*age | −0.01 (0.003)** | −0.01 (0.003)*** | |
| B-SEVLT-recall | |||
| MetS | −0.02 (0.022) | 0.47 (0.158)** | 0.58 (0.149)*** |
| Age | −0.03 (0.001)*** | −0.02 (0.002)*** | −0.02 (0.002)*** |
| MetS*age | −0.01 (0.003)** | −0.01 (0.003)*** | |
| WF | |||
| MetS | −0.15 (0.028)*** | −0.15 (0.185) | −0.09 (0.029)** |
| Age | −0.01 (0.002)*** | −0.01 (0.003)*** | −0.004 (0.002)* |
| MetS*age | 0.00 (0.003) | NA↟ | |
| DSS | |||
| MetS | −0.15 (0.022)*** | −0.23 (0.185) | −0.09 (0.021)*** |
| Age | −0.04 (0.002)*** | −0.04 (0.003)*** | −0.03 (0.001)*** |
| MetS*age | 0.0015 (0.003) | NA↟ | |
| Global cognition | |||
| MetS | −0.07 (0.023)** | 0.37 (0.158)* | 0.50 (0.144)*** |
| Age | −0.03 (0.002)*** | −0.03 (0.002)*** | −0.02 (0.002)*** |
| MetS*age | −0.01 (0.003)** | −0.01 (0.003)*** | |
| OR (95% CI) | |||
| Low mental status (SIS ≤4) | |||
| MetS | 1.13 (0.95; 1.34) | 1.71 (0.48; 6.16) | 1.07 (0.90; 1.29) |
| Age | 1.05 (1.04; 1.06)*** | 1.05 (1.03; 1.07)*** | 1.05 (1.04; 1.06)*** |
| MetS*age | 0.99 (0.97; 1.02) | NA↟ | |
Estimates based on survey-generalized linear regression models assuming a Gaussian distribution for continuous outcomes (Z scores) and logistic distribution for low mental status. MetS was defined using the IDF specifications. Model 1: age and sex adjusted; model 2: model 1 plus age*MetS interaction; model 3: model 2 plus education, Hispanic/Latino background, and CES-D-10 scale.
*P < 0.05;
**P < 0.01;
***P < 0.001;
↟Interaction excluded because not significant in model 2.
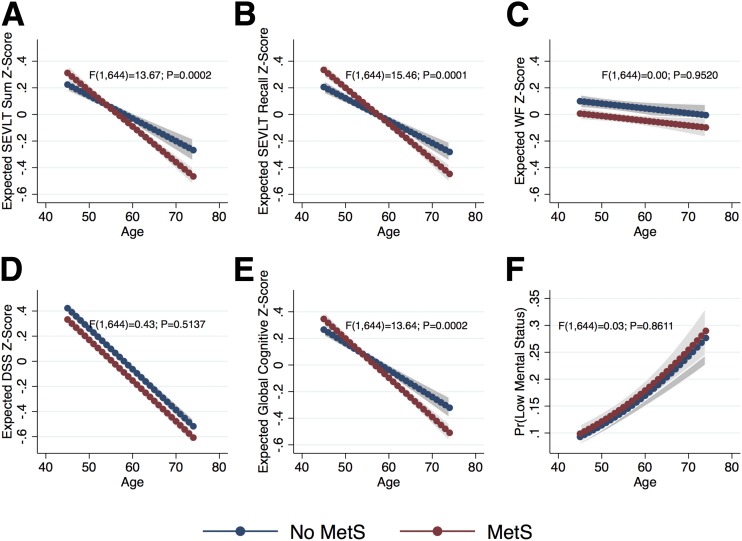
Figure 1.
A–F: Estimated marginal means (probabilities) of neurocognitive function over age by MetS status. Estimates based on survey-generalized linear regression models assuming a Gaussian distribution for continuous outcomes (Z scores) and logistic distribution for low mental status. MetS defined using the IDF specifications. Models include sex, age*MetS interaction, education, Hispanic/Latino background, and CES-D-10. Pr, predicted probability.
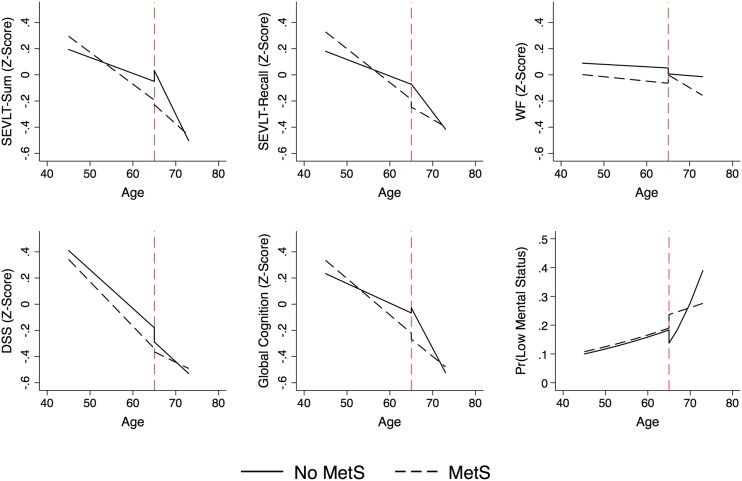
Additionally, we explicitly modeled and tested differential age slopes by MetS status for middle-aged and older adults using linear age splines with knots set at 65 years. Linear splines are piecewise regression models to examine prespecified hypotheses on differential nonlinear associations between outcomes and predictors. Theoretical discussions (27–29) and an applied perspective (30) that guided work in this manuscript have been published elsewhere. Briefly, the linear splines allow us to separately model, and subsequently test, the intercepts and slopes for age and age by MetS status interactions in the two distinct age groupings of middle-aged (45–64 years; n = 7,899) and older individuals (65–74 years; n = 1,237). Using 65 years as a threshold corresponds with and enables descriptive comparisons with published results on the associations between MetS and neurocognition from other cohort studies (13,14). The splines tests and P values are presented in Table 3, the estimated slopes underlying these tests are presented in Supplementary Table 1, and the predicted marginal means and probabilities by MetS status over age are presented in Fig. 2.
Table 3.
Tests of differences in slopes of neurocognitive function by age splines and MetS status
| Δ | SE | P value | |
|---|---|---|---|
| B-SEVLT-sum | |||
| Slope differences age <65 | −0.012 | 0.004 | 0.0066 |
| Slope differences age ≥65 | 0.038 | 0.020 | 0.0561 |
| No MetS: slope differences age <65 vs. age ≥65 | 0.055 | 0.016 | 0.001 |
| MetS: slope differences age <65 vs. age ≥65 | 0.005 | 0.013 | 0.711 |
| B-SEVLT-recall | |||
| Slope differences age <65 | −0.013 | 0.004 | 0.0027 |
| Slope differences age ≥65 | 0.024 | 0.020 | 0.2291 |
| No MetS: slope differences age <65 vs. age ≥65 | 0.031 | 0.016 | 0.053 |
| MetS: slope differences age <65 vs. age ≥65 | −0.007 | 0.014 | 0.62 |
| WF | |||
| Slope differences age <65 | −0.001 | 0.005 | 0.7519 |
| Slope differences age ≥65 | −0.017 | 0.025 | 0.5041 |
| No MetS: slope differences age <65 vs. age ≥65 | 0.001 | 0.021 | 0.97 |
| MetS: slope differences age <65 vs. age ≥65 | 0.016 | 0.016 | 0.307 |
| DSS | |||
| Slope differences age <65 | −0.004 | 0.004 | 0.2787 |
| Slope differences age ≥65 | 0.014 | 0.017 | 0.3929 |
| No MetS: slope differences age <65 vs. age ≥65 | 0.001 | 0.014 | 0.968 |
| MetS: slope differences age <65 vs. age ≥65 | −0.018 | 0.010 | 0.069 |
| Global cognition | |||
| Slope differences age <65 | −0.013 | 0.004 | 0.0036 |
| Slope differences age ≥65 | 0.035 | 0.020 | 0.0722 |
| No MetS: slope differences age <65 vs. age ≥65 | 0.047 | 0.016 | 0.003 |
| MetS: slope differences age <65 vs. age ≥65 | −0.001 | 0.014 | 0.926 |
| Low mental status | |||
| Slope differences age <65 | −0.002 | 0.018 | 0.9017 |
| Slope differences age ≥65 | −0.161 | 0.078 | 0.0383 |
| No MetS: slope differences age <65 vs. age ≥65 | −0.152 | 0.068 | 0.025 |
| MetS: slope differences age <65 vs. age ≥65 | 0.007 | 0.040 | 0.854 |
| Age <65 vs. age ≥65 (age65*MetS interaction) | |||
| B-SEVLT-sum | 0.0204 | ||
| B-SEVLT-recall | 0.0162 | ||
| WF | 0.7379 | ||
| DSS | 0.1223 | ||
| Global cognition | 0.0409 | ||
| Low mental status | 0.8723 |
Estimates based on survey-generalized linear regression models assuming a Gaussian distribution for continuous outcomes (Z scores) and logistic distribution for low mental status. MetS was defined using the IDF specifications. Models include sex, age splines (at age 65)*MetS interaction, education, Hispanic/Latino background, and CES-D-10 scale.
Figure 2.
Estimated marginal means (probabilities) of neurocognitive function by linear age splines over age by MetS status. Estimates based on survey-generalized linear regression models assuming a Gaussian distribution for continuous outcomes (Z scores) and logistic distribution for low mental status. MetS defined using the IDF specifications. Models include sex, age splines (at age 65)*MetS interaction, education, Hispanic/Latino background, and CES-D-10. Pr, predicted probability.
In order to provide additional information that could inform clinical and prevention practice, we examined individual MetS components in relation to neurocognitive function. Additionally, we examined neurocognitive performance as a function of a higher count of component indicators. To do so, we used similar survey-generalized linear models to separately examine the covariate-adjusted associations among the individual components of MetS, as well as a sum of the five individual components and our neurocognitive outcomes (Table 4). As with the overall syndrome models, we used interactions to test for differential nonadditive effects of continuous age on the associations between the individual predictors and neurocognition.
Table 4.
Association of individual MetS components and components count with neurocognitive function among Hispanics/Latinos of diverse backgrounds
| β (SE) |
OR (95% CI) |
|||||
|---|---|---|---|---|---|---|
| B-SEVLT-sum | B-SEVLT-recall | WF | DSS | Global cognition | Low mental status | |
| Component 1 | ||||||
| Abdominal obesity | 0.45 (0.232) | 0.33 (0.216) | −0.01 (0.033) | 0.05 (0.028) | 0.4 (0.212) | 0.9 (0.72; 1.12) |
| Age | −0.02 (0.003)*** | −0.02 (0.003)*** | −0.00 (0.002)** | −0.03 (0.001)*** | −0.02 (0.003)*** | 1.05 (1.04; 1.06)*** |
| Obesity*age | −0.01 (0.004) | −0.01 (0.004) | NA | NA | −0.01 (0.004) | NA |
| Component 2 | ||||||
| TGs | 0.06 (0.165) | 0.37 (0.160)* | −0.04 (0.024) | −0.05 (0.021)* | 0.14 (0.154) | 0.93 (0.77; 1.11) |
| Age | −0.02 (0.002)*** | −0.02 (0.002)*** | −0.00 (0.002)** | −0.03 (0.001)*** | −0.02 (0.002)*** | 1.05 (1.04; 1.06)*** |
| TGs*age | −0.002 (0.003) | −0.01 (0.003)* | NA | NA | −0.003 (0.003) | NA |
| Component 3 | ||||||
| HDL cholesterol | −0.08 (0.152) | 0.14 (0.156) | −0.07 (0.027)** | −0.06 (0.020)** | −0.04 (0.145) | 1.03 (0.87; 1.23) |
| Age | −0.02 (0.002)*** | −0.02 (0.002)*** | −0.00 (0.002)** | −0.03 (0.001)*** | −0.03 (0.002)*** | 1.05 (1.04; 1.06)*** |
| HDL*age | 0.001 (0.003) | −0.002 (0.003) | NA | NA | 0.00 (0.003) | NA |
| Component 4 | ||||||
| Elevated BP | 0.34 (0.161)* | 0.06 (0.152) | −0.09 (0.026)*** | −0.06 (0.021)** | 0.24 (0.150) | 1.08 (0.89; 1.31) |
| Age | −0.02 (0.003)*** | −0.02 (0.002)*** | 0 (0.002) | −0.03 (0.001)*** | −0.02 (0.002)*** | 1.05 (1.04; 1.06)*** |
| Elevated BP*age | −0.01 (0.003)* | −0.002 (0.003) | NA | NA | −0.01 (0.003) | NA |
| Component 5 | ||||||
| IFG | 0.53 (0.164)** | 0.42 (0.159)** | −0.07 (0.029)* | −0.06 (0.020)** | 0.47 (0.156)** | 1.12 (0.92; 1.36) |
| Age | −0.02 (0.002)*** | −0.02 (0.002)*** | −0.00 (0.002)* | −0.03 (0.001)*** | −0.02 (0.002)*** | 1.05 (1.04; 1.06)*** |
| IFG*age | −0.01 (0.003)*** | −0.01 (0.003)** | NA | NA | −0.01 (0.003)** | NA |
| Sum components | ||||||
| Sum | 0.16 (0.058)** | 0.17 (0.058)** | −0.04 (0.010)*** | −0.03 (0.008)*** | 0.15 (0.055)** | 1.01 (0.95; 1.08) |
| Age | −0.01 (0.003)*** | −0.01 (0.003)*** | −0.00 (0.002)* | −0.03 (0.001)*** | −0.02 (0.003)*** | 1.05 (1.04; 1.06)*** |
| Sum*age | −0.003 (0.001)** | −0.003 (0.001)** | NA | NA | −0.003 (0.001)** | NA |
| MetS | ||||||
| MetS | 0.53 (0.153)*** | 0.58 (0.149)*** | −0.09 (0.029)** | −0.09 (0.021)*** | 0.50 (0.144)*** | 1.07 (0.90; 1.29) |
| Age | −0.02 (0.002)*** | −0.02 (0.002)*** | −0.00 (0.002)* | −0.03 (0.001)*** | −0.02 (0.002)*** | 1.05 (1.04; 1.06)*** |
| MetS*age | −0.01 (0.003)*** | −0.01 (0.003)*** | NA | NA | −0.01 (0.003)*** | NA |
Estimates based on survey-generalized linear regression models assuming a Gaussian distribution for continuous outcomes (Z scores) and logistic distribution for low mental status. MetS and its components are defined using IDF specifications. Models adjust for age, sex, education, Hispanic/Latino background, and CES-D-10 scale. IFG, impaired fasting glucose; NA, interaction not included in the model.
*P < 0.05;
**P < 0.01;
***P < 0.001.
Finally, to test for possible differentiation by inflammation severity, we refit the models as described above and tested for differential effects in the association between MetS and neurocognition by normal (<3) and high (≥3) CRP groups. To do so, we used a joint modeling approach interacting the primary predictor and all covariates with the binary CRP indicator. The results for these analyses are presented in Supplementary Table 2, and the predicted marginal means and probabilities for each CRP group by MetS status over age are presented in Supplementary Fig. 2.
Results
Descriptive Statistics
More than half of Hispanics/Latinos in the target subpopulation satisfied criteria for MetS (54%), and the prevalence varied by age (50.4 vs. 67.2% among 45–64 and 65–74-year-olds, respectively). Descriptive characteristics for the target subpopulation and by MetS status are provided in Table 1. Slightly more than half of the target subpopulation (54.6%) was female, and the average age was 56.4 years. Individuals meeting criteria for MetS were older and more likely to be female, have less than a high school education, and use antihypertensive and antidiabetic medications.
Crude Differences in Neurocognitive Function
Individuals with MetS had lower average global neurocognitive performance and were more likely to satisfy criteria for low mental status. Additionally, MetS was uniformly associated with lower performances on B-SEVLT-sum, B-SEVLT-recall, WF, and DSS (Table 1).
Adjusted Associations
In age- and sex-adjusted models, MetS status was inversely associated with global neurocognition (β = −0.07 [SE 0.023]), B-SEVLT-sum (β = −0.07 [SE 0.025]), WF (β = −0.15 [SE 0.028]), and DSS (β = −0.15 [SE 0.022]) but not with B-SEVLT-recall or mental status (Table 2). Given our stated interest in midlife associations, we accounted for interactions between age and MetS, which provided evidence for significant modification effects with global neurocognition (βMain = 0.37 [SE 0.158]; βInteraction = −0.01 [SE 0.003]), particularly the memory measures, namely B-SEVLT-sum (βMain = 0.42 [SE 0.162]; βInteraction = −0.01 [SE 0.003]) and B-SEVLT-recall (βMain = 0.47 [SE 0.158]; βInteraction = −0.01 [SE 0.003]) (Table 2).
Adjusting for the additional covariates did not considerably change the associations between MetS and global neurocognition (βMain = 0.50 [SE 0.144]; βInteraction = −0.01 [SE 0.003]), B-SEVLT-sum (βMain = 0.53 [SE 0.153]; βInteraction = −0.01 [SE 0.003]), and B-SEVLT-recall (βMain = 0.58 [SE 0.149]; βInteraction = −0.01 [SE 0.003]). The associations between MetS and WF (β = −0.09 [SE 0.029]) and DSS (β = −0.09 [SE 0.021]) were attenuated by 36 and 39%, respectively. The estimated coefficients, SEs (for the continuous outcomes), and CIs (for the dichotomous SIS) are presented in Table 2. Figure 1F presents the fully adjusted marginal means for the neurocognitive scores and probability of low mental status over age by MetS status and their 95% CIs.
Age (Linear) Splines Models
We found that the effect of MetS on neurocognition was modified by age-groups (45–64 vs. ≥65 years) for global neurocognition and in particular for memory outcomes. We found that MetS status was associated with more pronounced age slopes among younger individuals (45–65 years), but not so among older individuals (≥65 years). Specifically, the age slope differences among younger individuals with MetS were steeper relative to those not meeting MetS criteria with respect to global cognition (Δ = −0.013 [SE 0.004]), B-SEVLT-sum (Δ = −0.012 [SE 0.004]), and B-SEVLT-recall (Δ = −0.013 [SE 0.004]). The differences in slopes between individuals with and without MetS were not statistically distinguishable among those in the ≥65 years group. Furthermore, among those not meeting criteria for MetS, the age slopes were more pronounced among older adults (≥65 years) relative to younger individuals with respect to global cognition (Δ = 0.047 [SE 0.016]), B-SEVLT-sum (Δ = 0.055 [SE 0.016]), and, to a slightly lesser extent, B-SEVLT-recall (Δ = 0.031 [SE 0.016]). The interaction tests and their P values for the age-groups and the slope contrasts for the linear age splines by MetS status are included in Table 3, and the corresponding slopes are plotted in Fig. 2. The estimated slopes for the linear age splines, their SEs, and P values are included in Supplementary Table 1 to facilitate a clearer reading of the plots included in Fig. 2.
Associations with MetS Components
There were notable variations in the associations between the individual components and neurocognitive outcomes (Table 4). We found significant age-modified effects of fasting glucose in terms of performance on global neurocognition (βMain = 0.47 [SE 0.156]; βInteraction = −0.01 [SE 0.003]), learning memory (βMain = 0.53 [SE 0.164]; βInteraction = −0.01 [SE 0.003]), and recall (βMain = 0.42 [SE 0.159]; βInteraction = −0.01 [SE 0.003]). We also found age-modified effects of elevated BP on learning and memory scores (βMain = 0.34 [SE 0.161]; βInteraction = −0.01 [SE 0.003]). Elevated TGs (β = −0.05 [SE 0.021]), HDL (β = −0.06 [SE 0.020]), BP (β = −0.06 [SE 0.021]), and fasting glucose levels (β = −0.06 [SE 0.020]) were linked to lower, non–age-modified performance on DSS. HDL (β = −0.07 [SE 0.027]), BP (β = −0.09 [SE 0.026]), and fasting glucose levels (β = −0.07 [SE 0.029]) were also linked to lower performance on the WF test. Finally, a higher count of MetS components was consistently associated with a more pronounced decrease over age in global cognition and the two memory measures (Table 3) and with lower, non–age-modified scores on the DSS (β = −0.03 [SE 0.008]) and WF (β = −0.04 [SE 0.010]). As with the overall syndrome, there were no statistically significant associations between the individual components or their count and lower mental status scores.
CRP-Differentiated Models
We found no effect modifications by inflammation severity in the relationships between MetS and neurocognition (Supplementary Table 2). The adjusted marginal means for the neurocognitive scores and probability of low mental status over age by MetS status for each CRP group and their 95% CIs are presented in Supplementary Fig. 2.
Conclusions
Based on this large sample of diverse middle-aged and older Hispanics/Latinos, we found that MetS was related to lower neurocognitive function in midlife and, in a less pronounced way, among older-aged adults. Our finding supports and extends current hypotheses that midlife may be a particularly vulnerable period for the development of neurocognitive decline. Secondly, we found that midlife episodic learning and memory were particularly vulnerable to MetS effects, which has direct implications for the development of memory impairment, which is the hallmark of AD. Thirdly, we found no evidence that inflammation (hsCRP) moderated associations between MetS and neurocognitive performance in middle age or in later life, which differs from previous studies of older Hispanics/Latinos (14). In our large and representative sample of middle-aged and older Hispanics/Latinos, over half met MetS criteria, which is consistent with national estimates from National Health and Nutrition Examination Survey (NHANES) (4). It is noteworthy that nationally, the rates of MetS among Hispanics/Latinos have steadily increased over the past decade (4). At the population level, our findings suggest that these increasing rates of MetS could have negative implications for neurocognitive aging among Hispanics/Latinos.
Previous studies have report mixed results on lower neurocognitive function among older adults meeting criteria for MetS (13,14,31–33). Authors of a recent systematic review concluded that the inconsistent findings could be explained by the age-modifying effects of MetS on lower neurocognitive function. That is, the MetS effects on neurocognition were pronounced among the young-old (i.e., <70 years) compared with older (i.e., ≥70 years) adults (34). Furthermore, among adults >70 years, MetS was not found to be associated with lower neurocognitive function and was reportedly protective against neurocognitive decline (35,36). We found that those meeting criteria for MetS age-associated neurocognitive decrements were consistent in both middle age and older adulthood. In contrast, among participants not meeting criteria for MetS, there were more marked neurocognitive decrements among older adults relative to those in middle age. This suggests that MetS is a trigger for neurocognitive change and decline starting in middle age. An alternative explanation for the less pronounced neurocognitive MetS effects in very old adults comes from a recent meta-analysis of aggregated (1.3 million participants), longitudinal cohort data of BMI (a MetS component). Firstly, higher midlife BMI was associated with increased all-cause mortality, whereas higher BMI was related to lower all-cause mortality among very old adults (≥85 years) (37). Secondly, weight loss is commonly observed clinically in very old patients with concomitant neurocognitive decline, particularly among those in terminal decline. Both explanations could provide insights into the lack of a robust negative association between neurocognition and MetS we observed among older adults in this study. The age-related MetS associations with neurocognition findings in our Hispanic/Latino cohort study were consistent with the conclusion of this systematic review and other previous studies. Middle age has been often overlooked in the existing literature due to common misconceptions that neurocognitive problems are restricted to older age. Our findings provide additional evidence and support for recognizing that midlife may be a particularly vulnerable period of neurocognitive aging. As such, our study results indicate that there may be a window of opportunity for MetS intervention in midlife. Although preliminary, additional biomarker (e.g., neuroimaging) and clinical information will help elucidate the nature of our age-related observations among Hispanics/Latinos.
In this study, episodic learning and memory were most notably related to MetS, which is important because memory impairment is a hallmark symptom of AD, the most common form of dementing disorders. As noted above, the relationship between neurocognitive function and MetS was modified by age. Secondly, executive function was lower among individuals with MetS compared with the MetS-free group, which is consistent with previous work (34). Unlike episodic learning and memory, the associations between executive function and MetS were not significantly modified by age. That is, the associations between MetS with executive function were similar between middle-aged and older Hispanics/Latinos in HCHS/SOL. This finding also suggests executive function may be more continuously vulnerable to MetS throughout middle age and older adulthood.
A closer examination of the relationships between the individual neurocognitive tests and MetS components revealed a pattern in which the associations between age and lower episodic learning and memory scores were most strongly modified by impaired fasting glucose. Additionally in our study, verbal fluency, processing speed, and executive function were associated with multiple MetS components. Nevertheless, our finding that having a higher count of impaired MetS components was associated with lower neurocognitive performances emphasizes the value of examining this cluster of symptoms in relation to brain function and health. As a whole, MetS effect sizes in relation to neurocognition were small. However, MetS was associated with the equivalent of ≥2 years of global neurocognitive aging when compared with MetS-free participants in this study. At the population level, such savings in neurocognitive aging in this highly vulnerable population could potentially translate into more years free of neurocognitive impairment and its associated social, physical, and economic burdens.
In contrast to previous studies (13,14), inflammation was not related to neurocognition and MetS in HCHS/SOL. It is unclear why inflammation had no effects on the relationships between MetS and neurocognitive function in this study. One likely explanation derives from the relative youth of the HCHS/SOL sample compared with previous studies, which primarily consisted of older adults. It may be that MetS is an early and primary catalyst for lower neurocognitive function, whereas inflammation is a secondary response that may be more apparent later in life. Clearly, our single cross-sectional study must be replicated in other populations. However, the biological brain mechanism in middle age merits further investigation in humans and nonhuman models, which may provide additional insights for earlier preventive and therapeutic opportunities. Nevertheless, our results point to MetS as a potential target for interventions aimed at maintaining neurocognitive health in diverse Hispanics/Latinos beginning in middle age.
There are several limitations that readers should note when interpreting these study results. Firstly and as mentioned above, this was a cross-sectional study, which precludes causal inferences. As such, we were unable to observe neurocognitive decline. Additionally, we did not ascertain mild cognitive impairment or ADRD. Secondly, although large, our sample was representative of Hispanics/Latinos in our targeted communities. Our sample is not nationally representative. HCHS/SOL is a large prospective cohort study that is focused primarily on heart disease, and the neurocognitive battery was limited. This limitation contributed to our inability to identify participants meeting criteria for mild cognitive impairment or ADRD. Thirdly, the HCHS/SOL cohort is relatively young (18–74 years), and the number of participants >65 years of age is relatively small compared with those in middle age, which could have limited our ability to detect significant associations between neurocognition and MetS among older participants. Fourthly, we did not objectively measure English/Spanish language proficiency and relied exclusively on study participants’ preferred language for test administration, which could introduce some imprecision to our results. It is noteworthy, however, that >85% of participants preferred testing in Spanish. Fifthly, we only examined one inflammatory marker (hsCRP) to replicate previous findings, and other markers could yield divergent results. Sixthly, HCHS/SOL did not include older adults >74 years of age. Consequently, we may have been unable to find any MetS-protective effects reported in other studies of very old adults. Furthermore, HCHS/SOL is a unique population of middle-aged and older Hispanics/Latinos, a diverse population representing nearly one-fifth of the U.S. population that has a very high prevalence of MetS. Future studies are planned to examine Hispanic/Latino diversity in relation neurocognition and MetS, including sex differences by Hispanic/Latino background. Diverse Hispanics/Latinos have been overlooked and long neglected in any health science, but particularly neurocognitive aging and ADRD research.
Conclusion
Based on this representative sample of middle-aged and older Hispanics/Latinos, >54% met MetS criteria (24). We found that MetS was associated with lower neurocognitive function, particularly among middle-aged adults (4,9). As such, preventing and managing MetS in this vulnerable population has important public health implications.
Supplementary Material
Article Information
Acknowledgments. The authors thank the staff of and participants in HCHS/SOL for important contributions (see http://www.cscc.unc.edu/hchs/ for a list of investigators).
Funding. H.M.G. and W.T. received support for this work from National Institute on Aging (NIA) grants R01-AG048642, RF1-AG054548, and R21-AG053760. H.M.G. also received support from NIA grant P30-AG005131 and W.T. from NIA grant P30-AG053760. They previously received support from National Heart, Lung, and Blood Institute (NHLBI) grant HC65233. The HCHS/SOL was carried out as a collaborative study supported by contracts from the NHLBI to the University of North Carolina (N01-HC65233), University of Miami (N01-HC65234), Albert Einstein College of Medicine (N01-HC65235), Northwestern University (N01-HC65236), and San Diego State University (N01-HC65237). The following institutes/centers/offices contributed to the HCHS/SOL through a transfer of funds to the NHLBI: National Institute on Minority Health and Health Disparities, National Institute on Deafness and Other Communication Disorders, National Institute of Dental and Craniofacial Research, National Institute of Diabetes and Digestive and Kidney Diseases, National Institute of Neurological Disorders and Stroke, and the National Institutes of Health (NIH) Office of Dietary Supplements. This work was supported by the NIH.
The contents of this work are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. H.M.G. conceived and researched the field. W.T. cowrote the manuscript and conducted the analyses. P.V. and A.H.S. assisted in drafting and editing the manuscript. N.I.R., S.D., C.J.R., L.C.G., B.T., M.D., T.K., and N.S. reviewed and edited the manuscript. J.C. edited the manuscript and provided statistical expertise. H.M.G. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc17-1896/-/DC1.
References
- 1.Grundy SM, Cleeman JI, Daniels SR, et al.; American Heart Association; National Heart Lung, and Blood Institute . Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement: Executive Summary. Circulation 2005;112:e285–e290 [Google Scholar]
- 2.Grundy SM, Brewer HB Jr, Cleeman JI, Smith SC Jr, Lenfant C; National Heart, Lung, and Blood Institute; American Heart Association . Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Arterioscler Thromb Vasc Biol 2004;24:e13–e18 [DOI] [PubMed] [Google Scholar]
- 3.Arenillas JF, Moro MA, Dávalos A. The metabolic syndrome and stroke: potential treatment approaches. Stroke 2007;38:2196–2203 [DOI] [PubMed] [Google Scholar]
- 4.Aguilar M, Bhuket T, Torres S, Liu B, Wong RJ. Prevalence of the metabolic syndrome in the United States, 2003-2012. JAMA 2015;313:1973–1974 [DOI] [PubMed] [Google Scholar]
- 5.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med 2004;256:183–194 [DOI] [PubMed] [Google Scholar]
- 6.Mueller SG, Weiner MW, Thal LJ, et al. Ways toward an early diagnosis in Alzheimer’s disease: the Alzheimer’s Disease Neuroimaging Initiative (ADNI). Alzheimers Dement 2005;1:55–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vemuri P, Knopman DS, Lesnick TG, et al. Evaluation of amyloid protective factors and Alzheimer disease neurodegeneration protective factors in elderly individuals. JAMA Neurol 2017;74:718–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Misiak B, Leszek J, Kiejna A. Metabolic syndrome, mild cognitive impairment and Alzheimer’s disease--the emerging role of systemic low-grade inflammation and adiposity. Brain Res Bull 2012;89:144–149 [DOI] [PubMed] [Google Scholar]
- 9.Henry J. Kaiser Family Foundation. Uninsured rates for the nonelderly by race/ethnicity, U.S. [Internet]. Available from https://www.kff.org/uninsured/state-indicator/rate-by-raceethnicity. Accessed 12 March 2018 [Google Scholar]
- 10.Bokura H, Nagai A, Oguro H, Kobayashi S, Yamaguchi S. The association of metabolic syndrome with executive dysfunction independent of subclinical ischemic brain lesions in Japanese adults. Dement Geriatr Cogn Disord 2010;30:479–485 [DOI] [PubMed] [Google Scholar]
- 11.Panza F, Frisardi V, Capurso C, et al. Metabolic syndrome and cognitive impairment: current epidemiology and possible underlying mechanisms. J Alzheimers Dis 2010;21:691–724 [DOI] [PubMed] [Google Scholar]
- 12.Taylor VH, MacQueen GM. Cognitive dysfunction associated with metabolic syndrome. Obes Rev 2007;8:409–418 [DOI] [PubMed] [Google Scholar]
- 13.Yaffe K, Haan M, Blackwell T, Cherkasova E, Whitmer RA, West N. Metabolic syndrome and cognitive decline in elderly Latinos: findings from the Sacramento Area Latino Study of Aging study. J Am Geriatr Soc 2007;55:758–762 [DOI] [PubMed] [Google Scholar]
- 14.Yaffe K, Kanaya A, Lindquist K, et al. The metabolic syndrome, inflammation, and risk of cognitive decline. JAMA 2004;292:2237–2242 [DOI] [PubMed] [Google Scholar]
- 15.Feng L, Chong MS, Lim WS, et al. Metabolic syndrome and amnestic mild cognitive impairment: Singapore Longitudinal Ageing Study-2 findings. J Alzheimers Dis 2013;34:649–657 [DOI] [PubMed] [Google Scholar]
- 16.Lavange LM, Kalsbeek WD, Sorlie PD, et al. Sample design and cohort selection in the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol 2010;20:642–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sorlie PD, Avilés-Santa LM, Wassertheil-Smoller S, et al. Design and implementation of the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol 2010;20:629–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Callahan CM, Unverzagt FW, Hui SL, Perkins AJ, Hendrie HC. Six-item screener to identify cognitive impairment among potential subjects for clinical research. Med Care 2002;40:771–781 [DOI] [PubMed] [Google Scholar]
- 19.González HM, Mungas D, Reed BR, Marshall S, Haan MN. A new verbal learning and memory test for English- and Spanish-speaking older people. J Int Neuropsychol Soc 2001;7:544–555 [DOI] [PubMed] [Google Scholar]
- 20.González HM, Mungas D, Haan MN. A verbal learning and memory test for English- and Spanish-speaking older Mexican-American adults. Clin Neuropsychol 2002;16:439–451 [DOI] [PubMed] [Google Scholar]
- 21.Lezak M, Howieson DB, Loring DW. Neuropsychological Assessment. New York, Oxford University Press, 2004 [Google Scholar]
- 22.Benton AL, Hamsher K. Multilingual Aphasia Examination. 2nd ed. Iowa City, IA, AJA Associates, 1989 [Google Scholar]
- 23.Wechsler D. WAIS-R Manual: Wechsler Adult Intelligence Scale - Revised. San Antonio, TX, Psychological Corporation, 1981 [Google Scholar]
- 24.González HM, Tarraf W, Gouskova N, et al. Neurocognitive function among middle-aged and older Hispanic/Latinos: results from the Hispanic Community Health Study/Study of Latinos. Arch Clin Neuropsychol 2015;30:68–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alberti KG, Eckel RH, Grundy SM, et al.; International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; International Association for the Study of Obesity . Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009;120:1640–1645 [DOI] [PubMed] [Google Scholar]
- 26.Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation 2003;107:363–369 [DOI] [PubMed] [Google Scholar]
- 27.Suits DB, Mason A, Chan L. Spline functions fitted by standard regression methods. Rev Econ Stat 1978;60:132–139 [Google Scholar]
- 28.Smith PL. Splines as a useful and convenient statistical tool. Am Stat 1979;33:57–62 [Google Scholar]
- 29.Marsh LC, Cormier DR. Spline regression models [Internet], 2002. Available from http://methods.sagepub.com/book/spline-regression-models. Accessed 10 March 2018
- 30.Mitchell MN. Interpreting and Visualizing Regression Models Using Stata. Philadelphia, Taylor & Francis, 2012 [Google Scholar]
- 31.Liu CL, Lin MH, Peng LN, et al. Late-life metabolic syndrome prevents cognitive decline among older men aged 75 years and over: one-year prospective cohort study. J Nutr Health Aging 2013;17:523–526 [DOI] [PubMed] [Google Scholar]
- 32.Liu M, He Y, Jiang B, et al. Association between metabolic syndrome and mild cognitive impairment and its age difference in a Chinese community elderly population. Clin Endocrinol (Oxf) 2015;82:844–853 [DOI] [PubMed] [Google Scholar]
- 33.Watts AS, Loskutova N, Burns JM, Johnson DK. Metabolic syndrome and cognitive decline in early Alzheimer’s disease and healthy older adults. J Alzheimers Dis 2013;35:253–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siervo M, Harrison SL, Jagger C, Robinson L, Stephan BC. Metabolic syndrome and longitudinal changes in cognitive function: a systematic review and meta-analysis. J Alzheimers Dis 2014;41:151–161 [DOI] [PubMed] [Google Scholar]
- 35.Harrison SL, Stephan BC, Siervo M, et al. Is there an association between metabolic syndrome and cognitive function in very old adults? The Newcastle 85+ Study. J Am Geriatr Soc 2015;63:667–675 [DOI] [PubMed] [Google Scholar]
- 36.van den Berg E, Biessels GJ, de Craen AJM, Gussekloo J, Westendorp RGJ. The metabolic syndrome is associated with decelerated cognitive decline in the oldest old. Neurology 2007;69:979–985 [DOI] [PubMed] [Google Scholar]
- 37.Kivimäki M, Luukkonen R, Batty GD, et al. Body mass index and risk of dementia: analysis of individual-level data from 1.3 million individuals. Alzheimers Dement 2018;14:601–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.