Abstract
OBJECTIVE
Evidence for long-term translational effectiveness of lifestyle interventions in minority populations is scarce. This article reports long-term outcomes, for up to 10 years, of such an intervention to prevent diabetes in American Indian and Alaska Native (AI/AN) communities.
RESEARCH DESIGN AND METHODS
From January 2006 to July 2016, the Special Diabetes Program for Indians Diabetes Prevention Program implemented the Diabetes Prevention Program lifestyle intervention among 46 AI/AN health care programs. Enrolled participants underwent a thorough clinical assessment at baseline, after completing the Lifestyle Balance Curriculum (postcurriculum assessment), and annually thereafter. Proportional hazards regression was used to estimate the association between diabetes incidence and postcurriculum weight loss status.
RESULTS
Of 8,652 enrolled participants, 65% finished the postcurriculum assessment. The assessment completion rate diminished over time to 13% in year 10. Among those with postcurriculum weight measurements, 2,028 (36%) lost >5% of their initial weight, 978 (17%) lost 3–5%, whereas 2,604 (47%) had <3% weight loss (average weight loss 3.8%). Compared with those with <3% weight loss, participants with >5% weight loss had a 64% (95% CI 54–72) lower risk of developing diabetes during the first 6 years of follow-up, whereas those with 3–5% weight loss had 40% (95% CI 24–53) lower risk.
CONCLUSIONS
Moderate to small weight loss was associated with substantially reduced long-term risk of diabetes in diverse AI/AN communities. High participant attrition rates and nonoptimal postcurriculum weight loss are important challenges found in this translational effort implemented in an underserved population.
Introduction
Type 2 diabetes, a serious global epidemic, disproportionately affects disadvantaged populations. Minority groups constitute 25% of all adult patients with diabetes in the U.S. and represent the majority of children and adolescents with type 2 diabetes (1). In particular, American Indians and Alaska Natives (AI/ANs) have the highest rates of diabetes in the nation: adult prevalence was 15.1% in 2015, more than twice that of non-Hispanic whites (2). Given the daunting diabetes disparities that AI/ANs face, successful intervention strategies are urgently needed to prevent diabetes in this population. Landmark clinical trials such as the U.S. Diabetes Prevention Program (DPP) have shown that lifestyle interventions can effectively prevent or delay the onset of diabetes among those at risk (3–6). Furthermore, long-term follow-up of these randomized clinical trials has demonstrated that lifestyle intervention can yield sustained risk reduction in diabetes incidence over a long time period, even 15–20 years after the intensive phase of the intervention (7–10).
Several diabetes prevention initiatives have attempted to implement lifestyle interventions in real-world settings in order to inform practice with evidence-based methods (11–13). Most previous translational efforts were small in scope and only reported short-term intervention outcomes without examining the primary outcome of interest, diabetes incidence. There is little evidence for their long-term, sustained effectiveness. Recently, a few studies reported reduction in diabetes incidence, notably a text-messaging intervention in India (14) and the U.S. Department of Veterans Affairs (VA) MOVE! Weight Management Program (15). The first study found that, compared with those receiving standard advice, male participants in India who received regular motivational text messages had a 36% lower incidence of diabetes 2 years postbaseline. The VA MOVE! Program observed that individuals with more frequent and sustained participation exhibited a 33% reduction in diabetes risk after an average follow-up of 5 years. In both studies, the magnitude of weight loss was strongly correlated with diabetes risk reduction.
The Special Diabetes Program for Indians Diabetes Prevention (SDPI-DP) Program (16) was a congressionally mandated initiative designed to prevent diabetes among AI/ANs by implementing the DPP lifestyle intervention. It began enrolling AI/AN adults with prediabetes in January 2006 and followed the initial cohort of participants for >10 years. With a total of 8,652 enrolled AI/ANs, the SDPI-DP is one of the largest DPP translational efforts in the U.S., especially among racial/ethnic minority populations. This article reports the long-term outcomes of the SDPI-DP participants over a follow-up period up to 10 years.
Research Design and Methods
From January 2006 to July 2016, 46 AI/AN local health care programs received funding to participate in the SDPI-DP program. A diverse mix of grantees served ≥80 tribes across 18 states and 11 of the 12 Indian Health Service (IHS) administrative areas. The participating programs were required to implement the 16-session Lifestyle Balance Curriculum drawn from the DPP (5) and participate in the evaluation of the effectiveness of their prevention activities. The inclusion of a control group was deemed unethical due to strong evidence supporting the efficacy of the lifestyle intervention in preventing diabetes (3–6). Rather, the goal of SDPI-DP was to pursue a comprehensive public health evaluation of the translation of a proven intervention in diverse AI/AN communities.
Participants
SDPI-DP programs identified potential participants through community events, local clinics, or provider referral. Eligibility criteria were being AI/AN (based on eligibility to receive IHS services), being at least 18 years old, and having prediabetes. Prediabetes was defined as having either a previous diagnosis of prediabetes or a fasting blood glucose (FBG) between 100 and 125 mg/dL, a 2-h oral glucose tolerance test (OGTT) result between 140 and 199 mg/dL, or an A1C of 5.7–6.4% at baseline (in the month before starting the intervention). The definition of prediabetes and eligibility criteria changed slightly after 31 July 2009. Details of the eligibility criteria before and after that date are presented in Supplementary Fig. 1. Exclusion criteria included: 1) a previous diagnosis of diabetes, 2) current pregnancy, 3) end-stage renal disease on dialysis, and 4) any condition that could affect successful participation based on provider judgment.
Enrollment began 1 January 2006, and centralized data submission ended on 31 July 2016. The analyses here include baseline and follow-up data for up to 10 years from 8,556 participants who completed the baseline assessment and started the intervention by 31 January 2016 or completed the postcurriculum assessment by 31 July 2016. The SDPI-DP protocol was approved by the institutional review boards of the University of Colorado Denver and the national IHS. When required, grantees obtained approval from other entities overseeing research in their programs (e.g., tribal review boards). All participants provided Health Insurance Portability and Accountability Act authorization and written informed consent in accordance with local authority.
Intervention
As in the DPP lifestyle intervention arm (17), the primary goal of the intervention was to achieve and maintain a weight reduction of at least 7% of initial body weight through a healthy diet and increased physical activity. Grantees used the 16-session DPP curriculum covering diet, exercise, and behavior modification to help participants achieve this goal. Adaptation for local culture and situation was allowed provided the same basic information was presented and adaptation was documented. Many grantees drew upon their local culture to translate educational concepts and curriculum into tribal languages and incorporated, for instance, talking circles or indigenous foods into intervention sessions. The curriculum was delivered in group settings within 16–24 weeks after baseline assessment and typically taught by a local program dietitian and/or health educator. It was supplemented by monthly individual lifestyle coaching sessions that used motivational interviewing strategies to personalize goals and care plans and address barriers to participation. Upon completing the curriculum, grantees offered continued quarterly individual lifestyle coaching as well as group and community diabetes-prevention activities, guided by the DPP Lifestyle Balance after-core manual. After-core group activities focused on different behavioral/motivational topics (e.g., physical activity or healthy eating) and were often combined with community-based activities to involve families and youth. On average, each participant attended 3.1 individual lifestyle coaching sessions and 2.1 after-core group activities per year. Initially, participants were informed that the program would last 3 years; as funding was extended, the intervention continued to be offered to all participants.
Outcome Measures
At baseline, within a month of completing the last lifestyle class (usually 4–6 months after baseline, hereafter called the postcurriculum assessment) and annually after baseline for up to 10 years, participants underwent a comprehensive clinical assessment to evaluate diabetes risk and incidence. At the same time, each participant completed a questionnaire including items regarding health-related behavior (exercise and diet) and comorbidities. Participants underwent an additional glycemic measurement midway between annual assessments to assess possible diabetes conversion. The primary outcome was incidence of diabetes, diagnosed by an annual or semiannual glycemic measurement conducted in local or regional laboratories. Before 31 July 2009, an annual OGTT and a semiannual FBG test were conducted for each participant. After 31 July 2009, each site conducted an A1C, FBG, or OGTT at each assessment. An A1C ≥6.5%, an FBG ≥126 mg/dL, or a 2-h OGTT result ≥200 mg/dL after a 75-g oral glucose load required confirmation by a second test, preferably within 6 weeks of the first test, to establish the diagnosis of diabetes. If diabetes was diagnosed, the participant was informed and referred to his/her doctor for treatment, and all data collection for that participant was discontinued. If a participant was diagnosed by a provider outside of the SDPI-DP, diagnostic information was obtained, and data collection was discontinued.
Secondary outcomes included weight loss, blood pressure (BP), lipid profile, and diet. At each clinical assessment, body weight was measured with participants wearing light clothing and no shoes; BP was measured by a grantee staff member. Laboratory assays of FBG, HDL cholesterol (HDL-C), LDL cholesterol (LDL-C; often calculated), and triglycerides were conducted after 9–12 h of fasting. Height and demographic information were obtained at baseline. Diet information was acquired using a set of culturally adapted questions for self-reported frequency of eating a variety of foods (18).
Statistical Analysis
Participant characteristics were compared between subgroups using χ2 tests for categorical variables and ANOVA for continuous variables. Product-limit curves were used to examine the primary outcome of the intervention (cumulative incidence of diabetes) by participant’s weight loss status at the postcurriculum assessment. Participants were divided into three groups based on their weight change between baseline and postcurriculum assessment: 1) <3% weight loss, 2) 3–5% weight loss, and 3) >5% weight loss.
Proportional hazards (Cox) regression models were used to estimate the hazard ratio (HR) of diabetes incidence by weight loss status, after controlling for baseline demographic characteristics (age and sex) and clinical diabetes risk factors (baseline glucose status, BMI, HDL-C, and smoking status). Baseline glucose status was dichotomized as normal versus nonnormal, in which normal glucose status was defined as having an FBG <100 mg/dL, a 2-h OGTT <140 mg/dL, and/or an A1C <5.7% at baseline. (Glycemic measure requirements changed over time; all baseline assessments included at least one of those three measures.) Other clinical risk factors initially considered included systolic and diastolic BP, triglycerides, and LDL-C, which were not retained in the final model during the backward model selection process because their P values were >0.2. The proportional hazards assumption of the Cox regression models was examined by including interaction terms of time with each of the independent variables. Weight loss status did not satisfy the proportional hazards assumption. Consequently, the interaction of time with weight loss status was retained in the Cox models, and time-varying HRs were calculated and presented graphically. In an additional model, a dichotomous time variable (≤6 vs. >6 years) was used in the interaction to estimate the average HRs of weight loss groups before and after 6 years of follow-up. For secondary outcomes, pairwise comparisons are displayed graphically, and paired t tests (or sign tests for triglycerides) were used to assess the significance of the paired changes.
Sensitivity analyses were conducted with the participants recruited before and after 31 July 2009 analyzed separately. The results were very similar between these two cohorts. Therefore, the analysis results for all SDPI-DP participants are presented in this study as the main findings. All data analyses were conducted using SAS 9.4 (SAS Institute Inc., Cary, NC).
Results
Baseline Characteristics and Participant Retention
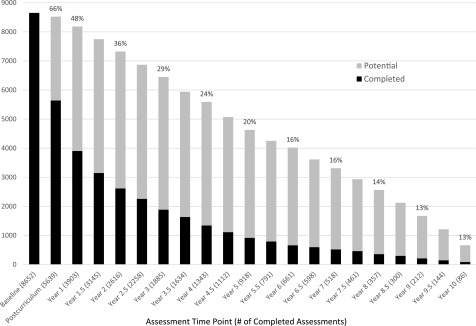
As shown in Fig. 1, 8,652 AI/AN participants met the inclusion criteria, enrolled, and finished the baseline assessment. Because of rolling enrollment, not every participant is expected to be followed the same number of years. Among the participants anticipated to complete the assessment, 66%, 48%, 16%, and 13% of them completed the postcurriculum, 1st, 6th, and 10th annual assessment, respectively. Some participants missed assessments and then returned to the program; 837 participants remained in the program for ≥6 years. When centralized data submission ended, 34% of enrolled participants were still active (defined as not formally dropped out, with attendance or assessments in the 18 months before closeout). The reasons for SDPI-DP participants becoming inactive are illustrated in Supplementary Fig. 2. Loss of contact and scheduling difficulties were the most common reasons cited; however, the reason was often listed as “other” or “unknown.”
Figure 1.
SDPI-DP assessment completion rates (percentage of potential participants completing the assessment, in which potential participants are defined as the participants who initiated the intervention early enough to reach the time point of a specific assessment by 31 July 2016).
Table 1 compares SDPI-DP participant characteristics by follow-up and postcurriculum weight loss status. About three-fourths of SDPI-DP participants with at least one postbaseline assessment (i.e., those included in subsequent analyses) were female, with a mean age of 48 years and average BMI of 35.8 at baseline. Compared with participants who did not achieve ≥3% weight loss at the postcurriculum assessment, those who lost more weight were older, more likely to be male and nonsmokers, and had higher systolic BP and lower unhealthy diet scores at baseline. On average, the participants with at least one postbaseline assessment were followed for 3.0 years (0.5–10 years) in the data reported in this study. Those who achieved more postcurriculum weight loss were more likely to attend all 16 DPP classes and completed more assessments. The average follow-up time for the three weight loss groups (<3, 3–5, and >5%) were 2.8, 3.0, and 3.2 years, respectively (P < 0.0001).
Table 1.
SDPI-DP sample characteristics by postcurriculum weight loss status
| Baseline only (N = 2,575) | Any postbaseline (N = 5,981) | P value | Weight loss at postcurriculum |
P value | |||
|---|---|---|---|---|---|---|---|
| <3% (N = 2,604) | 3–5% (N = 978) | >5% (N = 2,028) | |||||
| Baseline characteristics | |||||||
| Sex (% male) | 28 | 24 | 0.0006 | 23 | 23 | 27 | 0.0012 |
| Age (years) | 44.4 | 48.2 | <0.0001 | 47.0 | 48.3 | 50.0 | <0.0001 |
| Weight (lb) | 221.4 | 217.1 | 0.0007 | 216.9 | 216.5 | 217.4 | NS |
| BMI (kg/m2) | 36.2 | 35.8 | 0.03 | 35.9 | 35.8 | 35.6 | NS |
| Waist (inches) | 45.1 | 44.1 | <0.0001 | 44.2 | 43.9 | 44.1 | NS |
| Systolic BP (mmHg) | 126.2 | 127.0 | 0.03 | 126.3 | 127.4 | 127.7 | <0.005 |
| Diastolic BP (mmHg) | 78.4 | 78.6 | NS | 78.6 | 78.6 | 78.6 | NS |
| HDL-C (mg/dL) | 45.6 | 46.3 | 0.02 | 46.7 | 45.9 | 46.4 | NS |
| LDL-C (mg/dL) | 109.2 | 110.2 | NS | 109.9 | 110.6 | 110.5 | NS |
| Triglycerides (mg/dL) | 156.3 | 156.3 | NS | 156.3 | 159.6 | 154.7 | NS |
| Total cholesterol (mg/dL) | 181.9 | 184.9 | 0.0007 | 184.8 | 185.2 | 184.8 | NS |
| Glycemic measure in normal range (%) | 15 | 15 | NS | 16 | 15 | 14 | NS |
| Healthy diet scorea | 3.5 | 3.5 | NS | 3.5 | 3.5 | 3.5 | NS |
| Unhealthy diet scoreb | 3.0 | 2.9 | <0.0001 | 2.9 | 2.9 | 2.8 | <0.0001 |
| Nonsmoker (%) | 72 | 78 | <0.0001 | 77 | 79 | 81 | 0.02 |
| Physically active (%) | 30 | 31 | NS | 31 | 29 | 31 | NS |
| Attendance and retention | |||||||
| Completed 16 DPP classes (%) | 10 | 87 | — | 87 | 88 | 91 | <0.0001 |
| Postbaseline assessments submitted | 0 | 4.8 | — | 4.6 | 5.0 | 5.2 | <0.0001 |
| Follow-up years in program | 0.2 | 3.0 | — | 2.8 | 3.0 | 3.2 | <0.0001 |
Data are means or percentages. A total of 96 participants who started the program after 31 January 2016 with no postcurriculum assessment were excluded. A total of 342 participants did not complete the postcurriculum assessment but returned for at least one annual or midyear assessment, and 29 postcurriculum assessments were missing a weight measurement.
aHigher healthy diet score indicates healthier diet.
bLower unhealthy diet score indicates healthier diet.
Primary Outcome
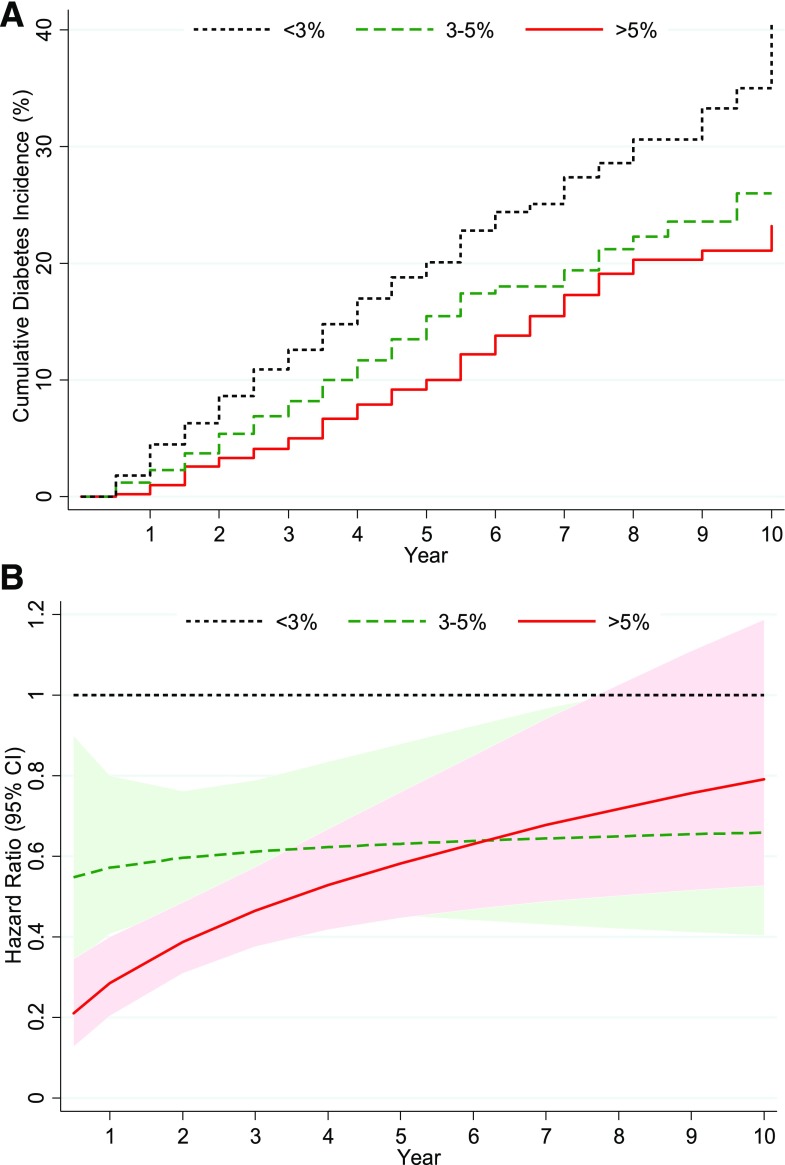
Between 1 January 2006 and 31 July 2016, a total of 625 SDPI-DP participants were diagnosed with diabetes, corresponding to a crude diabetes incidence rate of 3.5 cases/100 person-years. Among the participants with postcurriculum weight measurements, 2,028 (36%) lost >5% of their initial weight, and 978 (17%) lost 3–5% weight, whereas 2,604 (47%) did not achieve a weight loss of ≥3%. As presented in Fig. 2A, the unadjusted cumulative diabetes incidence decreased with greater postcurriculum weight loss. Cox regression models revealed that, in addition to postcurriculum weight loss, the following factors also significantly or marginally correlated with diabetes conversion: baseline age, BMI, HDL-C, glucose status, and smoking status.
Figure 2.
A: SDPI-DP cumulative incidence of diabetes by weight loss groups at postcurriculum assessment. B: Adjusted (by sex, baseline age, glucose status, BMI, HDL-C, and smoking status) HRs of weight loss groups for diabetes incidence.
The relationships of weight loss status with the hazard functions varied over time. Figure 2B and Supplementary Table 1 illustrate the time-varying HRs of diabetes incidence comparing weight loss groups after adjusting for demographic characteristics and baseline clinical diabetes risk factors. Although the adjusted HRs over 10 years for those with >5% or 3–5% weight loss were significantly lower than for those with <3% weight loss for the most part, the statistical significance declined over time (as did the sample size), and the advantage of >5% weight loss versus 3–5% weight loss also dissipated over time. On average, compared with those who did not achieve a weight loss of ≥3%, those who lost >5% of their initial weight had a 64% (95% CI 54–72; P < 0.0001) lower risk of developing diabetes during the first 6 years of follow-up, whereas those with 3–5% weight loss had a 40% (95% CI 24–53; P < 0.0001) lower risk on average. After year 6, the >5% weight loss group had a 38% (95% CI 14–56; P = 0.005) lower risk of incident diabetes than the <3% weight loss group, but its diabetes risk was not significantly different from the 3–5% weight loss group (40% lower risk than the <3% weight loss group). When weight loss was entered as a continuous variable into the multivariate Cox regression model, every additional 1% weight loss was associated with 13% reduction in diabetes risk in the first 6 years of follow-up (data not shown).
Secondary Outcomes
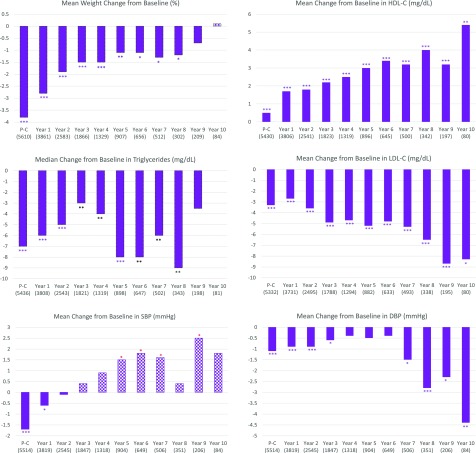
Figure 3 depicts changes from baseline in secondary outcome variables using paired data. On average, the participants who had postcurriculum weight measurements lost 3.8% of their initial weight (8.3 ± 10.6 lb). The average weight loss was attenuated to 2.8%, 1.5%, and 1.1% at years 1, 3, and 6, respectively. Yet, except for years 9 and 10, the paired weight changes at all time points were statistically significant. Overall, compared with their baseline data, the majority of participants had small but consistent improvements in triglycerides, HDL-C, and LDL-C, but not in BP. Consistent and significant improvements over baseline were also seen at most time points with respect to glucose status, smoking status, exercise levels, and dietary habits (Supplementary Fig. 3).
Figure 3.
Changes in secondary outcomes among SDPI-DP participants based on paired data. Means compared with paired t tests; medians compared with signed rank test. Numbers in parentheses in the second row of the horizontal axis are sample sizes. *P < 0.05; **P < 0.001; ***P < 0.0001. DBP, diastolic BP; P-C, postcurriculum assessment; SBP, systolic BP.
Conclusions
Ten years after the launch of the SDPI-DP, the data collected from ≥8,000 participants yielded a diabetes incidence of 3.5 cases/100 person-years among these AI/ANs with prediabetes. This is similar to the pooled rate of 3.4 cases/100 person-years (95% CI 2.2–5.6) based on eight other translational lifestyle intervention projects that reported incident diabetes (13) and is lower than the crude incidence rate of diabetes among participants with prediabetes in the Strong Heart Study (6.6 cases/100 person-years), an observational cardiovascular disease project conducted in 13 Native American communities/tribes (19). Thus, although without a concurrent control group, the evidence for the diabetes risk reduction effect of the SDPI-DP lifestyle intervention is strong. Further, our results confirmed the long-term effects of moderate weight loss achieved through an intensive lifestyle intervention in reducing the risk of type 2 diabetes among AI/ANs. Compared with those who did not attain ≥3% weight loss immediately after the intensive phase of the intervention, those who lost more weight had a substantially lower risk of developing diabetes during the follow-up period. Again, these findings are consistent with other studies showing that weight change is strongly associated with incident diabetes (20).
SDPI-DP participants also achieved small to moderate but consistent long-term improvements in most secondary outcome variables except BP. The average levels of both systolic and diastolic BP of SDPI-DP participants were in the normal range, with more than half of participants meeting the intervention goals at baseline, which may be a potential reason for the lack of improvement in BP. Overall, the participants who completed the postcurriculum assessment lost an average of 3.8% of their baseline weight immediately after the DPP curriculum. This amount of weight loss was lower than that in the lifestyle group of DPP (6.9% weight loss over the core curriculum) but comparable to the meta-analysis results of DPP translational studies (∼4% weight loss over 12 months) (11). The initial weight losses attenuated over time, consistent with observations from the DPP and several other lifestyle intervention translational projects (8,9,21,22). Although previous studies have shown successful initial weight loss is strongly associated with reduced risk of incident diabetes over relatively long follow-up periods (15,23), the DPP study found 2-year weight loss was the strongest predictor of diabetes risk (24). How much weight loss needs to be maintained over how long in order to effectively prevent type 2 diabetes remains unknown and warrants further inquiry.
Despite the encouraging results of SDPI-DP, especially among those with moderate weight loss, our findings also revealed important challenges in the widespread translation of intensive lifestyle intervention to prevent diabetes in real-world settings. First, although the SDPI-DP was successful at recruiting a large number of participants, it only reached a small proportion of potentially eligible AI/ANs who could benefit from a diabetes prevention program. For example, similar to many other lifestyle intervention projects (13,15,25,26), the majority of recruited participants (>70%) were women, indicating a critical challenge for this kind of program is to reach men. To overcome the challenges in the attempt to reach all potentially eligible participants, many grantees encouraged further expansion of local culture activities, such as drumming or powwow dancing, to be included in future recruitment efforts, which may well increase the representation of men and other hard-to-reach groups.
Second, the attrition rates of SDPI-DP participants ranged from 64% to 87% after year 1, indicating a huge challenge for participant retention. Although long-term retention was not a primary objective of the SDPI initiative, the high attrition rates pose an important limitation in our ability to soundly interpret the long-term results of the current study. Specifically, changes in secondary outcomes were based on paired data at each time point, relying upon a small and highly self-selected group for the long-term time points. Thus, the overall impact of the intervention on all participants of the program remains unknown. Similar difficulties in participant retention have been reported by several other large-scale real-world implementations of lifestyle interventions. For example, 43% of the Centers for Disease Control and Prevention’s National DPP completed 16 sessions, with most session attendance occurring in the first 6 months (26). In the Australian lifestyle intervention program Life!, 37% of 8,412 program starters completed the 8-month program (25). The Finnish National Diabetes Prevention Program acquired follow-up data from 38% of the 10,149 individuals who met initial eligibility criteria (27). The VA MOVE! Program (15) found that, compared with nonparticipants, low-intensity participants only lost 0.5–1% of their initial weight and had a 20% risk reduction for incident diabetes, whereas intensive and sustained participants (attending ≥8 sessions within 6 months) had 2–3% weight loss and 33% lower diabetes risk. Yet, in most DPP translational efforts, including the SDPI-DP, only a small fraction could be classified as intensive and sustained participants. This calls for additional research on the sustainability of the intervention when implemented in real-world settings.
Another challenge facing DPP translational projects is the relatively small percentage of participants achieving meaningful weight loss (i.e., ≥3%). As illustrated by the DPP (23) and confirmed by this study, weight loss is the dominant predictor of diabetes risk reduction. Yet, in most translational efforts, the magnitude of weight loss is lower than that realized in the evidence-establishing clinical trials (12). For instance, 81% of the DPP intensive lifestyle intervention participants lost ≥3% of their initial weight at the end of the DPP curriculum (28), whereas only 53% of SDPI-DP participants achieved such weight loss. Our previous study has shown strong socioeconomic disparities in postcurriculum weight loss among SDPI-DP participants, in which those with lower annual household income lost significantly less weight than participants with higher income. These income disparities were partially explained by difficulties in improving dietary scores in low-income participants (29). As the average socioeconomic status of the SDPI-DP participants was substantially lower than DPP participants, the smaller proportion of meaningful weight loss achieved in SDPI-DP may be partially caused by socioeconomic differences between the two cohorts. This emphasizes the practical challenges faced by many participants of lifestyle intervention with respect to adopting recommended behavioral changes in the real world. To maximize the effectiveness of lifestyle intervention in future widespread implementation, it may be important to not only target individual behavioral changes but also address the social context of the diabetes pandemic, such as improving the availability and affordability of healthy foods and other community resources for participants with disadvantaged socioeconomic status (30).
In addition to the practical challenges discussed above, the results of the current study need to be interpreted in light of several limitations. First, due to high attrition rates, our estimate of the crude diabetes incidence and conclusion based on the Cox regression model highly depends on the random censoring assumption, which cannot be verified. As shown in Table 1, participants who did not achieve ≥3% weight loss were more likely to be censored early, which means diabetes incident cases were likely to be underreported in that weight loss group, indicating diabetes incidence rate might be underestimated in this study. Meanwhile, it also implies our estimate for the association of weight loss with diabetes risk may be conservative. Second, residual confounding tempers our conclusion regarding the association between weight loss status and diabetes risk. However, we adjusted for many potential confounders in regression models, and our conclusion is consistent with previous studies (15,23). Third, the eligibility criteria, data collection methods, and diabetes diagnosis all had slight changes in the middle of SDPI-DP, which complicated the analytic strategy and interpretation of results. For example, OGTT results were not available for all participants recruited after 31 July 2009. However, sensitivity analysis exhibited no substantial differences between early and late cohorts, reducing the concern of cohort heterogeneity.
In summary, as one of the largest DPP translational efforts implemented in a racial/ethnic minority population, the SDPI-DP collected data for 10 years to demonstrate the feasibility of a lifestyle intervention for preventing diabetes in diverse AI/AN communities. Moderate to small weight loss immediately after the completion of the curriculum was associated with significantly reduced risk of incident diabetes, highlighting the importance of weight loss in diabetes prevention among AI/ANs. Although these results are encouraging, they also underscore important challenges facing the field as we move from clinical trials to real-world implementation. As have other similar efforts, this large-scale translational lifestyle intervention encountered difficulties in reaching all potentially eligible participants, retaining participants in the long-term, and achieving optimal weight loss. Future research is needed to examine the means by which to broaden the reach of the intervention, ensure long-term program engagement in diabetes risk reduction, and cost-effectively adapt the intervention to motivate more individuals to achieve weight loss goals in practical, real-world settings. Although central data submission of SDPI-DP stopped on 31 July 2016, the intervention continues to be offered to many eligible AI/AN participants, with the potential of health insurance reimbursement. As the SDPI-DP continues to be deployed in AI/AN communities, the lessons revealed by the current study will greatly inform the diffusion of this evidence-based intervention to health care systems to combat the diabetes disparities that plague AI/ANs and other underserved populations.
Supplementary Material
Article Information
Acknowledgments. The authors thank the IHS as well as tribal and urban Indian health programs and participants involved in the SDPI-DP.
Funding. Funding for the SDPI-DP project was provided by the Indian Health Service (grant HHSI242200400049C to S.M.M.). Manuscript preparation was supported in part by the National Institute of Diabetes and Digestive and Kidney Diseases (grants 1P30DK092923 to S.M.M. and R21DK108187 to L.J.). Grant programs participating in the SDPI-DP Demonstration Project: Central Oklahoma American Indian Health Council Inc., Chapa-De Indian Health Program, Inc., Cherokee Nation, Cheyenne River Sioux Tribe, Chickasaw Nation, Coeur d’Alene Tribe, Colorado River Indian Tribes, Confederated Tribes of the Colville Reservation, SDPI-DP Program Confederated Tribes of the Chehalis Reservation with Shoalwater Bay Tribe, Skokomish Indian Tribe, and Squaxin Island Tribe, Southern Oregon Tribal Diabetes Prevention Consortium with Cow Creek Band of Umpqua Tribe of Indians, Klamath Tribes, and Coquille Indian Tribe, Fond du Lac Reservation, Gila River Health Care, Kansas Consortium Diabetes Prevention Program with Haskell Indian Health Center, Kickapoo Tribe, and Prairie Band Potawatomi Nation, Ho-Chunk Nation, Indian Health Board of Minneapolis, Inc., Urban Native Diabetes Prevention Consortium with Indian Health Center of Santa Clara Valley, Native American Rehabilitation Association of the Northwest, Inc., and Hunter Health Clinic, Kenaitze Indian Tribe IRA, Ketchikan Indian Community, K’ima:w Medical Center–Hoopa, Lake County Tribal Health Consortium, Lawton IHS Service Unit/7 Tribes Consortium, Menominee Indian Tribe of Wisconsin, Mississippi Band of Choctaw Indians, Native Americans for Community Action, Inc., Norton Sound Health Corporation, Omaha Tribe of Nebraska, Oneida Nation of Wisconsin, Pine Ridge Service Unit of IHS, Middle Rio Grande Pueblo Tribal Consortium with the San Felipe Pueblo, Pueblo of Santa Ana, and Pueblo of Cochiti, Quinault Indian Nation, Rapid City IHS Diabetes Prevention Initiative, Red Lake Comprehensive Health Services, Rocky Boy Health Board, Rosebud Sioux Tribe, Seneca Nation of Indians, Sonoma County Indian Health Project, Sisseton Wahpeton Oyate Diabetes Program, Southeast Alaska Regional Health Consortium, Southcentral Foundation, Trenton Indian Service Area in consortium with Sac & Fox Tribe of the Mississippi in Iowa, Tuba City Regional Health Care Corporation, United American Indian Involvement, Inc., United Indian Health Services, Inc. in consortium with K’ima:w Medical Center (Hoopa Valley Tribe), Warm Springs Health & Wellness Center, Winnebago Tribe of Nebraska, and the Pueblo of Zuni.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. L.J. designed the study, researched the data, contributed to the discussion, and wrote, reviewed, and edited the manuscript. A.J. and K.P. researched the data, performed data analysis, and reviewed and edited the manuscript. J.B. participated in the design of the SDPI-DP project, contributed to the discussion, and reviewed and edited the manuscript. A.B. contributed to the discussion and reviewed and edited the manuscript. S.M.M. conceptualized and designed the SDPI-DP project, contributed to the discussion, and reviewed and edited the manuscript. L.J. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc17-2685/-/DC1.
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Indian Health Service.
References
- 1.U.S. Department of Health and Human Services Healthy People 2020: Improving the Health of Americans. Washington, DC, U.S. Government Printing Office, 2010 [Google Scholar]
- 2.U.S. Department of Health and Human Services, Centers for Disease Control and Prevention National Diabetes Statistics Report, 2017. Atlanta, GA, Centers for Disease Control and Prevention, 2017 [Google Scholar]
- 3.Pan XR, Li GW, Hu YH, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care 1997;20:537–544 [DOI] [PubMed] [Google Scholar]
- 4.Tuomilehto J, Lindström J, Eriksson JG, et al.; Finnish Diabetes Prevention Study Group . Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343–1350 [DOI] [PubMed] [Google Scholar]
- 5.Knowler WC, Barrett-Connor E, Fowler SE, et al.; Diabetes Prevention Program Research Group . Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Norris SL, Zhang X, Avenell A, et al. Long-term effectiveness of weight-loss interventions in adults with pre-diabetes: a review. Am J Prev Med 2005;28:126–139 [DOI] [PubMed] [Google Scholar]
- 7.Li G, Zhang P, Wang J, et al. The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20-year follow-up study. Lancet 2008;371:1783–1789 [DOI] [PubMed] [Google Scholar]
- 8.Lindström J, Ilanne-Parikka P, Peltonen M, et al.; Finnish Diabetes Prevention Study Group . Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet 2006;368:1673–1679 [DOI] [PubMed] [Google Scholar]
- 9.Knowler WC, Fowler SE, Hamman RF, et al.; Diabetes Prevention Program Research Group . 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009;374:1677–1686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diabetes Prevention Program Research Group Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: the Diabetes Prevention Program Outcomes Study. Lancet Diabetes Endocrinol 2015;3:866–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ali MK, Echouffo-Tcheugui J, Williamson DF. How effective were lifestyle interventions in real-world settings that were modeled on the Diabetes Prevention Program? Health Aff (Millwood) 2012;31:67–75 [DOI] [PubMed] [Google Scholar]
- 12.Cefalu WT, Buse JB, Tuomilehto J, et al. Update and next steps for real-world translation of interventions for type 2 diabetes prevention: reflections from a Diabetes Care Editors’ Expert Forum. Diabetes Care 2016;39:1186–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunkley AJ, Bodicoat DH, Greaves CJ, et al. Diabetes prevention in the real world: effectiveness of pragmatic lifestyle interventions for the prevention of type 2 diabetes and of the impact of adherence to guideline recommendations: a systematic review and meta-analysis. Diabetes Care 2014;37:922–933 [DOI] [PubMed] [Google Scholar]
- 14.Ramachandran A, Snehalatha C, Ram J, et al. Effectiveness of mobile phone messaging in prevention of type 2 diabetes by lifestyle modification in men in India: a prospective, parallel-group, randomised controlled trial. Lancet Diabetes Endocrinol 2013;1:191–198 [DOI] [PubMed] [Google Scholar]
- 15.Jackson SL, Long Q, Rhee MK, et al. Weight loss and incidence of diabetes with the Veterans Health Administration MOVE! lifestyle change programme: an observational study. Lancet Diabetes Endocrinol 2015;3:173–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang L, Manson SM, Beals J, et al.; Special Diabetes Program for Indians Diabetes Prevention Demonstration Project . Translating the Diabetes Prevention Program into American Indian and Alaska Native communities: results from the Special Diabetes Program for Indians Diabetes Prevention demonstration project. Diabetes Care 2013;36:2027–2034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diabetes Prevention Program (DPP) Research Group The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care 2002;25:2165–2171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teufel-Shone NI, Jiang L, Beals J, et al. Demographic characteristics and food choices of participants in the Special Diabetes Program for American Indians Diabetes Prevention Demonstration Project. Ethn Health 2015;20:327–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang H, Shara NM, Calhoun D, Umans JG, Lee ET, Howard BV. Incidence rates and predictors of diabetes in those with prediabetes: the Strong Heart Study. Diabetes Metab Res Rev 2010;26:378–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Delahanty LM. Weight loss in the prevention and treatment of diabetes. Prev Med 2017;104:120–123 [DOI] [PubMed] [Google Scholar]
- 21.Sepah SC, Jiang L, Ellis RJ, McDermott K, Peters AL. Engagement and outcomes in a digital Diabetes Prevention Program: 3-year update. BMJ Open Diabetes Res Care 2017;5:e000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gilis-Januszewska A, Lindström J, Tuomilehto J, et al. Sustained diabetes risk reduction after real life and primary health care setting implementation of the diabetes in Europe prevention using lifestyle, physical activity and nutritional intervention (DE-PLAN) project. BMC Public Health 2017;17:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamman RF, Wing RR, Edelstein SL, et al. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care 2006;29:2102–2107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Delahanty LM, Pan Q, Jablonski KA, et al.; Diabetes Prevention Program Research Group . Effects of weight loss, weight cycling, and weight loss maintenance on diabetes incidence and change in cardiometabolic traits in the Diabetes Prevention Program. Diabetes Care 2014;37:2738–2745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dunbar JA, Jayawardena A, Johnson G, et al. Scaling up diabetes prevention in Victoria, Australia: policy development, implementation, and evaluation. Diabetes Care 2014;37:934–942 [DOI] [PubMed] [Google Scholar]
- 26.Ely EK, Gruss SM, Luman ET, et al. A national effort to prevent type 2 diabetes: participant-level evaluation of CDC’s National Diabetes Prevention Program. Diabetes Care 2017;40:1331–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saaristo T, Moilanen L, Korpi-Hyövälti E, et al. Lifestyle intervention for prevention of type 2 diabetes in primary health care: one-year follow-up of the Finnish National Diabetes Prevention Program (FIN-D2D). Diabetes Care 2010;33:2146–2151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perreault L, Ma Y, Dagogo-Jack S, et al.; Diabetes Prevention Program . Sex differences in diabetes risk and the effect of intensive lifestyle modification in the Diabetes Prevention Program. Diabetes Care 2008;31:1416–1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang L, Huang H, Johnson A, et al.; Special Diabetes Program for Indians Diabetes Prevention Demonstration Project . Socioeconomic disparities in weight and behavioral outcomes among American Indian and Alaska Native participants of a translational lifestyle intervention project. Diabetes Care 2015;38:2090–2099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spencer Bonilla G, Rodriguez-Gutierrez R, Montori VM. What we don’t talk about when we talk about preventing type 2 diabetes—addressing socioeconomic disadvantage. JAMA Intern Med 2016;176:1053–1054 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.